Abstract
We investigated the effects of single nucleotide polymorphisms (SNPs) of the hepatic lipase gene (LIPC) on plasma HDL-cholesterol (HDL-C) levels in Turks, a population with low levels of HDL-C. All exons and six evolutionarily conserved regions from 28 Turkish subjects were sequenced. We found 51 SNPs, nine of which were novel. Those 51 SNPs and SNPs from the National Center for Biotechnology Information dbSNP were evaluated by bioinformatics approaches. The population frequencies and linkage disequilibrium among SNPs from HapMap were combined with results from transcriptional factor prediction tools and the literature to select SNPs for genotyping. We found that five tagging LIPC SNPs, two reported here for the first time, were significantly associated with plasma HDL-C levels in both men and women (n = 2,612). These results were replicated in a separate Turkish cohort (n = 1,164). Plasma HDL-C levels were higher in subjects homozygous for the minor alleles of rs4775041, rs1800588 (–514C>T), and rs11858164 and lower in subjects homozygous for the minor alleles of rs11856322 and rs2242061. These SNPs seemed to have independent and additive effects on plasma HDL-C levels (1.5–5.2 mg/dl). Hepatic lipase activity in a subset (n = 260) of the main cohort was also significantly associated with all five SNPs. Thus, five LIPC SNPs, two novel, are associated with plasma HDL-C levels and hepatic lipase activity in two cohorts of Turkish subjects.
Keywords: single nucleotide polymorphisms, lipoprotein metabolism, heart disease risk
Low levels of plasma HDL-cholesterol (HDL-C) are an independent risk factor for coronary heart disease (1, 2), the leading cause of death worldwide (3). Plasma HDL-C levels are strongly influenced by genetics, as demonstrated in extensive epidemiological, family, and association studies. Heritability estimates of total HDL-C have been reported as 0.20–0.69 (4–6). Recently, a genome scan for atherogenic dyslipidemia by the multinational Genetic Epidemiology of Metabolic Syndrome project (7) found significant linkage to plasma HDL-C levels on chromosome 15q22-23 in Turkish families; the reported heritability estimate for this trait in these families was 0.80 (6).
The hepatic lipase gene (LIPC) is located on chromosome 15q22, suggesting that variation(s) within this gene might be responsible for or contribute to the linkage peak in Turkish families. Hepatic lipase is primarily synthesized by hepatocytes and is at the surface of liver sinusoids. It has both triglyceride lipase and phospholipase activities and is involved at different steps of lipoprotein metabolism (8). High hepatic lipase activity is associated with low plasma HDL-C levels (8). Several single nucleotide polymorphisms (SNPs) in LIPC showed significant associations with plasma HDL-C (9–14) and hepatic lipase activity (12, 15–17). The most frequently examined, –514C>T (rs1800588), is located in the promoter region and is in perfect linkage disequilibrium (LD) with the SNPs –763A>G (rs1077835), –710C>T (rs1077834), and –250G>A (rs2070895) (9), which are also associated with plasma HDL-C levels (9). Other linkage studies also suggested the importance of the LIPC locus on chromosome 15q22 in determining HDL-C levels (9, 18, 19).
Recent genome-wide association (GWA) studies (20–22) showed the importance of variants at the LIPC locus; five SNPs, rs4775041, rs261332, rs10468017 (20), rs1800588 (21), and rs11858164 (22), were associated with plasma HDL-C levels. The rs4775041 variant was in strong LD with rs10468017 and rs1800588 (–514C>T) was in strong LD with rs261332, but rs11858164 was not in LD with any of them. Thus, three unlinked tagging-SNPs in the LIPC locus were strongly associated with plasma HDL-C levels (20–22). Although hundreds of thousands of SNPs can be examined simultaneously in GWA studies, significant SNPs might be markers for known or unknown functional SNPs (23).
Turks, whether living in Turkey or abroad, have very low plasma HDL-C levels (24–28) and 25–30% higher hepatic lipase activity and mass (28, 29). Plasma HDL-C levels and hepatic lipase activity association with the promoter variant, –514C>T (rs1800588), have been reported in the Turkish population (14, 29). In this study, we investigated in detail the association between LIPC SNPs and plasma HDL-C levels in over 3,750 participants in two separate cohorts in the Turkish Heart Study (THS), a large, cross-sectional epidemiological survey of the Turkish population (24, 25). All exons and six evolutionarily conserved regions of LIPC were sequenced to detect polymorphisms. There are more than 1,000 SNPs (dbSNP 128) in the LIPC locus. To assess and choose the SNPs for genotyping, HapMap and other resources were used for frequencies and LD among SNPs, and the results were combined with those from comparative genomic resources and transcriptional factor prediction tools.
MATERIALS AND METHODS
Study population and biochemical analyses
Study samples with complete biodata were randomly selected from THS participants. The first cohort (n = 2,612) included subjects whose samples were collected between 1990 and 1995 (24). The second cohort (n = 1,164) included subjects whose samples were collected between 2000 and 2003 (25) and were used mainly to verify results obtained with the first cohort. Detailed biodata and blood samples were collected for each subject after an overnight fast. Plasma lipids were measured as described (24). The protocols were approved by the Committee on Human Research of the University of California, San Francisco, and were in accordance with the Helsinki Declaration. Informed consent was obtained. Subjects who were taking lipid-lowering medication, had a history of diabetes mellitus, or had a plasma triglyceride level >800 mg/dl were excluded.
Detection and selection of LIPC polymorphisms
Primers (supplementary Table I) were designed to amplify across the LIPC promoter and all exons, including intron/exon splicing boundaries. Six evolutionarily conserved regions (ECRs; two upstream of the gene and four in intron 1) were also selected for sequencing (see below for selection criteria). DNA from 28 subjects (16 with low HDL-C and 12 with high HDL-C levels) was sequenced to identify polymorphisms in LIPC. DNA sequences were aligned and analyzed with Sequencher DNA analysis software (GeneCodes, Ann Arbor, MI).
LIPC is a very large gene and over 1,000 SNPs are found in and around LIPC with different frequencies and validation status (Ensembl BioMart, dbSNP 128). We sequenced about 9–10% of the LIPC locus. To choose and prioritize SNPs in the unsequenced parts of the LIPC locus, we used several bioinformatics tools. First, we used information from HapMap (30), Perlegen (31), and Applied Biosystems SNP Browser 3.5 (32), in which the frequency and LD information among SNPs were available for various populations. Data from other projects in our laboratory show that Turks more closely resemble Europeans than other populations with respect to allele frequency and we used the relevant populations from these sources. Second, because over half of the bases in ECRs in mammals appear to be functional (33), we examined SNPs in those regions with an ECR browser (34) and the UCSC browser for PhastCon conservation scores (35). Third, the transcriptional factor binding prediction tool (TRANSFAC) (36) was used to identify SNPs that may associate with allele-specific transcription factor recruitment. To speed up this process, we developed a web-based database resource, Delta-MATCH, which can determine in silico whether a SNP alters transcription factor binding sites between two sets of alleles. We also used PReMod, which analyzes information from phylogenetically conserved regions and transcription factor binding sites to predict regulatory modules (37). Fourth, possible microRNA binding sites were also evaluated (UCSC genome browser), and all results and data were harmonized to prioritize the selection of regions for sequencing or SNPs for genotyping. SNPs from published reports were also evaluated in detail.
Genotyping
Polymorphisms were genotyped with TaqMan genotyping assays (ABI, Applied Biosystems, Foster City, CA) or by restriction fragment length analysis. Methodological details and primer sequences are available on request. The average genotyping call rate exceeded 97% and the estimated genotyping error rate, determined by analysis of duplicate samples, was about 1%.
