Abstract
Over the past several years, proprotein convertase subtilisin kexin type 9 (PCSK9) has gained significant attention as a key regulator of serum LDL-cholesterol (LDL-C) levels. In humans, gain-of-function mutations in PCSK9 cause a form of familial hypercholesterolemia, whereas loss-of-function mutations result in significantly decreased LDL-C and cardiovascular risk. Our laboratory was the first to demonstrate that atorvastatin increases PCSK9 serum levels, an observation that has since been confirmed by at least two other groups. In light of these observations, we studied the effect of another common lipid-lowering medication, fenofibrate, on circulating PCSK9 protein levels in patients treated with fenofibrate or placebo for 12 weeks. We observed that fenofibrate (200 mg per day) significantly increased circulating PCSK9 levels by 25% compared with baseline. Placebo treatment, in comparison, had no effect on PCSK9 levels. Interestingly, fenofibrate-induced increases in serum PCSK9 levels were highly correlated with fenofibrate-induced changes in HDL-C and triglyceride levels, as well as with fenofibrate-induced changes in LDL-C levels. These results suggest an explanation for why fibrates do not achieve as much LDL-C lowering as might otherwise be expected and indicate that the addition of a PCSK9 inhibitor to fibrate therapy may result in additional beneficial LDL-C lowering.
Keywords: PCSK9, fibrates, LDL-cholesterol, LDL receptor, HDL-cholesterol
Proprotein convertase subtilisin kexin type 9 (PCSK9) has gained significant attention as a key regulator of serum LDL-cholesterol (LDL-C) levels (1–4). PCSK9 is a protease made and secreted by the liver into the plasma, which then binds to and causes the degradation of hepatic LDL receptors (LDLR) (5–10). The mechanism by which PCSK9 degrades LDLR is very complex and is only beginning to be understood. It has recently been observed that the protease itself does not have to be enzymatically active to cause degradation of the LDLR but rather, that PCSK9 binds to the LDLR and subsequently targets it for lysosomal destruction within the hepatocyte (11–14). This concept that PCSK9 acts as a secreted protein to bind the hepatic LDLR and causes its degradation is supported by recent findings that disruption of this binding using anti-PCSK9 antibody results in preserved LDLR and decreased LDL-C (15–17). As a result of LDLR levels being decreased through this action of PCSK9, the liver has decreased ability to bind LDL from the circulation and serum LDL-C levels increase. Therefore, functional mutations in PCSK9 have dramatic effects on serum LDL-C levels in humans.
Patients with gain-of-function mutations of PCSK9 present with severe familial hypercholesterolemia and accompanying increased cardiovascular risk (18–21). These gain-of-function mutations in PCSK9 account for some of the familial dominant hypercholesterolemia cases that could not be explained by mutations in either the LDLR or apolipoprotein B (18–21). In contrast, heterozygous subjects with loss-of-function mutations in PCSK9, including mutations that prevent the self-cleavage and secretion of the protein, have significantly decreased levels of serum LDL-C and dramatically decreased cardiovascular risk (22–24). Approximately 2% of African-Americans carry such mutations with an accompanying 80–90% decreased risk of serious cardiovascular disease (22). A compound heterozygote for PCSK9 loss-of-function mutations was also recently described. This subject, a healthy 32-year-old female, had an extremely low serum LDL-C level of 14 mg/dl (24).
Statins have been shown to increase the activity/nuclear translocation of sterol-regulatory element binding protein-2 (SREBP-2), a transcription factor that activates both the LDLR and PCSK9 genes (25, 26). Statins were reported to increase PCSK9 mRNA expression (25, 26), and we hypothesized that statin treatment in humans should increase circulating PCSK9 protein levels. Subsequently, our laboratory, using our novel dual monoclonal antibody PCSK9 sandwich ELISA (27), was the first to demonstrate that atorvastatin treatment significantly increased PCSK9 serum levels (28), an observation that has since been confirmed by at least two other laboratories (29, 30). These observations of statin-induced increases in serum PCSK9 levels may help explain the rule of 6% for statins, which indicates that each doubling of the statin dose results in only about a 6% further decrease in LDL-C. These observations also suggested that the addition of a PCSK9 inhibitor to statin therapy may result in even further LDL-C lowering. In light of these interesting observations with atorvastatin, we used our recently developed PCSK9 ELISA (27) to investigate the effect of another common lipid lowering medication, fenofibrate, on circulating PCSK9 protein levels in patients treated with fenofibrate or placebo over a 12 week period.
MATERIALS AND METHODS
Serum samples
Human serum samples were obtained from patients in a recently described phase 2 clinical trial who gave permission for their samples to be banked for future exploratory lipid analyses (31). After obtaining protocol approval from an Institutional Review Board and the proper informed consent from each patient, samples were collected, banked, and deidentified to protect patient privacy so that only lipid data and dose group could be linked to the PCSK9 ELISA results. The specific clinical trial studied the effect of 12 weeks of treatment with a novel peroxisome proliferator-activated receptor (PPAR)-α agonist LY518674. In addition, the study also included a placebo group and an active comparator group (200 mg per day of fenofibrate). The results of this trial as well as its sister study, which examined the effect of LY518674 on patients treated with atorvastatin, have been previously disclosed (31). Samples from placebo and fenofibrate-treated patients were analyzed to determine the effect of fenofibrate on PCSK9 levels compared with placebo.
In order to be included in the study, patients had to have a diagnosis of atherogenic dyslipidemia characterized by both a low HDL-C (<45 mg/dl for men or <50 mg/dl for women) and an elevated triglyceride level (150–600 mg/dl). Patients also had to have a LDL-C level <160 mg/dl and had to be between 18 and 80 years old to participate in the study. Patients with a HbA1c >8% were excluded from the study. In the placebo group, 72% of the patients were male, 28% were female, the mean age was 57, and the mean body mass index was 31. In the fenofibrate group, 65% of the patients were male, 35% were female, the mean age was 52, and the mean body mass index was 32. For this research, only patients who received placebo or fenofibrate therapy alone were studied, and only patients who contributed a complete set of baseline and endpoint banked samples were analyzed for PCSK9 levels. This resulted in 17 patients in the placebo group who received placebo only for 12 weeks, and 22 patients in the fenofibrate group receiving 200 mg per day of fenofibrate only for 12 weeks. Serum samples were shipped on dry ice and stored at −70°C prior to subsequent analysis.
