Abstract
A physiological atherogenic human diet consists of 0.1% cholesterol, fat, as well as high levels of methionine, which is the precursor to homocysteine. The pathological effects of a diet enriched with physiologically high levels of cholesterol, methionine and fat over a short period on the aorta are unknown. In this regard, we sought to determine the effects of a 0.1% cholesterol diet in combination with a 1% methionine over a 4-week period on endothelial function and artery pathology and the expression of endothelial nitric oxide synthase as well as nitrosative stress by nitrotyrosine (NT), oxidative stress by heat shock protein 70 (HSP70) and endoplasmic reticulum stress by glucose regulated protein 78 (GRP78). Rabbits were fed for 4 weeks a diet supplemented with 1% methionine + 0.1% cholesterol + 5% peanut oil (MC). The endothelial function of the abdominal aorta was examined using organ bath techniques, atherosclerosis determined in each artery by microscopy and eNOS, NT, GRP78 and HSP70 by standard immunohistochemistry. Endothelium dependent relaxation in response to acetylcholine significantly decreased by 63% at 1 μM acetylcholine (P < 0.001) compared with control arteries. There was no evidence of atherosclerosis formation in any artery studied, however, eNOS, NT and GRP78 was clearly present in all arteries studied but HSP70 was not easily detectable. Severe endothelial dysfunction is present in the abdominal aorta of rabbits within 4 weeks of physiological dietary manipulation, possibly due to NT formation and endoplasmic reticulum stress. This model could be used to study the early onset of endothelial dysfunction prior to the initiation of atherosclerosis.
Keywords: atherosclerosis, cholesterol, homocysteine
The rabbit, as an animal model for human atherosclerosis, has been widely used to study the mechanisms of atherosclerosis. However, these studies often used massive doses of cholesterol (>1% wt/wt cholesterol) which are way beyond normal physiological levels and lead to irrelevant plasma cholesterol levels. A human diet consisting of 1 g cholesterol/day has been shown to increase the risk of cardiovascular disease. Considering a human diet is approximately 500–1000 g of food weight per day, this equates to a 0.1–0.2% cholesterol diet (Shekelle & Stamler 1989; McNamara 2000). Such a diet can lead to an increase in plasma cholesterol level above 7 mmol/l, but rarely above10 mmol/l (Hegsted & Nicolosi 1987; Lewis 1987). However, patients suffering from genetic defects in cholesterol metabolism, such as familial hypercholesterolaemia, could show cholesterol levels between 10 and 13 mmol/l (Alrasadi et al. 2009) and can reach 17 mmol/l in some patients (Lind et al. 2004).
Genetically modified murine models to study atherosclerosis include the popular ApoE double knockout mouse. This model develops spontaneous lesions that are exacerbated by a high fat (10–20%), high cholesterol (0.15–1.5%) diet (Meir & Leitersdorf 2004; Getz & Reardon 2006). However, this model can develop severe hypercholesterolaemia above 50 mmol/l, which is similar to the total cholesterol levels observed in studies where rabbits were fed a 1–2% cholesterol diet in the 1950s (Kritchevsky et al. 1973; Prior et al. 1998). Furthermore, the increase in total cholesterol in the ApoE mouse is due to increased VLDL and IDL (Getz & Reardon 2006), which are triglyceride-rich particles and not the atherogenic LDL form which is comprised of 50% cholesterol. This lipid profile is commonly ignored and might not be relevant to human atherosclerosis, as LDL is the accepted ‘bad’ cholesterol. Another ignored problem inherent to the ApoE model is the lack of response of conduit arteries to angiotensin II, which is one of the most important peptide stimulants for human cardiovascular disease. To overcome this issue, assumptions have been made on model validity based on the anti-atherosclerotic effects of angiotensin II receptor blockade or angiotensin converting enzyme inhibition (Keidar et al. 1997; Hayek et al. 1998). However, our understanding of the complex interactions between the receptors of the renin-angiotensin system is still unfolding (Castro et al. 2005), and so other effects unrelated to angiotensin II could be involved.
