Abstract
In addition to providing mechanical stability, growing evidence suggests that surfactant lipid components can modulate inflammatory responses in the lung. However, little is known of the molecular mechanisms involved in the immunomodulatory action of surfactant lipids. This study investigates the effect of the lipid-rich surfactant preparations Survanta®, Curosurf®, and the major surfactant phospholipid dipalmitoylphosphatidylcholine (DPPC) on interleukin-8 (IL-8) gene and protein expression in human A549 lung epithelial cells using immunoassay and PCR techniques. To examine potential mechanisms of the surfactant lipid effects, Toll-like receptor 4 (TLR4) expression was analyzed by flow cytometry, and membrane lipid raft domains were separated by density gradient ultracentrifugation and analyzed by immunoblotting with anti-TLR4 antibody. The lipid-rich surfactant preparations Survanta®, Curosurf®, and DPPC, at physiological concentrations, significantly downregulated lipopolysaccharide (LPS)-induced IL-8 expression in A549 cells both at the mRNA and protein levels. The surfactant preparations did not affect the cell surface expression of TLR4 or the binding of LPS to the cells. However, LPS treatment induced translocation of TLR4 into membrane lipid raft microdomains, and this translocation was inhibited by incubation of the cells with the surfactant lipid. This study provides important mechanistic details of the immune-modulating action of pulmonary surfactant lipids.
Keywords: lipopolysaccharide, cytokines, inflammation
As a primary interface between the lung and pathogens, the epithelial cells lining the airways and the alveoli are crucial sites for innate immune responses. In addition to providing a physico-chemical barrier, the respiratory epithelium can respond to inhaled micro-organisms and irritants by releasing a variety of inflammatory mediators (1–4). Inappropriate or excessive innate immune responses of respiratory epithelia could likely contribute to the development of respiratory diseases, as activation of these cells is documented in a variety of human diseases, such as asthma, chronic obstructive pulmonary disease, cystic fibrosis, and respiratory infections (2, 5–7).
Stimulation of respiratory epithelial cells with inflammatory mediators and infectious stimuli causes the increased expression and secretion of a number of cytokines with chemoattractant and pro-inflammatory functions (1, 3, 4, 8, 9). Interleukin-8 (IL-8), a principal chemokine released by lung epithelial cells, plays a pivotal role in the recruitment of inflammatory cells into the lung (10, 11). In addition, this cytokine upregulates the expression of adhesion molecules on neutrophils (12), promotes their transendothelial migration (13), and stimulates the oxidative burst and the release of lysosomal enzymes (14). Thus, IL-8 plays a pivotal role in the recruitment of neutrophils into the lung and their activation to clear infection.
However, several studies have suggested that overexpression of IL-8 is central to pathophysiologic changes in the airways and to the severity of airflow obstruction in chronic inflammatory lung diseases, such as chronic obstructive pulmonary disease, cystic fibrosis, and bronchiectasis (9, 15–17). There is also accumulating evidence that Gram-negative bacterial infections play a role in the pathology and exacerbation of the inflammatory responses in some of these chronic lung diseases (18–20). Regulation of epithelial cell IL-8 responses is thus crucial to prevent excessive inflammatory reactions that are injurious to the lung.
The epithelial cells of the alveoli and the lower airways secrete pulmonary surfactant, a complex mixture of lipids and proteins (21). Although the major physiologic function of pulmonary surfactant is to confer mechanical stability to the alveoli and the small airways (22–24), there is growing evidence that pulmonary surfactant also has a potential role in modulating inflammation in normal and injured lungs (25–30). Our previous work (31, 32) and that of others (33–36) have shown that surfactant lipids modulate the release of oxidative and inflammatory mediators from inflammatory cells. However, the potential role of surfactant in modulating inflammatory responses from lung epithelial cells, which synthesize and secrete surfactant components, and the mechanisms by which surfactant lipids affect the immune response are poorly understood.
This study investigates the role of pulmonary surfactant, and in particular, the major phospholipid component, in modulating IL-8 production by alveolar epithelial cells. The molecular mechanisms by which surfactant downregulates the inflammatory responses of these cells are also determined.
MATERIALS AND METHODS
All of the reagents were obtained from Sigma (Poole, UK) unless otherwise stated.
Cell lines
The human lung epithelial cell line A549 (CCL-185) and Calu-3 (HTB-55) were obtained from American Type Culture Collection and maintained as described by the supplier. The A549 cell line was used because it has been shown to produce IL-8 and many other mediators in response to lipopolysaccharide (LPS) (37) and human lung epithelial cells are difficult to obtain. However, it should be noted that although these cells retain many features of type II alveolar epithelial cells, they exhibit some differences from primary human type II cells and show alterations in surfactant production (38).
Surfactant preparations
Survanta® (Abott Laboratories, Kent, UK), a modified bovine lung surfactant, contains 79.1 mol% phosphatidylcholine (PC) [of which dipalmitoyl PC (DPPC) constitutes 75%] and only one-tenth of the surfactant protein (SP)-B and half of SP-C of native surfactant. Curosurf® (Chiesi Farmaceutici, Parma, Italy), a modified porcine surfactant, contains only 30–50% SP-B and SP-C of the natural porcine surfactant. The major lipid species is PC (73 mol%), of which 50% is DPPC (39).
DPPC (Avanti Polar Lipids, Alabaster, AL) was provided by the supplier in chloroform and was aliquoted in silanized bijoux vials and dried in a fume hood under a gentle stream of nitrogen gas. The dried film of DPPC was resuspended in RPMI medium, which was prewarmed to 50°C in a water bath for 15 min. The resuspended DPPC was kept for 1 h at 50°C while being vortexed intermittently. After the phospholipid preparation was hydrated, it was sonicated for three 20 s bursts. DPPC prepared in this way is taken up by cells and intercalates into the cell membrane (31, 40).
LPS
LPS from Escherichia coli O111.B4 was made up as stock solutions at 10 μg/ml in sterile phosphate buffered saline with vortex mixing and kept in depyrogenated glass vials at −80°C. This commercial LPS preparation was found to be free from protein and nucleic acid contamination by Coomassie Blue binding and 260/280 nm absorbance, respectively, and from proteinase and DNase/RNase treatment and cellular responses as described recently (41).
Induction of IL-8
Confluent monolayers of cells (105 cells/ml) were stimulated with LPS (100 ng/ml) in the presence of recombinant human sCD14 (250 μg/ml) for various times (see figure legends). After incubation, the total RNA was isolated from cell pellets for RT-PCR, and the supernatants were collected and stored at −80°C for cytokine determination (see below). To investigate the effect of surfactants on IL-8 expression, cells were preincubated with DPPC (500 μg/ml) or Curosurf® or Survanta® (both at 250 μg/ml) for 2 h, followed by washing with PBS three times before being stimulated with LPS (100 ng/ml, 18 h). Preliminary dose-response experiments and our previous data (31, 32) showed that preincubation of cells with these concentrations of lipids were optimal for the inhibition of cytokine expression in these cells. In some experiments to investigate the reversibility of the surfactant effects, cells were preincubated with surfactants or DPPC, which were then washed off as above, but the cells were incubated in fresh medium for various times (0–24 h) before stimulation with LPS.
ELISA for IL-8 production
IL-8 in the cell supernatants was assayed by ELISA using a DuoSet Kit (R and D Systems, Oxford, UK) according to the manufacturer's instructions.
Expression of Toll-like receptor 4
Surface expression of Toll-like receptor 4 (TLR4) was assessed in A549 cells by flow cytometry using anti-TLR4-PE conjugated antibody and isotype control (H-80; eBioscience, CA) on a FACS calibur flow cytometer (BD Biosciences). The data were acquired using CELLQuest (BD Biosciences) and analyzed using WinMDI 2.8 software (Joe Trotter, Pharmingen, CA). Mean fluorescence index (mfi) was calculated as a percentage as: mfi% = fluorescence (test) – fluorescence (isotype control)/fluorescence (isotype control) × 100%.