Routine lipid analysis
Concentrations of serum triglycerides and total cholesterol were measured using Hitachi Chemistry Systems (Roche Diagnostics, Indianapolis, IN). HDL-C was measured via a dextran sulfate precipitation method in which a 10 g/L dextran sulfate (Mr 50,000) Mg2+ solution was used to precipitate apoB-containing particles. Following centrifugation, the cholesterol content of the HDL-C-containing supernatant was measured on a Roche Hitachi 717 analyzer. LDL-C was measured using an ultracentrifugation assay in which 3 ml of serum was centrifuged using a Beckman L8-70M ultracentrifuge at 25,000 g for 20.25 h. Afterward, a Beckman tube slicer was used to remove the bottom fraction of the tube. Following volume correction, cholesterol content of the bottom fraction of the tube was measured on a Roche Hitachi 717 analyzer. LDL-C was then determined as cholesterol present in the bottom fraction minus HDL-C.
PCSK9 ELISA
PCSK9 levels in the serum samples were measured using our recently described PCSK9 dual monoclonal antibody sandwich ELISA (27, 28) with minor modifications, including the use of a nonHis-tagged recombinant PCSK9 standard. The exact epitopes recognized by the antibodies used in the ELISA are not known at this time. Human PCSK9 used as a standard in the ELISA was cloned from a human liver cDNA library with a resulting construct used to generate an HEK293 stable cell line over-expressing PCSK9. The cDNA sequence used did not code for a His-tag. Cells were grown in serum free media, and the secreted PCSK9 protein was purified using an ion-exchange column followed by size-exclusion chromatography. Identity of the protein was confirmed by N-terminal sequencing, and purity was judged to be greater than 95% based on SDS-PAGE followed by Coomassie blue staining. ELISA wells were coated overnight with anti-PCSK9 monoclonal antibody at a concentration of 5 μg/ml. The following day, wells were aspirated, washed three times with assay buffer (50 mM HEPES, pH 7.40, 150 mM NaCl, 1% Triton X-100, 5 mM EDTA, 5 mM EGTA), and blocked for 1 h with TBS-casein blocking buffer (Pierce). Next, 100 μl of nonHis-tagged recombinant PCSK9 standards (varying concentrations of recombinant protein in assay buffer) was added to the wells as a standard curve. Afterward, serum samples were diluted 1:20 in assay buffer, added to their respective wells, and the ELISA plate was incubated for 2 h at room temperature. Following aspiration, wells were washed three times with assay buffer, and 100 μl of a 1:1000 dilution of conjugate antibody (HRP-labeled anti-PCSK9 monoclonal antibody, 1 mg/ml) was added to the wells for a 1 h incubation at room temperature. Following aspiration, wells were washed three times with Tris buffered saline + Tween (TBST). After the last aspiration of TBST, 100 μl of TMB development substrate (Pierce) were added to the wells and allowed to incubate for 30 min at room temperature. The reaction was stopped with an equal volume of 2N phosphoric acid and plates were read at 450 nm. SigmaPlot, version 8.0, was used for fitting of the calibration curves. Reproducibility of the ELISA on frozen serum samples was tested by looking at the effect of up to four freeze-thaw cycles on samples, with at least 90% recovery observed for all samples after four freeze-thaw cycles.
Immunoprecipitation and Western blotting of PCSK9
Analysis of PCSK9 levels in serum samples by immunoprecipitation and Western blotting was performed as previously described (27) with minor modifications, including the use of a nonHis-tagged recombinant PCSK9 standard. For each immunoprecipitation, 100 μl of serum was added to 900 μl of immunoprecipitation buffer (50 mmol/L HEPES, pH 7.40, 150 mmol/L NaCl, 10 ml/L Triton X-100, 5 mmol/L EDTA, 5 mmol/L EGTA). Next, PCSK9 was immunoprecipitated overnight with 1 μg of anti-PCSK9 monoclonal antibody covalently coupled to trisacryl beads (Pierce). Afterward, beads were washed twice with immunoprecipitation buffer, and 40 μl of 2× sample buffer (100 mmol/L Tris, pH 6.80, 40 g/L SDS, 200 ml/L glycerol, 20 mg/L bromophenol blue, 15 g/L dithiothreitol) was added to each tube. Samples were vortexed, boiled for 5 min, and stored at −20°C prior to electrophoretic analysis. Western blotting was performed using SDS-polyacrylamide gels with colored molecular weight markers (Invitrogen) run on each gel. Proteins were separated for 1 h at 175 V at room temperature and transferred to ECL nitrocellulose paper (Amersham) for 1 h (100 V, 4°C). Nitrocellulose blots were blocked for 1 h at room temperature in Tris-buffered saline (TBS)-casein blocking buffer (Pierce) containing 1 ml Tween 20/L. After blocking, blots were probed with HRP-labeled polyclonal anti-PCSK9 antibody in blocking buffer for 1 h at room temperature. Blots were washed three times (10 min each) with TBST (10 mmol/L Tris pH 7.40, 150 mmol/L NaCl with 1 ml Tween 20/L). After washing, blots were developed with ECL reagent (Amersham), air-dried, and exposed to Bio-Max X-ray film (Kodak).
Data analysis
SigmaPlot, version 8.0, was used for fitting of the calibration curves for the PCSK9 ELISA. Data were analyzed and plotted using the program FigP (Biosoft, St. Louis, MO). Statistical analysis was performed using the same program. A p-value of less than 0.05 was considered to indicate statistical significance.
