Abstract
The lipid phase of the photoreceptor outer segment membrane is essential to the photon capturing and signaling functions of rhodopsin. Rearrangement of phospholipids in the bilayer accompanies the formation of the active intermediates of rhodopsin following photon absorption. Furthermore, evidence for the formation of a condensation product between the photolyzed chromophore all-trans-retinal and phosphatidylethanolamine indicates that phospholipid may also participate in the movement of the retinoid in the membrane. The downside of these interactions is the formation of bisretinoid-phosphatidylethanolamine compounds that accumulate in retinal pigment epithelial cells with age and that are particularly abundant in some retinal disorders. The propensity of these compounds to negatively impact on the cells has been linked to the pathogenesis of some retinal disorders including juvenile onset recessive Stargardt disease and age-related macular degeneration.
Keywords: bisretinoids, photooxidation, fluorescence, lipofuscin, retina, light, visible spectrum, absorbance, all-trans-retinal dimer, retinal pigment epithelium
PHOSPHOLIPID ASSOCIATIONS WITH RHODOPSIN
Throughout the outer segment compartment of the photoreceptor cell, visual pigments (rhodopsin in rods and cone pigments) are densely packed within the plasma membrane and disc membranes; low levels of rhodopsin are also present in the plasma membrane of the rod inner segments (1). The lipid phase of the disc membrane is dominated by phospholipids (87 mol % of total lipid in human outer segment membrane) with the major species being phosphatidylcholine (PC) (32.5 mol %), phosphatidylethanolamine (PE) (37.6 mol %), and phosphatidylserine (PS) (12.1 mol %) (2). A remarkable feature of the phospholipids of the outer segment disc membrane is their unusually high content of long-chain polyunsaturated (e.g., 22:6n-3) fatty acids; in human outer segments, 22:6n-3 accounts for 34.2% of the fatty acid in PE, 19.5% of the fatty acid in PC and 34.1% of the fatty acid in PC (2). Indeed, docosahexaenoic acid (DHA) is more abundant in photoreceptor outer segments that in any other mammalian cell membrane (2). These unsaturated acyl chains provide the high fluidity necessary for proper signal transduction in the disc membrane.
Following absorption of a photon, the 11-cis-retinal chromophore of visual pigment rhodopsin isomerizes to all-trans-retinal and initiates a series of conformational rearrangements leading to formation of metarhodopsin II, a 380-nm absorbing species that consists of all-trans-retinal bound to opsin by a deprotonated Schiff base linkage (Fig. 1). Metarhodopsin II is distinguished by its ability to activate the G protein transducin (3). This activation initiates the phototransduction cascade leading to hyperpolarization of the photoreceptor cell and altered neurotransmitter release. Movement of phospholipid species during the rhodopsin to metarhodopsin II transition has been reported by multiple groups (4, 5). For instance, this transition is marked by a redistribution of PS to the membrane leaflet facing the cytoplasm and for each molecule of rhodopsin, two PC and two PE molecules form associations that render them less extractable. The phospholipids of the outer segment are also essential for the formation of a stable complex between photoactivated rhodopsin and the G-protein transducin (6).
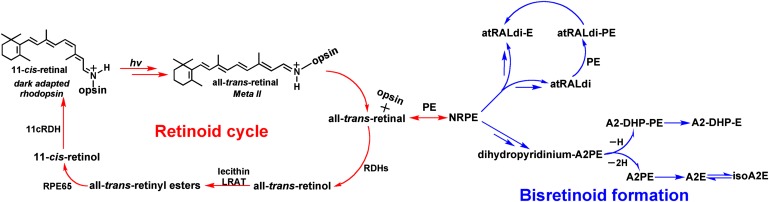
Fig. 1.
Retinoid cycling in the eye. The visual chromophore 11-cis-retinal forms a covalent Schiff base bond with lysine 296 (Lys 296) of opsin. Vision is initiated when a photon (hv) is captured by 11-cis-retinal; as a result, the chromophore is isomerized to all-trans-retinal. With all-trans-retinal still covalently bound to opsin, the activated pigment transitions to the metarhodopsin II conformation, the Schiff base is hydrolyzed, and all-trans-retinal is reduced to all-trans-retinol by retinol dehydrogenases (RDHs). Alternatively some all-trans-retinal reacts with phosphatidylethanolamine (PE) in the lipid bilayer to form N-retinylidene-PE, which is transported by ABCA4 and then hydrolyzes to release PE and all-trans-retinal. The latter is subsequently reduced to all-trans-retinol. Within the retinal pigment epithelium (RPE) cell, all-trans-retinol is esterified by the enzyme lecithin retinol acyl transferase (LRAT) and is isomerized from the all-trans configuration to the 11-cis-retinol by RPE65. The alcohol is then oxidized by 11-cis retinol dehydrogenase (11cRDH) to 11-cis-retinal. The bisretinoid pathway is initiated when N-retinylidene-PE, rather than hydrolyzing to all-trans-retinal and PE, reacts with a second molecule of all-trans-retinal. A multi-step pathway leads to formation of the intermediate dihydropyridinium-A2PE. Automatic oxidation of dihydropyridinium-A2PE with loss of two hydrogens (−2H) generates A2PE, the immediate precursor of A2E. Loss of one hydrogen (−H) generates A2-dihydropyridine-PE (A2-DHP-PE); phosphate hydrolysis of the latter produces A2-DHP-E. Via an alternative path, all-trans-retinal dimer forms from the condensation of two all-trans-retinal. Reaction all-trans-retinal dimer with PE with formation of a protonated Schiff base linkage generates all-trans-retinal dimer-PE (atRALdi-PE), and phosphate hydrolysis of the latter yields all-trans-retinal dimer-ethanolamine (atRALdi-E).
ALL-TRANS-RETINAL AND PHOSPHOLIPID
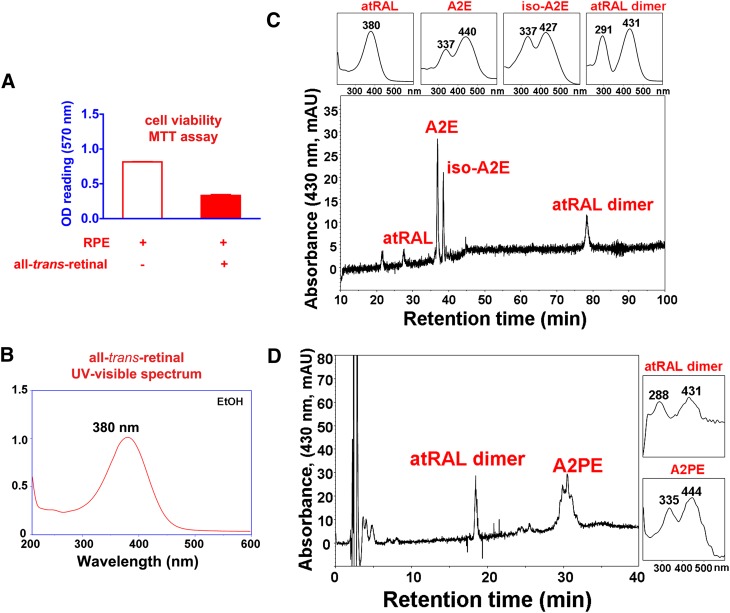
As noted above, photoisomerization of 11-cis-retinal leads to the generation of all-trans-retinal (Fig. 1). As an aldehyde-bearing agent that can permeabilize and kill cells (Fig. 2A) (7), all-trans-retinal necessitates careful handling by the photoreceptor cell. Accordingly, it has been shown that in addition to the well-known internal protein pocket (site I) wherein 11-cis-retinal is bound covalently via a Schiff base linkage, opsin contains two additional hydrophobic cavities housing retinoid, one that serves as entrance site for 11-cis-retinal and the other that is occupied by all-trans-retinal after its release from site I upon deactivation of metarhodopsin II (8). The implication of this work is that, as opposed to being released freely into the lipid phase of the photoreceptor membrane, all-trans-retinal remains bound to opsin while being reduced to all-trans-retinol by NADPH-dependent retinol dehydrogenases (RDHs) (8, 9) (Fig. 1). An aldehyde can react with a primary amine to form an adduct via a Schiff base linkage (C = C−N); accordingly, all-trans-retinal, upon release from photoactivated rhodopsin, has been shown to react with PE in the disc membrane to form the Schiff base adduct, N-retinylidene-PE (10). Thus, both the opsin protein and PE appear to chaperone all-trans-retinal, thereby preventing the toxicity that would otherwise be associated with free aldehyde. As will be discussed in the next section, N-retinylidene-PE is recognized as the ligand that binds the photoreceptor-specific ATP-binding cassette transporter (ABCA4/ABCR) in outer segments (11–14).
Fig. 2.
All-trans-retinal is generated within photoreceptor cell outer segments upon visual pigment photoisomerization. A: Free all-trans-retinal bears a reactive aldehyde that is toxic to cells. All-trans-retinal (30 microM) was incubated with ARPE-19 cell at 37°C for 16 h after which the cells were incubated with fresh media for 3 days. Cell viability was measured by MTT assay as described in ref. 95. B: Free all-trans-retinal has an absorbance maxima at 380 nm. C, D: In the presence of lipid bilayers, all-trans-retinal reacts to form several bisretinoid compounds including A2E, isoA2E, all-trans-retinal dimer, and A2PE. All-trans-retinal was incubated with ARPE-19 cells as in A; cells were extracted in chloroform/methanol and analyzed on reverse phase C18 and C8 columns as described in ref. 33.
THE SCHIFF BASE ADDUCT OF PHOSPHATIDYLETHANOLAMINE AND ALL-TRANS-RETINAL IS THE SUBSTRATE FOR ABCA4
The translocation of lipid across cell membranes is essential to cell structure and function. One well known family of lipid transporters is the ABCA4 subgroup of the ABC transporters. A number of these transporters have been identified in association with genetic disorders (15). ABCA4/ABCR (11–14), in particular, is primarily expressed in photoreceptor cells of retina and is the gene that is defective in recessive Stargardt disease, a form of macular dystrophy with usual onset in the second decade of life (16). More severe mutations in ABCA4 are also responsible for some cases of cone-rod dystrophy and a subset of cases of autosomal recessive RP (17–19).
Measurements of the ATPase activity of ABC4 in lipid vesicles revealed that the most pronounced activity occurred in the presence of all-trans-retinal and within PE-enriched vesicles, thus identifying the ligand as N-retinylidene-PE, the condensation product formed from PE and all-trans-retinal (13, 14, 20–23). Recent experiments indicate that the Schiff base linkage of N-retinylidene-PE is unprotonated when transported by ABCA4 (15). It is generally considered that the function of ABCA4 is to transport or flip N-retinylidene-PE across the lipid bilayer from the interior of the disk to the cytoplasmic face of the disc membrane. With exposure to water in the cytosol, N-retinylidene-PE is expected to undergo hydrolysis to all-trans-retinal and PE (Fig. 1) and the terminal aldehyde of all-trans-retinal is subsequently reduced to the less reactive alcohol (all-trans-retinol) by NADPH-dependent RDHs such as RDH8, RDH11, and RDH12 (24, 25). However, only a portion of all-trans-retinal that is generated by photoisomerization of 11-cis-retinal is handled via the ABCA4 pathway; it is estimated that this fraction could be 30% (9).
Although ATP-dependent transport of N-retinylidene-PE across the membrane has not yet been demonstrated (26), evidence for this role is provided by studies of Abca4 knock-out mice wherein elevated levels of N-retinylidene-PE are observed in retina (27, 28). PE is also increased in these mutant mice, although, as compared with wild-type mice, there is little difference in all-trans-retinal levels in the retinas of light exposed Abca4 null mutant mice (29).
N-RETINYLIDENE-PE IS AN INTERMEDIATE IN THE BISRETINOID BIOSYNTHETIC PATHWAY
Under some circumstances, not all of which are fully understood, N-retinylidene-PE reacts with a second molecule of all-trans-retinal instead of hydrolyzing to PE and all-trans-retinal; one condition under which this occurs is reduced or absent ABCA4 activity. This second condensation reaction initiates a nonenzymatic synthetic pathway that leads to the formation of fluorescent di-retinal compounds within the lipid bilayers of the photoreceptor outer segment. These compounds include the phosphatidyl-pyridinium bisretinoid, A2PE, the phosphatidyl-dihydropyridine bisretinoid, A2-DHP-PE, and both all-trans-retinal dimer and the related PE conjugate, all-trans-retinal dimer-phosphatidylethanolamine (30–34). It is probably, at least in part, to prevent the build up of these chromophores that the outer segment undergoes constant turnover with the membrane that is shed being cleared by retinal pigment epithelial (RPE) cell phagocytosis (35). As a result, the bisretinoid chromophores that form in the outer segment are deposited in the adjacent RPE cell where they accumulate (36). In addition to elevated levels of N-retinylidene-PE, mice deficient in Abca4 exhibit an abundant accumulation of the bisretinoids that constitute the lipofuscin of RPE cells (28, 29, 33, 37).