LPS binding assay
A549 cells were preincubated for 2 h with DPPC or Survanta®; they were then thoroughly washed with PBS and treated with trypsin solution. The cells were resuspended in fresh medium (with serum), and 105 cells in 15 μl of staining buffer (PBS-1% BSA) were transferred to 96 well plates. FITC-conjugated LPS (100 ng/ml) [E. coli O111.B4 LPS (Sigma), shown to be free from protein and nucleic acids as described for LPS above] was added, and the cells were incubated at 4°C in the dark for 30 min. The cells were then washed and resuspended in fresh buffer, and binding of FITC-LPS to the cells was determined by flow cytometry. The data were acquired using CELLQuest (BD Biosciences) and analyzed with WinMDI 2.8 software (Joe Trotter). The binding of LPS to the lung epithelial cells was analyzed after gating on side and forward scatters. The mfi was calculated as a percentage as: mfi% = fluorescence (LPS) – fluorescence (control)/fluorescence (control) × 100%.
Isolation of lipid rafts
The lipid raft domains were isolated from A549 cells as described by Triantafilou et al. (42), with slight modifications. Briefly, cells were lysed in 300 µl MEB buffer (150 mM NaCl and 20 mM MES, pH 6.5) containing 200 mM Na3VO4, 1% Triton X-100, and protease inhibitor cocktail for 1 h on ice. Lysates were homogenized by sonicating for three 10 s bursts and mixed with an equal volume of 90% sucrose in MEB and placed at the bottom of polyallomer centrifuge tubes. Samples were overlaid with 1 ml of 30% sucrose and 500 μl 5% sucrose in MEB buffer and centrifuged at 100,000 g for 16 h using TLS-55 rotor and Optima™ TL ultracentrifuge. Fractions (250 μl) were gently removed from the top of the gradient, and 4 µl of 5% sodium deoxycholate was added to each fraction to solubilize rafts.
Lipid raft containing fractions were identified by dot-blotting for GM-1 ganglioside with HRP-conjugated cholera toxin (Sigma, Poole, UK). Proteins in lipid raft fractions were concentrated by methanol precipitation as previously described [43].
TLR4 detection in membrane fractions by Western blotting
Cell membrane fractions with equal amounts of protein were separated on 10% SDS-PAGE and blotted onto a nitrocellulose membrane using a blot module (Invitrogen, Paisley, UK). TLR4 in each fraction was determined by immunoblotting with anti-TLR4 antibody (H-80; Santa Cruz Biotechnology, CA) and detected using an ECL Plus detection kit (Amersham Biosciences, Buckinghamshire, UK) as a major band at 96 kDa.
RT-PCR
Total RNA was isolated using an Ambion RNAqueous™ kit (Cambridgeshire, UK) according to the manufacturer's instructions. The total RNA (2 μg) was reverse transcribed, and cDNA was amplified by PCR using oligonucleotide primers specific for IL-8 (5′-CGATGTCAGTGCATAAAGACA-3′and 5′-GAATTCTCAGCCCTCTTCAAAAA-3′) and GAPDH (5′-TGCTGGGGCTGGTGGTC-3′ and 5′-TCAAGTGGGGCGATGCTG-3′). As a negative control, 2 μg of RNA was subjected to PCR without the RT step. PCR products were separated by agarose gel electrophoresis and detected with ethidium bromide.
Data analysis
Statistical comparisons among groups were determined by one-way ANOVA. To determine differences between groups, Tukey's all pairwise comparison was used. A P value of <0.05 was considered significant. Minitab software version 14 (Minitab, UK) was used for all statistical analyses.
RESULTS
Effect of surfactants on the viability of A549 cells
The viability of the cells was determined after 2–24 h incubation with the surfactants. It was found that none of the preparations significantly affected cell viability (data not shown). Cell viability after surfactant treatment was always >90% in all experiments.
Effect of surfactant on LPS-induced IL-8 expression in A549 cells
Recent observations show that A549 epithelial cells do not respond to low but physiologically relevant concentrations of LPS (∼ng/ml) unless accessory molecules, such as sCD14, are provided (3). Thus, all experiments with LPS stimulation of A549 cells in this study were done in the presence of recombinant sCD14 or pooled human serum.
We have previously shown that surfactant lipids have a suppressive effect on monocyte inflammatory responses when coincubated with the cells for up to 9 days (44). However, the same study also showed that the surfactants are effective when preincubated with cells for 2 h prior to LPS stimulation. Since the use of 2 h point simplifies the setup of experiments, this time was routinely used here. To determine the optimal concentrations for the anti-inflammatory actions of the surfactant preparations on LPS-induced response, a dose-dependent experiment was set up using a concentration from 0–500 μg/ml of surfactant phospholipids preparations (Fig. 1A).
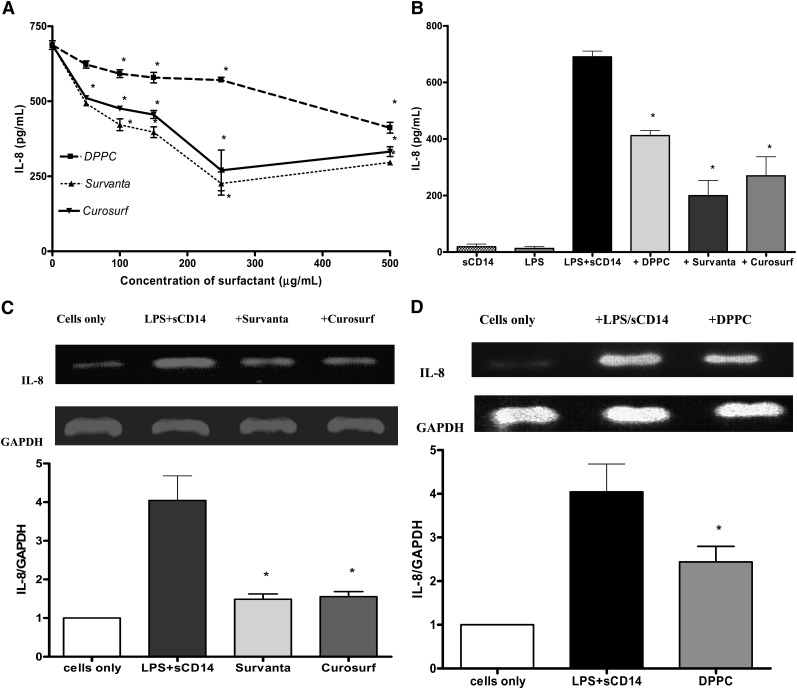
Fig. 1.
Surfactant lipids downregulate the production of LPS-induced IL-8 in A549 cells. A: ELISA results for IL-8 released by A549 cells pretreated with Survanta®, Curosurf®, or DPPC for 2 h at 0 to 500 μg/ml and stimulated with LPS (100 ng/ml) in the presence of sCD14 (250 ng/ml) for 18 h. Pretreatment of cells with Survanta® (≥50μg/ml), Curosurf® (≥50 μg/ml), or DPPC (≥100 μg/ml) significantly suppressed the release of IL-8. * P < 0.05 versus no surfactant. B: IL-8 released by A549 cells pretreated with Survanta® (250 μg/ml), Curosurf® (250 μg/ml), or DPPC (500 μg/ml) for 2 h and then stimulated with LPS (100 ng/ml) in the presence of sCD14 (250 ng/ml) for 18 h. Induction of IL-8 was significantly reduced when the cells were preincubated with the surfactant preparations. C and D: RT-PCR for IL-8 mRNA expression in A549 cells pretreated with Survanta® or Curosurf® (250 μg/ml) (C) or liposomal DPPC (500μg/ml) (D) for 2 h followed by stimulation with LPS for 4 h in the presence of sCD14. The graphs show the densitometric analyses of the IL-8 mRNA relative to GAPDH mRNA control. Values are mean ± SD (n = 4). *P < 0.05 versus cells without surfactant pretreatment.
When A549 were preincubated for 2 h with Survanta® or Curosurf® at a concentration ≥50 μg/ml, the amount of LPS-induced IL-8 was markedly reduced. DPPC alone also had a significant suppressive effect on LPS-induced IL-8 production from A549 epithelial cells when used at concentration of >100 μg/ml. For most of this work, a concentration of 500 μg/ml DPPC, which is within the physiological ranges for surfactants (45, 46) and showed a higher reduction of LPS-induced response, has been used. The comparable level of reduction in the level of LPS-mediated IL-8 release was seen when Survanta® or Curosurf® were used at a concentration of 250 μg/ml.
The effect of DPPC (500 μg/ml), Survanta® (250 μg/ml), or Curosurf® (250 μg/ml) on the expression of LPS-induced IL-8 mRNA was also determined. The results of the semiquantitative PCR experiments (Fig. 1C, D) show that all these surfactant preparations significantly downregulated the expression of LPS-stimulated IL-8 mRNA expression.