RESULTS
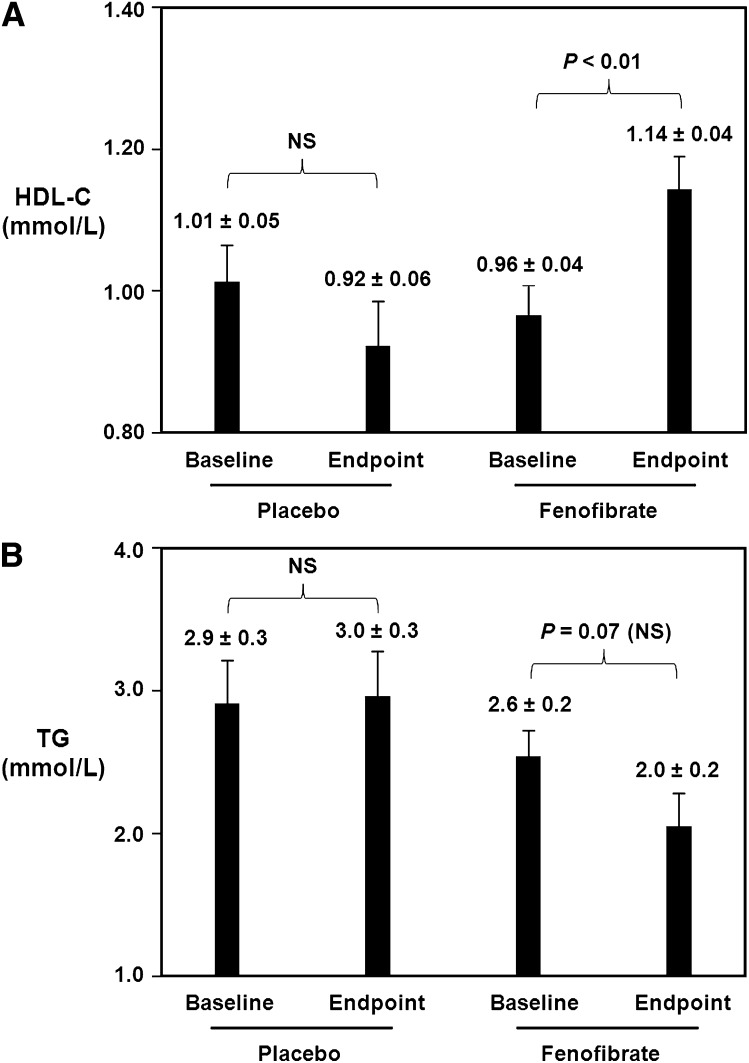
Figure 1A demonstrates the effect of fenofibrate, 200 mg per day for 12 weeks, versus placebo on serum HDL-C levels. In the placebo group, there was no significant change in HDL-C from baseline to endpoint. At baseline in the placebo group, HDL-C levels were 1.01 ± 0.05 mmol/L. Following 12 weeks of placebo only treatment, there was no significant change with endpoint HDL-C levels being 0.92 ± 0.06 mmol/L. In contrast, in the fenofibrate group, there was a significant increase in HDL-C from baseline to endpoint. At baseline in the fenofibrate-treated group, HDL-C levels were 0.96 ± 0.04 mmol/L. Following 12 weeks of fenofibrate treatment at a dose of 200 mg/day, there was a significant 19% increase with endpoint HDL-C levels of 1.14 ± 0.04 mmol/L (p < 0.01 versus baseline).
Fig. 1.
Effect of fenofibrate on serum HDL-C and triglyceride levels. A: Baseline and endpoint samples from patients receiving placebo only for 12 weeks or fenofibrate only (200 mg per day) for 12 weeks were analyzed for HDL-C levels. Levels at baseline and endpoint were plotted for each group. Data are expressed as the mean ± SEM. Fenofibrate treatment caused a significant 19% increase in serum HDL-C. B: Patient samples described in Fig. 1A were analyzed for serum triglyceride levels. Data are expressed as the mean ± SEM. Fenofibrate treatment caused a 23% decrease in serum triglycerides, which did not achieve statistical significance.
With regard to triglyceride levels, fenofibrate treatment caused a 23% decrease although this decrease did not reach statistical significance (Fig. 1B). Specifically, in the placebo group, there was no significant change in triglycerides (TGs) from baseline to endpoint. At baseline in the placebo group, TG levels were 2.9 ± 0.3 mmol/L. Following 12 weeks of placebo only treatment, there was no significant change with endpoint TG levels being 3.0 ± 0.3 mmol/L. At baseline in the fenofibrate-treated group, TG levels were 2.6 ± 0.2 mmol/L. Following 12 weeks of fenofibrate treatment at a dose of 200 mg/day, there was a 23% decrease with endpoint TG levels of 2.0 ± 0.2 mmol/L, although this decrease did not achieve statistical significance (p = 0.07 vs. baseline). Interestingly, further analysis of the individual data revealed that there was one patient who had a paradoxical 97% increase in serum triglyceride levels. When this patient's data were removed from the analysis, the fenofibrate-induced decrease in triglyceride became significant with a baseline level of 2.5 ± 0.2 mmol/L and an endpoint level of 1.8 ± 0.2 mmol/L (p = 0.02 vs. baseline).
Recent contradictory observations regarding the effects of fibrates on hepatocyte PCSK9 secretion (32, 33) led us to investigate the effect of fenofibrate on PCSK9 levels. We hypothesized that fenofibrate treatment might increase circulating PCSK9 protein levels based on our own previous observations with statins, which can increase the activity/nuclear translocation of SREBP-2, a transcription factor that activates both the LDLR and PCSK9 genes (25, 26). To test this hypothesis, we used our recently developed PCSK9 dual monoclonal antibody sandwich ELISA (27, 28) to measure serum PCSK9 levels in fenofibrate-treated patients.
