Abstract
Objective
Primary treatment of spinal metastasis has been external beam radiotherapy. Recent advance of technology enables radiosurgery to be extended to extracranial lesions. The purpose of this study was to determine the clinical effectiveness and safety of stereotactic radiosurgery using Cyberknife in spinal metastasis.
Methods
From June, 2002 to December, 2007, 129 patients with 167 spinal metastases were treated with Cyberknife. Most of the patients (94%) presented with pain and nine patients suffered from motor deficits. Twelve patients were asymptomatic. Fifty-three patients (32%) had previous radiation therapy. Using Cyberknife, 16-39 Gy in 1-5 fractions were delivered to spinal metastatic lesions. Radiation dose was not different regarding the tumor pathology or tumor volume.
Results
After six months follow-up, patient evaluation was possible in 108 lesions. Among them, significant pain relief was seen in 98 lesions (91%). Radiological data were obtained in 83 lesions. The mass size was decreased or stable in 75 lesions and increased in eight lesions. Radiological control failure cases were hepatocellular carcinoma (5 cases), lung cancer (1 case), breast cancer (1 case) and renal cell carcinoma (1 case). Treatment-related radiation injury was not detected.
Conclusion
Cyberknife radiosurgery is clinically effective and safe for spinal metastases. It is true even in previously irradiated patients. Compared to conventional radiation therapy, Cyberknife shows higher pain control rate and its treatment process is more convenient for patients. Thus, it can be regarded as a primary treatment modality for spinal metastases.
Keywords: Spinal metastasis, Radiosurgery, Cyberknife
INTRODUCTION
Spinal metastases are the most common tumors of the spinal column. Up to 10% of cancer patients develop symptomatic spinal metastases, with multiple lesions more common14,17) . In addition, spinal symptoms can represent initial manifestation of cancer in 12 to 20% of symptomatic spinal metastases17,22). The rising incidence of cancer development and the improved survival of cancer patients are likely to be associated with an increase of spinal metastases. The goals of the treatment for spinal metastasis are palliative : pain control, maintenance of locomotive function and sphincter function, and prevention of spinal pathologic fractures.
Radiation therapy is considered as the primary treatment for spinal metastases. The role of radiation therapy in the treatment of spinal metastasis is well established and it is often used as the initial treatment modality. And, surgery is reserved for a small portion of patients with adequate indications. Conventional external beam radiotherapy is the classic form of spinal irradiation with hyperfractionation. In conventional radiotherapy, 20-40 Gy is delivered to the spine including the spinal cord over 5-20 daily fractionations. A primary factor that limits radiation dose with conventional radiotherapy is the relatively low tolerance of the spinal cord to radiation8). In stereotactic radiosurgery (SRS), a conformal high dose of radiation is delivered in one to three fractions to a well-defined target with a sharp dose fall-off within the target volume. SRS has been demonstrated to be an effective treatment for brain metastases with 85-95% control rate3,8). Recent technological developments, including image guidance for 3-dimensional localization, the advent of intensity modulated radiation therapy and a higher degree of accuracy in achieving target dose conformation while sparing normal surrounding tissue have enabled clinicians to perform SRS in spinal diseases.
Since Hamilton et al.16) firstly described the possibility of linear-accelerator based spinal SRS in 1995, several types of radiosurgical technology, such as Novalis, Cyberknife and Proton beam, have been developed. CyberKnife Image-Guided Radiosurgery System (Accuray, Sunnyvale, CA, USA) was first used for spinal metastasis at Stanford University in 1996. There has been a rapid increase in use of CyberKnife radiosurgery (CKR) as a treatment for malignant tumors involving the spinal column. And, many CyberKnife centers worldwide have demonstrated the safety, the feasibility and clinical efficacy of CKR4,5,11,13). The combination of a steep dose gradient and high conformity of the CyberKnife system allows such high dose to be delivered close to the adjacent spinal cord. Our institute adopted Cyberknife system in 2002 and cumulative treatment cases amount over 2,000 cases. The purpose of this study was to evaluate the clinical and radiological result of CKR for spinal metastases in our institute.