To investigate whether pulmonary surfactant also regulates the release of IL-8 induced by inflammatory cytokines, the release of IL-8 from A549 cells stimulated with tumor necrosis factor-α (TNF-α) or IL-1β was studied. There was no marked reduction in the level of IL-8 induced by TNF-α or IL-1β when the cells were preincubated with either whole surfactant preparations or DPPC prior to being stimulated with these cytokines (data not shown). This result implies that the IL-8-inhibitory effects of the surfactants might be specific for LPS-induced responses.
In order to show that the immunomodulatory role of the surfactant preparations and DPPC was not specific to the A549 cell line, another bronchial epithelial cell line, Calu-3, was used. The LPS-induced chemokine release from Calu-3 cells was also downregulated by Survanta® or DPPC to a similar extent as that observed with the A549 cell line (data not shown).
The effect of surfactants on the expression of TLR4 and LPS binding to A549 cells
As surfactant lipids showed no effect on TNFα- or IL-1β-induced IL-8, they must have been modulating LPS- induced responses at the receptor level, as these cytokines share common signaling pathways with LPS downstream of their receptors (47–50). To elucidate the possible mechanisms by which the surfactant preparations and DPPC were regulating the LPS-induced IL-8 responses, we first investigated whether the surfactants had any effect on the binding of LPS to the cells or the expression of the LPS receptor TLR4 on the cell surface. It has been recently demonstrated that A549 epithelial cells express functional TLR4 on their surfaces (51, 52). When the A549 cells were incubated with Survanta® or DPPC and the expression of TLR4 was studied by flow cytometry, it was found that the surfactants had no effect on expression of this LPS receptor (Fig. 2). The effect of surfactant preparations or DPPC on the LPS binding to A549 was also investigated by flow cytometry using FITC-conjugated LPS. The pretreatment of A549 cells with Survanta®, Curosurf®, or DPPC had no effect on the binding of LPS to A549 cells (Fig. 3).
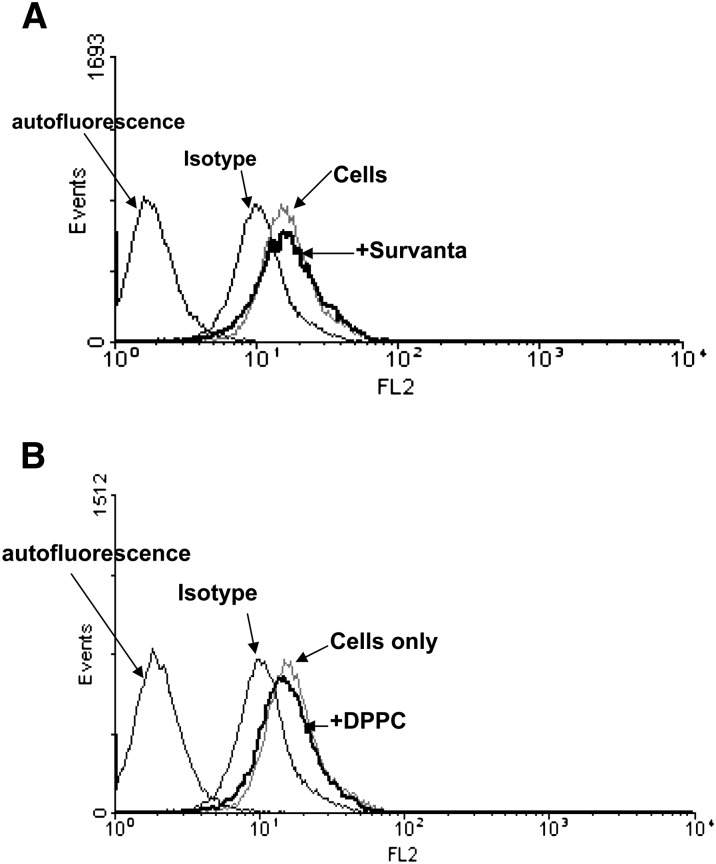
Fig. 2.
Effect of surfactant on the surface TLR-4 expression in A549 cells. The cells were preincubated with surfactant lipid preparations Survanta® (250 μg/ml) (A) or DPPC (500 μg/ml) (B) and the surface TLR4 expression determined by flow cytometry with phycoerythrin-conjugated anti-human TLR4. “Cells only” indicates untreated A549 cells stained with anti-TLR4 antibody. Mean fluorescence index (%); cells only 67.02 ± 17.9; DPPC-treated cells 70.08 ± 19.2 and Survanta®-treated cells 72.3 ± 20.4 (NS). Background autofluorescence was produced by A549 cells without antibody. Representative flow cytometry histograms from three independent experiments are shown.
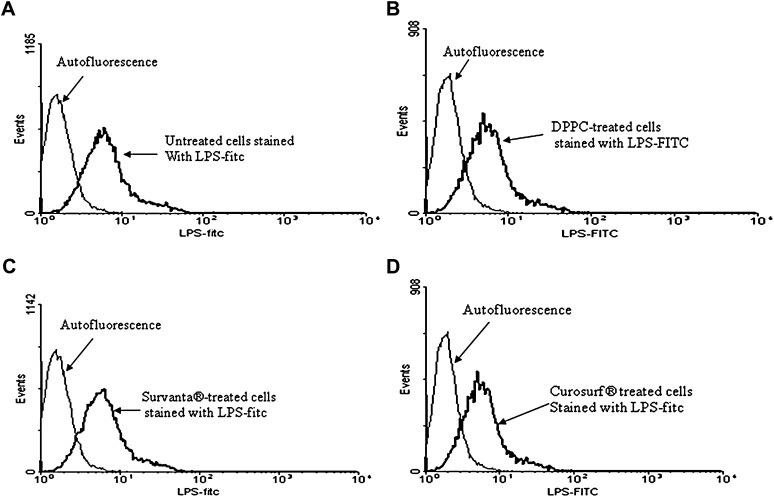
Fig. 3.
Effect of surfactant lipid preparations on the LPS binding to A549 cells. Flow cytometry of A549 cells incubated with FITC-conjugated LPS for 30 min after either no treatment (A) or preincubation with DPPC (500 μg/ml) (B), Survanta® (250 μg/ml) (C), or Curosurf® (D) for 2 h. Pretreatment of the cells with surfactant preparations did not affect the binding of LPS to the cells. Mean fluorescence percentage values: cells alone = 59.1 ± 11; DPPC-treated = 58.2 ± 27; Survanta®-treated = 64.9 ± 18, and Curosurf®-treated = 61.49 ± 14.2 (NS). Representative data from three independent experiments are shown.
Pulmonary surfactants regulate LPS-induced IL-8 by preventing translocation of TLR4 into lipid raft domains
It has been recently shown that membrane microdomains known as lipid rafts are used as platforms for the assembly of a functional receptor complex for LPS. The lateral mobility of TLR4 into the membrane lipid raft domains has been shown to be crucial for LPS signaling (42, 53). Molecules that disrupt lipid raft integrity, such as mycostatin, are known to inhibit signal transduction in response to LPS in monocytic cell lines (42).