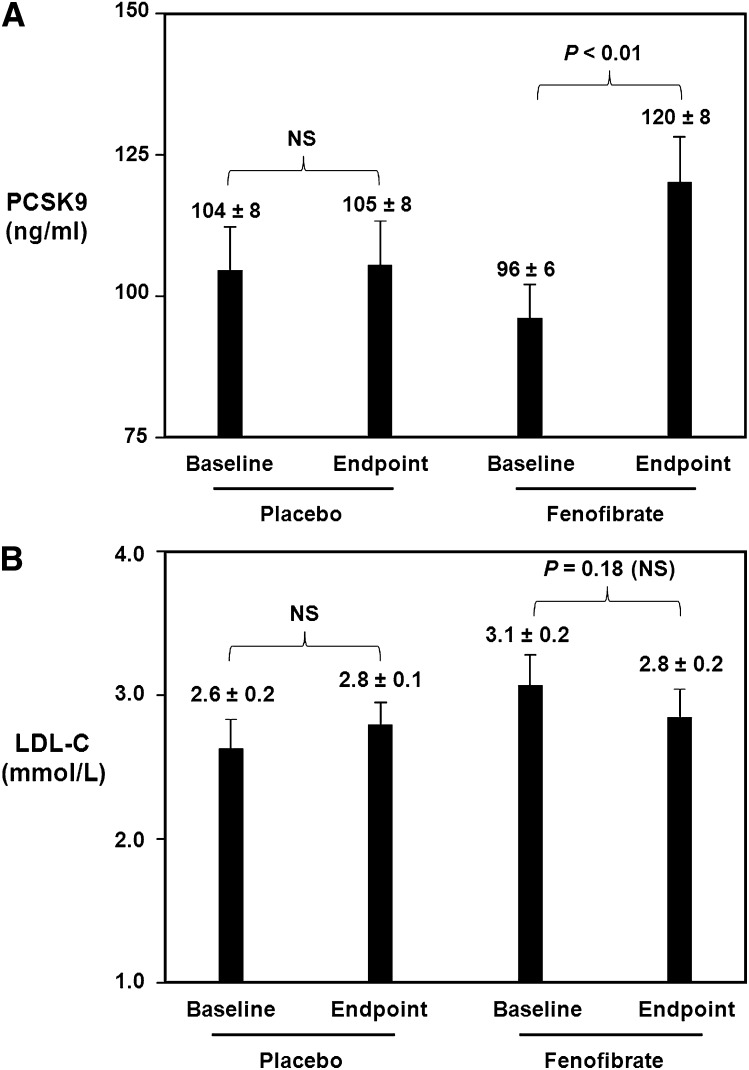
Figure 2A demonstrates that fenofibrate treatment significantly increased serum PCSK9 levels. In the placebo group, there was no significant change in PCSK9 from baseline to endpoint. At baseline in the placebo group, PCSK9 levels were 104 ± 8 ng/ml. Following 12 weeks of placebo treatment, there was no significant change with endpoint PCSK9 levels being 105 ± 8 ng/ml. In contrast, in the fenofibrate group, there was a significant increase in serum PCSK9 levels from baseline to endpoint. At baseline in the fenofibrate-treated group, PCSK9 levels were 96 ± 6 ng/ml. Following 12 weeks of fenofibrate treatment at a dose of 200 mg/day, there was a significant 25% increase with endpoint PCSK9 levels of 120 ± 8 ng/ml (p < 0.01 vs. baseline).
Fig. 2.
Effect of fenofibrate on serum PCSK9 and LDL-C levels. A: Baseline and endpoint samples from patients receiving placebo only for 12 weeks or fenofibrate only (200 mg per day) for 12 weeks were analyzed for PCSK9 levels using a dual monoclonal antibody PCSK9 sandwich ELISA method. Data are expressed as the mean ± SEM. Fenofibrate treatment caused a significant 25% increase in serum PCSK9 levels. B: Patient samples described in Fig. 2A were analyzed for serum LDL-C levels. Data are expressed as the mean ± SEM. Fenofibrate treatment caused a 10% decrease in serum LDL-C, which did not achieve statistical significance.
With regard to LDL-C levels, there were no significant changes from baseline to endpoint. At baseline in the placebo group, LDL-C levels were 2.6 ± 0.2 mmol/L. Following 12 weeks of placebo only treatment, there was no significant change with endpoint LDL-C levels being 2.8 ± 0.1 mmol/L. At baseline in the fenofibrate-treated group, LDL-C levels were 3.1 ± 0.2 mmol/L. Following 12 weeks of fenofibrate treatment at a dose of 200 mg/day, there was a 10% decrease, with endpoint LDL-C levels of 2.8 ± 0.2 mmol/L, although this decrease did not reach statistical significance (p = 0.18 vs. baseline).
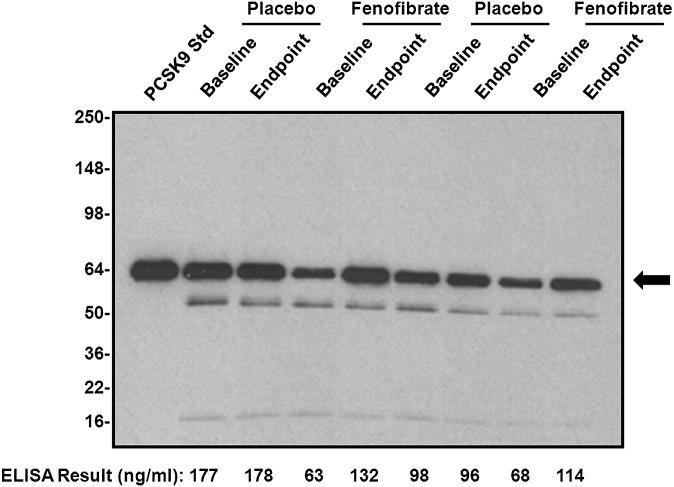
In light of these interesting results showing that fenofibrate treatment significantly increased serum PCSK9 levels as measured by our sandwich ELISA method, as well as a recent report of a furin breakdown product of PCSK9 protein present in plasma migrating as a band below the PCSK9 band (29), we further investigated the fenofibrate-induced increase in PCSK9 by performing immunoprecipitation and Western blotting analyses of some representative placebo and fenofibrate-treated patient samples included in Fig. 2A, which still had adequate volume remaining for immunoprecipitation (at least 100 μl). Results of these experiments are shown in Fig. 3, which demonstrates that fenofibrate treatment resulted in specific increases in the intact PCSK9 protein band that comigrated with the recombinant PCSK9 protein standard and that correlated well with results obtained using our sandwich ELISA.
Fig. 3.