Therefore, new animal models to study human cardiovascular disease are warranted. Such animal models will be required to develop endothelial dysfunction and atherosclerosis under physiological conditions and within a short period of time. In this regard, we have added physiologically relevant high dietary methionine to a high fat/cholesterol diet to mimic a natural human diet. Methionine is an essential amino acid ingested through dietary protein (Verhoef et al. 2005). An excess intake of methionine coupled with low intake of folic acid and vitamin B12 raises plasma homocysteine in humans and rabbits. Hyperhomocysteinemia induces endothelial dysfunction, is pro-atherogenic and is associated with cardiovascular disease (Guthikonda & Haynes 2006). We have previously used a diet consisting of 1% methionine in rabbits to induce endothelial dysfunction, hyperhomocysteinemia and intimal thickening, (Zulli et al. 2003) and when coupled with high dietary cholesterol, this abolishes endothelial function and exacerbates atherosclerosis formation (Zulli et al. 2003, 2004), and also induces myocardial fibrosis (Zulli et al. 2006a).
This study was designed to use physiological doses of cholesterol (0.1%), methionine (1%) and fat (5% peanut oil) over a short period (4 weeks) to establish a new animal model for severe endothelial dysfunction. As well, to determine the type of stress in the endothelial milieu, we sought to immunolocalize endothelial nitric oxide synthase (for eNOS deficiency), nitrotyrosine (nitrosative stress) (Halliwell 1997), heat shock protein 70 (HSP70) (Zhou et al. 2004) for oxidative stress and glucose regulated protein 78 (GRP78) (Outinen et al. 1999) for endoplasmic reticulum stress.
Methods
Two groups of male New Zealand White rabbits at 3 months of age received either a normal rabbit chow diet or a normal rabbit chow diet supplemented with 0.1% cholesterol (Teixeira et al. 1991; Sugano & Makino 1996; Kritchevsky et al. 2000), 1% methionine and 5% peanut oil. The peanut oil aids in the absorption of cholesterol as well as providing extra fat to the diet to resemble human diet, and it does not affect aortic pathology or endothelial function (Zulli et al. 2003). The animals were housed in individual cages and maintained at a constant temperature of approximately 21 °C. Food and water were supplied ad libitum. The animals were fed their respective diet for 4 weeks (n = 4). Age-matched controls were used in this experiment (n = 5). The experiments were carried out according to the National Health and Medical Research Council ‘Australian Code of Practice for the Care and Use of Animals for Scientific Purposes’ (6th Edition, 1997). The animals were then euthanized by an overdose intravenous injection of ketamine and xylazine via the main ear vein, as previously described elsewhere (Zulli et al. 2003, 2004, 2006a). The aorta was then excised, cleaned of connective tissue and fat and used for isometric tension studies (Zulli et al. 2003).
Diet composition of normal chow
Calcium Pantothenate 10 mg/kg; Vitamin B6 (Pyridoxine) 2.7 mg/kg; Niacin (Nicotinic acid) 20 mg/kg; Vitamin B2 (Riboflavin) 2.7 mg/kg; Vitamin B1 (Thiamine) 4 mg/kg; Vitamin E 25 mg/kg; (a Tocopherol acetate); Vitamin K (Menadione) 1.3 mg/kg; Vitamin A (Retinol) 13,000 IU/kg; Choline 2,200 mg/kg; Vitamin B12 7 mg/kg; Folic acid 0.6 mg/kg; Biotin 140 ug/kg; Pantothenic acid 19 mg/kg; Vitamin B6 (Pyridoxine) 5.8 mg/kg; Niacin (Nicotinic acid) 56 mg/kg; Vitamin B2 (Riboflavin) 6.6 mg/kg; Vitamin B1 (Thiamine) 5.6 mg/kg; Vitamin K (Menadione) 3 mg/kg; Vitamin E (Tocopherol) 60 mg/kg; Vitamin A (Retinol) 49,000 IU/kg; Selenium 0.1 mg/kg; Zinc 60 mg/kg; Cobalt 0.7 mg/kg; Manganese 90 mg/kg; Iodine 1.7 mg/kg; Copper 13 mg/kg; Iron 40 mg/kg; Tryptophan 0.2%; Tyrosine 0.5%; Phenylalanine 0.8%; Lysine 0.8%; Cystine 0.2%; Methionine 0.2%; Threonine 0.6%; Isoleucine 0.7%; Leucine 1.2%; Valine 0.8%; Arachadonic Acid 20:4 n6 0.01%; a Linolenic Acid 18:3 n3 0.1%; Linoleic Acid 18:2 n6 0.8%; Gadoleic Acid 20:1 0.02%; Oleic Acid 18:1 1.4%; Palmitoleic Acid 16:1 0.02%; Stearic Acid 18:0 0.1%; Palmitic Acid 16:0 0.3%; Myristic Acid 14:0 0.01%; Cadmium 0.01 mg/kg; Selenium 0.3 mg/kg; Molybdenum 1 mg/kg; Zinc 97 mg/kg; Cobalt 0.7 mg/kg; Manganese 130 mg/kg; Iodine 1.8 mg/kg; Copper 23 mg/kg; Iron 290 mg/kg; Sulphur 0.2%; Potassium 1.0%; Chloride 0.4%; Sodium 0.2%; Magnesium 0.3%; Phosphorous 0.7%; Calcium 1.1%.
