Abstract
Niemann-Pick type C1 disease (NPC1) is an autosomal recessive lysosomal storage disorder characterized by neonatal jaundice, hepatosplenomegaly, and progressive neurodegeneration. The present study provides the lipid profiles, mutations, and corresponding associations with the biochemical phenotype obtained from NPC1 patients who participated in the National NPC1 Disease Database. Lipid profiles were obtained from 34 patients (39%) in the survey and demonstrated significantly reduced plasma LDL cholesterol (LDL-C) and increased plasma triglycerides in the majority of patients. Reduced plasma HDL cholesterol (HDL-C) was the most consistent lipoprotein abnormality found in male and female NPC1 patients across age groups and occurred independent of changes in plasma triglycerides. A subset of 19 patients for whom the biochemical severity of known NPC1 mutations could be correlated with their lipid profile showed a strong inverse correlation between plasma HDL-C and severity of the biochemical phenotype. Gene mutations were available for 52 patients (59%) in the survey, including 52 different mutations and five novel mutations (Y628C, P887L, I923V, A1151T, and 3741_3744delACTC). Together, these findings provide novel information regarding the plasma lipoprotein changes and mutations in NPC1 disease, and suggest plasma HDL-C represents a potential biomarker of NPC1 disease severity.
Keywords: plasma lipoproteins, lysosomal storage disease, high density lipoproteins, biomarker
Niemann-Pick type C1 disease (NPC1) is an autosomal recessive lipid storage disorder characterized by clinical manifestations involving primarily the liver and brain (1). The onset of signs or symptoms can occur at any age and have a variable phenotype. The classic clinical phenotype is also variable and includes mid-to-late childhood onset of gait disturbance followed by progressive neurodegeneration with vertical gaze palsy, seizures, and dementia, resulting in death during the second or third decade (2–4). The clinical phenotype of NPC1 disease has been categorized according to the age of onset of symptoms (5, 6), including an early-onset, rapidly progressive form associated with hepatic dysfunction and psychomotor delay during infancy, the classic form, and a late-onset type characterized by a slowly progressive intellectual impairment in adolescence or adulthood.
The gene responsible for NPC1 disease, NPC1, was localized to chromosome 18 using linkage analysis and subsequently identified using positional mapping and molecular cloning techniques (7, 8). The encoded product, the Niemann-Pick C1 (NPC1) protein, contains several specialized regions (9–11), including a sterol-sensing domain also present in other key proteins regulating cholesterol metabolism, including 3-hydroxy-3-methyl-glutaryl-CoA reductase and sterol regulatory element-binding protein (SREBP) cleavage-activating protein (12, 13). Recent studies indicate cholesterol binds to luminal loop-1 of NPC1 (14), and that a separate N-terminal helical subdomain of NPC1 is required for cholesterol transfer between NPC2 and the cholesterol-binding domain of NPC1 (15). At the cellular level, decreased NPC1 protein function results in an accumulation of both cholesterol and glycosphingolipids within late endosomes and lysosomes (16–19). As a result, it is believed that the NPC1 protein has a central role in regulating the transport of these lipids out of late endosomes/lysosomes to other cellular compartments, including the Golgi apparatus, plasma membrane, and endoplasmic reticulum (20–23). Consistent with this proposed function, studies performed in human and mouse fibroblasts have demonstrated that the NPC1 protein is primarily localized to a novel late endosome-like compartment capable of transiently interacting with LDL-derived cholesterol-enriched late endosomes/lysosomes (24, 25).
Since the identification of the NPC1 gene, a number of studies have characterized various mutations and attempted to associate these mutations with a biochemical and clinical phenotype (26–29). To date, more than 243 different loss-of-function mutations of NPC1 have been reported (30, 31), in addition to 60 different nondisease-causing polymorphisms (31–34). Although the ability to establish meaningful genotype and clinical phenotype associations has been difficult, primarily due to the fact that most NPC1 patients are compound heterozygous for different mutations, some associations have been established for NPC1 patients with homozygous mutations. In particular, the relatively common I1061T mutation, present in approximately 20% of all known mutations for NPC1 and prominent among individuals of Western European descent, predisposes patients to the classic NPC1 clinical phenotype (34, 35).
Defects in cholesterol trafficking out of lysosomes might be expected to produce changes in plasma lipoprotein levels. We previously reported low plasma HDL cholesterol (HDL-C) levels in 17 of 21 NPC disease patients studied, and provided evidence using human NPC1−/− fibroblasts that this is due to defective upregulation of the key transporter mediating new HDL particle formation, ABCA1, as a consequence of impaired release of cholesterol from lysosomes (36). We also found a tendency toward reduced plasma LDL cholesterol (LDL-C) and increased plasma triglycerides in these patients (36). In the current study, we sought to determine whether these abnormalities are a consistent feature of NPC1 disease, and whether the reduction in HDL-C might be a reflection of the biochemical severity of the disease.
Recently, two independent reports, including ours, have been published providing an in depth analysis of the natural history, clinical features, and disabilities associated with NPC1 disease (37, 38). These reports confirm the heterogeneous nature of NPC1 disease, and have identified particular symptoms including cataplexy and epilepsy being more common than previously reported. The present analysis of the National NPC1 Disease Database reports i) further characterization of plasma lipoprotein abnormalities in NPC1 disease, ii) associations between the lipid profiles, gene mutations, and biochemical phenotypes in NPC1 patients, iii) possible correlations between the lipid profiles, age of diagnosis and death, and the average abilities for NPC1 patients, and iv) the NPC1 mutations including five novel mutations present in the database cohort.
METHODS
National NPC1 Disease Database
The development and initial information obtained for the National NPC1 Disease Database was provided in an earlier report (38). The plan to establish a National NPC1 Disease Database was introduced during the annual National Niemann-Pick Disease Foundation Family Conference, sponsored in part by the Ara Parseghian Medical Research Foundation (APMRF), in 2003. Those families expressing an interest in such a study were asked to provide their name, home address, and phone number for later contact by the APMRF. The study design was reviewed and approved by the University of Arizona Institutional Review Board through the Human Subjects Protection Program. The families were then mailed the NPC1 disease clinical questionnaire, in addition to a parental consent form, a subject's consent form, and a minor's assent form, each of which required the proper signatures and dates for the information to be entered into the database and used for the present study.
National NPC1 disease clinical questionnaire
An NPC1 disease clinical questionnaire, previously prepared and graciously provided by Dr. Mercè Pineda (Hospital Sant Joan de Déu, Barcelona), was translated into English and modified for use in creating an American National NPC1 Disease Database. The questionnaire consisted of 83 questions, including one for the specific gene mutations and four for the lipid profile components (total cholesterol, HDL-C, LDL-C, and triglycerides). The study was conducted over a one-year period from December 2003 to December 2004. The questionnaire was completed by parents/caregivers and/or physicians responsible for patients with NPC1 disease living in the United States. The questionnaire did not specifically request information concerning who provided the initial diagnosis of NPC1 disease (primary care physician, pediatrician, neurologist, or other specialist). However, in all cases the parents/caregivers and/or patients were informed of the diagnosis for NPC1 disease after either genotype analysis, enhanced filipin staining, and/or decreased cholesterol esterification using cultured fibroblasts derived from patients, consistent with the age at diagnosis reported in the questionnaire and the time of identification of the NPC1 gene.
