Abstract
This article reviews the development and the advances of print-and-peel (PAP) microfabrication. PAP techniques provide means for facile and expedient prototyping of microfluidic devices. Therefore, PAP has the potential for broadening the microfluidics technology by bringing it to researchers who lack regular or any accesses to specialized fabrication facilities and equipment. Microfluidics have, indeed, proven to be an indispensable toolkit for biological and biomedical research and development. Through accessibility to such methodologies for relatively fast and easy prototyping, PAP has the potential to considerably accelerate the impacts of microfluidics on the biological sciences and engineering. In summary, PAP encompasses: (1) direct printing of the masters for casting polymer device components; and (2) adding three-dimensional elements onto the masters for single-molding-step formation of channels and cavities within the bulk of the polymer slabs. Comparative discussions of the different PAP techniques, along with the current challenges and approaches for addressing them, outline the perspectives for PAP and how it can be readily adopted by a broad range of scientists and engineers.
Keywords: PAP, LaserJet, Solid-ink, Wax, Printer, Lithography, Biosensors, Poly(dimethylsiloxane), PDMS, μ-TAS
Introduction
Microfluidics has gained significance as an interdisciplinary technology with applications in many important areas such as biosensing, diagnostics and drug discovery.10,22,29,56,62,83,87 The huge attraction toward microfluidics results from its capabilities to achieve significant reduction in reagent volumes, in performance time and in power consumption while allowing massive parallelism.62,67,69,70 Over the last two decades, microfluidic systems have been developed for a broad range of application in biology, chemistry and physics.2,5,8,15,40,51,55,59,61,65,68,73,77,81
Due to its availability and to the relative simplicity of its molding, polydimethylsiloxane (PDMS) has become one of the preferred materials for fabrication of microfluidic devices.21,36,49,52,54,57,66 The masters for molding the PDMS components of the devices encompass the microchannel patterns as positive relief features on the smooth surfaces.23,28,81,86 The fabrication of such masters involves a series of lithographic and etching steps,7,9,24,60 most of which require a clean-room environment (with long-wavelength lighting) and specialized equipment. As an alternative, nonlithographic, or print-and-peel (PAP), procedures allow for facile and expedient fabrication of masters for molding polymer components for microfluidic devices.6,31,33,35,45,76
The PAP fabrication techniques allow for direct printing of the masters, using regular office equipment (Scheme 1).6,31,33,35,45,76 Any printing process that deposits ink or toner on the surface of smooth and non-absorptive substrate leaves positive-relief printout features.11,26,32,33,35,43,85 Therefore, LaserJet or solid-ink prints on overhead transparency films have proven their utility for PAP fabrication of masters for microfluidic devices.6,31,33,35,45,76
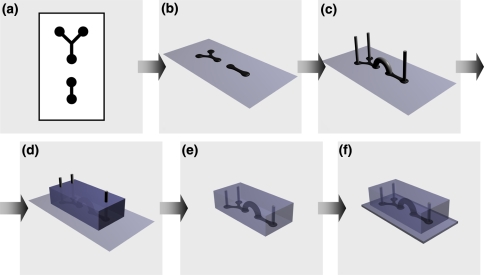
Scheme 1.
Print-and-peel fabrication of a microfluidic device. (a) CAD pattern; (b) printed master (e.g., solid-ink printout on an overhead transparency film); (c) printed master with 3D elements attached onto it; (d) polymer (e.g., PDMS) cast over the printed master; (e) cured polymer slab with negative-relief channels, connecting channels and a chambers molded in it; and (f) microfluidic device obtain via adhering the polymer slab to a flat substrate slide
Inkjet (bubble jet) printing offers another alternative for PAP. Via a “regular” printing process, however, the ink for bubble jet printers, when deposited, is absorbed by the substrates and does not leave relief features that exceed the roughness of the printed surfaces. Modifying the inkjet printing process and allowing the controlled formation of micrometer-size relief features,47,85 on the other hand, can prove beneficial for PAP. Martin et al.47 demonstrated the fabrication of 120-μm wide hydrophobic barriers on a chip by depositing polymer-containing droplets via inkjet printing. Xia and Friend85 demonstrated patterning of submicrometer-high relief features on polymer surfaces via controlled deposition of organic solvent with an inkjet printer.
An addition of three-dimensional (3D) elements to the masters allows for a single-step molding of device components with increased complexity76: i.e., a network of channels on multiple planes can be readily introduced to such device components and molded in a single step (Scheme 1c–e).3,46,74,75 Furthermore, molding the microfluidic components with 3D elements, such as inlet and outlet connecting channels, eliminates the need for drilling through the cured polymer.33,76 Drilling through PDMS not only produces channels with considerably rough walls, but also places a risk of cracking the cured polymer slab.
Due to its simplicity, expedience, and cost efficiency, PAP techniques offer significant advantages for fast and facile prototyping of microfluidic devices.6,31,76 Although PAP is less than a decade old, the recently developed PAP procedures for fabrication of biosensor,76 microelectrodes,33 devices for capillary electrophoresis,6,35,72 and lateral-gradient chemotaxis bioanalyzers31 demonstrate the feasibility of this fabrication approach for microfluidic biological applications. PAP, indeed, offers capabilities for bringing microfluidics technology to researchers, for whom access to specialized microfabrication facilities is not readily available.
Herein, we review the advances in PAP and their implication for microfluidics. Discussions of the limitations of PAP, along with approaches for addressing these limitations, introduce possible venues for expansion of these fabrication techniques.
What’s in it for Biomedical Applications?
Microfluidics provides a set of indispensable tools for cell biology, biochemistry, neuroscience, bioanalysis, drug testing, biomechanics and other areas of biology and biomedical engineering.22,51,54,81 Microfluidics, therefore, provides a liaison for integration of engineering and biology. PAP allows for facile and expedient fabrication of microfluidic devices. Because it does not require specialized facilities and equipment, PAP has the immense potential for broadening the impact of microfluidics as a driving force for innovativeness in biology and medicine.
Due to the simplicity of the channel design required for electrophoresis, electrophoretic separation has been by far the most targeted application for PAP-fabricated microfluidic devices.6,19,20,26,27,32,35,72 Employing nonlithographically fabricated microdevices for capillary gel electrophoretic separation of polynucleotide mixtures and achieving separation efficiencies exceeding 2 × 105 theoretical plates per meter,89 presents an excellent proof for the feasibility of pursuing PAP for development of microfluidic devices for clinical diagnosis and biomedical research.
Mixing of microflows is another key application for which PAP-fabricated devices have demonstrated their feasibility.43,45,48,76 Due to the laminar nature of microflows (resultant from the prevalent viscous forces at relatively low Reynolds numbers), achieving efficient mixing, faster than the inherent diffusion times, presents a set of design and fabrication challenges for microfluidic devices.71 PAP allows a facile and expedient preparation of masters with relatively complex features and transferring of these features in the polymer device components via a single molding step. Therefore, as an alternative to multiple fabrication and alignment steps, required for lithographic fabrication of micromixers, PAP offers venues for relatively simple incorporation of passive mixers in microfluidic devices.45,48,76
Microfluidic generators of concentration gradients, utilizing the laminar nature of the microflows, are promising tools for cell-biology and biomedical applications.25,84 Utilization of microfluidic gradient generators for stem-cell17 and cancer research64 presents examples for the potential impact of such devices on biomedical science and engineering. PAP, indeed, allows for facile and expedient fabrication of such microfluidic concentration-gradient generators.31,80
Micro-total-analytical systems (μTAS) have an immense potential for positive impact on clinical diagnosis, and on point-of-care research and development.30,34,58,82 The challenging fabrication of highly integrated μTAS devices, however, has impeded the realization of their full potential for health care and applied bioengineering. The simplicity and the expediency that PAP offers for prototyping of microfluidic devices, allow for bringing the μTAS research and development closer to the biomedical field. Utilizing PAP, medical teams (lacking extensive engineering expertise) can be directly involved in the development of μTAS devices targeted for specific clinical needs.