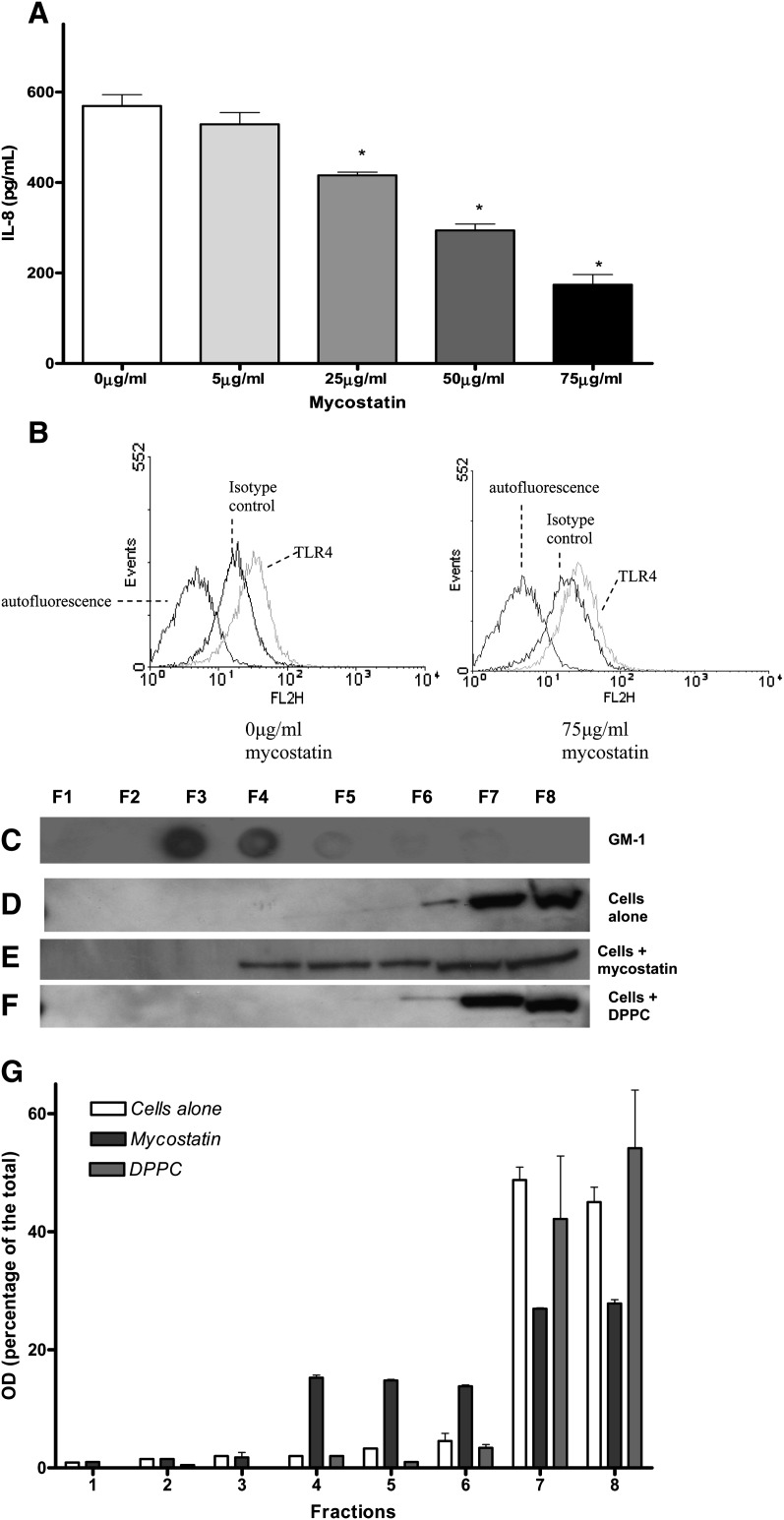
The role of lipid raft involvement in the induction of chemokine release by A549 cells was examined in this study first by disrupting the raft microdomains with mycostatin. The cells were preincubated with increasing concentrations of mycostatin (5–75 μg/ml) for 30 min and stimulated with LPS (100 ng/ml) for 18 h in the presence of sCD14 (250 ng/ml). Mycostatin treatment (75 μg/ml) disrupted membrane rafts as confirmed by the distribution of the raft marker, GM-1, into all membrane fractions (data not shown), as has been reported previously (42). The disruption of the lipid rafts caused a dose-dependent inhibition of LPS-mediated IL-8 production, which suggested that raft microdomains are important in LPS signaling in lung epithelial cells (Fig. 4A). Experiments showed that mycostatin did not affect the cell surface expression of TLR4 (Fig. 4B) but disrupts the partitioning of TLR-4 into raft and nonraft domains (Fig. 4C). The disrupting effect of mycostatin on the lipid rafts was independent of LPS treatment of the cells (data not shown). Mycostatin was therefore inhibiting the LPS response via disruption of lipid raft assemblies rather than direct effects on TLR4.
Fig. 4.
The role of lipid raft microdomain integrity on LPS-induced cytokine release from A549 cells. A: IL-8 ELISA results from A549 cells preincubated with mycostatin (0–75 μg/ml) for 30 min before stimulation with LPS for 18 h. Mycostatin significantly inhibited the LPS-induced IL-8 production in A549 cells (* P < 0.05 versus untreated cells). Values are mean ± SD, n = 3. B: Flow cytometry results showing that mycostatin treatment did not affect the cell surface TLR4 expression. Mean fluorescence index (%); 0 μg/ml mycostatin = 63.6 ± 19; 75 μg/ml mycostyatin = 62.9 ± 16 (NS). Representative data from three independent experiments are shown. C and D: Immunoblot results showing that the marker GM-1 is exclusively located in lipid raft domains (C), while TLR-4 is exclusively found in the nonraft fractions in untreated cells untreated (D). When cells were preincubated with mycostatin (75 μg/ml) for half an hour, it disrupted the partition of TLR-4 into lipid raft and nonraft domains (E), whereas DPPC (500 μg/ml) alone did not disrupt the partition of TLR-4 into lipid raft and nonraft fractions (F). G: Densitometric analysis of the TLR-4 protein in the membrane fractions 1–8 for D–F.
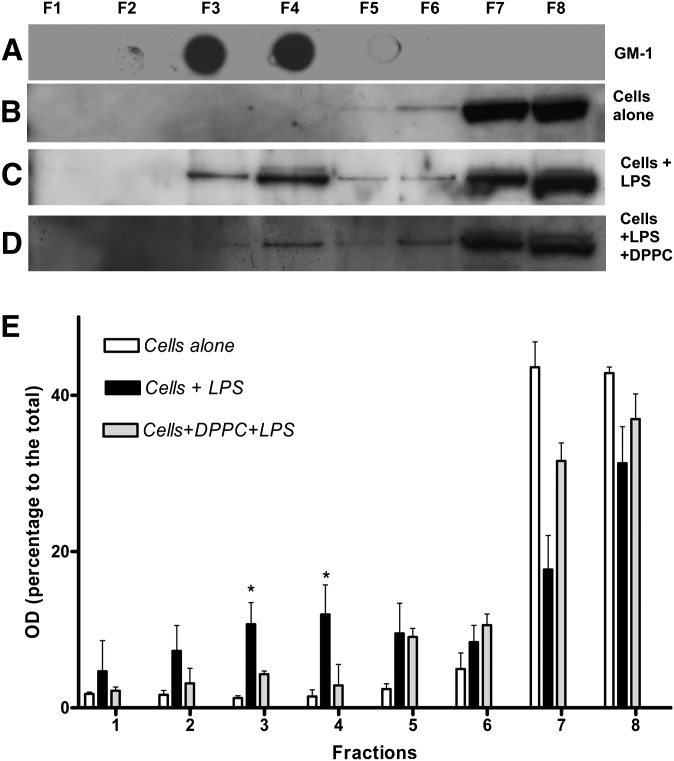
The effect of the surfactant preparations on the translocation of TLR-4 into lipid raft was next examined as described in Materials and Methods. Membrane fractions were prepared from A549 cells treated with LPS/pooled human serum for an hour with or without preincubation with the surfactants. The fractions containing the lipid rafts were identified by expression of the ganglioside GM1, known to be located in lipid raft domains (42). All the membrane fractions were then immunoblotted for TLR4 to visualize the location of the LPS receptor. Figure 5A–E shows the results of these experiments. It can be seen that in control untreated A549 cells, TLR4 is normally found outside the lipid raft domains (Fig. 5B). However, on stimulation of the cells with LPS, TLR4 translocates into the lipid raft domains to form the signaling receptor complex (Fig. 5C). When the cells are preincubated with DPPC, TLR4 is prevented from moving into lipid raft domains and remains in membrane fractions outside the rafts (Fig. 5D). Densitometric analysis of the blots in Fig. 5 was performed to confirm the amounts of TLR4 in each membrane fraction (Fig. 5E). This shows that in unstimulated cells, 94% of TLR4 is in the nonraft fractions (fractions 5–8), whereas this falls to 66% in cells stimulated with LPS (as TLR4 translocates to the raft fractions) and is 88% in LPS-stimulated cells pretreated with DPPC. These results show that DPPC inhibits the translocation of a significant proportion of TLR4 into raft fractions.
Fig. 5.
