Abstract
The aim of this study was to estimate the relationship of endothelial dysfunction induced by intracellular S-adenosylhomocysteine (SAH) accumulation and DNA methylation in human umbilical vein endothelial cells (HUVEC). The isolated HUVEC were incubated with 3-deazaadenosine (DZA) to induce experimental intracellular SAH accumulation. The impairment of HUVEC function was assessed by changes in morphology and proliferative ability. The expression of DNA methyltransferase-1 (DNMT1) and the atherosclerosis related genes [oestrogen receptor-alpha (ER-α), extracellular superoxide dismutase (EC-SOD) and monocyte chemoattractant protein-1 (MCP-1)] were analysed using quantitative real-time PCR. Global DNA methylated status was measured using the cytosine extension assay. The methylated patterns of ER-α, EC-SOD and MCP-1 genes were determined with methylation-specific PCR. We found that DZA administration increased intracellular SAH levels progressively and simultaneously decreased Hcy content in medium. Moreover, the supplementation induced HUVEC apoptosis, inhibited proliferation ability and DNMT1 mRNA expression (P<0.05) and furthermore reduced global DNA methylation status (P<0.05). Correlation analysis showed the presence of a negative correlation between intracellular SAH concentration, proliferative ability, and expression of ER-α, EC-SOD, and DNMT1 (r = −0.89, −0.86, −0.92 and −0.88 respectively, P<0.001); and a positive correlation with MCP-1 expression and DNA [3H]-dCTP incorporation (r = 0.89 and 0.93 respectively, P<0.001). Our results showed that endothelial dysfunction induced by intracellular SAH accumulation is mediated by regulating the expression of atherosclerosis related genes in HUVEC, which is not related with gene promoter methylated patterns, but may be associated with altered global DNA hypomethylated status. These findings suggest that SAH can act as the potential molecular biological marker in the promotion of atherogenesis.
Keywords: 3-deazaadenosine, DNA methyltransferase-1, extracellular superoxide dismutase, methylation, monocyte chemoattractant protein-1, oestrogen receptor-alpha, S-adenosylhomocysteine
The pathogenesis of atherosclerosis is complicated and many mechanisms have been proposed (Kinlay & Egido 2006; Riccioni et al. 2007). Previous research has shown that hyperhomocysteinaemia is an independent risk factor for vascular diseases (Boushey et al. 1995; Clarke 1998; Boers 2000; Pandey et al. 2006; Naushad et al. 2007). However, there is a controversy about the association between hyperhomocysteinaemia and vascular disease. Endothelial dysfunction plays a crucial role in homocysteine (Hcy)-induced vascular pathobiology (Weiss et al. 2002; Hadi et al. 2005). Both in vitro and in vivo studies failed to provide a satisfying explanation for increased susceptibility of endothelial cells to the adverse effect of increased levels of circulating total homocysteine (tHcy) (Weiss et al. 2002; Castro et al. 2003, 2005). Increasing attention is being paid to an indirect mechanism for Hcy toxicity (Christopher et al. 2002; Castro et al. 2003, 2005; Perna et al. 2003), secondary to the increment of S-adenosylhomocysteine (SAH), which can inhibit cellular methyltransferases regulating DNA methylation (James et al. 2002; Perna et al. 2003; Castro et al. 2006; Lee & Wang 2006).
Aberrant DNA methylation, leading to inappropriate gene expression and promotion of disease, has been recognised increasingly in atherogenesis during the past few years (Dong et al. 2002; Hiltunen & Ylä-Herttuala 2003; Lund et al. 2004; Zaina et al. 2005). The concomitance of global genome hypomethylation and specific gene hypermethylation is the notable characteristic in the development of atherosclerosis (Dong et al. 2002). It has been documented that SAH affects DNA methylated status, and the endothelial dysfunction induced by hyperhomocysteinaemia is positive correlated with increment of SAH in experimental animals (Chen et al. 2001; Dayal et al. 2001; Caudill et al. 2006) and in human (Yi et al. 2000; Perna et al., 2001; Castro et al. 2005). Therefore, SAH has been regarded as a likely primary determinant for the causal relationship between hyperhomocysteinaemia and vascular disease (Yi et al. 2000; Chen et al. 2001; Dayal et al. 2001; Perna et al., 2001,; Castro et al. 2005; Caudill et al. 2006). However, whether or not intracellular SAH accumulation damages vascular endothelial cells via its influence on DNA methylated patterns has not yet been substantiated fully by experimental evidence.
In the present study, isolated primary HUVEC were selected as an experimental model because primary endothelial cells have been used as in vitro models to study the ready properties of vascular endothelium, mainly because of availability of umbilical cords (Castro et al. 2005). Because mammalian cells can not transfer SAH into cells (Christopher et al. 2002), we used 3-deazaadenosine (DZA), a potent inhibitor of SAH hydrolase (SAHH), to elevate the intracellular SAH level. This study was designed to evaluate the impairment of intracellular SAH accumulation induced by DZA on vascular endothelial cells, and the relationship effect of this to DNA methylation in vitro.
Materials and methods
Cell culture and treatment
Human umbilical vein endothelial cells were obtained from umbilical cords of healthy foetuses from uncomplicated pregnancies and vaginal deliveries from healthy mothers as previously described (Jaffe et al. 2006). The investigation was approved by ethics committees for human set by Sun Yat-sen University and conformed to the principles outlined in the Declaration of Helsinki. Then, HUVEC were successively seeded until confluent monolayer was reached in 75-cm2 culture flasks to enable the access to proper amount of cells for study. After the removal of the culture medium, fresh medium without (control) or with 5, 10 and 20 μmol/l DZA (Sigma-Aldrich, St Louis, MO, USA) was added and incubated for another 24–72 h. The possible cytotoxicity of the tested DZA was excluded. For this purpose, after 72 h of incubation, lactate dehydrogenase (LDH) release was evaluated in the cell culture medium using the Cytotoxicity Detection Kit (Roche Molecular Biochemicals, Mannheim, Germany). After that, the incubation medium was collected at 24, 48 or 72 h of incubation for tHcy quantification, the cells were harvested and two aliquots were taken, one of which was exposed to cell denaturation buffer for SAH evaluation, and the other was for further RNA and DNA extraction.
Determination of total homocysteine and SAH
The incubation medium (2 ml) were collected at 24, 48 and 72 h of incubation for tHcy quantification using fluorescence polarization immunoassay (FPIA) with homocysteine reagent pack (abbott AXSYM™ system, Chicago, IL, USA). For intracellular SAH quantification, the cells were digested with 0.25% trypsinase and exposed to cell denaturation buffer. The cytosol was deproteinized with an equal volume of 10% perchloric acid, and the obtained supernatant was analysed by stable-isotope dilution LC-tandem mass spectrometry (MS/MS) as previously described (Struys et al. 2000).
