Abstract
Skeletal muscle triglyceride accumulation is associated with insulin resistance in obesity. Recently, it has been suggested that α lipoic acid (ALA) improves insulin sensitivity by lowering triglyceride accumulation in nonadipose tissues via activation of skeletal muscle AMP-activated protein kinase (AMPK). We examined whether chronic ALA supplementation prevents muscular lipid accumulation that is associated with high-fat diets via activation of AMPK. In addition, we tested if ALA supplementation was able to improve insulin sensitivity in rats fed low- and high-fat diets (LFD, HFD). Supplementing male Wistar rats with 0.5% ALA for 8 weeks significantly reduced body weight, both on LFD and HFD (−24% LFD+ALA vs. LFD, P < 0.01, and −29% HFD+ALA vs. HFD, P < 0.001). Oil red O lipid staining revealed a 3-fold higher lipid content in skeletal muscle after HFD compared with LFD and ALA-supplemented groups (P < 0.05). ALA improved whole body glucose tolerance (∼20% lower total area under the curve (AUC) in ALA supplemented groups vs. controls, P < 0.05). These effects were not mediated by increased muscular AMPK activation or ALA-induced improvement of muscular insulin sensitivity. To conclude, the prevention of HFD-induced muscular lipid accumulation and the improved whole body glucose tolerance are likely secondary effects due to the anorexic nature of ALA.
Keywords: AMP-activated protein kinase, intramyocellular lipid accumulation, glucose tolerance
Obesity has reached epidemic proportions globally and the situation is likely to deteriorate further. It is well known that obesity predisposes individuals to a range of serious health complications with high morbidity rates including insulin resistance, type 2 diabetes mellitus, and cardiovascular diseases (1). The mechanisms underlying the progression from obesity to insulin resistance and, ultimately, type 2 diabetes are not fully elucidated (2). It is speculated that excessive FFA mobilization from adipose tissue leads to an increase in plasma FFA with ectopic deposition of triglycerides in muscle, the liver, and the pancreas as a result. The increased intramyocellular lipid (IMCL) content could disturb muscle insulin signaling, which ultimately results in muscle insulin resistance (3).
Alpha lipoic acid (ALA), also known as thioctic acid, is a naturally occurring short-chain fatty acid with a powerful antioxidant capacity. It is synthesized in small amounts by plants and animals, including humans (4, 5). Endogenously synthesized ALA is an essential cofactor for several mitochondrial enzyme complexes that catalyze critical reactions related to energy production (6). ALA can also be exogenously derived from various food sources such as tomatoes, spinach, broccoli, and Brussels sprouts (7). ALA has been suggested to have influence on glucose homeostasis. Short-term incubations of L6 muscle cells with a rather high concentration of 10 mM of ALA increased glucose uptake by 40–80%, and ex vivo incubation of muscles derived from ob/ob mice with 10 mM ALA showed a substantial increase in glucose uptake (8). Acute and chronic parenteral treatments with ALA in obese Zucker (fa/fa) rats, an animal model of insulin resistance, significantly improved insulin-stimulated 2-deoxyglucose uptake in epitrochlearis muscles by 62% and 64% (9). In an uncontrolled study of 20 patients with type 2 diabetes, intravenous infusion of 500 mg/day of racemic ALA for 10 days improved insulin-stimulated glucose disposal measured 24 h after the last infusion (10). Moreover, in a placebo-controlled, multi-center study, 74 patients with type 2 diabetes were randomized to receive 600 mg ALA or placebo orally once, twice, or three times daily for 4 weeks. It was shown that patients who received ALA significantly improved their insulin-stimulated glucose disposal compared with those on the placebo although no significant differences regarding the various doses of ALA were observed (11). These studies suggest that ALA has the potential to improve insulin sensitivity via an effect on skeletal muscle glucose uptake.
In that respect, recent experimental research indicates that ALA might improve insulin sensitivity by lowering triglyceride accumulation in nonadipose tissues like muscle. Feeding diabetic Otsuka Long Evans Tokushima Fatty (OLETF) rats with ALA-supplemented chow reduced triglyceride accumulation in skeletal muscle and pancreatic islets compared with their untreated counterparts (12). The reduced triglyceride accumulation in skeletal muscle was attributed to the activation of skeletal muscle AMP-activated protein kinase (AMPK) (13), an enzyme that is activated when the cellular energy is depleted (14). Previous experiments in cells and animals showed that AMPK could stimulate glucose uptake independently of insulin, lower triglyceride accumulation in nonadipose tissues via increased fat oxidation, and increase mitochondrial biogenesis (15–19). Thus, activation of AMPK by ALA may lead to a lower accumulation of IMCL via an increased oxidation of long-chain fatty acids and might improve whole body insulin sensitivity.
To test this hypothesis, we studied whether supplementation of ALA to a high-fat diet prevented diet-induced obesity and muscular lipid accumulation. In addition, we studied if ALA supplementation could improve insulin sensitivity over time and if this was mediated by changed activity of skeletal muscle AMPK.
