Abstract
Murine desnutrin/human ATGL is a triacylglycerol (TAG) hydrolase with a predicted catalytic dyad within an α-β hydrolase fold in the N-terminal region. In humans, mutations resulting in C-terminal truncation cause neutral lipid storage disease with myopathy. To identify critical functional domains, we measured TAG breakdown in cultured cells by mutated or truncated desnutrin. In vitro, C-terminally truncated desnutrin displayed an even higher apparent Vmax than the full-length form without changes in Km, which may be explained by our finding of an interaction between the C- and N-terminal domains. In live cells, however, C-terminally truncated adenoviral desnutrin had lower TAG hydrolase activity. We investigated a role for the phosphorylation of C-terminal S406 and S430 residues but found that these were not necessary for TAG breakdown or lipid droplet localization in cells. The predicted N-terminal active sites, S47 and D166, were both critical for TAG hydrolysis in live cells and in vitro. We also identified two overlapping N-terminal motifs that predict lipid substrate binding domains, a glycine-rich motif (underlined) and an amphipathic α-helix (bold) within amino acid residues 10–24 (ISFAGCGFLGVYHIG). G14, F17, L18, and V20, but not G16 and G19, were important for TAG hydrolysis, suggesting a potential role for the amphipathic α-helix in TAG binding. This study identifies for the first time critical sites in the N-terminal region of desnutrin and reveals the requirement of the C-terminal region for TAG hydrolysis in cultured cells.
Keywords: adipose triglyceride lipase, mutant, lipid substrate binding domain, C-terminal region, N-terminal region, neutral lipid storage disease with myopathy, hydrolase, patatin-like phospholipase A domain containing 2
Triacylglycerol (TAG) serves as the major energy reserve in higher eukaryotic organisms (1). FFAs are mobilized from TAG through the hydrolytic action of lipases to provide substrates for oxidative metabolism in tissues, as well as substrates for synthesis of complex lipids and signaling molecules. Lipolysis, the breakdown of TAG to FFA and glycerol, occurs in an orderly and regulated manner, with different enzymes acting sequentially at each step (1–3). Hydrolysis of TAG to diacylglycerol is the first step (4–6), and our laboratory, and others, recently identified a novel TAG hydrolase in mice that we called desnutrin (5) [also known as TTS2.2, patatin-like phospholipase A domain containing 2, iPLAζ (4), or in humans, adipose triglyceride lipase (ATGL) (6)]. Desnutrin is highly expressed in white and brown adipose tissue, where it is a major TAG lipase, but is also found at lower levels in most other tissues where it also plays an important role in TAG hydrolysis (5, 7, 8).
Desnutrin contains an N-terminal patatin-like domain, spanning amino acids 8–180, that is characteristic of many plant lipid acyl hydrolases (5, 9). Enzymatic activity of desnutrin is predicted to be derived from an S47-D166 catalytic dyad that lies within an α-β hydrolase fold in the patatin-like domain (5, 9). Mutation of the S47 residue to alanine has been shown to result in a complete loss of function in vitro, confirming the predicted serine-esterase activity of this enzyme (10). However, the requirement of the D166 site has not yet been tested. Four individuals with point mutations in desnutrin/ATGL have been identified, and all developed neutral lipid storage disease with myopathy (NLSDM) (11, 12). In one, a duplication mutation within the N-terminal patatin-like domain caused a frameshift after L159 that is predicted to truncate the enzyme at amino acid position 178 as well as to cause a D166R mutation (11). In the other three individuals, mutations occurred distal to the patatin-like domain, causing truncation of the C-terminal region of desnutrin in one or both alleles (12). Surprisingly, however, truncated desnutrin/ATGL lacking the C-terminal region has been shown in vitro to have similar or even higher activity than the full-length form (13, 14).
To identify and characterize functional sites within desnutrin, we tested activity of a series of C-terminal truncations in adenovirus and C- and N-terminal mutants by expressing them in cultured cells and measuring TAG breakdown. We found that truncation of the C-terminal region of desnutrin increases TAG hydrolase activity in vitro by increasing the apparent Vmax without changing substrate affinity (Km), which we show may be attributed to removal of an inhibitory interaction of the C-terminal region with the N-terminal region. Conversely, we also found that C-terminal truncation impairs TAG hydrolase activity in live cells. While care should be used in extrapolating between effects observed with murine and human forms of desnutrin/ATGL, demonstration that the C-terminal region of desnutrin is required for TAG breakdown in vivo may help to explain the NLSDM phenotype of human C-terminal mutations. Two C-terminal region serines in full-length desnutrin have previously been reported as sites that can be phosphorylated (S406 and S430) (15). We investigated whether loss of phosphorylation at these sites may help to explain impaired activity of C-terminally truncated desnutrin in cells, but found that desnutrin TAG breakdown and localization to lipid droplets were unaffected by serine-to-alanine mutation of these residues. We also examined the N-terminal region and found that both S47 and D166 catalytic dyad residues located within the α-β hydrolase fold are critical for TAG hydrolysis within cells and in vitro. Within the proximal N-terminal region, we identified two overlapping motifs, a glycine-rich motif and an amphipathic α-helix that are predicted by sequence analysis to be neutral lipid substrate-binding domains (16, 17). We show by site-directed mutational analysis that this region, especially the amphipathic α-helix, is critical for TAG hydrolysis.
EXPERIMENTAL PROCEDURES
Materials
[U-14C]Palmitic acid (specific activity, 850 mCi/mmol) and [3H]triolein (specific activity, 53 Ci/mmol) were from PerkinElmer Life Sciences. Lipofectamine 2000 was from Invitrogen. HPLC-grade solvents were from Fisher. TLC plates were from Whatman. Unless otherwise indicated, all other chemical reagents were from Sigma.
