Abstract
Atherosclerosis has been associated with increased oxidative stress and monocyte recruitment by endothelial cells. Sub-endothelial basement membrane proteins, such as laminins that play a central role in cell adhesion, are exposed to reactive oxygen species. In the present study monocyte attachment on human umbilical cord vein endothelial cells (HUVEC) that were preattached to oxidized or native laminin, was investigated. Intracellular cell adhesion molecule-1 (ICAM-1) expression by HUVEC was estimated by an enzyme-linked immunosorbent assay. HUVEC attachment to oxidized or native laminin-1 was examined using the Hemacolor kit. Anti-alphaL, anti-alphaM, anti-alpha2 and anti-beta2 integrin subunit antibodies were used in order to further investigate the above phenomena. HUVEC that were preattached to oxidized laminin expressed higher levels of ICAM-1 and monocytes attached at a higher degree to these cells as compared to HUVEC that were preattached to native laminin. Incubation of monocytes with monoclonal antibodies against the alphaM and beta2 integrin subunits equalized the above mentioned differences. Moreover, HUVEC attached to oxidized laminin at a higher degree as compared to native laminin. This difference was equalized after incubation with the antibody against the alpha2 integrin subunit. These results indicate a modified interaction between HUVEC and the basement membranes in cases where laminin is oxidatively modified. This modified interaction results in increased ICAM-1 expression by endothelial cells and consequently increased monocyte recruitment capacity.
Keywords: attachment, human umbilical cord vein endothelial cells, laminin-1, monocytes, oxidation
Basement membrane (BM) proteins can be oxidized after their exposure to reactive oxygen species such as superoxide anions, hydrogen peroxide and hydroxyl radicals (Ahmed et al. 2003). This oxidation may alter protein configuration and properties, thus affecting its interactions with several types of cells, including monocytes and endothelial cells and therefore may represent a novel mechanism in the initiation of atherosclerosis.
Laminins are multidomain and multifunctional cross-shaped glycoproteins which are key structural and functional components of the basement membranes. Moreover, they play a central role in many biological functions such as cell adhesion, differentiation and migration of several cell types (Castronovo 1993; De Arcangelis et al., 1996). Previous studies performed in our laboratory indicated that laminin as well as collagen IV carbonylation strongly influenced its interactions with monocytes (Kostidou et al. 2007, 2008). Accordingly, laminin oxidation/carbonylation may also change its interactions with endothelial cells affecting their subsequent interactions with monocytes and contributing in this way to the formation of the atheromatic lesion.
Monocyte attachment to and migration through the activated endothelium represents the first crucial step which leads to the initiation of atherosclerosis (Osterud & Bjorklid 2003). Firm adhesion between monocytes and endothelial cells is mediated by the interactions between the supergene immunoglobins (ICAM-1, ICAM-2, VCAM-1) located on endothelial cells with their ligands, the beta2 integrins (β2, CD18) located on most leukocytes (Anderson 1995). Intracellular cell adhesion molecule-1 (ICAM-1) promotes monocyte attachment to endothelial cells through interaction with the beta2 (CD11/CD18) integrins. CD11 subunits, alphaL (aL, CD11a) and alphaM (aM, CD11b) are expressed on monocytes and bind to different domains on ICAM-1 (Anderson 1995; Huo & Ley 2001). ICAM-1 release and expression is induced by several cytokines (Carley et al. 1999) or ROS, such as hydrogen peroxide (Lo et al. 1993) and is regulated transcriptionally (Panes et al. 1995). It has been reported that chemotactic factors, ROS and phorbol esters induce increased attachment of neutrophils to endothelial cells by the involvement of the beta2 (CD11/CD18) integrins (Anderson et al. 1986; Seliak et al. 1994). However, the involvement of the endothelial cell BM interaction to the above phenomenon has never been studied.
In the present study, monocyte attachment to HUVEC that were preattached to either native or oxidized laminin has been investigated.
Materials and methods
Materials
Laminin-1 was isolated from EHS (Engelbreth-Holm-Swarm) tumour. Medium Earle 199, basal Iscove medium (IMDM) plus NaHCO3 and Percoll were purchased from Biochrom (Cambridge, UK). 3,3′,5,5′-Tetramethylbenzidine dihydrochloride (TMB) tablet, anti-mouse antibody HRP-conjugated IgG and o-dianisidinedihydrochloride tablet were from Sigma (St. Louis, MO, USA). Hydrogen peroxide (30%), ascorbic acid and Hemacolor staining kit were from Merck (Darmstadt, Germany). Anti-ICAM-1 monoclonal antibody [Mouse anti-human IgG1, epitope FL (h)] was purchased from Santa Cruz Biotechnology, Inc (Heidelberg, Germany). Ferrous ammonium sulphate and hexadecyltrimethylammonium bromide were from Fluka (Seelze, Germany). Acetic acid was from Applichem (Darmstadt, Germany) and anti-CD49b-FITC (Mouse anti-human IgG 2ak, clone HAS6) was from Ancell (Bayport, MN, USA). Anti-CD11a (Mouse anti-human IgG2a, clone 38), anti-CD11b (Mouse anti-human IgG1, clone ICRF44) and anti-CD18 (Rat anti-human IgG2b, clone YFC 118,3) were from AbD Serotec (Düsseldorf, Germany). All other reagents were of analytical grades and were obtained from commercial sources.