METHODS
Animals and diets
A total of 32 eleven-week-old male Wistar rats (Charles River Laboratories) were individually caged and randomly assigned to one of the four dietary treatments for 8 weeks. The first two groups were fed a semi-purified diet containing 10% of kcal as fat with an energy density of 3.85 kcal/g (D12450B; 20% energy (% E) protein, 70% E carbohydrate, and 10% E fat consisting of 225% E soybean oil and 180% E lard; Research Diets, Inc., New Brunswick, NJ) supplemented with or without 0.5% DL-ALA (Sigma-Aldrich Chemie B.V., Zwijndrecht, The Netherlands). The other two groups received a semi-purified diet containing 45% of kcal as fat with an energy density of 4.73 kcal/g (D12451; 20% E protein, 35% E carbohydrate, 45% E fat consisting of 225% E of soybean oil and 1598% E lard; Research Diets) supplemented with or without 0.5% DL-ALA. Rats were maintained at ambient temperature (22 ± 1°C), with 12:12 h light-dark cycles and allowed ad libitum access to food and water. Food consumption and body weight were recorded weekly. To calculate net absorption of nutrients during the diet intervention, fecal samples were collected during the last week of the intervention period, freeze-dried, and together with samples from the diet, analyzed for gross energy content using adiabatic bomb calorimetry (Ika-calorimeter system C4000 Heitersheim, Germany). All experiments were approved by the Institutional Animal Care and Use Committee of the Maastricht University and complied with the principles of laboratory animal care.
Intraperitoneal glucose tolerance tests
To investigate the effect of DL-ALA on glucose tolerance, rats were subjected to an intraperitoneal glucose tolerance test (ipGTT) after 4 and 8 weeks of the dietary treatment. Rats were administered 1.5 g/kg glucose (ICN Biomedicals, Inc., Aurora, OH) after a 6 h fast. A baseline blood sample was immediately taken (t = 0), and this was repeated at t = 15, 30, 60, and 120 min. Samples were collected in Na-EGTA coated Eppendorf tubes and centrifuged immediately at 4,000 rpm for 10 min. Plasma was then separated and stored at −80°C for the determination of insulin concentrations by Insulin (Rat) Ultrasensitive EIA (Alpco Diagnostics, Salem, NH).
Tissue collection
After 8-1/2 weeks of ALA supplementation, rats were euthanized after a 6 h fast. Rats received an intraperitoneal injection of insulin (10u/kg) and were anesthetized after 9 min by a mixture of 79% of CO2 and 21% of O2 for 1 min, followed by cervical dislocation. For histological analysis, the medial region of the left gastrocnemius muscle (mostly compromised of type 2 muscle fibers) was dissected and freed from any visible fat and blood, embedded in Tissue-Tek (Sakura Finetek, Zoeterwoude, The Netherlands), and frozen in liquid nitrogen-cooled isopentane (2-methyl-butane, Fluka, Zwijndrecht, The Netherlands). The right gastrocnemius muscle was rapidly dissected and immediately frozen in liquid nitrogen-cooled isopentane for Western blot analyses. All samples were stored at −80°C until further analysis.
Histological analysis of IMCL
Briefly, cryosections (5 μm) from the midbelly region of the gastrocnemius were thaw mounted on uncoated precleaned (96% ethanol) glass slides. Immediately after mounting, air-dried, fresh cryosections were fixed in 3.7% formaldehyde for 1 h. Then, the sections were treated with 0.5% Triton X-100 in PBS for 5 min and washed three times with PBS. Thereafter, sections were incubated for 30 min with a polyclonal rabbit antibody against the basement membrane protein laminin (Sigma-Aldrich, St. Louis, MO; 1:50 dilution in PBS) and a monoclonal antibody raised against adult human slow myosin heavy chain (A4.840, developed by Dr. Blau; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA) (20) to visualize individual cell membranes and distinguish between type 1 and 2 muscle fibers. This was followed by 30 min incubation with the appropriate secondary antibodies conjugated with Alexa Fluor488 and Alexa Fluor555 (Invitrogen, Groningen, The Netherlands). After washing with PBS, glass slides were immersed in the ORO working solution for 30 min for the detection of lipid droplets (21) and rinsed three times with deionized water for 30 s followed by 10 min of washing with running tap water. Stained sections were embedded in Mowiol.
All sections were examined using a Nikon E800 fluorescence microscope (Nikon Instruments Europe B.V., Badhoevedorp, The Netherlands) coupled to a Basler A101C progressive scan color charge-coupled device camera. All sections were processed and analyzed using Lucia GF 4.80 software (Nikon, Düsseldorf, Germany). Special care was taken to use the same camera settings (gain and exposure time) while grabbing all images. For IMCL, >800 cells per muscle were analyzed.
All images were analyzed for the lipid droplet over myocyte area fraction. To this end, a semiautomatic macro was written that allowed: 1) autodetection of the cellular membrane (identified in the blue channel), 2) measurement of the area covered by the measured myocytes with distinction between type 1 and type 2 fibers (identified in the green area), and 3) measurement of the area covered by lipid droplets (identified in the red channel). The area fraction was computed by dividing the area covered by the lipid droplets (in μm2) by the cell surface of the measured myocytes (in μm2). Thus, the mean area fraction reflects the percentage of total measured cell surface covered by lipid droplets.
