Abstract
Vitamin A (VA) is essential for fetal lung development and postnatal lung maturation. VA is stored mainly as retinyl esters (REs), which may be mobilized for production of retinoic acid (RA). This study was designed 1) to evaluate several acidic retinoids for their potential to increase RE in the lungs of VA-supplemented neonatal rats, and 2) to determine the expression of retinoid homeostatic genes related to retinol uptake, esterification, and catabolism as possible mechanisms. When neonatal rats were treated with VA combined with any one of several acidic retinoids (RA, 9-cis-RA, or Am580, a stable analog of RA), lung RE increased ∼5–7 times more than after an equal amount of VA alone. Retinol uptake and esterification during the period of absorption correlated with increased expression of both STRA6 (retinol-binding protein receptor) and LRAT (retinol esterification), while a reduction in RE after 12 h in Am580-treated, VA-supplemented rats correlated with a strong and persistent increase in CYP26B1 (RA hydroxylase). We conclude that neonatal lung RE can be increased synergistically by VA combined with both natural and synthetic acidic retinoids, concomitant with induction of the dyad of STRA6 and LRAT. However, the pronounced and prolonged induction of CYP26B1 by Am580 may counteract lung RE accumulation after the absorption process is completed.
Keywords: Am580, CYP26, retinol esterification, lecithin:retinol acyltransferase, plasma retinol, retinoic acid oxidation, postnatal lung development, vitamin A supplementation
Vitamin A (VA) is an essential micronutrient that is required for fetal lung development and alveolar septation in the postnatal period (1–3). The active metabolite of VA, retinoic acid (RA), fulfills nearly all of the functions of VA, but unlike VA, which is stored in tissues as retinyl ester (RE) and mobilized to produce RA (4), no significant amount of RA is stored and the pool of RA turns over rapidly (5). RA and other retinoids have been shown to enhance alveolar septation in neonatal rats and mice (as reviewed in Refs. 2, 3, 6, 7) and in some cases to improve lung repair after injury in adults (3, 8, 9). Most of the VA present in the lungs is in the form of RE (10–12). In full-term infants, a process of significant RE accumulation in the lungs has begun from the third trimester of fetal life, after which the stored RE becomes quickly depleted during late gestation and early postnatal life (10). By contrast, preterm infants often have low VA status at birth (13–15), which may contribute to poor lung maturation and increased susceptibility to respiratory diseases (16). Therefore, ways to improve RE storage in the lungs in the postnatal period could be useful clinically for supporting retinoid-requiring metabolic functions and aiding postnatal lung development.
Previously, we tested a combination of VA (retinol) and RA, referred to as VARA (10:1 molar mixture of VA and RA) as an oral supplement for promoting lung RE formation (12). Lung RE increased synergistically, at least 4-fold more than for an equal amount of VA alone (17). As RA is not reduced to retinol in vivo and thus is not a substrate for tissue RE synthesis, the increase in RE in the lungs implies that RA plays a regulatory role in this organ, in some manner facilitating RE formation. The synergistic effect of VARA was selective for the lungs, as RE formation in the liver did not differ between VARA and an equal dose of VA only (12, 17). In a metabolic study using [3H]retinol to trace the uptake of newly absorbed retinol, we found that RA served to direct more of the [3H]retinol tracer and, thus, the oral VA supplement, into the neonatal lung (12). The inclusion of RA in the diet of adult rats also increased retinol uptake kinetics into the lung (18). However, the mechanisms involved in the VARA synergy are still unknown.
Of the many genes that are regulated in vivo by RA, several play prominent roles in retinoid homeostasis (19). Lecithin:retinol acyltransferase (LRAT) and RA hydroxylases of the CYP26 family of cytochrome P450 genes, which catalyze the esterification of retinol and the oxidation of RA, respectively, are known to be regulated by RA in certain tissues (20). The expression of these enzymes varies with VA status and appears to provide a level of control over VA metabolism (20). During VA sufficiency, the continued presence of RA, a signal of high VA status, maintains the expression of LRAT (20). CYP26A1 and CYP26B1 also increase in some tissues when the concentration of RA rises (21). Conversely, in the situation of VA deficiency, when the concentration of RA is very low, LRAT is downregulated (22), and CYP26 is maintained at a very low level (23, 24). Therefore, to a certain extent, the self-regulatory mechanisms involving RA render the body able to modulate its retinol metabolism and thus to avoid both VA deficiency and toxicity. Another protein that may contribute to retinoid homeostasis is STRA6 (stimulated by retinoic acid gene 6), a transmembrane receptor for retinol-binding protein (RBP), and mediator of retinol uptake from plasma and extracellular fluid into cells (25). STRA6 is expressed at high levels in the retina and at lower levels in a variety of embryonic and adult cells or organs (26), including adult lung tissue (27). But whether the STRA6 gene is expressed in the neonatal lung and involved in VA uptake in the postnatal period is still unclear.
In this study, we first determined whether acidic retinoids besides all-trans-RA can produce the VARA synergy. We then tested the hypothesis that RA and Am580, a retinobenzoic acid analog of RA, which is known both for its resistance to metabolism and its selective activation of the nuclear receptor RAR-α (28, 29), can acutely alter neonatal lung retinoid metabolism. We thus determined changes in lung RE formation, [3H]retinol uptake, and the expression of some important retinoid homeostatic genes, including LRAT, CYP26A1, CYP26B1, and STRA6, in neonatal rats treated with VA, RA, VARA, Am580, or VA+Am580 (VAAm).
MATERIALS AND METHODS
Dose preparation
VA, in the form of all-trans-retinyl palmitate, all-trans-RA (at-RA), and 9-cis-RA, was purchased from Sigma-Aldrich (St. Louis, MO). Am580 was a gift of H. Kagechika, University of Tokyo. VARA and related treatments were prepared as described previously (12). The dose concentration of VA alone and after combination with an acidic retinoid was 0.05 M, and the dose concentration of the acidic retinoids (all-trans-RA, 9-cis-RA, or Am580) was 0.005 M. In practice, solutions were prepared by weight rather than volume for accuracy and were confirmed by UV spectrophotometry. Canola oil only was used as placebo (control).
Animals and experimental designs
Animal procedures were approved by the Institutional Animal Use and Care Committee, Pennsylvania State University. In each of three experiments, Sprague-Dawley rat pups with their dams were assigned to treatments across litters, with pups of both sexes included in each treatment group. The average body weight of each group was similar (data not shown). Before each treatment, the pups were weighed and the dose adjusted to 0.4 µl/g body weight (20 nmol retinol and 2 nmol of acidic retinoid, depending on treatment group, per gram of body weight). The dose was delivered directly into the pup's mouth by a micropipette. In experiment 1 (3 day study, n = 4/group) 5 day old neonates were treated once a day for 3 days with oil (control), VA alone, RA, 9-cis-RA, both isomers mixed in a 1:1 ratio, or VA combined with each the same acidic retinoids. Twenty-four hours after the last treatment, neonates were euthanized and tissues collected (12). In experiments 2 and 3 (acute studies lasting 6 and 12 h), 6–7 day old pups were treated with oil (control), VA alone, RA alone, Am580 alone, VA combined with RA (VARA), or with Am580 (VAAm). In the 12 h study, 2 µCi of [3H]retinol, prepared as previously described (12), was administered orally just after each treatment dose to provide a tracer for the tissue uptake and metabolism of newly absorbed retinol.
Tissue collection
At designated times, pups were killed with carbon dioxide. Heparinized blood was collected from the vena cava or heart for preparation of plasma, and the lungs and liver were removed, trimmed, weighed, and frozen immediately in liquid nitrogen.
Retinoid analysis
Tissue lipids were extracted by the procedure of Folch, Lees, and Sloane Stanley (30). A portion of the extracts underwent an alkaline hydrolysis to convert RE to retinol and, after addition of a known amount of an internal standard, trimethylmethoxyphenyl-retinol, samples in 100 µl of methanol were subjected to analysis by reverse-phase HPLC (31), monitored by a Waters 960 photodiode array detector (Milford, MA). The areas of the peaks for trimethylmethoxyphenyl-retinol and retinol were analyzed by Millenium-32 (Waters) software, and tissue total retinol concentrations were calculated. For some samples, the concentration of tissue RE was determined without saponification (31). Because RE constituted >90% of total retinol for each treatment group, we have referred to total retinol as RE in the figures and text, unless otherwise indicated.