NPC1 lipid profiles and gene mutations
Fasting lipid profiles, all of which were obtained from a certified clinical diagnostic laboratory, were either mailed or faxed from parents/caregivers or their physicians responsible for the patient with NPC1 disease. Components of the lipid profile obtained from patients in the NPC1 Database were compared with normal ranges for children between 5 and 9 years of age and adults between 25 and 29 years of age of both genders, representing the average range of ages for children and adults in the NPC1 Disease Database, using The Lipid Research Clinics Population Studies Data Book published by the National Institutes of Health (39). NPC1 gene mutations were determined at the Mayo Clinic (Rochester, MN) and provided to the APMRF. Through written parental or patient consent, these mutations were then made available for the National NPC1 Disease Database. In some cases, amino acid but not nucleotide changes were provided.
Analysis of NPC1 mutations, biochemical phenotype, and lipid profiles
The majority of known NPC1 mutations have been associated with specific biochemical phenotypes as defined by measuring the esterification of LDL-derived cholesterol in skin fibroblasts obtained from NPC1 patients (28, 31, 34, 40, 41). To further analyze NPC1 mutations and biochemical phenotypes in relation to different components of the lipid profile, the biochemical phenotypes were assigned a severity score based on the reduction in cholesterol esterification by ACAT as previously described (31). For example, a variant biochemical phenotype, represented by a mild mutation with a near normal biochemical phenotype, was given a score of one. A moderate biochemical phenotype, represented by an intermediate mutation and biochemical phenotype, was given a score of two. A classical biochemical phenotype, represented by a classical mutation that was more severe than the moderate mutation but less severe than a severe mutation, was given a score of three. A severe biochemical phenotype, represented by a severe mutation and biochemical phenotype, was given a score of four. In cases where the biochemical phenotype was unknown, no score was assigned (unknown = U). In order to assign a biochemical phenotype score for the combination of two different NPC1 mutations, the following rules were applied: 1) One moderate mutation always conferred a moderate biochemical phenotype, 2) classical mutations known to be variable always depended upon the other mutation, and 3) one severe mutation always conferred a severe biochemical phenotype.
Statistical analysis
The clinical information, lipid profiles, and gene mutations were entered into a Microsoft ACCESS database and analyzed by the Data Analysis Unit, University of Arizona Program Site of the Arizona University Center on Disabilities. Pearson correlation coefficients were computed to evaluate relationships among most of the variables in this report. However, Spearman's rank correlation coefficient was substituted whenever the biochemical phenotype score, which represents an ordinal scale variable, was used in an analysis. Significance was determined by P-values < 0.05. All statistics and significance tests were computed using SPSS version 14.0.
RESULTS
Lipid profiles of NPC1 patients
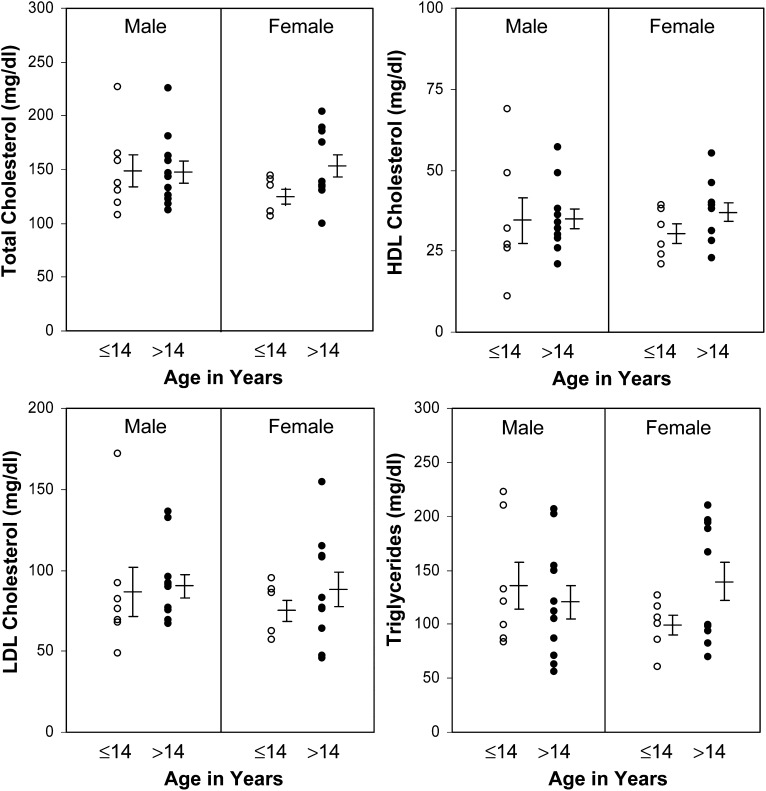
For the present study, a total of 136 questionnaires were mailed to NPC1 families living in the United States with 88 of these questionnaires (65%) being returned. For the 88 questionnaires returned, the lipid profiles from 34 patients (39%) were made available. The patients in this sample were represented by an approximately equal number of males (N = 18) and females (N = 16), ranging in age between 1.5 and 45 years and at different stages and severity of disease. For these patients, the average concentrations (± SE) of total cholesterol were 146 ± 5.6 mg/dl (3.78 ± 0.14 mmol/l), HDL-C 35 ± 2.0 mg/dl (0.91 ± 0.05 mmol/l), LDL-C 86 ± 5.0 mg/dl (2.22 ± 0.13 mmol/l), and triglycerides 126 ± 8.7 mg/dl (1.42 ± 0.10 mmol/l). The lipid profiles were grouped by gender and age ≤14 or >14 to determine whether there were gender or age-specific differences among these patients (Fig. 1). No significant differences were found among any of the lipid parameters when comparing male or female NPC1 patients based on age <14 or >14. However, several significant differences were found when comparing the NPC1 patient lipid levels with a control population represented by normal males and females 5–9 and 25–29 years of age as reported in the Lipid Research Clinics Population Studies Data Book (39) (Table 1). Total cholesterol levels were found to be significantly lower in female children and adult men with NPC1 disease but not in male children or adult females. LDL-C was significantly lower than controls in female children and adult males and females with NPC1 disease but not male children. Triglycerides were found to be significantly higher in male and female children and adult women with NPC1 disease but not adult males. HDL-C was found to be significantly lower in all male and female NPC1 patient groups when compared with controls, regardless of age, with HDL-C ranging from 56% to 78% lower than controls across the groups. In comparison with the lipid levels reported in our initial study in 2003 (36), where 17 of 21 patients (81%) had an HDL-C level below 40 mg/dl or 1.03 mmol/l (all of whom are included in the current analysis), the present study group contained 28 of 34 subjects (82%) with HDL-C levels below this cutoff. Using currently accepted lower limits of HDL-C for adults based on the National Cholesterol Education Program ATP III guidelines definition of the metabolic syndrome (<40 mg/dl or 1.0 mmol/l for men and <50 mg/dl or 1.29 mmol/l for women) (42), 14 of 18 or 78% of NPC1 male patients and 15 of 16 or 94% of female NPC1 patients had HDL-C levels below these norms.
Fig. 1.
Lipid profiles of NPC1 patients. The lipid profiles of 34 NPC1 patients were obtained from 18 males and 16 females, ranging in age between 1.5 and 45 years and at different stages and severity of disease. The concentrations of total cholesterol, HDL cholesterol, LDL cholesterol, and triglycerides are grouped according to gender and age ≤14 and >14. The mean values ± SE for males and females in each age group are indicated to the right of the data points.
TABLE 1.