Western blot analyses
Muscle homogenates for Western blot analysis of p-Akt protein were performed as described before (22). Equal amounts of protein were loaded on SDS-PAGE and after Western blotting, incubated with Akt and p-Akt antibodies (#9271 and #9272, respectively, Cell Signaling Technology, Bioké, Leiden, The Netherlands). After incubation with the appropriate secondary antibodies, specific protein bands were visualized by chemiluminescence and analyzed by Chemidoc XRS system (Bio-Rad, Veenendaal, The Netherlands).
For GLUT4 detection equal amounts of muscle membrane and muscle cytosol protein fractions were loaded on SDS-PAGE and after Western blotting, incubated with a GLUT4-antibody (sc-1608, Santa Cruz, Bio-Connect, Huissen, The Netherlands). After incubation with the appropriate secondary IRDye680- labeled antibody (Licor, Westburg, Leusden, The Netherlands), the specific GLUT4 protein was detected and analyzed using the Odyssey near Infrared Scanner (Licor).
AMPK activity and ACC phosphorylation
Kinase assays of total AMPK activity have been described previously (23). Briefly, total AMPK activity was measured in the tissue lysates by immunoprecipitating with a combination of α1- and α2-specific antibodies. Activities are presented in units per milligram protein, where a unit is defined as nanomoles of phosphate incorporation into the SAMS peptide per minute.
Western blot analyses of acetyl-CoA carboxylase (ACC) was carried out as follows. Muscle samples were powdered under liquid nitrogen and homogenized at a ratio of 1:4 (w/v) in the following buffer 0.05 M Tris-HCl pH 7.4, 0.25 M Sucrose, 0.001 M EDTA, 0.001 M EGTA, 1% TX-100, 0.001 M NaVO4 0.05 M NaF, 0.005 M Na2PO4, 0.1% DTT, and protease inhibitor cocktail tablet (1 tablet/10 ml) (Roche, Hertfordshire, UK). A total of 15 μg protein was loaded on a NuPage 3–8% Tris-Acetate gel (Invitrogen, Paisley, UK) and separated by SDS-PAGE followed by transfer onto nitrocellulose membrane. Membranes were blocked for 1 h with Odyssey blocking buffer (Licor, UK) and probed overnight with sheep polyclonal phosphospecific ACC1/2 (P-S79/ P-S221) antibody and total ACC antibody (Cell Signaling Technology, Beverly, MA). The phosphospecific polyclonal antibody was raised against a 13-amino-acid peptide that comprised amino acid 221 of human ACC2 coupled to KLH, and a 12-amino-acid peptide that comprised amino acid 79 of rat ACC1. After immunization of sheep with the peptides, antibodies were extracted from sera by Sephadex affinity column. Columns were washed with tris-buffered saline with 1% Tween-20 and eluted in 50 mM glycine (pH 2.5). A total of 75 mM tris pH 8 was added to eluted antibodies. Membranes were washed in TBS-T and incubated for 1 h with secondary antibodies IRDye 800 conjugated to streptavidin (picks up total ACC), and sheep IRDye 680 (picks up P-ACC) (Rockland Inc., Lorne Laboratories, Reading, UK). Membranes were washed again in TBS-T and analyzed using the Licor Odyssey near Infrared Scanner.
Statistical analysis
Results are presented as the mean ± SE. Differences between groups were analyzed by one-way ANOVA with a Bonferroni's post hoc correction. The accepted level of statistical significance was P < 0.05 for all analyses. All calculations were done using the Statistical Package for the Social Sciences (SPSS 11.0 software).
RESULTS
Body weight gain and composition
Body weights gradually increased over the 8 weeks of diet intervention in the low-fat diet (LFD) and the high-fat diet (HFD) rats. However, compared with animals on the LFD, rats receiving the HFD gained more weight during the intervention period (Fig. 1A). Supplementation of ALA to LFD or HFD attenuated weight gain, resulting in significantly lower final body weights (–24% in LFD+ALA vs. LFD, P < 0.01 and –29% in HFD+ALA vs. HFD, P < 0.001, Fig. 1A). Epididymal and perirenal fat pad weights were significantly higher in HFD animals compared with rats on the LFD (P < 0.001). ALA treatment resulted in an ∼50% lower epididymal and perirenal fat mass in the ALA-treated animals compared with their controls (P < 0.01, Fig. 1B). Thus, these data suggest that the differences in body weight between the groups are, at least in part, due to differences in adiposity (Fig. 1B).
Fig. 1.
ALA prevents high-fat diet-associated obesity. A: Body weight (n = 8). B: Epididymal and perirenal fat mass at sacrifice (n = 8). C: Food intake (n = 8). Values are expressed as means ± SEM. * P < 0.05 versus LFD; ** P < 0.05 versus HFD.