Participating subjects
Endothelial cells were isolated from the umbilical cords of 15 healthy newborns. Monocytes were prepared by differential centrifugation and solid face attachment from blood samples taken from 15 healthy volunteers. All samples were tested for each parameter/experiment.
Laminin oxidation
Laminin-1 was isolated from EHS (Engelbreth-Holm-Swarm) tumour and it was oxidized as described elsewhere (Alamdari et al. 2005). In brief, laminin in PBS was incubated with 100 mM EDTA, 833 mM ascorbic acid and 100 mM ferrous ammonium sulphate for 1 h at 37 °C. An overnight dialysis against PBS at 4 °C with two buffer changes followed and then oxidized laminin was stored at −80 °C. For the estimation of laminin oxidation, a high-sensitivity ELISA (enzyme-linked immunosorbent assay) method first established in our laboratory was used (Alamdari et al. 2005), while the level of in vitro-produced oxidized laminin was quantified using a colorimetric carbonyl assay (data not shown) (Alamdari et al. 2005).
Endothelial cell preparation and culture
Endothelial cells were isolated from the umbilical cords of healthy newborns by the collagenase perfusion method as previously described by Jaffe et al. with minor modifications (Jaffe et al. 1973). In brief, the umbilical cord veins were carefully washed with sterile phosphate buffered saline (PBS) (0.9% NaCl, pH 7.4) and were then filled with Earle 199 medium that contained 0.5 μg/ml collagenase, for 10 min at room temperature. The content was then emptied in a clean, sterile falcon and the umbilical cord vein was washed with Earle 199 medium (without collagenase). The total content was then centrifuged for 10 min at 260 g and finally resuspended in complete Earle 199 medium (20% FCS, 25 mM HEPES, 50 μl Pen/Strep, 0.25 μg/ml fungizon, 50 μg/ml gentamycin, 90 μg/ml heparin). The cells were then placed in flasks precoated with 0.2% w/v gelatin in PBS and cultured at 37 °C until they reach confluence. Before use, the cells were trypsinised with 0.05% trypsin and 0.02% EDTA, centrifuged for 10 min at 260 g and finally resuspended in complete Earle 199 medium.
Monocyte preparation
Monocytes were isolated from blood samples drawn in the morning before breakfast from healthy donors through a modification of a previously described method (Seager Danciger et al. 2004). In brief, heparinized whole blood was diluted with PBS 1× (1 mM EDTA, pH 7.2) and underlayered with the use of an 18-gauge spinal needle with Ficoll-Paque Plus (1.077 g/ml) in 50 ml falcon tubes. After centrifugation (400 g/20 min/RT/no brake) the peripheral blood mononuclear cell (PBMC) layer was collected and three washes with PBS 1× (1 mM EDTA, pH 7.2) followed. PBMCs were then diluted with IMDM and overlayered on 46% Percoll in 50 ml falcon tubes. After centrifugation (550 g/30 min/RT/no brake) the monocyte layer was collected, diluted with PBS 1× (1 mM EDTA, pH 7.2) and washed with cold PBS 1× before use in experiments. The number of the isolated monocytes was estimated by measurement on a Neubauer plate after a 1:1 dilution with 0.4% trypan blue solution. A total of 3–4 million cells/ml were isolated each time with a viability of at least 98%. Flow cytometry using a CD14 monoclonal antibody labelled with phycoerythrin revealed that more than 85% of the cells were monocytes.
Endothelial cell adhesion assay
Endothelial cell attachment was measured on microwells. 80,000 cells resuspended in Earle medium (10% FCS) were placed in each well of polystyrene plates that had been precoated with 37.5 μg/ml oxidized or native laminin-1. The cells were incubated in the wells for 30 min at 37 °C and after incubation, non-adherent cells were discarded by aspiration and the wells were rinsed three times with sterile PBS (pH 6.0). Endothelial cells that attached to laminin were stained with the Hemacolor staining kit, immersed in 200 μl 10% acetic acid added to each well in order to remove the bound dye and incubated on an orbital shaker for 3–4 min. The optical density of the stained solution was finally measured at 590 nm.