PCSK9 immunoprecipitation and Western blotting analysis of patient serum samples. In order to confirm the effect of fenofibrate treatment on serum PCSK9 levels, representative patient serum samples that still had adequate volume remaining were immunoprecipitated with the same anti-PCSK9 capture monoclonal antibody used in the ELISA. Afterward, immunoprecipitates were analyzed via Western blotting with HRP-labeled polyclonal anti-PCSK9 antibody. Both baseline and endpoint samples from representative placebo and fenofibrate treated patients were analyzed. The predominant band indicated with the arrow represents intact PCSK9 protein. The mobility of the band immediately below is consistent with the furin cleavage product of PCSK9 while the mobility of the band at the bottom of the blot is consistent with PCSK9 propeptide (29). Results were compared with those obtained using the PCSK9 ELISA described in Fig. 2A. Results obtained via immunoprecipitation and Western blotting compared well with those obtained by ELISA, confirming the effect of fenofibrate treatment on PCSK9 levels.
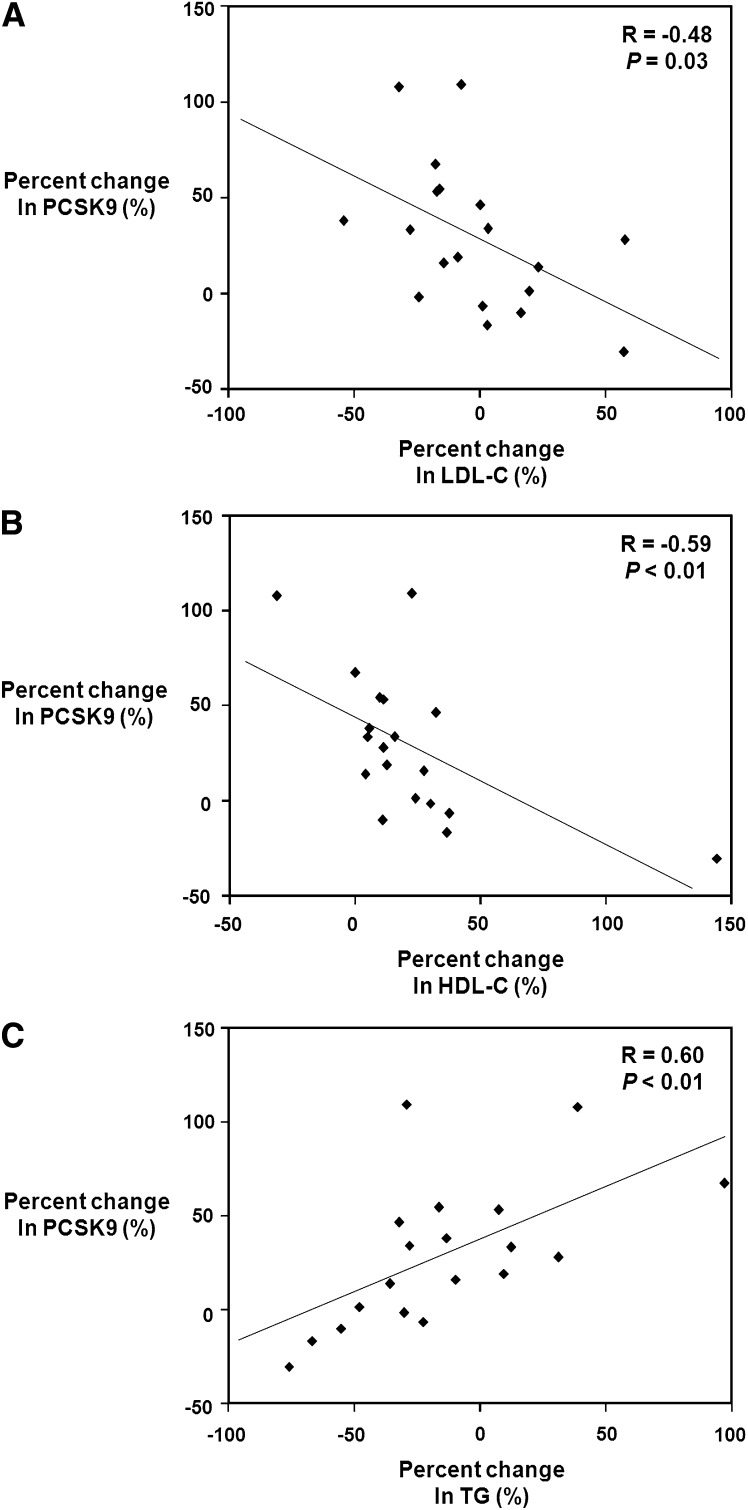
After obtaining these data, we next investigated the correlation in percent changes in serum PCSK9 levels versus the percent changes in LDL-C, HDL-C, and TG levels in the fenofibrate-treated patients. Figure 4A shows the correlation between percent changes in LDL-C and PCSK9 for the fenofibrate-treated patients. Interestingly, percent changes in PCSK9 levels were negatively significantly correlated with percent changes in serum LDL-C levels (r = −0.48, p = 0.03). Figure 4B shows the relationship between the percent changes in PCSK9 levels and the percent changes in HDL-C levels. This figure demonstrates that changes in PCSK9 levels were also negatively significantly correlated with percent changes in serum HDL-C levels (r = −0.59, p < 0.01). In contrast, as shown in Fig. 4C, when the relationship between percent changes in PCSK9 levels and percent changes in serum TG was examined, it was observed that percent changes in PCSK9 were significantly positively correlated with percent changes in serum TG (r = 0.60, p < 0.01). Additional analyses of these data shown in Fig. 4 revealed that the patient who had the paradoxical 97% increase in triglycerides corresponded to the point located at the far right of Fig. 4C. This point corresponds to the point in Fig. 4B showing a 0% increase in HDL-C. In contrast, in Fig. 4B, the patient indicated by the point at the far right who had a 144% increase in HDL-C had a 76% reduction in triglycerides and is indicated by the farthest left point in Fig. 4C. Thus, the patient who had the largest increase in HDL-C also had the greatest reduction in triglycerides.
Fig. 4.