Statistics and data analysis
Data were analyzed with SPSS 10.0, PLINK v1.05 (38), Microsoft Access, and Microsoft Excel. Associations between genotypes, lipids, and other parameters were analyzed separately for males and females. Because triglyceride levels were not normally distributed, log-transformed values were used for statistical comparison; untransformed mean values are reported here. Univariate ANOVA was used to calculate adjusted HDL-C levels. Log-transformed triglyceride level, body mass index (BMI), smoking (number of cigarettes/day), alcohol consumption (nondrinkers, 1–5 drinks/week, >5 drinks/week), and age were included as covariates, and genotype score was included as a fixed factor in the model (GLM Univariate, SPSS 10.0). Genotype-lipid association analyses were conducted with an additive model, including covariates, for genotypic effects implemented in PLINK. Corrections for multiple testing were conducted with a permutation test (50,000 permutations) using PLINK. The proportion of variation in plasma HDL-C level from each SNP was estimated from partial regression coefficients (39). Unadjusted mean values and association results with an additive model for genotypic effects, including permutation results, are presented in the supplementary tables. Hardy-Weinberg equilibrium was tested with Haploview 4.1 (40). Mean values and frequencies between males and females were compared with the t-test and chi-square analysis, respectively. P < 0.05 was considered significant.
RESULTS
Population characteristics
The demographic and biochemical characteristics of the 1990–1995 and 2000–2003 cohorts are shown in Table 1. In both groups and in men and women, the plasma HDL-C levels were low and total cholesterol/HDL-C ratios were high.
TABLE 1.
Demographic and biochemical characteristics of THS participants by gender (samples collected between 1990–1995 and 2000–2003)
| 1990–1995 Cohort |
2000–2003 Cohort |
|||||
|---|---|---|---|---|---|---|
| Males(n = 1549) | Females(n = 1063) | P | Males(n = 474) | Females(n = 690) | P | |
| Age (years) | 42.1 ± 13.2 | 42.2 ± 14.9 | NS | 44.1 ± 13.1 | 44.4 ± 13.9 | NS |
| BMI (kg/m2) | 26.1 ± 3.9 | 26.6 ± 5.4 | <0.05 | 28.1 ± 3.8 | 29.9 ± 5.4 | <0.05 |
| HDL-C (mg/dl) | 35.7 ± 7.5 | 41.2 ± 9 | <0.001 | 38.7 ± 8.5 | 47.0 ± 9.4 | <0.001 |
| Total cholesterol (mg/dl) | 183 ± 44 | 183 ± 42 | NS | 182 ± 37 | 184 ± 43 | NS |
| Total cholesterol/HDL-C ratio | 5.8 ± 2.9 | 4.5 ± 1.4 | <0.01 | 5.0 ± 1.6 | 4.0 ± 1.9 | <0.01 |
| LDL-C (mg/dl) | 126 ± 41 | 116 ± 39 | <0.05 | 112 ± 33 | 113 ± 35 | NS |
| Triglycerides (mg/dl) | 153 ± 107 | 110 ± 70 | <0.001 | 155 ± 105 | 118 ± 67 | <0.001 |
| Systolic blood pressure (mm Hg) | 125 ± 23 | 122 ± 21 | NS | 132 ± 20 | 133 ± 22 | NS |
| Diastolic blood pressure (mm Hg) | 82 ± 14 | 81 ± 13 | NS | 84 ± 12 | 85 ± 13 | NS |
| Consumption of alcohol (%)a | 29.8 | 5.6 | <0.001 | 36.7 | 9.6 | <0.001 |
| Cigarette smoking (%)b | 56.6 | 24 | <0.001 | 67.2 | 25.2 | <0.001 |
Values shown are means ± SD or percentages. Means were compared by t-test, and percentages were analyzed by chi-square test. NS, not significant.
One or more drinks per week.
One or more cigarettes per day.
Identification and selection of LIPC polymorphisms
Fifty-one SNPs with rare allele frequencies (<1% to 49%) were identified by sequencing DNA from 28 subjects. Nine of these SNPs were novel. Four additional SNPs were selected with other approaches. Two SNPs (rs2242061 and rs11632627) were selected using Delta-MATCH, a program that uses TRANSFAC database matrices (36) and aims to predict which polymorphisms may modulate transcription factor binding in an allele-specific manner. The rs2242061 (C/T) SNP is predicted to modulate the binding of the vitamin D receptor (VDR) transcription factor (C score = 0. 8594; T score = 0. 8075; VDR threshold score = 0.8590). Similarly, rs11632627 (A/G) is predicted to modulate the binding of the GATA transcription factor (A score = 1.0000; G score = 0. 7263; GATA threshold score = 1.0000). The other two tagging SNPs (rs4775041 and rs11858164) were selected for genotyping because they were significantly associated with plasma HDL-C levels in GWA studies (21, 22). Rs numbers, chromosomal and gene locations, allele frequencies, and nucleotide changes for all 55 SNPs are presented in supplementary Table II.
After examining the rare allele frequencies and LD (D′, r2) among all these SNPs, we selected 35 SNPs for follow-up. LD among the SNP pairs and the frequency data from available sources were also evaluated in the selection process (30–32). The 35 SNPs included all 10 coding sequence variants (synonymous and nonsynonymous), the four SNPs identified from bioinformatics approaches and GWA studies, six SNPs in the promoter (mean rare allele frequency >2%), and 15 SNPs in the evolutionarily conserved, promoter, and intronic regions (mean rare allele frequency >5%).
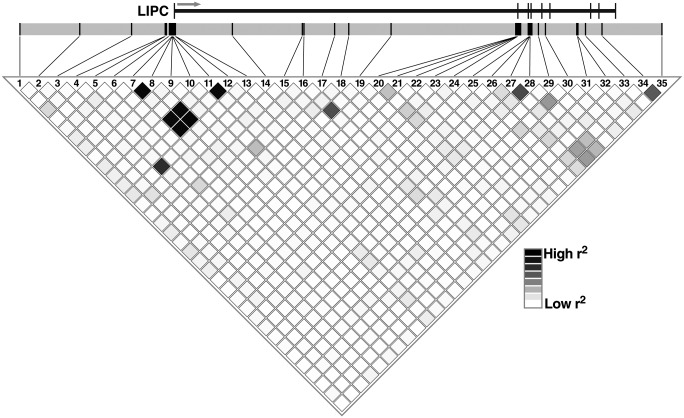
To gain additional insight into the frequencies of rare alleles and LD (D′, r2) among them, we genotyped these 35 SNPs in 260 subjects for whom hepatic lipase activity data (and lipid biodata) were available (supplementary Table III). Analysis of pairwise LD parametrics with Haploview software revealed low LD among those SNPs (Fig. 1). This is mostly because we chose SNPs with low LD to increase the coverage of the LIPC gene structure. The frequencies of the rare alleles of described polymorphisms did not differ between males and females. For all polymorphisms genotyped, the distribution of alleles was consistent with the Hardy-Weinberg equilibrium.
Fig. 1.
Linkage disequilibrium (r2) plot from Haploview for 35 LIPC SNPs in Turks. Darker diamonds represent regions of high pairwise r2; lighter diamonds represent regions of low pairwise r2. The exons on LIPC are shown as tick marks in scale. The arrow indicates the direction of expression of LIPC.
Evaluation of functional relevance of LIPC SNPs
Nine intronic polymorphisms close to the intron/exon boundaries were identified (supplementary Table II). Examination of mRNAs, expressed sequence tags, and alternative splicing of mRNA from the Swiss Institute of Bioinformatics through the UCSC genome browser suggested that they created no cryptic splice sites. The four synonymous variants, V155V (rs690), G197G (rs6082), T224T (rs6084), and T479T (rs6074), were not predicted to create any cryptic splice sites, either.