Analysis of [3H]retinol metabolism
Extracts of lung and liver total lipids, prepared as described above, were evaporated and subjected to liquid scintillation spectrometry to determine the total 3H (percent of dose) in the lungs and liver 12 h after dosing. Another portion of the organic solvent extract was dried, redissolved in hexanes, subjected to alumina column chromatography, and counted for [3H]RE to determine uptake as the percentage of the oral dose (12). The upper aqueous phases from the Folch wash were pooled, and a small portion was counted to assess the formation of aqueous [3H]labeled polar metabolites of retinol (32). The percentage of the oral dose in each tissue fraction was then calculated. The aqueous phase contributed <0.05 and 0.15% of the total 3H in lung and liver, respectively, and thus only total 3H and the percentage of 3H as [3H]RE are reported.
Gene mRNA level determination
Total RNA from the lungs of individual pups was extracted using a guanidine extraction method, and cDNA was prepared using reverse transcriptase (33). The equivalent of 0.05 µg RNA, as cDNA, was used for real-time PCR analysis. Primers designed to detect mRNA expression were as follows: rat LRAT (GenBank accession number AF255060), 5′-ATAGGATCCTGACCAACACTACATCCTCTC-3′ (forward) and 5′-ATTCTCGAGTCTAAGTTTATTGAAACCCCAGA-3′ (reverse); rat CYP26B1 (NM_181087), 5′-TTGAGGGCTTGGAGTTGGT-3′ (forward) and 5′-AACGTTGCCATACTTCTCGC-3′ (reverse); rat CYP26A1 (DQ266888), 5′-GTGCCAGTGATTGCTGAAGA-3′ (forward) and 5′-GGAGGTGTCCTCTGGATGAA-3′ (reverse); rat STRA6 (NM_0010029924.1), 5′-CCGATCCTGGACAGTTCCTA-3′ (forward) and 5′-CCACCTGGTAAGTGGCTGTT -3′ (reverse). The mRNA expression level of each sample was corrected by calculating mRNA-to-ribosomal 18S RNA ratio. Data were normalized to the average value for the control group, set at 1.00, prior to statistical analysis.
Statistical analysis
Data are presented as the group mean ± SE. Depending on the experiment, differences were tested by one-factor ANOVA or two-factor ANOVA followed by Fisher's protected least significant difference test and least squares means test. When variances were unequal, data were transformed to log10 values before statistic analysis. Differences with P < 0.05 were considered significant.
RESULTS
All-trans-RA and 9-cis-RA and both combined each promote RE formation
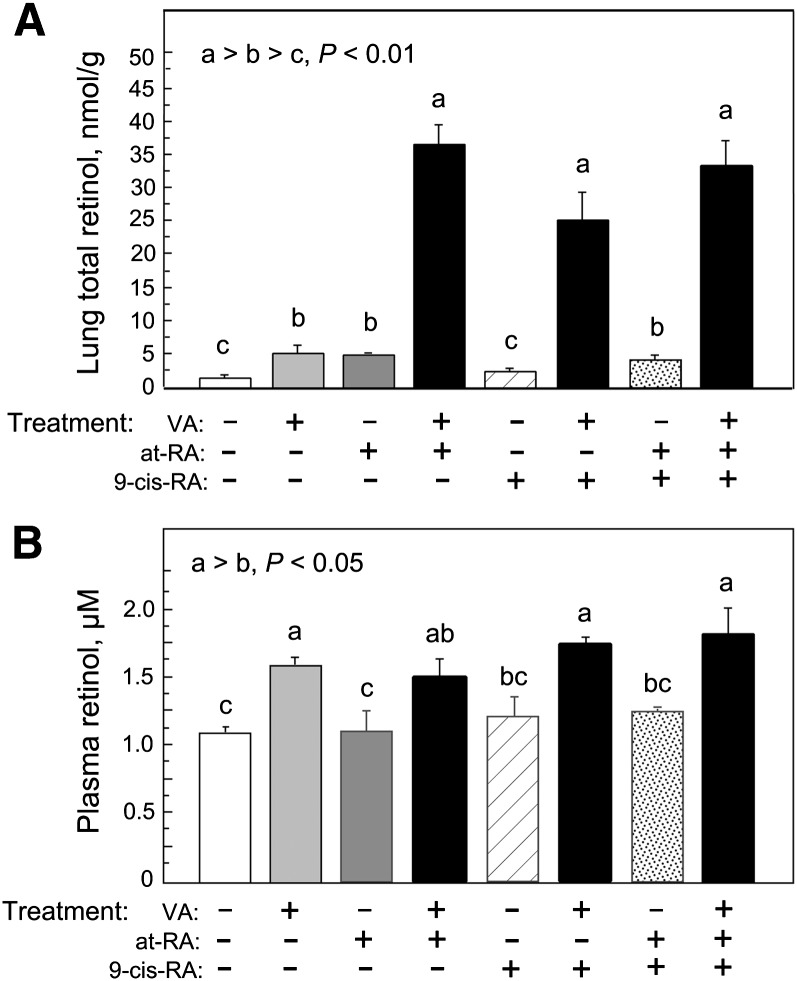
All-trans-RA is considered the major form of RA with endogenous activity as a ligand for nuclear receptors of the RAR family (RAR-α, RAR-β, and RAR-γ) (34). Although the status of 9-cis-RA as an endogenously produced retinoid is uncertain, it is known as an effective activator for receptors of the RXR family, when added exogenously. In some situations, both ligands in combination promote gene transactivation. Therefore, we first determined the response of the neonatal rat lung to treatments with all-trans-RA, 9-cis-RA, and a 1:1 combination of both isomers, administered in the absence of VA or combined with a supplement of VA (molar ratio of 10 VA:1 acidic retinoid). Treatments were given daily on postnatal days 5, 6, and 7, and lung and plasma retinol were determined on day 8. Fig. 1A shows that RA produced a small increase in lung RE when given alone, as we have shown previously (12), indicating that RA influences the metabolism of endogenous retinol in the absence of VA supplementation. When RA was combined with VA as VARA, lung RE increased ∼7 times higher compared with treatment with VA alone. In contrast to all-trans-RA, 9-cis-RA in the absence of VA had no effect on lung RE, suggesting this isomer does not significantly regulate the metabolism of endogenous retinol in the lungs. However, similar to all-trans-RA, 9-cis-RA produced a significant although slightly lower synergy when given with VA. The combination of at-RA and 9-cis-RA (mixed 1:1, with total RA equal to that given as either isomer alone) had no additional effect.
Fig. 1.
RE concentration in lung (A) and plasma (B) after three daily treatments with VA alone or combined with RA, 9-cis-RA, or an equimolar mixture (1:1) of at-RA and 9-cis-RA. Rat pups (n = 4/group) received oral doses on postnatal days 5, 6, and 7. A: Lung total retinol (>90% RE) was determined (see Materials and Methods) on day 8. Groups with different letters differed significantly, P < 0.05. B: Plasma total retinol. Only groups that received VA differed (mean increase for all groups that received VA = 0.49 µmol/l compared with groups that did not receive VA, P < 0.001).
The concentration of plasma retinol (Fig. 1B) was within the normal range for neonates in all treatment groups. Plasma retinol levels were ∼30% higher for each treatment that included VA, regardless of whether an acidic retinoid was coadministered (all P < 0.05), whereas none of the acidic retinoids alone had a significant effect. Therefore, plasma retinol responded only to the VA component of the dose.
From this initial study, we confirmed that at-RA is able to provide a significant boost to VA (e.g., as VARA) by increasing lung RE content ∼5–7 times. To further test the interaction of VA and acidic retinoids on lung RE formation and to examine regulatory processes, we next compared all-trans-RA, as the predominant natural regulatory isomer, and a retinobenzoic acid analog of RA, Am580, which has been shown to relatively resistant to metabolism and to bind selectively to the RAR-α form of nuclear retinoid receptors (29). All-trans-RA and Am580, with and without VA, were studied for their ability to promote lung RE formation, enhance the uptake of newly absorbed retinol, and alter the expression of genes potentially important for retinol uptake, esterification, and homeostasis.