Average concentration of total cholesterol, HDL cholesterol, LDL cholesterol, and triglycerides for normal individuals and NPC1 patients based on both gender and age
| Total Cholesterol | HDL Cholesterol | LDL Cholesterol | Triglycerides | |
|---|---|---|---|---|
| Normal Males | 159.9 ± 0.7 | 55.8 ± 1.0 | 92.5 ± 1.8 | 55.7 ± 0.6 |
| Age 5–9 Years | 4.13 ± 0.0 | 1.44 ± 0.0 | 2.39 ± 0.0 | 0.63 ± 0.0 |
| N = 1253 | N = 145 | N = 132 | N = 1253 | |
| NPC1 Males | 149.3 ± 15.0 | 34.4 ± 7.1 | 86.9 ± 15.0 | 136.3 ± 21.6 |
| Age 7.9 ± 1.4 Years | 3.86 ± 0.4 | 0.89 ± 0.8 | 2.25 ± 0.4 | 1.54 ± 0.2 |
| N = 7 | N = 7 | N = 7 | N = 7 | |
| Significance | NS | P = 0.02 | NS | P = 0.01 |
| Normal Females | 163.7 ± 0.7 | 53.2 ± 1.0 | 100.4 ± 2.1 | 60.3 ± 0.8 |
| Age 5–9 Years | 4.23 ± 0.0 | 1.38 ± 0.0 | 2.60 ± 0.07 | 0.68 ± 0.0 |
| N = 1118 | N = 127 | N = 114 | N = 1118 | |
| NPC1 Females | 124.6 ± 7.0 | 30.3 ± 3.0 | 75.0 ± 6.7 | 99.3 ± 9.5 |
| Age 9.9 ± 0.9 Years | 3.22 ± 0.3 | 0.78 ± 0.1 | 1.94 ± 0.2 | 1.12 ± 0.1 |
| N = 6 | N = 6 | N = 6 | N = 6 | |
| Significance | P = 0.003 | P = 0.001 | P = 0.01 | P = 0.01 |
| Normal Males | 182.2 ± 0.8 | 44.7 ± 0.7 | 116.7 ± 1.9 | 115.8 ± 2.3 |
| Age 25–29 Years | 4.71 ± 0.0 | 1.16 ± 0.0 | 3.02 ± 0.0 | 1.31 ± 0.0 |
| N = 2042 | N = 253 | N = 253 | N = 2042 | |
| NPC1 Males | 148.0 ± 10.0 | 34.9 ± 3.1 | 90.2 ± 7.3 | 120.5 ± 15.7 |
| Age 28.9 ± 3.0 Years | 3.83 ± 0.3 | 0.90 ± 0.1 | 2.33 ± 0.2 | 1.36 ± 0.2 |
| N = 11 | N = 11 | N = 11 | N = 11 | |
| Significance | P = 0.01 | P = 0.01 | P = 0.005 | NS |
| Normal Females | 175.8 ± 0.6 | 56.0 ± 0.8 | 110.2 ± 1.6 | 88.7 ± 1.0 |
| Age 25–29 Years | 4.55 ± 0.0 | 1.45 ± 0.0 | 2.85 ± 0.0 | 1.0 ± 0.0 |
| N = 2189 | N = 314 | N = 314 | N = 2189 | |
| NPC1 Females | 153.0 ± 10.4 | 37.0 ± 2.9 | 87.9 ± 2.6 | 139.7 ± 17.6 |
| Age 26.4 ± 2.6 Years | 3.96 ± 0.3 | 0.96 ± 0.1 | 2.27 ± 0.1 | 1.58 ± 0.2 |
| N = 10 | N = 10 | N = 10 | N = 10 | |
| Significance | NS | P = 0.0001 | P = 0.0001 | P = 0.02 |
The average lipid levels (mean ± S.E.) for normal individuals were obtained from a control population of males and females between the ages of 5–9 and 25–29 years as published in the Lipid Research Clinics Population Studies Data Book by the National Institutes of Health (38). Values in bold are in mg/dl; equivalent values in mmol/l are shown in italics. One-sample t-tests were performed using the mean values for both the gender and age of normal individuals and NPC1 patients to determine significant differences.
Association among components of the lipid profile, age, and average ability score of NPC1 patients
We next analyzed associations among individual components of the lipid profiles with each other, age, and average ability score using the 34 lipid profiles available in the database. Significant associations were found between total cholesterol and LDL-C (P < 0.0005), between total cholesterol and triglycerides (P = 0.001), and between LDL-C and triglycerides (P = 0.037) (Table 2). In contrast, we did not find a significant correlation between plasma HDL-C and triglycerides in these NPC1 patients (P = 0.059), suggesting the low HDL-C in NPC1 disease is a consequence of factors other than triglyceride replacement of cholesterol on HDL particles. When components of the lipid profile, age, and average ability scores were analyzed for possible associations, the results indicated that the concentration of LDL-C was directly and significantly associated with the age of patients but inversely related to the average ability score (Table 3). HDL-C level was not found to correlate with average ability score. Although information was made available from only five patients, the results indicated that the concentration of HDL-C was directly and significantly associated with the age at death.
TABLE 2.
Pearson correlation coefficients of the lipid profile components for NPC1 patientsa
| Total Cholesterol | Triglycerides | LDL Cholesterol | HDL Cholesterol | |
|---|---|---|---|---|
| Total Cholesterol | 0.53b | 0.92c | 0.07 | |
| Triglycerides | 0.36d | −0.33 | ||
| LDL Cholesterol | −0.20 | |||
| HDL Cholesterol |
N = 34.
P = 0.001.
P < 0.0005.
P = 0.037.
TABLE 3.
Pearson correlation coefficients of the lipid profile components with age, age at diagnosis, age at death, and average ability score for NPC1 patients
| Total Cholesterol | HDL Cholesterol | LDL Cholesterol | Triglycerides | |
|---|---|---|---|---|
| Age (years) | NS | NS | 0.41b(N = 28) | NS |
| Age at diagnosis | NS | NS | NS | NS |
| Age at death | NS | 0.96c(N = 5) | NS | NS |
| Average ability scorea | NS | NS | −0.35d(N = 34) | NS |
The average ability score was computed by taking the mean of the four individual abilities as assessed on the questionnaire. These abilities included walking, movement, language, and swallowing. Each of the individual abilities were ranked from 1 to 4, or 1 to 5, with a score of “1” being normal and scores of “4” or “5” being the most severe level of disability. NS = Not significant.
P = 0.032.
P = 0.011.
P = 0.045.
Molecular and biochemical characterization of the NPC1 gene mutations
Of the 88 questionnaires returned, the specific NPC1 gene mutations from 52 patients (59%) were made available (Table 4). Thirty-one of the patients were male (60%) and 21 were female (40%). The patients ranged in age from 1.5 to 48.5 years and were at different stages and severity of disease. The mutations were arranged according to exon or intron beginning near the 5′-portion of the NPC1 gene. Additional information for each mutation was included that corresponded to the i) nucleotide change, ii) mutation type, iii) amino acid change, iv) protein domain, v) biochemical phenotype, and vi) number of mutations in the sample. Of the 52 patients that sent this information, a total of 101 mutations from a possible 104 were provided, as three patients knew only one of their two mutations. Together, there were 52 total different mutations. Five of these have not previously been reported (Y628C, P887L, I923V, A1151T, and 3741_3744delACTC), and are therefore believed to represent novel mutations. The remaining 49 were present in more than one patient. Consistent with previous studies describing the I1061T mutation as a frequent mutant allele common among NPC1 patients of Western European descent (34), I1061T was present in 20% of the patients in this survey.
TABLE 4.