While the μTAS high level of integration may prove challenging for personnel lacking microfabrication expertise even if PAP is employed, a modular approach for building microfluidic devices presents an alternative.63 The current PAP technology permits facile and expedient fabrication of microfluidic modules: i.e., centimeter-large polymer blocks containing micromixers, valves, gradient generators, optical waveguides, microelectrodes and other key components for μTAS devices. Assembly of devices from such modules can yield systems with a broad range of functionalities.63
Overall, the accessibility to microfluidics technology that PAP provides, has a range of yet unrealized potentials for the biomedical field. In addition to research and development at the interface of biology, medicine and engineering, PAP has the incomparable potential for bringing microfluidics to early (e.g., high school and college) education. Via providing hands-on microfluidics and μTAS experience to high-school, biology and medical students, PAP will aid the development of experts who in the long run will catalyze the bridging between biomedical sciences and engineering.
Aside from the recent demonstrations of PAP-fabricated devices for capillary electrophoresis, biosensing, micromixers and gradient generators,31,33,35,72,74,76,80 the utilization of PAP for biological and biomedical applications remains largely unexplored. With this review we aim to demonstrate the potentials and perspectives of this relatively new microfabrication methodology. In a series of comparative discussions, we outline the advances and the limitations of PAP, along with recent demonstrations of innovative approaches for addressing these limitations. Simplicity and speed are some of the principal advantages of PAP that make it feasible for broadening the accessibility to microfluidics.
PAP vs. Photolithography
Photolithography has been the preferred method for fabrication of masters for molding polymer components of microfluidic devices.49,57,81 This method involves five steps:
Computer-aided design (CAD) software allows for the preparation of the microchannel patterns with submicrometer resolution. Transferring (printing) these patterns on thin films of transparent substrate produces the CAD microfabrication masks. (As an alternative, chromium masks allow for submicron resolution.)
Silicon wafers are coated with uniformly thick positive or negative photoresist polymer and thermally treated. The thickness of the deposited photoresist determines the height of the relief features of the master, and hence, the depth of the microchannels.
Exposure of the photoresist to collimated UV light through the CAD mask transfers the lateral features of the patterns onto the photoresist coating.
Treatment with organic solvents removes the loose polymer, leaving solely the positive-relief features on the surface of the silicon wafer. Thus, the produced masters comprise positive-relief microchannel patterns of photoresist on flat silicon/silicon dioxide surfaces (the exposure of silicon to air always results in oxidation producing layers of silicon dioxide).
To suppress permanent adhesion with the molded PDMS, the surfaces of the silicon-wafer masters are coated with perfluoroalkyl layers via gas-phase silanization.
Because PAP allows for direct printing of the masters from the CAD generated patterns, it eliminates the lithographic fabrication steps 2 to 4.31,33,35,45,76 In addition to requiring specialized equipment and environment, these steps are usually the most costly and labor-intense in the procedures for fabrication of PDMS microfluidic devices. Eliminating them, therefore, opens venues for rapid prototyping.6,31,76,88
Casting PDMS prepolymer over the masters and allowing it to cure, produce PDMS slabs with negative-relief patterns imprinted on their smooth surfaces. The silanization step, i.e., step 5 of the lithographic procedure, assures reliable separation of the polymer slabs from the surface of the master, without compromising the integrity of the imprinted relief features. After drilling inlet and outlet connecting channels through the PDMS slabs, they are exposed to oxygen plasma and brought against the smooth surface of another silica-based substrate to form the microfluidic devices.53 The oxygen-plasma treatment activates the PDMS surface allowing it to permanently adhere to other silica-based substrates (such as quartz, glass and PDMS) via formation of covalent bonds with high-density across the interface between the two materials.53
Drilling channels through the cured polymer components is essential for connecting the microfluidic devices with inlet and outlet tubing. Such drilled channels, however, have walls with considerable roughness making the sealing of the tubing quite problematic. In addition, cracks in the cured PDMS slabs, resultant from the mechanical drilling, are another source for leaking and compromised sealing.
Alternatively, PAP offers two additional advantageous venues for simplification of the device fabrication and assembly procedures:
Because PDMS does not adhere to the materials composing the PAP-fabricated masters, the perfluoroalkylation step 5 becomes unnecessary.6,31,33,35,45,76
3D elements allow for the formation of inlet and outlet channels while molding the PDMS slab, eliminating the requirement for drilling the cured polymer.33,76
Using PAP, we demonstrated the fabrication of smooth inlet and outlet channels that provide superior sealing with the connecting tubing.33,76 These round channels, formed during the molding of the elastomer, are resultant from polyethylene rods (or wires) attached to the masters. Such polyethylene 3D elements prove instrumental not only for formation of inlet and outlet channels, but also for creation of cavities within the bulk of the PDMS components of the microfluidic devices.76
The nature of processing results in distinct differences between the cross sections of microchannels fabricated using PAP and lithography. While photolithographically fabricated channels have close to rectangular cross sections, the channels obtained from LaserJet-printed and solid-ink-printed masters have, respectively, trapezoidal and round-bottom cross sections.6,76 Thermal treatment of LaserJet-printed masters, composed of pre-stressed polymer matrices, and etching of LaserJet-masked substrates, also produce channels with round cross sections.20,31
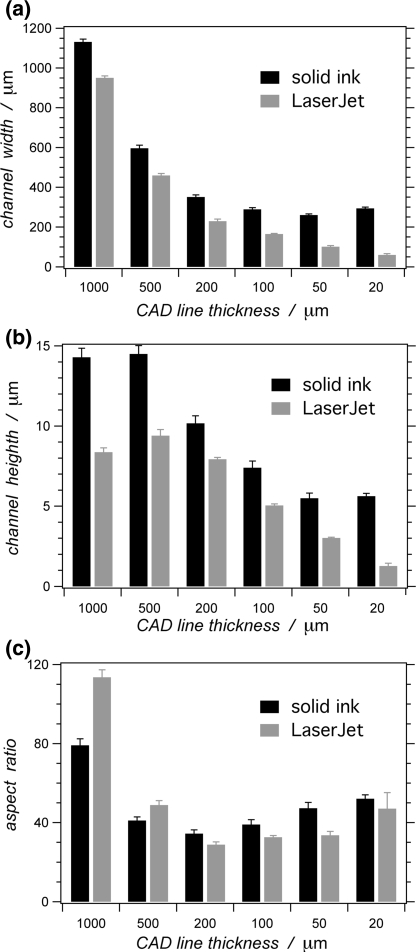
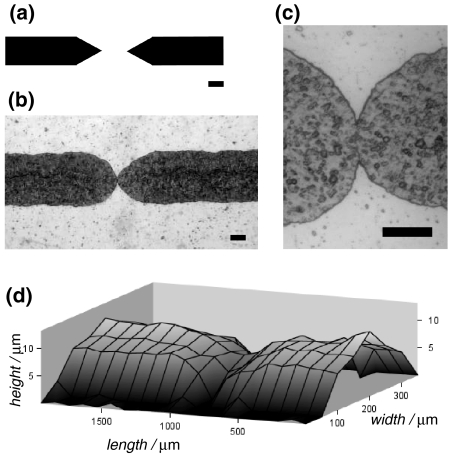
PAP, however, shows certain disadvantages in comparison with the established photolithographic fabrication approaches. The smallest lateral dimensions of features reproduced on printed masters, for example, are just a little narrower than 100 μm (Fig. 1a). The heights of the features on the masters printed with LaserJet or solid-ink printers do not exceed 15 μm (Fig. 1b). Therefore, PAP tends to produce microchannels with large aspect ratios: i.e., with aspect ratios between the widths and heights of the channels ranging from about 20 to 100 (Fig. 1c).6,35,72,76
Figure 1.