Surfactant inhibits the translocation of TLR4 into lipid raft microdomains. A549 cells were treated with MBE buffer with 1% Triton X-100 for 1 h on ice and then subjected to zonal centrifugation at 100,000 g. Fractions were collected from the top of the gradient (F1–F8); 5 µl of 5% sodium deoxycolate was added to each fraction, and equal amounts of each fractions were analyzed by dot blotting and Western blotting after the proteins in each fraction was concentrated. A:The lipid raft marker, GM-1, was detected by dot blot using HRP-conjugated cholera toxin. B: TLR4 receptor in nonraft fractions in resting cells. C and D: TLR4 receptor translocates into raft fractions in cells stimulated with LPS (C) and effect of DPPC preincubation on TLR4 receptor translocation in cells that were stimulated with LPS (D). E: Densitometric analysis of the TLR4 protein in the membrane fractions 1–8 from the above experiments. The blots shown are representative of four independent experiments. (* P < 0.05 versus DPPC pretreated).
The effect of surfactant preparation on the LPS-induced response is reversible
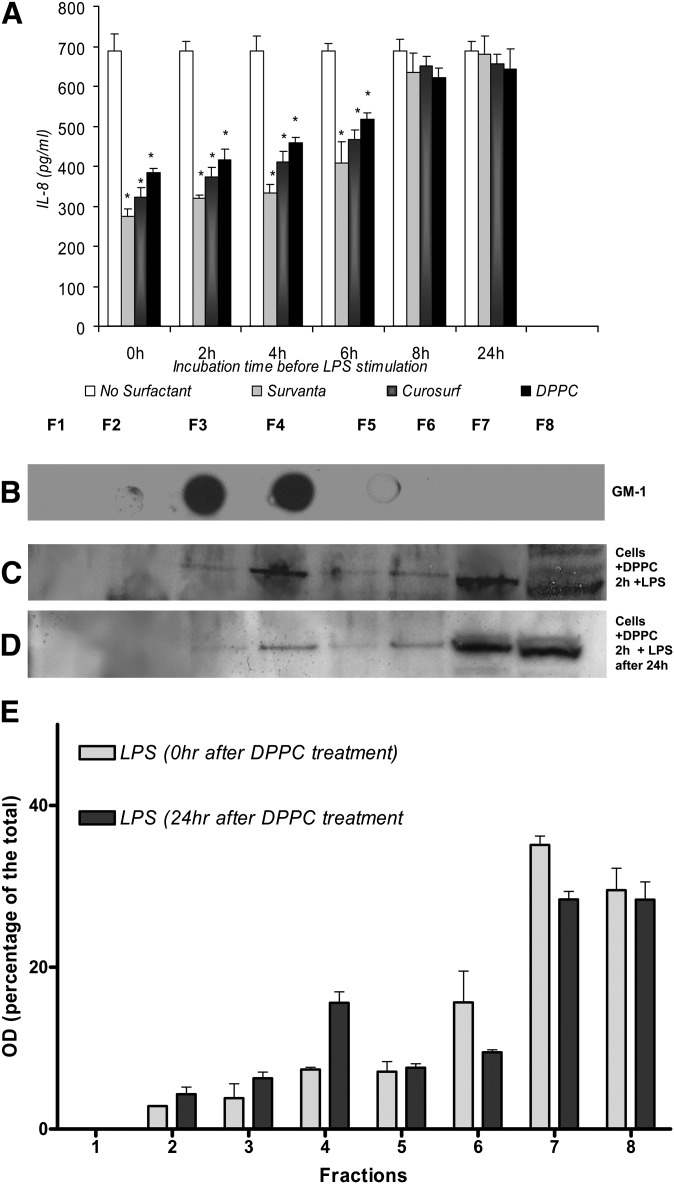
Previously, we found that surfactant lipids were most effective in inhibiting LPS-induced cytokine responses if added 2 h prior to LPS stimulation of cells, and their effectiveness decreased when added 15 min after LPS stimulation (31, 32). To determine how long the A549 cells treated with the surfactants remained refractory to LPS stimulation, cells pretreated with surfactant preparations were washed and placed in fresh medium for various times between 0 and 24 hours before stimulation with LPS. It was found that the immunomodulatory effect of the surfactant preparations on IL-8 production was reversible with time and decreased after 8 h (Fig. 6A). The immunoblot data also suggested that the effect of surfactant phospholipids on LPS-induced TLR4 translocation into raft fractions would be lost when cells were preincubated in fresh media for 24 h before being stimulated with LPS, indicating that the effect of the surfactant lipids is reversible (Fig. 6C, D), correlating the ELISA results (Fig. 6A).
Fig. 6.
The inhibition of IL-8 production in A549 cells treated with surfactant lipid preparations is reversible. A: A549 cells were preincubated with Survanta® (250 μg/ml), Curosurf® (250 μg/ml), or DPPC (500 μg/ml) for 2 h. Following this, the cells were washed and placed in fresh medium for various times (0–24 h) before stimulation with LPS. The inhibitory effects of the surfactant lipids on IL-8 were lost with longer incubation time (>6 h). Immunoblot results showing the raft marker GM-1 (B), cells preincubated with DPPC for 2 h and stimulated with LPS immediately (C), and cells preincubated with DPPC for 2 h washed and incubated for a further 24 h in fresh medium before being stimulated with LPS (D). The effect of DPPC on the translocation of TLR-4 was lost compared to when cells were incubated for 24 h in fresh medium before stimulated with LPS. Values are mean ± SD, n = 3. *P < 0.05 versus cells without surfactant pretreatment. E: Densitometric analysis of the TLR-4 blots in C and D.
DISCUSSION
In addition to its biophysical properties, pulmonary surfactant has been suggested to play an important immunomodulatory role in the lung (21). It has been hypothesized that under normal conditions, pulmonary surfactant might suppress excessive inflammatory responses to inhaled stimuli and thus prevents inflammatory tissue damage within the lung. Indeed, alterations in surfactant composition have been measured in several inflammatory lung diseases and may play a part in exacerbating the resultant lung damage (54, 55). Although defined antimicrobial and immunoregulatory properties have been attributed to the surfactant proteins, little is known of the immunomodulating properties of the surfactant lipids.
In this study, we have investigated, for the first time, the immunoregulatory actions of lipid-rich surfactants and the surfactant phospholipid, DPPC, on LPS-induced IL-8 responses in lung epithelial cells. Furthermore, we demonstrate that the immunoregulatory properties of surfactants on LPS-induced epithelial cell responses are mediated, at least partly, via inhibition of TLR4 translocation into membrane lipid raft domains. The results of this study have several important implications: First, they demonstrate that the inflammatory responses of lung epithelial cells may be downregulated, at least partly, by surfactant phospholipid components in the absence of surfactant proteins. Second, they suggest that these surfactant phospholipid components act by disrupting the translocation of TLRs into membrane lipid raft microdomains. Moreover, these results support the idea that under normal conditions, pulmonary surfactant, and particularly the lipid components, plays an immunoregulatory role, preventing excessive inflammatory responses in the delicate gas-exchanging regions of the lung (32–36, 46).
Recent reports suggest that SPs, particularly SP-A and SP-D, regulate a variety of immune cell functions in vitro, including enhanced chemotaxis and phagocytosis, and alterations in the production of reactive oxygen species and cytokines (56, 57). However, work from our laboratories and those of others indicate that surfactant lipids, particularly the major surfactant lipid species DPPC, consistently suppress inflammatory and oxidative responses of innate immune cells (31, 32 40, 46, 58). Moreover, in this study, the artificial surfactant preparations used are devoid of the hydrophilic proteins SP-A and SP-D and contain reduced amounts of the hydrophobic proteins SP-B and SP-C (39). Furthermore, we show that equivalent anti-inflammatory activity was produced by the major surfactant phospholipid species, DPPC, at concentrations that have been measured for this lipid in natural surfactant (44, 45). Previous studies, from our laboratories and others, have shown that DPPC has an immunoregulatory role on leukocytes (31–36, 40, 46). In this study, we extend this potential immunoregulatory role of surfactant lipids to lung epithelial cells. Although a few studies have investigated the role of DPPC in group B Streptococcus-induced injury to A549 cells (59, 60) none, that we are aware, have demonstrated a relation between DPPC and LPS-induced IL-8 gene expression.