Observation of morphology and ultrastructural organization
Human umbilical vein endothelial cells were plated on sterile chamber slides in 12-well microtitre plates at 1 × 106 per well and allowed to attach for 24 h. The tested DZA was added to the wells and incubated for an additional 24–72 h. For observation of morphology with phase contrast microscope, HUVEC were fixed with 4% paraformaldehyde in PBS at pH7.4 for 30 min and stained with haematoxylin-eosin (HE). For observation of ultrastructural organization, cells were fixed with glutaraldehyde and osmic acid, and then observed under a Hitachi H300 (Tokyo, Japan) electron microscope.
Proliferation ability assessment
In 96-well microtitre plates, 1 × 104 cells/well were seeded and treated with tested DZA for another 24–72 h. Thereafter, 20 μl of 5 mg/ml methyl thiazolyl tetrazolium (MTT) in phosphate buffered solution (PBS) were added to each well and the cultures were incubated for an additional 4 h at 37 °C. The supernatant was aspirated and formazan crystals were dissolved in 150 μl dimethyl sulphoxide. Absorbance at 490 nm was read by an automated plate reader. The proliferation inhibitory rate = (1−ODexperimental group/ODcontrol group) × 100%.
Cell cycle distribution analysis
After treatment without (control) or with the tested DZA for 24–72 h, the cells were collected to centrifugal tubes, centrifuged at 35,000 g for 5 min, and washed twice with PBS. The distribution of cell cycle phases was assessed by CycleTEST™ PLUS DNA kit (BD Biosciences Pharmingen, San Diego, CA, USA) with a BD FACScan (Macintosh operation platform, BD CellQuest1.0 analysis software).
Apoptosis assay
To determine the levels of apoptosis by TUNEL, the cells seeded on chamber slides were incubated with/without 20 μmol/l 3-deazaadenosine (DZA) for 24, 48 and 72 h, and fixed with 4% paraformaldehyde in PBS at pH7.4 for 30 min. Then the apoptotic cells were detected with an In Situ Cell Death Detection kit (Boehringer Mannheim GmbH, Mannheim, Germany) according to the manufacturer’s instructions. The TUNEL technique consists of labelling the free 3′-hydroxyl ends of a DNA fragment by enzymatically adding a nucleotide (i.e., digoxigenin-dUTP) covalently attached to digoxigenin using terminal deoxynucleotidyl transferase (TdT). Detection of the digoxigenin molecule covalently attached to the nucleotide can be accomplished by using an anti-digoxigenin antibody conjugated to fluorescein on a confocal microscope, which fluoresces green. Thus, DNA-breaks that are labelled by TdT with the digoxigenin-dUTP are tagged with the fluorescent antibody causing apoptotic cells to fluoresce green.
Genes expression analysed with quantitative real-time PCR
To analyse the expression of genes, a quantitative real-time PCR (qRT-PCR) was used as in our previous study (Yu et al. 2007). The primer and probe sequences used were as follows: for ER-α gene, forward 5′-CGCGCCAAAACAGTCATG-3′, reverse 5′-AGGTACTGCCCGCACTGAAT-3′, and probe 5′-FAM-ACTCCAAGACCCACCCTCCCAAGTGTAMRA-3′; for EC-SOD gene, forward 5′-GCGGAGCCCAACTCTGACT-3′, reverse 5′-TGCCAGATCTCCGTGACCTT-3′, and probe 5′-FAM-CGGAGTGGATCCGAGACATGTACGC-TAMRA-3′; for MCP-1 gene, forward 5′-GCTGTGATCT-TCAAGACCATTGTG-3′, reverse 5′-TGGAATCCTGAACCCACTTCTG -3′, and probe 5′-FAM-CCAAGGAGATCTGTGCTGACCCCAA-TAMRA-3′; for DNMT1 gene, forward 5′-CGCGCCAAAACAGTCATG -3′, reverse 5′-AGGTACTGCCCGCACTGAAT-3′, and probe 5′-FAM-ACTCCAAGACCCACCCTCCCAAGTG-TAMRA-3′; for β-actin, forward 5′-GCGCGGCTACAGCTTCA-3′, reverse 5′-TCTCCTTAATGTCACGCACGAT-3′, and probe 5′-FAM-CACCACGGCCGAGCGGGA-TAMRA-3′. Levels of the different mRNAs were subsequently normalized to β-actin mRNA levels.
Assessment of global DNA methylated status
Human umbilical vein endothelial cells were incubated without (control) or with the tested DZA and collected at 24, 48 or 72 h of incubation. Genomic DNA was extracted from the HUVEC nucleus using Universal Genomic DNA Extraction Kit Ver. 3.0 (Takara bioengineering Inc, Dalian, China). All the DNA samples had a high molecular weight of >20 kb as assessed by gel electrophoresis and an A260/A280 absorbance ratio of >1.8. Assessment of the global DNA methylated status was accomplished using the cytosine extension assay as described previously (Pogribny et al. 1999). The results were expressed as [3H]-dCTP incorporation/0.5 μg DNA. The radiolabel incorporation was considered proportional to the number of unmethylated sites in DNA. Therefore, an increase in relative [3H]-dCTP incorporation reflects a hypomethylated status.
Genes promoter methylated patterns analysed with methylation-specific PCR
To address the fact that the altered expression of atherosclerosis-related genes is associated with their promoter methylated patterns, methylation-specific PCR (MSP) was performed in CpG-rich regions of ER-α, EC-SOD and MCP-1 promoters as previous procedure (Herman et al. 1996). Bisulfite modification protocols of DNA were completed with EZ DNA Methylation-Gold Kit™ (ZYMO RESEARCH CORP, Orange, CA, USA). The oligonucleotide primers were designed from http://www.ucsf.edu/urogene/methprimer and were synthesized by Takara Biotechnology Inc. The unmethylation (U) and methylation (M) primer sequences used were as follows: for ER-α, unmethylation forward (Uf) 5′-GAATGAGTTGGAGTTTTTGAATTGT-3′ and unmethylation reverse (Ur) 5′-AAAAACCAATCTAACCATAAACCTACA-3′, methylation forward (Mf) 5′-GAACGAGTTGGAGTTTTTGAATC-3′ and methylation reverse (Mr) 5′-CCGATCTAACCGTAAACCTACG-3′, which afforded 176 and 171 bp fragments respectively; for EC-SOD, Uf 5′-GGGATGATGATGGTATGTTTTATG-3′ and Ur 5′-ACCAAAATCACCCAAATACTACAAA-3′, Mf 5′-GACGACGACGGTACGTTTTAC-3′ and Mr 5′-GAAATCGCCCGAATACTACG-3′, which produced 284 and 279 bp fragments respectively; for MCP-1, Uf 5′-GTGGTTTGAAGGTAAGTTGGTAGT-3′ and Ur 5′-AAAAAAAACAAAAAATCAAAACAAA-3′, Mf 5′-TGGTTTGAAGGTAAGTTGGTAGC-3′ and Mr 5′-AAAAAAAACAAAAAATCAAAACGAA-3′, which gave 229 and 228 bp fragments respectively. Their corresponding sequences were located in promoter region 457–483 bp for ER-α, 670–1428 bp for EC-SOD, 2890–3050 bp for MCP-1. The MSP products were directly loaded onto 1.5% agarose gels and electrophoresed for observation.