Relationship of fenofibrate-induced percent changes in serum PCSK9 levels compared with percent changes in LDL-C, HDL-C, and triglyceride levels. A: Percentage changes in serum PCSK9 levels from baseline to endpoint in patients receiving fenofibrate (200 mg per day) for 12 weeks were correlated with percentage changes in serum LDL-C levels. Percent changes in PCSK9 levels were negatively significantly correlated with percent changes in LDL-C levels. (r = −0.48, p = 0.03). B: Percentage changes in serum PCSK9 levels from baseline to endpoint in patients receiving fenofibrate (200 mg per day) for 12 weeks were correlated with percentage changes in serum HDL-C levels. Percent changes in PCSK9 levels were negatively significantly correlated with percent changes in HDL-C levels. (r = −0.59, p < 0.01). C: Percentage changes in serum PCSK9 levels from baseline to endpoint in patients receiving fenofibrate (200 mg per day) for 12 weeks were correlated with percentage changes in serum triglyceride levels. Percent changes in PCSK9 levels were positively significantly correlated with percent changes in triglyceride levels. (r = 0.60, p < 0.01).
After performing these analyses, we next examined the correlation of baseline PCSK9 levels with fenofibrate-induced percentage changes in triglycerides, LDL-C, and HDL-C in the fenofibrate-treated patients. There was little correlation between baseline PCSK9 levels and fenofibrate-induced changes in serum triglycerides (r = −0.05, p = 0.80). Interestingly, there was a negative correlation between baseline PCSK9 levels and fenofibrate-induced changes in LDL-C (r = −0.27, p = 0.26), although this correlation did not achieve statistical significance. Likewise, there was a negative correlation between baseline PCSK9 levels and fenofibrate-induced changes in HDL-C (r = −0.29, p = 0.22), although similar to what was observed for LDL-C, this correlation did not achieve statistical significance.
DISCUSSION
The above results demonstrate that fenofibrate treatment (200 mg per day) significantly increases circulating PCSK9 protein levels in humans while at the same time raising serum HDL-C levels. Interestingly, increases in PCSK9 in fenofibrate-treated patients were negatively significantly correlated with changes in LDL-C and HDL-C and positively significantly correlated with changes in triglycerides. Recent conflicting observations concerning the effect of fibrates on hepatocyte PCSK9 synthesis and secretion make these quantitative results obtained at the protein level in actual human patients treated with fenofibrate particularly important (32–34).
Previously, Mayne et al. (32) reported that fibrates increased human serum PCSK9 levels by 17%; however, all of the PCSK9 analyses in this study were performed by immunoprecipitation and Western blotting, which is a qualitative rather than a quantitative technique. In addition, the absolute levels of PCSK9 present in human serum as reported by this method were approximately 10- to 100-fold greater than what our laboratory as well as several other groups have reported (27–30). Also, the group of patients analyzed in this study was relatively small and consisted of a mixture of patients on gemfibrozil and fenofibrate.
In contrast to this report, Kourimate et al. (33) demonstrated that fibrate treatment resulted in reduced PCSK9 mRNA levels in hepatocytes. This group also went on to measure levels of PCSK9 protein expression in hepatocytes and concluded that PCSK9 protein expression in hepatocytes was also reduced. Again, similar to the work by Mayne and coworkers, PCSK9 protein levels were not measured via a truly quantitative technique but by qualitative Western blotting. Kourimate and coworkers concluded from these data that addition of a fibrate to preexisting statin therapy would result in suppression of circulating PCSK9 levels and, thus, enhanced LDL-C lowering activity of the statin.
In contrast to the observations of Kourimate and coworkers, Lambert et al. (34) used an ELISA method to report that fenofibrate treatment (200 mg per day for 6 weeks) decreased plasma PCSK9 levels by 8% and that this decrease correlated with the fenofibrate-induced decreases in circulating triglycerides. Similar to the observations by Mayne and colleagues, the absolute plasma levels of PCSK9 as reported by this method were approximately 10- to 100-fold greater than what our laboratory as well as several other groups have reported (27–30). The same researchers concluded that fenofibrate-induced decreases in PCSK9 might account for the modest LDL-C reduction observed with fenofibrate (34).
In comparing these different previously reported results for PCSK9 levels, one possibility for the discrepancies may be due to differences in analytical technique. In addition, however, the type of patient population being studied may also partly explain differences in reported absolute values for serum PCSK9 levels. For instance, the type of dyslipidemia present in patients being studied may account for differences in reported PCSK9 levels. Similarly, patient demographics such as age, sex, and ethnicity may play a role as well as whether or not patients had been previously treated for a lipid abnormality.
Our present data suggest that fenofibrate acts to increase PCSK9 levels because fenofibrate treatment significantly increased circulating PCSK9 levels by 25%. The mechanism by which fenofibrate increased PCSK9 levels is unclear. Fenofibrate belongs to a class of drugs called PPAR-α agonists, which affect lipid levels by altering transcription of a number of different genes involved in lipoprotein and fatty acid metabolism (35). As a PPAR-α agonist, fenofibrate reduces triglyceride synthesis in the liver and increases breakdown of triglyceride rich lipoproteins, while enhancing cholesterol efflux from the liver (36). It is possible that these effects on cholesterol and lipoprotein metabolism may work indirectly within the hepatocyte to decrease intracellular cholesterol levels, thus, leading to increased PCSK9 expression and secretion. Alternatively, in light of the multiple pleiotropic effects of fenofibrate (37), it may also be possible that fenofibrate exerts a direct effect within hepatocytes to stimulate increased PCSK9 synthesis and secretion.
Whatever the exact mechanism may be, it is clear from our data that fenofibrate treatment increases circulating PCSK9 levels. These data may explain why the addition of fenofibrate to statin therapy may not result in as much additional LDL lowering as might otherwise be anticipated. In other words, the combined effect of a statin-fenofibrate combination on LDL-C lowering may be modestly additive in spite of the observed opposing effects on PCSK9 levels for both statins and fenofibrate. To further investigate the combined effects of fenofibrate with statins, it will be important to analyze samples from patients treated with both compounds to see if the increase in PCSK9 levels is additive or synergistic. Unfortunately, our clinical trial did not include any dose arms in which patients were treated with both fenofibrate and atorvastatin; thus, we did not have the ability to analyze the combined effect of these agents on PCSK9 levels. Therefore, testing of this hypothesis will have to await future studies in which PCSK9 levels can be measured in patients treated with a statin-fibrate combination. Our current study does suggest, however, that addition of a PCSK9 inhibitor to fibrate therapy presents the possibility of further improving the anti-hyperlipidemic effect of fibrates.