Six nonsynonymous variants were found in Turks: V95M (rs6078), P111L (novel), N215S (rs6083), R344Q (novel), L356F (rs3829462), and R444C (novel). To evaluate their possible effects on protein function, we used two web-based tools. SIFT (41) categorized all six variants as tolerated. PolyPhen (42) categorized five as benign and R444C as possibly damaging.
Association of LIPC SNPs with plasma HDL-C levels and hepatic lipase activity in Turks
Data for 260 Turkish subjects and 35 SNPs are summarized for HDL-C level and hepatic lipase activity in males and females separately (supplementary Table III). Mean values and preliminary statistical results along with possible functional relevance were examined for both genders and for both phenotypes, and 21 SNPs were selected for genotyping in the 1990–1995 cohort, including three novel coding variants (P111L, R344Q, and R444C) (supplementary Table III). The results were evaluated after about half the cohort had been genotyped. The rare allele frequencies of these novel coding variants were <%1 (supplementary Table II); data for each subject are presented in supplementary Table IV. The LD (r2) among the SNP pairs was very low (supplementary Table V). We decided not to follow up 10 SNPs, inasmuch as there seemed to be no association with plasma HDL-C levels (supplementary Table VI). The remaining 11 SNPs were genotyped in full in the 1990–1995 cohort (Table 2) and the 2000–2003 cohort (Table 3). We chose these step-wise approaches to reduce the possibility of spurious association results and to reduce genotyping costs and labor. We considered these 11 SNPs as a potentially important final list, and multiple test corrections were based on these 11 SNPs.
TABLE 2.
LIPC SNPs and adjusted plasma HDL-C levels in the 1990–1995 cohort
| AA | AB | BB | P | Permuted P | AA | AB | BB | P | Permuted P | |
|---|---|---|---|---|---|---|---|---|---|---|
| SNP | Males | Females | ||||||||
| rs4775041 | 35.2 ± 6.4 (827) | 36.3 ± 6.6 (591) | 36.9 ± 7.3 (106) | 0.0071 | 0.028 | 41.0 ± 8.4 (557) | 40.8 ± 8.0 (396) | 44.4 ± 8.2 (76) | 0.0022 | 0.012 |
| rs16940212 | 35.4 ± 7.2 (991) | 36.0 ± 8.0 (504) | 34.9 ± 7.7 (49) | NS | NS | 41.6 ± 11.0 (708) | 40.5 ± 9.9 (328) | 42.1 ± 10.1 (25) | NS | NS |
| rs1800588 | 35.3 ± 6.5 (962) | 36.5 ± 6.5 (493) | 36.8 ± 6.7 (66) | 0.0110 | 0.048 | 40.5 ± 8.2 (655) | 41.8 ± 8.2 (329) | 45.7 ± 8.1 (46) | 0.0009 | 0.008 |
| rs11858164 | 34.6 ± 5.7 (412) | 36.0 ± 6.2 (772) | 36.5 ± 6.4 (353) | 0.0041 | 0.019 | 40.9 ± 8.0 (297) | 41.7 ± 8.1 (506) | 43.8 ± 7.9 (252) | 0.0025 | 0.017 |
| rs7182229 | 35.7 ± 7.4 (1049) | 35.7 ± 6.9 (435) | 35.4 ± 7.1 (44) | NS | NS | 41.1 ± 9.2 (721) | 41.5 ± 9.2 (286) | 41.5 ± 8.6 (37) | NS | NS |
| rs11856322 | 36.4 ± 6.5 (916) | 35.7 ± 6.5 (535) | 34.3 ± 6.3 (78) | 0.0021 | 0.011 | 42.5 ± 7.5 (627) | 41.4 ± 7.9 (364) | 40.6 ± 6.2 (53) | 0.0028 | 0.013 |
| rs6076 | 36.1 ± 7.4 (1047) | 35.4 ± 7.0 (436) | 35.7 ± 7.2 (49) | NS | NS | 40.9 ± 9.1 (718) | 41.5 ± 9.0 (282) | 41.3 ± 9.5 (44) | NS | NS |
| rs2242061 | 36.0 ± 6.6 (831) | 35.6 ± 6.1 (588) | 34.1 ± 6.1 (106) | 0.0033 | 0.017 | 42.7 ± 8.4 (550) | 41.3 ± 8.4 (402) | 40.7 ± 8.3 (87) | 0.0037 | 0.020 |
| rs690 | 35.7 ± 7.1 (549) | 35.9 ± 7.2 (758) | 35.3 ± 6.9 (219) | NS | NS | 40.5 ± 9.0 (356) | 41.6 ± 8.8 (500) | 41.1 ± 9.1 (188) | NS | NS |
| rs6083 | 36.1 ± 7.2 (680) | 35.6 ± 7.0 (678) | 35.7 ± 7.4 (171) | NS | NS | 41.3 ± 9.1 (490) | 41.2 ± 9.6 (438) | 41.5 ± 10.1 (121) | NS | NS |
| rs11632627 | 36.2 ± 7.3 (845) | 35.5 ± 7.7 (563) | 36.2 ± 7.8 (104) | NS | NS | 41.4 ± 8.9 (564) | 40.9 ± 9.5 (403) | 41.2 ± 8.4 (61) | NS | NS |
All means ± SD were calculated by ANCOVA using general linear models, and adjusted for log triglyceride, age, BMI, smoking, and alcohol consumption. Total number of subjects: 1549 males, 1063 females. Number of subjects for each group is shown in parentheses. P-values were calculated using an additive genetic model in PLINK. Permutation P-values were calculated from 50,000 random iterations in PLINK. NS, not significant. A, common allele; B, rare allele. Significant SNPs are in bold.
TABLE 3.
LIPC SNPs and adjusted plasma HDL-C levels in the 2000–2003 cohort
| AA | AB | BB | P | Permuted P | AA | AB | BB | P | Permuted P | |
|---|---|---|---|---|---|---|---|---|---|---|
| SNP | Males | Females | ||||||||
| rs4775041 | 36.0 ± 7.4 (260) | 37.1 ± 9.0 (174) | 39.0 ± 6.7 (33) | 0.0041 | 0.018 | 45.1 ± 9.1 (377) | 46.8 ± 8.2 (264) | 48.3 ± 8.9 (40) | 0.0056 | 0.028 |
| rs16940212 | 37.5 ± 8.8 (325) | 38.2 ± 10.2 (131) | 37.8 ± 9.0 (13) | NS | NS | 46.4 ± 10.3 (478) | 47.1 ± 9.2 (189) | 46.8 ± 9.1 (19) | NS | NS |
| rs1800588 | 36.3 ± 7.8 (298) | 38.6 ± 9.1 (152) | 40.6 ± 8.5 (21) | 0.0011 | 0.008 | 44.9 ± 9.2 (435) | 47.1 ± 8.7 (222) | 49.1 ± 7.9 (28) | 0.0016 | 0.010 |
| rs11858164 | 35.9 ± 8.0 (131) | 38.0 ± 9.4 (234) | 39.1 ± 7.1 (105) | 0.0035 | 0.018 | 44.8 ± 9.1 (196) | 47.0 ± 8.6 (341) | 48.1 ± 9.4 (148) | 0.0031 | 0.013 |
| rs7182229 | 38.5 ± 9.4 (333) | 38.2 ± 10.5 (121) | 39.0 ± 8.8 (20) | NS | NS | 47.0 ± 11.6 (478) | 48.2 ± 11.2 (180) | 45.9 ± 11.4 (28) | NS | NS |
| rs11856322 | 37.2 ± 8.6 (295) | 36.7 ± 8.5 (146) | 33.3 ± 9.0 (32) | 0.0024 | 0.012 | 46.2 ± 8.1 (411) | 44.6 ± 8.0 (236) | 43.3 ± 7.7 (28) | 0.0074 | 0.031 |
| rs6076 | 39.1 ± 9.6 (319) | 38.0 ± 9.6 (136) | 40.2 ± 12.7 (15) | NS | NS | 48.0 ± 12.6 (461) | 46.5 ± 11.0 (202) | 46.9 ± 11.3 (22) | NS | NS |
| rs2242061 | 37.4 ± 7.8 (260) | 37.1 ± 8.8 (174) | 33.3 ± 9.1 (40) | 0.0017 | 0.011 | 46.5 ± 9.1 (372) | 45.7 ± 9.5 (250) | 42.2 ± 9.3 (58) | 0.0022 | 0.012 |
| rs690 | 38.1 ± 10.0 (175) | 37.9 ± 9.2 (215) | 39.0 ± 11.1 (80) | NS | NS | 46.4 ± 11.7 (230) | 47.5 ± 12.0 (315) | 45.5 ± 11.3 (123) | NS | NS |
| rs6083 | 38.4 ± 9.8 (195) | 37.9 ± 10.0 (212) | 38.9 ± 8.1 (65) | NS | NS | 47.3 ± 12.0 (276) | 47.2 ± 12.1 (327) | 48.2 ± 11.8 (85) | NS | NS |
| rs11632627 | 38.4 ± 10.1 (261) | 37.2 ± 10.2 (178) | 39.3 ± 10.2 (35) | NS | NS | 47.5 ± 11.5 (375) | 46.8 ± 12.1 (254) | 48.4 ± 13.2 (46) | NS | NS |
All means ± SD were calculated by ANCOVA using general linear models, and adjusted for log triglyceride, age, BMI, smoking, and alcohol consumption. Total number of subjects: 474 males, 690 females. Number of subjects for each group is shown in parentheses. P-values were calculated using an additive genetic model in PLINK. Permutation P-values were calculated from 50,000 random iterations in PLINK. NS, not significant. A, common allele; B, rare allele. Significant SNPs are in bold.