Endothelial cell attachment to laminin in the presence of the anti-human alpha2 (a2, CD49b) integrin subunit
One to two million HUVEC were washed and preincubated on ice for 5 min with 20 μl of 250 μg/ml human IgG in order to block non-specific binding and were then incubated for 45 min on ice and in the dark with 80 μl of a monoclonal anti-alpha2 integrin subunit (initial concentration: 0.25 mg/ml) at 1:50 dilution. Cells that were incubated with 250 μg/ml human IgG in the absence of the anti-alpha2 integrin subunit used as controls. Cells were then washed three times with a specific buffer that contained 50 mM sodium phosphate pH 7.5, 100 mM potassium chloride, 150 mM NaCl, 5% glycerol and 0.2% BSA. For the dilution of the antibody the same buffer was used.
Human umbilical cord vein endothelial cells attachment to native and oxidized laminin was then estimated as described above.
ELISA assay for measuring ICAM-1 expression in endothelial cells
One hundred microliters of cell suspension (80,000 cells in Earle 199 medium, 10% FCS) were placed in each well of polystyrene plates that had been precoated with 37.5 μg/ml either native or oxidized laminin-1. Cells were then incubated in the wells overnight at 37 °C. In pilot experiments the number of cells attached to either oxidized or native laminin under these conditions (overnight incubation) was estimated using the Hemacolor kit as described above and was found equal.
After incubation, culture medium was discarded by aspiration and the wells were rinsed one time with 100 μl sterile PBS 1×. ICAM-1 expression in endothelial cells attached to native or oxidized laminin was estimated through modifications of a previously described method (Shingu et al. 1994). In brief, 50 μl of 0.2 μg/well mouse anti-ICAM MoAb in Earle 199 medium were added to each well and incubated for 1 h at 37 °C. After incubation, the wells were washed three times with PBS 1× and 100 μl of a horseradish peroxidase-conjugated anti-mouse IgG 1:10,000 diluted in Earle 199 medium were added to each well and incubated for 1 h at 37 °C. The wells were then washed four times with PBS 1× and 100 μl of a 3,3′,5,5′-tetramethylbenzidine (TMB) solution in substrate buffer (1.455 g Na2HPO4, 1.91 g citric acid, pH 5 and 2 μl 30% H2O2, final volume: 200 ml) were added to each well and incubated for 50 min at 37 °C. The optical density was finally read at 450 nm using an ELISA reader.
Monocyte adhesion assay
Monocyte attachment was measured on microwells. 80,000 cells in IMDM, (10% FCS) were placed in each well of polystyrene plates precoated with endothelial cells (106/ml) that had been preattached to 37.5 μg/ml oxidized or native laminin-1 as described above. Monocytes were incubated in the wells for 30 min at 37 °C and after incubation, non-adherent cells were discarded by aspiration and the wells were rinsed three times with sterile PBS (pH 6.0). Monocyte attachment was quantified using the myeloperoxidase (MPO) assay as previously described (Koliakos et al., 2004). In brief, monocytes were lysed with 0.5% (w/v) hexadecyltrimethylammonium bromide in PBS (pH 6.0) for 30 min at 37 °C. After lysis, 50 μl 0.2 mg/ml dianisidinedihydrochloride in PBS (pH 6.0) containing 0.4 mM H2O2 were added to each well. After 15 min, the MPO activity of the lysate was measured spectrophotometrically at 405 nm, using an ELISA reader.
Monocyte attachment to HUVEC that had been preattached to either oxidised or native laminin, in the presence of the anti-human alphaL (aL, CD11a), alphaM (aM, CD11b) and beta2 (b2, CD18) integrin subunits
One to two million monocytes were washed and preincubated on ice for 5 min with 20 μl of 250 μg/ml human IgG in order to block non-specific binding and were then incubated for 45 min on ice and in the dark with 10 μl of 0.1 mg/ml anti-human alphaL (CD11a), or 0.1 mg/ml anti-human alphaM (CD11b) or 0.1 mg/ml anti-human beta2 (CD18) integrin subunits. Cells were then washed three times with PBS. Monocyte attachment to HUVEC that had been preattached to either oxidized or non-oxidized laminin was then estimated as described above.
Statistical analysis
For the statistical evaluation, the statistical software GraphPad InStat version 3.00 for Windows was used (GraphPad Software Inc., San Diego, CA, USA). Values are expressed as means ± standard error of means (SEMs). The statistical significance of the differences between the sets of data was estimated by the Student’s t-test (paired or un-paired). In addition, when appropriate, one-way anova with Bonferroni or Dunnett post-test was used. The two-tailed P < 0.05 was used as the significance level.