VA combined with RA or Am580 increases lung RE significantly at 6 and 12 h
We conducted two studies, one of 6 h and one of 12 h duration, each with groups of 6–7 day old neonates that were treated orally with oil (control), VA alone, RA alone, VARA, Am580 alone, and VAAm. The values for the control groups in the two experiments were very similar; thus, treatment responses for both experiments are presented together for comparison.
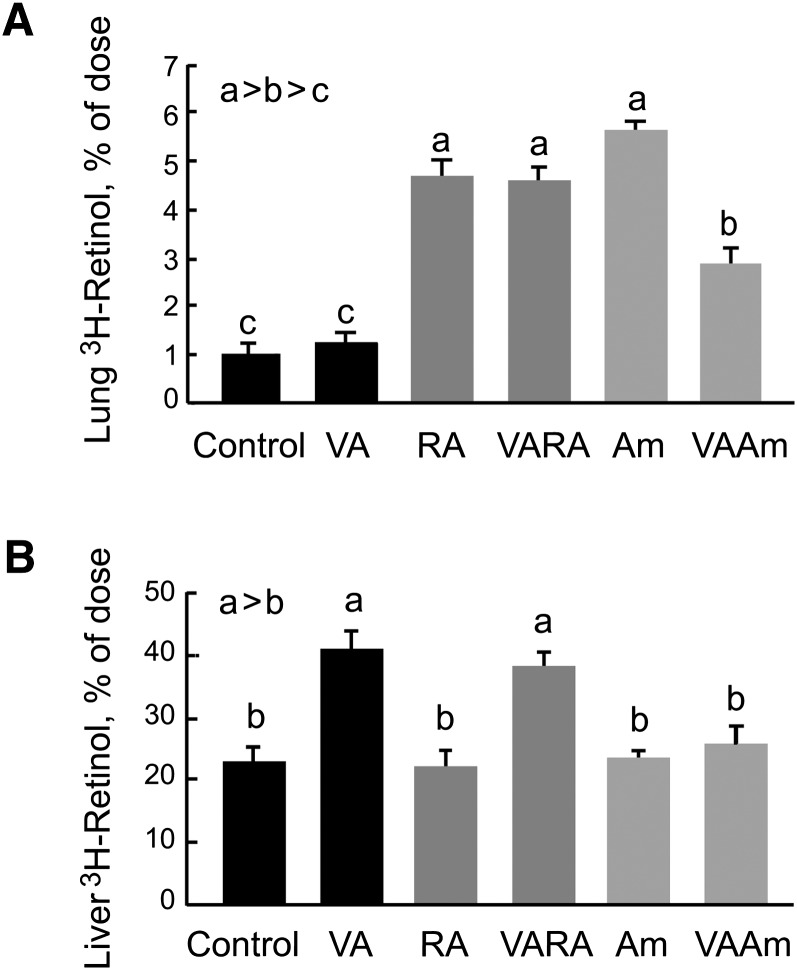
Lung RE increased after VA supplementation alone, which was evident as early as 6 h after dosing (Fig. 2A). Notably, the magnitude of increase was the same at both 6 and 12 h (Fig. 2B) for the VA, RA, and VARA groups, implying that the uptake of VA and its storage as RE in the lungs is very rapid after oral dosing, reaching a steady state by 6 h. Am580 alone produced a small increase in lung RE at both times (Fig. 2A, B). Thus, Am580, like RA, influences the metabolism of endogenous retinol. However, whereas the synergy with VAAm was significant at both 6 and 12 h, lung RE in the VAAm group was significantly lower at 12 h than at 6 h and lower for the VAAm group than for the VARA group at 12 h. These results indicated that the stable analog Am580 is as effective as RA for promoting RE formation in the very early absorptive period (6 h), while Am580 appeared to have the effect of quickening retinol metablism in the lung, as evidenced by the fall in lung RE in the postabsorptive period, measured at 12 h.
Fig. 2.
Lung RE concentration 6 h (A) and 12 h (B) after a single oral treatment with VA alone, all-trans-RA alone, VARA, Am580 alone, and combination of VA and Am580 (VAAm). Rat pups (n = 4–6/group) received a single oral dose on postnatal day 7. Lung total retinol (>90% RE) was determined. Groups with different letters differed significantly, P < 0.05.
RA and Am580 promote higher lung uptake of newly absorbed 3H-retinol
In the 12 h study, we included a tracer of [3H]retinol with each treatment to investigate how newly absorbed [3H]retinol is partitioned between the neonatal rat lung and liver, the major storage organ for VA, and whether treatments with VARA and VAAm may promote the uptake of newly absorbed retinol into the lung, which in turn could be a mechanism for the observed increase in lung RE content after VARA or VAAm treatment. The 12 h time point was chosen because we expected intestinal absorption of the [3H]retinol tracer and the VA dose it traced to have been completed by 12 h; thus, the tissue distribution of newly absorbed retinol for the entire dose could be assessed. The percentage of newly absorbed 3H in the lungs did not differ between the VA group and the control group (Fig. 3A), indicating that the supplement of VA alone did not increase the fractional uptake of retinol from the oral dose. However, the uptake of [3H]retinol was significantly higher in the lungs of neonates treated with at-RA, VARA, Am580, and VAAm (all P < 0.05 versus control). From these results, it can be inferred that VA itself did not affect the uptake of newly absorbed retinol, whereas both at-RA and Am580 promoted a higher uptake of newly absorbed retinol, whether or not the mass of VA being absorbed at the time was low, i.e., without VA, or high, after the VA supplement. We noted, however, that more 3H was present in the lungs of noenates treated with VARA group than with VAAm. This result might be due to a difference in the rate of retinol metabolism as affected by at-RA and Am580.
Fig. 3.
Uptake and metabolism of newly absorbed [3H]retinol and conversion to [3H]RE in lung and liver. Rat pups received a tracer dose of 3H-labeled retinol by mouth at the time of administration of VA alone, RA alone, VARA, Am580 alone, and the combination of VA and Am580 (VAAm). Tissues were collected 12 h later. Groups with different letters differed significantly, P < 0.05. A: Lung total [3H] as percent of dose. B: Lung [3H]RE as a percentage of lung total [3H], representing conversion of retinol to RE.
We also measured the uptake of the orally administered [3H]retinol into the liver, the major storage organ for VA, for comparison to lung. The percentage of [3H]retinol taken up by the liver was higher, as expected; however, in addition, we noted that the treatment effect in liver differed from that in lung. In the liver, [3H]RE was increased only by VA and VARA (Fig. 3B). We did not expect VARA to increase the mass of liver RE more than VA alone because the VARA synergy has not been observed for liver (12, 17). Similarly, VAAm did not increase liver RE content more than VA alone. However, liver from the VAAm-treated group did not show the same increase in [3H-retinol as in the VA and the VARA groups. We surmised that this might be due to a higher rate of retinol metabolism in the VAAm group. The difference between VARA and VAAm did not appear to be due to a difference in the fractional conversion of newly absorbed retinol to RE, as the percentage of 3H present as [3H]RE was similar in the VARA and VAAm groups (data not shown).
The expression of retinoid homeostatic genes is differentially regulated in neonatal lung by at-RA and Am580
The rapid effect of VARA and VAAm on retinol uptake and RE accumulation in the lungs suggested that if changes in gene expression are responsible, they must occur early after treatment. We thus determined the expression of several genes implicated in retinoid homeostasis in the lungs of neonates in the 6 and 12 h studies. Gene expression was determined by quantitative PCR for STRA6, LRAT, and two members of the CYP26 family, CYP26A1 and CYP26B1, as well as the cellular retinol-binding protein (CRBP1).