For genotype-lipid association analysis for these 11 SNPs, an additive model with covariates was used, and corrections for multiple testing were conducted with a permutation test implemented in PLINK. Five SNPs (rs4775041, rs1800588, rs11858164, rs11856322, and rs2242061) were associated significantly with plasma HDL-C levels (Tables 2, 3). All five SNPs showed a gene-dose effect in which the mean HDL-C values of heterozygous subjects were roughly halfway between those of subjects with rare and common homozygous genotypes. In the two cohorts and in both genders, the rare alleles of rs4775041, rs1800588 (–514C>T), and rs11858164 were associated with increased plasma HDL-C levels, and the rare alleles of rs11856322 and rs2242061 were associated with decreased plasma HDL-C levels (Tables 2, 3). These SNPs individually explained 0.7–1.1% of the variance in plasma HDL-C levels in the Turkish population. The mean differences in HDL-C levels between the rare and common homozygous genotypes were 1.5–5.2 mg/dl, a range similar to that obtained by GWA (20–22) for the rs1800588, rs4775041, and rs11858164 SNPs. Unadjusted results showed similar significance (supplementary Tables VII and VIII).
All five SNPs were associated with hepatic lipase activity, and the effects were stronger and had lower P-values in males than females (Table 4). The differences in hepatic lipase activity between subjects with rare and common homozygous genotypes were 15–50%. The rare alleles of rs4775041, rs1800588, and rs11858164 were associated with decreased activity, and the rare alleles of rs11856322 and rs2242061 were associated with increased activity. Because hepatic lipase activity is inversely correlated with plasma HDL-C levels in our samples (Spearman correlation, r = 0.362, P < 0.001, n = 260) as in the literature (8), these results are consistent with the association of the five SNPs with plasma HDL-C levels.
TABLE 4.
LIPC SNPs and adjusted hepatic lipase activity (mmol/ml/h ± SD) in the Turkish population
| Hepatic Lipase Activity |
||||||
|---|---|---|---|---|---|---|
| rs# | AA | AB | BB | P | Permuted P | |
| Males | rs4775041 | 52.8 ± 14.6 (64) | 51.2 ± 15.2 (56) | 42.0 ± 12.4 (9) | 0.002 | 0.012 |
| rs1800588 | 54.7 ± 14.3 (87) | 45.5 ± 13.8 (38) | 25.8 ± 10.7 (3) | 0.00002 | 0.0001 | |
| rs11858164 | 57.5 ± 14.1 (41) | 50.6 ± 14.9 (64) | 44.3 ± 15.9 (21) | 0.0014 | 0.007 | |
| rs11856322 | 44.3 ± 13.6 (78) | 47.1 ± 12.4 (46) | 52.8 ± 9.0 (6) | 0.0085 | 0.031 | |
| rs2242061 | 49.4 ± 12.5 (72) | 52.4 ± 13.7 (51) | 58.3 ± 9.6 (7) | 0.0101 | 0.043 | |
| Females | rs4775041 | 37.5 ± 12.9 (54) | 34.8 ± 13.1 (59) | 29.4 ± 9.1 (13) | 0.003 | 0.022 |
| rs1800588 | 36.7 ± 12.0 (94) | 33.2 ± 15.4 (28) | 30.9 ± 6.2 (5) | 0.0202 | 0.081 | |
| rs11858164 | 37.8 ± 13.3 (35) | 35.9 ± 13.4 (58) | 31.4 ± 9.7 (29) | 0.014 | 0.055 | |
| rs11856322 | 32.8 ± 11.1 (81) | 34.6 ± 14.0 (40) | 39.9 ± 6.4 (7) | 0.0155 | 0.069 | |
| rs2242061 | 30.1 ± 11.2 (69) | 33.3 ± 15.5 (49) | 39.5 ± 6.2 (10) | 0.0038 | 0.023 | |
All means ± SD were calculated by ANCOVA using general linear models, and adjusted for log triglyceride, age, BMI, and HDL-C. Total number of subjects: 132 males, 128 females. Number of subjects for each group is shown in parentheses. P values were calculated using an additive genetic model in PLINK. Permutation P values were calculated from 50,000 random iterations in PLINK. A, common allele; B, rare allele.
Combined effect of LIPC SNPs on HDL-C levels in Turkish populations
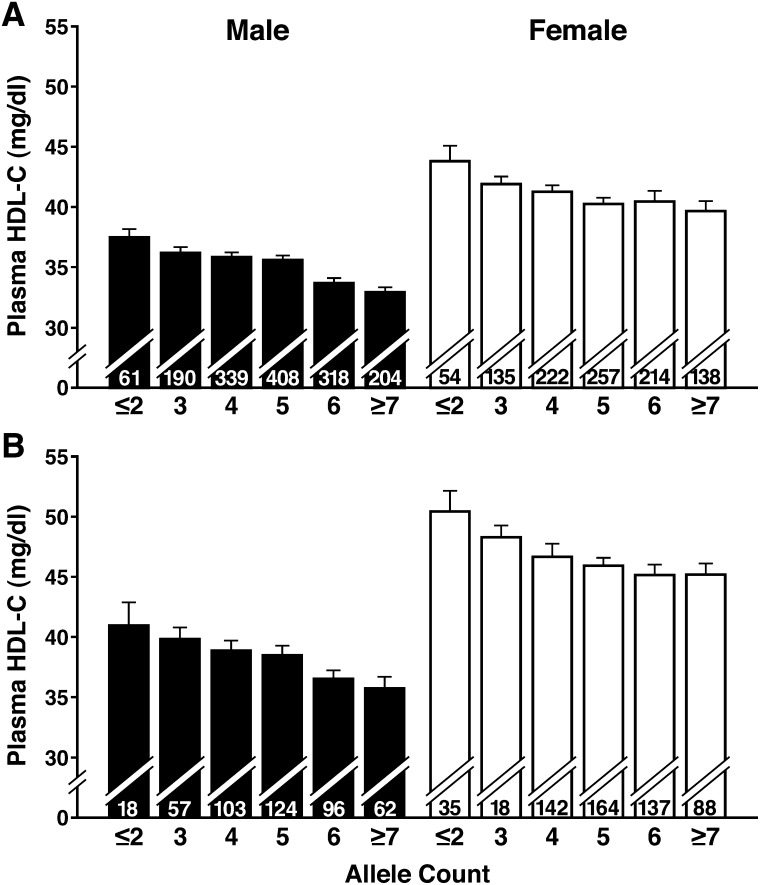
There was generally low LD across the five significant LIPC SNPs spanning the 160 kb between rs4775041 and rs2242061 (Table 5 and supplementary Table V). Because of the low LD and distance among SNPs, it was not reasonable to perform a haplotype analysis. To examine the combined effect of the five SNPs on plasma HDL-C levels, we developed a scoring system based on the number of alleles associated with low HDL-C. Common alleles of rs4775041, rs1800588, and rs11858164 and rare alleles of rs11856322 and rs2242061 were counted for each participant for whom complete genotyping information at these five SNPs was available (0 to 10 alleles). Individuals with ≤2 or ≥7 alleles associated with low HDL-C were grouped. In both men and women, an increased number of such alleles were associated with lower plasma HDL-C levels (Fig. 2). On average, among subjects with ≥7 alleles, HDL-C levels were 4.6 and 5.2 mg/dl lower in males and 4.2 and 5.1 mg/dl lower in females than in those with ≤2 alleles in the 1990–1995 and 2000–2003 cohorts, respectively.