Food intake and net absorption
Analysis of food intake and fecal energy content over the last week revealed that gross energy absorption was 30% lower in the ALA supplemented groups compared with control groups (P < 0.01) (data not shown). This reduction in gross energy absorption was completely accounted for by a 30% lower food intake in animals treated with ALA (P < 0.001, Fig. 1C), with no differences in fecal energy content between the groups (data not shown).
Glucose and insulin levels following ipGTT
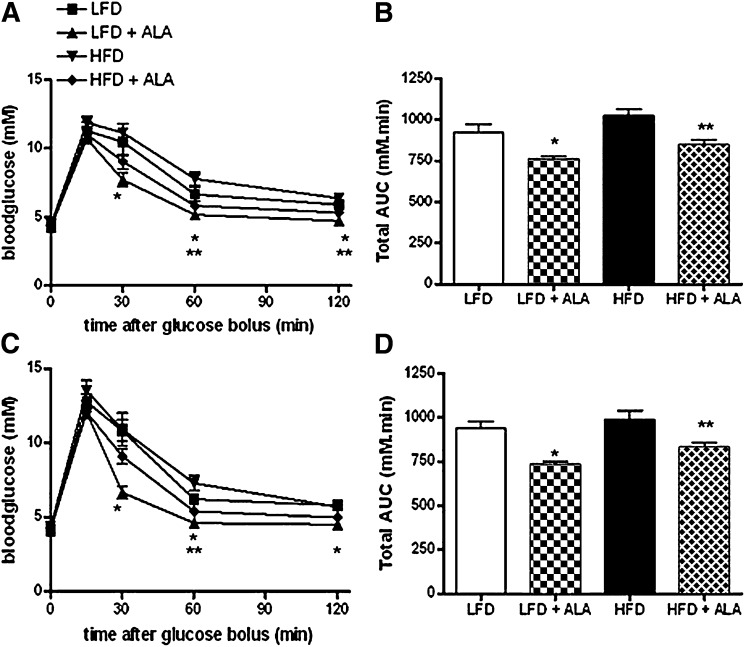
IpGTTs were performed 4 and 8 weeks after commencement of dietary treatment to study time-dependent effects of the diet on whole body glucose tolerance. Fasting blood glucose values were not different between ALA and control animals after 4 and 8 weeks (Fig. 2A, C). Glucose injection in the animals after 4 weeks diet intervention resulted in similar peak blood glucose values at time-point 15 min between groups. Glucose clearance in the ALA treated animals was faster than in the corresponding LFD and HFD fed rats (∼17% lower total area under the curve (AUC), P < 0.05, Fig. 2B). After 8 weeks of treatment, glucose levels peaked again at time point 15 min after injection of the glucose bolus and values were not different between groups. Glucose clearance remained faster in the LFD+ALA versus LFD group and HFD+ALA versus HFD group (∼ 20% lower total AUC, P < 0.05, Fig. 2D).
Fig. 2.
Effect of dietary ALA in response to a 2 h intraperitoneal glucose tolerance test (ipGTT) (1.5g/kg). Blood glucose concentrations in time (A) and total area under curve (AUC) (B) during ipGTT after 4 weeks of diet intervention (n = 8). Blood glucose concentrations in time (C) and total AUC (D) during ipGTT after 8 weeks of diet intervention (n = 8). D: Values are expressed as means ± SEM. * P < 0.05 versus LFD; ** P < 0.05 versus HFD.
Fasting plasma insulin levels were lower in the groups receiving ALA compared with controls after 4 weeks of treatment (1.08 ± 0.19 vs. 2.43 ± 0.30 ng/ml·min in LFD+ALA vs. LFD, P < 0.001, and 0.66 ± 0.06 vs. 1.60 ± 0.34 ng/ml·min in HFD+ALA vs. HFD). Total area under the curve was ∼30% lower in the ALA supplemented animals compared with the control LFD and HFD groups (P < 0.05, Fig. 3A). After 8 weeks of ALA supplementation, fasting plasma insulin levels remained lower compared with the control matched animals (1.36 ± 0.31 vs. 2.52 ± 0.36 ng/ml·min in LFD+ALA vs. LFD, P < 0.05, and 0.87 ± 0.10 vs. 1.90 ± 0.21 ng/ml·min in HFD+ALA vs. HFD, P < 0.001). Total AUC was again ∼30% lower in the ALA versus control treated animals; however, due to the high variance this did not reach statistical significance (P = 0.34, Fig. 3B).
Fig. 3.
Effect of dietary ALA on insulin concentrations during a 2 h intraperitoneal glucose tolerance test (ipGTT). Total area under the insulin curve after 4 (n = 5) (A) and 8 (n = 6) (B) weeks of diet intervention (n = 5). Values are expressed as means ± SEM. * P < 0.05 versus LFD; ** P < 0.05 versus HFD.
Intramyocellular lipid levels
After 8 weeks of dietary intervention, IMCL content was 3-fold higher in the animals receiving the HFD (P < 0.05). There were no differences in IMCL accumulation between type 1 and 2 muscle fibers (data not shown). Supplementation of ALA in rats on the HFD reduced lipid accumulation significantly, resulting in an intramyocellular lipid content that was comparable to animals receiving the LFD (P < 0.05, Fig. 4). ALA did not further reduce IMCL in the LFD group. Representative images demonstrating differences in staining between the four different diets are included as supplementary.