Construction of plasmids
Full-length desnutrin cDNA tagged with hemagluttinin epitope (HA) in the pEGFP-N1 vector (5) was used as a template for the production of desnutrin cDNA truncations for subcloning. Full-length desnutrin-green-fluorescent protein (GFP) cDNA was amplified by PCR (forward primer: 5′-CTCGAGACCATGTTCCCGAGGGAGACCAAG-3′; reverse primer: 5′-AAGCTTTTACTTGTACAGCTCGTC-3′) and subcloned into XhoI-HindIII sites of pShuttle-CMV (Stratagene). The W367× truncation was produced by PCR amplification (forward primer: 5′- GTCGASHYCACCASHYTGTTCCCGAGGGAGACCAAG-3′; reverse primer: 5′-GGSHYGSHYCCSHYCTCCACCGGATATCTTCAGGGACATC-3′), and the cDNA was subcloned in-frame with GFP into the SalI/ApaI sites of pEGFP-N1, and then the fusion product was subcloned into SalI/NotI sites of pShuttle-CMV. The Q289× truncation was amplified (forward primer: 5′-GTCGACACCATGTTCCCGAGGGAGACCAAG-3′; reverse primer: 5′-GGGCCCTATCCTCCTCTCCAGCCCTCTCCTC-3′), and the resulting cDNA was subcloned into SalI/ApaI sites of pEGFP-N1, and then the GFP fusion product was subcloned into SalI/NotI sites of pShuttle-CMV. The 1-246 HA-tagged N-terminal truncation was produced by PCR amplification of desnutrin (forward primer: 5′-AGATCTACGATGTTCCCGAGGGSHYAGACCAAGTGG-3′; reverse primer: 5′-CTCGAGTSHYCAGTAATCTSHYGGAACATCGTATGGGTASHYAAGTCCATSHYCTCTSHYGTSHYASHYSHYGSHYCSHYCCTG-3′), and the resulting cDNA was subcloned into the BamHI/XhoI site of pcDNA3.1. The 237–486 FLAG-tagged truncation was produced by PCR amplification of desnutrin (forward primer: 5′-AAGCTTCACCATGTGCAAACAGGGCTACAGA-3′; reverse primer: 5′-CTCGAGTCACTTGTCATCGTCGTCCTTGTAGTCGCAAGGCGSHYGGAGGCCAGG-3′) with the resulting cDNA subcloned into HindIII/XhoI sites of pcDNA3.1. All insert fragments were fully sequenced. Site-directed mutagenesis was employed to generate point mutations. Briefly, HA-tagged desnutrin in pcDNA3.1 or pEGFP-N1 was amplified by PCR (for primers, see supplementary ) under the following conditions: 95°C for 30s (one cycle), then 16 cycles of denaturation (95°C for 30s), annealing (52°C for 1 min), and extension (72°C for 8 min), with a final extension cycle of 10 min at 72°C. The resulting product was digested with DpnI to linearize methylated template DNA, and remaining cDNA was transformed. Plasmid DNA prepared from isolated colonies was sequenced to identify mutants.
Production of recombinant adenoviruses
Adenoviruses were produced using the Ad-Easy adenovirus system according to the manufacturer's instructions (Stratagene). Briefly, pShuttle-CMV targeting vectors containing full-length or truncated desnutrin tagged with GFP were linearized by digestion with PmeI and electroporated into BJ5183 electrocompetant cells. pShuttle-IRES-hrGFP-2 was used to generate control GFP adenovirus. Adenoviral vectors containing insert DNA were linearized with PacI and transfected into 293FT cells using Lipofectamine 2000. Following amplification in 293FT cells, infectious adenovirus was titred in 293FT cells using the Adeno-X Rapid Titer Kit (Clontech).
Cell culture
COS-7 cells and 293FT cells were from ATCC. 3T3-L1CARΔ cells were a kind gift from D. Orlicky, University of Colorado Cancer Center (18). Cells were maintained in DMEM containing 10% FBS with 50 units/ml of penicillin and 50 μg/ml of streptomycin (complete medium). G418 was added to 293FT cells and 3T3-L1CARΔ (500 μg/ml). 3T3-L1CARΔ cells were differentiated to adipocytes as previously described (19).
Confocal microscopy
3T3-L1CARΔ cells were grown and differentiated on glass coverslips as previously described (19). COS-7 cells were grown in 6-well plates on glass coverslips in complete medium containing 200 μM oleic acid bound to BSA (to promote lipid droplet formation). Cell were infected at a multiplicity of infection of ∼10 and then maintained for a further 48–72 h. Cells were labeled in culture with nile red (1 μg/ml), washed with PBS, fixed with 4% paraformaldehyde, and then stained with diamidino-2-phenylindole (1 μg/ml). Coverslips were mounted on glass slides using the ProLong Antifade kit (Invitrogen) and imaged using a Zeiss LSM 510 Meta confocal microscope.
In vitro TAG hydrolase assay
Lysates were prepared from COS-7 cells overexpressing full-length, truncated, or mutated desnutrin by lysis in 50 mM Tris, pH 7.4, 0.1 M sucrose, and 1 mM EDTA, followed by centrifugation at 16,000 g for 15 min at 4°C.
Reactions were started by addition of supernatants containing 50 μg of protein in 50 μl volumes to 50 μl of substrate containing 2× concentrations of [3H]triolein (ranging from 10–100 μM as indicated in Fig. 2A) sonicated into mixed micelles with 25 μM egg yolk lecithin, 100 μM taurocholate, 2% BSA (w/v), 2 mM EDTA, 1 mM DTT, and 50 mM potassium phosphate, pH 7.2. Reactions were allowed to proceed for 20 min at 37°C and were terminated by the addition of 1.63 ml of methanol: chloroform:heptane (10:9:7). Fatty acids were extracted with 0.5 ml of 0.1 M potassium carbonate, 0.1 M boric acid, pH 10.5, and radioactivity in 0.7 ml of upper phase obtained after centrifugation for 20 min at 800 g was quantified by liquid scintillation counting. Radioactivity in GFP control samples was taken as a baseline of TAG hydrolase activity in lysates at each concentration and subtracted from test values. Activities in mutants were normalized relative to full-length desnutrin levels as assessed by Western blotting.
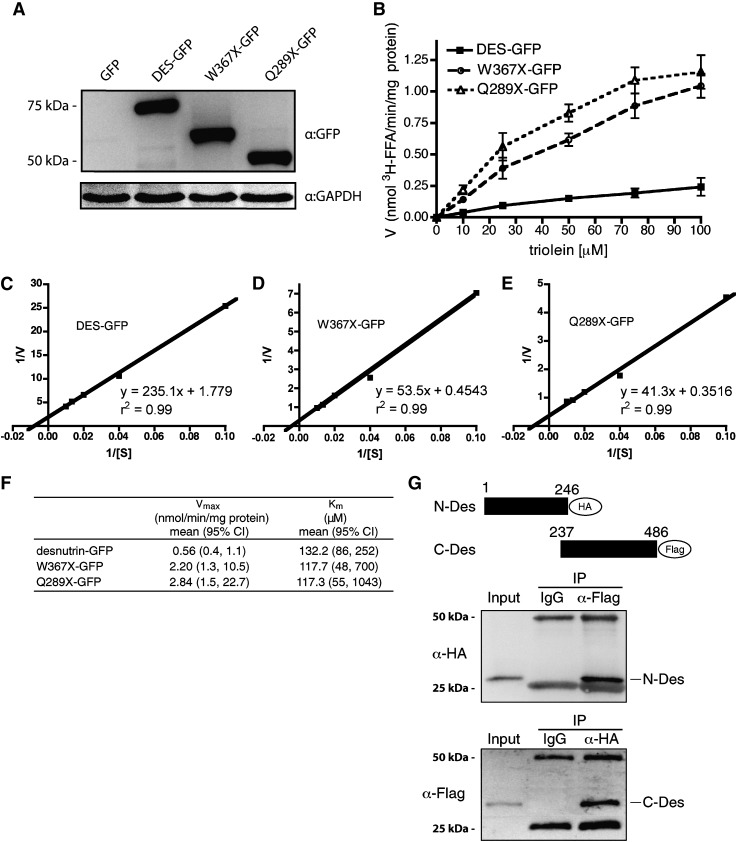
Fig. 2.