Results
Endothelial cell attachment to oxidized and native laminin-1. The role of the alpha2 integrin subunit
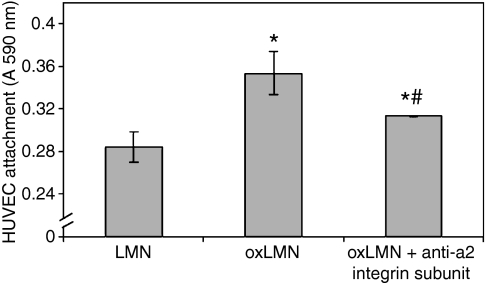
In an attempt to investigate whether laminin oxidation could affect its interactions with endothelial cells, endothelial cell attachment to oxidized and non-oxidized laminin after 30 min of incubation was studied. Our results indicated that HUVEC attach to oxidized laminin at a higher degree in relation to the non-oxidized molecule after 30 min of incubation (P ≤ 0.05) (Figure 1). Moreover, incubation of HUVEC with the antibody against the alpha2 integrin subunit equalized the previously observed differences (P ≤ 0.05) (Figure 1).
Figure 1.
Endothelial cell attachment to oxidized or native laminin-1 in the presence or absence of the antibody against the alpha2 (a2) integrin subunit (anti-CD49b). Bars represent mean value ± standard error of means of at least 15 independent experiments. * indicates significant difference with the value obtained in endothelial cells that attached to non-oxidized laminin-1. # indicates significant difference with the value obtained in endothelial cells that attached to oxidized laminin-1.
On the other hand, after overnight incubation all the cells attached to the matrix either native or oxidized. Therefore in the following experiments preattached endothelial cells were used after overnight incubation.
ICAM-1 expression in endothelial cells attached to oxidized and native laminin-1
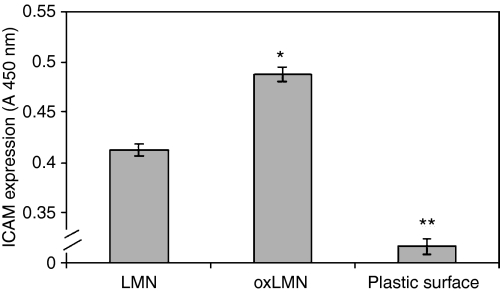
In order to quantitate any differences in relation to ICAM-1 expression between endothelial cells attached to oxidized laminin and those attached to the native molecule we used a sensitive ELISA assay (Shingu et al. 1994). ICAM expression was also estimated to HUVEC attached to plastic surface. Plastic surface is referred to the bottom of the well of the ELISA plate used to estimate ICAM expression of HUVEC attached to either oxidized or native laminin. These samples were used as negative controls to estimate HUVEC attachment to a non-protein substrate. HUVEC attachment to plastic surface was estimated to 0.316 (A450 nm).
Our results indicated that endothelial cells that attached to oxidized laminin-1 expressed higher levels of ICAM-1 as compared to those attached to the non-oxidized molecule (P < 0.0001) (Figure 2).
Figure 2.
ICAM-1 expression in endothelial cells that attached to either oxidized or native laminin-1. Bars represent mean value ± standard error of means of at least 15 independent experiments. * indicates significant difference with the value obtained in endothelial cells that attached to non-oxidized laminin-1. ** indicates significant difference with the value obtained in endothelial cells that attached to either oxidized or native laminin-1.
Monocyte attachment to endothelial cells that were attached to oxidized or native laminin-1
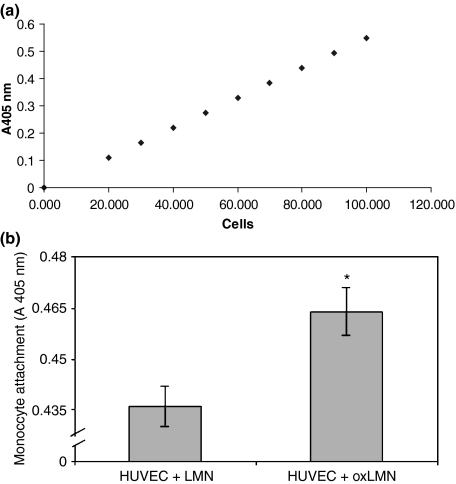
As we found that HUVEC attached to oxidized laminin expressed higher levels of ICAM-1, therefore we investigated whether their monocyte recruitment capacity was increased accordingly. For the quantification of unknown monocyte samples attached to HUVEC that were preattached to oxidized or native laminin, a standard curve used, based on the absorbance (A405 nm) of different proportions of MPO, which reflects the different amount of cells (100,000 = 100% MPO) (Figure 3a).
Figure 3.