Isometric tension studies
Abdominal aortae were dissected into 6 × 3 mm rings and sequentially mounted between two metal hooks in organ baths attached to force displacement transducers (Grass FT03) coupled to a data acquisition system (MacLab, ADInstruments, Australia). The baths were filled with Krebs solution, kept at a constant temperature of 37 °C and continuously bubbled with 95% O2/5% CO2. After 1 h, the vessels were gently stretched to a resting tension of 2 g. After another hour, maximum constriction was determined by a high potassium Krebs solution (124 mM K+). After plateau (6 min), vessels were rinsed with Krebs solution and 1 hour later the vessel rings were pre-contracted with phenylephrine to approximately 30–40% of maximal contraction. After the contraction became stable, an acetylcholine concentration response curve (10−8–10−6 M Ach, half log units) was performed (Zulli et al. 2003).
Wall pathology and immunohistochemistry
Excess rings and the rings used in the organ baths were removed from the organ bath and placed in freshly prepared 4% paraformaldehyde in PBS, pH 7.3, overnight, after which the vessels were placed in PBS. Once all experiments were finished, all blood vessels were processed for paraffin embedding in one batch. This is to keep the shrinking of vessels constant in all vessels. All blood vessels were then mounted vertically in two paraffin blocks. A minimum of eight 3 mm vessels were used per animal. After this, 5 micron sections were cut, mounted on microscope slides and immunohistochemistry performed. eNOS was purchased from BD Transduction Laboratories (Cat# 610296), HSP70 (Cat# MAB3516) and nitrotyrosine (Cat# MAB5404) were purchased from Chemicon International and GRP78 from Santa Cruz (Cat# sc-1050). All primary antibody dilutions were 1:100 and immunohistochemistry performed as previously described elsewhere (Zulli et al. 2003, 2006b). Wall pathology was determined by standard microscopy.
Data analysis
Unpaired student’s t test was used to compare the lipid profile of the experimental group with that of the control. All endothelial function data points were grouped and analysed by anova (non-repeated measures), followed by a Newman–Keuls multiple comparison post hoc test. A P < 0.05 was accepted in all cases as significant. All data are expressed as mean ± SEM.
Results
Blood total cholesterol levels significantly increased to 11.8 ± 2.5 mmol/l in the experimental group compared to 1.3 ± 0.1 mmol/l (P < 0.01) in the control. This increase was mainly due to a marked increase in LDL cholesterol (9.5 ± 2.2 mmol/l vs. 0.38 ± 0.05, P < 0.01) and a small, significant increase in HDL cholesterol (1.9 ± 0.15 mmol/l vs. 0.8 ± 0.1 mmol/l, P < 0.01). Blood triglycerides also significantly increased in the experimental group (1.8 ± 0.5 mmol/l vs. 0.7 ± 0.1 mmol/l, P < 0.05). Homocysteine was increased by the dietary regimen (28.8 ± 13 vs. 8.5 ± 1.3 μmol/l, P = 0.1).
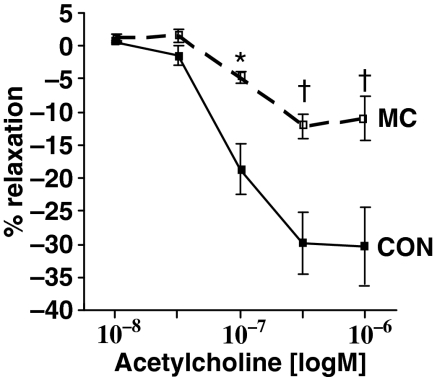
Endothelium-dependent relaxation in response to acetylcholine was significantly reduced by 72% at 0.1 μM acetylcholine (P < 0.05). Such dysfunction continued to be significant at 0.3 μM acetylcholine (60% decrease, P < 0.001) and also at 1 μM acetylcholine (63% decrease, P < 0.001) when compared to control arteries (Figure 1).
Figure 1.