Molecular and biochemical characterization of the NPC1 genetic mutations
| Exon or Intron | Nucleotide Change | Mutation Type | Amino Acid Change | Protein Domainab | Biochemical Phenotypec | Sample Numberd |
|---|---|---|---|---|---|---|
| 2 | 72delC | Frameshift | 1 | |||
| 3 | 221G>A | Missense | C74Y | LM A | Severe | 2 |
| 3 | 275A>G | Missense | Q92R | LM A | Severe | 1 |
| 4 | 410C>T | Missense | T137M | LM A | Variable | 3 |
| 4 | 395delC | Frameshift | 1 | |||
| 4 | 451_452delAG | Frameshift | 2 | |||
| 5 | 496C>T | Missense | P166S | LM A | 1 | |
| 6 | 688_693del6bp | Deletion/Insertion | S230_V231del | LM A | Classical | 3 |
| 6 | 740G>A | Missense | C247Y | LM A | Severe | 1 |
| 8 | 1201C>A | Missense | P401T | LM C | 1 | |
| 8 | 974_975insGA | Frameshift | 1 | |||
| 8 | 1211G>A | Missense/Splicing | R404Q | LM C | Severe | 3 |
| 8 | 1261C>T | Nonsense | Q421X | 1 | ||
| 8 | 1298C>T | Missense | P433L | LM C | 1 | |
| 9 | 1421C>T | Missense | P474L | LM C | Classical/Variant | 1 |
| 9 | 1526A>C | Missense | Y509S | LM C | 1 | |
| 10 | 1628C>T | Missense | P543L | LM C | 2 | |
| 12 | 1836A>C | Missense | E612D | LM C | Severe | 1 |
| 12 | Missense | Y628C | TM III, SSD | 1 (N) | ||
| 13 | 1955C>G | Missense | S652W | CP D, SSD | Severe | 3 |
| 13 | 1978G>A | Missense | G660S | TM IV, SSD | Moderate | 1 |
| 13 | Missense | G673V | TM IV, SSD | 1 | ||
| 13 | 2098G>A | Missense | D700N | TM V, SSD | Severe | 2 |
| 15 | 2336_2337insT | Frameshift | 2 | |||
| 15 | 2365C>T | Missense | R789G | CP H, SSD | Severe | 1 |
| 16 | 2474A>G | Missense | Y825C | CP H | Severe | 2 |
| 18 | 2621A>T | Missense | D874V | LM I | Classical/Severe | 2 |
| 18 | Missense | P887L | LM I | 1 (N) | ||
| 18 | 2669G>A | Missense | Y890C | LM I | Classical | 2 |
| 19 | Missense | I923V | LM I | 1 (N) | ||
| 19 | 2819C>T | Missense | S940L | LM I | Variable | 3 |
| 19 | 2833G>A | Missense | D945N | LM I | Severe | 2 |
| 19 | 2848G>A | Missense | V950M | LM I | Variant | 2 |
| 20 | 2926T>C | Splicing | C976R | 2 | ||
| IVS20 | IVS20(-2)insG | Splicing | 1 | |||
| 20 | 3019C>G | Missense | P1007A | LM I | Moderate/Variant | 3 |
| IVS21 | IVS21(-10)delTCC | Splicing | 1 | |||
| 21 | 3107C>T | Missense | T1036M | LM I | Severe | 4 |
| 21 | 3182T>C | Missense | I1061T | LM I | Classical/Variable | 21 |
| 22 | 3259T>C | Missense | F1087L | LM I | 1 | |
| 22 | 3265G>A | Missense | E1089K | LM I | 2 | |
| 22 | Missense | A1151T | TM XI | 1 (N) | ||
| 22 | 3467A>G | Missense | N1156S | TM XI | Moderate | 2 |
| 23 | 3493G>A | Missense | V1165M | CP L | 1 | |
| 23 | 3566A>G | Missense | E1189G | CP L | Severe | 1 |
| 23 | 3573_3574insACTT | Frameshift | 1 | |||
| 24 | 3612_3613insGdelTA | Frameshift | 1 | |||
| 24 | 3662delT | Frameshift | 2 | |||
| 24 | 3741_3744delACTC | Frameshift | 1 (N) | |||
| 24 | 3742_3745delCTCA | Frameshift | 1 | |||
| 24 | 3745A>G | Missense | S1249G | TM XIII | Severe | 1 |
| 25 | 3797G>A | Missense | R1266Q | CP N | 1 (P) |
Novel mutations are indicated in bold-face type.
Protein domains are provided for missense and deletion/insertion amino acid changes where known, as previously indicated (30).
The lumen (LM), transmembrane (TM), cytoplasmic (CP), and sterol sensing (SSD) protein domains are provided as previously indicated (30, 53).
The biochemical phenotypes of respective nucleotide and/or amino acid changes are based upon the degree of cholesterol esterification catalyzed by acyl-CoA:cholesterol acyltransferase (ACAT), and were provided for known nucleotide and/or amino acid changes as previously indicated (30).
Number of respective variations within the sample of NPC1 patients are provided, whereby polymorphisms (P) and novel (N) mutations are indicated.
Gene mutations, biochemical phenotype, and lipid profile
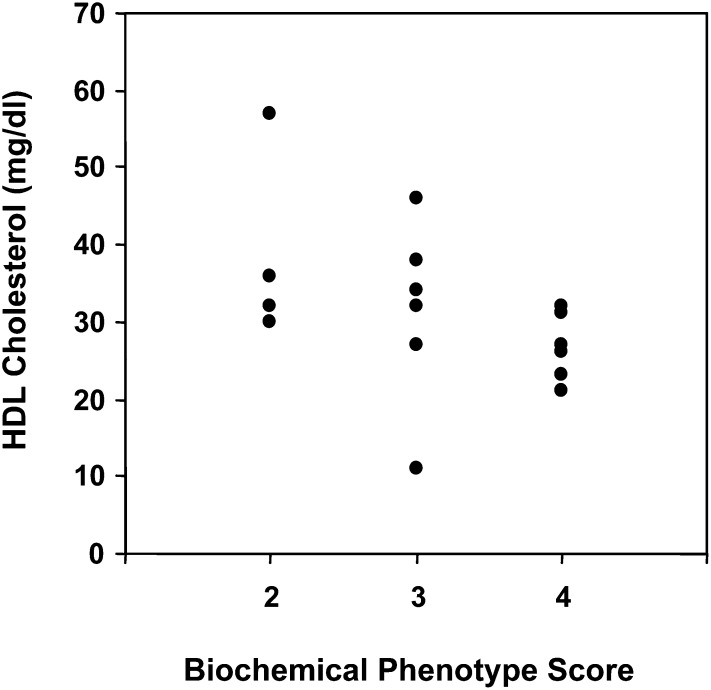
Of the 88 questionnaires received, both NPC1 mutation and lipid profile information were provided for 19 patients (22%), which were then used for independent analysis (Table 5). This sample consisted of 13 males and six females, ranging in age between 1.5 and 45 years and at different stages and severity of disease. The mean concentrations ± SE of total cholesterol (141 ± 6.32 mg/dl, 3.65 ± 0.2 mmol/l), HDL-C (34 ± 3.2 mg/dl, 0.88 ± 0/1 mmol/l), LDL-C (83 ± 6.1 mg/dl, 2.15 ± 0.2 mmol/l), and triglycerides (127 ± 12.5 mg/dl, 1.43 ± 0.1 mmol/l) in this smaller sample of 19 patients were not significantly different from the larger sample of 34 NPC1 patients. For these 19 patients, a biochemical phenotype score was assigned based on the gene mutations and the previously determined biochemical phenotype associated with those mutations. Because the biochemical phenotype scores represented ordinal scale variables, nonparametric correlations were used to determine the existence of associations between the biochemical phenotype and lipid profiles in this sample of patients. Using Spearman's rank correlation coefficient analysis, the results indicated no significant correlation between the biochemical phenotype score and the concentration of plasma total cholesterol, LDL-C, or triglycerides. However, the results indicated a significant inverse association (−0.66, P = 0.005) between the biochemical phenotype score and concentration of HDL-C (Fig. 2), indicating that a more severe biochemical phenotype results in a lower level of plasma HDL cholesterol.