Fig. 4.
Intramyocellular lipid levels in left gastrocnemius muscle after 8 weeks of diet intervention (n = 8). Values are expressed as means ± SEM. * P < 0.05 versus LFD; ** P < 0.05 versus HFD.
Insulin signaling
Muscle insulin signaling was analyzed to see whether the ALA-induced increase in whole body glucose tolerance, together with the lower muscular lipid accumulation, resulted in improved insulin-stimulated glucose disposal.
Among the different dietary interventions there were no differences in insulin-induced Akt phosphorylation (Fig. 5A). In parallel, ALA treatment failed to increase insulin-stimulated GLUT4 translocation (Fig. 5B).
Fig. 5.
Effect of 8 week treatment with dietary ALA on (A) Akt phosphorylation and (B) GLUT4 translocation in right gastrocnemius muscle (n = 8). Values are expressed as means ± SEM.
AMPK activity
No differences in total AMPK α-isoform activity were observed after 8 weeks supplementation with 0.5% ALA to rats on both a LFD or HFD(Fig. 6A). Consistent with this finding, no effect of ALA on phosphorylation of ACC on either LFD or HFD was observed (Fig. 6B).
Fig. 6.
Effect of 8 week treatment with dietary ALA on (A) AMPK activity and (B) S79/S221 ACC phosphorylation in right gastrocnemius muscle (n = 8). Values are expressed as means ± SEM.
DISCUSSION
Recently, it has been suggested that ALA may improve insulin sensitivity by lowering triglyceride accumulation in nonadipose tissues (12). The reduced triglyceride accumulation in skeletal muscle was suggested to be attributed to activation of skeletal muscle AMPK (13). Therefore, it is possible that ALA supplementation can prevent the lipid accumulation that is associated with high-fat diets via activation of AMPK and thereby, improve insulin sensitivity. Here, we indeed show that ALA supplementation reduces high-fat diet-induced accumulation of body fat and intramyocellular lipid content and improves whole body glucose tolerance, but these effects are not associated with increased muscular AMPK activation.
ALA supplementation prevented high-fat diet-associated obesity, as evidenced by significantly lower gain in total body weight as well as lower fat mass. Measuring fat pad weights at euthanasia indicated that animals receiving the HFD had significantly more fat mass than animals on the LFD, and this diet-induced increase in fat mass was completely prevented by supplementation of ALA. Interestingly, the net loss of fat is independent of the amount of fat in the diet. These effects were most likely due to pronounced effects of ALA on food intake and body weight gain of the animals. Already after one week of supplementation with ALA, animals had significant lower food intake and body weight gain compared with control groups. This pattern persisted over the entire 8 week intervention period. The reduction in food intake was not compensated by changes in food absorption as fecal energy loss was unaffected by ALA supplementation. These results are in agreement with those of Kim et al. (24), who found that feeding male Sprague-Dawley rats standard rat chow containing ALA for 2 weeks significantly reduced food intake and body weight in a dose-dependent manner. They suggested that the anorexic effect of ALA is the direct result of suppression of hypothalamic AMPK activity because intracerebrovascular injection of small doses of ALA reduced food intake. Indeed, several reports showed that inhibition of hypothalamic AMPK activity has an anorexic effect (25, 26). Furthermore, the ALA-induced anorexic effects were not due to illness, as no adverse pathology was seen during the study or at time of euthanasia of the animals. It also seems unlikely that the decreased food intake is the result of taste aversion, because intraperitoneal injection of ALA in Sprague-Dawley rats caused a similar decrease in food consumption, and a conditioned taste aversion test showed that there was no difference in preference ratio of saccharine intake between ALA and saline injected animals (24).