Statistical analysis
Results are given as mean and standard deviation ( ± SD). Statistical differences of the levels of tHcy and SAH, proliferation ability, genes mRNA expression and DNA [3H]-dCTP incorporation between incubations with the tested DZA and control were calculated using Student’s t test. Correlations of intracellular SAH content with proliferation capability, DNA [3H]-dCTP incorporation and genes mRNA expression were analysed using the least-squares method. P values were two-tailed. Differences were considered significant if P<0.05. SPSS version 13.0 (SPSS, Chicago, IL, USA) was used for all statistical analysis.
± SD). Statistical differences of the levels of tHcy and SAH, proliferation ability, genes mRNA expression and DNA [3H]-dCTP incorporation between incubations with the tested DZA and control were calculated using Student’s t test. Correlations of intracellular SAH content with proliferation capability, DNA [3H]-dCTP incorporation and genes mRNA expression were analysed using the least-squares method. P values were two-tailed. Differences were considered significant if P<0.05. SPSS version 13.0 (SPSS, Chicago, IL, USA) was used for all statistical analysis.
Results
Lactate dehydrogenase
There was no statistical difference in cell LDH release after 72 h of incubation, in the absence (100 ± 6.6%) and presence of the tested DZA concentrations (5 μmol/l: 99.1 ± 8.7%; 10 μmol/l: 103.5 ± 11.3%; and 20 μmol/l: 102.2 ± 9.6%) (n = 6, P>0.05). Thus, any severe cell cytotoxic effect for the DZA concentrations used was excluded in this study.
The levels of total homocysteine and SAH
Under normal culture medium, there was a progressive increase of tHcy concentration in the medium over culture time in a time- and dose-dependent manner (P<0.05). This is a normal property of cultured endothelial cells (Castro et al. 2005). In the presence of DZA, tHcy concentration was lower than that of incubation at the same culture time with normal culture medium (Figure 1a). But the intracellular concentration of SAH in HUVEC incubated with normal culture medium maintained stable levels with culture time, even incubating for 72 h (P>0.05). This is a normal homeostatic property of endothelial cells to SAH. In the presence of DZA, as expected, there was a progressive increase in intracellular SAH concentration in a time- and dose-dependent manner (P<0.05) (Figure 1b).
Figure 1.
The levels of total homocsteine (tHcy) in culture medium (a) and intracellular S-adenosylhomocysteine (SAH) (b) in HUVEC incubated with 0 (white), 5 (light grey), 10 (deep grey) and 20 (black) μmol/l 3-deazaadenosine (DZA) for 24–72 h. Values are means for three determinations for each time point, with standard deviations represented by vertical bars. *P<0.05, **P<0.01. Mean value was significantly different from that for HUVEC at the same culture time incubated without DZA (Student’s t-test).
Morphology and ultrastructural organization
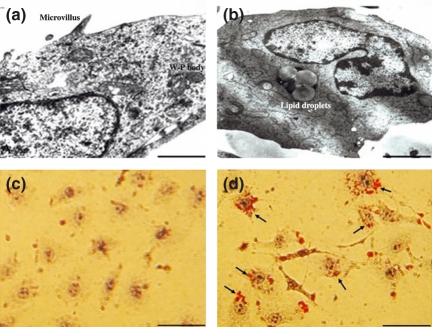
The HUVEC incubated with normal culture medium was cobblestone-like and arranged compactly when observed under phase contrast microscopy. The special organelles of vascular endothelial cells, including Weibel-Palade bodies (W-P), plasmalemmal vesicles, and microvilli around cells, were observed by electron microscopy (Figure 2a). Supplementation of the culture medium with DZA, cell shrinkage and the cell density also decreased. Its arrangement became sparse, many slender apophyses appeared at the two poles and the shape became irregular. Under electron microscopy, lipid droplets were presented around the nucleaus, and there was karyopyknosis, margined and swollen chromatin, dilated endocytoplasmic reticulum, folded nuclear membrane and degeneration of nucleus were observed (Figure 2b).
Figure 2.
Examples of HUVEC incubated without (a, c) or with (b, d) 20 μmol/l 3-deazaadenosine (DZA) for 72 h showing the changes of ultrastructural organization (Bar = 2.5 μm) detected with electronmicroscope (a, b) and lipid droplets accumulation (Bar = 100 μm) with oil red O dyeing (c, d). Images are representative of fields from three experiments.
It was interesting that many lipid droplets gathered around the nucleaus in the presence of DZA. Reinforcing this phenomenon, we validated it by oil red O dyeing (Figure 2). We found that there was negative staining in HUVEC in the absence of DZA (Figure 2c), but there were many red strained areas in HUVEC in the presence of DZA (Figure 2d).
Proliferative ability and apoptosis
Cell proliferative capability was documented as absorbency at OD570 nm (Table1). There was a significant decrease under the supplement with DZA at the same culture time (P<0.05). The inhibition ratio of DZA on HUVEC proliferation was from 10.8% to 77.6% ranging with different concentrations of DZA. The percentage of cells in the G1/G0 phase of the cell cycle was increased progressively from 53.46 ± 12.35% in normal culture medium to 86.20 ± 13.57% in the tested DZA culture medium (P<0.05), but the percentage of S phase was decreased from 35.19 ± 8.96% to 8.10 ± 2.46% by flow cytometry (P<0.05). There was a negative correlation between intracellular SAH concentration and the proliferation capability (r = −0.89, P<0.001). The data demonstrates that intracellular SAH accumulation inhibits HUVEC growth and mainly arrests in the G1/G0 phase.
Table 1.