After 4 weeks of a diet supplemented with 0.1% cholesterol, 1% methionine and 5% peanut oil, rabbit abdominal aorta showed severe endothelial dysfunction. Endothelium-dependent relaxation in response to acetylcholine significantly decreased by 72% at 0.1 μM acetylcholine (P < 0.05), by 60% at 0.3 μM acetylcholine (P < 0.001) and by 63% at 1 μM acetylcholine (P < 0.001) compared to control arteries. Mean ± SEM. n = 4 per group. *P < 0.05 vs. control and †P < 0.001 vs. control.
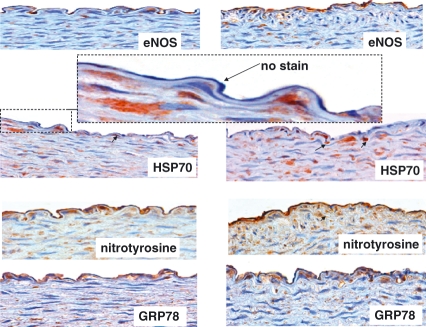
There was no evidence of atherosclerosis formation in any artery studied (Figure 2). However, intimal thickening was present in one animal fed high dietary cholesterol plus methionine (Figure selected and shown).
Figure 2.
Photomicrographs of eNOS, HSP70, nitrotyrosine and GRP78 in the abdominal aorta of rabbits fed 0.1% cholesterol, 1% methionine and 5% peanut oil. Left panel shows endothelium overlying normal aortic wall, whereas the right panel shows endothelium overlying intimal thickening. All photos are taken from serial adjacent sections of one vessel that is representative of all arteries. eNOS is clearly present in the arteries in both endothelia (overlying normal wall and intimal thickening). It is not clear whether the aortic wall contains some cells that also stain for eNOS. HSP70 immunohistochemistry shows positive cells in the media (arrows), but the endothelium is virtually clear of immunoreactivity (enlarged dashed figure). However, nitrotyrosine immunoreactivity was clearly evident throughout all vessels, as well as in the media. This was paralleled with GRP78, indicating a possible association between nitrosative stress and endoplasmic reticulum stress.
The examination of eNOS showed that the enzyme was clearly present in all endothelium of arteries studied in both groups. In fact, there were cells binding to many areas of endothelia that showed eNOS immunoreactivity. Examination of oxidative stress by HSP70 shows virtually no expression in the endothelia. However, endothelial nitrotyrosine was clearly visible throughout the endothelium, indicating that the endothelial milieu is one of nitrosative stress rather than oxidative stress. As well, the endothelium clearly showed expression of GRP78, indicating that the endothelium is also undergoing endoplasmic reticulum stress.
Discussion
The major findings in this investigation are that high dietary cholesterol plus methionine for 4 weeks leads to physiologically relevant atherogenic lipid profile and severe endothelial dysfunction with a minor intimal thickening. The severe endothelial dysfunction did not appear to be due to a lack of eNOS protein or oxidative stress as detected by HSP70, but possibly because of increased endoplasmic reticulum stress, as detected by GRP78 and nitrosative stress, as detected by nitrotyrosine.
Nitric oxide is produced by three different isoforms of nitric oxide synthase (NOS) and is widely distributed in virtually all vascular cell types. The endothelial isoform (eNOS) plays a crucial role in vascular tone and structure regulation. It also exerts an anti-inflammatory influence, inhibits platelet adhesion and aggregation and prevents proliferation and migration of smooth muscle cells (Rekka & Chrysselis 2002). Several lines of evidence link endothelial dysfunction, characterized by decreased bioavailability of nitric oxide, with the development of many pathological conditions, such as heart failure, hypertension, diabetes and atherosclerosis (Wennmalm 1994). Our results suggests that the lack of endothelial function in the experimental group is not related to a lack of eNOS enzyme, as immunoreactivity to this protein was clearly visible throughout all the endothelial layer, and this supports our previous work in a similar model (Zulli et al. 2006b).
Experimental and clinical studies suggest that oxidative stress contributes to the development and progression of cardiovascular disease. However, clinical trials with classic vitamin antioxidants failed to demonstrate any benefit in cardiovascular outcomes. Oxidative stress in tissue can be detected by studying the 70-kDa HSP70s. These proteins are well-studied and characterized HSPs which constitute essential components of a quality control system of protein synthesis, and function as molecular chaperones to prevent proteins from misfolding and aggregating during both new protein synthesis and under conditions of cellular stress. Current research suggests that upregulation of HSP70 can be caused by oxidative stress (McDuffee et al. 1997; Callahan et al. 2002; Antunes-Neto et al. 2006), and thus provides a novel approach to study oxidative stress by immunohistochemistry and Western blot analysis. Indeed, Zhou and collegues have recently shown that increased HSP70 immunohistochemistry correlates with the more commonly used dihydroethidium staining in murine atherosclerotic plaques (Zhou et al. 2004). In our study, we failed to demonstrate high levels of the oxidative stress marker HSP70 in the endothelial layer; however, high levels were found in atherosclerotic plaques (personal observations). These results suggest that oxidative stress might not be the cause of such dysfunction observed in the arteries in this model.