Increasing evidence in humans and animal models indicates that accumulation of muscle triglyceride is associated with decreased insulin induction of glucose disposal into skeletal muscle due to interference of fatty acid metabolites with insulin signaling at multiple levels. Here, we found that ALA supplementation completely prevented the high-fat diet-induced increase in muscular lipid content. Animals receiving the HFD experienced 3-fold higher IMCL levels distributed both in the type 1 and type 2 fibers. Supplementing ALA in the HFD group reduced lipid accumulation significantly, resulting in an intramyocellular lipid content that is comparable to animals receiving the LFD. In accordance with the reduction of muscular lipid content, whole body glucose tolerance was improved after 4 and 8 weeks of ALA treatment. At both time points, rats receiving the LFD and HFD supplemented with ALA had lower AUC compared with their control groups. Together with the improved glucose tolerance, plasma insulin levels were significantly lower in the groups receiving ALA, albeit that these results did not reach statistical significance after 8 weeks of treatment. Together, these findings seem to indicate that ALA supplementation is able to improve whole body insulin sensitivity even when high-fat diets are consumed. The mechanisms by which ALA decreases ectopic lipid accumulation and improves whole body glucose tolerance may be multifactorial. First of all, it has been speculated that ALA can activate skeletal muscle AMPK. AMPK is known to induce GLUT4 translocation in skeletal muscle, thereby improving muscular glucose uptake independent of insulin signaling. In addition, AMPK increases fatty acid oxidation by inhibiting ACC. Thus, ALA-induced AMPK activation in muscle would decrease IMCL levels by stimulating fatty acid oxidation as well as directly stimulating glucose uptake via increased GLUT4 translocation, thereby bypassing insulin resistance induced by muscular lipid accumulation. Indeed, 3 days of ALA supplementation to diabetic OLETF rats activated AMPK in skeletal muscle and improved insulin sensitivity, whereas adenoviral infection of dominant-negative AMPK prevented ALA-induced increase in insulin-stimulated glucose uptake and failed to increase fatty acid oxidation and ACC phosphorylation (13). Also, numerous studies have shown that ALA can improve glucose tolerance in both diabetic animal models as well as individuals with type 2 diabetes (8, 10, 11, 27). However, here we could not confirm these results. Despite the fact that ALA affected insulin sensitivity at the whole body level, no change in muscle specific insulin-stimulated Akt phosphorylation and GLUT4 translocation was noticed. Thus, skeletal muscle insulin sensitivity was not affected by ALA treatment. Moreover, we could not detect a positive effect of ALA treatment on skeletal muscle AMPK activity nor ACC phosphorylation. It is possible that the effects of ALA treatment on whole body glucose tolerance are due to the anorexic effects of ALA, thereby reducing not only the triglyceride accumulation in the different fat depots but also targeting ectopic lipid accumulation. It is well known that moderate reduction in caloric intake, without causing malnutrition, is effective in obese diabetic rats to improve insulin stimulated glucose uptake and lower insulin levels during a GTT (28, 29).
At first sight, these results are in contrast with previous findings showing that ALA improves insulin sensitivity possibly by increasing AMPK activity (13, 30). Although the study of Lee et al. (13) applied the same dosage of ALA (0.5%) and route of administration, the duration of ALA supplementation was shorter. So, the different outcome of our study may be explained by the acute versus chronic treatment with ALA. AMPK activity might only be influenced by short-term ALA administration. On the other hand, Gupte et al. (30) reported that chronic ALA supplementation increased AMPK activity and improved insulin signaling. However, a major difference in study protocol is the route of administration of ALA. ALA was dissolved in Tris HCL (120 mM, pH 7.4) and administered through daily injections intraperitoneally at a dosage of 30 mg/kg body weight. By investigating the metabolism of ALA through administration of radioactively labeled ALA to rats, Harrison and McCormick (31) reported that the retention of the radioactive label in the body of rats was approximately twice as high after oral administration than after intraperitoneal administration. This was as expected given the slower rate of excretion of radioactivity in urine and 14CO2 after oral administration (31). Bioavailability and excretion of ALA seems to be influenced by the route of administration, making comparison between studies difficult. The anesthesia used and the method of euthanasia of animals is often different in study protocols investigating the effect of ALA on insulin sensitivity and AMPK activity. AMPK activity can be increased if animals suffer from hypoxia (32). Both CO2 and barbiturates have the same working principle and with an overdose can depress the respiratory center, which is followed by cardiac arrest (33). So, studies that euthanize the animals by intraperitoneal injection of sodium pentobarbital may influence AMPK activity. We only sedated the rats with CO2 for one minute, after which they were euthanized by cervical dislocation. So, it is unlikely that hypoxia occurred and affected the AMPK activity measurements.
In conclusion, we have confirmed that ALA supplementation can prevent the lipid accumulation that is associated with high-fat diets. Moreover, we have shown that the reduction in ectopic lipid accumulation is associated with an improved whole body glucose tolerance. However, these effects of ALA are not mediated via ALA-induced activation of muscle AMPK or ALA-induced improvement in muscular insulin sensitivity. Possibly, ALA could have affected AMPK activity in the food-regulating center in the hypothalamus. Therefore, we propose that the prevention of HFD-induced muscular lipid accumulation and the improved whole body glucose tolerance may partly result from the anorexic nature of ALA.
Supplementary Material
Footnotes
Abbreviations:
- ACC
- acetyl-CoA carboxylase
- ALA
- α lipoic acid
- AMPK
- AMP-activated protein kinase
- AUC
- area under the curve
- % E
- % energy
- HFD
- high-fat diet
- IMCL
- intramyocellular lipid
- IpGTT
- intraperitoneal glucose tolerance test
- LFD
- low-fat diet
- OLETF
- Otsuka Long Evans Tokushima Fatty
The study was funded by Top Institute Food and Nutrition. TI Food and Nutrition, formerly known as WCFS, is a unique public/private partnership that generates vision on scientific breakthroughs in food and nutrition, resulting in the development of innovative products and technologies that respond to consumer demands for safe, tasty, and healthy foods. Partners are major Dutch food companies and research organizations. D.G.H. was supported by a Programme Grant (080982) from the Wellcome Trust.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of a figure.