RPE lipofuscin bisretinoids originate in photoreceptors cells and are deposited secondarily in RPE
Although the bisretinoids of RPE lipofuscin (Fig. 3) are particularly abundant in ABCA4-associated retinal dystrophy, these pigments also accumulate in the RPE of healthy eyes, albeit at lower levels (37, 38). Indications that RPE lipofuscin formation occurs in photoreceptor outer segments were first provided by studies of a blind strain of rat (Royal College of Surgeon rat, RCS) in which RPE cells are unable to phagocytose shed outer segment discs; under these conditions, RPE is devoid of lipofuscin (39, 40). Lipofuscin was also found to be diminished when photoreceptor cells were caused to degenerate (41).
Early investigators also considered the possibility that lipofuscin fluorophores of RPE cells might form within the acidic environment of the lysosome. However, an origin from photoreceptor cells is indicated by the detection of RPE lipofuscin bisretinoids in photoreceptor outer segments (Fig. 4). Moreover, all-trans-retinal, the precursor essential to the formation of these bisretinoids, is not available in RPE cells. Specifically, the flow of retinoid is such that all-trans-retinal is reduced to all-trans-retinol before transfer of retinoid from photoreceptor cells to RPE (9, 24, 42). In addition, all-trans-retinal generated by retinol dehydrogenase activity in RPE is complexed to RGR (retinal G protein-coupled receptor) and is of low abundance (43).
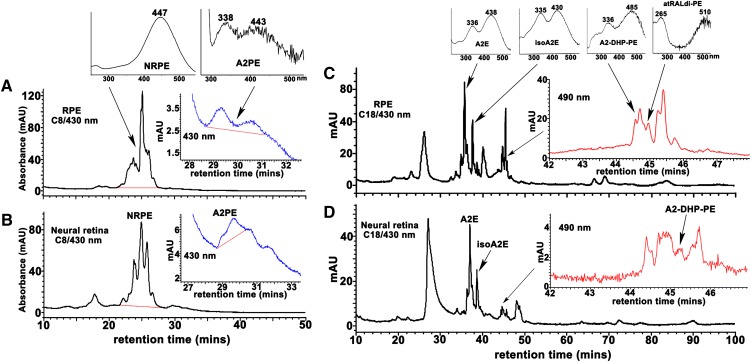
Fig. 4.
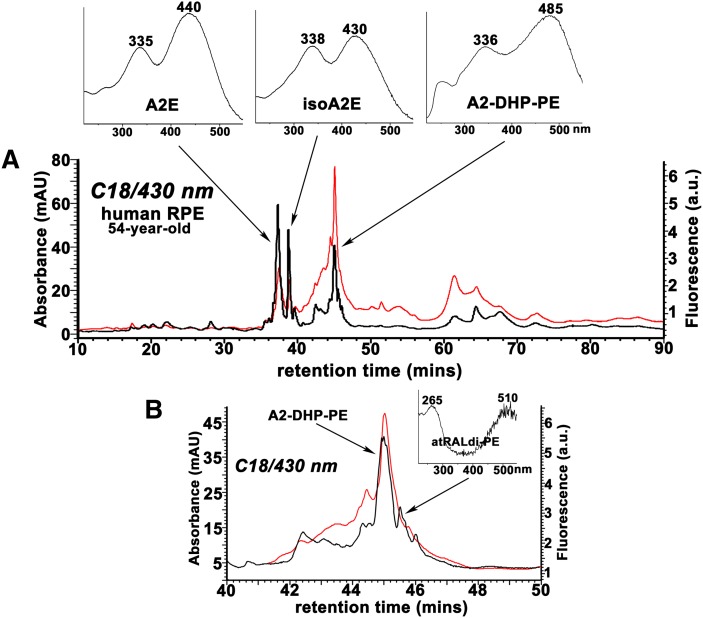
Detection of bisretinoids in RPE and neural retina from bovine eyes. Bovine RPE/choroid (A, C) and neural retina (B, D) were extracted with chloroform/methanol and analyzed by reverse-phase HPLC with C8 (A, B) and C18 (C, D) columns and monitoring at 430 and 490 nm. Tissue extracts pooled and concentrated from 15 eyes for RPE analysis and 20 eyes for neural retina analysis. Top insets in A and C: UV-visible absorbance spectra of the indicated bisretinoids. Insets on the right in A–D: Chromatograms expanded between retention times indicated. C: The shorter wavelength absorbance of atRAL dimer-PE presents as ∼265 nm; a strong absorbance in this region from unsaturated fatty acids (such as DHA) in the phosphatidic acid moiety can sometimes mask the 290 nm absorbance generated from the shorter of the two retinoid-derived side-arms of the molecule.
Evidence for the involvement of the visual cycle as the major source of RPE lipofuscin originated with experiments documenting that the deposition of lipofuscin granules and the presence of lipofuscin autofluorescence is dependent on dietary vitamin A (44, 45). Additionally, when the 11-cis- and all-trans-retinal chromophores of visual pigment are not generated, as in Rpe65−/− mice, RPE lipofuscin measured as fluorescence intensity is severely diminished (46) and the many lipofuscin chromophores absorbing in the visible range of the spectrum are absent (34, 47). Similarly, in patients with early-onset retinal dystrophy associated with mutations in RPE65, the absence of fundus autofluorescence (48) reflects RPE cells that are devoid of lipofuscin.
The lipofuscin of RPE is amassed within organelles of the lysosomal compartment of the cells; because of their ultrastructural appearance, these bodies are known as lipofuscin granules (49–52). Other macromolecules phagocytosed by the RPE are degraded by lysosomal enzymes to small molecules that leave the lysosome assisted by permeases (53). However, after an initial phosphate cleavage that occurs to varying degrees (discussed below), the bisretinoid compounds deposited in RPE appear to largely defy further hydrolytic degradation. The failure to degrade is evident from the propensity of these bisretinoids to accumulate. Due to the complex structures of these bisretinoid compounds, it is conceivable that the lysosomal enzymes of the RPE cell do not recognize them.
It has been assumed that the lipofuscin material in RPE includes partially degraded or oxidized protein, but in a recent proteomic study of purified lipofuscin granules, amino acid analyses revealed very little protein (∼2%). On the other hand, several previously identified bisretinoid compounds (discussed below) were detected that likely accounted for the phototoxicity measured in the isolated granules (54). Also present were carboxyethyl pyrrole protein (CEP)-adducts, modifications that are generated from the oxidation of docosahexaenoate- containing lipids in photoreceptor cells. Presumably, the photooxidative process responsible for generating these products of lipid oxidation could occur in the photoreceptor cells or within the lipofuscin granule or both.
A2E, isomers, and precursors.
A prominent component of RPE lipofuscin is the di-retinal conjugate A2E (55) (Figs. 1, 3). The polar head of A2E consists of an aromatic ring carrying a permanent positive charge conferred by a quaternary amine nitrogen. The charge on the pyridinium nitrogen is neutralized by a counter ion, probably chloride. Two side-arms extend from the ring, a long arm and a short. In this unprecedented structure, each arm is derived from a molecule of all-trans-retinal (55). The alternating double and single bonds that extend the length of the long arm and into the pyridinium and ionone rings of A2E provide the extended conjugation system that imparts absorbance at wavelengths in the visible range of the spectrum (∼440 nm) (Fig. 3). The absorbance at ∼335 nm is generated within the short arm.
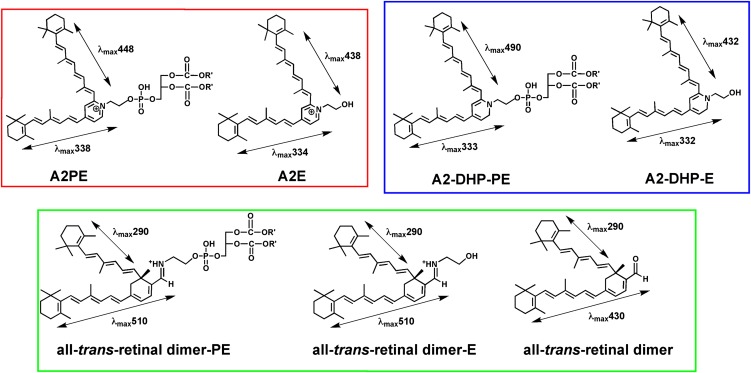
Fig. 3.
Structures and absorbance maxima (λmax) of the fluorophores that constitute the bisretinoids of retina lipofuscin. Phosphate cleavage of A2PE, A2-DHP-PE, and all-trans-retinal dimer-PE releases A2E, A2-DHP-E, and all-trans-retinal dimer-E, respectively. Absorbance maxima of these bischromophores can be assigned to the shorter and longer side-arms of the molecules. For all-trans-retinal dimer-PE and all-trans-retinal dimer-E, the absorbance generated from the long-arm exhibits a bathochromic shift (red-shift) due to protonation of the imine functional group (— CH = N —).
We have proposed that the biosynthesis of A2E (30, 31, 38) begins in photoreceptor outer segments with condensation reactions between PE and all-trans-retinal (Fig. 1) that generate N-retinylidene-PE, the Schiff base conjugate that is the substrate of ABCA4. Within the eye, N-retinylidene-PE is generally found to be protonated. N-retinylidene-PE likely then undergoes a [1,6]proton tautomerization generating the phosphatidyl analog of enamine. After reaction with a second molecule of all-trans-retinal, we suggested that an iminium salt would form and, following electrocyclization, a phosphatidyl dihydropyridinium molecule (dihydro-pyridinium-A2PE) (56). Subsequent automatic oxidation under a drive to aromaticity (57) would eliminate two hydrogens to yield A2PE (Fig. 1 and 3), a phosphatidyl pyridinium bisretinoid that is the immediate precursor of A2E. Cleavage of A2PE to generate A2E occurs in RPE cell lysosomes and is likely mediated by the enzymatic activity of phospholipase D (PLD) (31, 58) (discussed below).
Analysis of A2PE, the immediate precursor of A2E, has shown that this bisretinoid originates in photoreceptor outer segments. For example, the formation of [14C2]A2PE was measured in outer segments isolated from excised whole retinas submitted to [14C2]ethanolamine incorporation and irradiation to release endogenous all-trans-retinal. A2PE also formed in outer segments incubated with exogenous all-trans-retinal (30, 31). Indeed, it can be readily demonstrated that exposure of cell membranes to exogenous all-trans-retinal results in the facile production of A2PE and other bisretinoids such as all-trans-retinal dimer, which will be discussed below (Fig. 2C, D). In addition, bisretinoid precursors have been identified in outer segments isolated from Abca4−/− mice (29), and mass spectrometric analysis verified that bisretinoid compounds account for at least some of the autofluorescent pigments that accumulate in the orange-colored degenerating photoreceptor outer segment debris in RCS rats (31, 40), the strain having an inability to phagocytose shed outer segment membrane.
The double bonds along the side-arms of A2E are all in the trans (E) position, a lower energy configuration (Fig. 3). However, photoisomerization of A2E generates several cis-isomers, the most abundant of which is iso-A2E, wherein the double bond at the C13-14 position assumes the cis (Z) configuration (38). A2E and isoA2E are present in the eye in approximately a 4:1 ratio. Other cis-isomers having Z-olefins at the C9/9’-10/10’ and C11/11’-12/12’ positions, are also detected in eye extracts (31). For all of these photoisomers of A2E, absorbance spectra are slightly blue shifted relative to A2E (e.g., A2E: λmax 334, 438; iso-A2E: λmax 337, 428). A2E and its isomers have been detected in isolated human RPE (38) and in eyecups harvested from mice (33, 34, 37). The levels of A2E and corresponding isomers are increased at least 5-fold in the Abca4 null mutant mouse, a model of recessive Stargardt macular degeneration (27–29, 37, 59).
A2-DHP-PE.
We have recently shown that oxidation of dihydropyridinium-A2PE, the intermediate discussed above, can lead to a second pathway (Fig. 1). Here hydrogen transfer and one hydrogen elimination leads to the formation of an uncharged dihydropyridine compound that we refer to as A2-dihydropyridine-phosphatidylethanolamine (A2-DHP-PE) (Fig. 3), to indicate both its structure and its formation from two vitamin A-aldehyde (A2) (34). That the core of this compound is a dihydropyridine ring was confirmed by high performance liquid chromatography-electrospray ionization-tandem mass spectrometry with corroboration by Fourier transform infrared spectroscopy and modeling using density functional theory.