Peroxynitrite (ONOO-) is a ‘reactive nitrogen species’ that can be formed by combination of superoxide and nitric oxide. It is an assumption that formation of nitrotyrosine is a biomarker specifically diagnostic of ONOO- production. Nitrotyrosine can also be formed by tyrosyl radicals, NO·2, NO−3, NO2Cl and HOCl reacting with tyrosine (Halliwell 1997). In our study, nitrotyrosine was clearly detectable in the endothelium in the arteries, suggesting nitrated endothelial proteins. Thus, our results suggest that oxidative stress (as measured by HSP70) might not be the cause of the endothelial dysfunction, but the removal of bio-available NO by other mechanisms could be possible.
The endoplasmic reticulum (ER) is responsible for governing protein production and conformation. On one hand, properly folded proteins must be guided from the ER to their final destination within the cell, and on the other hand, misfolded proteins that may be toxic to the cell must be disposed of without compromising normal cell function. This quality control system is based on common structural and biophysical features that distinguish native from non-native protein conformations. Folding is assisted by a variety of folding enzymes and chaperones with different properties and functions. The main role of these components is to prevent protein misfolding and aggregation, which could cause cellular dysfunction (Sitia & Braakman 2003). Accumulation of misfolded proteins in the ER has been shown to cause ER stress and activation of a protective response known as the unfolded protein response (UPR). Homocysteine (Outinen et al. 1999), LDL (Sorensen et al. 2006) and type II diabetes (Tsiotra & Tsigos 2006) all cause ER stress and thus UPR. Glucose-regulated protein (GRP) 78 is a molecular chaperone involved in the UPR and it has been suggested that elevated GRP78 levels could have favourable anti-cancer properties (Wu et al. 2005). GRP78 is an accurate marker of ER stress, being elevated by homocysteine (Outinen et al. 1999) and diabetes (Parfett et al. 1990). In our study, GRP78 was clearly visible in the endothelium overlying arteries, suggesting that the endothelial milieu is undergoing endoplasmic reticulum stress. This is supported by our current research, indicating that GRP78 positive cells do decrease after the cessation of the atherogenic diet, indicating a decrease in ER stress (Zulli & Hare 2009).
We have previously reported that the combination of high dietary cholesterol (0.5%) plus methionine (1%) fed to rabbits for 12 weeks abolishes endothelial function and exacerbates atherosclerosis formation compared with high dietary cholesterol or methionine alone (Zulli et al. 2003, 2004). In this study, we also show that severe endothelial dysfunction can be induced with physiologically elevated levels of cholesterol and methionine, but atherosclerosis was not present at this time point.
The 0.1% cholesterol-fed rabbit has been extensively studied by Kolodgie et al. They showed that this diet produced healthy animals, although they had hypercholesterolaemia and atherosclerosis formation after 31–32 weeks of dietary manipulation (Kolodgie et al. 1996). Plasma levels of liver enzymes ALT and AST were also normal over this period. This extensive study shows that a 0.1% cholesterol diet will induce atherosclerosis formation if the animals are maintained on this dietary regimen for a longer period. High dietary methionine, on the other hand, does not cause atherosclerosis in the abdominal aorta or left main coronary artery or arterioles even after 3 months of dietary manipulation (Zulli et al. 2003, 2004).
The high cholesterol fed rabbit has not always been accepted as an adequate model to represent atherosclerosis in humans. The evidence of early studies suggested that the atheromatous plaques in animals fed 1–2% cholesterol did not resemble human atherosclerosis, but appeared to be related to a ‘cholesterol storage disease’ because of lipid deposition in almost all organs and tissues of the body (Clarkson 1971). These conclusions have proved the rabbit as an adequate model; however, recent studies, including those from our laboratory, support the hypothesis that the rabbit can be used to study atherogenesis.