Desnutrin C-terminal deletion mutants have increased catalytic activity in vitro. A: Immunoblot of full-length and truncated desnutrin expressed in COS-7 cells. B: Substrate velocity plot showing in vitro [3H]triolein hydrolase activity of COS-7 cell lysates overexpressing desnutrin-GFP (DES-GFP) or C-terminal deletion mutants of desnutrin-GFP truncated at W367 (W367×-GFP) or Q289 (Q289×-GFP). Lysates from cells expressing GFP alone were taken as a baseline measure at each substrate concentration and subtracted from test values to derive net TAG hydrolase activity that was expressed per mg of lysate protein (n = 3). C–E: Double reciprocal plots for desnutrin-GFP and C-terminal truncations of desnutrin-GFP. F: Km and apparent Vmax derived from double reciprocal plots. G: Immunoblot showing the interaction of N-terminally and C-terminally truncated desnutrin. Lysates from COS-7 cells cotransfected with plasmids expressing the HA-tagged N-terminal region of desnutrin (amino acids 1–246) and the FLAG-tagged C-terminal region of desnutrin (amino acids 237–486) were immunoprecipitated with IgG (negative control) or with anti-FLAG (top) or anti-HA (bottom) antibodies, subjected to SDS-PAGE, and probed to determine the presence of the interacting partner. Upper and lower nonspecific bands are IgG heavy and light chains, respectively.
TAG breakdown and separation of lipids
Tracer levels of radiolabeled [U-14C]palmitic acid were complexed with BSA in FBS prior to dilution with DMEM (final concentration 0.15 μCi/ml) and then added to COS-7 cells, 3T3-L1CARΔ adipocytes, or 293FT cells grown in 12-well plates for 4 h to allow for incorporation into complex lipids including TAG. After 4 h, COS-7 and 293FT cells accumulated approximately 8,000 dpm per well of [U-14C]palmitic acid into cellular TAG stores, and approximately 60,000 dpm of [U-14C]palmitic acid into phospholipid. During the labeling period, 3T3-L1CARΔ adipocytes accumulated approximately 10,000 dpm per well of [U-14C]palmitic acid into the pool of cellular TAG and approximately 18,000 dpm per well of [U-14C]palmitic acid into cellular phospholipid. Cells were then washed with unlabeled complete medium and infected with full-length or C-terminally truncated adenoviral GFP-tagged desnutrin at a multiplicity of infection = 10. Cells were maintained in unlabeled complete medium for an additional 60 h chase period. TAG hydrolysis in transfected 293FT cells was performed as we have previously described (5). Lipids from cells were extracted by the method of Bligh and Dyer and separated by TLC using hexane/diethyl ether/acetic acid (80:20:2) (20), after which radioactive lipids were detected by autoradiography and 14C-TAG was quantified by scraping of the corresponding band into CytoScint (Fisher Scientific) followed by scintillation counting. After 60 h, an average of approximately 55% of radioactivity was remaining in TAG stores of COS-7 and 293FT cells expressing GFP control and 40% in 3T3-L1CARΔ adipocytes. The dpms were normalized to protein content per well and expressed as a percentage of control.
Immunoblotting
Cells were washed with ice-cold PBS, scraped into RIPA lysis buffer [50 mM Tris, pH 7.4, 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.5% SDS, and 1% (v/v) protease inhibitor cocktail (Sigma)], incubated on ice for 30 min with frequent vortexing, and then centrifuged at 4°C for 20 min at 12,000 g. Fifty to 100 µg of protein in supernatants were mixed with 2× Laemmeli sample buffer (125 mM Tris-HCl, pH 6.8, 20% glycerol, 4% SDS, 10% 2-mercaptoethanol, and 0.04% bromophenol blue) and denatured by heating for 5 min to 95°C and then subjected to 8% SDS-PAGE and transferred to nitrocellulose membranes. Blots were blocked for 1 h and then probed overnight at 4°C, first with antibodies to GFP (Sigma), HA (Covance), FLAG (Sigma), or GAPDH (Santa Cruz) and then with HRP-conjugated anti-mouse or anti-rabbit secondary antibodies (Santa Cruz). Signals were detected by enhanced chemiluminescence.
Coimmunoprecipitaton
COS-7 cells were cotransfected with an N-terminal truncation of desnutrin tagged with HA and a C-terminal truncation of desnutrin tagged with FLAG. Lysates prepared in RIPA buffer were centrifuged at 4°C for 20 min at 12,000 g and then rotated overnight at 4°C with antibodies to either HA or FLAG, or with a nonspecific control IgG, followed by addition of 30 μl of protein A/G agarose beads for 1 h. Beads were collected by pulse centrifugation and washed four times with lysis buffer prior to resolution by SDS-PAGE and immunoblotting.
Statistical analyses
Results are means ± SEM. Differences among groups were analyzed by one-way ANOVA with Dunnett's posthoc test.