(a) Standard curve for the quantification of unknown monocyte samples attached to HUVEC that were preattached to oxidized or native laminin. (b) Monocyte attachment to endothelial cells that had been preattached to either oxidized or non-oxidized laminin-1. Bars represent mean value ± standard error of means of at least 15 independent experiments. * indicates significant difference with the value obtained in monocytes that attached to endothelial cells that had been preattached to native laminin-1.
According to our results, monocytes attached at a higher degree to endothelial cells that were attached to oxidized laminin, as compared to those attached to the native molecule (P = 0.005) (Figure 3). Moreover, as monocyte attachment to endothelium is mainly mediated by ICAM-1 interaction with the integrins alphaLbeta2 (aLb2) and alphaMbeta2 (aMb2), we studied monocyte attachment on HUVEC that were attached to oxidized or native laminin, in the presence or absence of anti-alphaM and anti-beta2 integrin subunit antibodies. Our results indicated that incubation of monocytes with the antibody against the alphaM and beta2 integrin subunits equalized the previously observed differences (P < 0.0001 in both cases), while incubation with the antibody against the alphaL integrin subunit did not affect HUVEC attachment to oxLMN (Table 1) (Dunnett post-test: P < 0.0001, Bonferroni post-test: P < 0.0001). Moreover, our results showed that the differences on monocyte attachment were not attributed to differences in the number of endothelial cells attached to either oxidized or native laminin, as under our experimental conditions HUVEC attached at the same degree to either oxidized or native laminin (data not shown).
Table 1.
Monocyte attachment on HUVEC that were attached to oxidized or native laminin, in the presence of the anti-alphaL (CD11a), or alphaM (CD11b) or beta2 (CD18) integrin subunits
| Treatment | Number of cells ± SD# (×103) |
|---|---|
| LMN | 91.7 ± 3.6 |
| LMN + anti-alphaL (CD11a) | 95.3 ± 9* |
| LMN + anti-alphaM (CD11b) | 90.5 ± 0.2 |
| LMN + anti-beta2 (CD18) | 91.7 ± 3.7 |
| oxLMN | 95.2 ± 0.26* |
| oxLMN + anti-alphaL (CD11a) | 95.8 ± 0.2 |
| oxLMN + anti-alphaM (CD11b) | 75.4 ± 1.5** |
| oxLMN + anti-beta2 (CD18) | 90.1 ± 0.3** |
Mean of at least 10 independent experiments.
Significant difference with the LMN value (P < 0.05).
Significant difference with the oxLMN value (P < 0.05).
Discussion
The results of the present study indicate an increased monocyte attachment rate to surface of the endothelial cells (HUVEC) that were attached to the oxidized laminin as compared to the same cells attached to native laminin. This is the first time that increased attachment of monocytes to endothelial cells is associated with laminin oxidation.
Incubation of monocytes with antibodies against the alphaM and beta2 integrin but not alphaL integrin subunits equalized the previously observed differences. In accordance with our results, previous studies showed that stimulated monocyte adherence to endothelial cells was mediated by the alphaM (CD11b) (Prieto et al. 1988; Hassall et al. 1991), but not the alphaL (CD11a) integrin subunits (Prieto et al. 1988) when specific antibodies against the above integrins were used.
Moreover, it has been reported that monocyte affinity to the surface of endothelial cells is dependent on ICAM-1 expression as ICAM-1 is one of the major monocyte attachment molecules on the endothelium cell surface (Huo & Ley 2001).
Accordingly, the data of the present study indicate that ICAM-1 expression was higher on the surface of HUVEC cells attached to the oxidized laminin in comparison to HUVEC attached to the native molecule. For the estimation of monocyte attachment and ICAM-1 expression on the surface of HUVEC, overnight incubation of HUVEC to laminin was performed in order to achieve the attachment of all the cells (100%) to either oxidized or native laminin. When monocytes were added to the endothelial cell substrate, the endothelial cells had already completely attached to either oxidized or non-oxidized laminin. According to our knowledge, after overnight incubation, no differentiation of HUVEC cells has been reported. Furthermore, the population of the HUVEC cells used was homogenous, while the cells added to either oxidized or native laminin were derived from the same sample.
Accordingly, the differences observed could not be attributed to the number of cells attached to laminin, but to the oxidative modification of the protein itself.
A further finding of the present study was that HUVEC cells attach to oxidized laminin with a higher affinity as compared to the native molecule after 30 min of incubation. To our knowledge this is the first time that laminin oxidation is associated with endothelial cell attachment. It should be noted that previous studies performed in our laboratory showed that monocytes attach at a higher degree to oxidized laminin as compared to the non-oxidized molecule (Kostidou et al. 2007; Kostidou et al., 2009). For the estimation of differences in HUVEC affinity to laminin, the cells were incubated with laminin for only 30 min. It is conceivable that HUVEC require more time to completely attach to laminin. Accordingly, after overnight incubation all seeded cells attached to both forms of laminin, native and oxidized. Based on the above, it could be conjectured that there is time dependency on the initial HUVEC attachment to laminin that reflects differences in the affinity between cells and oxidized or native laminin.