Acknowledgments
The authors thank Dr. Kathy Landschulz and Jayne Talbot for their support.
Footnotes
Abbreviations:
- LDL-C
- LDL cholesterol
- LDLR
- LDL receptor
- PCSK9
- proprotein convertase subtilisin kexin type 9
- PPAR
- peroxisome proliferator-activated receptor
- SREBP-2
- sterol-regulatory binding protein-2
- TG
- triglyceride
REFERENCES
- 1.Cao G., Qian Y. W., Kowala M. C., Konrad R. J. 2008. Further LDL cholesterol lowering through targeting PCSK9 for coronary artery disease. Endocr. Metab. Immune Disord. Drug Targets. 8: 238–243. [DOI] [PubMed] [Google Scholar]
- 2.Horton J. D., Cohen J. C., Hobbs H. H. 2007. Molecular biology of PCSK9: its role in LDL metabolism. Trends Biochem. Sci. 32: 71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qian Y. W., Schmidt R. J., Zhang Y., Chu S., Lin A., Wang H., Wang X., Beyer T. P., Bensch W. R., Li W., et al. 2007. Secreted proprotein convertase subtilisin/kexin-type 9 reduces low-density lipoprotein receptor through receptor-mediated endocytosis. J. Lipid Res. 48: 1488–1498. [DOI] [PubMed] [Google Scholar]
- 4.Lambert G., Krempf M., Costet P. 2006. PCSK9: a promising therapeutic target for dyslipidemias. Trends Endocrinol. Metab. 17: 79–81. [DOI] [PubMed] [Google Scholar]
- 5.Graham M. J., Lemonidis K. M., Whipple C. P., Subramaniam A., Monia B. P., Crooke S. T., Crooke R. M. 2007. Antisense inhibition of proprotein convertase subtilisin kexin 9 reduces serum LDL in hyperlipidemic mice. J. Lipid Res. 48: 763–767. [DOI] [PubMed] [Google Scholar]
- 6.Lagace T. A., Curtis D. E., Garuti R., McNutt M. C., Park S. W., Prather H. B., Anderson N. N., Ho Y. K., Hammer R. E., Horton J. D. 2006. Secreted PCSK9 decreases the number of LDL receptors in hepatocytes and in livers of parabiotic mice. J. Clin. Invest. 116: 2995–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maxwell K. N., Fisher E. A., Breslow J. L. 2005. Overexpression of PCSK9 accelerates the degradation of the LDLR in a post- endoplasmic reticulum compartment. Proc. Natl. Acad. Sci. USA. 102: 2069–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park S. W., Moon Y. A., Horton J. D. 2004. Post-transcriptional regulation of low density lipoprotein receptor protein by proprotein convertase subtilisin/kexin type 9a in mouse liver. J. Biol. Chem. 279: 50630–50638. [DOI] [PubMed] [Google Scholar]
- 9.Benjannet S., Rhainds D., Essalmani R., Mayne J., Wickham L., Jin W., Asselin M. C., Hamelin J., Varret M., Allard D., et al. 2004. NARC-1/PCSK9 and its natural mutants: zymogen cleavage and effects on the low density lipoprotein (LDL) receptor and LDL cholesterol. J. Biol. Chem. 279: 48865–48875. [DOI] [PubMed] [Google Scholar]
- 10.Maxwell K. N., Breslow J. L. 2004. Adenoviral-mediated expression of Pcsk9 in mice results in a low-density lipoprotein receptor knockout phenotype. Proc. Natl. Acad. Sci. USA. 101: 7100–7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmidt R. J., Beyer T. P., Bensch W. R., Qian Y. W., Lin A., Kowala M., Alborn W. E., Konrad R. J., Cao G. 2008. Secreted proprotein convertase subtilisin/kexin type 9 reduces both hepatic and extrahepatic low-density lipoprotein receptors in vivo. Biochem. Biophys. Res. Commun. 370: 634–640. [DOI] [PubMed] [Google Scholar]
- 12.Li J., Tumanut C., Gavigan J. A., Huang W. J., Hampton E. N., Tumanut R., Suen K. F., Trauger J. W., Spraggon G., Lesley S. A., et al. 2007. Secreted PCSK9 promotes LDL receptor degradation independently of proteolytic activity. Biochem. J. 406: 203–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McNutt M. C., Lagace T. A., Horton J. D. 2007. Catalytic activity is not required for secreted PCSK9 to reduce low density lipoprotein receptors in HepG2 cells. J. Biol. Chem. 282: 20799–20803. [DOI] [PubMed] [Google Scholar]
- 14.Zhang D. W., Lagace T. A., Garuti R., Zhao Z., McDonald M., Horton J. D., Cohen J. C., Hobbs H. H. 2007. Binding of proprotein convertase subtilisin/kexin type 9 to epidermal growth factor-like repeat A of low density lipoprotein receptor decreases receptor recycling and increases degradation. J. Biol. Chem. 282: 18602–18612. [DOI] [PubMed] [Google Scholar]
- 15.Chan J. C., Piper D. E., Cao Q., Liu D., King C., Wang W., Tang J., Liu Q., Higbee J., Xia Z., et al. 2009. A proprotein convertase subtilisin/kexin type 9 neutralizing antibody reduces serum cholesterol in mice and nonhuman primates. Proc. Natl. Acad. Sci. USA. 106: 9820–9825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McNutt M. C., Kwon H. J., Chen C., Chen J. R., Horton J. D., Lagace T. A. 2009. Antagonism of secreted PCSK9 increases low density lipoprotein receptor expression in HepG2 cells. J. Biol. Chem. 284: 10561–10570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duff C. J., Scott M. J., Kirby I. T., Hutchinson S. E., Martin S. L., Hooper N. M. 2009. Antibody-mediated disruption of the interaction between PCSK9 and the low-density lipoprotein receptor. Biochem. J. 419: 577–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maxwell K. N., Breslow J. L. 2005. Proprotein convertase subtilisin kexin 9: the third locus implicated in autosomal dominant hypercholesterolemia. Curr. Opin. Lipidol. 16: 167–172. [DOI] [PubMed] [Google Scholar]