The stability of this lipofuscin bisretinoid is indicated by its detection in mouse eyecups, in human and bovine retina (Fig. 4), and by studies demonstrating that A2-DHP-PE accumulates with age (34). In human RPE, A2-DHP-PE was observed at levels that were similar to A2E; however, in Abca4−/− mice, the content of A2E was greater than that of A2-DHP-PE. This finding could be explained by either accelerated formation of A2E versus A2-DHP-PE in Abca4−/− mice or greater loss of A2-DHP-PE such as could occur due to photooxidation (discussed below).
As with the other bisretinoid compounds, A2-DHP-PE presents with two side-arms and has two absorbance maxima (λmax 490 and 333 nm) (Fig. 3). The conjugation system present within the long arm of A2-DHP-PE extends into the dihydropyridine ring, thereby allowing for a system with six double bonds. The short arm of A2-DHP-PE also extends into the dihydropyridine ring giving five conjugated double bonds. With this configuration, the 490 nm absorbance can be assigned to the long arm of A2-DHP-PE and the 333 nm absorbance to the short arm (34) (Fig. 3).
The all-trans-retinal dimer series of lipofuscin fluorophores.
Although A2E absorbs in the visible spectrum at about 440 nm, the ‘blue’ region, at least two bisretinoids in RPE lipofuscin have ∼510 nm absorbance (Fig. 3). One of these, the pigment all-trans-retinal dimer-phosphatidylethanolamine (all-trans-retinal dimer-PE; λmax ∼290, 510 nm) is produced when two molecules of all-trans-retinal condense to form an aldehyde-bearing dimer (all-trans-retinal dimer) (Figs. 1, 3) that can proceed to form a conjugate with PE via a Schiff base linkage that exhibits pH-dependent protonation (32, 33) (Figs. 1, 3). The second ∼510 nm absorbing species, all-trans-retinal dimer-ethanolmine (all-trans-retinal dimer-E) (Figs. 1, 3) can be generated subsequently by phosphate cleavage of all-trans-retinal dimer-PE. Moreover, with deprotonation and Schiff base hydrolysis of all-trans-retinal dimer-PE and -E, unconjugated all-trans-retinal dimer can reform. As with the other lipofuscin pigments, the all-trans-retinal dimer series of compounds are housed in lysosomes (pH ∼5). We detected the protonated (all-trans-retinal dimer-PE and -E) and unprotonated unconjugated (all-trans-retinal dimer) forms of this group of compounds in extracts of RPE lipofuscin from humans and mice (32, 33). Thus, conditions in the lysosome appear to support both protonated and unprotonated forms with the relative levels of these three pigments probably being pH dependent.
The pigments all-trans-retinal dimer-PE and all-trans-retinal dimer-E are composed of long (seven double-bond conjugations) and short (four conjugations) polyene arms extending from a cyclohexadiene ring (Fig. 3) (60). The relatively long wavelength absorbance of all-trans-retinal dimer-PE and all-trans-retinal dimer-E in the visible spectrum (λmax ∼510 nm) is attributable to protonation of the Schiff base linkage in these compounds. Conversely, the absorbance spectrum of unconjugated all-trans-retinal dimer exhibits maxima at ∼290 and 430 nm. In the lipofuscin-filled RPE of Abca4 null mutant mice, all-trans-retinal dimer-PE and all-trans-retinal dimer-E are present at elevated levels (33).
Photooxidized forms of bisretinoid pigments.
The complex mixture of lipofuscin pigments in the RPE includes several photooxidized species of A2E and all-trans-retinal dimer (33, 61) that form by established mechanisms. In particular, A2E, when irradiated at an excitation maximum in the blue region of the spectrum, serves as a photosensitizer generating various reactive forms of oxygen with singlet oxygen adding to A2E at carbon-carbon double bonds along the side-arms of the molecule (61–65). Indeed, the photooxidation of A2E can involve the incorporation of as many as nine oxygens into the retinoid-derived side-arms of A2E resulting in multiple oxygen-containing moieties situated within an A2E molecule. The reactive species generated within photooxidized A2E include endoperoxides, epoxides, and furanoid moieties that have been identified in hydrophobic extracts of human RPE and Abca4−/− mouse eyecups and that likely account for the adverse effects of A2E photoreactivity (61). Oxidized all-trans-retinal dimer is readily detectable in mouse eyecups. Indeed, in both wild-type and Abca4−/− mice, the levels of oxidized all-trans-retinal dimer exceed the amounts of all-trans- retinal dimer. Moreover, as compared with wild-type, oxidized all-trans-retinal dimer is almost 3-fold higher in the Abca4−/− mice.
Oxidized forms of A2E and all-trans-retinal dimer exhibit hypsochromic absorbance shifts reflecting oxidation-associated loss of double bond conjugations. For every double bond lost, a shift of ∼ 30 nm results. The oxidation of these compounds also confers increased polarity. Accordingly, mono- and bis-peroxy-A2E, mono- and bis-furano-A2E, mono- and bis-peroxy- all-trans-retinal dimer, and mono-and bis-furano- all-trans-retinal dimer can be detected in extracts from human and mouse eyes (33, 61).
PHOSPHATIDYL-BISRETINOID SPECIES DEMONSTRATE DIFFERENT RESISTANCES TO HYDROLYTIC CLEAVAGE
Because shedding of outer segment membrane with deposition in RPE cells leads to complete replacement of the outer segment every 10–14 days (66), the bisretinoids that form in photoreceptor cells are not continually amassed in outer segments; instead, these pigments are transferred to RPE cells within the phagocytosed outer segment material.
We have cumulated evidence that once the pigment A2PE is deposited in RPE cells together with the phagocytosed outer segment membrane, the bisretinoid undergoes phosphate hydrolysis within RPE lysosomes to generate A2E (30, 31, 58). For instance, although acid hydrolysis of A2PE may occur at a slow rate (30, 31), the lysosomal enzyme PLD can readily generate A2E from A2PE. The appropriate hydrolytic activity is also present in lysosomal fractions from RPE and liver cells and the lysosomal activity that is able to release A2E from A2PE is efficiently suppressed by the PLD inhibitor calphostin C and by a protease inhibitor cocktail (58). Hydrolytic cleavage of A2PE appears to be quite facile because A2E is always a substantial peak in RPE extracts and A2PE is present at relatively low levels (Fig. 4). For instance, in a sample of pooled RPE from four bovine eyes, A2E and A2PE levels were found to be 279.8 and 23.1 pmole/eye, respectively (J. Sparrow and Y. Wu, unpublished observations).
A2-DHP-PE also serves as a substrate for PLD-mediated cleavage, the resulting phosphate hydrolysis releasing A2-DHP-E (Figs. 1, 3) and presumably phosphatidic acid (34). Cleavage of A2-DHP-PE can take place in the eye because we also detected the product A2-DHP-E in mouse eyecups, albeit at low levels. Moreover, the cleavage presumably occurs within RPE, as A2-DHP-E was not detected in concentrated extracts of pooled bovine neural retina (data not shown) whereas A2-DHP-PE was (Fig. 4). Conversely, the relative abundance of A2-DHP-PE in mouse eyecups and human and bovine RPE indicates that A2-DHP-PE is more refractory to cleavage. This is also the case for all-trans- retinal dimer-PE (33); PLD-mediated cleavage of all-trans-retinal dimer-PE was found to be less efficient than PLD-mediated hydrolysis of A2PE (33). The reasons for these differences are not known but could reflect the ease with which the enzyme gains access to the phosphate moiety.
PROPERTIES OF LIPOFUSCIN BISRETINOIDS
Amphiphilic properties
Properties of A2E that may be injurious to the RPE cell include an ability to destabilize cell membranes (67–70). An amphiphilic structure conferred by a hydrophilic head group and a pair of hydrophobic side-arms accounts for this behavior (Fig. 3). Accordingly, A2E has a tendency to aggregate (55, 71). This behavior was initially predicted from the broadening of the 1H-NMR signal with A2E in deuterated chloroform (CDCl3); this observation indicated that the protonated pyridinium moieties of A2E were packed within the interior of micelles whereas the hydrophobic chains extended within the solvent. When confronted with a membrane bilayer, A2E, because of its cationic head group, may distribute preferentially in the inner leaflet of the membrane due to an attraction to negatively charged PS that is concentrated on the cytoplasmic side of the bilayer (72). Fluorescence anisotropy studies (71) indicate that electrostatic attractions between A2E and PS may operate despite the presence of a counterion. Within the cytoplasmic leaflet of the membrane, wedge-shaped A2E would orient with its polar pyridinium moiety interacting with the polar heads of the phospholipids and its broadly-spaced hydrophobic side-arms intermingling with the phospholipid hydrocarbon tails (67). Accordingly, the bulky side-arms of A2E could force a large separation of the lipid acyl chains and expand the inner leaflet relative to the outer, the negative curvature of the inner leaflet producing a surface protrusion or bleb. Thus, it is not surprising that A2E has been shown to provoke membrane blebbing (69). Further evidence of the detergent-like behavior of A2E has been revealed in experiments demonstrating the ability of A2E to induce concentration-dependent membrane leakage (67). Work with unilamellar vesicles has also demonstrated that A2E at critical micellar concentrations can solubilize membranes (71). Because A2E is housed within lysosomal organelles of the cell, A2E at sufficient concentration may exert similar effects on the lysosomal membrane. Accordingly, the ability of A2E to act like a detergent may explain other A2E-mediated effects that have been observed such as the detachment of pro-apoptotic proteins from mitochondria (73), inhibition of the lysosomal proton pump (74), alkalinization of lysosomes (75), and reduced degradative function (76). A direct effect of A2E on activities of lysosomal enzymes is not observed (77).
Protonation/deprotonation that is pH dependent
Efforts to explain the tendency of A2E to accumulate in lysosomes lead to the suggestion that A2E may behave as a deprotonated alkyl amine that crosses membranes, is protonated in the acidic environment of the lysosome, and becomes trapped in these organelles (78). However, A2E is a pyridinium salt carrying a quaternary amine nitrogen that confers a permanent positive charge on the head group; A2E cannot deprotonate or reprotonate (38, 67). Interestingly, however, another bisretinoid lipofuscin pigment, all-trans-retinal dimer-PE (Figs. 1, 3), is a Schiff base with an imine nitrogen that is protonated. The detection of protonated all-trans-retinal dimer conjugates (all-trans-retinal dimer-PE and all-trans-retinal dimer-E) and unprotonated unconjugated all-trans-retinal dimer, though at lesser abundance, in extracts of RPE lipofuscin suggests conditions permitting both protonated and unprotonated forms. On the other hand, experiments in vitro have demonstrated that increasing pH in the environment of all-trans-retinal dimer-PE is associated with a hypsochromic shift in absorbance as the Schiff base deprotonates. Thus, all-trans-retinal dimer-PE and all-trans-retinal dimer-E have the potential to undergo pH-dependent deprotonation in response to a change in the acidity of lysosomes (33).
Spectroscopic properties
For all of the bisretinoid compounds of RPE lipofuscin, the two-armed polyene structures ending in β-ionone rings at one end and six-membered rings at the other provide the extended conjugation systems that confer absorbance maxima in both the UV and visible ranges of the spectrum: all-trans-retinal dimer-PE/ all-trans-retinal dimer-E, λmax ∼510, 290 nm; all-trans-retinal dimer, λmax ∼430, 290 nm; A2E, λmax ∼438, 334 nm; and A2-DHP-PE, λmax ∼490, 333 nm (Fig. 3). When present, the phospholipid moieties associated with these bisretinoids make contributions to the UV-visible absorbance at wavelengths less than 250 nm.