The increased affinity of endothelium to oxidized laminin as compared to the native molecule may reflect an in vivo tendency for faster coverage of lesions exposed to oxidative stress by endothelial cells. Oxidative stress is a common feature of atherosclerosis and therefore, extracellular matrix oxidation may be also common in atherosclerosis.
Human umbilical cord vein endothelial cells interact with extracellular matrix mainly through various integrin receptors, each one recognizing specific extracellular sequences (Cines et al. 1998; Garmy-Susini & Varner 2008). Τhe differences on HUVEC attachment to laminin observed between the oxidized and the native molecule could be attributed to cell-laminin interactions via different integrin receptors. Accordingly, it could be assumed that the addition of negative charged residues by laminin oxidation could change the shape and conformation of the molecule leading to modified interactions with the cells. Oxidation could release some new integrin binding sites on laminin’s surface, which were cryptic under non-oxidative conditions.
Our data also indicate that the a2 integrin subunit plays a specific role for the attachment of HUVEC to the oxidized but not to the native laminin. Alpha2beta1 integrin is known to be involved in atherosclerosis (Grenache et al. 2003), and was found increased in diabetes mellitus and metabolic syndrome (Jin et al. 1996; Kostidou et al. 2007; Kostidou et al., 2009) however apart from the a2 integrin subunit our data do not exclude the involvement of other cell surface molecules on the described phenomenon.
Laminin-1, used in the present paper, is considered the prototype laminin because it can be easily isolated from the matrix of the Engelbreth-Holm-Swarm (EHS) tumour (Timpl et al. 1979). The vascular-specific laminin isoforms that encounter with monocytes during extravasation are laminin-8 and laminin-10 (Pedraza et al. 2000). Interestingly, laminin-1, laminin-8 and laminin-10 share the same beta and gamma chains (beta1-gamma1) (Miner et al. 1997). Accordingly, it could be assumed that the above chains, which are prone to phosphorylation (Trachana et al. 2005), may also be prone to other types of post-translational modification, such as oxidation.
Summarizing our data, it is suggested that the oxidatively modified laminin activates endothelial cells, possibly via alpha 2 integrins and triggers them to express increased levels of ICAM-1 and other cell surface molecules, recruiting blood monocytes on the endothelium surface. Upregulation of ICAM-1 expression on endothelial cell surface has been also reported after endothelial cell binding on oxidized fibrinogen (Shcheglovitova et al. 2006).and fibronectin (Orr et al. 2005). An increased production of ICAM by dysfunctional endothelial cells was reported to cause an increased attachment of monocytes and T lymphocytes in atherosclerosis (Gimbrone & Topper 1999). However, this is the first time that increased ICAM expression by endothelium and monocyte attachment is associated with oxidized laminin. Activated endothelial cells are known to release a variety of molecules including chemokines, which have been reported to activate integrins that are required for a more sufficient binding of mononuclear cells to endothelium (Weber et al. 1996). So, activated endothelium could induce in this way alphaMbeta2 (CD11b/CD18) integrin activation in monocytes, while increased ICAM-1 expression could recruit monocytes through interaction with the activated alphaMbeta2 (CD11b/CD18) integrins.
Our data do not exclude the involvement of other adhesion molecules, such as VCAM-1 and selectins in the above phenomenon. It is noteworthy that VCAM-1 was found to play a key role in the initiation of the atherosclerotic process in mice, while its expression in luminal endothelium has been reported to precede subendothelial accumulation of macrophages (Dansky et al. 2001). However, the present study was focused on the ICAM-1 expression in endothelial cells that attached to oxidized laminin.
In conclusion, our data indicate for the first time that in cases where basement membrane proteins such as laminins are oxidatively modified the interaction between endothelia and the basement membranes can be also modified with the involvement of the alpha2 integrin subunit. This modified interaction can result in increased monocyte recruitment capacity attributed to increased ICAM-1 expression with the involvement of the alphaM and beta2 integrin subunits. These initial observations, further substantiated with future studies, may lead to novel insights on the initiation and progress mechanism of atherosclerotic lesions.
Acknowledgments
This paper is part of the 03ED29 research project, implemented within the framework of the “Reinforcement Programme of Human Research Manpower” (PENED) and co-financed by National and Community Funds (25% from the Greek Ministry of Development-General Secretariat of Research and Technology and 75% from E.U.-European Social Fund).