Despite many studies highlighting a role for pulmonary surfactant lipids in controlling inflammatory responses, little data exist on the potential mechanisms by which this is accomplished. Our previous studies on the modulation of monocyte respiratory burst by DPPC suggest a membrane-perturbing effect that alters PKC activation (46). Such a notion is supported by a recent study demonstrating that surfactant modulates cAMP accumulation in monocytes through a membrane-controlled mechanism (61). Alterations of plasma membrane phospholipids, resulting from incorporation of surfactant lipids that may change transmembrane receptor signaling, has been hypothesized as a possible mechanism of action for surfactant lipids (36, 62). In support of this hypothesis, previous studies from our laboratory on monocytes (40) and those of others on lymphocytes (63) have shown membrane perturbations and altered membrane fluidity after exposure to liposomes containing PC.
Consistent with this membrane-centered view of surfactant lipid action, it was noted in this study that the suppressive effects of the surfactants on IL-8 production was apparent after 30 min of incubation with the lipids prior to cell stimulation with LPS (data not shown). However, incubation of the epithelial cells with the surfactants prior to stimulation with the inflammatory cytokines IL-1β or TNF-α failed to suppress IL-8 production (data not shown). This result implies that the surfactants do not affect IL-8 expression or processing per se. In support of this, it was noted that surfactant lipids do not inhibit the basal release of IL-8 from the lung epithelial cell line Calu3, although DPPC could inhibit release of this cytokine in response to LPS stimulation (data not shown). Furthermore, signaling in response to LPS and IL-1β/TNF-α has been shown to share the same molecular pathways downstream from their receptors (47–50), suggesting that the inhibitory effects of the surfactants are specifically acting at the level of the LPS receptor complex. Although DPPC and surfactant lipids significantly downregulated IL-8 production in lung epithelial cells, the inhibition was not total. This probably reflects that the relationship between TLR4 translocation and cytokine production is not directly linear or that other nonraft-dependent mechanisms of stimulation are also operative.
Interference with efficient LPS signaling by surfactant lipids at the level of the LPS membrane receptor could occur by several mechanisms. These include direct interaction with TLR4 and inhibition of LPS binding, downregulation of the expression of membrane TLR4 or accessory molecules, and interference with the assembly of the TLR4 receptor complex. Each of these possibilities was investigated in this study.
Results of flow cytometry experiments with fluorescent-labeled LPS indicate that the surfactants used did not prevent binding of LPS to the epithelial cells. This suggests that the surfactant lipid components do not directly compete with LPS for binding to TLR4 or other membrane receptor components. In agreement with this, it has been previously demonstrated in our laboratory that DPPC has no effect on the binding of LPS to monocyte cells lines (40). Moreover, in our experiments, surfactant or DPPC was removed prior to stimulation of the cells with LPS, making a direct interaction between the surfactant lipid and LPS or LPS binding proteins unlikely. A recent study by Mueller et al. (64) demonstrates that several phospholipid species inhibit the interaction between LPS and LBP, although PC was not an effective inhibitor. This further supports our findings that DPPC does not directly inhibit the binding of LPS to receptor components and suggests that inhibition of LPS-induced cellular responses by this lipid must be by alternative mechanisms. Further flow cytometry experiments also showed that the surfactants and DPPC did not alter the cell surface expression of TLR4, indicating that decreased TLR4 expression was not the mechanisms for the downregulation of IL-8 expression by these lipid agents.
The pivotal role of lipid rafts in the innate immune response has recently been demonstrated (42, 53). In this study, we showed that disruption of lipid raft microdomain significantly reduced the release of IL-8 release from A549 cells. It has also been further elucidated that upon LPS stimulation, TLR4 translocates into membrane lipid raft domains in these cells. The disruption of the lipid raft by mycostatin resulted in a marked reduction of the LPS-induced cytokine release from A549 cells in this work in agreement with previous finding in a monocytic cell line (42). Following mycostatin treatment, the lipid raft, which provides a signaling platform and also has a focusing effect for the initiation of LPS signaling (42), will be disrupted, and there is no partition of TLR4 into lipid raft and nonraft fractions (Fig. 4D). However, the preincubation of A549 cells with DPPC inhibits the movement of TLR4 into the membrane raft domains without disrupting the partition of the two domains. Based on these observations, we hypothesize that surfactant lipids downregulate the production of LPS-induced IL-8 synthesis in lung epithelial cells by interfering with the translocation of TLR4 from the nonraft to raft domains subsequent to LPS stimulation. This may be an important mechanism by which a variety of lipid structures can influence cellular responses.
Several phospholipid metabolites and free fatty acids have recently been shown to modulate LPS-induced cell responses via interaction with the TLR4 receptor complex. Hwang (65) suggested that saturated and unsaturated free fatty acids can differentially regulate COX-2 expression through effects on TLR4, although the mechanistic details were not given. A cationic lipid (lipofectamine) was shown to uncouple LPS binding and internalization from signaling by preventing CD14-TLR4 interactions (57). It has also been demonstrated recently that preincubation of endothelial cells with oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine can inhibit subsequent LPS-induced IL-8 synthesis by altering lipid raft/caveolar processing (66). Recently, we demonstrated that alterations in membrane PC composition can affect translocation of TLR4 into raft fractions in human MonoMac6 monocytes (67).
In summary, we have demonstrated that surfactant preparations with increased phospholipid content, as well as DPPC alone, can downregulate the synthesis of IL-8 by an alveolar epithelial cell line in response to LPS. We suggest that the mechanism of this modulation is at least partly via inhibition of TLR4 translocation into membrane lipid raft domains. Understanding the molecular mechanisms by which inflammation is controlled in the lungs is a prerequisite to the development of more effective therapies for inflammatory lung disease. Pulmonary surfactant is normally present in the lung, and it is also well tolerated as a replacement therapy (39, 68). Moreover, the surfactants used here, Survanta® and Curosurf®, have already been used as surfactant replacement treatments in infant respiratory distress syndrome and bronchiolitis (69). The results from this study suggest that artificial surfactant preparations based on DPPC might be effective for restoring the homeostatic anti-inflammatory balance in lung inflammatory diseases in which natural surfactant compositions might be altered.
Footnotes
Abbreviations:
- DPPC
- dipalmitoyl phosphatidylcholine
- IL-8
- interleukin-8
- LPS
- lipopolysaccharide
- mfi
- mean fluorescence index
- PC
- phosphatidylcholine
- SP
- surfactant protein
- TLR4
- Toll-like receptor 4
- TNF-α
- tumor necrosis factor-α
REFERENCES
- 1.Becker M. N., Diamond G., Verghese M. W., Randell S. H. 2000. CD14-dependent lipopolysaccharide-induced beta-defensin-2 expression in human tracheobronchial epithelium. J. Biol. Chem. 275: 29731–29736. [DOI] [PubMed] [Google Scholar]