TABLE 5.
Genetic mutations, biochemical phenotype, biochemical phenotype score, and lipid profile for patients with NPC1 disease
| Cholesterol (mg/dl) |
|||||||
|---|---|---|---|---|---|---|---|
| Patient | Nucleotide/Amino Acid Change | Biochemical Phenotypea | Biochemical Phenotype Score | Total | HDL | LDL | Triglycerides (mg/dl) |
| 1 | G660S, 3573_3574insACTT | M, U | 2 | 163 | 36 | 96 | 154 |
| 2 | I1061T, V1165M | C/VB, U | 3 | 122 | 32 | 69 | 105 |
| 3 | R789G, I1061T | S, C/VB | 4 | 204 | 31 | 154 | 98 |
| 4 | C247Y, P401T | S, U | 4 | 131 | 32 | 82 | 83 |
| 5 | G673V, I1061T | U, C/VB | 3 | 119 | 27 | 68 | 121 |
| 6 | P887L, 3741_3744delACTC | U, U | U | 165 | 69 | 76 | 99 |
| 7 | I923V, A1151T | VB, U | U | 111 | 39 | 61 | 61 |
| 8 | P543L, E612D | U, S | 4 | 137 | 27 | 69 | 210 |
| 9 | V950M, N1156S | VT, M | 2 | 133 | 32 | 91 | 87 |
| 10 | V950M, N1156S | VT, M | 2 | 118 | 30 | 77 | 63 |
| 11 | S1249G, 3742_3745delCTCA, | S, U | 4 | 112 | 21 | 67 | 121 |
| 12 | S940L, I1061T | VB, C/VB | 3 | 227 | 11 | 172 | 222 |
| 13 | T1036M, I1061T | S, C/VB | 4 | 141 | 21 | 95 | 127 |
| 14 | T1036M, I1061T | S, C/VB | 4 | 139 | 23 | 83 | 166 |
| 15 | T1036M, I1061T | S, C/VB | 4 | 158 | 26 | 92 | 202 |
| 16 | P1007A, IVS21(-10)delTCC | M/VT, U | 2 | 143 | 57 | 75 | 56 |
| 17 | S230_V231del, 974_975insGA | C, U | 3 | 146 | 34 | 90 | 112 |
| 18 | I1061T, I1061T | C/VB, C/VB | 3 | 131 | 46 | 46 | 196 |
| 19 | P166S, P474L | U, C/VT | 3 | 134 | 38 | 76 | 99 |
Abbreviations for the biochemical phenotype are defined as follows: U = unknown, M = moderate, VB = variable, VT = variant, C = classical, S = severe as previously described (30). The biochemical phenotype score was calculated as described in the Methods, and defined as: U = unknown, 2 = moderate, 3 = classical, 4 = severe.
Fig. 2.
Association between the concentration of HDL cholesterol and severity of the NPC1 biochemical phenotype. The concentration of HDL cholesterol and biochemical phenotype score from 19 NPC1 patients, as presented in Table 5, was analyzed using Spearman's rank correlation coefficient analysis. The biochemical phenotype score was calculated as described in the Methods and defined as: 2 = moderate, 3 = classical, 4 = severe. The results indicated a significant inverse correlation (−0.66, P = 0.005) between the concentration of HDL cholesterol and biochemical phenotype score.
DISCUSSION
The National Niemann-Pick type C1 Disease Database was established to investigate the clinical phenotype of this disease in relation to known and novel mutations of the NPC1 gene, the biochemical phenotype of cultured fibroblasts determined by measuring ACAT-mediated esterification of LDL-derived cholesterol, and the plasma lipid profile obtained from NPC1 patients. For the 88 patients who participated in this study, the current results indicate i) a significantly decreased concentration of LDL-C for the majority of patient groups, ii) a significantly increased concentration of triglycerides for the majority of patient groups, iii) a significantly decreased concentration of HDL-C for all patient groups that was independent of changes in plasma triglyceride levels, iv) a significant inverse correlation between the concentration of plasma HDL-C and severity of the biochemical phenotype, and between HDL-C and age of death among patients, and v) five previously unreported disease-causing NPC1 mutations. These findings indicate previously unrecognized and widespread changes in the lipid profile components in NPC1 patients compared with normal controls, and provide novel evidence that plasma HDL-C levels represent a potential biomarker of the biochemical severity of NPC1 disease.
Although the lipoprotein profiles of NPC disease patients have previously been reported to be normal (1), one study did report a reduction in plasma total cholesterol in a small group of male NPC subjects but did not report the distribution of cholesterol among lipoprotein fractions (43). Our previous study found a preponderance of low HDL-C in 81% of a group of 21 male and female NPC1 disease patients of ages ranging from 3 to 42 (36). In the current study, the most consistent lipoprotein abnormality in male and female NPC1 patients of varying ages was again low plasma HDL-C. Plasma HDL-C in these individuals did not correlate significantly with plasma triglycerides, suggesting the reduction in HDL-C is not on the basis of replacement of cholesterol by triglyceride on HDL particles, or more rapid clearance of HDL particles from plasma as is reported to occur with triglyceride-enriched HDL (44). Consistent with this conclusion, Shamburek et al. (43) also reported no increased rate of appearance of HDL-derived cholesterol in bile in NPC disease subjects. We previously found impaired upregulation of ABCA1, the rate-limiting step in new HDL particle formation, in response to loading of human NPC1 disease fibroblasts with either LDL or nonlipoprotein cholesterol (36), and that HDL formation by NPC1 disease cells could be corrected by the addition of exogenous agonists of liver X receptor to increase ABCA1 expression (45). These findings, plus the demonstrated close relationship between cellular ABCA1 activity and plasma HDL-C (46), strongly suggest that the reduced HDL-C in NPC1 patients is a consequence of impaired ABCA1 upregulation in response to lipoprotein cholesterol uptake by cells with impaired NPC1 activity. Patients with another lysosomal cholesterol storage disorder, cholesteryl ester storage disease, caused by mutations in lysosomal acid lipase, have also been shown to exhibit low plasma HDL-C (46). Consistent with impaired ABCA1 activation as a cause of low HDL-C in NPC1 disease, we have also found impaired upregulation of ABCA1 in response to LDL loading of human cholesteryl ester storage disease fibroblasts (G. Francis, unpublished observations). The direct inverse correlation found between plasma HDL-C and severity of the NPC1 mutation and biochemical phenotype in 19 patients in this study for which mutation, biochemical phenotype, and lipid profiles were all available (Table 5 and Fig. 2), and between HDL-C and age at death in a small subset of patients, further supports the conclusion that plasma HDL-C is a reflection of the degree of biochemical defect in NPC1 disease and may be useful as a biomarker of the disease as well as response to potential treatments.
Other lipoprotein abnormalities including reduced plasma LDL-C and increased triglycerides were seen in the majority but not all NPC1 disease patients who provided lipid profiles for the database. The cause of reduced LDL (and total) cholesterol is presently unknown but could be explained by increased LDL receptor expression (48, 49) and increased clearance of plasma LDL particles. Increased expression of SREBP-1 (50, 51) and SREBP-1-dependent upregulation of fatty acid synthase in the liver might in part explain the increased plasma triglycerides seen in the majority of NPC1 disease patients in this study. Further studies are required to resolve these questions. The possibility that illness alone is responsible for the lipoprotein abnormalities in NPC1 disease is unlikely, given that the findings were consistent across a range of NPC1 patients of varying degree of disease and illness.