TABLE 5.
Pair-wise linkage disequilibrium coefficients (r2, bottom-left and D′, up-right) between LIPC SNPs in the Turkish population
| r2/D′ | rs4775041 | rs1800588 | rs11858164 | rs11856322 | rs2242061 |
|---|---|---|---|---|---|
| rs4775041 | 0.48 | 0.09 | 0.20 | 0.08 | |
| rs1800588 | 0.05 | 0.03 | 0.01 | 0.31 | |
| rs11858164 | 0.00 | 0.00 | 0.08 | 0.32 | |
| rs11856322 | 0.01 | 0.00 | 0.00 | 0.12 | |
| rs2242061 | 0.00 | 0.01 | 0.02 | 0.01 |
Fig. 2.
Analysis of combined effect of alleles associated with low HDL-C. For each participant in the 1990–1995 cohort (A) and the 2000–2003 cohort (B) with complete genotype for 5 LIPC SNPs, alleles associated with low HDL-C were counted (G of rs4775041, C of rs1800588, G of rs11858164, G of rs11856322, and T of rs2242061). Subjects with ≤2 or ≥7 alleles were grouped. N numbers are indicated inside the columns.
DISCUSSION
This study shows that five common LIPC SNPs are significantly associated with plasma HDL-C levels in the Turkish population. In two cohorts of men and women, the plasma HDL-C levels were higher in subjects homozygous for the minor alleles of the rs4775041, rs1800588 (–514C>T), or rs11858164 SNP and lower in subjects homozygous for the rs11856322 and rs2242061 minor alleles (Tables 2, 3). As expected, these SNP alleles had the opposite associations with hepatic lipase activity (Table 4), which is inversely correlated with plasma HDL-C levels (8). Because there was very low LD among the SNPs and plasma HDL-C levels declined as the number of alleles associated with low HDL-C increased, it is logical to consider that these five SNPs might have independent effects. However, any SNPs in strong LD with them might be functional SNPs or markers for the association we observed. Supplementary Fig. I illustrates the relationship of significant SNP(s) with the other SNPs in the region with respect to r2 values in the HapMap Centre d'Etude du Polymorphisme Humaine from Utah (CEU) population (rs2242061 was not genotyped in HapMap so there are no data for it) and shows the possibility of other SNPs being responsible for the signal that we observed. On the other hand, we could not totally eliminate the possibility that ungenotyped or unknown SNP markers were moderately linked to two or more of those five SNPs and that particular SNPs had an association signal, and therefore, the observed association signals might not be independent.
The –514C>T promoter SNP (rs1800588) has been repeatedly associated with HDL-C levels and hepatic lipase activity, including recent GWA studies and previous studies of Turkish samples (9–14, 20, 21). Functional studies of this SNP have yielded contradictory results. The T allele of –514C>T has significantly lower transcriptional activity than the C allele (43, 44), and there was a differential binding for the upstream stimulatory factor that mediates glucose and lipid metabolism in the liver (44). On the other hand, none of the four SNPs in the promoter region (–514C>T, –763A>G, –710C>T, and –250G>A) contributes to the basal transcription rate of LIPC (45). In a very recent study, a large number of human liver samples were analyzed for whole-genome expression levels and whole-genome DNA variations to characterize the genetic architecture of gene expression in the human liver. Of the SNPs genotyped within 1 megabase of the LIPC locus, rs261332 (strong LD with rs1800588) was most strongly associated with hepatic lipase expression (46). It is unclear whether the –514C>T (rs1800588) SNP is functional or in LD with one or more functional SNPs in the LIPC locus.
The three SNPs (rs1800588, rs4775041, and rs11858164) that were significantly associated with plasma HDL-C levels in the Turkish population have similar effect size in recent GWA studies (1.4–1.8 mg/dl per allele) (20–22). These GWA studies also identified two additional significant SNPs that were not analyzed in this study. These two SNPs are rs261332 (20) (pairwise r2 = 0.92 to rs1800588 in CEU) and rs10468017 (21) (pairwise r2 = 0.81 to rs4775041 in CEU), suggesting they are markers for the other SNPs in the pairs we had already genotyped in the Turkish population. None of those four SNPs (rs4775041, rs11858164, rs261332, and rs10468017) reside in conserved intronic or intergenic regions, and no allele-specific transcriptional factor binding sites are predicted. Although these SNPs may not be functional (but might be markers for functional SNPs), they demonstrate the importance of the LIPC locus for HDL-C variation.
For the first time, in this study, significant results are reported for rs11856322 and rs2242061 (Tables 2, 3). The rs11856322 SNP may modulate the binding of hepatic nuclear factor 4α (HNF4α). Reporter analysis showed that HNF4α directly regulates the expression of the LIPC promoter through two direct repeat elements, DR1 and DR4 (47). According to the TRANSFAC database (36), HNF4α is predicted to bind the DR1 DNA sequence when the LIPC rs11856322 minor A allele is present, but not when the major G allele is present (A score = 0.8957; G score = 0.8116; HNF4α threshold score = 0.8390). We hypothesize that increased expression with the A allele (presumably increased enzymatic activity) is associated with low HDL-C levels, as we observed in subjects homozygous for the rare allele of rs11856322. However, this has to be experimentally proven for the rs11856322 SNP, located in intron 1. Similarly, the rs2242061 minor C allele is predicted to bind to the VDR transcription factor with higher affinity than the major A allele (C score = 0. 8594; T score = 0. 8075; VDR threshold score = 0. 8590) (36). This receptor has an important function in lipid metabolism, as it suppressed apolipoprotein AI gene expression (48) and modulated bile acid and cholesterol homeostasis interaction through the liver X receptor α (49); its effects on LIPC regulation have not been established.