Comparison between relief features printed with a solid-ink printer (Xerox 8550D Phaser) and a LaserJet printer (HP 1320), reproducing CAD (FreeHand, v. 10) horizontal lines with different thicknesses. Dependence of: (a) feature width; (b) feature height; and (c) the width-to-height aspect ratio of the features, on the lines on the CAD images. The data are collected from profilometry measurements (six measurements per a data point; error bars represent 0.9 confidence limits, based on Student’s t-distribution)
In comparison with their photolithographically fabricated counterparts, LaserJet-printed masters manifest stronger tendency for deterioration from multiple uses.76 Therefore, while PAP is perfectly suitable for fast and facile prototyping, durability of the printed masters needs to be improved for PAP to impact mass-production applications.
Overall the limitations of PAP fabrication are set by the resolution of the printers used for printing the masters, by the amount of toner or ink deposited, and by the printing materials (i.e., the toner or the ink for the printer and the substrate, on which the master is printed). Therefore, software or hardware improvement of currently used office printers presents a plausible way for addressing these limitations of PAP.
Development of PAP
In the beginning of this century, Glennon et al.72 reported the use of xerography for fabrication of masters for PDMS microfluidic devices. The following year, Whitesides et al.48 demonstrated the versatility of direct three-dimensional (3D) printing of masters using a solid-object printer. Utilizing this nonlithographic approach allowed for achieving, for example, 3D crossing of non-intercepting microchannels on different planes. Also, for passive micromixers, “fishbone patterns” within a channel were achieved in a single printing microfabrication step.48 The cost of the printing equipment, however, was still a drawback.
In 2002, Shyy et al.11 reported replication of positive-relief patterns printed with an office LaserJet printer. The patterns were printed on overhead transparencies, over which PDMS was cast. The reproduction of patterns of lines with different widths was tested.11 Several years later, Chen et al.6 demonstrated a microfluidic electrophoresis PDMS device, with a relatively simple two-dimensional configuration, fabricated using a LaserJet-printed master.
Following these advances, we investigated the feasibility and the limitation of the LaserJet PAP microfabrication.76 The depth of the microchannels, formed from LaserJet-printed features, manifested dependence not only on the printer and on the toner, but also on their width (Fig. 1).76
As we demonstrated, attaching 3D elements onto the printed features of the masters presented a facile approach for achieving connecting channels and cavities within the PDMS slabs via a single molding step.33,76 Such 3D elements allow for achieving features, demonstrated with solid-object printing,48 in a cost efficient manner (considering the price of the solid-object printing equipment). Based on our PAP findings, we designed, fabricated and demonstrated a continuous-flow microfluidic biosensor that utilizes emission enhancement as a handle for detection.76
Ghatak et al.75 investigated PDMS molding around 3D elements with different size, shape and complexity. Such 3D elements allowed for a single-step molding of multihelical passive micromixers,74 and of channel arrays for bioinspired adhesive elastomer slabs.3,46 Following these advancements, Kim et al.4 utilized 3D elements for a single-step fabrication procedure for devices with multilevel channel configuration.
The submicrometer reproduction of the features (including the defects) of the LaserJet printed masters onto the surface of the PDMS slabs,11,76 suggests that the casting of the polymer does not set the resolution limits of PAP. Instead, the LaserJet printers set the lateral resolution of PAP.76
Utilization of pre-stressed thermoplastic polymer material for a master substrate, allowed Khine et al.31,45 to fabricate microchannels with widths that were narrower than what the printing resolution permitted. Upon thermal relaxation of the pre-stressed polymer, the lateral dimension of the LaserJet-printed features decreased.31 Concurrently, such shrinking of the printed area led to an increase in the height of the positive-relief lines.31 Khine et al.31,45 demonstrated the application of this PAP approach for the fabrication of micromixers and of microfluidic gradient generator for chemotaxis analysis.
Others and we have referred to microfabrication utilizing LaserJet-printed masters as “nonlithographic.” PAP, indeed, does not involve photolithography or other lithographic steps requiring specialized equipment and clean-room environment, qualifying it as “nonlithographic” in this sense. LaserJet printing, however, is a form of lithography, allowing for transferring the toner patterns from the drum onto the printed surfaces.
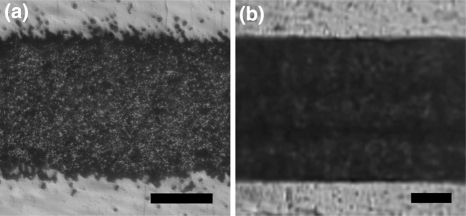
Solid-ink (wax) printers present an alternative to LaserJet printers for PAP applications.33,35 The solid-ink printers have the principle advantage for generating features with improved smoothness (Fig. 2), while the granular structure of the LaserJet toners is quite conspicuous on the produced PDMS replicas (Fig. 3). The relatively low melting point of the waxy solid ink, however, prevents the cast PDMS from curing at elevated temperature.
Figure 2.
Microscope images of horizontal lines printed with (a) a LaserJet printer (HP 1320) and (b) a solid-ink printer (Xerox 8550D Phaser). Both lines are reproduction of the same CAD (FreeHand, v. 10) image, in which the line thickness was 200 μm. The scale bars correspond to 100 μm
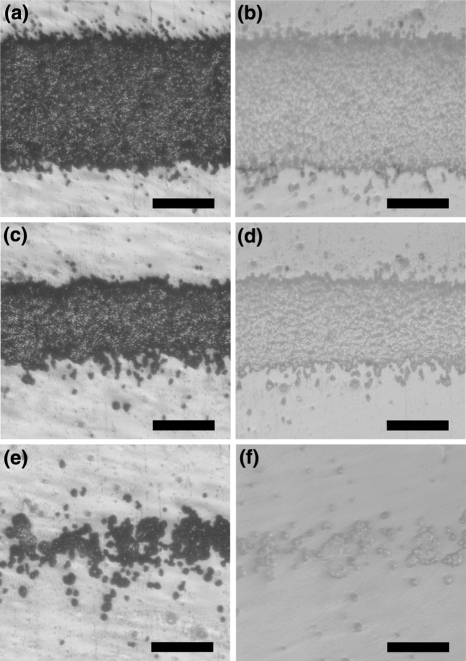
Figure 3.
Microscope images of: (a, c, e) horizontal lines printed with a LaserJet printer (HP 1320); and (b, d, f) the corresponding PDMS replica of the printed lines. The printed features are reproduction of CAD (FreeHand, v. 10) images with line thickness: (a, b) 200 μm; (c, d) 100 μm; and (e, f) 20 μm. The scale bars correspond to 100 μm
As a principal disadvantage, Backhouse et al.35 and we demonstrated that the smallest lateral dimensions achievable with office-grade solid-ink printers are in the order of 300 μm (Fig. 1a). Had utilized an improved printer model, however, Pan et al.80 recently reported 170-μm thick lines, printed at edge-to-edge separation of 70 μm.