MATERIALS AND METHODS
The Cyberknife system
The Cyberknife system consists of 6-MV compact linear accelerator, robotic manipulator, two diagonal X-ray cameras, image detector, treatment couch and treatment planning computer. Linear accelerator is the machinery which accelerates electron and produces X-ray energy. It is smaller and lighter in weight than linear accelerators used in conventional radiotherapy. It has variable size of collimators ranging from 5 mm to 60 mm. The smaller size allows it to be mounted on a robotic manipulator. A computer-controlled robotic manipulator has six degrees of freedom and provides 1,200 different directions of beam. Two diagnostic X-ray cameras are positioned orthogonally to acquire real-time images of the patient's internal anatomy. The images are processed to identify radiographic features and then automatically compared with the patient's computed tomography (CT) treatment planning study. The precise tumor position is communicated through a real-time control loop to a robotic manipulator that aligns the radiation beam with the target.
Treatment procedure
CKR was performed as an outpatient procedure in most cases. The patient underwent percutaneous placement of six gold seed fiducials into the posterior element of the adjacent vertebrae of the lesion under fluoroscopic guidance one week before treatment. CT scan of 1.5 mm slice was done one day before treatment. During CT scan, a custom-made non-rigid immobilization device consisting of Aquaplast face mask (WFR/Aquaplast Crop., Wyckoff, NJ, USA) or a vacuum foam body cradle was used. The target lesion and critical structures including the spinal cord were contoured on axial CT slices to obtain 3-dimensional reconstruction using the CyberKnife treatment planning soft ware (Accuray, Inc.). Treatment dose and fractionation number were determined by the doctors. Prescription dose of radiation was determined based on the shape of the tumor and radiation distribution of the spinal cord. The patient was placed on the treatment couch in a supine position with appropriate immobilization. During treatment, real-time digital X-ray images of the implanted fiducial markers were obtained. The location of the vertebral body being treated was established from these images and was used to determine tumor location.
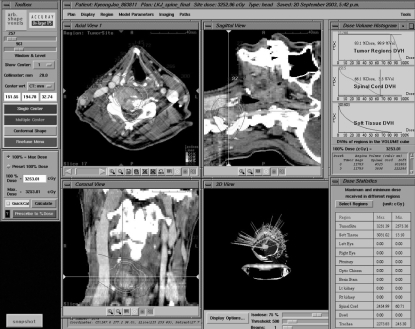
A typical treatment plan is shown in Fig 1. Using predetermined treatment plan, approximately 100-150 non-isocentric beams were delivered sequentially. Treatment time usually lasted 40-90 minutes. Patients were followed clinically and radiographically with CT or MRI at 3-6 months intervals. The change of pain manifestation was evaluated by means of visual analogue scale (VAS).
Fig. 1.
Treatment planning illustration; A 41-year-old male patient of renal cell carcinoma with C5 metastasis. Tumor mass (C5 vertebral body right side) and adjacent spinal cord are marked. Lesion volume was 11, 789 mm3 and exposed spinal cord volume was 4, 515 mm3. Spinal cord dose volume histogram shows that 3.5% volume of exposed spinal cord received 66.1% of maximal radiation dose (32.53 Gy).
Patient population
During past five-and-half years (2002, 06-2007, 12), a total of 233 procedures of spinal radiosurgery were done with Cyberknife® Robotic Radiosurgery System in Korean Cancer Center Hospital Cyberknife Center. Among them, 167 lesions were metastatic spinal tumors in 129 patients (Table 1). Some patients had multiple treatments due to successive occurrence of metastases. All patients had a histological diagnosis of malignant neoplasm and had either synchronous or metachronous metastasis to spine with or without cord compression.
Table 1.