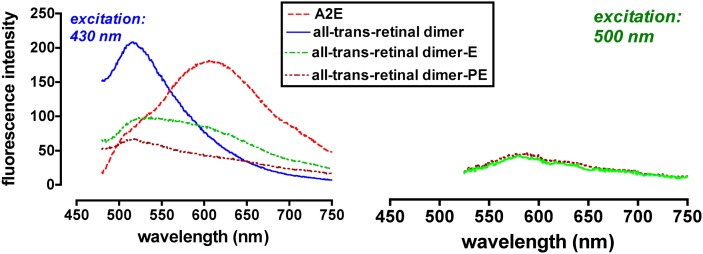
Of course, all of the known bisretinoids of RPE lipofuscin are fluorescent compounds. When excited at 430 nm, A2E and isoA2E have relatively broad emission spectra with maxima at ∼600 nm (67), an orange fluorescence (Fig. 5). Excitation of all-trans-retinal dimer-PE and all-trans-retinal dimer-E at approximately their absorbance maxima (500 nm) also produces an emission centered at ∼600 nm but the fluorescence intensity is weaker and the maxima are less clearly defined. The emission spectra of A2E/isoA2E and all-trans-retinal dimer-PE/all-trans-retinal dimer-E also correspond well to the emission maximum (590–620 nm) of native RPE lipofuscin. The excitation spectrum of A2E/isoA2E peaks at ∼440 nm and is narrower than that of whole lipofuscin (F. Delori and J. R. Sparrow, unpublished observations) (36). Unconjugated all-trans-retinal dimer presents with greater fluorescence emission than A2E and all-trans-retinal dimer-PE when excited at 430 but peak emission occurs at ∼510 nm and the spectral width is reduced (Fig. 5). When considered relative to the corresponding absorbance, A2-DHP-PE also exhibits fluorescence (emission monitored at 630 nm; excitation at 430 nm) of greater intensity than A2E and all-trans-retinal dimer-PE (Fig. 6).
Fig. 5.
Fluorescence emission spectra of A2E, all-trans-retinal dimer, and all-trans-retinal dimer-ethanolamine (all-trans-retinal dimer-E), all-trans-retinal dimer-phosphatidylethanolamine (all-trans-retinal dimer-PE). Spectra were recorded at excitation wavelengths of 430 nm (left) and 500 nm (right). Fluorescence intensity is presented in arbitrary units.
Fig. 6.
The bisretinoid A2-DHP-PE exhibits fluorescence intensity that is greater than that of A2E when considered relative to the absorbance peaks of each compound. Reverse-phase HPLC chromatogram using C18 column. Overlay of UV-visible absorbance (black) and fluorescence (red; excitation, 430 nm, emission, 600 nm) profiles. AU, absorbance units; a.u., arbitrary units of fluorescence intensity. Insets above, absorbance spectra of A2E, isoA2E, and A2-DHP-PE. B: Chromatogram in A is expanded between retention times 40–50 min; Inset above, absorbance spectra of all-trans-retinal dimer-PE (atRALdi-PE).
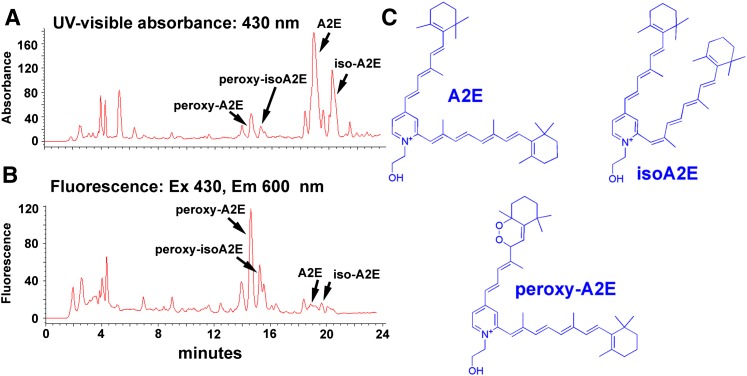
Interestingly, photooxidized forms of A2E and all-trans-retinal dimer can also display fluorescence emission of greater intensity than the parent compounds (Fig. 7). When the oxidation occurs on the short arm of the molecule, the increase in fluorescence emission can occur without a change in the absorbance maximum in the visible range (S. Kim, Y. Yang, and J. Sparrow, unpublished observations).
Fig. 7.
Oxidation of A2E is associated with increased autofluorescence emission. Monitoring of UV-visible absorbance (A) and fluorescence (B) in a mixture of A2E, isoA2E, peroxy-A2E, and peroxy-isoA2E by reverse-phase HPLC. Peroxy-A2/isoA2E was produced by oxidation with endoperoxide of 1,4-dimethylnaphthalene. Presented as absorbance units (A) and arbitrary units of fluorescence intensity (B). Comparison of the absorbance and fluorescence traces for A2E/isoA2E with the same traces for peroxyA2E/peroxy-isoA2E reveals that the fluorescence intensities (peak heights) of bisperoxyA2E and peroxy-isoA2E were considerably increased relative to A2E and isoA2E. C: Structures of A2E, isoA2E, and peroxy-A2E.
Photoreactivity
Given their origin from all-trans-retinal, it is perhaps not surprising that the bisretinoid lipofuscin pigments are photoreactive. For instance, when A2E is photoexcited, singlet oxygen is generated. Quantum yields of singlet oxygen produced are low when measured in polar solvents (0.0008–0.03) (79–82). The use of nonpolar solvents in such studies is more relevant because spectroscopic studies indicate that within its native environment, A2E most likely associates with the hydrophobic interior of phospholipid membranes (67). With outcome measured as cell death in an in vitro model, the release of singlet oxygen is corroborated by the modulating effects of an enhancer (D2O) and quenchers (histidine, DABCO, azide) of singlet oxygen (62). The photooxidative processes initiated in RPE cells through blue light-induced sensitization of A2E can lead to DNA base lesions (83), modification of protein by lipid peroxidation products and advanced glycation end products (AGEs), proteasome stress and accumulation of endogenous ubiquitin-conjugates (84), upregulation of RAGE (the cell surface receptor that transduces the effects of AGEs) (85), elevations in vascular endothelial growth factor (VEGF) (85), and cell death (63, 86, 87). The greater damage evoked by blue light as compared with green light reflects a wavelength dependency that is consistent with the excitation spectra of A2E (63) and is of interest as work in animal models has shown that RPE cells are particularly susceptible to injury from short wavelength visible light (type 2 retinal light damage) (88–93).
As noted above, A2E also undergoes photochemical changes that involve the insertion of oxygens at carbon-carbon double bonds to generate reactive oxo-A2E compounds (62, 64) that by mass spectroscopy are observed as a series of peaks, the sizes of which increase by increments of mass 16, starting from the M+ 592 peak attributable to A2E (62, 64). The participation of singlet oxygen in the photooxidation of A2E is indicated by the phosphorescence detection of singlet oxygen upon 430 nm irradiation of A2E; by the potentiation of A2E photooxidation in deuterium solvent and by experiments demonstrating that, as a surrogate for 430 nm light irradiation, endoperoxide-produced singlet oxygen can serve to oxidize A2E (64). Because singlet oxygen with its short life-time cannot account for damage distant from the lysosome (e.g., DNA damage) (62, 83, 94), photooxidation products of A2E are also implicated.
Although A2E has been the most intensively studied of the bisretinoid compounds of RPE lipofuscin, unconjugated all-trans-retinal dimer is a more efficient generator of singlet oxygen than A2E and the all-trans-retinal dimer series is also more readily photooxidized (33). Differences in electron distribution within the molecules likely account for the greater susceptibility to oxidation exhibited by these compounds.
The antioxidants vitamins E and C and resveratrol have been shown to reduce A2E photooxidation, at least in part, by quenching singlet oxygen (94). Phytochemicals, specifically anthocyanin and sulforaphane offer similar protection, the latter by inducing cellular increases in glutathione and the phase 2 enzymes NAD(P)H:quinone reductase and glutathione-S-transferase (95, 96). Protection offered by the iron chelator salicylaldehyde isonicontinoyl hydrazone suggests that iron-mediated Fenton chemistry and particularly, hydrogen peroxide toxicity, may also be operating during the process of bisretinoid photooxidation (97).
PHOTORECEPTOR LIPIDS AND LIGHT DAMAGE
The lipids of photoreceptor cells also participate in the propagation of retinal light damage. For instance, acute light exposure to rats and mice causes photoreceptor and retinal pigment epithelial cell damage, including lipid peroxidation (85, 98), which leads to the modification of proteins by reactive aldehydes (4-hydroxynonenal, 4-HNE; 4-hydroxyhexenal, 4-HHE) (99) that are the products of light-induced oxidation of n-6 and n-3 polyunsaturated fatty acids. In addition, experimentally induced increases in the DHA (22:6n-3) content of photoreceptor outer segment membrane are associated with a heightened vulnerability to light damage that is evidenced by enhanced lipid aldehyde-adducted proteins and increased photoreceptor cell apoptosis (100). Conversely, rats raised on a diet deficient in DHA precursor (α-linolenic acid, 18:3n-3) are protected against retinal light damage (101).
Oxidation of DHA, the most abundant of the long-chain polyunsaturated fatty acids in photoreceptor outer segments, also gives rise to aldehyde bearing seven-carbon CEP fragments that forms adducts with amino groups on proteins. As noted above, CEP-adducts are present in lipofuscin granules (54). These adducts have also been identified in drusen and elevated levels of CEP-adducted proteins are also found in the plasma of age-related macular degeneration (AMD) patients (102, 103). Conditions in the outer retina that are permissive for CEP formation include high oxygen levels and light but photosensitizing molecules in the outer segments, molecules that can transfer energy from photons to generate reactive oxygen species, are a corequisite. As known photosensitizers in the outer segments, bisretinoids such as A2PE and all-trans-retinal dimer are candidates for this role as they are also in light damage.
Retinal light damage can ensue from primary injury to photoreceptor cells or to RPE cells but secondary photoreceptor cell death will follow RPE cell loss. Action spectra that peak at both ∼500 nm and ∼440 nm have been described (104–112). The site of origin of short wavelength (∼440 nm) damage in primates appears to be both photoreceptor outer segments and RPE cells, although the greatest damage is reported in RPE cells (88–90, 108, 113, 114). Photooxidative mechanisms are clearly involved, because antioxidants can protect against photic damage and increased oxygen availability lowers the threshold (89, 115, 116). A comparative study of the damaging effects of narrow band light of wavelengths between 408 and 485 nm in rabbits revealed that RPE cells sustain maximal injury at 439 nm and melanin played neither a damaging or protective role (92).
Light exposure must be accompanied by 11-cis-retinal isomerization in order for photoreceptor cell degeneration to occur but phototransduction is not necessary (110, 117). All-trans-retinal, the product of photoisomerization of 11-cis-retinal, has been suggested as the damaging agent, the mechanism being either the toxicity associated with the aldehyde moiety in the chromophore or the photoreactivity of all-trans-retinal (7, 118). Because the concentration of rhodopsin is approximately 3 mM, there is the potential for release of a considerable amount of all-trans-retinal under conditions of intense light and yet, a single extensive bleach of rhodopsin, for instance, in a wild-type mouse, does not induce photoreceptor cell death (110); instead, repetitive photon absorption seems to be required, perhaps because of the need to produce the photoreactive bisretinoid byproducts generated by reaction of the aldehyde moiety in all-trans-retinal. As noted above, compelling evidence indicates that the photoreceptor cell has processes in place to chaperone all-trans-retinal upon its formation by photoisomerization of 11-cis-retinal (8, 10). Furthermore, all-trans-retinal has an absorbance maximum at 380 nm (Fig. 2B); as the primate lens and cornea block wavelengths below 400 nm, all-trans-retinal is largely shielded from light in the human eye (119, 120). However, as a cautionary note, it should be mentioned that lenses of both the mouse and rat transmit a significant amount of UV light to the retina (∼80% at 360 nm) (121); thus, findings related to all-trans-retinal in these rodents do not necessarily translate to the human.
A number of chromophores in RPE have been suggested as candidates responsible for short-wavelength damage to this cell; these include melanin, the mitochondrial enzyme cytochrome-c oxidase, and riboflavin (105, 122–125). All-trans-retinal cannot account for light damage to RPE because, as discussed above, retinoid processing is such that all-trans-retinal is reduced to all-trans-retinol within photoreceptor cells and is essentially absent from the RPE (9). Melanin has been excluded as a mediator on the basis that light damage can be induced in albino animals (125, 126); in addition, the action spectrum of blue-light damage does not correspond to the action spectrum for uptake of oxygen by melanin (105). Given that light damage is selective for RPE and photoreceptor cells of retina, it is difficult to attribute the effect to chromophores such as cytochrome-c oxidase (λmax 445 nm) and riboflavin (λmax 445 nm) that are not specific to these retinal cells. Moreover, short wavelength irradiation (430 nm) of cultured ARPE-19 cells devoid of melanin and lipofuscin does not result in cell death (63, 86). The requirement of a functioning visual cycle is indicated by the observation that exposure of retina to 430 nm light in Rpe65 null mutant mice does not result in a damage response in the RPE (127). Photoreactive compounds that are specific to RPE and photoreceptor cells, associated with a functioning visual cycle, and photoexcitable at wavelengths responsible for retinal light damage are the bisretinoids of RPE and photoreceptor cells.