Furthermore, although murine models of atherosclerosis are now established (Getz & Reardon 2006) and offer a variety of advantages, such as low husbandry cost, ease of which atherosclerosis develops and ease of genetic manipulation, the major drawbacks of using rodents to study atherosclerosis are commonly ignored (Zulli & Hare 2009). These can include an irrelevant lipid profile (increased VLDL and IDL), massive increase in total blood cholesterol and that the aorta of these animals do not constrict to the powerful vasoconstrictor and pro-atherogenic peptide, angiotensin II (personal observations). In this case, many scientists use either phenylephrine (Wassmann et al. 2004) or thromboxane analogues. Therefore, the extrapolation of results obtained from these models must be viewed with caution, as the mechanisms of atherogenesis in murine models that do not respond to angiotensin II might well be different to that observed in humans, where angiotensin II is a potent vasoconstrictor and atherogenic peptide. Thus, we need to recognize that all current studies might not be predictive of effects in humans (Getz & Reardon 2006). In this regard, we have now developed a new model for the study of severe endothelial dysfunction in rabbit arteries using physiologically relevant levels of dietary cholesterol, methionine and fat, which leads to physiologically relevant blood lipid profile and homocysteine levels. As well, rabbit arteries constrict very well to angiotensin II and thus provide a new model for the study of the renin-angiotenin system in severe endothelial dysfunction.
In conclusion, we show that in only 4 weeks of dietary manipulation, rabbits fed a normal chow diet supplemented with physiologically relevant levels of cholesterol (0.1%) and methionine (1%) and fat exhibit marked endothelial dysfunction and a physiologically relevant atherogenic lipid profile, but not atherosclerosis at this time point. The endothelial dysfunction appears to be due to nitrosative and endoplasmic reticulum stress rather than oxidative stress or lack of eNOS. We suggest this model could be used to study human endothelial dysfunction with similar lipid and homocysteine profile.
Acknowledgments
This work was supported by the National Health Medical Research Council and National Heart Foundation of Australia.
References
- Alrasadi K, Alwaili K, Awan Z, Valenti D, Couture P, Genest J. Aortic calcifications in familial hypercholesterolemia: potential role of the low-density lipoprotein receptor gene. Am. Heart J. 2009;157:170–176. doi: 10.1016/j.ahj.2008.08.021. [DOI] [PubMed] [Google Scholar]
- Antunes-Neto JM, Toyama MH, Carneiro EM, Boschero AC, Pereira-da-Silva L, Macedo DV. Circulating leukocyte heat shock protein 70 (HSP70) and oxidative stress markers in rats after a bout of exhaustive exercise. Stress. 2006;9:107–115. doi: 10.1080/10253890600772211. [DOI] [PubMed] [Google Scholar]
- Callahan MK, Chaillot D, Jacquin C, Clark PR, Menoret A. Differential acquisition of antigenic peptides by Hsp70 and Hsc70 under oxidative conditions. J. Biol. Chem. 2002;277:33604–33609. doi: 10.1074/jbc.M202890200. [DOI] [PubMed] [Google Scholar]
- Castro CH, Santos RA, Ferreira AJ, Bader M, Alenina N, Almeida AP. Evidence for a functional interaction of the angiotensin-(1-7) receptor Mas with AT1 and AT2 receptors in the mouse heart. Hypertension. 2005;46:937–942. doi: 10.1161/01.HYP.0000175813.04375.8a. [DOI] [PubMed] [Google Scholar]
- Clarkson TB. Animal models for atherosclerosis. A review. N. C. Med. J. 1971;32:88–98. [PubMed] [Google Scholar]
- Getz GS, Reardon CA. Diet and murine atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2006;26:242–249. doi: 10.1161/01.ATV.0000201071.49029.17. [DOI] [PubMed] [Google Scholar]
- Guthikonda S, Haynes WG. Homocysteine: role and implications in atherosclerosis. Curr. Atheroscler. Rep. 2006;8:100–106. doi: 10.1007/s11883-006-0046-4. [DOI] [PubMed] [Google Scholar]