PAP methodology offers more than just printing the masters for molding the polymer components of devices. Using LaserJet printouts on substrate (i.e., wax paper), to which the toner does not adhere strongly, Carrilho et al.20 fabricated glass components for microfluidic devices. Thermal pressing allowed for transferring the LaserJet-printed features from the wax paper onto the glass surface, forming an etch mask from the deposited toner.20 Treatment of the toner-coated glass with hydrofluoric acid etched the exposed areas, forming the negative-relief features of the designed microchannels.20
Double PAP methodology allowed us to fabricate arrays of microelectrodes.33 In the first PAP step, we fabricated PDMS slabs with negative-relieve channel patterns of the microelectrode arrays: i.e., the PDMS slab could be viewed as a microelectrode fabrication mask.33 For the second PAP step, we reversibly adhered the PDMS electrode-array mask to a smooth substrate, glass. Electroless metal deposition within the formed channel (i.e., printing of the metal features), and peeling of the PDMS slab, left patterns of conductive material (microelectrodes) on the glass surface.33
Alternative approaches, which are not truly “print-and-peel,” also demonstrate considerable contributions toward facilitating microfluidics technology and improving the accessibility to it. Employing only “print” without “peel,” for example, do Lago et al.26 utilized polyester transparency films, with LaserJet-printed patterns on them, for components of microfluidic devices (rather than for molding masters). Consequently, the authors extended this approach and demonstrated the fabrication of glass microfluidic devices.27 They thermally transferred the toner relief features onto glass substrate, thus, producing microchannels from printed toner patterns sandwiched between glass slides.27 The electrophoretic performance of such printed devices was compatible to the performance of microfluidic devices made of PDMS.19
This approach for direct printing of device components was adopted by Xia et al.,32 allowing them to utilize the height-differentiation of patterns, printed at different gray-scale settings, for the fabrication of passive micromixers.43 Adopting such a “lab-on-a-print” approach, Pan et al.80 demonstrated the fabrication of a microfluidic gradient generator via a series of solid-ink printing selective etching of the printed polymer substrate.
Micromachining (which is a relatively old technology) presents another alternative to lithography and PAP for fabrication of microfluidic devices.38,50,89 Recent reports demonstrate the feasibility of micromachining for:
Fabrication of masters for molding polymer components of microfluidic devices,50,89
Direct fabrication of components of microfluidic devices.38
Micromachining requires a different set of specialized equipment. As such, therefore, micromachining brings the microfluidics technology to members of the research-and-development community who have access to machine shops with capability for fabrication of relief features with submillimeter resolution.
In addition to mechanical machining, non-contact (e.g., laser and ion-beam) milling offers venues for fabrication of microfluidic devices not only with submicrometer lateral resolution, but also with relief features having small width-to-depth aspect ratios.12,41,42,78,79 Infrared lasers (such as carbon dioxide lasers, widely used for machining and processing) for example, have proven feasible for fabrication of polymer and glass components for microfluidic devices.16,18,37,42,44 Employing ultraviolet lasers for micromachining, on the other hand, allows not only for improved resolution (due to the several-fold decrease in the diffraction limit), but also for utilization of photochemical ablation (due to the photoexcitation at relatively high frequencies).14,37
The requirement for specialized equipment, however, presents a severe cost limitation for the wide adoption of contact and non-contact machining in the fabrication of microfluidic devices. Alternatively, Khine et al.13 directly created negative-relief microchannel features on smooth surfaces of biaxially pre-stressed thermoplastic polymer slabs. Thermal relaxation of the polymer slabs led to a decrease in the lateral dimension, and to an increase in the depth, of the negative-relief features.13
Via a modular approach, Rhee and Burns demonstrated an alternative for facile and expedient prototyping of microfluidic devices.63 Mass-produced sets of standard modules (comprising, for example, channels, chambers, valves and inlets/outlets with different configurations) present the possibility for cost-efficient broadening of the accessibility to the microfluidics technology. The assembly of such modules into different configurations yields mirofluidic devices that can address a variety of needs and requirements.63
Printing Masters with Office Equipment
For fabrication procedures, computer-aided design (CAD) software allows for representation of a planar network of microfluidic channels and wells as a two-dimensional map (Scheme 1a). In the case of PAP, printing the CAD patterns on a smooth substrate directly yields the masters: i.e., without any modification, LaserJet and solid-ink office printers allow for producing the master relief features on the smooth surfaces of overhead transparency films (Scheme 1).6,33,35,76
Office printers, overall, yield relief features with size ranges comparable to the sizes of the relief features nonlithographically produced by costly and specialized equipment, employing, for example, solid-object printing and micromachining (Table 1). Among the commercially available office printers, LaserJets achieve relief features with smaller lateral dimensions than the solid-ink printers (Fig. 1a). LaserJet printers, however, produce patterns of toner particles molten together. Therefore, the LaserJet-printed relief patterns have granular structures determined by the size of the toner particles (Fig. 3).6,11,76
Table 1.
Reported lateral and vertical dimensions of relief feathers achievable via various nonlithographic techniques
| Master fabrication technique | Dimensions | Reference | |
|---|---|---|---|
| Width | Height | ||
| LaserJet printing | From 50 to >500 μm | From 2 to 9 μma | 6,76 |
| Solid-ink/wax printing | From 170 μm to >1 mmb | From 6 to 14 μm | 33,35,80 |
| Inkjet printingc | From 120 μm | From submicrometer | 47,85 |
| Solid-object printingd | From 250 μm to 10 cm | From 250 μm to 10 cm | 48 |
| Micromachiningd | From ~25 to >450 μm | Tens of micrometers | 50,89 |
| Ion-beam millingd | From 40 nm to >1 μm | From 60 nm to 8 μm | 12,78,79 |
| Laser millingd | From 20 μm to >1 mm | From <100 μm to >1 mm | 14,16,18,37 |
aMultiple printing allows for achieving relief heights exceeding 20 μm27,76; using xerography, allows for features up to 14-μm high72; and the use of pre-stressed thermoplastic polymer for master material, allows for vertical heights exceeding 50 μm31
bContacts between spreading molten wax ink on the printed substrate allow for achieving dimensions below 100 μm (Fig. 4)
cThis technique is still largely unexplored, probably due to the requirement for the development of inks that upon deposition with an inkjet printer leave substantially high positive-relief features
dThese techniques require relatively expensive specialized equipment
Alternatively, utilizing wax-based ink with dye dispersed in it, solid-ink printers deposit patterns of molten wax (pre-heated to 135 °C) on the printed substrates. The wax ink rapidly solidifies before the substrate (e.g., the overhead transparency film) rolls out of the printing zone. As a result, the microchannel patterns, produced from solid-ink printers, have considerably smoother walls in comparison with the granular walls of the LaserJet-produced channels (Fig. 2).33,35 The current commercially available solid-ink office printers, however, do not have the capability for producing lines much thinner than ~0.2 mm, which presents a principal disadvantage in comparison with LaserJet printers.
It should be emphasized that the dots-per-inch (dpi) resolution (reported by the printer manufactures) does not represent the smallest dimensions of patterns reproducible by a printer. Forcing a LaserJet to print lines with widths close to their published resolution, results in discontinuity in the patterns (Figs. 3e and 3f). For example, 1200 dpi suggests for the deposition of dots at ~21 μm center-to-center separation distance from each other. Features with lateral dimensions below ~50 μm, or even below ~100 μm, printed with 1200 dpi LaserJets, however, manifest deteriorated quality and are not feasible for microfabrication applications.
Alternatively, the current office-grade solid-ink printers do not have the capability to print features smaller than about 0.2–0.3 mm.35 Lines thinner than ~0.2 mm from the CAD designed images, for example, can result into 0.3-mm-thick lines when printed even with 2400-dpi solid-ink printer (Fig. 1a). This observation suggests that even if a solid-ink printer may have the resolution to deposit “dots” with ~11 μm center-to-center separation distance from each other (for 2400 dpi), the size of the “dots” significantly exceeds (about 25 fold) the separation distance between them.
The achievable height of the printed features shows correlation with their lateral dimensions and is dependent on the model of the printer.76 Furthermore, while the cross section of LaserJet-printed channels is trapezoidal,76 solid-ink printers produce microchannels with rounded cross sections.
Overall the outlined drawbacks of the office-grade printers are not necessarily a corollary of the inherent limitation of the LaserJet or solid-ink printing technologies. Rather, the lack of development of such “office” printers, geared toward microfabrication applications, appears to be the principal hurdle for pushing the technology beyond its current limitations.