RPE LIPOFUSCIN BISRETINOIDS IN HUMAN RETINAL DISORDERS AND MOUSE MODELS
ABCA4-related retinal disorders
As noted above, elevated levels of bisretinoid lipofuscin are a feature of diseases caused by ABCA4 gene mutations in humans. Bisretinoid pigments likely also account for the lipofuscin-like autofluorescence that can be visualized in the photoreceptor cell membrane in some forms of ABCA4-linked disease (128–130). More than 500 different mutations in the ABCA4 gene have been described and depending on the severity of the mutation, the ABCA4 gene is responsible for multiple related retinal degenerative diseases including recessive Stargardt macular degeneration, recessive cone-rod dystrophy, and recessive retinitis pigmentosa (131). Individuals heterozygous for some disease-causing mutations in ABCA4 may also exhibit increased susceptibility to AMD (132). A model has been proposed whereby the severity of the disease phenotype is inversely proportional to the level of residual protein activity with excessive production of bisretinoid RPE lipofuscin causing the degeneration (16). Nevertheless, given that some mutations, particularly those in the C terminus, are associated with misfolded protein that is retained in the endoplasmic reticulum, the possibility remains that simple loss of function may not account for the disease process in all cases (19, 133).
Studies in the Abca4−/− mice also point to an association between excessive RPE lipofuscin accumulation and photoreceptor cell death. Specifically, by morphometric analysis of outer nuclear layer thickness combined with counting of photoreceptor cell nuclei spanning the outer nuclear layer, it has been shown that albino Abca4−/− mice display progressive photoreceptor cell loss that is clearly detectable at 8 months of age and that has worsened by 12 and 13 months of age (134). Photoreceptor cell degeneration was independently reported in 11-month-old albino Abca4−/− mice (135).
Dysfunction related to RPE65 mutation
As opposed to the abundant accumulation that occurs in ABCA4 disease, in Lebers congenital amaurosis due to RPE65 mutations, there is an absence of RPE lipofuscin as demonstrated by fundus autofluorescence imaging (48). In the visual cycle, RPE65 serves as the isomerase that converts all-trans-retinyl esters to 11-cis-retinol (136–138). The absence of RPE65 results in a failure to produce the 11-cis-retinal chromophore, so all-trans-retinal, which is the essential precursor for bisretinoid formation, is not generated and bisretinoid lipofuscin does not form. Even an amino acid variant that slows the visual cycle because of reduced levels of Rpe65 in mouse RPE (139, 140) can modulate A2E formation (37) as can small molecule inhibitors that target RPE65 function (59, 141).
RDH deficiency
Mutant mice deficient in enzymes such as Rdh8 and Rdh12, which reduce all-trans-retinal, exhibit several-fold increases in A2E as compared with wild-type mice (25, 120). This abnormality occurs because the photoreceptor cell is deprived of avenues for disarming all-trans-retinal. Mutations in human RDH genes are responsible for Fundus albipunctatus, a mild form of night blindness (RDH5) (142) and for a subset of cases of Leber Congenital Amaurosis (RDH12), a severe autosomal recessive retinal disorder with onset in children (143).
ELOVL4-related macular dystrophy
Mutations in ELOVL4, the gene responsible for early-onset dominant Stargardt-like macular degeneration, are also reported to result in increased levels of RPE lipofuscin. Frame-shift mutations in ELOVL4 result in premature termination of the protein, aggregate-formation with the wild-type protein, and mis-localization of the protein (144–147). Transgenic mice expressing the mutant protein exhibit modest elevations in RPE lipofuscin measured as A2E (146, 148, 149). ELOVL4 is required for the synthesis of C28 and C30 saturated fatty acids and the synthesis of C28-C38 very long-chain polyunsaturated fatty acids, the latter being abundant in retina (150). Nevertheless, the link to enhanced bisretinoid formation is not understood.
AMD
Complement activation that is insufficiently regulated is considered to underlie the susceptibility to AMD that occurs with certain genetic variants in complement factors (151–154). Thus, it is of interest that photooxidation products of A2E and all-trans-retinal dimer can activate complement, whereas depletion of factor B reduces complement activation as does an inhibitor of C3 (155, 156). The addition of C-reactive protein in these assays also suppresses complement activation. Although AMD has onset in the elder years, it likely develops for several years before diagnosis. Complement activation triggered by photooxidation products of RPE bisretinoid lipofuscin could begin early in life and generate low-grade inflammatory processes that gradually predispose the macula to disease.
SUMMARY
By forming a reversible Schiff base linkage with all-trans-retinal, PE in the lipid bilayer of photoreceptor cells aids in removal of a share of the all-trans-retinal that is generated as a consequence of the light capturing function of the cell. If instead of hydrolyzing back to PE and all-trans-retinal, N-retinylidene-PE reacts with a second molecule of all-trans-retinal, the reactants commit to a pathway culminating in the synthesis of bisretinoid fluorophores. The extent to which the fatty acid composition of the photoreceptor membrane contributes to the production of these bisretinoid compounds is not known. The formation of these compounds could be viewed as a reflection of the photoreceptor cell's efforts to protect against acute aldehyde damage from all-trans-retinal and/or as a by-product of the cell's efforts to shepherd all-trans-retinal (in the form of N-retinylidene-PE) so that it is retained within the visual cycle. Whichever the case, the bisretinoids are ultimately deposited in RPE cells where they accumulate as the lipofuscin of the cell. These pigments vary in structure and in their tendency to retain their phosphatidic acid moiety. Some of these compounds exhibit detergent-like behavior and all are photoreactive when excited with photons having energies in the visible range of the spectrum. Given the likelihood that these pigments contribute to some retinal disorders, efforts are ongoing to develop therapies aimed at limiting their formation (59, 141, 157, 158). The characterization of the various bisretinoid constituents of RPE lipofuscin is fundamental to our understanding of the burden placed on the RPE cell by the deposition of this vitamin A-aldehyde-derived material.
Footnotes
Abbreviations:
- ABCA4/ABCR
- ATP-binding cassette transporter
- all-trans-retinal dimer-E
- all-trans-retinal dimer-ethanolamine
- all-trans-retinal dimer-PE
- all-trans-retinal dimer-phosphatidylethanolamine
- AMD
- age-related macular degeneration
- CEP
- carboxyethyl pyrrole
- DHA
- docosahexaenoic acid
- PC
- phosphatidylcholine
- PE
- phosphatidylethanolamine
- PLD
- phospholipase D
- PS
- phosphatidylserine
- RCS
- Royal College of Surgeon rat
- RDH
- NADPH-dependent retinol dehydrogenase
- RPE
- retinal pigment epithelium
- VEGF
- vascular endothelial growth factor
This work was supported by the National Institutes of Health Grant EY 12951 (J.R.S.), the Kaplen Foundation (J.R.S.), and unrestricted funds from Research to Prevent Blindness to the Department of Ophthalmology, Columbia University. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. J.R.S. is a recipient of an RPB Senior Scientist Award.
REFERENCES
- 1.Hicks D., Sparrow J. R., Barnstable C. J. 1989. Immunoelectron microscopical examination of the surface distribution of opsin in rat rod photoreceptor cells. Exp. Eye Res. 49: 13–29. [DOI] [PubMed] [Google Scholar]
- 2.Fliesler S. J., Anderson R. E. 1983. Chemistry and metabolism of lipids in the vertebrate retina. Prog. Lipid Res. 22: 79–131. [DOI] [PubMed] [Google Scholar]
- 3.McBee J. K., Palczewski K., Baehr W., Pepperberg D. R. 2001. Confronting complexity: the interlink of phototransduction and retinoid metabolism in the vertebrate retina. Prog. Retin. Eye Res. 20: 469–529. [DOI] [PubMed] [Google Scholar]
- 4.Beck M., Siebert F., Sakmar T. P. 1998. Evidence for the specific interaction of a lipid molecule with rhodopsin which is altered in the transition to the active state metarhodopsin II. FEBS Lett. 436: 304–308. [DOI] [PubMed] [Google Scholar]
- 5.Hessel E., Muller P., Hermann A., Hofmann K. P. 2001. Light-induced reorganization of phospholipid in rod disc membrane. J. Biol. Chem. 276: 2538–2543. [DOI] [PubMed] [Google Scholar]
- 6.Jastrzebska B., Goc A., Golczak M., Palczewski K. 2009. Phospholipids are needed for the proper formation, stability, and function of the photoactivated rhodopsin-transducin complex. Biochemistry. 48: 5159–5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maeda A., Maeda T., Golczak M., Chou S., Desai A., Hoppel C. L., Matsuyama S., Palczewski K. 2009. Involvement of all-trans-retinal in acute light-induced retinopathy of mice. J. Biol. Chem. 284: 15173–15183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schadel S. A., Heck M., Maretzki D., Filipek S., Teller D. C., Palczewski K., Hofmann K. P. 2003. Ligand channeling with a G-protein-coupled receptor: the entry and exit of retinal in native opsin. J. Biol. Chem. 278: 24896–24903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lamb T. D., Pugh E. N. 2004. Dark adaptation and the retinoid cycle of vision. Prog. Retin. Eye Res. 23: 307–380. [DOI] [PubMed] [Google Scholar]
- 10.Anderson R. E., Maude M. B. 1970. Phospholipids of bovine outer segments. Biochemistry. 9: 3624–3628. [DOI] [PubMed] [Google Scholar]
- 11.Papermaster D. S., Schneider B. G., Zorn M. A., Kraehenbuhl J. P. 1978. Immunocytochemical localization of a large intrinsic membrane protein to the incisures and margins of frog rod outer segment disks. J. Cell Biol. 78: 415–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Illing M., Molday L. L., Molday R. S. 1997. The 220-kDa rim protein of retinal rod outer segments is a member of the ABC transporter superfamily. J. Biol. Chem. 272: 10303–10310. [DOI] [PubMed] [Google Scholar]
- 13.Sun H., Molday R. S., Nathans J. 1999. Retinal stimulates ATP hydrolysis by purified and reconstituted ABCR, the photoreceptor-specific ATP-binding cassette transporter responsible for Stargardt disease. J. Biol. Chem. 274: 8269–8281. [DOI] [PubMed] [Google Scholar]
- 14.Sun H., Nathans J. 1997. Stargardt's ABCR is localized to the disc membrane of retinal rod outer segments. Nat. Genet. 17: 15–16. [DOI] [PubMed] [Google Scholar]
- 15.Molday R. S., Zhong M., Quazi F. 2009. The role of the photoreceptor ABC transporter ABCA4 in lipid transport and Stargardt macular degeneration. Biochim. Biophys. Acta. 1791: 573–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shroyer N. F., Lewis R. A., Allikmets R., Singh N., Dean M., Leppert M., Lupski J. R. 1999. The rod photoreceptor ATP-binding cassette transporter gene, ABCR, and retinal disease: from monogenic to multifactorial. Vision Res. 39: 2537–2544. [DOI] [PubMed] [Google Scholar]