- Halliwell B. What nitrates tyrosine? Is nitrotyrosine specific as a biomarker of peroxynitrite formation in vivo? FEBS Lett. 1997;411:157–160. doi: 10.1016/s0014-5793(97)00469-9. [DOI] [PubMed] [Google Scholar]
- Hayek T, Attias J, Smith J, Breslow JL, Keidar S. Antiatherosclerotic and antioxidative effects of captopril in apolipoprotein E-deficient mice. J. Cardiovasc. Pharmacol. 1998;31:540–544. doi: 10.1097/00005344-199804000-00011. [DOI] [PubMed] [Google Scholar]
- Hegsted DM, Nicolosi RJ. Individual variation in serum cholesterol levels. Proc. Natl. Acad. Sci. U. S. A. 1987;84:6259–6261. doi: 10.1073/pnas.84.17.6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keidar S, Attias J, Smith J, Breslow JL, Hayek T. The angiotensin-II receptor antagonist, losartan, inhibits LDL lipid peroxidation and atherosclerosis in apolipoprotein E-deficient mice. Biochem. Biophys. Res. Commun. 1997;236:622–625. doi: 10.1006/bbrc.1997.6844. [DOI] [PubMed] [Google Scholar]
- Kolodgie FD, Katocs AS, Jr, Largis EE, et al. Hypercholesterolemia in the rabbit induced by feeding graded amounts of low-level cholesterol. Methodological considerations regarding individual variability in response to dietary cholesterol and development of lesion type. Arterioscler. Thromb. Vasc. Biol. 1996;16:1454–1464. doi: 10.1161/01.atv.16.12.1454. [DOI] [PubMed] [Google Scholar]
- Kritchevsky D, Tepper SA, Vesselinovitch D, Wissler RW. Cholesterol vehicle in experimental atherosclerosis. 13. Randomized peanut oil. Atherosclerosis. 1973;17:225–243. doi: 10.1016/0021-9150(73)90090-7. [DOI] [PubMed] [Google Scholar]
- Kritchevsky D, Tepper SA, Wright S, Tso P, Czarnecki SK. Influence of conjugated linoleic acid (CLA) on establishment and progression of atherosclerosis in rabbits. J. Am. Coll. Nutr. 2000;19:472S–477S. doi: 10.1080/07315724.2000.10718950. [DOI] [PubMed] [Google Scholar]
- Lewis B. Plasma lipid concentrations: the concept of “normality” and its implications for detection of high cardiovascular risk. J. Clin. Pathol. 1987;40:1118–1127. doi: 10.1136/jcp.40.9.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind S, Olsson AG, Eriksson M, Rudling M, Eggertsen G, Angelin B. Autosomal recessive hypercholesterolaemia: normalization of plasma LDL cholesterol by ezetimibe in combination with statin treatment. J. Intern. Med. 2004;256:406–412. doi: 10.1111/j.1365-2796.2004.01401.x. [DOI] [PubMed] [Google Scholar]
- McDuffee AT, Senisterra G, Huntley S, et al. Proteins containing non-native disulfide bonds generated by oxidative stress can act as signals for the induction of the heat shock response. J. Cell. Physiol. 1997;171:143–151. doi: 10.1002/(SICI)1097-4652(199705)171:2<143::AID-JCP4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- McNamara DJ. Dietary cholesterol and atherosclerosis. Biochim. Biophys. Acta. 2000;1529:310–320. doi: 10.1016/s1388-1981(00)00156-6. [DOI] [PubMed] [Google Scholar]
- Meir KS, Leitersdorf E. Atherosclerosis in the apolipoprotein-E-deficient mouse: a decade of progress. Arterioscler. Thromb. Vasc. Biol. 2004;24:1006–1014. doi: 10.1161/01.ATV.0000128849.12617.f4. [DOI] [PubMed] [Google Scholar]
- Outinen PA, Sood SK, Pfeifer SI, et al. Homocysteine-induced endoplasmic reticulum stress and growth arrest leads to specific changes in gene expression in human vascular endothelial cells. Blood. 1999;94:959–967. [PubMed] [Google Scholar]
- Parfett CL, Brudzynski K, Stiller C. Enhanced accumulation of mRNA for 78-kilodalton glucose-regulated protein (GRP78) in tissues of nonobese diabetic mice. Biochem. Cell Biol. 1990;68:1428–1432. doi: 10.1139/o90-206. [DOI] [PubMed] [Google Scholar]
- Prior MJ, Williams EV, Shukla HS, Phillips S, Vig S, Lewis M. Prospective randomized controlled trial comparing Lichtenstein with modified Bassini repair of inguinal hernia. J. R. Coll. Surg. Edinb. 1998;43:82–86. [PubMed] [Google Scholar]
- Rekka EA, Chrysselis MC. Nitric oxide in atherosclerosis. Mini Rev. Med. Chem. 2002;2:585–593. doi: 10.2174/1389557023405666. [DOI] [PubMed] [Google Scholar]