- 19.Dubuc G., Chamberland A., Wassef H., Davignon J., Seidah N. G., Bernier L., Prat A. 2004. Statins upregulate PCSK9, the gene encoding the proprotein convertase neural apoptosis-regulated convertase-1 implicated in familial hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. 24: 1454–1459. [DOI] [PubMed] [Google Scholar]
- 20.Abifadel M., Varret M., Rabes J. P., Allard D., Ouguerram K., Devillers M., Cruaud C., Benjannet S., Wickham L., Erlich D., et al. 2003. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat. Genet. 34: 154–156. [DOI] [PubMed] [Google Scholar]
- 21.Allard D., Amsellem S., Abifadel M., Trillard M., Devillers M., Luc G., Krempf M., Reznik Y., Girardet J. P., Fredenrich A., et al. 2005. Novel mutations of the PCSK9 gene cause variable phenotype of autosomal dominant hypercholesterolemia. Hum. Mutat. 26: 497. [DOI] [PubMed] [Google Scholar]
- 22.Cohen J. C., Boerwinkle E., Mosley T. H., Jr., Hobbs H. H. 2006. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N. Engl. J. Med. 354: 1264–1272. [DOI] [PubMed] [Google Scholar]
- 23.Fasano T., Cefalu A. B., Di Leo E., Noto D., Pollaccia D., Bocchi L., Valenti V., Bonardi R., Guardamagna O., Averna M., et al. 2007. A novel loss-of-function mutation of PCSK9 gene in white subjects with low-plasma low-density lipoprotein cholesterol. Arterioscler. Thromb. Vasc. Biol. 27: 677–681. [DOI] [PubMed] [Google Scholar]
- 24.Zhao Z., Tuakli-Wosornu Y., Lagace T. A., Kinch L., Grishin N. V., Horton J. D., Cohen J. C., Hobbs H. H. 2006. Molecular characterization of loss-of-function mutations in PCSK9 and identification of a compound heterozygote. Am. J. Hum. Genet. 79: 514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rashid S., Curtis D. E., Garuti R., Anderson N. N., Bashmakov Y., Ho Y. K., Hammer R. E., Moon Y. A., Horton J. D. 2005. Decreased plasma cholesterol and hypersensitivity to statins in mice lacking Pcsk9. Proc. Natl. Acad. Sci. USA. 102: 5374–5379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berge K. E., Ose L., Leren T. P. 2006. Missense mutations in the PCSK9 gene are associated with hypocholesterolemia and possibly increased response to statin therapy. Arterioscler. Thromb. Vasc. Biol. 26: 1094–1100. [DOI] [PubMed] [Google Scholar]
- 27.Alborn W. E., Cao G., Careskey H. E., Qian Y. W., Subramaniam D. R., Davies J., Conner E. M., Konrad R. J. 2007. Serum proprotein convertase subtilisin kexin type 9 is correlated directly with serum LDL cholesterol. Clin. Chem. 53: 1814–1819. [DOI] [PubMed] [Google Scholar]
- 28.Careskey H. E., Davis R. A., Alborn W. E., Troutt J. S., Cao G., Konrad R. J. 2008. Atorvastatin increases human serum levels of proprotein convertase subtilisin/kexin type 9. J. Lipid Res. 49: 394–398. [DOI] [PubMed] [Google Scholar]
- 29.Dubuc G., Tremblay M., Pare G., Jacques H., Hamelin J., Benjannet S., Boulet L., Genest J., Bernier L., Seidah N. G., et al. 2009. A new method for measurement of total plasma PSCK9 - clinical applications. J. Lipid Res.; Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lakoski S. G., Lagace T. A., Cohen J. C., Horton J. D., Hobbs H. H. 2009. Genetic and metabolic determinants of plasma PCSK9 levels. J. Clin. Endocrinol. Metab. 94: 2537–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nissen S. E., Nicholls S. J., Wolski K., Howey D. C., McErlean E., Wang M. D., Gomez E. V., Russo J. M. 2007. Effects of a potent and selective PPAR-alpha agonist in patients with atherogenic dyslipidemia or hypercholesterolemia: two randomized controlled trials. JAMA. 297: 1362–1373. [DOI] [PubMed] [Google Scholar]
- 32.Mayne J., Dewpura T., Raymond A., Cousins M., Chaplin A., Lahey K. A., Lahaye S. A., Mbikay M., Ooi T. C., Chrétien M. 2008. Plasma PCSK9 levels are significantly modified by statins and fibrates in humans. Lipids Health Dis. 7: 22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kourimate S., Le May C., Langhi C., Jarnoux A. L., Ouguerram K., Zaïr Y., Nguyen P., Krempf M., Cariou B., Costet P. 2008. Dual mechanisms for the fibrate-mediated repression of proprotein convertase subtilisin/kexin type 9. J. Biol. Chem. 283: 9666–9673. [DOI] [PubMed] [Google Scholar]
- 34.Lambert G., Ancellin N., Charlton F., Comas D., Pilot J., Keech A., Patel S., Sullivan D. R., Cohn J. S., Rye K. A., et al. 2008. Plasma PCSK9 concentrations correlate with LDL and total cholesterol in diabetic patients and are decreased by fenofibrate treatment. Clin. Chem. 54: 1038–1045. [DOI] [PubMed] [Google Scholar]
- 35.Farnier M. 2008. Update on the clinical utility of fenofibrate in mixed dyslipidemias: mechanisms of action and rational prescribing. Vasc. Health Risk Manag. 4: 991–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keating G. M., Croom K. F. 2007. Fenofibrate. A review of its use in primary dyslipidemia, the metabolic syndrome and type 2 diabetes mellitus. Drugs. 67: 121–153. [DOI] [PubMed] [Google Scholar]
- 37.Paumelle R., Staels B. 2008. Cross-talk between statins and PPARα in cardiovascular diseases: clinical evidence and basic mechanisms. Trends Cardiovasc. Med. 18: 73–78. [DOI] [PubMed] [Google Scholar]