Characteristics of 129 patients with 167 tumors (between June, 2002 and December, 2007)
Preoperative evaluation tool was MRI or CT with or without PET scan. Pretreatment neurological estimation was performed and severity of pain was assessed with VAS. Primary sites were most prevalent in hepatocellular carcinoma and breast cancer (Table 1). Treatment group information was summarized in Table 3, including involved spinal levels, treatment indications and previous treatment. All the symptomatic spinal metastases can be indication for CKR with few exceptions. Exclusion criteria were overt spinal instability, paraparesis worse than motor grade IV and three column involvement cases.
Table 3.
Radiotherapeutical characteristics
RESULTS
Patient characteristics
The age distribution ranged from 19 to 81 (mean age; 52). Most of the patients (94%) presented with pain. Motor deficit was seen in nine patients (5%). In 12 patients, spinal metastasis was diagnosed in routine follow-up check without any symptoms. In 53 patients (32%), previous radiation therapy had been performed on the lesion site. CKR was done under several clinical situations (Table 1). CKR was the initial treatment after spinal metastases were diagnosed in 98 patients (primary treatment). In 58 patients, other treatment modalities were tried and followed up. When tumor growth was detected, CKR was performed (tumor progression). In the remaining cases, CKR was applied as an adjuvant treatment for postoperative residual mass or booster treatment after radiation therapy (4 lesions and 7 lesions). The spinal levels involved were cervical 17%, thoracic 41%, lumbar 28% and sacral 14% lesions (Table 1). Primary cancer sites were liver 21%, breast 20%, colo-rectal 10%, soft tissue sarcoma 9%, kidney 8%, lung 7%, cervix 4% and others 30% (Table 2)
Table 2.
Histologies of primary tumors
Radiotherapeutic parameters
The lesion volume ranged from 1.87 cm3 to 399 cm3 with a mean of 59.00 cm3. The difference of lesion volume was not significant between primary tumors, but the largest volume was found in the colon cancer group (148 cm3) and the cervix cancer group (90.7 cm3). The reason for the largest volume in these cancer patients is attributed to the preference of metastatic location to lower lumbar and sacral regions. A dose of 16-39 Gy in 1-5 fractions was prescribed (Table 3). Mean dose of 26.46 Gy was delivered with a mean of 3.1 fractionation. Administered radiation dose can be calculated as biological equivalent dose (BED) for comparison. Using the assumption of the linear-quadratic model, where n, number of fractions; d, dose per fraction; α/β, alpha beta ratio (10 for early responding tissue, 2 or 3 for late responding tissue), BED is calculated as follows15).
BED = nd (1 + d /α / β)
Mean value of BED was estimated to be 57.4 Gy. When irradiated doses as BED were compared according to the lesion volume, significant relation was not observed (Fig. 2). When the BED was compared between primary cancers, it ranged from 53.1 Gy (hepatocellular carcinoma) to 73 Gy (cervix cancer). In the remaining groups, BED values were from 53.2 Gy to 55.9 Gy (Fig. 3). Mean value of BED was 36.8 Gy in the primary treatment group and 34.7 Gy in tumor progression group. When BED was analyzed according to the lesion location, a mean of 52.3 Gy was delivered to spinal cord level (cervical and thoracic level) and 64.7 Gy to cauda equina level (lumbar and sacral level). Isodose line was determined from 75% to 88%. The quality of the treatment plan as a radiosurgery is evaluated in terms of dose homogeneity, target coverage, and dose conformity23). Homogeneity index was measured as a ratio of the maximum dose to the prescription dose. Mean value of our data was 1.31. Target coverage was calculated by dividing minimum radiation dose in target volume by the prescription radiation dose. Our mean value was 95.18. Dose conformity index was defined as the total volume enclosed by the prescription isodose divided by the target volume. Mean value of our data was 1.4. These radiotherapeutical parameters were within acceptable range with regard to Radiation therapy Oncology Group (RTOG) radiosurgery guidelines18).