- Shekelle RB, Stamler J. Dietary cholesterol and ischaemic heart disease. Lancet. 1989;1:1177–1179. doi: 10.1016/s0140-6736(89)92759-1. [DOI] [PubMed] [Google Scholar]
- Sitia R, Braakman I. Quality control in the endoplasmic reticulum protein factory. Nature. 2003;426:891–894. doi: 10.1038/nature02262. [DOI] [PubMed] [Google Scholar]
- Sorensen S, Ranheim T, Bakken KS, Leren TP, Kulseth MA. Retention of mutant low density lipoprotein receptor in endoplasmic reticulum (ER) leads to ER stress. J. Biol. Chem. 2006;281:468–476. doi: 10.1074/jbc.M507071200. [DOI] [PubMed] [Google Scholar]
- Sugano M, Makino N. Changes in plasma lipoprotein cholesterol levels by antisense oligodeoxynucleotides against cholesteryl ester transfer protein in cholesterol-fed rabbits. J. Biol. Chem. 1996;271:19080–19083. doi: 10.1074/jbc.271.32.19080. [DOI] [PubMed] [Google Scholar]
- Teixeira F, Beja ML, Serra e Silva P, et al. Experimental atherogenesis and vascular noradrenaline content in NZW rabbits and activity of a new nicotinic acid derivative (L 44-0) J. Lipid Mediat. 1991;3:167–175. [PubMed] [Google Scholar]
- Tsiotra PC, Tsigos C. Stress, the endoplasmic reticulum, and insulin resistance. Ann. N. Y. Acad. Sci. 2006;1083:63–76. doi: 10.1196/annals.1367.007. [DOI] [PubMed] [Google Scholar]
- Verhoef P, van Vliet T, Olthof MR, Katan MB. A high-protein diet increases postprandial but not fasting plasma total homocysteine concentrations: a dietary controlled, crossover trial in healthy volunteers. Am. J. Clin. Nutr. 2005;82:553–558. doi: 10.1093/ajcn.82.3.553. [DOI] [PubMed] [Google Scholar]
- Wassmann S, Czech T, van Eickels M, Fleming I, Bohm M, Nickenig G. Inhibition of diet-induced atherosclerosis and endothelial dysfunction in apolipoprotein E/angiotensin II type 1A receptor double-knockout mice. Circulation. 2004;110:3062–3067. doi: 10.1161/01.CIR.0000137970.47771.AF. [DOI] [PubMed] [Google Scholar]
- Wennmalm A. Endothelial nitric oxide and cardiovascular disease. J. Intern. Med. 1994;235:317–327. doi: 10.1111/j.1365-2796.1994.tb01081.x. [DOI] [PubMed] [Google Scholar]
- Wu Y, Zhang H, Dong Y, Park YM, Ip C. Endoplasmic reticulum stress signal mediators are targets of selenium action. Cancer Res. 2005;65:9073–9079. doi: 10.1158/0008-5472.CAN-05-2016. [DOI] [PubMed] [Google Scholar]
- Zhou J, Werstuck GH, Lhotak S, et al. Association of multiple cellular stress pathways with accelerated atherosclerosis in hyperhomocysteinemic apolipoprotein E-deficient mice. Circulation. 2004;110:207–213. doi: 10.1161/01.CIR.0000134487.51510.97. [DOI] [PubMed] [Google Scholar]
- Zulli A, Hare DL. High dietray methionine plus cholesterol stimulates early atherosclerosis and late fibrous cap development which is associated with a decrease in GRP78 positive plaque cells. Int. J. Exp. Pathol. 2009 doi: 10.1111/j.1365-2613.2009.00649.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zulli A, Widdop RE, Hare DL, Buxton BF, Black MJ. High methionine and cholesterol diet abolishes endothelial relaxation. Arterioscler. Thromb. Vasc. Biol. 2003;23:1358–1363. doi: 10.1161/01.ATV.0000080686.39871.54. [DOI] [PubMed] [Google Scholar]
- Zulli A, Hare DL, Buxton BF, Black MJ. High dietary methionine plus cholesterol exacerbates atherosclerosis formation in the left main coronary artery of rabbits. Atherosclerosis. 2004;176:83–89. doi: 10.1016/j.atherosclerosis.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Zulli A, Hare DL, Buxton BF, Black MJ. The combination of high dietary methionine plus cholesterol induces myocardial fibrosis in rabbits. Atherosclerosis. 2006a;185:278–281. doi: 10.1016/j.atherosclerosis.2005.06.036. [DOI] [PubMed] [Google Scholar]
- Zulli A, Buxton BF, Black MJ, Ming Z, Cameron A, Hare DL. The immunoquantification of caveolin-1 and eNOS in human and rabbit diseased blood vessels. J. Histochem. Cytochem. 2006b;54:151–159. doi: 10.1369/jhc.5A6677.2005. [DOI] [PubMed] [Google Scholar]