- 17.Maugeri A., Klevering B. J., Rohrschneider K., Blankenagel A., Brunner H. G., Dentman A. F., Hoyng C. B., Cremers F. P. 2000. Mutations in the ABCA4 (ABCR) gene are the major cause of autosomal recessive cone-rod dystrophy. Am. J. Hum. Genet. 67: 960–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shroyer N. F., Lewis R. A., Yatsenko A. N., Lupski J. R. 2001. Null missense ABCR (ABCA4) mutations in a family with Stargardt disease and retinitis pigmentosa. Invest. Ophthalmol. Vis. Sci. 42: 2757–2761. [PubMed] [Google Scholar]
- 19.Cideciyan A. V., Swider M., Aleman T. S., Tsybovsky Y., Schwartz S. B., Windsor E. A., Roman A. J., Sumaroka A., Steinberg J. D., Jacobson S. G., et al. 2009. ABCA4 disease progression and a proposed strategy for gene therapy. Hum. Mol. Genet. 18: 931–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahn J., Wong J. T., Molday R. S. 2000. The effect of lipid environment and retinoids on the ATPase activity of ABCR, the photoreceptor ABC transporter responsible for Stargardt macular dystrophy. J. Biol. Chem. 275: 20399–20405. [DOI] [PubMed] [Google Scholar]
- 21.Beharry S., Zhong M., Molday R. S. 2004. N-retinylidene-phosphatidylethanolamine is the preferred retinoid substrate for the photoreceptor-specific ABC transporter ABCA4 (ABCR). J. Biol. Chem. 279: 53972–53979. [DOI] [PubMed] [Google Scholar]
- 22.Molday L. L., Rabin A. R., Molday R. S. 2000. ABCR expression in foveal cone photoreceptors and its role in Stargardt macular dystrophy. Nat. Genet. 25: 257–258. [DOI] [PubMed] [Google Scholar]
- 23.Sun H., Nathans J. 2001. Mechanistic studies of ABCR, the ABC transporter in photoreceptor outer segments responsible for autosomal recessive Stargardt disease. J. Bioenerg. Biomembr. 33: 523–530. [DOI] [PubMed] [Google Scholar]
- 24.Travis G. H., Golczak M., Moise A. R., Palczewski K. 2007. Diseases caused by defects in the visual cycle: retinoids as potential therapeutic agents. Annu. Rev. Pharmacol. Toxicol. 47: 469–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chrispell J. D., Feathers K. L., Kane M. A., Kim C. Y., Brooks M., Khanna R., Kurth I., Huebner C. A., Gal A., Mears A. J., et al. 2009. RDH12 activity and effects on retinoid processing in the murine retina. J. Biol. Chem. 284: 21468–21477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Molday R. S. 2007. ATP-binding cassette transporter ABCA4: molecular properties and role in vision and macular degeneration. J. Bioenerg. Biomembr. 39: 507–517. [DOI] [PubMed] [Google Scholar]
- 27.Mata N. L., Tzekov R. T., Liu X., Weng J., Birch D. G., Travis G. H. 2001. Delayed dark adaptation and lipofuscin accumulation in abcr+/− mice: implications for involvement of ABCR in age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 42: 1685–1690. [PubMed] [Google Scholar]
- 28.Weng J., Mata N. L., Azarian S. M., Tzekov R. T., Birch D. G., Travis G. H. 1999. Insights into the function of Rim protein in photoreceptors and etiology of Stargardt's disease from the phenotype in abcr knockout mice. Cell. 98: 13–23. [DOI] [PubMed] [Google Scholar]
- 29.Mata N. L., Weng J., Travis G. H. 2000. Biosynthesis of a major lipofuscin fluorophore in mice and humans with ABCR-mediated retinal and macular degeneration. Proc. Natl. Acad. Sci. USA. 97: 7154–7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu J., Itagaki Y., Ben-Shabat S., Nakanishi K., Sparrow J. R. 2000. The biosynthesis of A2E, a fluorophore of aging retina, involves the formation of the precursor, A2-PE, in the photoreceptor outer segment membrane. J. Biol. Chem. 275: 29354–29360. [DOI] [PubMed] [Google Scholar]
- 31.Ben-Shabat S., Parish C. A., Vollmer H. R., Itagaki Y., Fishkin N., Nakanishi K., Sparrow J. R. 2002. Biosynthetic studies of A2E, a major fluorophore of RPE lipofuscin. J. Biol. Chem. 277: 7183–7190. [DOI] [PubMed] [Google Scholar]
- 32.Fishkin N. E., Sparrow J. R., Allikmets R., Nakanishi K. 2005. Isolation and characterization of a retinal pigment epithelial cell fluorophore: an all-trans-retinal dimer conjugate. Proc. Natl. Acad. Sci. USA. 102: 7091–7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim S. R., Jang Y. P., Jockusch S., Fishkin N. E., Turro N. J., Sparrow J. R. 2007. The all-trans-retinal dimer series of lipofuscin pigments in retinal pigment epithelial cells in a recessive Stargardt disease model. Proc. Natl. Acad. Sci. USA. 104: 19273–19278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu Y., Fishkin N. E., Pande A., Pande J., Sparrow J. R. 2009. Novel lipofuscin bisretinoids prominent in human retina and in a model of recessive Stargardt disease. J. Biol. Chem. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bok D. 1993. The retinal pigment epithelium: a versatile partner in vision. J. Cell Sci. Suppl. 17: 189–195. [DOI] [PubMed] [Google Scholar]
- 36.Sparrow J. R., Boulton M. 2005. RPE lipofuscin and its role in retinal photobiology. Exp. Eye Res. 80: 595–606. [DOI] [PubMed] [Google Scholar]
- 37.Kim S. R., Fishkin N., Kong J., Nakanishi K., Allikmets R., Sparrow J. R. 2004. The Rpe65 Leu450Met variant is associated with reduced levels of the RPE lipofuscin fluorophores A2E and iso-A2E. Proc. Natl. Acad. Sci. USA. 101: 11668–11672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parish C. A., Hashimoto M., Nakanishi K., Dillon J., Sparrow J. R. 1998. Isolation and one-step preparation of A2E and iso-A2E, fluorophores from human retinal pigment epithelium. Proc. Natl. Acad. Sci. USA. 95: 14609–14613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katz M. L., Drea C. M., Eldred G. E., Hess H. H., Robison W. G., Jr 1986. Influence of early photoreceptor degeneration on lipofuscin in the retinal pigment epithelium. Exp. Eye Res. 43: 561–573. [DOI] [PubMed] [Google Scholar]
- 40.Sparrow J. R. 2007. RPE lipofuscin: formation, properties and relevance to retinal degeneration. Retinal Degenerations: Biology, Diagnostics and Therapeutics. Tombran-Tink J., Barnstable C. J., Humana Press, Totowa, NJ: 213–236. [Google Scholar]
- 41.Katz M. L., Eldred G. E. 1989. Retinal light damage reduces autofluorescent pigment deposition in the retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 30: 37–43. [PubMed] [Google Scholar]
- 42.Rando R. R. 2001. The biochemistry of the visual cycle. Chem. Rev. 101: 1881–1896. [DOI] [PubMed] [Google Scholar]
- 43.Maeda T., Van Hooser J. P., Driessen C. A. G. G., Filipek S., Janssen J. J. M., Palczewski K. 2003. Evaluation of the role of the retinal G protein-coupled receptor (RGR) in the vertebrate retina in vivo. J. Neurochem. 85: 944–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Robison W. G. J., Kuwabara T., Bieri J. G. 1980. Deficiencies of vitamins E and A in the rat. Retinal damage and lipofuscin accumulation. Invest. Ophthalmol. Vis. Sci. 19: 1030–1037. [PubMed] [Google Scholar]
- 45.Katz M. L., Drea C. M., Robison W. G., Jr 1986. Relationship between dietary retinol and lipofuscin in the retinal pigment epithelium. Mech. Ageing Dev. 35: 291–305. [DOI] [PubMed] [Google Scholar]
- 46.Katz M. L., Redmond T. M. 2001. Effect of Rpe65 knockout on accumulation of lipofuscin fluorophores in the retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 42: 3023–3030. [PubMed] [Google Scholar]
- 47.Sparrow J. R., Kim S. R., Jang Y. P., Zhou J. 2008. The lipofuscin of retinal pigment epithelial cells: learning from mouse models of retinal disease. Eye, Retina, and Visual System of the Mouse. Chalupa L. M., editor MIT Press, Cambridge, MA: 539–546. [Google Scholar]
- 48.Lorenz B., Wabbels B., Wegscheider E., Hamel C. P., Drexler W., Presing M. N. 2004. Lack of fundus autofluorescence to 488 nanometers from childhood on in patients with early-onset severe retinal dystrophy associated with mutations in RPE65. Ophthalmology. 111: 1585–1594. [DOI] [PubMed] [Google Scholar]
- 49.Clancy C. M. R., Krogmeier J. R., Pawlak A., Rozanowska M., Sarna T., Dunn R. C., Simon J. D. 2000. Atomic force microscopy and near-field scanning optical microscopy measurements of single human retinal lipofuscin granules. J. Phys. Chem. B. 104: 12098–12101. [Google Scholar]
- 50.Feeney-Burns L., Eldred G. E. 1983. The fate of the phagosome: conversion to ‘age pigment’ and impact in human retinal pigment epithelium. Trans. Ophthalmol. Soc. U. K. 103: 416–421. [PubMed] [Google Scholar]
- 51.Boulton M., Docchio F., Dayhaw-Barker P., Ramponi R., Cubeddu R. 1990. Age-related changes in the morphology, absorption and fluorescence of melanosomes and lipofuscin granules of the retinal pigment epithelium. Vision Res. 30: 1291–1303. [DOI] [PubMed] [Google Scholar]
- 52.Haralampus-Grynaviski N. M., Lamb L. E., Clancy C. M. R., Skumatz C., Burke J. M., Sarna T., Simon J. D. 2003. Spectroscopic and morphological studies of human retinal lipofuscin granules. Proc. Natl. Acad. Sci. USA. 100: 3179–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cuervo A. M. 2008. Autophagy and aging: keeping that old broom working. Trends Genet. 24: 604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ng K. P., Gugiu B. G., Renganathan K., Davies M. W., Gu X., Crabb J. S., Kim S. R., Rozanowska M. B., Bonilha V. L., Rayborn M. E., et al. 2008. Retinal pigment epithelium lipofuscin proteomics. Mol. Cell. Proteomics. 7: 1397–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sakai N., Decatur J., Nakanishi K., Eldred G. E. 1996. Ocular age pigment “A2E“: an unprecedented pyridinium bisretinoid. J. Am. Chem. Soc. 118: 1559–1560. [Google Scholar]
- 56.Kim S. R., He J., Yanase E., Jang Y. P., Berova N., Sparrow J. R., Nakanishi K. 2007. Characterization of dihydro-A2PE: an intermediate in the A2E biosynthetic pathway. Biochemistry. 46: 10122–10129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dalvis D., Zhao Z., Castagnoli N. 1992. Characterization of an unexpected product from a monoamine oxidase B generated 2,3-dihydropyridinium species. J. Org. Chem. 57: 7321–7324. [Google Scholar]
- 58.Sparrow J. R., Kim S. R., Cuervo A. M., Bandhyopadhyayand U. 2008. A2E, a pigment of RPE lipofuscin is generated from the precursor A2PE by a lysosomal enzyme activity. Adv. Exp. Med. Biol. 613: 393–398. [DOI] [PubMed] [Google Scholar]
- 59.Maiti P., Kong J., Kim S. R., Sparrow J. R., Allikmets R., Rando R. R. 2006. Small molecule RPE65 antagonists limit the visual cycle and prevent lipofuscin formation. Biochemistry. 45: 852–860. [DOI] [PubMed] [Google Scholar]
- 60.Fishkin N., Pescitelli G., Sparrow J. R., Nakanishi K., Berova N. 2004. Absolute configurational determination of an all-trans-retinal dimer isolated from photoreceptor outer segments. Chirality. 16: 637–641. [DOI] [PubMed] [Google Scholar]
- 61.Jang Y. P., Matsuda H., Itagaki Y., Nakanishi K., Sparrow J. R. 2005. Characterization of peroxy-A2E and furan-A2E photooxidation products and detection in human and mouse retinal pigment epithelial cells lipofuscin. J. Biol. Chem. 280: 39732–39739. [DOI] [PubMed] [Google Scholar]
- 62.Sparrow J. R., Zhou J., Ben-Shabat S., Vollmer H., Itagaki Y., Nakanishi K. 2002. Involvement of oxidative mechanisms in blue light induced damage to A2E-laden RPE. Invest. Ophthalmol. Vis. Sci. 43: 1222–1227. [PubMed] [Google Scholar]
- 63.Sparrow J. R., Nakanishi K., Parish C. A. 2000. The lipofuscin fluorophore A2E mediates blue light-induced damage to retinal pigmented epithelial cells. Invest. Ophthalmol. Vis. Sci. 41: 1981–1989. [PubMed] [Google Scholar]