Several other synonymous, nonsynonymous, and promoter variants of LIPC are potentially important. The nonsynonymous variants L334F (50), R186H (51), T383M (52), and S267F (52) cause hepatic lipase deficiency in carrier subjects. The L334F variant is common in African-Americans (allelic frequency of 18%) and associated with low hepatic lipase activity but is rare in whites (1.3%) (16). It is also rare in Turks (1.4%) and seems not to be associated with hepatic lipase activity. R186H, T383M, and S267F were not observed in Turks. Other nonsynonymous (V95M and N215S) and synonymous (V155V and T224T) variants have been reported (17). The S allele of N215S was significantly associated with low hepatic lipase activity in whites (allelic frequency of 34%) but not in African-American men (64%) (16). However, although the frequency of the S allele was 33% in Turks, it was not associated with HDL-C levels or hepatic lipase activity. The frequency of T224T was similar in Finnish cases and controls (11), but was significantly lower in Italian coronary heart disease patients than controls (36% vs. 53%) (53). This variant was not associated with lipid parameters in Turks. Recently, three novel SNPs with high allelic frequencies (21–25%) were reported in a Han Chinese population (54–56). The –586C>T SNP was associated with increased HDL-C levels. Luciferase reporter assays suggested that the minor T allele decreased promoter activity (54). The two other variants, –2T>C (55) and R276L (56), had significantly higher allelic frequencies in coronary heart disease patients than control subjects. These variants were not observed in Turks.
We sequenced about 9–10% of the LIPC locus and found nine novel SNPs with relatively low frequencies (supplementary Table I). Recently, the full genomic sequences of three individuals (57–59) were published. All subjects had approximately three million SNPs, and 14–43% of them were novel (57–59). Examination of the LIPC locus for novel SNPs in these three subjects (18–59 novel SNPs, Ensembl Genome browser, BioMart tool) revealed very little overlap among those individuals, and none of those SNPs overlapped with the novel SNPs (n = 9) found in the Turkish population. Thus, it is likely that many more SNPs with relatively low frequencies remain to be found, and some might have important functional properties. Although we did not observe the R186H (51), T383M (52), S267F (52), and R276L (56) variants, we found three novel coding variants (P111L, R344Q, and R444C) with low allelic frequency; however, in silico analysis and plasma HDL-C levels of those subjects did not suggest a functional or biological consequence.
In summary, we found five independent SNPs in LIPC that are significantly associated with plasma HDL-C levels in Turks. Although the size of the effect was somewhat similar to that in previous association studies, it is unlikely that these variants explain the magnitude of low HDL-C in Turks. One possibility is that we have not found all the variants of LIPC that affect plasma HDL-C levels as rare variants may have a major effect (60, 61). Another possibility is that other genes within the 15q22-23 locus also affect HDL-C levels. We are currently examining the 15q22-23 locus more extensively.
Supplementary Material
Acknowledgments
DNA samples were provided through the THS and the Genetic Epidemiology of the Metabolic Syndrome study, a multinational collaborative project supported by GlaxoSmithKline. The authors acknowledge the support of GlaxoSmithKline scientists and project leaders, especially Vincent Mooser, M.D., and Dawn M. Waterworth, Ph.D. The authors also thank Drs. Thomas P. Bersot, Karl H. Weisgraber, and Yadong Huang for valuable input and critical reading of the manuscript. In addition, the authors are indebted to their associates at the American Hospital, Istanbul, especially Guy Pépin, Sibel Tanir, Judy Dawson-Pépin, Linda L. Mahley, and Drs. K. Erhan Palaoğlu, Oryal Gökdemir, Sinan Özbayrakcı, Kerem Özer, and Selçuk Can. The authors thank Sylvia Richmond for manuscript preparation and Stephen Ordway and Gary Howard for editorial assistance. The authors acknowledge the generous support of the American Hospital, especially Mr. George Rountree, and the J. David Gladstone Institutes.
Footnotes
Abbreviations:
- BMI
- body mass index
- CEU
- Centre d'Etude du Polymorphisme Humaine from Utah
- ECR
- evolutionarily conserved region
- GWA
- genome-wide association
- HDL-C
- HDL-cholesterol
- HNF4α
- hepatic nuclear factor 4α
- LD
- linkage disequilibrium
- LDL-C
- LDL-cholesterol
- LIPC
- hepatic lipase gene
- SNP
- single nucleotide polymorphism
- THS
- Turkish Heart Study
- VDR
- vitamin D receptor
This work was supported in part by grant R01 HL71027 from the National Institutes of Health. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of eight tables and one figure.
REFERENCES
- 1.Gordon D. J., Probstfield J. L., Garrison R. J., Neaton J. D., Castelli W. P., Knoke J. D., Jacobs D. R., Jr., Bangdiwala S., Tyroler H. A. 1989. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 79: 8–15. [DOI] [PubMed] [Google Scholar]
- 2.Jacobs D. R., Jr., Mebane I. L., Bangdiwala S. I., Criqui M. H., Tyroler H. A. 1990. High density lipoprotein cholesterol as a predictor of cardiovascular disease mortality in men and women: the follow-up study of the Lipid Research Clinics Prevalence Study. Am. J. Epidemiol. 131: 32–47. [DOI] [PubMed] [Google Scholar]
- 3.Murray C. J. L., Lopez A. D. 1997. Mortality by cause for eight regions of the world: Global Burden of Disease study. Lancet. 349: 1269–1276. [DOI] [PubMed] [Google Scholar]
- 4.Mahaney M. C., Blangero J., Rainwater D. L., Comuzzie A. G., VandeBerg J. L., Stern M. P., MacCluer J. W., Hixson J. E. 1995. A major locus influencing plasma high-density lipoprotein cholesterol levels in the San Antonio Family Heart Study: segregation and linkage analyses. Arterioscler. Thromb. Vasc. Biol. 15: 1730–1739. [DOI] [PubMed] [Google Scholar]
- 5.Knoblauch H., Bauerfeind A., Toliat M. R., Becker C., Luganskaja T., Günther U. P., Rohde K., Schuster H., Junghans C., Luft F. C., et al. 2004. Haplotypes and SNPs in 13 lipid-relevant genes explain most of the genetic variance in high-density lipoprotein and low-density lipoprotein cholesterol. Hum. Mol. Genet. 13: 993–1004. [DOI] [PubMed] [Google Scholar]
- 6.Yu Y., Wyszynski D. F., Waterworth D. M., Wilton S. D., Barter P. J., Kesäniemi Y. A., Mahley R. W., McPherson R., Waeber G., Bersot T. P., et al. 2005. Multiple QTLs influencing triglyceride and HDL and total cholesterol levels identified in families with atherogenic dyslipidemia. J. Lipid Res. 46: 2202–2213. [DOI] [PubMed] [Google Scholar]
- 7.Wyszynski D. F., Waterworth D. M., Barter P. J., Cohen J., Kesäniemi Y. A., Mahley R. W., McPherson R., Waeber G., Bersot T. P., Sharma S. S., et al. 2005. Relation between atherogenic dyslipidemia and the Adult Treatment Program-III definition of metabolic syndrome (Genetic Epidemiology of Metabolic Syndrome Project). Am. J. Cardiol. 95: 194–198. [DOI] [PubMed] [Google Scholar]