- 2.Diamond G., Legarda D., Ryan L. K. 2000. The innate immune response of the respiratory epithelium. Immunol. Rev. 173: 27–38. [DOI] [PubMed] [Google Scholar]
- 3.Schulz C., Farkas L., Wolf K., Kratzel K., Eissner G., Pfeifer M. 2002. Differences in LPS-induced activation of bronchial epithelial cells (BEAS-2B) and type II-like pneumocytes (A549). Scand. J. Immunol. 56: 294–302. [DOI] [PubMed] [Google Scholar]
- 4.Skerrett S. J., Liggitt H. D., Hajjar A. M., Ernst R. K., Miller S. I., Wilson C. B. 2004. Respiratory epithelial cells regulate lung inflammation in response to inhaled endotoxin. Am. J. Physiol. Lung Cell. Mol. Physiol. 287: L143–L152. [DOI] [PubMed] [Google Scholar]
- 5.Fehrenbach H. 2001. Alveolar epithelial type II cell: defender of the alveolus revisited. Respir. Res. 2: 33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pettersen C. A., Adler K. B. 2002. Airways inflammation and COPD: epithelial-neutrophil interactions. Chest. 121: 142S–150S. [DOI] [PubMed] [Google Scholar]
- 7.Bals R., Hiemstra P. S. 2004. Innate immunity in the lung: how epithelial cells fight against respiratory pathogens. Eur. Respir. J. 23: 327–333. [DOI] [PubMed] [Google Scholar]
- 8.Pechkovsky D. V., Zissel G., Ziegenhagen M. W., Einhaus M., Taube C., Rabe K. F., Magnussen H., Papadopoulos T., Schlaak M., Müller-Quernheim J. 2000. Effect of proinflammatory cytokines on interleukin-8 mRNA expression and protein production by isolated human alveolar epithelial cells type II in primary culture. Eur. Cytokine Netw. 11: 618–625. [PubMed] [Google Scholar]
- 9.Reddi K., Phagoo S. B., Anderson K. D., Warburton D. 2003. Burkholderia cepacia-induced IL-8 gene expression in an alveolar epithelial cell line: signalling through CD14 and mitogen-activated protein kinase. Pediatr. Res. 54: 297–305. [DOI] [PubMed] [Google Scholar]
- 10.Kunkel S. L., Standiford T., Kasahara K., Strieter R. M. 1991. Interleukin-8 (IL-8): the major neutrophil chemotactic factor in the lung. Exp. Lung Res. 17: 17–23. [DOI] [PubMed] [Google Scholar]
- 11.Murphy P. M. 1997. Neutrophil receptors for interleukin-8 and related CXC chemokines. Semin. Hematol. 34: 311–318. [PubMed] [Google Scholar]
- 12.Detmers P. A., Lo S. K., Olsen-Egbert E., Walz A., Baggiolini M., Cohn Z. A. 1990. Neutrophil-activating protein 1/interleukin 8 stimulates the binding activity of the leukocyte adhesion receptor CD11b/CD18 on human neutrophils. J. Exp. Med. 171: 1155–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huber A. R., Kunkel S. L., Todd R. F., III, Weiss S. J. 1991. Regulation of transendothelial neutrophil migration by endogenous interleukin-8. Science. 254: 99–102. [DOI] [PubMed] [Google Scholar]
- 14.Peveri P., Walz A., Dewald B., Baggiolini M. 1988. A novel neutrophil-activating factor produced by human mononuclear phagocytes. J. Exp. Med. 167: 1547–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keatings V. M., Collins P. D., Scott D. M., Barnes P. J. 1996. Differences in interleukin-8 and tumour necrosis factor-alpha in induced sputum from patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 153: 530–534. [DOI] [PubMed] [Google Scholar]
- 16.Mikami M., Llewellyn-Jones C. G., Bayley D., Hill S. L., Stockley R. A. 1998. The chemotactic activity of sputum from patients with bronchiectasis. Am. J. Respir. Crit. Care Med. 157: 723–728. [DOI] [PubMed] [Google Scholar]
- 17.Hill A., Gomertz S., Stockley R. 2000. Factors influencing airways inflammation in chronic obstructive pulmonary disease. Thorax. 55: 970–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sethi S. 2000. Infectious aetiology of acute exacerbations of chronic bronchitis. Chest. 117: 380S–385S. [DOI] [PubMed] [Google Scholar]
- 19.Sethi S., Murphy T. F. 2001. Bacterial infection in chronic obstructive pulmonary disease in 2000: a state-of-the-art review. Clin. Microbiol. Rev. 14: 336–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White A. J., Gompertz S., Stockley R. A. 2003. Chronic obstructive pulmonary disease. 6: The aetiology of exacerbations of chronic obstructive pulmonary disease. Thorax. 58: 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goerke J. 1998. Pulmonary surfactant: functions and molecular composition. Biochim. Biophys. Acta. 1408: 79–89. [DOI] [PubMed] [Google Scholar]
- 22.Bastacky J., Lee C. Y., Goerke J., Koushafar H., Yager D., Kenaga L., Speed T. P., Chen Y., Clements J. A. 1995. Alveolar lining layer is thin and continuous: low-temperature scanning electron microscopy of rat lung. J. Appl. Physiol. 79: 1615–1628. [DOI] [PubMed] [Google Scholar]
- 23.Bernhard W., Haagsman H. P., Tschernig T., Poets C. F., Postle A. D., van Eijk M. E., von Der H. H. 1997. Conductive airway surfactant: surface-tension function, biochemical composition, and possible alveolar origin. Am. J. Respir. Cell Mol. Biol. 17: 41–50. [DOI] [PubMed] [Google Scholar]
- 24.Hills B. A. 1999. An alternative view of the role(s) of surfactant and the alveolar model. J. Appl. Physiol. 87: 1567–1583. [DOI] [PubMed] [Google Scholar]
- 25.Coonrod J. D. 1987. Role of surfactant free fatty acids in antimicrobial defences. Eur. J. Respir. Dis. 71 (Suppl.): 209–214. [PubMed] [Google Scholar]
- 26.Hills B. A. 1996. Asthma: is there an airway receptor barrier? Thorax. 51: 773–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Widdicombe J. G. 1997. Airway liquid: a barrier to drug diffusion? Eur. Respir. J. 10: 2194–2197. [DOI] [PubMed] [Google Scholar]
- 28.Phelps D. S. 2001. Surfactant regulation of host defense function in the lung: a question of balance. Pediatr. Pathol. Mol. Med. 20: 269–292. [PubMed] [Google Scholar]
- 29.Crouch E., Wright J. R. 2001. Surfactant proteins A and D and pulmonary host defense. Annu. Rev. Physiol. 63: 521–554. [DOI] [PubMed] [Google Scholar]
- 30.Wu H., Kuzmenko A., Wan S., Schaffer L., Weiss A., Fisher J. H., Kim K. S., McCormack F. X. 2003. Surfactant proteins A and D inhibit the growth of Gram-negative bacteria by increasing membrane permeability. J. Clin. Invest. 111: 1589–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tonks A., Morris R. H. K., Price A. J., Thomas A. W., Jones K. P., Jackson S. K. 2001. Dipalmitoylphosphatidylcholine modulates inflammatory functions of monocytic cells independently of mitogen activated protein kinases. Clin. Exp. Immunol. 124: 86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morris R. H. K., Price A. J., Tonks A., Jackson S. K., Jones K. P. 2000. Prostaglandin E (2) and tumour necrosis factor-alpha release by monocytes are modulated by phospholipids. Cytokine. 12: 1717–1719. [DOI] [PubMed] [Google Scholar]
- 33.Hayakawa H., Myrvik Q. N., St Clair R. W. 1989. Pulmonary surfactant inhibits priming of rabbit alveolar macrophage. Evidence that surfactant suppresses the oxidative burst of alveolar macrophage in infant rabbits. Am. Rev. Respir. Dis. 140: 1390–1397. [DOI] [PubMed] [Google Scholar]
- 34.Thomassen M. J., Meeker D. P., Antal J. M., Connors M. J., Wiedemann H. P. 1992. Synthetic surfactant (Exosurf) inhibits endotoxin-stimulated cytokine secretion by human alveolar macrophages. Am. J. Respir. Cell Mol. Biol. 7: 257–260. [DOI] [PubMed] [Google Scholar]
- 35.Thomassen M.J., Antal J. M., Connors M. J., Meeker D. P., Wiedemann H. P. 1994. Characterization of exosurf (surfactant)-mediated suppression of stimulated human alveolar macrophage cytokine responses. Am. J. Respir. Cell Mol. Biol. 10: 399–404. [DOI] [PubMed] [Google Scholar]
- 36.Walti H., Polla B. S., Bachelet M. 1997. Modified natural porcine surfactant inhibits superoxide anions and proinflammatory mediators released by resting and stimulated human monocytes. Pediatr. Res. 41: 114–119. [DOI] [PubMed] [Google Scholar]
- 37.Lee J. H., Del Sorbo L., Uhlig S., Porro G. A., Whitehead T., Voglis S., Liu M., Slutsky A. S., Zhang H. 2004. Intercellular adhesion molecule-1 mediates cellular cross-talk between parenchymal and immune cells after lipopolysaccharide neutralization. J. Immunol. 172: 608–616. [DOI] [PubMed] [Google Scholar]