- 64.Ben-Shabat S., Itagaki Y., Jockusch S., Sparrow J. R., Turro N. J., Nakanishi K. 2002. Formation of a nona-oxirane from A2E, a lipofuscin fluorophore related to macular degeneration, and evidence of singlet oxygen involvement. Agnew. Chem. Int. Ed. 41: 814–817. [DOI] [PubMed] [Google Scholar]
- 65.Kim S. R., Jockusch S., Itagaki Y., Turro N. J., Sparrow J. R. 2008. Mechanisms involved in A2E oxidation. Exp. Eye Res. 86: 975–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Young R. W. 1971. The renewal of rod and cone outer segments in the rhesus monkey. J. Cell Biol. 49: 303–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sparrow J. R., Parish C. A., Hashimoto M., Nakanishi K. 1999. A2E, a lipofuscin fluorophore, in human retinal pigmented epithelial cells in culture. Invest. Ophthalmol. Vis. Sci. 40: 2988–2995. [PubMed] [Google Scholar]
- 68.Eldred G. E. 1995. Lipofuscin fluorophore inhibits lysosomal protein degradation and may cause early stages of macular degeneration. Gerontology. 41: 15–28. [DOI] [PubMed] [Google Scholar]
- 69.Sparrow J. R., Cai B., Jang Y. P., Zhou J., Nakanishi K. 2006. A2E, a fluorophore of RPE lipofuscin, can destabilize membrane. Adv. Exp. Med. Biol. 572: 63–68. [DOI] [PubMed] [Google Scholar]
- 70.Sparrow J. R., Fishkin N., Zhou J., Cai B., Jang Y. P., Krane S., Itagaki Y., Nakanishi K. 2003. A2E, a byproduct of the visual cycle. Vision Res. 43: 2983–2990. [DOI] [PubMed] [Google Scholar]
- 71.De S., Sakmar T. P. 2002. Interaction of A2E with model membranes. Implications to the pathogenesis of age-related macular degeneration. J. Gen. Physiol. 120: 147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sheetz M. P., Singer S. J. 1974. Biological membranes as bilayer couples. A molecular mechanism of drug-erythrocyte interactions. Proc. Natl. Acad. Sci. USA. 71: 4457–4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Suter M., Reme C. E., Grimm C., Wenzel A., Jaattela M., Esser P., Kociok N., Leist M., Richter C. 2000. Age-related macular degeneration. The lipofuscin component in N-retinyl-N-retinylidene ethanolamine detaches proapoptotic proteins from mitochondria and induces apoptosis in mammalian retinal pigment epithelial cells. J. Biol. Chem. 275: 39625–39630. [DOI] [PubMed] [Google Scholar]
- 74.Bergmann M., Schutt F., Holz F. G., Kopitz J. 2004. Inhibition of the ATP-driven proton pump in RPE lysosomes by the major lipofuscin fluorophore A2E may contribute to the pathogenesis of age-related macular degeneration. FASEB J. 18: 562–564. [DOI] [PubMed] [Google Scholar]
- 75.Holz F. G., Schutt F., Kopitz J., Eldred G. E., Kruse F. E., Volcker H. E., Cantz M. 1999. Inhibition of lysosomal degradative functions in RPE cells by a retinoid component of lipofuscin. Invest. Ophthalmol. Vis. Sci. 40: 737–743. [PubMed] [Google Scholar]
- 76.Finnemann S. C., Leung L. W., Rodriguez-Boulan E. 2002. The lipofuscin component A2E selectively inhibits phagolysosomal degradation of photoreceptor phospholipid by the retinal pigment epithelium. Proc. Natl. Acad. Sci. USA. 99: 3842–3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bermann M., Schutt F., Holz F. G., Kopitz J. 2001. Does A2E, a retinoid component of lipofuscin and inhibitor of lysosomal degradative functions, directly affect the activity of lysosomal hydrolases. Exp. Eye Res. 72: 191–195. [DOI] [PubMed] [Google Scholar]
- 78.Eldred G. E., Lasky M. R. 1993. Retinal age pigments generated by self-assembling lysosomotropic detergents. Nature. 361: 724–726. [DOI] [PubMed] [Google Scholar]
- 79.Kanofsky J. R., Sima P. D., Richter C. 2003. Singlet-oxygen generation from A2E. Photochem. Photobiol. 77: 235–242. [DOI] [PubMed] [Google Scholar]
- 80.Lamb L. E., Ye T., Haralampus-Grynaviski N. M., Williams T. R., Pawlak A., Sarna T., Simon J. D. 2001. Primary photophysical properties of A2E in solution. J. Phys. Chem. B. 105: 11507–11512. [Google Scholar]
- 81.Ragauskaite L., Heckathorn R. C., Gaillard E. R. 2001. Environmental effects on the photochemistry of A2E, a component of human retinal lipofuscin. Photochem. Photobiol. 74: 483–488. [DOI] [PubMed] [Google Scholar]
- 82.Cantrell A., McGarvey D. J., Roberts J., Sarna T., Truscott T. G. 2001. Photochemical studies of A2E. J. Photochem. Photobiol. B: Biol. 64: 162–165. [DOI] [PubMed] [Google Scholar]
- 83.Sparrow J. R., Zhou J., Cai B. 2003. DNA is a target of the photodynamic effects elicited in A2E-laden RPE by blue light illumination. Invest. Ophthalmol. Vis. Sci. 44: 2245–2251. [DOI] [PubMed] [Google Scholar]
- 84.Zhang X., Zhou J., Fernandes A. F., Sparrow J. R., Pereira P., Taylor A., Shang F. 2008. The proteasome: a target of oxidative damage in cultured human retina pigment epithelial cells. Invest. Ophthalmol. Vis. Sci. 49: 3622–3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhou J., Cai B., Jang Y. P., Pachydaki S., Schmidt A. M., Sparrow J. R. 2005. Mechanisms for the induction of HNE- MDA- and AGE-adducts, RAGE and VEGF in retinal pigment epithelial cells. Exp. Eye Res. 80: 567–580. [DOI] [PubMed] [Google Scholar]
- 86.Schutt F., Davies S., Kopitz J., Holz F. G., Boulton M. E. 2000. Photodamage to human RPE cells by A2-E, a retinoid component of lipofuscin. Invest. Ophthalmol. Vis. Sci. 41: 2303–2308. [PubMed] [Google Scholar]
- 87.Sparrow J. R., Cai B. 2001. Blue light-induced apoptosis of A2E-containing RPE: involvement of caspase-3 and protection by Bcl-2. Invest. Ophthalmol. Vis. Sci. 42: 1356–1362. [PubMed] [Google Scholar]
- 88.Ham W. T. J., Allen R. G., Feeney-Burns L., Marmor M. F., Parver L. M., Proctor P. H., Sliney D. H., Wolbarsht M. L. 1986. The involvement of the retinal pigment epithelium. CRC Optical Radiation and Visual Health. Waxler M., Hitchins V. M., CRC Press, Inc., Boca Raton: 43–67. [Google Scholar]
- 89.Ham W. T., Mueller H. A., Ruffolo J. J., Millen J. E., Cleary S. F., Guerry R. K., Guerry D. 1984. Basic mechanisms underlying the production of photochemical lesions in the mammalian retina. Curr. Eye Res. 3: 165–174. [DOI] [PubMed] [Google Scholar]
- 90.Busch E. M., Gorgels T. G. M. F., Roberts J. E., van Norren D. 1999. The effects of two stereoisomers of N-acetylcysteine on photochemical damage by UVA and blue light in rat retina. Photochem. Photobiol. 70: 353–358. [PubMed] [Google Scholar]
- 91.Borges J., Li Z-Y., Tso M. O. 1990. Effects of repeated photic exposures on the monkey macula. Arch. Ophthalmol. 108: 727–733. [DOI] [PubMed] [Google Scholar]
- 92.Putting B. J., Van Best J. A., Vrensen G. F. J. M., Oosterhuis J. A. 1994. Blue-light-induced dysfunction of the blood-retinal barrier at the pigment epithelium in albino versus pigmented rabbits. Exp. Eye Res. 58: 31–40. [DOI] [PubMed] [Google Scholar]
- 93.Paultler E. L., Morita M., Beezley D. 1989. Reversible and irreversible blue light damage to the isolated mammalian pigment epithelium. Inherited and Environmental Induced Retinal Degeneration. La Vail M. M., Anderson R. E., Hollyfield J. G., Alan R. Liss, New York: 555–567. [PubMed] [Google Scholar]
- 94.Sparrow J. R., Vollmer-Snarr H. R., Zhou J., Jang Y. P., Jockusch S., Itagaki Y., Nakanishi K. 2003. A2E-epoxides damage DNA in retinal pigment epithelial cells. Vitamin E and other antioxidants inhibit A2E-epoxide formation. J. Biol. Chem. 278: 18207–18213. [DOI] [PubMed] [Google Scholar]
- 95.Zhou J., Gao X., Cai B., Sparrow J. R. 2006. Indirect antioxidant protection against photooxidative processes initiated in retinal pigment epithelial cells by a lipofuscin pigment. Rejuven. Res. 9: 256–263. [DOI] [PubMed] [Google Scholar]
- 96.Jang Y. P., Zhou J., Nakanishi K., Sparrow J. R. 2005. Anthocyanins protect against A2E photooxidation and membrane permeabilization in retinal pigment epithelial cells. Photochem. Photobiol. 81: 529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lukinova N., Iacovelli J., Dentchev T., Wolkow N., Hunter A., Amado D., Ying G-S., Sparrow J. R., Dunaief J. L. 2009. Iron chelation protects the retinal pigment epithelial cell line ARPE-19 against cell death triggered by diverse stimuli. Invest. Ophthalmol. Vis. Sci. 50: 1440–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wiegand R. D., Giusto N. M., Rapp L. M., Anderson R. E. 1983. Evidence for rod outer segment lipid peroxidation following constant illumination of the rat retina. Invest. Ophthalmol. Vis. Sci. 24: 1433–1435. [PubMed] [Google Scholar]
- 99.Tanito M., Elliot M. H., Kotake Y., Anderson R. E. 2005. Protein modifications by 4-hydroxynonenal and 4-hydroxyhexenal in light-exposed rat retina. Invest. Ophthalmol. Vis. Sci. 46: 3859–3868. [DOI] [PubMed] [Google Scholar]
- 100.Tanito M., Brush R. S., Elliott M. H., Wicker L. D., Henry K. R., Anderson R. E. 2009. High levels of retinal membrane docosahexaenoic acid increase susceptibility to stress-induced degeneration. J. Lipid Res. 50: 807–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Organisciak D. T., Darrow R. M., Jiang Y. L., Blanks J. C. 1996. Retinal light damage in rats with altered levels of rod outer segment docosahexaenoate. Invest. Ophthalmol. Vis. Sci. 37: 2243–2257. [PubMed] [Google Scholar]
- 102.Crabb J. W., Miyagi M., Gu X., Shadrach K., West K. A., Sakaguchi H., Kamei M., Hasan A., Yan L., Raybourn M. E., et al. 2002. Drusen proteome analysis: an approach to the etiology of age-related macular degeneration. Proc. Natl. Acad. Sci. USA. 99: 14682–14687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gu X., Meer S. G., Miyagi M., Rayborn M. E., Hollyfield J. G., Crabb J. W., Salomon R. G. 2003. Carboxyethylpyrrole protein adducts and autoantibodies, biomarkers for age-related macular degeneration. J. Biol. Chem. 278: 42027–42035. [DOI] [PubMed] [Google Scholar]
- 104.Noell W. K., Walker V. S., Kang B. S., Berman S. 1966. Retinal damage by light in rats. Invest. Ophthalmol. Vis. Sci. 5: 450–473. [PubMed] [Google Scholar]
- 105.Mellerio J. 1994. Light effects on the retina. Principles and Practice of Ophthalmology: Basic Science. Albert D. M., Jakobiec F. A., W.B. Saunders Co, Philadelphia: 1326–1345. [Google Scholar]
- 106.Williams T. P., Howell W. L. 1983. Action spectrum of retinal light damage in albino rats. Invest. Ophthalmol. Vis. Sci. 24: 285–287. [PubMed] [Google Scholar]
- 107.Algvere P. V., Marshall J., Seregard S. 2006. Age-related maculopathy and the impact of blue light hazard. Acta Ophthalmol. Scand. 84: 4–15. [DOI] [PubMed] [Google Scholar]
- 108.Ham W. T., Mueller H. A. 1976. Retinal sensitivity to damage from short wavelength light. Nature. 260: 153–154. [DOI] [PubMed] [Google Scholar]
- 109.Grimm C., Wenzel A., Williams T. P., Rol P. O., Hafezi F., Reme C. E. 2001. Rhodopsin-mediated blue-light damage to the rat retina: effect of photoreversal of bleaching. Invest. Ophthalmol. Vis. Sci. 42: 497–505. [PubMed] [Google Scholar]
- 110.Wenzel A., Grimm C., Samardzija M., Reme C. E. 2005. Molecular mechanisms of light-induced photoreceptor apoptosis and neuroprotection for retinal degeneration. Prog. Retin. Eye Res. 24: 275–306. [DOI] [PubMed] [Google Scholar]
- 111.Van Norren D., Schellekens P. 1990. Blue light hazard in rat. Vision Res. 30: 1517–1520. [DOI] [PubMed] [Google Scholar]
- 112.Gorgels T. G. M. F., van Norren D. 1995. Ultraviolet and green light cause different types of damage in rat retina. Invest. Ophthalmol. Vis. Sci. 36: 851–863. [PubMed] [Google Scholar]
- 113.Friedman E., Kuwabara T. 1968. The retinal pigment epithelium. IV. The damaging effects of radiant energy. Arch. Ophthalmol. 80: 265–279. [DOI] [PubMed] [Google Scholar]