The proliferation activity of HUVEC incubated with different concentrations of 3-deazaadenosine (DZA) for 24, 48 and 72 h detected by methyl thiazolyl tetrazolium (MTT) method
| Proliferation activity (OD570) |
|||
|---|---|---|---|
| DZA concentration (μmol/l) | 24 h | 48 h | 72 h |
| 0 (n = 8) | 0.352 ± 0.102 | 0.557 ± 0.198 | 1.106 ± 0.357 |
| 5 (n = 7) | 0.314 ± 0.115 | 0.471 ± 0.186* | 0.920 ± 0.324** |
| 10 (n = 7) | 0.201 ± 0.096* | 0.357 ± 0.137** | 0.428 ± 0.173** |
| 20 (n = 7) | 0.152 ± 0.074** | 0.298 ± 0.079** | 0.248 ± 0.061** |
Values are means ± SD for six determinations.
P<0.05
P<0.01.
Mean value was significantly different from that for HUVEC at the same culture time incubated with 0 μmol/l DZA (Student’s t-test).
Thereafter, we detected the presence of apoptosis in HUVEC incubated with/without 20 μmol/l DZA for 24–72 h using TUNEL technique (Figure 3). In the normal culture medium there was hardly any apoptotic cells (Figure 3a). However, supplementation of the culture medium with 20 μmol/l DZA, the apoptotic cells showing by flourescence emerged from at 24 h (Figure 3b), and the density of flourescence was progressively reinforced with the incubation time from 24 to 72 h (Figure 3b,c and d). The results based on the density of flourescence demonstrate that DZA administration induces apoptosis of HUVEC in time-dependent manner.
Figure 3.
TUNEL analysis of apoptosis presence in HUVEC treated with 20 μmol/l 3-deazaadenosine (DZA) for 0 (a), 24 (b), 48 (c) and 72 h (d) on a confocal microscope. Images are representative of fields from the same experiment (Bar = 200 μm).
Atherosclerosis-related genes and DNMT1 mRNA expression
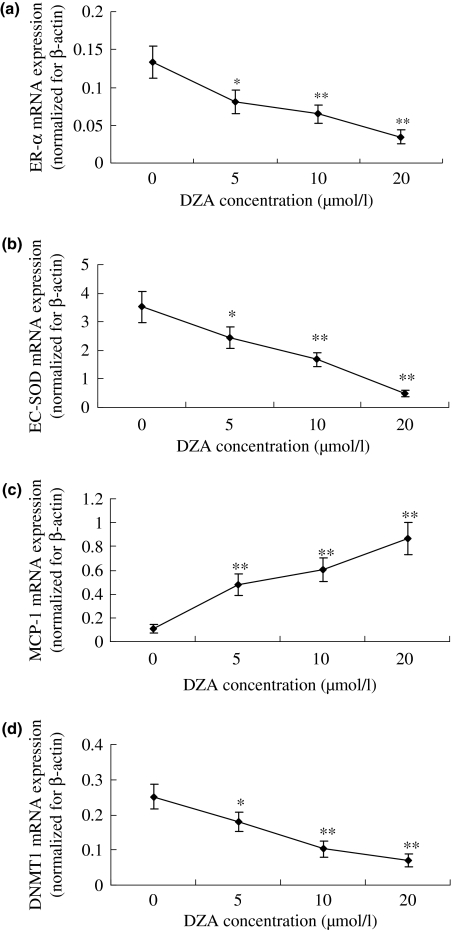
The relative expression of ER-α (Figure 4a), EC-SOD (Figure 4b), and DNMT1 (Figure 4d) mRNA in HUVEC cultured with DZA supplementation was lower than that seen after incubation with normal culture medium (P<0.05), but the MCP-1 (Figure 4c) mRNA expression was increased (P<0.05). The correlation coefficient of intracellular SAH concentration with ER-α, MCP-1, EC-SOD, DNMT1 expression were −0.86, 0.89, −0.92 and −0.88 respectively (P<0.001).The data indicate that the intracellular SAH accumulation inhibits DNMT-1, ER-α and EC-SOD expression, but stimulates MCP-1 expression.
Figure 4.
The relative ER-α (a), EC-SOD (b), MCP-1 (c) and DNMT1 (d) mRNA expression normalized for corresponding β-actin levels in HUVEC incubated with the tested 3-deazaadenosine (DZA) for 72 h detected with quantitative real-time PCR (qRT-PCR) as our previous method (Yu et al. 2007). Values are means for three determinations for each time point, with standard deviations represented by vertical bars. *P<0.05, **P<0.01. Mean value was significantly different from that for HUVEC at the same culture time incubated without DZA (Student’s t-test).
Global DNA methylated status
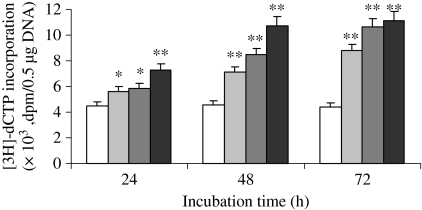
In the presence of DZA, there was a progressive increase of the [3H]-dCTP radiolabel incorporation in a dose- and time-dependent manner (P<0.05) (Figure 5). Correlation analysis showed the presence of a positive correlation between intracellular SAH concentration and DNA [3H]-dCTP incorporation (r = 0.93, P<0.001). The result suggests that intracellular SAH accumulation is associated with DNA hypomethylation.
Figure 5.
The global DNA methylated status in HUVEC incubated with 0 (white), 5 (light grey), 10 (deep grey) and 20 (black) μmol/l 3-deazaadenosine (DZA) for 24–72 h analysed with cytosine extension assay (Pogribny et al. 1999). Values are means for three determinations for each time point, with standard deviations represented by vertical bars. *P<0.05, **P<0.01. Mean value was significantly different from that for HUVEC at the same culture time incubated without DZA (Student’s t-test).