- 38.Mason R. J., William M. C. 1980. Phospholipid composition and ultrastructure of A549 cells and other cultured pulmonary epithelial cells of presumed type II cell origin. Biochim. Biophys. Acta. 617: 36–50. [DOI] [PubMed] [Google Scholar]
- 39.Bernhard W., Mottaghian J., Gebert A., Rau G. A., von Der H. A. R. D., Poets C. F. 2000. Commercial versus native surfactants. Surface activity, molecular components, and the effect of calcium. Am. J. Respir. Crit. Care Med. 162: 1524–1533. [DOI] [PubMed] [Google Scholar]
- 40.Tonks A. J., Tonks A., Morris R. H. K., Jones K. P., Jackson S. K. 2003. Regulation of platelet activating factor in human monocytes by dipalmitoylphosphatidylcholine. J. Leukoc. Biol. 74: 95–101. [DOI] [PubMed] [Google Scholar]
- 41.Bamford S., Ryley H., Jackson S. K. 2007. Highly purified LPS from B. cepacia clinical isolates induce inflammatory cytokine responses in human monocytes and epithelial cells by activation of TLR4-mediated MAPK signalling pathways. Cell. Microbiol. 9: 532–543. [DOI] [PubMed] [Google Scholar]
- 42.Triantafilou M., Miyake K., Golenbock D. T., Triantafilou K. 2002. Mediators of innate immune recognition of bacteria concentrate in lipid rafts and facilitate lipopolysaccharide-induced cell activation. J. Cell Sci. 115: 2603–2611. [DOI] [PubMed] [Google Scholar]
- 43.Wessel D., Flugge U. I. 1984. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal. Biochem. 138: 141–143. [DOI] [PubMed] [Google Scholar]
- 44.Tonks A., Parton J., Tonks A. J., Morris R. H. K., Finall A., Jones K. P., Jackson S. K. 2005. The surfactant phospholipid, DPPC, down regulates monocyte respiratory burst via modulation of PKC. Am. J. Physiol. Lung Cell. Mol. Physiol. 288: L1070–L1078. [DOI] [PubMed] [Google Scholar]
- 45.Rooney S. A., Canavan P. M., Motoyama E. K. 1974. The identification of phosphatidylglycerol in the rat, rabbit, monkey and human lung. Biochim. Biophys. Acta. 360: 56–67. [DOI] [PubMed] [Google Scholar]
- 46.Harwood J. L. 1987. Lung surfactant. Prog. Lipid Res. 26: 211–256. [DOI] [PubMed] [Google Scholar]
- 47.Maschera B., Ray K., Burns K., Volpe F. 1999. Overexpression of an enzymatically inactive interleukin-1-receptor-associated kinase activates nuclear factor-kappaB. Biochem. J. 339: 227–231. [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang F. X., Kirschning C. J., Mancinelli R., Xu X. P., Jin Y., Faure E., Mantovani A., Rothe M., Muzio M., Arditi M. 1999. Bacterial lipopolysaccharide activates nuclear factor-kappaB through interleukin-1 signaling mediators in cultured human dermal endothelial cells and mononuclear phagocytes. J. Biol. Chem. 274: 7611–7614. [DOI] [PubMed] [Google Scholar]
- 49.Zhang G., Ghosh S. 2001. Toll-like receptor-mediated NF-kappaB activation: a phylogenetically conserved paradigm in innate immunity. J. Clin. Invest. 107: 13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martin M. U., Wesche H. 2002. Summary and comparison of the signalling mechanisms of the Toll/interleukin-1 receptor family. Biochim. Biophys. Acta. 1592: 265–280. [DOI] [PubMed] [Google Scholar]
- 51.Monick M. M., Yarovinsky T. O., Powers L. S., Butler N. S., Carter A. B., Gudmundsson G., Hunninghake G. W. 2003. Respiratory syncytial virus up-regulates TLR4 and sensitizes airway epithelial cells to endotoxin. J. Biol. Chem. 278: 53035–53044. [DOI] [PubMed] [Google Scholar]
- 52.MacRedmond R., Greene C., Taggart C. C., McElvaney N., O'Neill S. 2005. Respiratory epithelial cells require Toll-like receptor 4 for induction of human beta-defensin 2 by lipopolysaccharide. Respir. Res. 6: 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pfeiffer A., Bottcher A., Orso E., Kapinsky M., Nagy P., Bodnar A., Spreitzer I., Liebisch G., Drobnik W., Gempel K. 2001. Lipopolysaccharide and ceramide docking to CD14 provokes ligand-specific receptor clustering in rafts. Eur. J. Immunol. 31: 3153–3164. [DOI] [PubMed] [Google Scholar]
- 54.Schmidt R., Meier U., Yabut-Perez M., Walmrath D., Grimminger F., Seeger W., Gunther A. 2001. Alteration of fatty acid profiles in different pulmonary surfactant phospholipids in acute respiratory distress syndrome and severe pneumonia. Am. J. Respir. Crit. Care Med. 163: 95–100. [DOI] [PubMed] [Google Scholar]
- 55.Hohlfeld J. M. 2002. The role of surfactant in asthma. Respir. Res. 3: 8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.LeVine A. M., Whitsett J. A., Gwozdz J. A., Richardson T. R., Fisher J. H., Burhans M. S., Korfhagen T. R. 2000. Distinct effects of surfactant protein A or D deficiency during bacterial infection on the lung. J. Immunol. 165: 3934–3940. [DOI] [PubMed] [Google Scholar]
- 57.Leon-Ponte M., Kirchhof M. G., Sun T., Stephens T., Singh B., Sandhu S., Madrenas J. 2005. Polycationic lipids inhibit the pro-inflammatory response to LPS. Immunol. Lett. 96: 73–83. [DOI] [PubMed] [Google Scholar]
- 58.Ahuja A., Oh N., Chao W., Spragg R. G., Smith R. M. 1996. Inhibition of the human neutrophil respiratory burst by native and synthetic surfactant. Am. J. Respir. Cell Mol. Biol. 14: 496–503. [DOI] [PubMed] [Google Scholar]
- 59.Nizet V., Gibson R. L., Chi E. Y., Framson P. E., Hulse M., Rubens C. E. 1996. Group B streptococcal beta-hemolysin expression is associated with injury of lung epithelial cells. Infect. Immun. 64: 3818–3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Doran K. S., Chang J. C., Benoit V. M., Eckmann L., Nizet V. 2002. Group B streptococcal beta-hemolysin/cytolysin promotes invasion of human lung epithelial cells and the release of interleukin-8. J. Infect. Dis. 185: 196–203. [DOI] [PubMed] [Google Scholar]
- 61.Pinot F., Walti H., Haagsman H. P., Polla B. S., Bachelet M. 2000. Curosurf modulates cAMP accumulation in human moncytes through a membrane controlled mechanism. Am. J. Physiol. Lung Cell. Mol. Physiol. 278: L99–L104. [DOI] [PubMed] [Google Scholar]
- 62.Wilsher M. L., Hughes D. A., Haslam P. L. 1988. Immunoregulatory properties of pulmonary surfactant: influence of variations in the phospholipid profile. Clin. Exp. Immunol. 73: 117–122. [PMC free article] [PubMed] [Google Scholar]
- 63.Rivnay B., Globerson A., Shinitzky M. 1978. Perturbation of lymphocyte response to concanavalin-A by exogenous cholsterol and lecithin. Eur. J. Immunol. 8: 185–189. [DOI] [PubMed] [Google Scholar]
- 64.Mueller M., Brandenburg K., Dedrick R., Schromm A. B., Seydel U. 2005. Phospholipids inhibit lipopolysaccharide (LPS)-induced cell activation: a role for LPS-binding protein. J. Immunol. 174: 1091–1096. [DOI] [PubMed] [Google Scholar]
- 65.Hwang D. 2001. Modulation of the expression of cyclooxygenase-2 by fatty acids mediated through TLR-4-derived pathways. FASEB J. 15: 2556–2564. [DOI] [PubMed] [Google Scholar]
- 66.Walton K. A., Hsieh X., Gharavi N., Wang S., Wang G., Yeh M., Cole A. L., Berliner J. A. 2003. Receptors involved in the oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine-mediated synthesis of interleukin-8. A role for Toll-like receptor 4 and a glycosylphosphatidylinositol-anchored protein. J. Biol. Chem. 278: 29661–29666. [DOI] [PubMed] [Google Scholar]
- 67.Jackson S. K., Abate W., Parton J., Jones S., Harwood J. L. 2008. Lysophospholipid metabolism facilitates Toll-like receptor 4 membrane translocation to regulate the inflammatory response. J. Leukoc. Biol. 84: 86–92. [DOI] [PubMed] [Google Scholar]
- 68.Postle A. D. 2000. The role of pulmonary surfactant in the asthmatic response. Clin. Exp. Allergy. 30: 1201–1204. [DOI] [PubMed] [Google Scholar]
- 69.Tibby S. M., Hatherill M., Wright S. M., Wilson P., Postle A. D., Murdoch I. A. 2000. Exogenous surfactant supplementation in infants with respiratory syncytial virus bronchiolitis. Am. J. Respir. Crit. Care Med. 162: 1251–1256. [DOI] [PubMed] [Google Scholar]