- 114.Ham W. T., Ruffolo J. J., Mueller H. A., Clarke A. M., Moon M. E. 1978. Histologic analysis of photochemical lesions produced in rhesus retina by short-wavelength light. Invest. Ophthalmol. Vis. Sci. 17: 1029–1035. [PubMed] [Google Scholar]
- 115.Tanito M., Kaidzu S., Anderson R. E. 2006. Protective effects of soft acrylic yellow filter against blue light-induced retinal damage in rats. Exp. Eye Res. 83: 1493–1504. [DOI] [PubMed] [Google Scholar]
- 116.Organisciak D. T., Darrow R. M., Jiang Y. I., Marak G. E., Blanks J. C. 1992. Protection by dimethylthiourea against retinal light damage in rats. Invest. Ophthalmol. Vis. Sci. 33: 1599–1609. [PubMed] [Google Scholar]
- 117.Hao W., Wenzel A., Obin M. S., Chen C. K., Brill E., Krasnoperova N. V., Eversole-Cire P., Kleyner Y., Taylor A., Simon M. I., Grimm C., Remé C. E., Jem L. 2002. Evidence for two apoptotic pathways in light-induced retinal degeneration. Nat. Genet. 32: 254–260. [DOI] [PubMed] [Google Scholar]
- 118.Rózanowska M., Sarna T. 2005. Light-induced damage to the retina: role of rhodopsin chromophore revisited. Photochem. Photobiol. 81: 1305–1330. [DOI] [PubMed] [Google Scholar]
- 119.Boettner E. A., Wolter J. R. 1962. Transmission of the ocular media. Invest. Ophthalmol. 1: 776–783. [Google Scholar]
- 120.Maeda A., Maeda T., Golczak M., Palczewski K. 2008. Retinopathy in mice induced by disrupted all-trans-retinal clearance. J. Biol. Chem. 283: 26684–26693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lei B., Yao G. 2006. Spectral attenuation of the mouse, rat, pig and human lenses from wavelengths 360 nm to 1020 nm. Exp. Eye Res. 83: 610–614. [DOI] [PubMed] [Google Scholar]
- 122.Noell W. K. 1980. Possible mechanisms of photoreceptor damage by light in mammalian eyes. Vision Res. 20: 1163–1171. [DOI] [PubMed] [Google Scholar]
- 123.Boulton M. 1998. Melanin and the retinal pigment epithelium. Oxford University Press, New York City. [Google Scholar]
- 124.Reme C. E., Hafezi F., Marti A., Munz K., Reinboth J. J. 1998. Light damage to retina and retinal pigment epithelium. Oxford University Press, New York City. [Google Scholar]
- 125.Organisciak D. T., Winkler B. S. 1994. Retinal light damage: practical and theoretical considerations. Prog. Retin. Eye Res. 13: 1–29. [Google Scholar]
- 126.Hoppeler T., Hendrickson P., Dietrich C., Reme C. E. 1988. Morphology and time-course of defined photochemical lesions in the rabbit retina. Curr. Eye Res. 7: 849–860. [DOI] [PubMed] [Google Scholar]
- 127.Rattner A., Toulabi L., Williams J., Yu H., Nathans J. 2008. The genomic response of the retinal pigment epithelium to light damage and retinal detachment. J. Neurosci. 28: 9880–9889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Birnbach C. D., Jarvelainen M., Possin D. E., Milam A. H. 1994. Histopathology and immunocytochemistry of the neurosensory retina in fundus flavimaculatus. Ophthalmology. 101: 1211–1219. [DOI] [PubMed] [Google Scholar]
- 129.Bunt-Milam A. H., Kalina R. E., Pagon R. A. 1983. Clinical-ultrastructural study of a retinal dystrophy. Invest. Ophthalmol. Vis. Sci. 24: 458–469. [PubMed] [Google Scholar]
- 130.Szamier R. B., Berson E. L. 1977. Retinal ultrastructure in advanced retinitis pigmentosa. Invest. Ophthalmol. Vis. Sci. 16: 947–962. [PubMed] [Google Scholar]
- 131.Cremers F. P., van de Pol D. J., van Driel M., den Hollander A. I., van Haren F. J., Knoers N. V., Tijmes N., Bergen A. A., Rohrschneider K., Blankenagel A., et al. 1998. Autosomal recessive retinitis pigmentosa and cone-rod dystrophy caused by splice site mutations in the Stargardt's disease gene ABCR. Hum. Mol. Genet. 7: 355–362. [DOI] [PubMed] [Google Scholar]
- 132.Allikmets R. 2000. Further evidence for an association of ABCR alleles with age-related macular degeneration. The international ABCR Screening Consortium. Am. J. Hum. Genet. 67: 487–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhong M., Molday L. L., Molday R. S. 2009. Role of the C terminus of the photoreceptor ABCA4 transporter in protein folding, function and retinal degenerative disease. J. Biol. Chem. 284: 3640–3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wu L., Nagasaki T., Sparrow J. R. 2009. Photoreceptor cell degeneration in Abcr−/− mice. Adv. Exp. Med. Biol. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Radu R. A., Yuan Q., Hu J., Peng J. H., Lloyd M., Nusinowitz S., Bok D., Travis G. H. 2008. Accelerated accumulation of lipofuscin pigments in the RPE of a mouse model for ABCA4-mediated retinal dystrophies following Vitamin A supplementation. Invest. Ophthalmol. Vis. Sci. 49: 3821–3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jin M., Li S., Moghrabi W. N., Sun H., Travis G. H. 2005. Rpe65 is the retinoid isomerase in bovine retinal pigment epithelium. Cell. 122: 449–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Moiseyev G., Chen Y., Takahashi Y., Wu B. X., Ma J. X. 2005. RPE65 is the isomerohydrolase in the retinoid visual cycle. Proc. Natl. Acad. Sci. USA. 102: 12413–12418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Redmond T. M., Poliakov E., Yu S., Tsai J. Y., Lu Z., Gentleman S. 2005. Mutation of key residues of RPE65 abolishes its enzymatic role as isomerohydrolase in the visual cycle. Proc. Natl. Acad. Sci. USA. 102: 13658–13663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nusinowitz S., Nguyen L., Radu R. A., Kashani Z., Farber D. B., Danciger M. 2003. Electroretinographic evidence for altered phototransduction gain and slowed recovery from photobleaches in albino mice with a MET450 variant in RPE6. Exp. Eye Res. 77: 627–638. [DOI] [PubMed] [Google Scholar]
- 140.Lyubarsky A. L., Savchenko A. B., Morocco S. B., Daniele L. L., Redmond T. M., Pugh E. N. 2005. Mole quantity of RPE65 and its productivity in the generation of 11-cis-retinal from retinyl esters in the living mouse eye. Biochemistry. 44: 9880–9888. [DOI] [PubMed] [Google Scholar]
- 141.Golczak M., Kuksa V., Maeda T., Moise A. R., Palczewski K. 2005. Positively charged retinoids are potent and selective inhibitors of the trans-cis isomerization in the retinoid (visual) cycle. Proc. Natl. Acad. Sci. USA. 102: 8162–8167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jang G. F., Van Hooser J. P., Kuksa V., McBee J. K., He Y. G., Janssen J. J., Driessen C. A., Palczewski K. 2001. Characterization of a dehydrogenase activity responsible for oxidation of 11-cis-retinol in the retinal pigment epithelium of mice with a disrupted RDH5 gene. A model for the human hereditary disease fundus albipunctatus. J. Biol. Chem. 276: 32456–32465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Janecke A. R., Thompson D. A., Utermann G., Becker C., Hübner C. A., Schmid E., McHenry C. L., Nair A. R., Rüschendorf F., Heckenlively J. R., et al. 2004. Mutations in RDH12 encoding a photoreceptor cell retinol dehydrogenase cause childhood-onset severe retinal dystrophy. Nat. Genet. 36: 850–854. [DOI] [PubMed] [Google Scholar]
- 144.Grayson C., Molday R. S. 2005. Dominant negative mechanism underlies autosomal dominant Stargardt-like macular dystrophy linked to mutations in ELOVL4. J. Biol. Chem. 280: 32521–32530. [DOI] [PubMed] [Google Scholar]
- 145.Karan G., Yang Z., Howes K., Zhao Y., Chen Y., Cameron D. J., Lin Y., Pearson E., Zhang K. 2005. Loss of ER retention and sequestration of the wild-type ELOVL4 by Stargardt disease dominant negative mutants. Mol. Vis. 11: 657–664. [PubMed] [Google Scholar]
- 146.Vasireddy V., Vijayasarathy C., Huang J., Wang X. F., Jablonski M. M., Petty H. R., Sieving P. A., Ayyagari R. 2005. Stargardt-like macular dystrophy protein ELOVL4 exerts a dominant negative effect by recruiting wild-type protein into aggresomes. Mol. Vis. 11: 665–676. [PubMed] [Google Scholar]
- 147.Zhang K., Kniazeva M., Han M., Li W., Yu Z., Yang Z., Li Y., Metzker M. L., Allikmets R., Zack D. J., et al. 2001. A 5-bp deletion in ELOVL4 is associated with two related forms of autosomal dominant macular dystrophy. Nat. Genet. 27: 89–93. [DOI] [PubMed] [Google Scholar]
- 148.Karan G., Lillo C., Yang Z., Cameron D. J., Locke K. G., Zhao Y., Thirumalaichary S., Li C., Birch D. G., Vollmer-Snarr H. R., et al. 2005. Lipofuscin accumulation, abnormal electrophysiology, and photoreceptor degeneration in mutant ELOVL4 transgenic mice: a model for macular degeneration. Proc. Natl. Acad. Sci. USA. 102: 4164–4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Vasireddy V., Jablonski M. M., Khan N. W., Wang X. F., Sahu P., Sparrow J. R., Ayyagari R. 2009. Elovl4 5-bp deletion knock-in mouse model for Stargardt-like macular degeneration demonstrates accumulation of ELOVL4 and lipofusin. Exp. Eye Res. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Agbaga M. P., Brush R. S., Mandal M. N., Henry K., Elliott M. H., Anderson R. E. 2008. Role of Stargardt-3 macular dystrophy protein (ELOVL4) in the biosynthesis of very long chain fatty acids. Proc. Natl. Acad. Sci. USA. 105: 12843–12848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Edwards A. O., Ritter R., Abel K. J., Manning A., Panhuysen C., Farrer L. A. 2005. Complement factor H polymorphism and age-related macular degeneration. Science. 308: 421–424. [DOI] [PubMed] [Google Scholar]
- 152.Hageman G. S., Anderson D. H., Johnson L. V., Hancox L. S., Taiber A. J., Hardisty L. I., Hageman J. L., Stockman H. A., Borchardt J. D., Gehrs K. M., et al. 2005. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc. Natl. Acad. Sci. USA. 102: 7227–7232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Haines J. L., Hauser M. A., Schmidt S., Scott W. K., Olson L. M., Gallins P., Spencer K. L., Kwan S. Y., Noureddine M., Gilbert J. R., et al. 2005. Complement factor H variant increases the risk of age-related macular degeneration. Science. 308: 419–421. [DOI] [PubMed] [Google Scholar]
- 154.Klein R. J., Zeiss C., Chew E. Y., Tsai J. Y., Sackler R. S., Haynes C., Henning A. K., SanGiovanni J. P., Mane S. M., Mayne S. T., et al. 2005. Complement factor H polymorphism in age-related macular degeneration. Science. 308: 385–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhou J., Jang Y. P., Kim S. R., Sparrow J. R. 2006. Complement activation by photooxidation products of A2E, a lipofuscin constituent of the retinal pigment epithelium. Proc. Natl. Acad. Sci. USA. 103: 16182–16187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhou J., Kim S. R., Westlund B. S., Sparrow J. R. 2009. Complement activation by bisretinoid constituents of RPE lipofuscin. Invest. Ophthalmol. Vis. Sci. 50: 1392–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Radu R. A., Han Y., Bui T. V., Nusinowitz S., Bok D., Lichter J., Widder K., Travis G. H., Mata N. L. 2005. Reductions in serum vitamin A arrest accumulation of toxic retinal fluorophores: a potential therapy for treatment of lipofuscin-based retinal diseases. Invest. Ophthalmol. Vis. Sci. 46: 4393–4401. [DOI] [PubMed] [Google Scholar]
- 158.Kong J., Kim S. R., Binley K., Pata I., Doi K., Mannik J., Zernant-Rajang J., Kan O., Iqball S., Naylor S., Sparrow J. R., Gouras P., Allikmets R. 2008. Correction of the disease phenotype in the mouse model of Stargardt disease by lentiviral gene therapy. Gene Ther. 15: 1311–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]