REFERENCES
- 1.Mokdad A. H., Ford E. S., Bowman B. A., Dietz W. H., Vinicor F., Bales V. S., Marks J. S. 2003. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 289: 76–79. [DOI] [PubMed] [Google Scholar]
- 2.Hill M. J., Metcalfe D., McTernan P. G. 2009. Obesity and diabetes: lipids, ‘nowhere to run to’. Clin. Sci. (Lond.). 116: 113–123. [DOI] [PubMed] [Google Scholar]
- 3.Dresner A., Laurent D., Marcucci M., Griffin M. E., Dufour S., Cline G. W., Slezak L. A., Andersen D. K., Hundal R. S., Rothman D. L., et al. 1999. Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J. Clin. Invest. 103: 253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carreau J. P. 1979. Biosynthesis of lipoic acid via unsaturated fatty acids. Methods Enzymol. 62: 152–158. [DOI] [PubMed] [Google Scholar]
- 5.Reed L. J. 2001. A trail of research from lipoic acid to alpha-keto acid dehydrogenase complexes. J. Biol. Chem. 276: 38329–38336. [DOI] [PubMed] [Google Scholar]
- 6.Bustamante J., Lodge J. K., Marcocci L., Tritschler H. J., Packer L., Rihn B. H. 1998. Alpha-lipoic acid in liver metabolism and disease. Free Radic. Biol. Med. 24: 1023–1039. [DOI] [PubMed] [Google Scholar]
- 7.Packer L., Witt E. H., Tritschler H. J. 1995. alpha-Lipoic acid as a biological antioxidant. Free Radic. Biol. Med. 19: 227–250. [DOI] [PubMed] [Google Scholar]
- 8.Eason R. C., Archer H. E., Akhtar S., Bailey C. J. 2002. Lipoic acid increases glucose uptake by skeletal muscles of obese-diabetic ob/ob mice. Diabetes Obes. Metab. 4: 29–35. [DOI] [PubMed] [Google Scholar]
- 9.Jacob S., Streeper R. S., Fogt D. L., Hokama J. Y., Tritschler H. J., Dietze G. J., Henriksen E. J. 1996. The antioxidant alpha-lipoic acid enhances insulin-stimulated glucose metabolism in insulin-resistant rat skeletal muscle. Diabetes. 45: 1024–1029. [DOI] [PubMed] [Google Scholar]
- 10.Jacob S., Henriksen E. J., Tritschler H. J., Augustin H. J., Dietze G. J. 1996. Improvement of insulin-stimulated glucose-disposal in type 2 diabetes after repeated parenteral administration of thioctic acid. Exp. Clin. Endocrinol. Diabetes. 104: 284–288. [DOI] [PubMed] [Google Scholar]
- 11.Jacob S., Ruus P., Hermann R., Tritschler H. J., Maerker E., Renn W., Augustin H. J., Dietze G. J., Rett K. 1999. Oral administration of RAC-alpha-lipoic acid modulates insulin sensitivity in patients with type-2 diabetes mellitus: a placebo-controlled pilot trial. Free Radic. Biol. Med. 27: 309–314. [DOI] [PubMed] [Google Scholar]
- 12.Song K. H., Lee W. J., Koh J. M., Kim H. S., Youn J. Y., Park H. S., Koh E. H., Kim M. S., Youn J. H., Lee K. U., et al. 2005. alpha-Lipoic acid prevents diabetes mellitus in diabetes-prone obese rats. Biochem. Biophys. Res. Commun. 326: 197–202. [DOI] [PubMed] [Google Scholar]
- 13.Lee W. J., Song K. H., Koh E. H., Won J. C., Kim H. S., Park H. S., Kim M. S., Kim S. W., Lee K. U., Park J. Y. 2005. Alpha-lipoic acid increases insulin sensitivity by activating AMPK in skeletal muscle. Biochem. Biophys. Res. Commun. 332: 885–891. [DOI] [PubMed] [Google Scholar]
- 14.Hardie D. G., Carling D. 1997. The AMP-activated protein kinase—fuel gauge of the mammalian cell? Eur. J. Biochem. 246: 259–273. [DOI] [PubMed] [Google Scholar]
- 15.Bergeron R., Ren J. M., Cadman K. S., Moore I. K., Perret P., Pypaert M., Young L. H., Semenkovich C. F., Shulman G. I. 2001. Chronic activation of AMP kinase results in NRF-1 activation and mitochondrial biogenesis. Am. J. Physiol. Endocrinol. Metab. 281: E1340–E1346. [DOI] [PubMed] [Google Scholar]
- 16.Hayashi T., Hirshman M. F., Fujii N., Habinowski S. A., Witters L. A., Goodyear L. J. 2000. Metabolic stress and altered glucose transport: activation of AMP-activated protein kinase as a unifying coupling mechanism. Diabetes. 49: 527–531. [DOI] [PubMed] [Google Scholar]
- 17.Ruderman N. B., Saha A. K., Vavvas D., Witters L. A. 1999. Malonyl-CoA, fuel sensing, and insulin resistance. Am. J. Physiol. 276: E1–E18. [DOI] [PubMed] [Google Scholar]
- 18.Winder W. W. 2001. Energy-sensing and signaling by AMP-activated protein kinase in skeletal muscle. J. Appl. Physiol. 91: 1017–1028. [DOI] [PubMed] [Google Scholar]