Atherosclerosis-related genes promoter methylated patterns
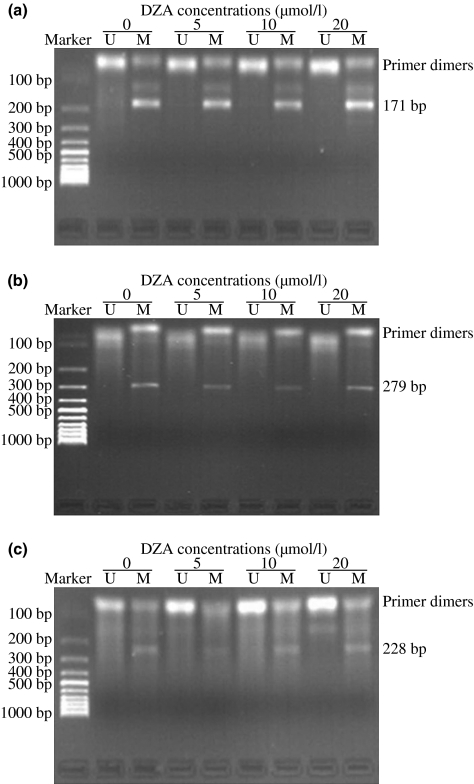
There was a bright zone of MSP product for methylation primer (M), not unmethylation primer (U), in ER-α (Figure 6a), MCP-1 (Figure 6b), EC-SOD (Figure 6c) gene in HUVEC cultured with normal medium. Their methylation patterns showed no obvious change in the presence of DZA, even after incubation for 72 h (Figure 6). Therefore, it appears that the altered expression of atherosclerosis-related genes upon intracellular SAH accumulation induced by DZA is not relative to gene promoter methylated patterns.
Figure 6.
The promoter methylation of ER-α (a), MCP-1 (b), EC-SOD (c) in HUVEC incubated with/without the tested 3-deazaadenosine (DZA) for 72 h detected with methylation specific PCR (MSP) (Herman et al. 1996). Images are representative of fields from three experiments. U, unmethylation primer; M, methylation primer.
Discussion
The most significant finding of this study is that, in HUVEC, intracellular SAH accumulation induced by DZA, under the normal level of tHcy in culture medium, inhibits proliferation (mainly arrested at the G1/G0 phase), and alters morphological and ultrastructural organization, even to early apoptosis. This suggests that intracellular SAH accumulation damages vascular endothelial cells.
Impairment of normal homeostatic properties of the vascular endothelium is an early event in the promotion of atherogenesis. HUVEC, like most other cells, lack some key enzymes in Hcy transsulfuration (van der Molen et al. 1997) and remethylation pathways (Zaina et al. 2005). Thus, HUVEC are expected to be susceptible to continuous exposure of high SAH level and to the effects induced by cellular methylation impairment (Castro et al. 2005). Though hyperhomocysteinaemia is an established risk factor for vascular disease (McCully 1969; Boushey et al. 1995; Clarke 1998; Boers 2000; Hadi et al. 2005; Pandey et al. 2006) and many kinds of mechanisms proposed have been (Harker et al. 1974; Hopkins et al. 1997; Damiel et al. 2000; Morita et al. 2001; Auer et al. 2002; Piolot et al. 2003), but the question whether homocysteine is a cause, a fellow traveller, or a marker of pathogenic mechanisms in vascular disease remains controversial (Christopher et al. 2002; Perna et al. 2003; Castro et al. 2005). Most animal models of hereditary severe hyperhomocysteinaemia, such as the cystathionine ß-synthase-null mouse, do not develop atherosclerotic lesions spontaneously (Watanabe et al. 1995). The positive correlation evidence of Hcy as an independent risk factor is not supported by the findings from clinical research (Kerins et al. 2000; Ridker et al. 2001; O’Grady et al. 2002), and the results from high pharmaco-concentration in vitro hardly reflect the real pathological effects of Hcy in vivo (O’Grady et al. 2002; Fruchart et al. 2004). In addition, such studies have demonstrated successfully that dietary supplementation with B vitamins or folic acid cannot prevent arterial lesions or thrombotic events, despite normalization of homocysteine levels, in rats, rabbits and pigs fed a methionine-rich diet (Ambrosi et al. 1999; Lentz et al. 2001; Zhou et al. 2001; Zhang et al. 2004). Therefore, plasma tHcy increase may be a concomitant phenomenon promoting atherosclerosis complications (Brattstrom & Wilcken 2000; Ueland et al. 2000; Troen et al. 2003).
The questionarises, what is the direct-acting molecule of methionine-rich dietary atherogenic action? By supplementing HUVEC culture medium with DZA, a potent inhibitor of SAH, there was progressive intracellular SAH increase and a concomitant decrease in tHcy in culture medium (r = −0.91, P<0.001). Castro et al. (Castro et al. 2005) also have demonstrated that supplementation of the culture medium with adenosine-2,3-dialdehyde, another SAHH inhibitor, increases the intracellular SAH concentration in a dose-dependent manner. Our results are consistent with these findings. Moreover, we found that intracellular SAH accumulation induced by DZA damages endothelial cells, as changes of morphology and proliferation ability, even to apoptosis. Based on these results, we deduced that the aetiological factor for vascular disease induced by hyperhomocysteinaemia is probably a result of the increment of SAH.
In this experiment, we also observed that, in HUVEC, an increase of intracellular SAH concentration is associated with the reduced methylation of global DNA (r = −0.93, P<0.001), probably as a result of an SAH-mediated inhibition of the DNMT1 expression (r = −0.88, P<0.001). These observations are also consistent with previous findings (Castro et al. 2005). Global DNA hypomethylation is an important mechanism involving in aged-related chronic diseases, including atherosclerosis (Hiltunen et al. 2002; Holliday 2006). Recently, increasing attention is being paid to DNA methyltransferase inhibition modulated by intracellular accumulation of SAH (Christopher et al. 2002; Perna et al. 2003; Castro et al. 2005). Published data, obtained under a variety of experimental conditions and using different models, suggest that an elevated SAH level has been used as a marker of impaired cellular methylation (Fu et al. 2000; Melnyk et al. 2000; Caudill et al. 2006). Our results showed that global DNA hypomethylated status is strictly dependent on intracellular SAH levels, and the cell must maintain the physiological SAH content accurately to prevent any alteration in the genomic DNA methylated status. These findings further confirm the theory that SAH acts as a major predictor of global genomic DNA hypomethylated status (Melnyk et al. 2000; Perna et al. 2003; Fruchart et al. 2004; Kortelainen & Huttunen 2004; Caudill et al. 2006).