References
- Ahmed S, Adamidis A, Jan LC, Gibbons N, Mattana J. Dexamethasone attenuates oxidation of extracellular matrix proteins by human monocytes. Exp. Mol. Pathol. 2003;75:137–143. doi: 10.1016/s0014-4800(03)00070-4. [DOI] [PubMed] [Google Scholar]
- Alamdari DH, Kostidou E, Paletas K, et al. A simplified and rapid ELISA method for measuring carbonyl in samples with low amounts of protein. Free Radic. Biol. Med. 2005;39:1362–1367. doi: 10.1016/j.freeradbiomed.2005.06.023. [DOI] [PubMed] [Google Scholar]
- Anderson DC. The role of the β2 integrins and intracellular adhesion molecule type 1 in inflammation. In: Granger DN, Schmid-Schobein GW, editors. Physiology and Pathophysiology of Leukocyte Adhesion. 1st edn. New York: Oxford University Press; 1995. pp. 3–42. [Google Scholar]
- Anderson DC, Miller LG, Schamaltstieg FC, Rothlein TA, Springer TA. Contribution of the Mac-1 glycoprotein family to adherence-dependence granulocyte functions: structure-function assessments employing subunit-specific monoclonal antibodies. J. Immunol. 1986;137:15–27. [PubMed] [Google Scholar]
- Carley W, Ligon G, Phan S, et al. Distinct ICAM-1 forms and expression pathways in synovial microvascular endothelial cells. Cell. Mol. Biol. 1999;45:79–88. [PubMed] [Google Scholar]
- Castronovo V. Laminin receptors and laminin-binding proteins during tumour invasion and metastasis. Inv. Met. 1993;13:1–30. [PubMed] [Google Scholar]
- Cines D, Pollak E, Buck C, et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91(10):3527–3561. [PubMed] [Google Scholar]
- Dansky M, Barlow B, Lominska C, et al. Adhesion of monocytes to arterial endothelium and initiation of atherosclerosis are critically dependent on vascular cell adhesion molecule-1 gene dosage. Arterioscler. Thromb. Vasc. Biol. 2001;21:1662–1667. doi: 10.1161/hq1001.096625. [DOI] [PubMed] [Google Scholar]
- De Arcangelis A, Neuville P, Boukamel R, Lefebvre O, Kediger M, Simon-Assmann P. Inhibition of laminin alpha 1-chain expression leads to alteration of basement membrane assembly and cell differentiation. J. Cell Biol. 1996;133:291–299. doi: 10.1083/jcb.133.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garmy-Susini B, Varner J. Roles of integrins in tumor angiogenesis and lymphangiogenesis. Lymphat. Res. Biol. 2008;6(3–4):155–163. doi: 10.1089/lrb.2008.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimbrone M, Topper J. Biology of the vessel wall endothelium. In: KR Chen., editor. Molecular Basis of Cardiovascular Disease. Philadelphia: WB Saunders; 1999. pp. 331–348. [Google Scholar]
- Grenache D, Coleman T, Semenkovich C, Santoro S, Zutter M. α2β1 integrin and development of atherosclerosis in a mouse model. Assessment of risk. Arterioscler. Thromb. Vasc. Biol. 2003;23:2104–2109. doi: 10.1161/01.ATV.0000097282.22923.EF. [DOI] [PubMed] [Google Scholar]
- Hassall D, Bath P, Gladwin A, Beesley J. CD11/CD18 cell surface adhesion glycoproteins: discordance of monocyte function and expression in response to stimulation. Exp. Cell Res. 1991;196:346–352. doi: 10.1016/0014-4827(91)90270-5. [DOI] [PubMed] [Google Scholar]
- Huo Y, Ley K. Adhesion molecules and atherogenesis. Acta Physiol. Scand. 2001;173:35–43. doi: 10.1046/j.1365-201X.2001.00882.x. [DOI] [PubMed] [Google Scholar]
- Jaffe EA, Nachman RL, Becker CG, Minick LR. Culture of human endothelial cells derived from umbilical cord vein. J. Clin. Invest. 1973;52:2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin DK, Fish AJ, Wayner EA, et al. Distribution of integrin subunits in human diabetic kidneys. J. Am. Soc. Nephrol. 1996;7(12):2636–2645. doi: 10.1681/ASN.V7122636. [DOI] [PubMed] [Google Scholar]
- Koliakos G, Zolota Z, Paletas K, Kaloyianni M. High glucose concentrations stimulate human monocyte sodium/hydrogen exchanger activity and modulate atherosclerosis-related functions. Eur. J. Physiol. 2004;449:298–306. doi: 10.1007/s00424-004-1340-z. [DOI] [PubMed] [Google Scholar]