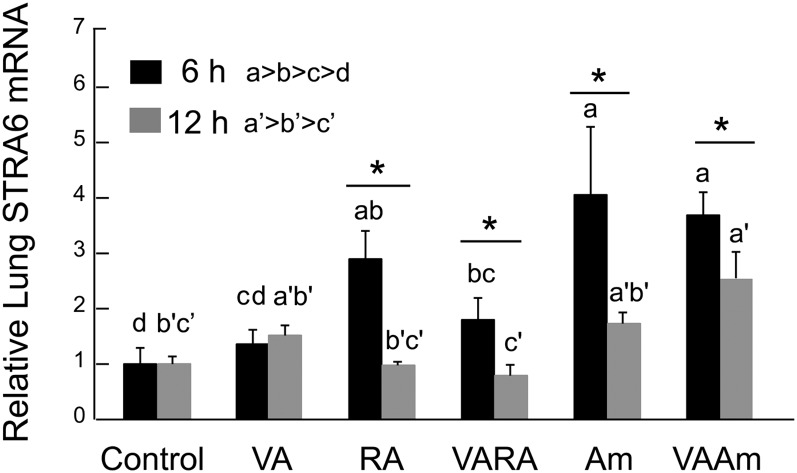
Results for STRA6, a transmembrane protein receptor for RBP that facilitates the uptake of retinol into cells (25), showed that both RA and Am580 induced expression to a level ∼3–4 times higher than the control group (Fig. 4). VA alone had no significant effect, while it slightly attenuated the induction of STRA6 by the acidic retinoids. The elevated level of STRA6 mRNA at 6 h suggests that an increase in retinol uptake from the extracellular RBP-retinol complex into the neonatal lungs via the STRA6 receptor could be a mechanism for the rapid response of the neonatal lungs to acidic retinoids. Interestingly, STRA6 expression at 12 h was lower than at 6 h for all but the control group and no longer differentially affected by the retinoid treatments. These results indicate that STRA6 expression is very dynamically regulated and suggest that STRA6 could play a more significant role in the uptake of retinol into the lungs especially during the postprandial period of maximal retinol absorption.
Fig. 4.
Expression level of STRA6 in neonatal lung 6 and 12 h after treatment with VA, RA, VARA, Am580, and VAAm580. Lung tissue from 7–8 day old rats treated with oil, VA, RA VARA, Am580 (Am), and VAAm580 was processed for total RNA isolation and subjected to quantitative PCR analysis using primers for rat STRA6 (see Materials and Methods). For each sample, gene expression levels were normalized to 18S rRNA measured in the same sample, and the average value for the control group was set to 1.00 for each experiment. Data shown are the means ± SE. Data were log10 transformed prior to ANOVA. Groups with different letters differed significantly (one-way ANOVA and Fisher's protected least significant difference test) P < 0.05. Asterisks denote difference between 6 h and 12 h (t-test), P < 0.05.
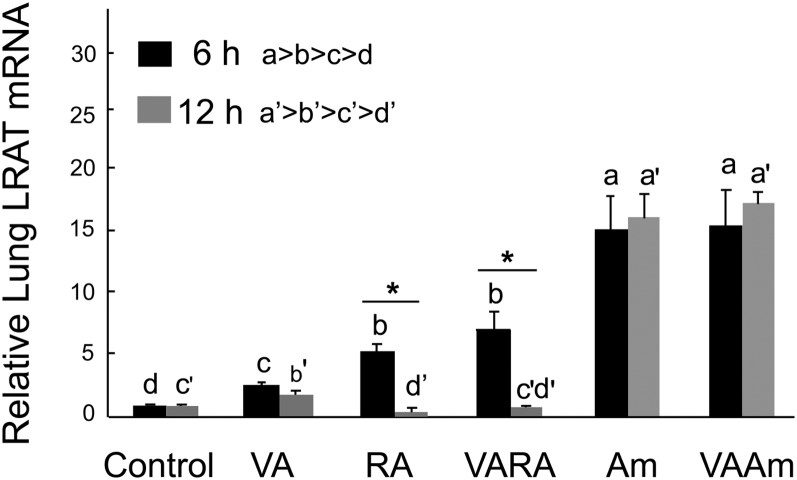
Because LRAT, CYP26B1, and CYP26A1 are important genes in VA homeostasis and they are known to be regulated by RA in tissue-specific patterns (20, 21), we next evaluated their expression in both the 6 and 12 h studies. LRAT expression was increased at 6 h by all treatments, in the order VA < at-RA = VARA << Am580 and VAAm580 (Fig. 5). By 12 h, LRAT mRNA was reduced toward basal levels in the VA group and had fallen entirely to or somewhat below basal levels in the lungs of RA and VARA-treated neonates. By comparison with RA, Am580 dramatically increased LRAT mRNA at 6 h and maintained this level at 12 h. The expression of CRBP1, a retinol chaperone that delivers retinol to LRAT, was readily detectable in neonatal lung, as reported by others (35), but its expression did not differ significantly with treatment (data not shown).
Fig. 5.
Expression levels of LRAT in neonatal lung 6 and 12 h after treatment with VA, RA, VARA, Am580, and VAAm580. Data were obtained as described in the legend to Fig. 4. Groups with different letters differed significantly, P < 0.05. Asterisks denote difference between 6 and 12 h (t-test), P < 0.05.
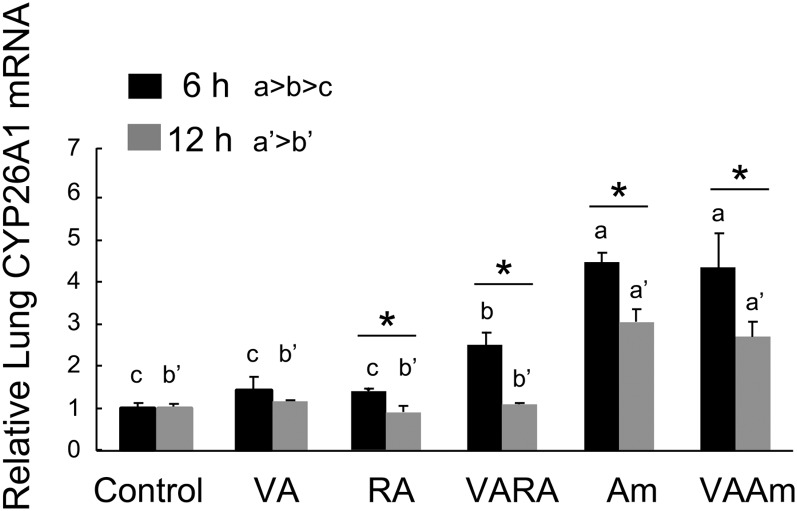
CYP26A1 expression did not increase in neonatal lung in response to VA or at-RA but was up-regulated ∼2-fold by VARA and 4–5-fold by Am580 at 6 h (Fig. 6). CYP26A1 was lower at 12 h compared with 6 h for all treatments. By 12 h, the VA, RA, and VARA groups did not differ from the control group. Although the expression of CYP26A1 was lower at 12 h than at 6 h in the Am580- and VAAm-treated groups, CYP26A1 was still significantly elevated in the lungs of neonates that received these treatments, compared with all other groups.
Fig. 6.
Expression levels of CYP26A1 in neonatal lung 6 and 12 h after treatment with VA, RA, VARA, Am580, and VAAm580. Data were obtained as described in the legend to Fig. 4. Groups with different letters differed significantly, P < 0.05. Asterisks denote difference between 6 and 12 h (t-test), P < 0.05.