Since identification of the NPC1 gene, several studies have reported specific disease-causing mutations among different populations and ethnic groups (27, 29, 31, 33, 40, 52, 53). In the present study, the 52 patients that provided gene mutations ranged in age between 1.5 and 48.5 years and were at different stages and severity of disease. Of the 101 total mutations reported, 52 were found to be different, whereas the remaining 49 were similar and shared among patients. As each patient would be expected to provide at least two mutations for a total of 104, three of the patients were only able to provide one mutation, thereby suggesting that three patients had mutations that may have existed outside the NPC1 gene coding region. Importantly, five novel NPC1 mutations (Y628C, P887L, I923V, A1151T, and 3741_3744delACTC) were identified that have not been reported previously. This relatively large and diverse sample of patients exhibited 101 mutations distributed throughout the entire length of the NPC1 gene, including most of the domains and structural motifs previously described for the NPC1 protein (54). Specifically, 15 mutations were found to reside within the first large luminal loop A, including one (P166S) contained within the N-terminal helical subdomain (amino acids 162–200) recently found to be required for transfer of cholesterol from NPC2 to NPC1 (15). Twelve mutations were found within the second large luminal loop C, and 11 within the sterol-sensing domain (amino acids 616–797) consisting of five transmembrane domains III-VII), two small luminal loops E and G, and three cytoplasmic loops D, F, and H). An additional 48 mutations were in the third large luminal loop I containing the NPC1-specific cysteine-rich region (amino acids 855–1098), along with an apparent “mutation hot spot” (amino acids 927–958), followed distally by the initial portion of a region sharing extensive homology with the Patched protein (amino acids 1038–1253). The remaining 13 mutations were found within transmembrane domains and both luminal and cytoplasmic loops located near the carboxy-terminus of the NPC1 protein. The most highly represented mutation for patients in the present study was the relatively common I1061T missense mutation, known to be a particularly frequent mutation among NPC1 patients of Western European decent (34). In contrast, other frequent mutations (P474L, G992W, and R518Q) commonly associated with NPC1 patients of Italian, Nova Scotian, or Japanese decent, respectively, were either represented once or absent from this sample of patients.
In summary, the current report of the National Niemann-Pick Type C1 Disease Database found i) a significantly decreased concentration of plasma LDL cholesterol and increased plasma triglycerides in the majority of NPC1 disease patients, ii) a significantly decreased concentration of HDL cholesterol for all patient groups that was not associated with the concentration of plasma triglycerides, iii) a significant inverse association between the concentration of HDL cholesterol and biochemical phenotype, and HDL cholesterol and age of death for NPC1 patients, and iv) five previously unreported disease-causing mutations in NPC1. These results indicate the presence of a unique NPC1-related dyslipidemia, characterized particularly by hypoalphalipoproteinemia, that may serve as a valuable and convenient biomarker of the biochemical severity of the disease, in addition to monitoring the potential benefit of therapies used for the eventual treatment of NPC disease.
Acknowledgments
The authors thank the Ara Parseghian Medical Research Foundation, the National Niemann-Pick Disease Foundation, and most importantly, the families of individuals with NPC1 disease for their generosity and willingness to provide the necessary information for establishment of the National Niemann-Pick Type C1 Disease Database.
Footnotes
Abbreviations:
- APMRF
- Ara Parseghian Medical Research Foundation
- HDL-C
- HDL cholesterol
- LDL-C
- LDL cholesterol
- NPC1
- Niemann-Pick type C1 disease
- SREBP
- sterol regulatory element-binding protein
This work was supported in part by an investigator award from the Ara Parseghian Medical Research Foundation (to W.S.G.), National Institutes of Health Grant R21-DK071544-01 (to W.S.G.), and Canadian Institutes of Health Research Grant MOP-79532 (to G.A.F.). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Patterson M. C., Vanier M. T., Suzuki K., Morris J. E., Carstea E. D., Neufeld E. B., Blanchette-Mackie E. J., Pentchev P. G. 2001. Niemann-Pick Disease Type C: a lipid trafficking disorder. The Metabolic and Molecular Bases of Inherited Disease. 8th edition C. R. Beaudet A. L., Sly W. S., Valle D., McGraw-Hill, New York: 3611–3633. [Google Scholar]
- 2.Vanier M. T., Wenger D. A., Comly M. E., Rousson R., Brady R. O., Pentchev P. G. 1988. Niemann-Pick disease group C: clinical variability and diagnosis based on defective cholesterol esterification. A collaborative study on 70 patients. Clin. Genet. 33: 331–348. [DOI] [PubMed] [Google Scholar]
- 3.Vanier M. T., Rodriguez-Lafrasse C., Rousson R., Gazzah N., Juge M. C., Pentchev P. G., Revol A., Louisot P. 1991. Type C Niemann-Pick disease: spectrum of phenotypic variation in disruption of intracellular LDL-derived cholesterol processing. Biochim. Biophys. Acta. 1096: 328–337. [DOI] [PubMed] [Google Scholar]
- 4.Vanier M. T., Rodriguez-Lafrasse C., Rousson R., Duthel S., Harzer K., Pentchev P. G., Revol A., Louisot P. 1991. Type C Niemann-Pick disease: biochemical aspects and phenotypic heterogeneity. Dev. Neurosci. 13: 307–314. [DOI] [PubMed] [Google Scholar]
- 5.Fink J. K., Filling-Katz M. R., Sokol J., Cogan D. G., Pikus A., Sonies B., Soong B., Pentchev P. G., Comly M. E., Brady R. O., et al. 1989. Clinical spectrum of Niemann-Pick disease type C. Neurology. 39: 1040–1049. [DOI] [PubMed] [Google Scholar]
- 6.Omura K., Suzuki Y., Norose N., Sato M., Maruyama K., Koeda T. 1989. Type C Niemann-Pick disease: clinical and biochemical studies on 6 cases. Brain Dev. 11: 57–61. [DOI] [PubMed] [Google Scholar]
- 7.Carstea E. D., Polymeropoulos M. H., Parker C. C., Detera-Wadleigh S. D., O'Neill R. R., Patterson M. C., Goldin E., Xiao H., Straub R. E., Vanier M. T., et al. 1993. Linkage of Niemann-Pick disease type C to human chromosome 18. Proc. Natl. Acad. Sci. USA. 90: 2002–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carstea E. D., Morris J. A., Coleman K. G., Loftus S. K., Zhang D., Cummings C., Gu J., Rosenfeld M. A., Pavan W. J., Krizman D. B., et al. 1997. Niemann-Pick C1 disease gene: homology to mediators of cholesterol homeostasis. Science. 277: 228–231. [DOI] [PubMed] [Google Scholar]