To explore the mechanism involved in the impairment of HUVEC after intracellular SAH accumulation, we demonstrated intracellular SAH accumulation induced by DZA causes a decrease of ER-α, EC-SOD expression (r = −0.86 and −0.92 respectively, P<0.001) and an increase of MCP-1 expression (r = 0.89, P<0.001). Both the decrease of ER-α, EC-SOD expression and the increase of MCP-1 expression accelerate the development of atherogenesis and blood vessel disease (Kortelainen & Huttunen 2004; Boyle 2005). In contrast to cancer research, the methylated pattern of special genes in the context of atherosclerosis has been the subject of relatively little experimental investigation. The previous data have demonstrated that promoter hypomethylation of ER-α and EC-SOD gene is associated with the development of atherosclerosis in vivo (Laukkanen et al. 1999; Post et al. 1999). But, in the present experience, no obvious changes of promoter methylated patterns of ER-α, EC-SOD and MCP-1 genes were detected after the increment of intracellular SAH. These findings suggest the altered expression of atherosclerosis-related genes induced by intracellular SAH accumulation is not related to their promoter methylated patterns in HUVEC treated with DZA. Besides being a potent inhibitor of SAHH, DZA also has a strong effect on NF-κB signalling, a pathway implicated in atherogenesis (Jeong et al. 1999). Hence, it may be possible that the altered gene expression is mediated by the NF-κB signalling pathway, but the underlying mechanism remains to be fully elucidated.
It is significant that, in the presence of DZA, the finding of many lipid droplets around nuclear observed with electron microscopy was supported by the results with oil red O stain. The reason for this finding is unclear at present. Its clarification may provide a new target for intervention in heart disease.
In conclusion, the present data confirmed that endothelial dysfunction induced by intracellular SAH accumulation is mediated by regulating atherosclerosis-related gene expression. This regulation is not related to their promoter methylated patterns, but may be associated with global DNA hypomethylated status. SAH can act as the potential biological marker in the promotion of atherogenesis. However, the underlying mechanisms remain to be determined whereby global methylated status is important but there is no promoter methylation in differentially expressed genes that are implicated in endothelial dysfunction.
Acknowledgments
This work is supported by grants from the National Natural Science Foundation of China (No: 30771794, 30571568), the Key Project of Chinese Ministry of Education (No: 208123), Youth Found of Department of Science and Technology, Sichuan Province, China (No: 08ZQ026-05), Emphasis Item of Department of Education, Sichuan Province, China (No: 07ZA015), and Found of Chengdu Medical College, China (No: CYZ07-001). We thank Dr. Min Xia and Yuan Zhang for their expert technical assistance and gratefully thank Professor Yixiang Su (Sun Yat-sen University) for most valuable comments on an earlier draft of this study.
References
- Ambrosi P, Rolland PH, Bodard H, et al. Effects of folate supplementation in hyperhomocysteinemic pigs. J. Am. Coll. Cardiol. 1999;34:274–279. doi: 10.1016/s0735-1097(99)00144-8. [DOI] [PubMed] [Google Scholar]
- Auer J, Rammer M, Berent R, Weber T, Lassnig E, Eber B. Lack of association between plasma lipoprotein(a) concentrations and the presence or absence of coronary atherosclerosis. Acta Cardiol. 2002;57:409–414. doi: 10.2143/AC.57.6.2005464. [DOI] [PubMed] [Google Scholar]
- Boers GH. Mild hyperhomocysteinemia is an independent risk factor of arterial vascular disease. Semin. Thromb. Hemost. 2000;26:291–295. doi: 10.1055/s-2000-8096. [DOI] [PubMed] [Google Scholar]
- Boushey CJ, Beresford SA, Omenn GS, Motulsky AG. A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. Probable benefits of increasing folic acid intakes. JAMA. 1995;274:1049–1057. doi: 10.1001/jama.1995.03530130055028. [DOI] [PubMed] [Google Scholar]
- Boyle JJ. Macrophage activation in atherosclerosis pathogenesis and pharmacology of plaque rupture. Curr. Vasc. Pharmacol. 2005;3:63–68. doi: 10.2174/1570161052773861. [DOI] [PubMed] [Google Scholar]
- Brattstrom L, Wilcken DE. Homocysteine and cardiovascular disease: cause or effect? Am. J. Clin. Nutr. 2000;72:315–323. doi: 10.1093/ajcn/72.2.315. [DOI] [PubMed] [Google Scholar]
- Castro R, Rivera I, Struys EA, et al. Increased homocysteine and S-adenosylhomocysteine concentrations and DNA hypomethylation in vascular disease. Clin. Chem. 2003;49:1292–1296. doi: 10.1373/49.8.1292. [DOI] [PubMed] [Google Scholar]
- Castro R, Rivera I, Martins C, et al. Intracellular S-adenosylhomocysteine increased levels are associated with DNA hypomethylation in HUVEC. J. Mol. Med. 2005;83:831–836. doi: 10.1007/s00109-005-0679-8. [DOI] [PubMed] [Google Scholar]
- Castro R, Rivera I, Blom HJ, Jakobs C, de Almeida IT. Homocysteine metabolism, hyperhomocysteinaemia and vascular disease: an overview. J. Inherit. Metab. Dis. 2006;29:3–20. doi: 10.1007/s10545-006-0106-5. [DOI] [PubMed] [Google Scholar]
- Caudill MA, Wang JC, Melnyk S, et al. Intracellular S-adenosylhomocysteine concentrations predict global DNA hypomethylation in tissues of methyl-deficient cystathionine beta-synthase heterozygous mice. J. Nutr. 2006;131:2811–2818. doi: 10.1093/jn/131.11.2811. [DOI] [PubMed] [Google Scholar]
- Chen Z, Karaplis AC, Ackerman SL, et al. Mice deficient in methylenetetrahydrofolate reductase exhibit hyperhomocysteinemia and decreased methylation capacity, with neuropathology and aortic lipid deposition. Hum. Mol. Genet. 2001;10:433–443. doi: 10.1093/hmg/10.5.433. [DOI] [PubMed] [Google Scholar]