3D Elements
Masters with three-dimensional (3D) elements on their positive-relief features allow for single-step molding of relatively complex components for microfluidic devices.33,76 Solid-object printers have the capability for 3D printing, and hence, for directly producing masters with such 3D elements on their surfaces.48 Attaching 3D elements onto the positive-relief features of the printed masters, however, offers an approach for achieving similar single-step 3D molding without a solid-object printer.33,76
Although there is no real limitation on the choice of materials for the 3D elements, they have to be readily processable into the desired shapes, non-adhesive to PDMS, and not interfering with the PDMS curing. Wires or fibers of polyethylene33,76 and nylon3,46,74,75 have proven to be an excellent choice for preparation of such 3D elements. Heating of the polymer wires above their glass-transition temperatures (and below their melting points) allows for forming the desired shapes of the 3D elements. Attaching the 3D elements to the masters is readily achieved via thermally melting the ends of the polyethylene wires and pressing the molten sections against the positive-relief features.76 Hot glue (i.e., thermoplastic adhesive), which adheres strongly to polyethylene and to the wax (or to the toner) of the printouts, but at the same time, does not adhere to PDMS, is an excellent alternative for attaching 3D elements onto the printed masters.33
In addition to allowing the formation of a complex network of chambers and channels on different planes,3,74–76 the 3D elements have proven immensely beneficial for formation of inlet and outlet round channels having walls with superior smoothness.33,76 The formation of the inlet and outlet channels during the molding process eliminates the requirement for drilling through the cured PDMS slabs. Furthermore, the smoothness of the 3D-molded inlet and outlet channels allows for superior sealing with the connecting tubing. For further enhancement of the quality of the sealing, we dip the end of the tubing, plugged with a dab of glue, into a PDMS prepolymer mixture and allow it to cure. We cut off the excess elastomer along with the plug, leaving at least 5 mm long section of PDMS-coated tubing. Inserting the PDMS-coated ends of the tubing into the smooth-wall inlet and outlet channels results in superiorly sealed connections.
Limitations
Employing office-grade printers for PAP sets limitations on the accessible lateral and vertical dimensions of the positive-relief features on the printed masters. Lateral dimensions smaller than ~50 and ~200 μm are not accessible by office-grade LaserJet and solid-ink printers, respectively.76,80 Concurrently, the same printers produce relief features with height between about 5 and 15 μm (Fig. 1b).
As others and we have demonstrated, LaserJets cannot print continuous lines narrower than about 50–100 μm.76 (This limit is strongly dependent on the printer model and on the quality of the toner.) Furthermore, the consistency of the printed features has inherent roughness that depends on the size of the toner particles (Fig. 3).
The solid-ink printers, on the other hand, do not suffer from leaving particulate patterns due to the waxy consistency of the deposited relief features (Fig. 2b). Similar to LaserJets, however, the office-grade solid-ink printers have a limit of about 200 μm for the minimum lateral dimensions of patterns that they can reproduce.35,80 Furthermore, the relatively low melting point of the wax ink places limitations on conducting thermal curing of the polymer cast over the solid-ink-printed masters.
The use of pre-stressed thermoplastic polymers for master substrate allows for decreasing the lateral dimensions of the positive-relief features after they are printed.31,45 The masters are printed while the polymer is in its pre-stressed state. Thermal treatment of the printed sheets allows the polymer to relax and shrink laterally along with the patterns printed on it.31
Printing CAD images, containing features, closer to each other than the printing resolution, with a solid-ink printer, presents an alternative for achieving patterns with relatively small lateral dimensions. Solid-ink printers cannot reproduce submillimeter features with sharp edges. During the printing process, as the molten wax ink is deposited on smooth non-absorptive substrates, it forms round droplets, which mask any sharp-edge CAD feature smaller than the drop size.33,35 The spread of the round droplets depends on the surface energies of the molten wax and of the printed substrate, as well as on the interfacial energy between these two materials. As a result, the ends of printed lines, with submillimeter widths, are always round, despite the CAD pattern shapes.35 This characteristic of solid-ink printing allows for generating, along the channels, “junctions,” narrower than the minimum printable lateral dimensions (Fig. 4).
Figure 4.
Narrow junction along printed microchannel relief features. (a) a CAD image for the junction connection. (b, c) Reproduction of the CAD image, printed with a solid-ink printer (Xerox 8550D Phaser) on a overhead transparency film. The molten wax ink spreads between the edges of the two lines to form a narrow junction connection. (d) Topography of a junction (reproduced from profilometry measurements). The scale bars for (a)–(c) correspond to 100 μm
The height of the relief features, printed with office-grade printers, is limited to less than about 15 μm.6,35,76 This restriction, along with the inability for printing shapes with lateral dimensions smaller than about 100 μm, limits PAP to making devices predominantly comprising microchannels with high width-to-height aspect ratios (Fig. 1c).6,35,76
Repeated LaserJet printing of the CAD image on the same master, allows for an increase in the amount of deposited toner material over the same area, and hence for an increase in the height of the positive relief features, for example, from ~8 to ~20 μm.76 Inherently to the printer, slight misalignments of the substrate for each consecutive printing step compromise the lateral resolution and result in widening of the printed features.76
Adjusting the gray scale (instead of using solely a binary black-and-white contrast) of the CAD image, allows for controlling the height of the LaserJet printed features.43 This gray-scale approach, however, permits only vertical dimensions that are smaller than the maximum relief height possible by the printer used. Furthermore, introduction of gray areas in the CAD image decreases the amount of toner deposited in these particular areas, and hence, may lead to discontinuity of the printed patterns.43
In addition to achieving relatively small lateral dimensions, the use of pre-stressed thermoplastic materials as master substrates allows for an increase in the heights of the printed positive relief features.31 While the thermal relaxation of the pre-stressed polymer sheets leads to a decrease in their surface areas, such induced lateral shrinking of the printed patterns crowds and pushes the deposited toner material upward, increasing the height of the relief features. As a result, thermal relaxation of biaxially pre-stressed polystyrene, with LaserJet-printed patterns on it, can yield relief features with heights as large as 80 μm.31 Such heights are comparable with feature heights on lithographically fabricated masters.
Despite certain disadvantages, such as porosity51 and a lack of resistance against numerous organic solvents39 (unless resistant surface coatings are introduced),1 PDMS is still the common material of choice for fabrication of components for micro and nanofluidic devices due to its ease of molding and physical properties.66,81 It is not surprising, therefore, that most of the efforts in the development of PAP involved PDMS.6,11,31,33,35,48,72,74–76 Aside from direct printing on the materials that compose the microfluidic devices (i.e., “print” without “peel”),20,26,32,43,80 utilizing PAP for materials different from PDMS is still unexplored.
Conclusions
Print-and-peel has proven to be a facile and expedient approach for fabrication and in particular, for fast prototyping of microfluidic devices. As such, PAP provides a venue for broadening the accessibility to microfluidics technology.