- Kostidou E, Koliakos G, Alamdari DH, Paletas K, Tsapas A, Kaloyianni M. Enhanced laminin carbonylation by monocytes in diabetes mellitus. Clin. Biochem. 2007;40:671–679. doi: 10.1016/j.clinbiochem.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Kostidou E, Koliakos G, Paletas K, Kaloyianni M. Monocyte attachment and migration through collagen IV in diabetes mellitus. Mol. Cells. 2008;25(3):452–456. [PubMed] [Google Scholar]
- Kostidou E, Koliakos G, Kaloyianni M. Increased monocyte alphaL, alphaM and beta2 integrin subunits in diabetes mellitus. Clin. Biochem. 2009;42:634–640. doi: 10.1016/j.clinbiochem.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Lo SK, Janakidevi K, Lai L, Malik A. Hydrogen peroxide-induced increase in endothelial adhesiveness in dependent on ICAM-1 activation. Am. J. Physiol. 1993;264:406–412. doi: 10.1152/ajplung.1993.264.4.L406. [DOI] [PubMed] [Google Scholar]
- Miner J, Patton B, Lentz S, et al. The laminin α chains: expression, developmental transitions, and chromosomal locations of α1-5, identification of heterotrimeric laminins 8-11, and cloning of a novel α3 isoform. J. Cell Biol. 1997;137(3):685–701. doi: 10.1083/jcb.137.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr W, Sanders J, Bevard M, Coleman E, Sarembock I, Schawartz A. The subendothelial extracellular matrix modulates NF-kB activation by flow: a potential role in atherosclerosis. J. Cell Biol. 2005;169(1):191–202. doi: 10.1083/jcb.200410073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterud B, Bjorklid E. Role of monocytes in atherogenesis. Physiol. Rev. 2003;83:1069–1112. doi: 10.1152/physrev.00005.2003. [DOI] [PubMed] [Google Scholar]
- Panes J, Perry MA, Anderson DC, et al. Regional differences in constitutive and induced ICAM-1 expression in vivo. Am. J. Physiol. 1995;269:1955–1964. doi: 10.1152/ajpheart.1995.269.6.H1955. (6 pt 2) [DOI] [PubMed] [Google Scholar]
- Pedraza C, Geberhiwot T, Ingerpuu S, et al. Monocytic cells synthesize, adhere to, and migrate on laminin-8 (alpha 4 beta 1 gamma 1) J. Immunol. 2000;165:5831–5838. doi: 10.4049/jimmunol.165.10.5831. [DOI] [PubMed] [Google Scholar]
- Prieto J, Beatty P, Clark A, Patarroyo M. Molecules mediating adhesion of T and B cells, monocytes and granulocytes to vascular endothelial cells. Immunology. 1988;63:631–637. [PMC free article] [PubMed] [Google Scholar]
- Seager Danciger J, Lutz M, Harna S, et al. Method for large scale isolation, culture and cryopreservation of human monocytes suitable for chemotaxis, cellular adhesion assays, macrophage and dendritic cell differentiation. J. Immunol. Methods. 2004;288:123–134. doi: 10.1016/j.jim.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Seliak H, Franzini E, Hakim J, Pasquier C. Reactive oxygen species rapidly increase endothelial ICAM-1 ability to bind neutrophils without detectable upregulation. Blood. 1994;83(9):2669–2677. [PubMed] [Google Scholar]
- Shcheglovitova ON, Azizova OA, Romanov Yu A, et al. Oxidized forms of fibrinogen induce expression of cell adhesion molecules by cultured endothelial cells from human blood vessels. Bull. Exp. Biol. Med. 2006;142(3):308–312. doi: 10.1007/s10517-006-0353-3. [DOI] [PubMed] [Google Scholar]
- Shingu M, Hashimoto M, Ezaki I, Nobunaga M. Effect of cytokine-induced soluble ICAM-1 from human synovial cells on synovial cell-lymphocyte adhesion. Clin. Exp. Immunol. 1994;97:46–51. doi: 10.1111/j.1365-2249.1994.tb06605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpl R, Rohde H, Robey PG, Rennard SI, Foidart JM, Martin GR. Laminin: a glycoprotein from basement membranes. J. Biol. Chem. 1979;254:9933–9937. [PubMed] [Google Scholar]
- Trachana V, Christophorides E, Kouzi-Koliakos K, Koliakos G. Laminin-1 is phosphorylated by ecto-protein kinases of monocytes. Int. J. Biochem. 2005;37:478–492. doi: 10.1016/j.biocel.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Weber C, Alon R, Moser B, Springer T. Sequential regulation of a4b1 and a5b1 integrin avidity by CC chemokines in monocytes: implications for transendothelial chemotaxis. J. Cell Biol. 1996;134:1063–1073. doi: 10.1083/jcb.134.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]