- 8.Mahley R. W., Weisgraber K. H., Bersot T. P. 2008. Disorders of lipid metabolism. Williams Textbook of Endocrinology. Kronenberg H. M., Melmed S., Polonsky K. S., Larsen P. R., Saunders, Philadelphia: 1589–1653. [Google Scholar]
- 9.Guerra R., Wang J., Grundy S. M., Cohen J. C. 1997. A hepatic lipase (LIPC) allele associated with high plasma concentrations of high density lipoprotein cholesterol. Proc. Natl. Acad. Sci. USA. 94: 4532–4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Couture P., Otvos J. D., Cupples L. A., Lahoz C., Wilson P. W. F., Schaefer E. J., Ordovas J. M. 2000. Association of the C–514T polymorphism in the hepatic lipase gene with variations in lipoprotein subclass profiles: the Framingham Offspring Study. Arterioscler. Thromb. Vasc. Biol. 20: 815–822. [DOI] [PubMed] [Google Scholar]
- 11.Murtomäki S., Tahvanainen E., Antikainen M., Tiret L., Nicaud V., Jansen H., Ehnholm C. 1997. Hepatic lipase gene polymorphisms influence plasma HDL levels. Results from Finnish EARS participants. Arterioscler. Thromb. Vasc. Biol. 17: 1879–1884. [DOI] [PubMed] [Google Scholar]
- 12.Andersen R. V., Wittrup H. H., Tybjærg-Hansen A., Steffensen R., Schnohr P., Nordestgaard B. G. 2003. Hepatic lipase mutations, elevated high-density lipoprotein cholesterol, and increased risk of ischemic heart disease: the Copenhagen City Heart Study. J. Am. Coll. Cardiol. 41: 1972–1982. [DOI] [PubMed] [Google Scholar]
- 13.Kathiresan S., Melander O., Anevski D., Guiducci C., Burtt N. P., Roos C., Hirschhorn J. N., Berglund G., Hedblad B., Groop L., et al. 2008. Polymorphisms associated with cholesterol and risk of cardiovascular events. N. Engl. J. Med. 358: 1240–1249. [DOI] [PubMed] [Google Scholar]
- 14.Vega G. L., Gao J., Bersot T. P., Mahley R. W., Verstraete R., Grundy S. M., White A., Cohen J. C. 1998. The –514 polymorphism in the hepatic lipase gene (LIPC) does not influence androgen-mediated stimulation of hepatic lipase activity. J. Lipid Res. 39: 1520–1524. [PubMed] [Google Scholar]
- 15.Nie L., Wang J., Clark L. T., Tang A., Vega G. L., Grundy S. M., Cohen J. C. 1998. Body mass index and hepatic lipase gene (LIPC) polymorphism jointly influence postheparin plasma hepatic lipase activity. J. Lipid Res. 39: 1127–1130. [PubMed] [Google Scholar]
- 16.Nie L., Niu S., Vega G. L., Clark L. T., Tang A., Grundy S. M., Cohen J. C. 1998. Three polymorphisms associated with low hepatic lipase activity are common in African Americans. J. Lipid Res. 39: 1900–1903. [PubMed] [Google Scholar]
- 17.Hegele R. A., Tu L., Connelly P. W. 1992. Human hepatic lipase mutations and polymorphisms. Hum. Mutat. 1: 320–324. [DOI] [PubMed] [Google Scholar]
- 18.Cohen J. C., Wang Z., Grundy S. M., Stoesz M. R., Guerra R. 1994. Variation at the hepatic lipase and apolipoprotein AI/CIII/AIV loci is a major cause of genetically determined variation in plasma HDL cholesterol levels. J. Clin. Invest. 94: 2377–2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allayee H., Dominguez K. M., Aouizerat B. E., Krauss R. M., Rotter J. I., Lu J., Cantor R. M., de Bruin T. W. A., Lusis A. J. 2000. Contribution of the hepatic lipase gene to the atherogenic lipoprotein phenotype in familial combined hyperlipidemia. J. Lipid Res. 41: 245–252. [PubMed] [Google Scholar]
- 20.Willer C. J., Sanna S., Jackson A. U., Scuteri A., Bonnycastle L. L., Clarke R., Heath S. C., Timpson N. J., Najjar S. S., Stringham H. M., et al. 2008. Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nat. Genet. 40: 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kathiresan S., Melander O., Guiducci C., Surti A., Burtt N. P., Rieder M. J., Cooper G. M., Roos C., Voight B. F., Havulinna A. S., et al. 2008. Six new loci associated with blood low-density lipoprotein cholesterol, high-density lipoprotein cholesterol or triglycerides in humans. Nat. Genet. 40: 189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kooner J. S., Chambers J. C., Aguilar-Salinas C. A., Hinds D. A., Hyde C. L., Warnes G. R., Gómez Pérez F. J., Frazer K. A., Elliott P., Scott J., et al. 2008. Genome-wide scan identifies variation in MLXIPL associated with plasma triglycerides. Nat. Genet. 40: 149–151. [DOI] [PubMed] [Google Scholar]
- 23.McCarthy M. I., Abecasis G. R., Cardon L. R., Goldstein D. B., Little J., Ioannidis J. P. A., Hirschhorn J. N. 2008. Genome-wide association studies for complex traits: consensus, uncertainty and challenges. Nat. Rev. Genet. 9: 356–369. [DOI] [PubMed] [Google Scholar]
- 24.Mahley R. W., Palaoğlu K. E., Atak Z., Dawson-Pepin J., Langlois A-M., Cheung V., Onat H., Fulks P., Mahley L. L., Vakar F., et al. 1995. Turkish Heart Study: lipids, lipoproteins, and apolipoproteins. J. Lipid Res. 36: 839–859. [PubMed] [Google Scholar]
- 25.Mahley R. W., Can S., Özbayrakçı S., Bersot T. P., Tanir S., Palaoğlu K. E., Pépin G. M. 2005. Modulation of high-density lipoproteins in a population in Istanbul, Turkey, with low levels of high-density lipoproteins. Am. J. Cardiol. 96: 547–555. [DOI] [PubMed] [Google Scholar]
- 26.Tezcan S., Altıntaş H., Sönmez R., Akinci A., Doğan B., Çakır B., Bilgin Y., Klör H. U., Razum O. 2003. Cardiovascular risk factor levels in a lower middle-class community in Ankara, Turkey. Trop. Med. Int. Health. 8: 660–667. [DOI] [PubMed] [Google Scholar]
- 27.Porsch-Oezçueruemez M., Bilgin Y., Wollny M., Gediz A., Arat A., Karatay E., Akinci A., Sinterhauf K., Koch H., Siegfried I., et al. 1999. Prevalence of risk factors of coronary heart disease in Turks living in Germany: the Giessen Study. Atherosclerosis. 144: 185–198. [DOI] [PubMed] [Google Scholar]
- 28.Bersot T. P., Vega G. L., Grundy S. M., Palaoğlu K. E., Atagündüz P., Özbayrakçi S., Gökdemir O., Mahley R. W. 1999. Elevated hepatic lipase activity and low levels of high density lipoprotein in a normotriglyceridemic, nonobese Turkish population. J. Lipid Res. 40: 432–438. [PubMed] [Google Scholar]
- 29.Shohet R. V., Vega G. L., Bersot T. P., Mahley R. W., Grundy S. M., Guerra R., Cohen J. C. 2002. Sources of variability in genetic association studies: insights from the analysis of hepatic lipase (LIPC). Hum. Mutat. 19: 536–542. [DOI] [PubMed] [Google Scholar]
- 30.The International HapMap Consortium. 2007. A second generation human haplotype map of over 3.1 million SNPs. Nature. 449: 851–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hinds D. A., Stuve L. L., Nilsen G. B., Halperin E., Eskin E., Ballinger D. G., Frazer K. A., Cox D. R. 2005. Whole-genome patterns of common DNA variation in three human populations. Science. 307: 1072–1079. [DOI] [PubMed] [Google Scholar]
- 32.De La Vega F. M., Isaac H. I., Scafe C. R. 2006. A tool for selecting SNPs for association studies based on observed linkage disequilibrium patterns. Pac. Symp. Biocomput. 11: 487–498. [PubMed] [Google Scholar]