RESULTS AND DISCUSSION
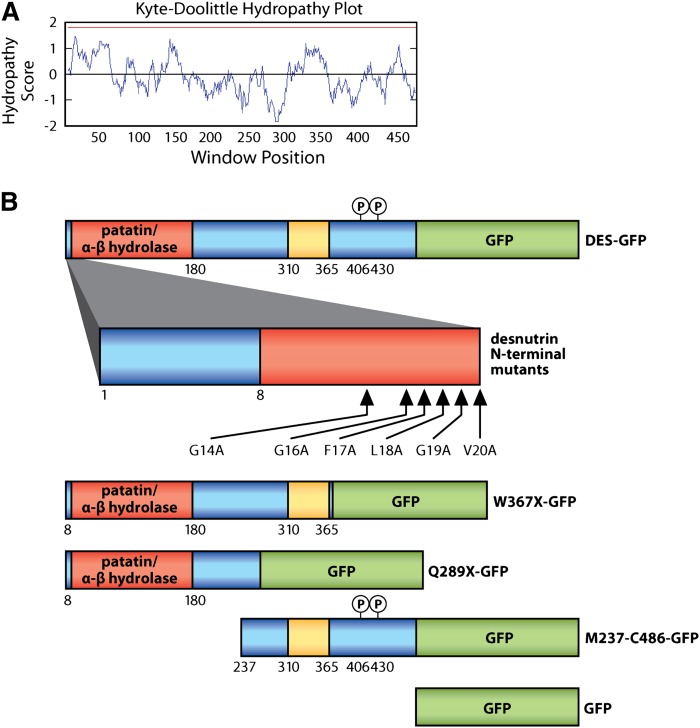
To identify and characterize critical functional domains, we performed a series of studies with mutated and truncated forms of murine desnutrin examining TAG hydrolysis in live cells. Desnutrin/ATGL is predicted to derive TAG hydrolase activity from an S47-D166 catalytic dyad located within an α-β hydrolase fold in the N-terminal region, called the patatin-like domain, named for its shared homology with the plant acyl hydrolase patatin. However, humans with NLSDM have been found harboring mutations that occur both within and distal to the patatin-like domain, suggesting the importance of other regions in desnutrin for activity in vivo. We constructed adenovirus expressing full-length desnutrin tagged with GFP, as well as GFP-tagged desnutrin lacking portions of the C-terminal region. These full-length and deletion mutants are shown in alignment with a Kyte-Doolittle plot of hydropathy scores (Fig. 1A, B). The Q289×-GFP construct was designed to mimic the truncated protein that results from a nonsense mutation reported in a patient with NLSDM (12). The W367×-GFP construct was designed to truncate the C-terminal region while leaving intact the putative hydrophobic span occurring between amino acids 310 and 365 of desnutrin (12).
Fig. 1.
Desnutrin mutants and truncations. A: Kyte-Doolittle hydropathy plot (window size = 19) with red line designating the cutoff for potential transmembrane domains. B: Schematic illustrating desnutrin mutants and truncations, shown in alignment with hydropathy plot. Red = patatin-like domain/α-β hydrolase fold. Yellow = putative hydrophobic span. Green = GFP tag. Blue = unstructured region.
We first tested the in vitro catalytic activity of full-length GFP-tagged murine desnutrin as well as the W367×-GFP and Q289×-GFP C-terminal region deletion mutants (Fig. 2A). Although TAG hydrolase activity of desnutrin/ATGL is routinely measured in vitro, kinetics for this enzyme have not been reported previously. All three forms of desnutrin displayed classic Michaelis-Menten kinetics (Fig. 2B). The Km for triolein for full-length desnutrin was determined to be 0.13 mM (Fig. 2C, F). In this regard, the Km for triolein for hormone-sensitive lipase has been reported to be 0.73 mM (21). Higher affinity of desnutrin for triolein compared with HSL is in accord with the recently accepted function of HSL as principally a diacylglycerol hydrolase, rather than a TAG hydrolase (22). All three desnutrin forms had similar Km values, indicating that affinity for TAG did not change with loss of the C-terminal region (Fig. 2CndashF). The apparent Vmax, however, was 4- to 5-fold higher for truncations of desnutrin than for the full-length form, indicating increased catalytic activity (Fig. 2F). This finding is in agreement with a previous report suggesting that the C-terminal region of desnutrin/ATGL possesses a negative regulatory function in vitro (14). To determine whether the C-terminal region may interact to mediate decreased catalytic activity of the N-terminal region, we differentially tagged and coexpressed these domains. Using either as bait, we were able to coimmunoprecipitate both, indicating an interaction between the C-terminal region and the N-terminal region (Fig. 2G). This finding suggests that the negative regulatory function of the C-terminal domain in vitro likely results from the masking of important sites in the catalytic N-terminal patatin domain through interaction.
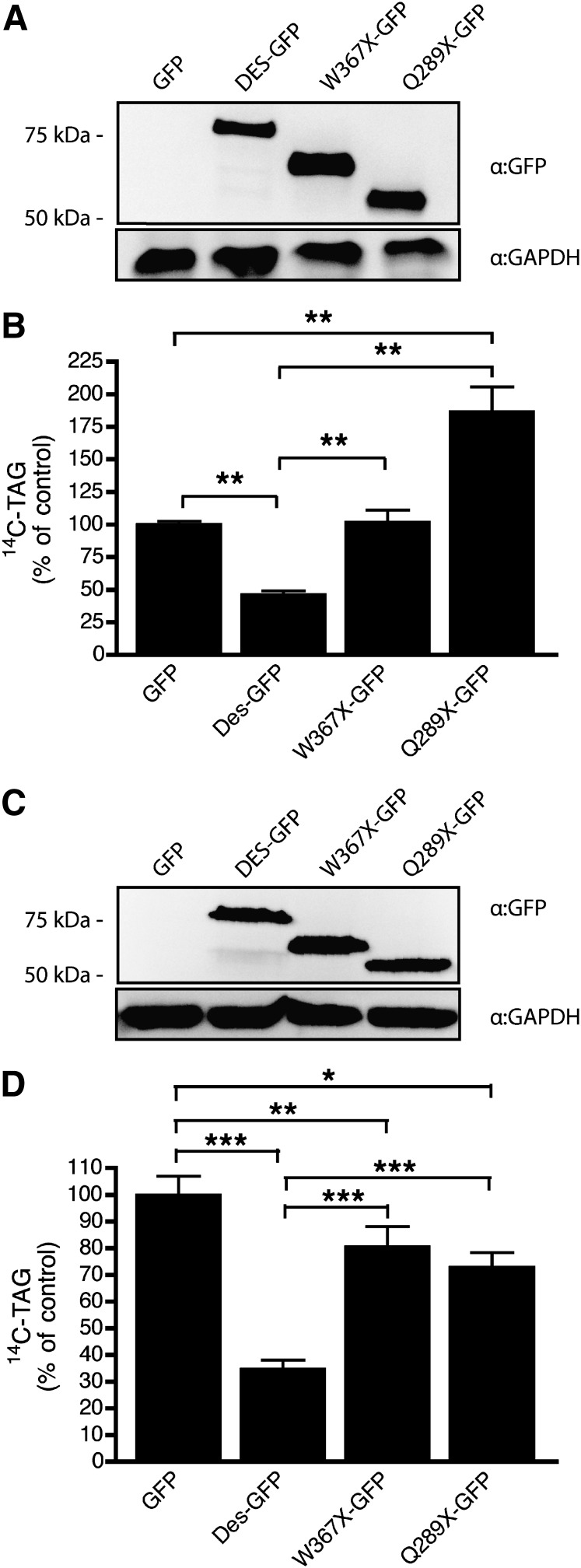
While the above in vitro results show enhanced activity for desnutrin C-terminal truncations, NLSDM in individuals with similar truncations implies impaired function in vivo. Therefore, we measured TAG hydrolase activity of C-terminal truncations in live cells. COS-7 cells or 3T3-L1CARΔ adipocytes were uniformly labeled with [U-14C]palmitate that readily incorporates into complex cellular lipids, including TAG, and then washed with unlabeled media before infection with various forms of adenoviral desnutrin. Cells were then maintained for a further 60 h to allow for viral expression and breakdown of radiolabeled TAG. We found that in COS-7 cells, full-length desnutrin-GFP (Fig. 3A) increased breakdown of radiolabeled TAG by >50% relative to control GFP, indicating abundant TAG hydrolase activity by desnutrin in this cell line (Fig. 3B). Truncated desnutrin missing portions of the C-terminal region, however, completely lacked TAG hydrolase activity