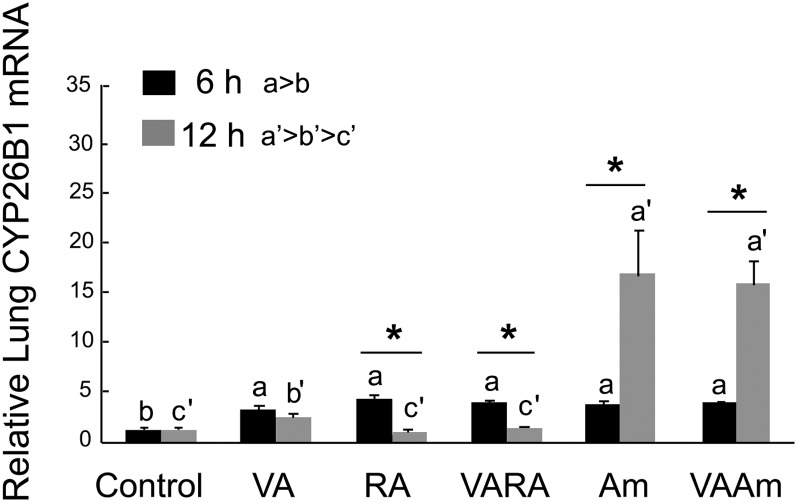
By contrast to CYP26A1, CYP26B1 exhibited much stronger induction (Fig. 7). The level of CYP26B1 mRNA was elevated 4–5-fold by all of the treatments, compared with control, at 6 h. Moreover, Am580 further and dramatically increased CYP26B1 mRNA at 12 h, whether given alone or as VAAm, reaching levels 16–17 times higher than the control group. Since CYP26B1 expression was higher and more responsive to retinoids compared with CYP26A1, CYP26B1 might play the major role in metabolizing RA in neonatal lungs.
Fig. 7.
Expression levels of CYP26B1 in neonatal lung 6 and 12 h after treatment with VA, RA, VARA, Am580, and VAAm580. Data were obtained as described in the legend to Fig. 4. Groups with different letters differed significantly, P < 0.05. Asterisks denote difference between 6 and 12 h (t-test), P < 0.05.
DISCUSSION
Human lung development goes through a long, progressive journey from as early as the fourth week of embryonic stage until birth, with continuing changes to 8 years of age (2, 16, 36–38). During the late fetal and postnatal period, VA has a major role in promoting lung development and maturation. In the rat, significant storage of VA in the lungs starts in late gestation just before the onset of alveolization and surfactant synthesis (39). These stores are rapidly depleted during late pregnancy and postnatal life as the lungs are still developing (10, 11, 40). Weanling rats fed VA-deficient diet showed the characteristics of keratinizing metaplasia in the trachea and the bronchopulmonary tree (36, 41). Supplementation with retinol or RA has been shown to repair epithelial lesions and increase surfactant phospholipid synthesis in fetal rat lung (39). In the population of preterm infants, premature delivery accompanied with VA deficiency usually has been associated with an increased susceptibility to lung injury, such as in neonatal respiratory distress syndrome and subsequent bronchopulmonary dysplasia, due to a deficit in pulmonary surfactant (38, 42). However, in clinical trials, neonatal supplementation with retinol reduced the risk of bronchopulmonary dysplasia (43–45). Since VA has shown its essential activity in neonatal lung development and has produced promising results for reducing lung injury and dysfunction (2, 3, 6–9), there could be a biological advantage in increasing or maintaining adequate VA storage in the neonatal lung. Supplementing VA to the offspring directly may be efficient in increasing lung VA. However, VA dosage is still a concern for the clinical use of VA due to the potential for toxicity of high amounts of VA (44, 46). Therefore, improving the efficiency and specificity of lung VA uptake by rapidly increasing lung retinol uptake and RE storage could be helpful in treating VA-deficient populations, such as very low birth weights infants, and in other clinical settings. Thus, it is important to have a clear understanding of the expression pattern of those genes affected by RA and other retinoids that may be used for therapy.
VA and its major bioactive metabolite RA exert their influence in development and cell differentiation through the binding of RA with nuclear retinoid receptors of the RAR family. These receptors typically form heterodimers with RXR proteins and activate, or repress, target genes possessing retinoic acid-responsive elements, thereby regulating the downstream genes (34). These direct or indirect signaling pathways set in motion by RA are known to modulate a large number of RA-responsive genes, some of which play important roles in lung development, cellular differentiation, and the metabolism of VA itself. Among these genes, LRAT and members of cytochrome P450 family CYP26 are considered among the most important genes for regulating VA metabolism (20, 21). By converting retinol to its RE storage form, or by oxidizing RA to inactive polar metabolites, LRAT and CYP26A1/CYP26B1 could control the balance of the metabolism of retinol and might thereby regulate the homeostasis of VA in lung tissue. Other factors, including retinoid-binding proteins, and receptors such as STRA6, could also be involved.
Our previous research had indicated that VA given orally in combination with at-RA (VARA) increased retinol uptake and lung RE formation much more effectively compared with VA given individually (12, 17). For reference, the VA dose we used in our studies of neonatal rats, described in more detail in (12), is representative of the 50,000 IU (15 mg) dose of retinol that has been administered to human newborns in studies that have shown reduced mortality in the first year of life (47, 48). The dose of RA we have used is similar to that first shown by Massaro and Massaro (49) to improve alveolar septation in postnatal rats. We have previously shown by conducting a dose dilution study that VARA has about 4–5 times the effect of the standard dose of VA alone (17). In this study, the synergy between VA and at-RA or VA and Am580 was as great or greater, with a 5–7-fold increase in lung RE content just 6 h after treatment with VARA or VAAm (Fig. 2). The administration of RA redirects part of the flow of supplemental VA in the neonate lungs, as supported by increased [3H]retinol uptake in RA or Am580-treated neonates (Fig. 3). This result also agrees with the results of a long-term feeding study in adult rats fed an RA-supplemented diet, in which the rates of retinol uptake into the lungs were increased, compared with control rats (18).
In this study, we examined the expression of LRAT, CYP26A1, CYP26B1, and STRA6 because we hypothesized that they could play a major role in redirecting the flow of VA into the lungs as well as influencing the turnover of RA. As LRAT functions to esterify retinol to its storage form, RE, while CYP26A1 and CYP26B1 oxidize RA to inactive polar metabolites (21), these genes could significantly affect RE levels in RA-responsive tissues. STRA6 was of interest because in studies of the retina and retinal pigment epithelial cells, STRA6 and LRAT cooperatively enhanced the uptake and accumulation of retinol as RE (25). STRA6 mRNA was shown by Northern blot to be present at relatively low abundance in adult lung tissue (25). Mutations in STRA6 have been associated with development defects, including lung hypoplasia (27), but the physiological regulation of STRA6 in the lung has not been reported. Indeed, prior demonstrations of STRA6 regulation by RA have been in cancer cells, originally in P19 embryonal carcinoma cells (50), and we believe our results may be the first evidence of the physiological regulation of STRA6 by RA coupled with increased retinol uptake in normal tissue. Our comparison of the expression of STRA6, LRAT, CYP26A1 and CYP26B1 provides insight into the treatment-initiated changes in expression in the period of most active VA absorption (6 h) as well as in the period after absorption has been completed and tissue retinoid turnover and homeostatic regulation may be observed (12 h). Since all-trans-RA can be rapidly metabolized by the 4-hydroxylase activity of CYP26A1 and CYP26B1 (21), while the methyl groups at C-4 of Am580 prevent oxidation, the use of Am580 in our studies allowed us to ask whether a more persistent retinoid might extend or exaggerate the effects observed for at-RA on lung VA metabolism.
VARA and VAAm did not differ from each other with respect to lung RE and STRA6 expression at 6 h. For STRA6, the increase in mRNA was transient and coincided with the time when the uptake of newly absorbed retinol from the oral VA supplement would be most active (Fig. 4). As illustrated in Fig. 8, STRA6 may therefore contribute to retinol uptake in the absorptive period (6 h), provided that sufficient VA is present (Fig. 8A for RA and 8C for Am580). However, by 12 h after dose administration, the input of VA and the level of STRA6 had both declined (Fig. 8B for RA and 8D for Am580); thus, the process of retinol uptake would no longer be likely to contribute to the VARA synergy. As the lung RE content is the result of integrated, cumulative changes over time, where gene expression has been measured at discrete times, it is possible that the reduction in lung RE in the VAAm group at 12 h results from a change in balance of several factors.
Fig. 8.