Fig. 2.
The relation between radiation dose (biological equivalent dose : BED) and lesion volume. A mean value of BED was 57.4 Gy. The lesion volume ranged from 1.87 cm3 to 399 cm3. No significant relation was found between two factors.
Fig. 3.
Biological equivalent dose of radiation and primary cancer type. It ranged from 53.1 Gy (hepatocellular carcinoma) to 73 Gy (cervix cancer).
Follow-up result
Spinal pain relief began within two weeks after the CKR treatment and was definite one month later in most cases. Follow-up period ranged from one month to 63 months and mean value was 14.3 months. On six month follow-up, patient evaluation was possible in 108 lesions. Among them, significant pain relief was seen in 98 lesions (91%) (Table 4). In 10 lesions, pain relief was minimal and another measure for pain control was required. In 59 lesions, the evaluation was impossible due to follow-up loss, aggravation of other metastatic lesions, and death before six months.
Table 4.
Pain control rate after Cyberknife radiosurgery
Radiological data were obtained in 83 lesions during six months follow-up. Within the data mass size increase was seen in eight lesions. In the other 75 lesions, follow-up images showed stable condition or decreased mass size (Table 5). The cases in which mass size increased were five HCCs, one lung cancer patient, one breast cancer patient and one renal cell cancer patient. Six out of eight cases were previously irradiated (Table 6). No radiation myelopathy was observed in associated with CKR. In breast cancer group, 25 patients were available on mean follow-up of 27.1 months. Most of breast cancer patients were stable during follow-up period. The earliest recurred case was on 24 months, the longest follow-up with no recurrence was 54 months (Fig. 4). In cases of HCC metastasis, 29 patients were followed up during a mean of 9.2 months. Two long-term survivors were found and recurrences were detected on 12 months and 27 months (Fig. 5).
Table 5.
Radiological control rate after Cyberknife radiosurgery
Table 6.
Summary of radiological regrowth cases
ca : cancer, HCC: hepatocellular carcinoma, RCC : renal cell carcinoma
Fig. 4.
A : Magnetic resonance image of a 42-year-old woman of breast cancer with L3 metastasis. Enhancing mass is seen in L3 vertebral body, left pedicle and left lamina. The patient received 21 Gy in three fractionations. B : Two years later, mass is shrunken on follow-up computed tomography. C : Four years and six months later, the mass is under control on follow-up computed tomography.
Fig. 5.
A : Magnetic resonance image of a 56-year-old man of hepatocellular carcinoma with T11 metastasis. Osteolytic lesion is in the T11 vertebral body and epidural mass shows cord compression. He received 27 Gy in three fractionations. B : After six months, epidural mass has disappeared on follow-up magnetic resonance image and pain relieved completely.
DISCUSSION
Advantages of Cyberknife radiosurgery
The CyberKnife (Accuray, Inc., Sunnyvale, CA, USA) is an image-guided, robotic radiosurgery system. It consists of a 6-MV compact linear accelerator (LINAC) used in conventional radiotherapy. First LINAC designed for radiosurgery was developed with a 3-dimensional treatment planning software called X-knife at Harvard2). The development of X-knife was followed by a prototype LINAC with features that included tighter tolerances for isocentric rotation, 360 of gantry rotation with a couch mount apparatus, and fixed primary and secondary collimation units that minimized gantry sag2). X-knife was commercially used in 1991 in the name of Varian 600SR. And, dynamic arc technique was interfaced with the Varian SR600, which was renamed the Novalis Shaped Beam Radiosurgery Unit. The CyberKnife is the first robotic LINAC capable of the precise delivery of radiation beyond the cranium. It is the third LINAC after the Varian 600SR and its successor, the Novalis. With Novalis using infrared markers and intensity modulation, Ryu et al. showed an isocenter accuracy of 1.36 mm. The Cyberknife system allows 1.1 ± 0.3 mm spatial accuracy using a 1.25-mm CT slice thickness9). The Cyberknife system differs from frame-based radiosurgery in three fundamental ways1,10,21). First, it refers the position of the treatment target to internal radiographic features such as the skull or implanted fiducials rather than a fixed frame. Second, it uses real-time X-ray images to establish the position of the lesion during treatment and then dynamically bring the radiation beam into alignment with the observed position of the treatment target. Third, it aims each beam independently, without a fixed isocenter. Changes in patient position during the treatment are compensated by adaptive beam pointing rather than controlled through rigid immobilization.