- 33.The ENCODE Project Consortium. 2007. Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature. 447: 799–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ovcharenko I., Nobrega M. A., Loots G. G., Stubbs L. 2004. ECR Browser: a tool for visualizing and accessing data from comparisons of multiple vertebrate genomes. Nucleic Acids Res. 32: W280–W286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siepel A., Bejerano G., Pedersen J. S., Hinrichs A. S., Hou M., Rosenbloom K., Clawson H., Spieth J., Hillier L. W., Richards S., et al. 2005. Evolutionarily conserved elements in vertebrate, insect, worm, and yeast genomes. Genome Res. 15: 1034–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matys V., Fricke E., Geffers R., Gößling E., Haubrock M., Hehl R., Hornischer K., Karas D., Kel A. E., Kel-Margoulis O. V., et al. 2003. TRANSFAC®: transcriptional regulation, from patterns to profiles. Nucleic Acids Res. 31: 374–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blanchette M., Bataille A. R., Chen X., Poitras C., Laganière J., Lefèbvre C., Deblois G., Giguère V., Ferretti V., Bergeron D., et al. 2006. Genome-wide computational prediction of transcriptional regulatory modules reveals new insights into human gene expression. Genome Res. 16: 656–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Purcell S., Neale B., Todd-Brown K., Thomas L., Ferreira M. A. R., Bender D., Maller J., Sklar P., de Bakker P. I. W., Daly M. J., et al. 2007. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81: 559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Corbex M., Poirier O., Fumeron F., Betoulle D., Evans A., Ruidavets J. B., Arveiler D., Luc G., Tiret L., Cambien F. 2000. Extensive association analysis between the CETP gene and coronary heart disease phenotypes reveals several putative functional polymorphisms and gene-environment interaction. Genet. Epidemiol. 19: 64–80. [DOI] [PubMed] [Google Scholar]
- 40.Barrett J. C., Fry B., Maller J., Daly M. J. 2005. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 21: 263–265. [DOI] [PubMed] [Google Scholar]
- 41.Ng P. C., Henikoff S. 2002. Accounting for human polymorphisms predicted to affect protein function. Genome Res. 12: 436–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sunyaev S., Ramensky V., Koch I., Lathe W., III, Kondrashov A. S., Bork P. 2001. Prediction of deleterious human alleles. Hum. Mol. Genet. 10: 591–597. [DOI] [PubMed] [Google Scholar]
- 43.Deeb S. S., Peng R. 2000. The C-514T polymorphism in the human hepatic lipase gene promoter diminishes its activity. J. Lipid Res. 41: 155–158. [PubMed] [Google Scholar]
- 44.Botma G-J., Verhoeven A. J. M., Jansen H. 2001. Hepatic lipase promoter activity is reduced by the C-480T and G-216A substitutions present in the common LIPC gene variant, and is increased by Upstream Stimulatory Factor. Atherosclerosis. 154: 625–632. [DOI] [PubMed] [Google Scholar]
- 45.van't Hooft F. M., Lundahl B., Ragogna F., Karpe F., Olivecrona G., Hamsten A. 2000. Functional characterization of 4 polymorphisms in promoter region of hepatic lipase gene. Arterioscler. Thromb. Vasc. Biol. 20: 1335–1339. [DOI] [PubMed] [Google Scholar]
- 46.Schadt E. E., Molony C., Chudin E., Hao K., Yang X., Lum P. Y., Kasarskis A., Zhang B., Wang S., Suver C., et al. 2008. Mapping the genetic architecture of gene expression in human liver. PLoS Biol. 6: e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rufibach L. E., Duncan S. A., Battle M., Deeb S. S. 2006. Transcriptional regulation of the human hepatic lipase (LIPC) gene promoter. J. Lipid Res. 47: 1463–1477. [DOI] [PubMed] [Google Scholar]
- 48.Wehmeier K., Beers A., Haas M. J., Wong N. C. W., Steinmeyer A., Zugel U., Mooradian A. D. 2005. Inhibition of apolipoprotein AI gene expression by 1, 25-dihydroxyvitamin D3. Biochim. Biophys. Acta. 1737: 16–26. [DOI] [PubMed] [Google Scholar]
- 49.Jiang W., Miyamoto T., Kakizawa T., Nishio S-I., Oiwa A., Takeda T., Suzuki S., Hashizume K. 2006. Inhibition of LXRα signaling by vitamin D receptor: possible role of VDR in bile acid synthesis. Biochem. Biophys. Res. Commun. 351: 176–184. [DOI] [PubMed] [Google Scholar]
- 50.Knudsen P., Antikainen M., Ehnholm S., Uusi-Oukari M., Tenkanen H., Lahdenperä S., Kahri J., Tilly-Kiesi M., Bensadoun A., Taskinen M-R., et al. 1996. A compound heterozygote for hepatic lipase gene mutations Leu334 → Phe and Thr383 → Met: correlation between hepatic lipase activity and phenotypic expression. J. Lipid Res. 37: 825–834. [PubMed] [Google Scholar]
- 51.Knudsen P., Antikainen M., Uusi-Oukari M., Ehnholm S., Lahdenperä S., Bensadoun A., Funke H., Wiebusch H., Assmann G., Taskinen M-R., et al. 1997. Heterozygous hepatic lipase deficiency, due to two missense mutations R186H and L334F, in the HL gene. Atherosclerosis. 128: 165–174. [DOI] [PubMed] [Google Scholar]
- 52.Hegele R. A., Little J. A., Vezina C., Maguire G. F., Tu L., Wolever T. S., Jenkins D. J. A., Connelly P. W. 1993. Hepatic lipase deficiency. Clinical, biochemical, and molecular genetic characteristics. Arterioscler. Thromb. 13: 720–728. [DOI] [PubMed] [Google Scholar]
- 53.Baroni M. G., Berni A., Romeo S., Arca M., Tesorio T., Sorropago G., Di Mario U., Galton D. J. 2003. Genetic study of common variants at the apo E, apo AI, apo CIII, apo B, lipoprotein lipase (LPL) and hepatic lipase (LIPC) genes and coronary artery disease (CAD): variation in LIPC gene associates with clinical outcomes in patients with established CAD. BMC Med. Genet. 4: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Su Z., Zhang S., Nebert D. W., Zhang L., Huang D., Hou Y., Liao L., Xiao C. 2002. A novel allele in the promoter of the hepatic lipase is associated with increased concentration of HDL-C and decreased promoter activity. J. Lipid Res. 43: 1595–1601. [DOI] [PubMed] [Google Scholar]
- 55.Su Z-G., Zhang S-Z., Hou Y-P., Zhang L., Huang D-J., Liao L-C., Xiao C-Y. 2002. Relationship between a novel polymorphism of hepatic lipase gene and coronary artery disease. Acta Biochim. Biophys. Sin. (Shanghai). 34: 780–785. [PubMed] [Google Scholar]
- 56.Su Z-G., Zhang S-Z., Zhang L., Tong Y., Xiao C-Y., Hou Y-P., Liao L-C. 2003. A novel polymorphism A+884 → G in the hepatic lipase gene and its association with coronary artery disease. Acta Biochim. Biophys. Sin. (Shanghai). 35: 606–610. [PubMed] [Google Scholar]
- 57.Wheeler D. A., Srinivasan M., Egholm M., Shen Y., Chen L., McGuire A., He W., Chen Y-J., Makhijani V., Roth G. T., et al. 2008. The complete genome of an individual by massively parallel DNA sequencing. Nature. 452: 872–876. [DOI] [PubMed] [Google Scholar]
- 58.Levy S., Sutton G., Ng P. C., Feuk L., Halpern A. L., Walenz B. P., Axelrod N., Huang J., Kirkness E. F., Denisov G., et al. 2007. The diploid genome sequence of an individual human. PLoS Biol. 5: e254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang J., Wang W., Li R., Li Y., Tian G., Goodman L., Fan W., Zhang J., Li J., Zhang J., et al. 2008. The diploid genome sequence of an Asian individual. Nature. 456: 60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cohen J. C., Kiss R. S., Pertsemlidis A., Marcel Y. L., McPherson R., Hobbs H. H. 2004. Multiple rare alleles contribute to low plasma levels of HDL cholesterol. Science. 305: 869–872. [DOI] [PubMed] [Google Scholar]
- 61.Cohen J. C., Pertsemlidis A., Fahmi S., Esmail S., Vega G. L., Grundy S. M., Hobbs H. H. 2006. Multiple rare variants in NPC1L1 associated with reduced sterol absorption and plasma low-density lipoprotein levels. Proc. Natl. Acad. Sci. USA. 103: 1810–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.