Model of VA metabolism in neonatal rat lung. The four panels represent the observed and proposed pathways of retinoid uptake, esterification, and oxidative metabolism in the lungs of neonates treated with RA for 6 h (A) and 12 h (B), and with Am580 for 6 h (C) and 12 h (D). Changes in gene expression represent the effects of the acidic retinoids, with and without VA, while retinol uptake and RE formation represent the treatments that included VA. A: When RA is administered to neonatal rats with a supplement of VA, RA upregulates the expression of LRAT and CYP26B1 to the same extent at 6 h, with the flow of retinol to RE formation or to polar metabolites kept in a balance. More dietary retinol is taken up by lung tissue due to upregulation of STRA6 and elevated plasma retinol at 6 h. B: At 12 h, RA is metabolized by CYP26B1. Although the biological activity of RA has declined, the pathway is still balanced as STRA6, LRAT, and CYP26B1 have all returned to basal levels. C: When Am580 is administered to neonatal rats with a supplement of VA, LRAT expression is induced to a higher level than CYP26B1 at 6 h, favoring RE formation. D: At 12 h, Am580 continues to exert its regulatory activity due to its resistance to oxidative metabolism, and CYP26B1 is dramatically upregulated, to a higher extent than LRAT. Thus, the pathway is unbalanced and retinoid metabolism flows toward the oxidative direction. The decline in the size of the lung RE pool suggests that retinol, released from the RE pool, is either oxidized directly to polar metabolites such as 4-oxo-retinol (denoted as “?” in D) or first oxidized to RA and then to polar metabolites of RA for elimination.
For LRAT, lung LRAT mRNA levels changed significantly with both time and treatment. Previous studies showed that LRAT mRNA and LRAT enzymatic activity are closely correlated (33, 51); thus, the higher expression of LRAT mRNA at 6 h in all groups (Figs. 5, 8A, 8C), coupled with the increased lung RE content in the VARA and VAAm groups, suggests that retinol substrate was the limiting factor in the VARA- and VAAm-treated neonates, while LRAT may have been the limiting factor in the neonates treated only with VA. At 12 h, LRAT expression had fallen to near basal levels in the RA and VARA groups but remained high in the Am580 and VAAm groups (Fig. 5 and summary in Fig. 8B, D). This result suggests that if a second supplement of VA were given around 12 h after treatment with Am580 or VAAm, it might synergize with the previously administered Am580 to further elevate lung RE because LRAT expression was already highly induced. We observed, however, that treatment with VAAm resulted in a lower lung RE concentration at 12 h compared with either VARA at 12 h or VAAm at 6 h (Fig. 2A, B and summary in Fig. 8B, D). The pattern of expression for CYP26B1 in particular appears to explain these differences, as Am580 further upregulated the expression of CYP26B1 at 12 h (Fig. 6). The high levels of both LRAT and CYP26B1 induced by Am580, especially dramatically for CYP26B1, could speed up the rate of metabolism of retinol and change the balance of the metabolic pathway toward the direction of retinoid oxidation. Although the substrate for CYP26A1 and CYP26B1 is usually considered to be at-RA, it is possible that one or both of these enzymes also oxidizes retinol because 4-hydroxy- and 4-oxo-retinol have been found as an endogenous metabolite in vivo (52–54), which increased in plasma after VA supplementation (55). We therefore speculate that the oxidative metabolism of retinol itself could have been increased in the lungs of the neonates treated with Am580 or VAAm, which may account for the reduction in RE mass at 12 h in the VAAm group, as compared both to the VARA group at 12 h and the VAAm group at 6 h (Fig. 8D). Although CYP26A1 was also upregulated by Am580, it did not increase in response to RA, and there was also no difference in its expression at 6 and 12 h (Fig. 7). Since our results show that CYP26B1 is much more highly expressed than CYP26A1 in the neonatal rat lung, even in the basal state before treatment (comparison not shown), CYP26B1 might play the major role in metabolizing RA in the neonatal lung. However, CYP26A1 mRNA in the liver was significantly increased by at-RA (data not shown), which suggests its importance in VA metabolism in the neonatal liver and in clearance of VA from the body through biliary excretion of polar retinoid metabolites. Based on these findings, we speculate that LRAT and CYP26B1 are the critical genes in regulating neonatal lung VA homeostasis. Figure 8 suggests how RA and Am580 act both similarly early on and in a differential manner after 12 h to affect lung RE content and retinoid homeostasis in the lungs. We also determined the expression of several other genes that could potentially contribute to retinol uptake, and metabolism was also measured in our study, including the LDL-receptor-related protein and lipoprotein lipase, which might influence the binding and uptake of chylomicron RE and CRBP1, a chaperone protein that delivers retinol to LRAT. However, while the mRNA for each of these genes was readily detected in neonatal lung, expression levels did not change with our treatments. Previous studies showed that CRBP1 in liver was not always saturated with retinol (56); thus, if this is the case in the lungs, there may be sufficient capacity for binding and metabolizing retinol after VARA treatment, without an increase in CRBP1 expression. Thus, overall, STRA6, LRAT, and CYP26B1 appear to be the key retinoid-regulated genes guiding retinoid homeostasis in the neonatal lung.
We also compared the metabolism of newly absorbed [3H]retinol in the 12 h metabolic study. This experiment revealed that more 3H was presented in the lung of RA- or Am580-treated neonates compared with the VA and control groups. In addition, the newly formed [3H]RE was proportional to the [3H]retinol taken up by lung tissue (data not shown), which implies an accordingly increased esterification of retinol in neonatal rat lung.
Although our study did not include an analysis of retinoid nuclear receptors, the importance of the RAR receptor family to lung development is well established (57). In particular, RAR-α is known to be important in the period of postnatal lung development (58). The ability of Am580, which binds selectively to RAR-α as compared with RAR-β or RAR-γ (29), to replicate the effects of all-trans-RA on lung RE accumulation in the neonatal lung suggests that RAR-α is active in this process. The induction of LRAT expression by Am580 was not surprising because Am580 had been shown to induce LRAT activity in the liver of VA-deficient rats, in the absence of other retinoids (59). However, the magnitude and persistence of the effect of Am580 in the neonatal lung was distinctive. Moreover, the marked effect of Am580 on CYP26B1 mRNA levels was unexpected. This large increase may explain the reduction in lung RE at 12 h in the VAAm-treated neonates. Although the regulation of CYP26A1 gene expression by RA is known to be directly mediated through RA-activated RAR-RXR binding to retinoic acid-responsive elements (60), it is still uncertain how LRAT and CYP26B1 gene expression is induced by retinoids. The very strong regulation of CYP26B1 by Am580 in the neonatal lung is both interesting and cautionary, as a high and sustained level of expression of CYP26B1 hydroxylase could potentially cause a local depletion of at-RA. Given the lack of affinity of Am580 for RAR-β or RAR-γ, if the induction of CYP26B1 resulted in a deprivation of at-RA in the lungs, those genes that depend on RA-induced activation RAR-β or RAR-γ for the maintenance of their expression could be especially affected. These potential differences warrant further study.
In summary, our studies have shown that several acidic retinoids are potent regulators of lung RE formation. The concomitant expression of several important genes, LRAT, CYP26B1, and STRA6 by RA and Am580, revealed a possible molecular mechanism for the synergistic effect of VARA, involving STRA6 and LRAT as early-regulated genes, which have already been shown to cooperate in the synthesis of RE the retina (25). The higher magnitude and prolonged increase in gene expression, especially CYP26B1 after induction by Am580, provided new evidence for a better understanding of the metabolism of VA in the lung. Considering the beneficial effects of VA in promoting lung maturation and the synergistic effect of VARA in increasing lung RE, VARA could be a promising therapeutic option in clinical medicine to induce RE formation in the lungs with lower doses of VA.
Acknowledgments
The authors thank Drs. Reza Zolfaghari, Nan-qian Li, and Qiuyan Chen for their helpful advice.