- Christopher SA, Melnyk S, James SJ, Kruger WD. S-adenosylhomocysteine, but not homocysteine, is toxic to yeast lacking cystathionine beta-synthase. Mol. Genet. Metab. 2002;75:335–343. doi: 10.1016/S1096-7192(02)00003-3. [DOI] [PubMed] [Google Scholar]
- Clarke R. Homocysteine and cardiovascular disease. Overview. J. Cardiovasc. Risk. 1998;5:213–215. doi: 10.1097/00043798-199808000-00001. [DOI] [PubMed] [Google Scholar]
- Damiel KW, Stanley JD, Baucer ES. Homocysteine stimulates MAP kinase in bovine aortic smooth muscle cells. Surgery. 2000;128:59–63. doi: 10.1067/msy.2000.106531. [DOI] [PubMed] [Google Scholar]
- Dayal S, Bottiglieri T, Arning E, et al. Endothelial dysfunction and elevation of S-adenosylhomocysteine in cystathionine beta-synthase-deficient mice. Circ. Res. 2001;88:1203–1209. doi: 10.1161/hh1101.092180. [DOI] [PubMed] [Google Scholar]
- Dong CM, Yoon WH, Goldschmidt-Clermont PJ. DNA methylation and atherosclerosis. J. Nutr. 2002;132:2406S–2409S. doi: 10.1093/jn/132.8.2406S. [DOI] [PubMed] [Google Scholar]
- Fruchart JC, Nierman MC, Stroes ES, Kastelein JJ, Duriez P. New risk factors for atherosclerosis and patient risk assessment. Circulation. 2004;109:15–19. doi: 10.1161/01.CIR.0000131513.33892.5b. [DOI] [PubMed] [Google Scholar]
- Fu W, Dudman NP, Perry MA, Young K, Wang XL. Interrelations between plasma homocysteine and intracellular S-adenosylhomocysteine. Biochem. Biophys. Res. Commun. 2000;271:47–53. doi: 10.1006/bbrc.2000.2587. [DOI] [PubMed] [Google Scholar]
- Hadi HA, Carr CS, Al Suwaidi J. Endothelial dysfunction: cardiovascular risk factors, therapy, and outcome. Vasc. Health. Risk. Manag. 2005;1:183–198. [PMC free article] [PubMed] [Google Scholar]
- Harker LA, Slichter SJ, Scott CR, Ross R. Homocystinemia. Vascular injury and arterial thrombosis. N. Engl. J. Med. 1974;291:537–543. doi: 10.1056/NEJM197409122911101. [DOI] [PubMed] [Google Scholar]
- Herman JG, Graff JR, Myohanen S, Nelkin BD, Baylin SB. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc. Natl Acad. Sci. USA. 1996;93:9821–9826. doi: 10.1073/pnas.93.18.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiltunen MO, Ylä-Herttuala S. DNA methylation, smooth muscle cells, and atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2003;23:1750–1753. doi: 10.1161/01.ATV.0000092871.30563.41. [DOI] [PubMed] [Google Scholar]
- Hiltunen MO, Turunen MP, Hakkinen TP, et al. DNA hypomethylation and methyltransferase expression in atherosclerotic lesions. Vasc. Med. 2002;7:5–11. doi: 10.1191/1358863x02vm418oa. [DOI] [PubMed] [Google Scholar]
- Holliday R. The inheritance of epigenetic defects. Science. 2006;238:163–170. doi: 10.1126/science.3310230. [DOI] [PubMed] [Google Scholar]
- Hopkins PN, Wu LL, Hunt SC, James BC, Vincent GM, Williams RR. Lipoprotein(a) interactions with lipid and nonlipid risk factors in early familial coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 1997;17:2783–2792. doi: 10.1161/01.atv.17.11.2783. [DOI] [PubMed] [Google Scholar]
- Jaffe EA, Nachman RL, Becker CG, Minick CR. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J. Clin. Invest. 2006;52:2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James SJ, Melnyk S, Pogribna M, Pogribny IP, Caudill MA. Elevation in S-adenosylhomocysteine and DNA hypomethylation: potential epigenetic mechanism for homocysteine-related pathology. J. Nutr. 2002;132:2361S–2366S. doi: 10.1093/jn/132.8.2361S. [DOI] [PubMed] [Google Scholar]
- Jeong SY, Ahn SG, Lee JH, et al. 3-deazaadenosine, a S-adenosylhomocysteine hydrolase inhibitor, has dual effects on NF-kappaB regulation. Inhibition of NF-kappaB transcriptional activity and promotion of IkappaBalpha degradation. J. Biol. Chem. 1999;274:18981–18988. doi: 10.1074/jbc.274.27.18981. [DOI] [PubMed] [Google Scholar]
- Kerins DM, Koury MJ, Capdevila A, Rana S, Wagner C. Plasma S-adenosylhomocysteine is a more sensitive indicator of cardiovascular disease than plasma homocysteine. Am. J. Clin. Nutr. 2000;74:723–729. doi: 10.1093/ajcn/74.6.723. [DOI] [PubMed] [Google Scholar]
- Kinlay S, Egido J. Inflammatory biomarkers in stable atherosclerosis. Am. J. Cardiol. 2006;98:2–8. doi: 10.1016/j.amjcard.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Kortelainen ML, Huttunen P. Expression of estrogen receptors in the coronary arteries of young and premenopausal women in relation to central obesity. Int. J. Obes. Relat. Metab. Disord. 2004;28:623–627. doi: 10.1038/sj.ijo.0802522. [DOI] [PubMed] [Google Scholar]
- Laukkanen MO, Mannermaa S, Hiltunen MO, et al. Local hypomethylation in atherosclerosis found in rabbit ec-sod gene. Arterioscler. Thromb. Vasc. Biol. 1999;19:2171–2178. doi: 10.1161/01.atv.19.9.2171. [DOI] [PubMed] [Google Scholar]
- Lee ME, Wang H. Homocysteine and hypomethylation. A novel link to vascular disease. Trends Cardiovasc. Med. 2006;9:49–54. doi: 10.1016/s1050-1738(99)00002-x. [DOI] [PubMed] [Google Scholar]
- Lentz SR, Piegors DJ, Malinow MR, Heistad DD. Supplementation of atherogenic diet with B vitamins does not prevent atherosclerosis or vascular dysfunction in monkeys. Circulation. 2001;103:1006–1011. doi: 10.1161/01.cir.103.7.1006. [DOI] [PubMed] [Google Scholar]
- Lund G, Andersson L, Lauria M, et al. DNA methylation polymorphisms precede any histological sign of atherosclerosis in mice lacking apolipoprotein E. J. Biol. Chem. 2004;279:29147–29154. doi: 10.1074/jbc.M403618200. [DOI] [PubMed] [Google Scholar]
- McCully KS. Vascular pathology of homocysteinemia: implications for the pathogenesis of arteriosclerosis. Am. J. Pathol. 1969;56:111–128. [PMC free article] [PubMed] [Google Scholar]
- Melnyk S, Pogribna M, Pogribny IP, Yi P, James SJ. Measurement of plasma and intracellular S-adenosylmethionine and S-adenosylhomocysteine utilizing coulometric electrochemical detection: alterations with plasma homocysteine and pyridoxal 5′-phosphate concentrations. Clin. Chem. 2000;46:265–272. [PubMed] [Google Scholar]