- 19.Zong H., Ren J. M., Young L. H., Pypaert M., Mu J., Birnbaum M. J., Shulman G. I. 2002. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc. Natl. Acad. Sci. USA. 99: 15983–15987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cho M., Webster S. G., Blau H. M. 1993. Evidence for myoblast-extrinsic regulation of slow myosin heavy chain expression during muscle fiber formation in embryonic development. J. Cell Biol. 121: 795–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brand M. D., Buckingham J. A., Esteves T. C., Green K., Lambert A. J., Miwa S., Murphy M. P., Pakay J. L., Talbot D. A., Echtay K. S. 2004. Mitochondrial superoxide and aging: uncoupling-protein activity and superoxide production. Biochem. Soc. Symp. (71): 203–213. [DOI] [PubMed] [Google Scholar]
- 22.Schrauwen P., Hoeks J., Schaart G., Kornips E., Binas B., Van De Vusse G. J., Van Bilsen M., Luiken J. J., Coort S. L., Glatz J. F., et al. 2003. Uncoupling protein 3 as a mitochondrial fatty acid anion exporter. FASEB J. 17: 2272–2274. [DOI] [PubMed] [Google Scholar]
- 23.Davies S. P., Carling D., Hardie D. G. 1989. Tissue distribution of the AMP-activated protein kinase, and lack of activation by cyclic-AMP-dependent protein kinase, studied using a specific and sensitive peptide assay. Eur. J. Biochem. 186: 123–128. [DOI] [PubMed] [Google Scholar]
- 24.Kim M. S., Park J. Y., Namkoong C., Jang P. G., Ryu J. W., Song H. S., Yun J. Y., Namgoong I. S., Ha J., Park I. S., et al. 2004. Anti-obesity effects of alpha-lipoic acid mediated by suppression of hypothalamic AMP-activated protein kinase. Nat. Med. 10: 727–733. [DOI] [PubMed] [Google Scholar]
- 25.Andersson U., Filipsson K., Abbott C. R., Woods A., Smith K., Bloom S. R., Carling D., Small C. J. 2004. AMP-activated protein kinase plays a role in the control of food intake. J. Biol. Chem. 279: 12005–12008. [DOI] [PubMed] [Google Scholar]
- 26.Minokoshi Y., Alquier T., Furukawa N., Kim Y. B., Lee A., Xue B., Mu J., Foufelle F., Ferré P., Birnbaum M. J., et al. 2004. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 428: 569–574. [DOI] [PubMed] [Google Scholar]
- 27.Estrada D. E., Ewart H. S., Tsakiridis T., Volchuk A., Ramlal T., Tritschler H., Klip A. 1996. Stimulation of glucose uptake by the natural coenzyme alpha-lipoic acid/thioctic acid: participation of elements of the insulin signaling pathway. Diabetes. 45: 1798–1804. [DOI] [PubMed] [Google Scholar]
- 28.Park S. Y., Choi G. H., Choi H. I., Ryu J., Jung C. Y., Lee W. 2005. Calorie restriction improves whole-body glucose disposal and insulin resistance in association with the increased adipocyte-specific GLUT4 expression in Otsuka Long-Evans Tokushima fatty rats. Arch. Biochem. Biophys. 436: 276–284. [DOI] [PubMed] [Google Scholar]
- 29.Okauchi N., Mizuno A., Yoshimoto S., Zhu M., Sano T., Shima K. 1995. Is caloric restriction effective in preventing diabetes mellitus in the Otsuka Long Evans Tokushima fatty rat, a model of spontaneous non-insulin-dependent diabetes mellitus? Diabetes Res. Clin. Pract. 27: 97–106. [DOI] [PubMed] [Google Scholar]
- 30.Gupte A. A., Bomhoff G. L., Morris J. K., Gorres B. K., Geiger P. C. 2009. Lipoic acid increases heat shock protein expression and inhibits stress kinase activation to improve insulin signaling in skeletal muscle from high-fat-fed rats. J. Appl. Physiol. 106: 1425–1434. [DOI] [PubMed] [Google Scholar]
- 31.Harrison E. H., McCormick D. B. 1974. The metabolism of dl-(1,6-14C)lipoic acid in the rat. Arch. Biochem. Biophys. 160: 514–522. [DOI] [PubMed] [Google Scholar]
- 32.Frederich M., Zhang L., Balschi J. A. 2005. Hypoxia and AMP independently regulate AMP-activated protein kinase activity in heart. Am. J. Physiol. Heart Circ. Physiol. 288: H2412–H2421. [DOI] [PubMed] [Google Scholar]
- 33.van Zupthen L. F. M., Baumans V., Beynen A. C. Anesthesie, analgesie en euthanasie. Handboek Proefdierkunde. Gezondheidszorg E., editor 2003: Maarssen, the Netherlands: p. 286–289. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.