Footnotes
Abbreviations:
- CRBP1
- cellular retinol-binding protein 1
- LRAT
- lecithin:retinol acyltransferase
- RA
- retinoic acid
- RBP
- retinol-binding protein
- RE
- retinyl ester
- Stra6
- stimulated by retinoic acid gene 6
- VA
- vitamin A
- VARA
- vitamin A and retinoic acid
This research was supported by National Institutes of Health Grants CA90214 and DK41479. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Roth-Kleiner M., Post M. 2005. Similarities and dissimilarities of branching and septation during lung development. Pediatr. Pulmonol. 40: 113–134. [DOI] [PubMed] [Google Scholar]
- 2.Massaro D., Massaro G. D. 2002. Pre- and postnatal lung development, maturation, and plasticity - Invited review: Pulmonary alveoli: formation, the “call for oxygen,” and other regulators. Am. J. Physiol. Lung Cell. Mol. Physiol. 282: L345–L358. [DOI] [PubMed] [Google Scholar]
- 3.Maden M., Hind M. 2004. Retinoic acid in alveolar development, maintenance and regeneration. Philos. Trans. R. Soc. Lond. B Biol. Sci. 359: 799–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ross A. C., Harrison E. H. 2007. Vitamin A: nutritional aspects of retinoids and carotenoids. Handbook of Vitamins. Zempleni J., Rucker R.B, McCormick D. B., Suttie J. W., Taylor & Francis Group, Boca Raton, FL: 1–40. [Google Scholar]
- 5.Rigas J. R., Francis P. A., Muindi J. R. F., Kris M. G., Huselton C., DeGrazia F., Orazem J. P., Young C. W., Warrell R. P., Jr 1993. Constitutive variability in the pharmacokinetics of the natural retinoid, all-trans-retinoic acid, and its modulation by ketoconazole. J. Natl. Cancer Inst. 85: 1921–1926. [DOI] [PubMed] [Google Scholar]
- 6.Hind M., Corcoran J., Maden M. 2002. Pre- and postnatal lung development, maturation, and plasticity - temporal/spatial expression of retinoid binding proteins and RAR isoforms in the postnatal lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 282: L468–L476. [DOI] [PubMed] [Google Scholar]
- 7.Hind M., Maden M. 2004. Retinoic acid induces alveolar regeneration in the adult mouse lung. Eur. Respir. J. 23: 20–27. [DOI] [PubMed] [Google Scholar]
- 8.Belloni P. N., Garvin L., Mao C. P., Bailey-Healy I., Leaffer D. 2000. Effects of all-trans-retinoic acid in promoting alveolar repair. Chest. 117 (Suppl. 1): 235S–241S. [DOI] [PubMed] [Google Scholar]
- 9.Massaro D., Massaro G. D. 2006. Toward therapeutic pulmonary alveolar regeneration in humans. Proc. Am. Thorac. Soc. 3: 709–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shenai J. P., Chytil F. 1990. Vitamin A storage in lungs during perinatal development in the rat. Biol. Neonate. 57: 126–132. [DOI] [PubMed] [Google Scholar]
- 11.Shenai J. P., Chytil F. 1990. Effect of maternal vitamin-A administration on fetal lung vitamin-A stores in the perinatal rat. Biol. Neonate. 58: 318–325. [DOI] [PubMed] [Google Scholar]
- 12.Ross A. C., Ambalavanan N., Zolfaghari R., Li N-q. 2006. Vitamin A combined with retinoic acid increases retinol uptake and lung retinyl ester formation in neonatal rats. J. Lipid Res. 47: 1844–1851. [DOI] [PubMed] [Google Scholar]
- 13.Shenai J. P., Chytil F., Jhaveri A., Stahlman M. T. 1981. Plasma vitamin A and retinol-binding protein in premature and term neonates. J. Pediatr. 99: 302–305. [DOI] [PubMed] [Google Scholar]
- 14.Shenai J. P., Rush M. G., Stahlman M. T., Chytil F. 1992. Vitamin A supplementation and bronchopulmonary dysplasia–revisited. J. Pediatr. 121: 399–401. [DOI] [PubMed] [Google Scholar]
- 15.Mactier H., Weaver L. T. 2005. Vitamin A and preterm infants: what we know, what we don't know, and what we need to know. Arch. Dis. Child. Fetal Neonatal Ed. 90: F103–F108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan V., Cheeseman P., Gamsu H. R. 1993. Vitamin A status in preterm and term infants at birth. J. Perinat. Med. 21: 59–62. [DOI] [PubMed] [Google Scholar]
- 17.Ross A. C., Li N., Wu L. 2006. The components of VARA, a nutrient-metabolite combination of vitamin A and retinoic acid, act efficiently together and separately to increase retinyl esters in the lungs of neonatal rats. J. Nutr. 136: 2803–2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cifelli C. J., Green J. B., Green M. H. 2005. Dietary retinoic acid alters vitamin A kinetics in both the whole body and in specific organs of rats with low vitamin A status. J. Nutr. 135: 746–752. [DOI] [PubMed] [Google Scholar]
- 19.Ross A. C., Zolfaghari R., Weisz J. 2001. Vitamin A: recent advances in the biotransformation, transport, and metabolism of retinoids. Curr. Opin. Gastroenterol. 17: 184–192. [DOI] [PubMed] [Google Scholar]
- 20.Ross A. C. 2003. Retinoid production and catabolism: role of diet in regulating retinol esterification and retinoic acid oxidation. J. Nutr. 133: 291S–296S. [DOI] [PubMed] [Google Scholar]
- 21.Petkovich P. M. 2001. Retinoic acid metabolism. J. Am. Acad. Dermatol. 45: S136–S142. [DOI] [PubMed] [Google Scholar]
- 22.Randolph R. K., Ross A. C. 1991. Vitamin A status regulates hepatic lecithin:retinol acyltransferase activity in rats. J. Biol. Chem. 266: 16453–16457. [PubMed] [Google Scholar]
- 23.Yamamoto Y., Zolfaghari R., Ross A. C. 2000. Regulation of CYP26 (Cytochrome P450RAI) mRNA expression and retinoic acid metabolism by retinoids and dietary vitamin A in liver of mice and rats. FASEB J. 14: 2119–2127. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y., Zolfaghari R., Ross A. C. 2002. Cloning of rat cytochrome P450RAI (CYP26) cDNA and regulation of its gene expression by all-trans-retinoic acid in vivo. Arch. Biochem. Biophys. 401: 235–243. [DOI] [PubMed] [Google Scholar]
- 25.Kawaguchi R., Yu J., Honda J., Hu J., Whitelegge J., Ping P., Wiita P., Bok D., Sun H. 2007. A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science. 315: 820–825. [DOI] [PubMed] [Google Scholar]
- 26.Bouillet P., Sapin V., Chazaud C., Messaddeq N., Décimo D., Dollé P., Chambon P. 1997. Developmental expression pattern of Stra6, a retinoic acid-responsive gene encoding a new type of membrane protein. Mech. Dev. 63: 173–186. [DOI] [PubMed] [Google Scholar]
- 27.Pasutto F., Sticht H., Hammersen G., Gillessen-Kaesbach G., Fitzpatrick D. R., Nürnberg G., Brasch F., Schirmer-Zimmermann H., Tolmie J., Chitayat D., et al. 2007. Mutations in STRA6 cause a broad spectrum of malformations including anophthalmia, congenital heart defects, diaphragmatic hernia, alveolar capillary dysplasia, lung hypoplasia, and mental retardation. Am. J. Hum. Genet. 80: 550–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hashimoto Y., Kagechika H., Shudo K. 1990. Expression of retinoic acid receptor genes and the ligand-binding selectivity of retinoic acid receptors (RAR'S). Biochem. Biophys. Res. Commun. 166: 1300–1307. [DOI] [PubMed] [Google Scholar]
- 29.Kagechika H. 2002. Novel synthetic retinoids and separation of the pleiotropic retinoidal activities. Curr. Med. Chem. 9: 591–608. [DOI] [PubMed] [Google Scholar]
- 30.Folch J., Lees M., Sloane Stanley G. H. 1957. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 226: 497–509. [PubMed] [Google Scholar]