Acknowledgments
This work was supported by UCR Academic Senate and by the U.C. Regents Faculty Development Award, as well as by U.S. Department of Education (for M.S.T.), U.C. LEADS (for J.M.C.), U.C. Energy Institute (for D.B.), and the National Science Foundation (for C.H.).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviations
- 3D
Three-dimensional
- CAD
Computer-aided design
- μTAS
Micro-total-analytical systems
- PAP
Print-and-peel
- PDMS
Poly(dimethylsiloxane)
References
- 1.Abate AR, Lee D, Do T, Holtze C, Weitz DA. Glass coating for PDMS microfluidic channels by sol-gel methods. Lab Chip. 2008;8:516–518. doi: 10.1039/b800001h. [DOI] [PubMed] [Google Scholar]
- 2.Abdelgawad M, Wheeler AR. The digital revolution: a new paradigm for microfluidics. Adv. Mater. 2009;21:920–925. doi: 10.1002/adma.200802244. [DOI] [Google Scholar]
- 3.Arul EP, Ghatak A. Bioinspired design of a hierarchically structured adhesive. Langmuir. 2009;25:611–617. doi: 10.1021/la803092d. [DOI] [PubMed] [Google Scholar]
- 4.Asthana A, Kim K-O, Perumal J, Kim D-M, Kim D-P. Facile single step fabrication of microchannels with varying size. Lab Chip. 2009;9:1138–1142. doi: 10.1039/b818987k. [DOI] [PubMed] [Google Scholar]
- 5.Atencia J, Beebe DJ. Controlled microfluidic interfaces. Nature. 2005;437:648–655. doi: 10.1038/nature04163. [DOI] [PubMed] [Google Scholar]
- 6.Bao N, Zhang Q, Xu J-J, Chen H-Y. Fabrication of poly(dimethylsiloxane) microfluidic system based on masters directly printed with an office laser printer. J. Chromatogr. A. 2005;1089:270–275. doi: 10.1016/j.chroma.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Becker H, Gartner C. Polymer microfabrication technologies for microfluidic systems. Anal. Bioanal. Chem. 2008;390:89–111. doi: 10.1007/s00216-007-1692-2. [DOI] [PubMed] [Google Scholar]
- 8.Beebe DJ, Mensing GA, Walker GM. Physics and applications of microfluidics in biology. Annu. Rev. Biomed. Eng. 2002;4:261–286. doi: 10.1146/annurev.bioeng.4.112601.125916. [DOI] [PubMed] [Google Scholar]
- 9.Beebe DJ, Moore JS, Yu Q, Liu RH, Kraft ML, Jo B-H, Devadoss C. Microfluidic tectonics: a comprehensive construction platform for microfluidic systems. Proc. Natl. Acad. Sci. USA. 2000;97:13488–13493. doi: 10.1073/pnas.250273097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhogal N, Balls M. Translation of new technologies: from basic research to drug discovery and development. Curr. Drug Discov. Technol. 2008;5:250–262. doi: 10.2174/157016308785739839. [DOI] [PubMed] [Google Scholar]
- 11.Branham ML, Tran-Son-Tay R, Schoonover C, Davis PS, Allen SD, Shyy W. Rapid prototyping of micropatterned substrates using conventional laser printers. J. Mater. Res. 2002;17:1559–1562. doi: 10.1557/JMR.2002.0231. [DOI] [Google Scholar]
- 12.Cannon DM, Jr, Flachsbart BR, Shannon MA, Sweedler JV, Bohn PW. Fabrication of single nanofluidic channels in poly(methylmethacrylate) films via focused-ion beam milling for use as molecular gates. Appl. Phys. Lett. 2004;85:1241–1243. doi: 10.1063/1.1780605. [DOI] [Google Scholar]
- 13.Chen C-S, Breslauer DN, Luna JI, Grimes A, Chin W-C, Lee LP, Khine M. Shrinky-Dink microfluidics: 3D polystyrene chips. Lab Chip. 2008;8:622–624. doi: 10.1039/b719029h. [DOI] [PubMed] [Google Scholar]
- 14.Chen T-C, Darling RB. Laser micromachining of the materials using in microfluidics by high precision pulsed near and mid-ultraviolet Nd:YAG lasers. J. Mater. Process. Technol. 2008;198:248–253. doi: 10.1016/j.jmatprotec.2007.06.083. [DOI] [Google Scholar]
- 15.Chen H, Fan ZH. Two-dimensional protein separation in microfluidic devices. Electrophoresis. 2009;30:758–765. doi: 10.1002/elps.200800566. [DOI] [PubMed] [Google Scholar]
- 16.Cheng J-Y, Wei C-W, Hsu K-H, Young T-H. Direct-write laser micromachining and universal surface modification of PMMA for device development. Sens. Actuators B. 2004;99:186–196. doi: 10.1016/j.snb.2003.10.022. [DOI] [Google Scholar]
- 17.Chung BG, Flanagan LA, Rhee SW, Schwartz PH, Lee AP, Monuki ES, Jeon NL. Human neural stem cell growth and differentiation in a gradient-generating microfluidic device. Lab Chip. 2005;5:401–406. doi: 10.1039/b417651k. [DOI] [PubMed] [Google Scholar]
- 18.Chung CK, Sung YC, Huang GR, Hsiao EJ, Lin WH, Lin SL. Crackless linear through-wafer etching of Pyrex glass using liquid-assisted CO2 laser processing. Appl. Phys. A. 2009;94:927–932. doi: 10.1007/s00339-008-4863-x. [DOI] [Google Scholar]
- 19.Coltro WKT, Lunte SM, Carrilho E. Comparison of the analytical performance of electrophoresis microchannels fabricated in PDMS, glass, and polyester-toner. Electrophoresis. 2008;29:4928–4937. doi: 10.1002/elps.200700897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coltro WKT, Piccin E, Fracassi da Silva JA, Lucio do Lago C, Carrilho E. A toner-mediated lithographic technology for rapid prototyping of glass microchannels. Lab Chip. 2007;7:931–934. doi: 10.1039/b702931d. [DOI] [PubMed] [Google Scholar]
- 21.Cong H, Pan T. Photopatternable conductive PDMS materials for microfabrication. Adv. Funct. Mater. 2008;18:1912–1921. doi: 10.1002/adfm.200701437. [DOI] [Google Scholar]
- 22.Davis G. Microfluidics: its impact on drug discovery. Innov. Pharm. Technol. 2008;25:24–27. [Google Scholar]
- 23.Delamarche E, Bernard A, Schmid H, Michael B, Biebuyck H. Patterned delivery of immunoglobulins to surfaces using microfluidic networks. Science. 1997;276:779–781. doi: 10.1126/science.276.5313.779. [DOI] [PubMed] [Google Scholar]
- 24.De La Rue R. Photonic crystals: microassembly in 3D. Nat. Mater. 2003;2:74–76. doi: 10.1038/nmat818. [DOI] [PubMed] [Google Scholar]
- 25.Dertinger SKW, Chiu DT, Jeon NL, Whitesides GM. Generation of gradients having complex shapes using microfluidic networks. Anal. Chem. 2001;73:1240–1246. doi: 10.1021/ac001132d. [DOI] [Google Scholar]
- 26.do Lago CL, da Silva HDT, Neves CA, Brito-Neto JGA, da Silva JAF. A dry process for production of microfluidic devices based on the lamination of laser-printed polyester films. Anal. Chem. 2003;75:3853–3858. doi: 10.1021/ac034437b. [DOI] [PubMed] [Google Scholar]
- 27.do Lago CL, Neves CA, Pereira de Jesus D, da Silva HDT, Brito-Neto JGA, Fracassi da Silva JA. Microfluidic devices obtained by thermal toner transferring on glass substrate. Electrophoresis. 2004;25:3825–3831. doi: 10.1002/elps.200406076. [DOI] [PubMed] [Google Scholar]