in COS-7 cells (Fig. 3B). Previous work in COS-7 cells has reported that loss of the C-terminal region of human ATGL partially impairs localization of this protein to lipid droplets (14), which may explain some of the impaired TAG breakdown by truncated desnutrin in cultured cells. However, our results show that breakdown of 14C-TAG was actually impaired in COS-7 cells infected with Q289×-GFP relative to GFP controls (Fig. 3B), suggesting that this truncated desnutrin may hinder TAG breakdown rather than merely lack activity. Previous work by others suggested that the C-terminal region of desnutrin may interfere with interaction with CGI-58 (14). Therefore, Q289×-GFP may act in a dominant inhibitory fashion in COS-7 cells by forming increased interactions with CGI-58 or other yet to be identified cofactor(s), which are nonfunctional. Alternatively, truncated desnutrin lacking the C-terminal region may displace other lipases from the lipid droplet but fail to make appropriate interactions required for activity at the lipid droplet surface. In 3T3-L1CARΔ adipocytes (Fig. 3C), unlike effects observed in COS-7 cells, both Q289×-GFP and W367×-GFP displayed a partial capacity to hydrolyze TAG, although this activity was significantly decreased relative to that of full-length desnutrin-GFP (Fig. 3D). The presence of residual activity by truncated desnutrin in adipocytes but not in COS-7 fibroblasts may help to explain the intriguing finding that individuals with truncated desnutrin in NLSDM are not obese, despite accumulating excessive TAG in other tissues, such as skin and muscle (12). Interestingly, we have observed by immunoblotting under nonreducing conditions that formation of higher molecular weight bands by desnutrin expressed in 3T3-L1CARΔ adipocytes is greatly diminished when the C-terminal region is deleted (data not shown). This indicates that the C-terminal domain of desnutrin may be involved in the formation of protein complexes and suggests that impaired protein–protein interactions may also contribute, at least in part, to decreased TAG hydrolase activity of truncated desnutrin in cultured cells.
Fig. 3.
Desnutrin C-terminal deletion mutants lack TAG hydrolase activity in cells. Immunoblots of full-length GFP-tagged desnutrin (DES-GFP) or GFP-tagged truncated desnutrin (W367×-GFP and Q289×-GFP) in COS-7 cells (A) or 3T3-L1CARΔ (C). TAG hydrolysis measured by 14C-TAG content remaining in COS-7 cells (B) or 3T3-L1CARΔ adipocytes (D) pulse labeled with [U-14C]palmitate, washed, and then infected with GFP, desnutrin-GFP, or GFP-tagged desnutrin C-terminal deletion mutants prior to chase with unlabeled medium for 60 h. Values are means ± SEM from two to three separate experiments. *P < 0.05,**P < 0.01, and ***P < 0.001.
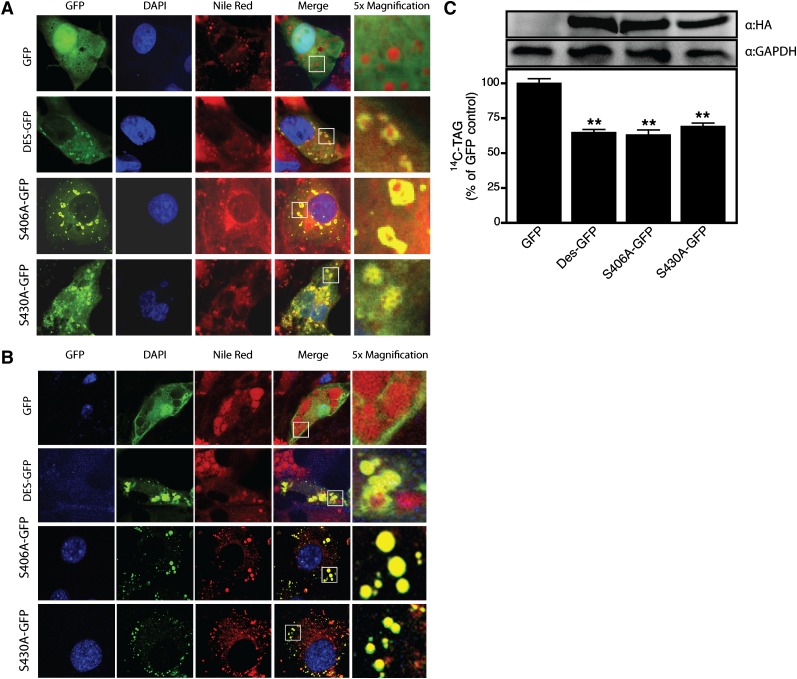
Our studies demonstrate that although the N-terminal region contains all residues necessary for catalytic activity in vitro, the C-terminal region is clearly critical for TAG breakdown in cells. The distal C-terminal region of human ATGL contains two previously identified residues (S406 and S430) that can be phosphorylated (15), both of which lie within motifs that are conserved in murine desnutrin. The function(s) of these sites, however, are unknown. Since both W367×-GFP and Q289×-GFP lack these residues, it is possible that loss of critical phosphosphorylation events at these sites may contribute to loss of TAG hydrolase activity of truncated desnutrin in cells. We investigated the effect of S406A and S430A mutations in full-length desnutrin on localization to the lipid droplet or TAG hydrolase activity in cells. Control GFP was found to localize diffusely throughout the cytoplasm and nucleus of both COS-7 cells and 3T3-L1CARΔ adipocytes (Fig. 4A, B, respectively). In contrast, desnutrin-GFP and GFP-tagged mutants of desnutrin (S406A and S430A) were easily visualized on lipid droplets, indicating that phosphorylation at these sites is not critical for localization (Fig. 4A, B). We next tested the effect of these mutations on TAG breakdown in live cells (Fig. 4C). 293FT cells transfected with desnutrin-GFP had significantly greater TAG breakdown relative to GFP-transfected control cells. S406A-GFP and S430A-GFP mutants showed TAG breakdown similar to wild-type desnutrin, indicating that phosphorylation at these sites is not necessary for TAG breakdown and that loss of phosphorylation at these sites does not explain impaired TAG breakdown by C-terminally truncated desnutrin.