- 31.Ross A. C. 1986. Separation and quantitation of retinyl esters and retinol by high-performance liquid chromatography. Methods Enzymol. 123: 68–74. [DOI] [PubMed] [Google Scholar]
- 32.Ross A. C. 1982. Retinol esterification by mammary gland microsomes from the lactating rat. J. Lipid Res. 23: 133–144. [PubMed] [Google Scholar]
- 33.Zolfaghari R., Ross A. C. 2000. Lecithin:retinol acyltransferase from mouse and rat liver: cDNA cloning and liver-specific regulation by dietary vitamin A and retinoic acid. J. Lipid Res. 41: 2024–2034. [PubMed] [Google Scholar]
- 34.Bastien J., Rochette-Egly C. 2004. Nuclear retinoid receptors and the transcription of retinoid-target genes. Gene. 328: 1–16. [DOI] [PubMed] [Google Scholar]
- 35.Dirami G., Massaro G. D., Clerch L. B., Ryan U. S., Reczek P., Massaro D. 2003. Lung retinol storing cells synthesize and secrete retinoic acid, an inducer of alveolus formation. Am. J. Physiol. Lung Cell. Mol. Physiol. 286: L249–L256. [DOI] [PubMed] [Google Scholar]
- 36.Chytil F. 1996. Retinoids in lung development. FASEB J. 10: 986–992. [DOI] [PubMed] [Google Scholar]
- 37.McGowan S. E. 2002. Contributions of retinoids to the generation and repair of the pulmonary alveolus. Chest. 121: 206S–208S. [DOI] [PubMed] [Google Scholar]
- 38.Jobe A. H., Ikegami M. 2000. Lung development and function in preterm infants in the surfactant treatment era. Annu. Rev. Physiol. 62: 825–846. [DOI] [PubMed] [Google Scholar]
- 39.Fraslon C., Bourbon J. R. 1994. Retinoids control surfactant phospholipid biosynthesis in fetal rat lung. Am. J. Physiol. 266: L705–L712. [DOI] [PubMed] [Google Scholar]
- 40.Bourbon J. R., Farrell P. M., Doucet E., Brown D. J., Valenza C. 1987. Biochemical maturation of fetal rat lung: a comprehensive study including surfactant determination. Biol. Neonate. 52: 48–60. [DOI] [PubMed] [Google Scholar]
- 41.Baybutt R. C., Hu L., Molteni A. 2000. Vitamin A deficiency injures lung and liver parenchyma and impairs function of rat type II pneumocytes. J. Nutr. 130: 1159–1165. [DOI] [PubMed] [Google Scholar]
- 42.Bry K., Lappalainen U. 2006. Pathogenesis of bronchopulmonary dysplasia: the role of interleukin 1beta in the regulation of inflammation-mediated pulmonary retinoic acid pathways in transgenic mice. Semin. Perinatol. 30: 121–128. [DOI] [PubMed] [Google Scholar]
- 43.Shenai J. P., Kennedy K. A., Chytil F., Stahlman M. T. 1987. Clinical trial of vitamin A supplementation in infants susceptible to bronchopulmonary dysplasia. J. Pediatr. 111: 269–277. [DOI] [PubMed] [Google Scholar]
- 44.Tyson J. E., Wright L. L., Oh W., Kennedy K. A., Mele L., Ehrenkranz R. A., Stoll B. J., Lemons J. A., Stevenson D. K., Bauer C. R., et al. 1999. Vitamin A supplementation for extremely-low-birth-weight infants. National Institute of Child Health and Human Development Neonatal Research Network. N. Engl. J. Med. 340: 1962–1968. [DOI] [PubMed] [Google Scholar]
- 45.Ambalavanan N., Tyson J. E., Kennedy K. A., Hansen N. I., Vohr B. R., Wright L. L., Carlo W. A., Natl Inst Child Hlth Human Dev N. R. N. 2005. Vitamin A supplementation for extremely low birth weight infants: Outcome at 18 to 22 months. Pediatrics. 115: E249–E254. [DOI] [PubMed] [Google Scholar]
- 46.Ambalavanan N., Wu T. J., Tyson J. E., Kennedy K. A., Roane C., Carlo W. A. 2003. Comparison of three vitamin A dosing regimens in extremely-low-birth-weight infants. J. Pediatr. 142: 656–661. [DOI] [PubMed] [Google Scholar]
- 47.Humphrey J. H., Agoestina T., Wu L., Usman A., Nurachim M., Subardja D., Hidayat S., Tielsch J., West K. P., Jr., Sommer A. 1996. Impact of neonatal vitamin A supplementation on infant morbidity and mortality. J. Pediatr. 128: 489–496. [DOI] [PubMed] [Google Scholar]
- 48.Rahmathullah L., Tielsch J. M., Thulasiraj R. D., Katz J., Coles C., Devi S., John R., Prakash K., Sadanand A. V., Edwin N., et al. 2003. Impact of supplementing newborn infants with vitamin A on early infant mortality: community based randomised trial in southern India. BMJ. 327: 254–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Massaro G. D., Massaro D. 1996. Postnatal treatment with retinoic acid increases the number of pulmonary alveoli in rats. Am. J. Physiol. 270: L305–L310. [DOI] [PubMed] [Google Scholar]
- 50.Bouillet P., Oulad-Abdelghani M., Vicaire S., Garnier J-M., Schuhbaur B., Dollé P., Chambon P. 1995. Efficient cloning of cDNAs of retinoic acid-responsive genes in P19 embryonal carcinoma cells and characterization of a novel mouse gene, Stra 1 (Mouse LERK-2/Eplg2). Dev. Biol. 170: 420–433. [DOI] [PubMed] [Google Scholar]
- 51.Matsuura T., Gad M. Z., Harrison E. H., Ross A. C. 1997. Lecthin:retinol acyltransferase and retinyl ester hydrolase activities are differentially regulated by retinoids and have distinct distributions between hepatocyte and nonparenchymal cell fractions of rat liver. J. Nutr. 127: 218–224. [DOI] [PubMed] [Google Scholar]
- 52.Leo M. A., Lieber C. S. 1985. New pathway for retinol metabolism in liver microsomes. J. Biol. Chem. 260: 5228–5231. [PubMed] [Google Scholar]
- 53.Rühl R., Hamscher G., Garcia A. L., Nau H., Schweigert F. J. 2005. Identification of 14-hydroxy-retro-retinol and 4-hydroxy-retinol as endogenous retinoids in rats throughout neonatal development. Life Sci. 76: 1613–1622. [DOI] [PubMed] [Google Scholar]
- 54.Achkar C. C., Derguini F., Blumberg B., Langston A., Levin A. A., Speck J., Evans R. M., Bolado J., Jr., Nakanishi K., Buck J., et al. 1996. 4-Oxoretinol, a new natural ligand and transactivator of the retinoic acid receptors. Proc. Natl. Acad. Sci. USA. 93: 4879–4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Penniston K. L., Tanumihardjo S. A. 2005. Elevated serum concentrations of β-glucuronide metabolites and 4-oxoretinol following treatment with vitamin A in lactating sows: a model for evaluating supplementation of lactating women. Am. J. Clin. Nutr. 81: 851–858. [DOI] [PubMed] [Google Scholar]
- 56.Harrison E. H., Blaner W. S., Goodman D. S., Ross A. C. 1987. Subcellular localization of retinoids, retinoid-binding proteins, and acyl-CoA:retinol acyltransferase in rat liver. J. Lipid Res. 28: 973–981. [PubMed] [Google Scholar]
- 57.McGowan S., Jackson S. K., Jenkins-Moore M., Dai H. H., Chambon P., Snyder J. M. 2000. Mice bearing deletions of retinoic acid receptors demonstrate reduced lung elastin and alveolar numbers. Am. J. Respir. Cell Mol. Biol. 23: 162–167. [DOI] [PubMed] [Google Scholar]
- 58.Massaro G. D., Massaro D., Chambon P. 2003. Retinoic acid receptor-alpha regulates pulmonary alveolus formation in mice after, but not during, perinatal period. Am. J. Physiol. Lung Cell. Mol. Physiol. 284: L431–L433. [DOI] [PubMed] [Google Scholar]
- 59.Shimada T., Ross A. C., Muccio D. D., Brouillette W. J., Shealy Y. F. 1997. Regulation of hepatic lecithin:retinol acyltransferase activity by retinoic acid receptor-selective retinoids. Arch. Biochem. Biophys. 344: 220–227. [DOI] [PubMed] [Google Scholar]
- 60.Loudig O., Maclean G. A., Dore N. L., Luu L., Petkovich M. 2005. Transcriptional co-operativity between distant retinoic acid response elements in regulation of Cyp26A1 inducibility. Biochem. J. 392: 241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]