- 28.Effenhauser CS, Bruin GJM, Paulus A, Ehrat M. Integrated capillary electrophoresis on flexible silicone microdevices: analysis of DNA restriction fragments and detection of single DNA molecules on microchips. Anal. Chem. 1997;69:3451–3457. doi: 10.1021/ac9703919. [DOI] [PubMed] [Google Scholar]
- 29.El-Ali J, Sorger PK, Jensen KF. Cells on chips. Nature. 2006;442:403–411. doi: 10.1038/nature05063. [DOI] [PubMed] [Google Scholar]
- 30.Greenwood PA, Greenway GM. Sample manipulation in micro total analytical systems. Trends Anal. Chem. 2002;21:726–740. doi: 10.1016/S0165-9936(02)01104-4. [DOI] [Google Scholar]
- 31.Grimes A, Breslauer DN, Long M, Pegan J, Lee LP, Khine M. Shrinky-Dink microfluidics: rapid generation of deep and rounded patterns. Lab Chip. 2008;8:170–172. doi: 10.1039/b711622e. [DOI] [PubMed] [Google Scholar]
- 32.He F-Y, Liu A-L, Yuan J-H, Coltro WKT, Carrilho E, Xia X-H. Electrokinetic control of fluid in plastified laser-printed poly(ethylene terephthalate)-toner microchips. Anal. Bioanal. Chem. 2005;382:192–197. doi: 10.1007/s00216-005-3200-x. [DOI] [PubMed] [Google Scholar]
- 33.Hong C, Bao D, Thomas MS, Clift JM, Vullev VI. Print-and-peel fabrication of microelectrodes. Langmuir. 2008;24:8439–8442. doi: 10.1021/la801752k. [DOI] [PubMed] [Google Scholar]
- 34.Janasek D, Franzke J, Manz A. Scaling and the design of miniaturized chemical-analysis systems. Nature. 2006;442:374–380. doi: 10.1038/nature05059. [DOI] [PubMed] [Google Scholar]
- 35.Kaigala GV, Ho S, Penterman R, Backhouse CJ. Rapid prototyping of microfluidic devices with a wax printer. Lab Chip. 2007;7:384–387. doi: 10.1039/b617764f. [DOI] [PubMed] [Google Scholar]
- 36.Kartalov EP, Anderson WF, Scherer A. The analytical approach to polydimethylsiloxane microfluidic technology and its biological applications. J. Nanosci. Nanotechnol. 2006;6:2265–2277. doi: 10.1166/jnn.2006.504. [DOI] [PubMed] [Google Scholar]
- 37.Kim J, Xu X. Excimer laser fabrication of polymer microfluidic devices. J. Laser Appl. 2003;15:255–260. doi: 10.2351/1.1585085. [DOI] [Google Scholar]
- 38.Lee J, Musyimi HK, Soper SA, Murray KK. Development of an automated digestion and droplet deposition microfluidic chip for MALDI-TOF MS. J. Am. Soc. Mass Spect. 2008;19:964–972. doi: 10.1016/j.jasms.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 39.Lee JN, Park C, Whitesides GM. Solvent compatibility of poly(dimethylsiloxane)-based microfluidic devices. Anal. Chem. 2003;75:6544–6554. doi: 10.1021/ac0346712. [DOI] [PubMed] [Google Scholar]
- 40.Leng J, Salmon J-B. Microfluidic crystallization. Lab Chip. 2009;9:24–34. doi: 10.1039/b807653g. [DOI] [PubMed] [Google Scholar]
- 41.Lim D, Santoso EG, Teh KM, Wan S, Zheng HY. Theoretical analysis for laser machining microchannels and demonstration of their uses in manufacture of a microfluidic optical switch. Surf. Rev. Lett. 2006;13:795–802. doi: 10.1142/S0218625X06008876. [DOI] [Google Scholar]
- 42.Lin, Y., K. Tang, and R. D. Smith. Laser-machined microdevices for mass spectrometry. Mass Spectrometry and Hyphenated Techniques in Neuropeptide Research, pp. 171–192, 2002.
- 43.Liu A-L, He F-Y, Wang K, Zhou T, Lu Y, Xia X-H. Rapid method for design and fabrication of passive micromixers in microfluidic devices using a direct-printing process. Lab Chip. 2005;5:974–978. doi: 10.1039/b502764k. [DOI] [PubMed] [Google Scholar]
- 44.Liu Y, Rauch CB. DNA probe attachment on plastic surfaces and microfluidic hybridization array channel devices with sample oscillation. Anal. Biochem. 2003;317:76–84. doi: 10.1016/S0003-2697(03)00051-4. [DOI] [PubMed] [Google Scholar]
- 45.Long M, Sprague MA, Grimes AA, Rich BD, Khine M. A simple three-dimensional vortex micromixer. Appl. Phys. Lett. 2009;94:133501/133501–133501/133503. [Google Scholar]
- 46.Majumder A, Ghatak A, Sharma A. Micro fluidic adhesion induced by subsurface microstructures. Science. 2007;318:258–261. doi: 10.1126/science.1145839. [DOI] [PubMed] [Google Scholar]
- 47.Martin C, Llobera A, Leichle T, Villanueva G, Voigt A, Fakhfouri V, Kim JY, Berthet N, Bausells J, Gruetzner G, Nicu L, Brugger J, Perez-Murano F. Novel methods to pattern polymers for microfluidics. Microelectron. Eng. 2008;85:972–975. doi: 10.1016/j.mee.2008.01.052. [DOI] [Google Scholar]
- 48.McDonald JC, Chabinyc ML, Metallo SJ, Anderson JR, Stroock AD, Whitesides GM. Prototyping of microfluidic devices in poly(dimethylsiloxane) using solid-object printing. Anal. Chem. 2002;74:1537–1545. doi: 10.1021/ac010938q. [DOI] [PubMed] [Google Scholar]
- 49.McDonald JC, Duffy DC, Anderson JR, Chiu DT, Wu H, Schueller OJA, Whitesides GM. Fabrication of microfluidic systems in poly(dimethylsiloxane) Electrophoresis. 2000;21:27–40. doi: 10.1002/(SICI)1522-2683(20000101)21:1<27::AID-ELPS27>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 50.Mecomber JS, Stalcup AM, Hurd D, Halsall HB, Heineman WR, Seliskar CJ, Wehmeyer KR, Limbach PA. Analytical performance of polymer-based microfluidic devices fabricated by computer numerical controlled machining. Anal. Chem. 2006;78:936–941. doi: 10.1021/ac051523y. [DOI] [PubMed] [Google Scholar]
- 51.Mehta G, Lee J, Cha W, Tung Y-C, Linderman JJ, Takayama S. Hard top soft bottom microfluidic devices for cell culture and chemical analysis. Anal. Chem. 2009;81:3714–3722. doi: 10.1021/ac802178u. [DOI] [PubMed] [Google Scholar]
- 52.Melin J, Quake SR. Microfluidic large-scale integration: the evolution of design rules for biological automation. Annu. Rev. Biophys. Biomol. Struct. 2007;36:213–231. doi: 10.1146/annurev.biophys.36.040306.132646. [DOI] [PubMed] [Google Scholar]
- 53.Millare B, Thomas M, Ferreira A, Xu H, Holesinger M, Vullev VI. Dependence of the quality of adhesion between polydimethyl siloxane and glass surfaces on the conditions of treatment with oxygen plasma. Langmuir. 2008;24:13218–13224. doi: 10.1021/la801965s. [DOI] [PubMed] [Google Scholar]
- 54.Mitrovski, S. M., S. Avasthy, E. M. Erickson, M. E. Stewart, J. A. Rogers, and R. G. Nuzzo. Soft lithography for microfluidic micro-electromechanical systems (MEMS) and optical devices. Unconventional Nanopatterning Techniques and Applications, pp. 295–323, 2009.