Fig. 4.
Phosphorylation of desnutrin at S406 and S430 is not essential for activity or localization. Confocal micrographs showing diffuse localization of GFP (control) or prominent lipid droplet localization of GFP-tagged desnutrin, S406A, and S430A desnutrin mutants in COS-7 cells (A) or 3T3-L1CARΔ adipocytes (B). TAG hydrolysis by desnutrin-GFP or serine to alanine mutants of desnutrin-GFP measured in live 293FT cells (C). Immunoblots show expression of desnutrin and mutant desnutrin and GAPDH (control). Values are means ± SEM from a representative experiment. **P < 0.01 versus GFP control.
It is likely that elucidation of the complete function and mechanism(s) underlying requirement of the C-terminal region of desnutrin/ATGL for TAG hydrolase activity in live cells will require considerable additional work. Of note, an important difference in the C-terminal domains of murine desnutrin and human ATGL is suggested by our studies. We found that TAG hydrolase activity of the W367×-GFP truncation was impaired in cells, although the putative hydrophobic span (Fig. 1A, B) was intact, whereas interruption of the hydrophobic span has previously been shown to be required to impair TAG hydrolase activity of C-terminally truncated human ATGL (13). Therefore, in mice, significantly more of the C-terminal domain appears to be critical for activity in cells. In this regard, others have also reported functional differences between the C-terminal regions of murine desnutrin and human ATGL in vitro (14). Using chimeric proteins, Schweiger et al. demonstrated that replacement of the C-terminal region of human ATGL with murine desnutrin significantly increased in vitro activity of the enzyme, whereas replacement of the C-terminal region of murine desnutrin with human ATGL significantly decreased activity (14). The C-terminal region of human ATGL, including the hydrophobic span, shares a high degree of identity with murine desnutrin. Future investigation of nonidentical sequences, including the regions spanning amino acids 266–295 and 467–484, and the additional 17 amino acid residues at the C terminus of the human sequence, may provide greater insight into the specific functions of the C-terminal domain.
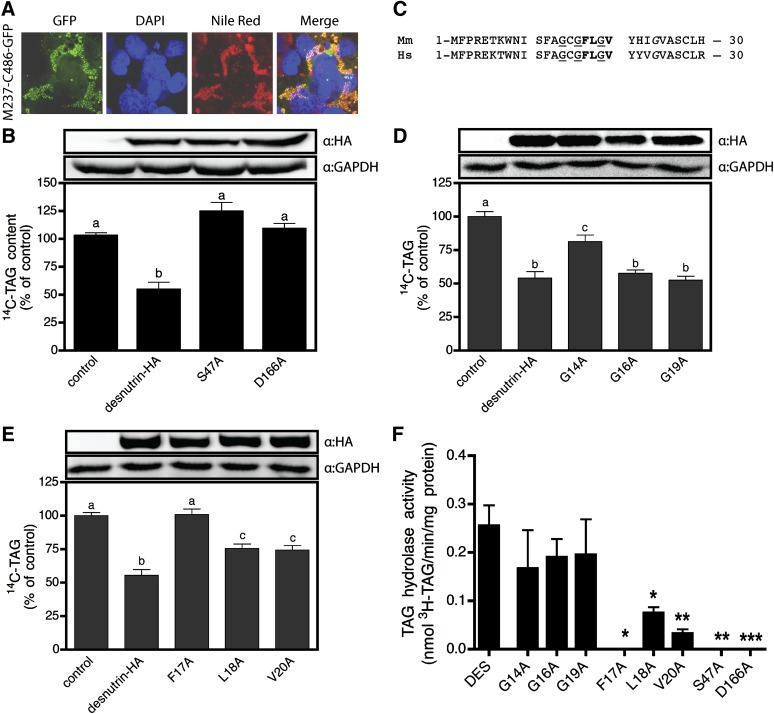
Since the above in vitro kinetic studies indicate that the N-terminal region contains all residues required for catalytic activity of desnutrin, we investigated the nature of predicted functional motifs in this domain for TAG breakdown in cells. We first tested the requirement of the N-terminal region for residency of desnutrin on the lipid droplet. Lipid droplets are complex organelles consisting of a lipid core rich in nonpolar TAG coated with a polar phospholipid layer as well as myriad proteins (23). Localization of desnutrin to the surface of lipid droplets likely requires complex stabilizing protein-lipid and protein-protein interactions essential for tethering of the lipase to this organelle, as well as for proper orientation to permit access of catalytically essential residues to TAG substrate in the core. Importantly, the domain required for lipid droplet localization in cells may be separate from the catalytic domain(s) required for TAG substrate-binding and hydrolysis; therefore, mutations in either domain may affect desnutrin activity separately. To test whether the catalytic N-terminal domain is required for lipid droplet localization, we transfected COS-7 cells with GFP-tagged desnutrin lacking almost the entire N-terminal region (M237-C486-GFP) and found that it was easily and strongly visualized on lipid droplets in COS-7 cells (Fig. 5A). The C-terminal domain alone is therefore sufficient for lipid droplet localization, while the N-terminal region and patatin-like domain contains the TAG substrate binding and hydrolysis sites that are critical for catalytic activity but are not necessary for lipid droplet residency.
Fig. 5.