The advantages of the Cyberknife radiosurgery are as follows. Treatment duration is short, ranging from one day to five days according to fractionation schedule. In most of our cases, three fraction schedules are applied, but current trend in metastasis treatment is toward single session radiosurgery4,5,20). Treatment course of radiosurgery is more comfortable to patient than prolonged fractionated radiotherapy. A large hypofraction radiation may be radiobiologically superior to conventional radiotherapy, providing improved local control and resulting in a more rapid and longer duration of pain control. Pain relief is seen between the first day and the fourteenth day after treatment begins and is maintained over one year. Radiosurgery avoids the irradiation to large segments of the spinal cord and spinal column, which is known to deleterious effect on bone marrow function. Spinal cord can be spared from irradiation in radiosurgery, which enables previously irradiated lesions to be treated. Our research includes 53 lesions which received conventional radiotherapy previously. In Gagnon et al.4)s report, they treated breast cancer patients with spinal metastasis recurrent within a previous irradiation field with Cyberknife and compared the result with the patients who received conventional external beam radiotherapy (CRT) up-front for spinal metastasis. The treatment outcomes were similar for patients in both groups with regard to ambulation, performance status and pain score.
When a patient presents multiple, noncontiguous lesions, such as C7, T4 and L3, simultaneous or consecutive treatment can be applied. In our series, 53-year-old woman with breast cancer received six successive Cyberknife radiosurgery to spine lesions without complication. In cases of postoperative treatment, radiosurgery can start earlier than radiotherapy, which usually begins three weeks later. The directions of irradiation beam are diverse and wound site can be avoided. Asymptomatic spinal metastasis, in which metastatic lesions are detected on regular check and lesion size is very small, is the best indication of Cyberknife radiosurgery. In these cases, treatment evaluation is done with positron emission tomography (PET) rather than MRI.
Pain control effect and radiological outcome
Gibbs12) reported that 84% of the patients who presented with pain at the time of treatment improved after CKR during follow up of 9 months. Long term pain improvement was observed in 290 of 336 cases (86%) and long term tumor control was demonstrated in 88% of lesions in the study of Gerszten et al.8). They thought that whether pain is successfully controlled or not does not depend on the tumor pathology and radiation dose.
Ryu et al.20) reported in the radiosurgery experience with Novalis that the overall rate of pain control was 84% for one year. Median duration of pain relief at the treated level was 13.3 months. Relapse of pain at the treated spinal segment was 6.9%20). They insisted that the strong trend was observed between increased pain control rate and higher radiation dose20). Higher dose than 14 Gy achieved more consistent pain control compared to the lower dose group. Pain control rate does not seem to be affected by the fractionation number, which is supported by a number of prospective randomized studies involving bone metastases25,26). In the study of conventional radiotherapy for spinal metastasis, partial pain relief is estimated from 55% to 89% and complete pain response from 21% to 58%. The median duration of pain relief was typically less than one year and median retreatment rate was as high as 44%4,24).