- van der Molen EF, Hiipakka MJ, van Lith-Zanders H, et al. Homocysteine metabolism in endothelial cells of a patient homozygous for cystathionine beta-synthase (CS) deficiency. Thromb. Haemost. 1997;78:827–833. [PubMed] [Google Scholar]
- Morita H, Kurihara H, Yoshida S, et al. Diet-induced hyperhomocysteinemia exacerbates neointima formation in rat carotid arteries after balloon injury. Circulation. 2001;103:133–139. doi: 10.1161/01.cir.103.1.133. [DOI] [PubMed] [Google Scholar]
- Naushad S, Jamal NJ, Angalena R, Prasad CK, Devi AR. Hyperhomocysteinemia and the compound heterozygous state for methylene tetrahydrofolate reductase are independent risk factors for deep vein thrombosis among South Indians. Blood Coagul. Fibrinolysis. 2007;18:113–117. doi: 10.1097/MBC.0b013e3280108e01. [DOI] [PubMed] [Google Scholar]
- O’Grady H, Kelly C, Bouchier-Hayes D, Leahy A. Homocysteine and occlusive arterial disease. Br. J. Surg. 2002;89:838–844. doi: 10.1046/j.1365-2168.2002.02108.x. [DOI] [PubMed] [Google Scholar]
- Pandey SN, Vaidya AD, Vaidya RA, Talwalkar S. Hyperhomocysteinemia as a cardiovascular risk factor in Indian women: determinants and directionality. J. Assoc. Physicians India. 2006;54:769–774. [PubMed] [Google Scholar]
- Perna AF, Castaldo P, De Santo NG, et al. Homocysteine and transmethylations in uremia. Kidney Int. 2001a;59:2299–2308. doi: 10.1046/j.1523-1755.2001.59780230.x. [DOI] [PubMed] [Google Scholar]
- Perna AF, Ingrosso D, Satta E, et al. Metabolic consequences of hyperhomocysteinemia in uremia. Am. J. Kidney Dis. 2001b;38:S85–S90. doi: 10.1053/ajkd.2001.27411. [DOI] [PubMed] [Google Scholar]
- Perna AF, Ingrosso D, Lombardi C, et al. Possible mechanisms of homocysteine toxicity. Kidney Int. 2003;84:S137–S140. doi: 10.1046/j.1523-1755.63.s84.33.x. [DOI] [PubMed] [Google Scholar]
- Piolot A, Blache D, Boulet L, et al. Effect of fish oil on LDL oxidation and plasma homocysteine concentrations in health. J. Lab. Clin. Med. 2003;141:41–49. doi: 10.1067/mlc.2003.3. [DOI] [PubMed] [Google Scholar]
- Pogribny I, Yi P, James SJ. A sensitive new method for rapid detection of abnormal methylation patterns in global DNA and within CpG islands. Biochem. Biophys. Res. Commun. 1999;262:624–628. doi: 10.1006/bbrc.1999.1187. [DOI] [PubMed] [Google Scholar]
- Post WS, Goldschmidt-Clermont PJ, Wilhide CC, et al. Methylation of the estrogen receptor gene is associated with aging and atherosclerosis in the cardiovascular system. Cardiovasc. Res. 1999;43:985–991. doi: 10.1016/s0008-6363(99)00153-4. [DOI] [PubMed] [Google Scholar]
- Riccioni G, Bucciarelli T, Mancini B, Di Ilio C, Capra V, D’Orazio N. The role of the antioxidant vitamin supplementation in the prevention of cardiovascular diseases. Expert. Opin. Investig. Drugs. 2007;16:25–32. doi: 10.1517/13543784.16.1.25. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Stampfer MJ, Rifai N. Novel risk factors for systemic atherosclerosis: a comparison of C-reactive protein, fibrinogen, homocysteine, lipoprotein(a), and standard cholesterol screening as predictors of peripheral arterial disease. JAMA. 2001;285:2481–2485. doi: 10.1001/jama.285.19.2481. [DOI] [PubMed] [Google Scholar]
- Struys EA, Jansen EE, de Meer K, Jakobs C. Determination of S-adenosylmethionine and S-adenosylhomocysteine in plasma and cerebrospinal fluid by stable-isotope dilution tandem mass spectrometry. Clin. Chem. 2000;46:1650–1656. [PubMed] [Google Scholar]
- Troen AM, Lutgens E, Smith DE, Rosenberg IH, Selhub J. The atherogenic effect of excess methionine intake. PNAS. 2003;100:15089–15094. doi: 10.1073/pnas.2436385100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueland PM, Refsum H, Beresford SA, Vollset SE. The controversy over homocysteine and cardiovascular risk. Am. J. Clin. Nutr. 2000;72:324–332. doi: 10.1093/ajcn/72.2.324. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Osada J, Aratani Y, et al. Mice deficient in cystathionine ß-synthase: animal models for mild and severe homocyst(e)inemia. Proc. Natl Acad. Sci. USA. 1995;92:1585–1589. doi: 10.1073/pnas.92.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss N, Keller C, Hoffmann U, Loscalzo J. Endothelial dysfunction and atherothrombosis in mild hyperhomocysteinemia. Vasc. Med. 2002;7:227–239. doi: 10.1191/1358863x02vm428ra. [DOI] [PubMed] [Google Scholar]
- Yi P, Melnyk S, Pogribna M, Pogribny IP, Hine RJ, James SJ. Increase in plasma homocysteine associated with parallel increases in plasma S-adenosylhomocysteine and lymphocyte DNA hypomethylation. J. Biol. Chem. 2000;275:29318–29323. doi: 10.1074/jbc.M002725200. [DOI] [PubMed] [Google Scholar]
- Yu X, Chen K, Wei N, Zhang Q, Liu J, Mi M. Dietary taurine reduces retinal damage produced by photochemical stress via antioxidant and anti-apoptotic mechanisms in Sprague-Dawley rats. Br. J. Nutr. 2007;98:711–719. doi: 10.1017/S0007114507744409. [DOI] [PubMed] [Google Scholar]
- Zaina S, Lindholm MW, Lund G. Nutrition and aberrant DNA methylation patterns in atherosclerosis: more than just hyperhomocysteinemia? J. Nutr. 2005;135:5–8. doi: 10.1093/jn/135.1.5. [DOI] [PubMed] [Google Scholar]
- Zhang R, Ma J, Xia M, Zhu H, Ling W. Mild hyperhomocysteinemia induced by feeding rats diets rich in methionine or deficient in folate promotes early atherosclerotic inflammatory processes. J. Nutr. 2004;134:825–830. doi: 10.1093/jn/134.4.825. [DOI] [PubMed] [Google Scholar]
- Zhou J, Moller J, Danielsen CC, et al. Dietary supplementation with methionine and homocysteine promotes early atherosclerosis but not plaque rupture in ApoE-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2001;21:1470–1476. doi: 10.1161/hq0901.096582. [DOI] [PubMed] [Google Scholar]