The proximal N-terminal region contains the S47-D166 catalytic dyad as well as a putative lipid substrate-binding domain. A: Confocal micrograph showing lipid droplet localization of GFP-tagged desnutrin lacking the N-terminal domain (M237-C486-GFP). B: TAG hydrolysis by S47A and D166A desnutrin mutants is decreased to control levels in live 293FT cells. C: Sequence alignment of the proximal N-terminal regions of murine and human desnutrin. Underlined glycines (G) comprise the glycine-rich motif (XhXhGXGXXG), while letters in bold or italics are part of a predicted amphipathic α-helix (FLXVXXXn), where X is any amino acid except proline, h is a hydrophobic residue, and n is any nonpolar amino acid. D: TAG hydrolysis by G14A, G16A, and G19A mutants of desnutrin in live 293FT cells. E: TAG hydrolysis by F17A, L18A, and V20A desnutrin mutants in live 293FT cells. Values are expressed as a percentage of pcDNA3.1 control vector. F: In vitro TAG hydrolase activity of wild-type and mutant desnutrin assayed in COS-7 cell lysates using 100 μM 3H-triolein. Values are means ± SEM from two to four separate experiments. Columns with different superscripts are significantly different, abcP < 0.01, *P < 0.05, **P < 0.01, and ***P < 0.001.
We next tested the requirement of predicted functional amino acids for TAG hydrolysis by desnutrin in cells. Although mutational analysis has previously confirmed requirement of S47 for activity in vitro (10), the D166 residue has not previously been tested, nor has the importance of either site been examined in live cells. Here, we report that both sites are critical for TAG hydrolysis in cells (Fig. 5B) and in vitro (Fig. 5F). Sequence alignment of murine desnutrin and human ATGL indicates that two motifs in the proximal N-terminal region are highly conserved (Fig. 5C). The first is a glycine-rich decapeptide ISFAGCGFL(G) corresponding to amino acids 10–19. This motif consists of invariant glycine residues in the sequence XhXhGXGXX(G), where X = any residue, h = a hydrophobic residue, and the final G is optional (17). This sequence has been identified in proteins as a dinucleotide binding motif (24), but it is also found in phospholipases A2 where it stabilizes the transition state during catalysis (17, 25). We found that G14A mutants showed partially impaired TAG breakdown in 293FT cells relative to wild-type desnutrin, retaining only about 40% activity (Fig. 5D). Conversely, however, G16A and G19A mutants showed similar TAG breakdown to wild-type desnutrin, indicating that these sites are not critical in cells. In vitro, none of the sites appeared to be required for activity (Fig. 5F), suggesting that the G14 residue may play an indirect role in TAG hydrolysis in living cells, possibly by participating in interactions with other cellular proteins. The second motif consists of the octapeptide FLGVYHIG, corresponding to amino acids 17–24, that is a nonconsensus version of the sequence FLXLXXXn, where X = any residue except proline and n = a nonpolar residue (Fig. 5C). This motif is predicted to form an amphipathic α-helix involved in binding of nonpolar lipid substrates and is found in a number of diverse enzymes with functions in lipid metabolism, including TAG hydrolase, which is a hepatic TAG lipase (16, 26). Our mutational analysis indicated that the phenylalanine at position 17 is critical to TAG breakdown by desnutrin in cells, since conversion of F17A completely abolished TAG breakdown to control levels (Fig. 5E). On the other hand, L18A or V20A mutations only partially blunted TAG breakdown to 55–60% of wild-type desnutrin levels (Fig. 5E). Similar effects of alanine conversions were observed for these mutants when assayed in vitro (Fig. 5F). Taken together, our results suggest an important function for the proximal N-terminal region of desnutrin in TAG hydrolysis, while sequence analysis and comparison with other lipid metabolizing enzymes suggests that this region may have a potential role in binding TAG substrate.
In conclusion, here, we attempted to define critical domains and residues of desnutrin required for TAG hydrolysis in cells. We show that the C-terminal region of desnutrin is required for TAG breakdown in live cells but not for hydrolase activity in vitro, where deletion mutants lacking the C-terminal region display a similar Km but increased apparent Vmax relative to the full-length enzyme. Indeed, we found binding of the C-terminal region of desnutrin to the N-terminal region, which likely explains the inhibitory role of the C-terminal domain on activity in vitro. Loss of phosphorylation at S406 or S430 did not alter cellular localization or TAG hydrolysis by desnutrin and therefore did not contribute to decreased activity of C-terminal deletion mutants in cells. By point mutation, we show the critical nature of D166 in the catalytic dyad of desnutrin as well as the importance of both S47 and D166 sites to TAG hydrolysis by desnutrin in cells. Point mutation of amino acids in the proximal N-terminal region also demonstrates the presence of catalytically important residues in an amphipathic α-helix that sequence analysis indicates may form a putative neutral lipid substrate-binding domain.
Supplementary Material
Acknowledgments
We thank Chris Lange for assistance with graphics.
Footnotes
Abbreviations:
- ATGL
- adipose triglyceride lipase
- GFP
- green fluorescent protein
- HA
- hemaglutinin
- NLSDM
- neutral lipid storage disease with myopathy
- TAG
- triacylglycerol
This work was supported in part by Grant DK-075682 from the National Institutes of Health to H.S.S. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. R.E.D. is a recipient of postdoctoral fellowships from the Natural Sciences and Engineering Research Council of Canada and from the Canadian Institutes of Health Research.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of one figure.