Radiological tumor control was achieved in 90% of tumors in our series, which is comparable to other reports8). In patients with breast cancer and lung cancer, tumor control rate is reported to be almost 100%8). In 68 spinal breast metastases, radiological tumor control was seen in all cases with a follow-up period of 6-48 months9). In 77 spinal metastasis of lung cancer, radiological tumor control rate was observed in nearly all patients on a follow-up of 6-40 months5). Tumor control rate declines to 90% in renal cell cancer patients. In Gerszten et al.7)s report, six out of sixty patients who had received radiosurgery showed radiological regrowth during the follow-up period (range 14-48 months)7). Radiological control failure cases in our series are those who had significant epidural mass in the spinal canal or insufficient radiation dose due to previous irradiation (Table 6).
Radiotherapeutical parameters
The appropriate dose for spinal radiosurgery for spinal metastasis varied depending on primary cancer pathology and technical factors such as proximity to spinal cord and previous irradiation. In our series, a mean of 26.4 Gy was delivered with a mean of 3.1 fractionations, which is equivalent of 57.4 Gy (BED). This value was somewhat higher than other reports, which ranged from 45 Gy to 55 Gy8,13,20,21). In lung cancer and renal cell cancer patients, 18 Gy of marginal dose (single session) is recommended for successful tumor control. In breast cancer, marginal dose (single session) can be lowered to 16 Gy. For complete tumor control, irradiation dose can be escalated over 18 Gy (single session) if the spinal cord is safely spared from significant amount of radiation. BED was the highest in our cervix cancer patients (72 Gy). Cervix cancer usually metastasizes to lower lumbar and sacrum. Six out of seven patients had L5 or sacral metastasis. In sacral metastasis, radiation dose can be increased over 80 Gy of BED, which is the highest value in spinal radiosurgery. A mean of 52.3 Gy (BED) was irradiated at spinal cord level and 64.7 Gy (BED) at cauda equine level. At cauda equine level, radiation dose can be increased because nerve roots are more resistant to radiation than spinal cord. Even though epidural mass compresses nerve roots at cauda equina level, spinal radiosurgery can be performed without neurological complication.
Spinal radiosurgery was found to be safe at similar doses to what was used for intracranial radosurgery without the occurrence of radiation induced spinal cord injury8). Radiation toxicity has been rarely reported in the patients who have received Cyberknife radiosurgery.6,12,13,19) Gibbs12) reported that three patients out of 74 patients with 102 metastatic lesions developed new spinal cord complications after radiosurgery. Their lesions were located at T1, T5 and T6 level respectively. Clinical symptoms of myelopathy presented at 6 months (two cases) and 10 months. There was no significant relationship found between radiation dose with myelopathy12). Their observation indicated that no complication occurred when the volume of the spinal cord receiving a biologically equivalent dose of 12 Gy in a single fraction (i.e., BED3 of 58 Gy) was less than 0.15 cm3,12,13). In another series of 73 patients who have been treated due to benign intradural spinal tumors, three patients have been reported to show radiation-induced spinal cord toxicity after treatment6). The presenting time of radiation myelopathy was between 6 months and 12 months after treatment. Clinical symptoms may be relieved or persistent. Their statistical estimation for radiation dose of the spinal cord suggests that the volume of the spinal cord receiving a dose of 10 Gy should be less than 0.3 cm3,12,13).
CONCLUSION
The authors treated 167 lesions of spinal metastases with Cyberknife radiosurgery system. The treatment group includes previously irradiated patients and non-irradiated patients. Overall pain control rate was 91% and radiological tumor control rate was 90% at the time of six months after treatment. No neurological complications were associated with radiosurgery. Local tumor control rate is higher and tumor control maintains for longer duration in radiosurgery than conventional radiotherapy. Moreover, treatment time is relatively short and patients can have a radiosurgery on outpatient setting. Cyberknife radiosurgery can be considered as a primary treatment modality in the management of spinal metastasis.