- 9.Watari H., Blanchette-Mackie E. J., Dwyer N. K., Glick J. M., Patel S., Neufeld E. B., Brady R. O., Pentchev P. G., Strauss J. F., 3rd 1999. Niemann-Pick C1 protein: obligatory roles for N-terminal domains and lysosomal targeting in cholesterol mobilization. Proc. Natl. Acad. Sci. USA. 96: 805–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watari H., Blanchette-Mackie E. J., Dwyer N. K., Watari M., Neufeld E. B., Patel S., Pentchev P. G., Strauss J. F., 3rd 1999. Mutations in the leucine zipper motif and sterol-sensing domain inactivate the Niemann-Pick C1 glycoprotein. J. Biol. Chem. 274: 21861–21866. [DOI] [PubMed] [Google Scholar]
- 11.Watari H., Blanchette-Mackie E. J., Dwyer N. K., Watari M., Burd C. G., Patel S., Pentchev P. G., Strauss J. F., 3rd 2000. Determinants of NPC1 expression and action: key promoter regions, posttranscriptional control, and the importance of a “cysteine-rich” loop. Exp. Cell Res. 259: 247–256. [DOI] [PubMed] [Google Scholar]
- 12.Ohgami N., Ko D. C., Thomas M., Scott M. P., Chang C. C., Chang T. Y. 2004. Binding between the Niemann-Pick C1 protein and a photoactivatable cholesterol analog requires a functional sterol-sensing domain. Proc. Natl. Acad. Sci. USA. 101: 12473–12478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu R., Lu P., Chu J. W., Sharom F. J. 2009. Characterization of fluorescent sterol binding to purified human NPC1. J. Biol. Chem. 284: 1840–1852. [DOI] [PubMed] [Google Scholar]
- 14.Infante R. E., Radhakrishnan A., Abi-Mosleh L., Kinch L. N., Wang M. L., Grishin N. V., Goldstein J. L., Brown M. S. 2008. Purified NPC1 protein: II. Localization of sterol binding to a 240-amino acid soluble luminal loop. J. Biol. Chem. 283: 1064–1075. [DOI] [PubMed] [Google Scholar]
- 15.Kwon H. J., Abi-Mosleh L., Wang M. L., Deisenhofer J., Goldstein J. L., Brown M. S., Infante R. E. 2009. Structure of N-terminal domain of NPC1 reveals distinct subdomains for binding and transfer of cholesterol. Cell. 137: 1213–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zervas M., Somers K. L., Thrall M. A., Walkley S. U. 2001. Critical role for glycosphingolipids in Niemann-Pick disease type C. Curr. Biol. 11: 1283–1287. [DOI] [PubMed] [Google Scholar]
- 17.te Vruchte D., Lloyd-Evans E., Veldman R. J., Neville D. C., Dwek R. A., Platt F. M., van Blitterswijk W. J., Sillence D. J. 2004. Accumulation of glycosphingolipids in Niemann-Pick C disease disrupts endosomal transport. J. Biol. Chem. 279: 26167–26175. [DOI] [PubMed] [Google Scholar]
- 18.Pentchev P. G., Comly M. E., Kruth H. S., Patel S., Proestel M., Weintroub H. 1986. The cholesterol storage disorder of the mutant BALB/c mouse. A primary genetic lesion closely linked to defective esterification of exogenously derived cholesterol and its relationship to human type C Niemann-Pick disease. J. Biol. Chem. 261: 2772–2777. [PubMed] [Google Scholar]
- 19.Liscum L., Ruggiero R. M., Faust J. R. 1989. The intracellular transport of low density lipoprotein-derived cholesterol is defective in Niemann-Pick type C fibroblasts. J. Cell Biol. 108: 1625–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blanchette-Mackie E. J., Dwyer N. K., Amende L. M., Kruth H. S., Butler J. D., Sokol J., Comly M. E., Vanier M. T., August J. T., Brady R. O., et al. 1988. Type-C Niemann-Pick disease: low density lipoprotein uptake is associated with premature cholesterol accumulation in the Golgi complex and excessive cholesterol storage in lysosomes. Proc. Natl. Acad. Sci. USA. 85: 8022–8026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garver W. S., Krishnan K., Gallagos J. R., Michikawa M., Francis G. A., Heidenreich R. A. 2002. Niemann-Pick C1 protein regulates cholesterol transport to the trans-Golgi network and plasma membrane caveolae. J. Lipid Res. 43: 579–589. [PubMed] [Google Scholar]
- 22.Puri V., Watanabe R., Dominguez M., Sun X., Wheatley C. L., Marks D. L., Pagano R. E. 1999. Cholesterol modulates membrane traffic along the endocytic pathway in sphingolipid-storage diseases. Nat. Cell Biol. 1: 386–388. [DOI] [PubMed] [Google Scholar]
- 23.Sagiv Y., Hudspeth K., Mattner J., Schrantz N., Stern R. K., Zhou D., Savage P. B., Teyton L., Bendelac A. 2006. Cutting edge: impaired glycosphingolipid trafficking and NKT cell development in mice lacking Niemann-Pick type C1 protein. J. Immunol. 177: 26–30. [DOI] [PubMed] [Google Scholar]
- 24.Neufeld E. B., Wastney M., Patel S., Suresh S., Cooney A. M., Dwyer N. K., Roff C. F., Ohno K., Morris J. A., Carstea E. D., et al. 1999. The Niemann-Pick C1 protein resides in a vesicular compartment linked to retrograde transport of multiple lysosomal cargo. J. Biol. Chem. 274: 9627–9635. [DOI] [PubMed] [Google Scholar]
- 25.Garver W. S., Heidenreich R. A., Erickson R. P., Thomas M. A., Wilson J. M. 2000. Localization of the murine Niemann-Pick C1 protein to two distinct intracellular compartments. J. Lipid Res. 41: 673–687. [PubMed] [Google Scholar]
- 26.Greer W. L., Riddell D. C., Murty S., Gillan T. L., Girouard G. S., Sparrow S. M., Tatlidil C., Dobson M. J., Neumann P. E. 1999. Linkage disequilibrium mapping of the Nova Scotia variant of Niemann-Pick disease. Clin. Genet. 55: 248–255. [DOI] [PubMed] [Google Scholar]
- 27.Greer W. L., Dobson M. J., Girouard G. S., Byers D. M., Riddell D. C., Neumann P. E. 1999. Mutations in NPC1 highlight a conserved NPC1-specific cysteine-rich domain. Am. J. Hum. Genet. 65: 1252–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meiner V., Shpitzen S., Mandel H., Klar A., Ben-Neriah Z., Zlotogora J., Sagi M., Lossos A., Bargal R., Sury V., et al. 2001. Clinical-biochemical correlation in molecularly characterized patients with Niemann-Pick type C. Genet. Med. 3: 343–348. [DOI] [PubMed] [Google Scholar]
- 29.Tarugi P., Ballarini G., Bembi B., Battisti C., Palmeri S., Panzani F., Di Leo E., Martini C., Federico A., Calandra S. 2002. Niemann-Pick type C disease: mutations of NPC1 gene and evidence of abnormal expression of some mutant alleles in fibroblasts. J. Lipid Res. 43: 1908–1919. [DOI] [PubMed] [Google Scholar]
- 30.Niemann-Pick Type C Disease Gene Variation Database. Available at http://npc.fzk.de/index.php?section=about.