- 55.Moorthy J, Beebe DJ. Organic and biomimetic designs for microfluidic systems. Anal. Chem. 2003;75:292A–301A. doi: 10.1021/ac0313567. [DOI] [PubMed] [Google Scholar]
- 56.Myers FB, Lee LP. Innovations in optical microfluidic technologies for point-of-care diagnostics. Lab Chip. 2008;8:2015–2031. doi: 10.1039/b812343h. [DOI] [PubMed] [Google Scholar]
- 57.Ng JMK, Gitlin I, Stroock AD, Whitesides GM. Components for integrated poly(dimethylsiloxane) microfluidic systems. Electrophoresis. 2002;23:3461–3473. doi: 10.1002/1522-2683(200210)23:20<3461::AID-ELPS3461>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 58.Ohno K-I, Tachikawa K, Manz A. Microfluidics: applications for analytical purposes in chemistry and biochemistry. Electrophoresis. 2008;29:4443–4453. doi: 10.1002/elps.200800121. [DOI] [PubMed] [Google Scholar]
- 59.Paguirigan AL, Beebe DJ. Microfluidics meet cell biology: bridging the gap by validation and application of microscale techniques for cell biological assays. BioEssays. 2008;30:811–821. doi: 10.1002/bies.20804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peeni BA, Lee ML, Hawkins AR, Woolley AT. Sacrificial layer microfluidic device fabrication methods. Electrophoresis. 2006;27:4888–4895. doi: 10.1002/elps.200600399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Psaltis D, Quake SR, Yang C. Developing optofluidic technology through the fusion of microfluidics and optics. Nature. 2006;442:381–386. doi: 10.1038/nature05060. [DOI] [PubMed] [Google Scholar]
- 62.Quake SR, Scherer A. From micro- to nanofabrication with soft materials. Science. 2000;290:1536–1540. doi: 10.1126/science.290.5496.1536. [DOI] [PubMed] [Google Scholar]
- 63.Rhee M, Burns MA. Microfluidic assembly blocks. Lab Chip. 2008;8:1365–1373. doi: 10.1039/b805137b. [DOI] [PubMed] [Google Scholar]
- 64.Saadi W, Wang S-J, Lin F, Jeon Noo L. A parallel-gradient microfluidic chamber for quantitative analysis of breast cancer cell chemotaxis. Biomed. Microdev. 2006;8:109–118. doi: 10.1007/s10544-006-7706-6. [DOI] [PubMed] [Google Scholar]
- 65.Sia SK, Kricka LJ. Microfluidics and point-of-care testing. Lab Chip. 2008;8:1982–1983. doi: 10.1039/b817915h. [DOI] [PubMed] [Google Scholar]
- 66.Sia SK, Whitesides GM. Microfluidic devices fabricated in poly(dimethylsiloxane) for biological studies. Electrophoresis. 2003;24:3563–3576. doi: 10.1002/elps.200305584. [DOI] [PubMed] [Google Scholar]
- 67.Situma C, Hashimoto M, Soper SA. Merging microfluidics with microarray-based bioassays. Biomol. Eng. 2006;23:213–231. doi: 10.1016/j.bioeng.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 68.Sorger PK. Microfluidics closes in on point-of-care assays. Nat. Biotechnol. 2008;26:1345–1346. doi: 10.1038/nbt1208-1345. [DOI] [PubMed] [Google Scholar]
- 69.Squires TM, Quake SR. Microfluidics: fluid physics at the nanoliter scale. Rev. Mod. Phys. 2005;77:977–1026. doi: 10.1103/RevModPhys.77.977. [DOI] [Google Scholar]
- 70.Stone HA, Kim S. Microfluidics: basic issues, applications, and challenges. AIChE J. 2001;47:1250–1254. doi: 10.1002/aic.690470602. [DOI] [Google Scholar]
- 71.Stone HA, Stroock AD, Ajdari A. Engineering flows in small devices: microfluidics towards a lab-on-a-chip. Ann. Rev. Fluid Mech. 2004;36:381–411. doi: 10.1146/annurev.fluid.36.050802.122124. [DOI] [Google Scholar]
- 72.Tan A, Rodgers K, Murrihy JP, O’Mathuna C, Glennon JD. Rapid fabrication of microfluidic devices in poly(dimethylsiloxane) by photocopying. Lab Chip. 2001;1:7–9. doi: 10.1039/b102905n. [DOI] [PubMed] [Google Scholar]
- 73.van Noort D, Ong SM, Zhang C, Zhang S, Arooz T, Yu H. Stem cells in microfluidics. Biotechnol. Prog. 2009;25:52–60. doi: 10.1002/btpr.171. [DOI] [PubMed] [Google Scholar]
- 74.Verma MKS, Ganneboyina SR, Rakshith VR, Ghatak A. Three-dimensional multihelical microfluidic mixers for rapid mixing of liquids. Langmuir. 2008;24:2248–2251. doi: 10.1021/la702895w. [DOI] [PubMed] [Google Scholar]
- 75.Verma MKS, Majumder A, Ghatak A. Embedded template-assisted fabrication of complex microchannels in PDMS and design of a microfluidic adhesive. Langmuir. 2006;22:10291–10295. doi: 10.1021/la062516n. [DOI] [PubMed] [Google Scholar]
- 76.Vullev VI, Wan J, Heinrich V, Landsman P, Bower PE, Xia B, Millare B, Jones G., II Nonlithographic fabrication of microfluidic devices. J. Am. Chem. Soc. 2006;128:16062–16072. doi: 10.1021/ja061776o. [DOI] [PubMed] [Google Scholar]
- 77.Walker GM, Zeringue HC, Beebe DJ. Microenvironment design considerations for cellular scale studies. Lab Chip. 2004;4:91–97. doi: 10.1039/b311214d. [DOI] [PubMed] [Google Scholar]
- 78.Wang K, Yue S, Wang L, Jin A, Gu C, Wang P, Wang H, Xu X, Wang Y, Niu H. Nanofluidic channels fabrication and manipulation of DNA molecules. IEEE Proc.: Nanobiotechnol. 2006;153:11–15. doi: 10.1049/ip-nbt:20050044. [DOI] [PubMed] [Google Scholar]
- 79.Wang K-G, Yue S, Wang L, Jin A, Gu C, Wang P-Y, Feng Y, Wang Y, Niu H. Manipulating DNA molecules in nanofluidic channels. Microfluid. Nanofluid. 2006;2:85–88. doi: 10.1007/s10404-005-0057-4. [DOI] [Google Scholar]
- 80.Wang W, Zhao S, Pan T. Lab-on-a-print: from a single polymer film to three-dimensional integrated microfluidics. Lab Chip. 2009;9:1133–1137. doi: 10.1039/b816287e. [DOI] [PubMed] [Google Scholar]
- 81.Weibel DB, Garstecki P, Whitesides GM. Combining microscience and neurobiology. Curr. Opin. Neurobiol. 2005;15:560–567. doi: 10.1016/j.conb.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 82.West J, Becker M, Tombrink S, Manz A. Micro total analysis systems: latest achievements. Anal. Chem. 2008;80:4403–4419. doi: 10.1021/ac800680j. [DOI] [PubMed] [Google Scholar]
- 83.Whitesides GM. The origins and the future of microfluidics. Nature. 2006;442:368–373. doi: 10.1038/nature05058. [DOI] [PubMed] [Google Scholar]
- 84.Wu H, Huang B, Zare RN. Generation of complex, static solution gradients in microfluidic channels. J. Am. Chem. Soc. 2006;128:4194–4195. doi: 10.1021/ja058530o. [DOI] [PubMed] [Google Scholar]
- 85.Xia Y, Friend RH. Nonlithographic patterning through inkjet printing via holes. Appl. Phys. Lett. 2007;90:253513/253511–253513/253513. [Google Scholar]
- 86.Xia Y, Kim E, Zhao X-M, Rogers JA, Prentiss M, Whitesides GM. Complex optical surfaces formed by replica molding against elastomeric masters. Science. 1996;273:347–349. doi: 10.1126/science.273.5273.347. [DOI] [PubMed] [Google Scholar]
- 87.Yager P, Edwards T, Fu E, Helton K, Nelson K, Tam MR, Weigl BH. Microfluidic diagnostic technologies for global public health. Nature. 2006;442:412–418. doi: 10.1038/nature05064. [DOI] [PubMed] [Google Scholar]
- 88.Zhao S, Cong H, Pan T. Direct projection on dry-film photoresist (DP2): do-it-yourself three-dimensional polymer microfluidics. Lab Chip. 2009;9:1128–1132. doi: 10.1039/b817925e. [DOI] [PubMed] [Google Scholar]
- 89.Zhao DS, Roy B, McCormick MT, Kuhr WG, Brazill SA. Rapid fabrication of a poly(dimethylsiloxane) microfluidic capillary gel electrophoresis system utilizing high precision machining. Lab Chip. 2003;3:93–99. doi: 10.1039/b300577a. [DOI] [PubMed] [Google Scholar]