References
- 1.Adler JR, Jr, Murphy MJ, Chang SD, Hancock SL. Image-guided robotic radiosurgery. Neurosurgery. 1999;44:1299–1306. discussion 1306-1307. [PubMed] [Google Scholar]
- 2.Andrews DW, Bednarz G, Evans JJ, Downes B. A review of 3 current radiosurgery systems. Surg Neurol. 2006;66:559–564. doi: 10.1016/j.surneu.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Flickinger JC, Kondziolka D, Lunsford LD, Coffey RJ, Goodman ML, Shaw EG, et al. A multi-institutional experience with stereotactic radiosurgery for solitary brain metastasis. Int J Radiat Oncol Biol Phys. 1994;28:797–802. doi: 10.1016/0360-3016(94)90098-1. [DOI] [PubMed] [Google Scholar]
- 4.Gagnon GJ, Henderson FC, Gehan EA, Sanford D, Collins BT, Moulds JC, et al. Cyberknife radiosurgery for breast cancer spine metastases : a matched-pair analysis. Cancer. 2007;110:1796–1802. doi: 10.1002/cncr.22977. [DOI] [PubMed] [Google Scholar]
- 5.Gerszten PC, Burton SA, Belani CP, Ramalingam S, Friedland DM, Ozhasoglu C, et al. Radiosurgery for the treatment of spinal lung metastases. Cancer. 2006;107:2653–2661. doi: 10.1002/cncr.22299. [DOI] [PubMed] [Google Scholar]
- 6.Gerszten PC, Burton SA, Ozhasoglu C, McCue KJ, Quinn AE. Radiosurgery for benign intradural spinal tumors. Neurosurgery. 2008;62:887–895. doi: 10.1227/01.neu.0000318174.28461.fc. discussion 895-896. [DOI] [PubMed] [Google Scholar]
- 7.Gerszten PC, Burton SA, Ozhasoglu C, Vogel WJ, Welch WC, Baar J, et al. Stereotactic radiosurgery for spinal metastases from renal cell carcinoma. J Neurosurg Spine. 2005;3:288–295. doi: 10.3171/spi.2005.3.4.0288. [DOI] [PubMed] [Google Scholar]
- 8.Gerszten PC, Burton SA, Ozhasoglu C, Welch WC. Radiosurgery for spinal metastases : clinical experience in 500 cases from a single institution. Spine. 2007;32:193–199. doi: 10.1097/01.brs.0000251863.76595.a2. [DOI] [PubMed] [Google Scholar]
- 9.Gerszten PC, Burton SA, Welch WC, Brufsky AM, Lembersky BC, Ozhasoglu C, et al. Single-fraction radiosurgery for the treatment of spinal breast metastases. Cancer. 2005;104:2244–2254. doi: 10.1002/cncr.21467. [DOI] [PubMed] [Google Scholar]
- 10.Gerszten PC, Ozhasoglu C, Burton SA, Vogel W, Atkins B, Kalnicki S, et al. Evaluation of CyberKnife frameless real-time image-guided stereotactic radiosurgery for spinal lesions. Stereotact Funct Neurosurg. 2003;81:84–89. doi: 10.1159/000075109. [DOI] [PubMed] [Google Scholar]
- 11.Gerszten PC, Ozhasoglu C, Burton SA, Vogel WJ, Atkins BA, Kalnicki S, et al. CyberKnife frameless stereotactic radiosurgery for spinal lesions : clinical experience in 125 cases. Neurosurgery. 2004;55:89–98. discussion 98-99. [PubMed] [Google Scholar]
- 12.Gibbs IC. Spinal and paraspinal lesions : the role of stereotactic body radiotherapy. Front Radiat Ther Oncol. 2007;40:407–414. doi: 10.1159/000106050. [DOI] [PubMed] [Google Scholar]
- 13.Gibbs IC, Kamnerdsupaphon P, Ryu MR, Dodd R, Kiernan M, Chang SD, et al. Image-guided robotic radiosurgery for spinal metastases. Radiother Oncol. 2007;82:185–190. doi: 10.1016/j.radonc.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 14.Grant R, Papadopoulos SM, Sandler HM, Greenberg HS. Metastatic epidural spinal cord compression : current concepts and treatment. J Neurooncol. 1994;19:79–92. doi: 10.1007/BF01051052. [DOI] [PubMed] [Google Scholar]