- 31.Park W. D., O'Brien J. F., Lundquist P. A., Kraft D. L., Vockley C. W., Karnes P. S., Patterson M. C., Snow K. 2003. Identification of 58 novel mutations in Niemann-Pick disease type C: correlation with biochemical phenotype and importance of PTC1-like domains in NPC1. Hum. Mutat. 22: 313–325. [DOI] [PubMed] [Google Scholar]
- 32.Scott C., Ioannou Y. A. 2004. The NPC1 protein: structure implies function. Biochim. Biophys. Acta. 1685: 8–13. [DOI] [PubMed] [Google Scholar]
- 33.Fernandez-Valero E. M., Ballart A., Iturriaga C., Lluch M., Macias J., Vanier M. T., Pineda M., Coll M. J. 2005. Identification of 25 new mutations in 40 unrelated Spanish Niemann-Pick type C patients: genotype-phenotype correlations. Clin. Genet. 68: 245–254. [DOI] [PubMed] [Google Scholar]
- 34.Millat G., Marcais C., Rafi M. A., Yamamoto T., Morris J. A., Pentchev P. G., Ohno K., Wenger D. A., Vanier M. T. 1999. Niemann-Pick C1 disease: the I1061T substitution is a frequent mutant allele in patients of Western European descent and correlates with a classic juvenile phenotype. Am. J. Hum. Genet. 65: 1321–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamamoto T., Ninomiya H., Matsumoto M., Ohta Y., Nanba E., Tsutsumi Y., Yamakawa K., Millat G., Vanier M. T., Pentchev P. G., et al. 2000. Genotype-phenotype relationship of Niemann-Pick disease type C: a possible correlation between clinical onset and levels of NPC1 protein in isolated skin fibroblasts. J. Med. Genet. 37: 707–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi H. Y., Karten B., Chan T., Vance J. E., Greer W. L., Heidenreich R. A., Garver W. S., Francis G. A. 2003. Impaired ABCA1-dependent lipid efflux and hypoalphalipoproteinemia in human Niemann-Pick type C disease. J. Biol. Chem. 278: 32569–32577. [DOI] [PubMed] [Google Scholar]
- 37.Imrie J., Dasgupta S., Besley G. T., Harris C., Heptinstall L., Knight S., Vanier M. T., Fensom A. H., Ward C., Jacklin E., et al. 2007. The natural history of Niemann-Pick disease type C in the UK. J. Inherit. Metab. Dis. 30: 51–59. [DOI] [PubMed] [Google Scholar]
- 38.Garver W. S., Francis G. A., Jelinek D., Shepherd G., Flynn J., Castro G., Walsh Vockley C., Coppock D. L., Pettit K. M., Heidenreich R. A., et al. 2007. The National Niemann-Pick C1 disease database: report of clinical features and health problems. Am. J. Med. Genet. A. 143A: 1204–1211. [DOI] [PubMed] [Google Scholar]
- 39.Lipid Metabolism Branch, National Heart, Lung, and Blood Institute, National Institutes of Health. 1980. The Lipid Research Clinics Population Studies Data Book, The prevalence study, NIC Publication No. 79–1527 ed US Department of Health and Human Services, Public Health Service, Bethesda, MD. [Google Scholar]
- 40.Millat G., Marcais C., Tomasetto C., Chikh K., Fensom A. H., Harzer K., Wenger D. A., Ohno K., Vanier M. T. 2001. Niemann-Pick C1 disease: correlations between NPC1 mutations, levels of NPC1 protein, and phenotypes emphasize the functional significance of the putative sterol-sensing domain and of the cysteine-rich luminal loop. Am. J. Hum. Genet. 68: 1373–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bauer P., Knoblich R., Bauer C., Finckh U., Hufen A., Kropp J., Braun S., Kustermann-Kuhn B., Schmidt D., Harzer K., et al. 2002. NPC1: complete genomic sequence, mutation analysis, and characterization of haplotypes. Hum. Mutat. 19: 30–38. [DOI] [PubMed] [Google Scholar]
- 42.Grundy S. M., Brewer H. B., Jr., Cleeman J. I., Smith S. C., Jr., Lenfant C. 2004. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 109: 433–438. [DOI] [PubMed] [Google Scholar]
- 43.Shamburek R. D., Pentchev P. G., Zech L. A., Blanchette-Mackie J., Carstea E. D., VandenBroek J. M., Cooper P. S., Neufeld E. B., Phair R. D., Brewer H. B., Jr, et al. 1997. Intracellular trafficking of the free cholesterol derived from LDL cholesteryl ester is defective in vivo in Niemann-Pick C disease: insights on normal metabolism of HDL and LDL gained from the NP-C mutation. J. Lipid Res. 38: 2422–2435. [PubMed] [Google Scholar]
- 44.Lamarche B., Uffelman K. D., Carpentier A., Cohn J. S., Steiner G., Barrett P. H., Lewis G. F. 1999. Triglyceride enrichment of HDL enhances in vivo metabolic clearance of HDL apo A-I in healthy men. J. Clin. Invest. 103: 1191–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boadu E., Choi H. Y., Lee D. W., Waddington E. I., Chan T., Asztalos B., Vance J. E., Chan A., Castro G., Francis G. A. 2006. Correction of apolipoprotein A-I-mediated lipid efflux and high density lipoprotein particle formation in human Niemann-Pick type C disease fibroblasts. J. Biol. Chem. 281: 37081–37090. [DOI] [PubMed] [Google Scholar]
- 46.Clee S. M., Kastelein J. J., van Dam M., Marcil M., Roomp K., Zwarts K. Y., Collins J. A., Roelants R., Tamasawa N., Stulc T., et al. 2000. Age and residual cholesterol efflux affect HDL cholesterol levels and coronary artery disease in ABCA1 heterozygotes. J. Clin. Invest. 106: 1263–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Assmann G., Seedorf U. 2001. Acid lipase deficiency: Wolman disease and cholesteryl ester storage disease. The Metabolic and Molecular Bases of Inherited Disease. 8th edition Scriver C. R., Beaudet A. L., Sly W. S., Valle D., McGraw Hill, New York: 3551–3572. [Google Scholar]
- 48.Pentchev P. G., Kruth H. S., Comly M. E., Butler J. D., Vanier M. T., Wenger D. A., Patel S. 1986. Type C Niemann-Pick disease. A parallel loss of regulatory responses in both the uptake and esterification of low density lipoprotein-derived cholesterol in cultured fibroblasts. J. Biol. Chem. 261: 16775–16780. [PubMed] [Google Scholar]
- 49.Liscum L., Faust J. R. 1987. Low density lipoprotein (LDL)-mediated suppression of cholesterol synthesis and LDL uptake is defective in Niemann-Pick type C fibroblasts. J. Biol. Chem. 262: 17002–17008. [PubMed] [Google Scholar]
- 50.Garver W. S., Jelinek D., Oyarzo J. N., Flynn J., Zuckerman M., Krishnan K., Chung B. H., Heidenreich R. A. 2007. Characterization of liver disease and lipid metabolism in the Niemann-Pick C1 mouse. J. Cell. Biochem. 101: 498–516. [DOI] [PubMed] [Google Scholar]
- 51.Kulinski A., Vance J. E. 2007. Lipid homeostasis and lipoprotein secretion in Niemann-Pick C1-deficient hepatocytes. J. Biol. Chem. 282: 1627–1637. [DOI] [PubMed] [Google Scholar]
- 52.Sun X., Marks D. L., Park W. D., Wheatley C. L., Puri V., O'Brien J. F., Kraft D. L., Lundquist P. A., Patterson M. C., Pagano R. E., et al. 2001. Niemann-Pick C variant detection by altered sphingolipid trafficking and correlation with mutations within a specific domain of NPC1. Am. J. Hum. Genet. 68: 1361–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang C. C., Su Y. N., Chiou P. C., Fietz M. J., Yu C. L., Hwu W. L., Lee M. J. 2005. Six novel NPC1 mutations in Chinese patients with Niemann-Pick disease type C. J. Neurol. Neurosurg. Psychiatry. 76: 592–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Davies J. P., Ioannou Y. A. 2000. Topological analysis of Niemann-Pick C1 protein reveals that the membrane orientation of the putative sterol-sensing domain is identical to those of 3-hydroxy-3-methylglutaryl-CoA reductase and sterol regulatory element binding protein cleavage-activating protein. J. Biol. Chem. 275: 24367–24374. [DOI] [PubMed] [Google Scholar]