REFERENCES
- 1.Duncan R. E., Ahmadian M., Jaworski K., Sarkadi-Nagy E., Sul H. S. 2007. Regulation of lipolysis in adipocytes. Annu. Rev. Nutr. 27: 79–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmadian M., Duncan R. E., Jaworski K., Sarkadi-Nagy E., Sul H. S. 2007. Triacylglycerol metabolism in adipose tissue. Future Lipidol. 2: 229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jaworski K., Sarkadi-Nagy E., Duncan R. E., Ahmadian M., Sul H. S. 2007. Regulation of triglyceride metabolism. IV. Hormonal regulation of lipolysis in adipose tissue. Am. J. Physiol. Gastrointest. Liver Physiol. 293: G1–G4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jenkins C. M., Mancuso D. J., Yan W., Sims H. F., Gibson B., Gross R. W. 2004. Identification, cloning, expression, and purification of three novel human calcium-independent phospholipase A2 family members possessing triacylglycerol lipase and acylglycerol transacylase activities. J. Biol. Chem. 279: 48968–48975. [DOI] [PubMed] [Google Scholar]
- 5.Villena J. A., Roy S., Sarkadi-Nagy E., Kim K. H., Sul H. S. 2004. Desnutrin, an adipocyte gene encoding a novel patatin domain-containing protein, is induced by fasting and glucocorticoids: ectopic expression of desnutrin increases triglyceride hydrolysis. J. Biol. Chem. 279: 47066–47075. [DOI] [PubMed] [Google Scholar]
- 6.Zimmermann R., Strauss J. G., Haemmerle G., Schoiswohl G., Birner-Gruenberger R., Riederer M., Lass A., Neuberger G., Eisenhaber F., Hermetter A., et al. 2004. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 306: 1383–1386. [DOI] [PubMed] [Google Scholar]
- 7.Haemmerle G., Lass A., Zimmermann R., Gorkiewicz G., Meyer C., Rozman J., Heldmaier G., Maier R., Theussl C., Eder S., et al. 2006. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 312: 734–737. [DOI] [PubMed] [Google Scholar]
- 8.Schweiger M., Schreiber R., Haemmerle G., Lass A., Fledelius C., Jacobsen P., Tornqvist H., Zechner R., Zimmermann R. 2006. Adipose triglyceride lipase and hormone-sensitive lipase are the major enzymes in adipose tissue triacylglycerol catabolism. J. Biol. Chem. 281: 40236–40241. [DOI] [PubMed] [Google Scholar]
- 9.Rydel T. J., Williams J. M., Krieger E., Moshiri F., Stallings W. C., Brown S. M., Pershing J. C., Purcell J. P., Alibhai M. F. 2003. The crystal structure, mutagenesis, and activity studies reveal that patatin is a lipid acyl hydrolase with a Ser-Asp catalytic dyad. Biochemistry. 42: 6696–6708. [DOI] [PubMed] [Google Scholar]
- 10.Lake A. C., Sun Y., Li J. L., Kim J. E., Johnson J. W., Li D., Revett T., Shih H. H., Liu W., Paulsen J. E., et al. 2005. Expression, regulation, and triglyceride hydrolase activity of Adiponutrin family members. J. Lipid Res. 46: 2477–2487. [DOI] [PubMed] [Google Scholar]
- 11.Akiyama M., Sakai K., Ogawa M., McMillan J. R., Sawamura D., Shimizu H. 2007. Novel duplication mutation in the patatin domain of adipose triglyceride lipase (PNPLA2) in neutral lipid storage disease with severe myopathy. Muscle Nerve. 36: 856–859. [DOI] [PubMed] [Google Scholar]
- 12.Fischer J., Lefevre C., Morava E., Mussini J-M., Laforet P., Negre-Salvayre A., Lathrop M., Salvayre R. 2007. The gene encoding adipose triglyceride lipase (PNPLA2) is mutated in neutral lipid storage disease with myopathy. Nat. Genet. 39: 28–30. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi K., Inoguchi T., Maeda Y., Nakashima N., Kuwano A., Eto E., Ueno N., Sasaki S., Sawada F., Fujii M., et al. 2008. The lack of the C-terminal domain of adipose triglyceride lipase causes neutral lipid storage disease through impaired interactions with lipid droplets. J. Clin. Endocrinol. Metab. 93: 2877–2884. [DOI] [PubMed] [Google Scholar]
- 14.Schweiger M., Schoiswohl G., Lass A., Radner F. P. W., Haemmerle G., Malli R., Wolfgang G., Cornaciu I., Oberer M., Salvayre R., et al. 2008. The C-terminal region of human adipose triglyceride lipase affects enzyme activity and lipid droplet binding. J. Biol. Chem. 283: 17211–17220. [DOI] [PubMed] [Google Scholar]
- 15.Kim S. C., Chen Y., Mirza S., Xu Y., Lee J., Liu P., Zhao Y. 2006. A clean, more efficient method for in-solution digestion of protein mixtures without detergent or urea. J. Proteome Res. 5: 3446–3452. [DOI] [PubMed] [Google Scholar]
- 16.Au-Young J., Fielding C. J. 1992. Synthesis and secretion of wild-type and mutant human plasma cholesteryl ester transfer protein in baculovirus-transfected insect cells: The carboxyl-terminal region is required for both lipoprotein binding and catalysis of transfer. Proc. Natl. Acad. Sci. USA. 89: 4094–4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geisow M. J. 1986. Common domain structure of Ca2+ and lipid-binding proteins. FEBS Lett. 203: 99–103. [DOI] [PubMed] [Google Scholar]
- 18.Orlicky D. J., DeGregori J., Schaack J. 2001. Construction of stable coxsackievirus and adenovirus receptor-expressing 3T3-L1 cells. J. Lipid Res. 42: 910–915. [PubMed] [Google Scholar]
- 19.Duncan R. E., Sarkadi-Nagy E., Jaworski K., Ahmadian M., Sul H. S. 2008. Identification and functional characterization of Adipose-specific Phospholipase A2 (AdPLA). J. Biol. Chem. 283: 25428–25436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bligh E. G., Dyer W. J. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37: 911–917. [DOI] [PubMed] [Google Scholar]
- 21.Krintel C., Osmark P., Larsen M. R., Resjo S., Logan D. T., Holm C. 2008. Ser649 and Ser650 are the major determinants of protein kinase A-mediated activation of human hormone-sensitive lipase against lipid substrates. PLoS One. 3: e3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haemmerle G., Zimmermann R., Hayn M., Theussl C., Waeg G., Wagner E., Sattler W., Magin T. M., Wagner E. F., Zechner R. 2002. Hormone-sensitive lipase deficiency in mice causes diglyceride accumulation in adipose tissue, muscle, and testis. J. Biol. Chem. 277: 4806–4815. [DOI] [PubMed] [Google Scholar]
- 23.Bartz R., Zehmer J. K., Zhu M., Chen Y., Serrero G., Zhao Y., Liu P. 2007. Dynamic activity of lipid droplets: protein phosphorylation and GTP-mediated protein translocation. J. Proteome Res. 6: 3256–3265. [DOI] [PubMed] [Google Scholar]
- 24.Bellamacina C. R. 1996. The nicotinamide dinucleotide binding motif: a comparison of nucleotide binding proteins. FASEB J. 10: 1257–1269. [DOI] [PubMed] [Google Scholar]
- 25.Dessen A., Tang J., Schmidt H., Stahl M., Clark J. D., Seehra J., Somers W. S. 1999. Crystal structure of human cytosolic phospholipase A2 reveals a novel topology and catalytic mechanism. Cell. 97: 349–360. [DOI] [PubMed] [Google Scholar]
- 26.Alam M., Gilham D., Vance D. E., Lehner R. 2006. Mutation of F417 but not L418 or L420 in the lipid binding domain decreases the activity of triacylglycerol hydrolase. J. Lipid Res. 47: 375–383. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.