- 15.Hall EJ, Brenner DJ. The radiobiology of radiosurgery : rationale for different treatment regimes for AVMs and malignancies. Int J Radiat Oncol Biol Phys. 1993;25:381–385. doi: 10.1016/0360-3016(93)90367-5. [DOI] [PubMed] [Google Scholar]
- 16.Hamilton AJ, Lulu BA, Fosmire H, Stea B, Cassady JR. Preliminary clinical experience with linear accelerator-based spinal stereotactic radiosurgery. Neurosurgery. 1995;36:311–319. doi: 10.1227/00006123-199502000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Jacobs WB, Perrin RG. Evaluation and treatment of spinal metastases : an overview. Neurosurg Focus. 2001;11:e10. doi: 10.3171/foc.2001.11.6.11. [DOI] [PubMed] [Google Scholar]
- 18.Lomax NJ, Scheib SG. Quantifying the degree of conformity in radiosurgery treatment planning. Int J Radiat Oncol Biol Phys. 2003;55:1409–1419. doi: 10.1016/s0360-3016(02)04599-6. [DOI] [PubMed] [Google Scholar]
- 19.Ryu S, Jin JY, Jin R, Rock J, Ajlouni M, Movsas B, et al. Partial volume tolerance of the spinal cord and complications of single-dose radiosurgery. Cancer. 2007;109:628–636. doi: 10.1002/cncr.22442. [DOI] [PubMed] [Google Scholar]
- 20.Ryu S, Jin R, Jin JY, Chen Q, Rock J, Anderson J, et al. Pain control by image-guided radiosurgery for solitary spinal metastasis. J Pain Symptom Manage. 2008;35:292–298. doi: 10.1016/j.jpainsymman.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 21.Ryu SI, Chang SD, Kim DH, Murphy MJ, Le QT, Martin DP, et al. Image-guided hypo-fractionated stereotactic radiosurgery to spinal lesions. Neurosurgery. 2001;49:838–846. doi: 10.1097/00006123-200110000-00011. [DOI] [PubMed] [Google Scholar]
- 22.Schiff D, O'Neill BP, Suman VJ. Spinal epidural metastasis as the initial manifestation of malignancy : clinical features and diagnostic approach. Neurology. 1997;49:452–456. doi: 10.1212/wnl.49.2.452. [DOI] [PubMed] [Google Scholar]
- 23.Shaw E, Kline R, Gillin M, Souhami L, Hirschfeld A, Dinapoli R, et al. Radiation therapy oncology group : radiosurgery quality assurance guidelines. Int J Radiat Oncol Biol Phys. 1993;27:1231–1239. doi: 10.1016/0360-3016(93)90548-a. [DOI] [PubMed] [Google Scholar]
- 24.Steenland E, Leer JW, van Houwelingen H, Post WJ, van den Hout WB, Kievit J, et al. The effect of a single fraction compared to multiple fractions on painful bone metastases : a global analysis of the dutch bone metastasis study. Radiother Oncol. 1999;52:101–109. doi: 10.1016/s0167-8140(99)00110-3. [DOI] [PubMed] [Google Scholar]
- 25.Tong D, Gillick L, Hendrickson FR. The palliation of symptomatic osseous metastases : final results of the study by the radiation therapy oncology group. Cancer. 1982;50:893–899. doi: 10.1002/1097-0142(19820901)50:5<893::aid-cncr2820500515>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 26.Tsai JT, Lin JW, Chiu WT, Chu WC. Assessment of image-guided CyberKnife radiosurgery for metastatic spine tumors. J Neurooncol. 2009;94:119–127. doi: 10.1007/s11060-009-9814-7. [DOI] [PubMed] [Google Scholar]