Abstract
Radiologic findings of Bing-Neel syndrome, which is an extremely uncommon complication resulting from malignant lymphocyte infiltration into the central nervous system (CNS) in patients with Waldenström's macroglobulinemia (WM), have been infrequently reported due to extreme rarity of the case. A 75-year-old man with WM presented at a neurology clinic with progressive gait and memory disturbances, and dysarthria of 2 months duration. Cerebrospinal fluid and serum protein electrophoresis and immunofixation electrophoresis showed IgM kappa-type monoclonal gammopathy. Brain magnetic resonance imaging revealed multifocal, hyperintense lesions on T2 weighted-images. Brain diffusion-weighted imaging (DWI) demonstrated hyperintensities in cerebral and cerebellar lesions that appeared isointense on apparent diffusion coefficient maps, which were compatible with vasogenic edema. Although histologic analysis is a confirmative study to prove direct cell infiltration into the brain, brain MRI with DWI may be a good supportive study to diagnose Bing-Neel syndrome.
Keywords: Bing-Neel syndrome, Waldenström's macroglobulinemia, MRI
INTRODUCTION
Waldenström's macroglobulinemia (WM) is a chronic malignant proliferative disorder of lymphoid cells that infiltrates bone marrow and produces IgM monoclonal protein in serum8). In 1936, Bing and Neel3) reported an association between hyperglobulinemia, central nervous system (CNS) symptoms, and brain infiltration comprised of plasma cells and lymphocytes in two patients. Since then, the eponym Bing-Neel syndrome, has been used to define the extremely rare cases of WM with CNS involvement. The mechanism underlying CNS involvement has been explained as direct CNS infiltration of malignant cells; histologic analysis is needed to confirm the direct cell infiltration6). With the recent dramatic advances in radiology, a key evaluation for diagnosis of many CNS diseases has moved from histologic studies to radiologic studies. However, the radiologic findings of CNS involvement in patients with WM have been rarely reported due to the extreme rarity of the combination. A recent case report, in which diffusion weighted image (DWI) findings have been presented, suggested cytotoxic edema representing multiple infarcts as a vascular obstruction mechanism1). Herein, we present a case of Bing-Neel syndrome with DWI findings demonstrating vasogenic edema, and suggest that a brain magnetic resonance imaging (MRI) is a good supportive study in diagnosing the Bing-Neel syndrome.
CASE REPORT
A 75-year-old man presented to our neurology clinic for evaluation of progressive gait and memory disturbances, and dysarthria of 2 months duration. Three years earlier, he was diagnosed with WM by a bone marrow biopsy, which showed lymphoplasmacytic cells with IgM kappa monoclonality. He was treated successfully by immunosuppressive chemotherapy. Two months prior to our evaluation, he began to experience slight dysarthria and memory impairment, which prevented social activities. These symptoms rapidly progressed, and he developed severe dizziness with an ataxic gait three weeks prior to the evaluation. On physical examination, his vital signs were stable, and neither lymphadenopathy nor organomegaly was present. On the neurologic examination, he appeared alert, but had slow verbal responses and reduced verbal fluency. His mini-mental status examination score was 24/30 (missing 2 points on orientation, 3 points on recall, and 1 point on his copy of the intersecting pentagon). He had a clinical dementia rating scale of 1.0. The frontal lobe function test was normal. Central-type right facial palsy and right side gaze-evoked nystagmus were observed. The motor and sensory examinations were normal, and there were no pathologic reflexes. On cerebellar function testing, the right upper and lower extremities showed dysmetria. He could not walk without assistance because of tilting to the right side.
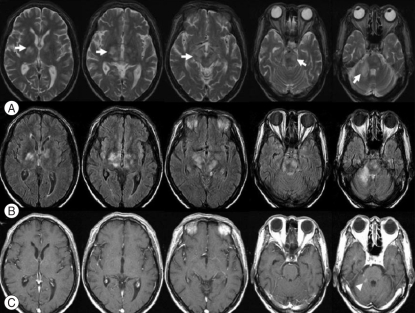
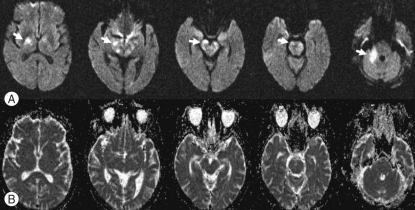
The hematologic laboratory values were as follows : hemoglobin, 9.2 g/dL; white blood cell count, 5.9 × 103/µL with neutrophilia (82.2%); and platelets, 136 × 103/µL. Blood chemistries, including albumin, was normal. Brain MRI showed hyperintensities involving the capsulostriatal area (anterior thalamus, hypothalamus, anterior midbrain, pons, and medulla oblongata) and the right middle cerebellar peduncle on T2-weighted and fluid attenuated inversion recovery (FLAIR) images (Fig. 1). Post-contrast T1-weighted images revealed focal enhancements in lesions of the right middle cerebellar peduncle (Fig. 1). DWI demonstrated hyperintensities in the cerebral and cerebellar lesions that appeared isointense on apparent diffusion coefficient (ADC) maps (Fig. 2).
Fig. 1.
Conventional brain magnetic resonance images. Serial axial T2-weighted images (A) show hyperintensities (arrows) involving the capsulostriatal area (anterior thalamus, hypothalamus, midbrain, pons, and medulla oblongata) and the right middle cerebellar peduncle, which are more well revealed in fluid attenuated inversion recovery images (B). Serial axial post-contrast T1-weighted images (C) reveal focal enhancements (arrow head) in lesions of the right middle cerebellar peduncle.
Fig. 2.
Diffusion weighted images. Serial diffusion weighted images of the brain (b = 1000 s/mm2, upper row) demonstrating hyperintensities (arrows) in cerebral and cerebellar lesions that appear isointense on apparent diffusion coefficient maps (lower row).
His serum IgM level was 1,490 mg/dL (normal range, 50-300 mg/dL), and this was significantly less than the 3,780 mg/dL recorded 3 years previously. His serum β2-microglobulin level was 2.46 mg/dL (normal range, 1-2.4 mg/dL), and this was also lower compared to the level found 3 years previously (4.46 mg/dL). A lumbar puncture (LP), which was performed to investigate possible malignant cell infiltration, revealed a slightly increased cell count and protein level with normal pressure as follows : pressure, 13 cm H2O; white blood cell count, 81/µL (neutrophils, 22%; lymphocytes, 50%; monocytes, 27%; and eosinophils, 1%), red blood cells, 3/µL; protein, 49 mg/dL; and glucose, 68 mg/dL. The serum microhemagglutination-Treponema pallidum test was negative. The cerebrospinal fluid (CSF) Venereal Disease Research Laboratory (VDRL) test, fungus culture, gram stain and culture, India ink, tuberculosis (Tb) culture, and Tb polymerase chain reaction were all negative, which ruled out an opportunistic infection of the CNS, a common complication of patients with undergoing immunosuppressive treatment. CSF and serum protein electrophoresis and immunofixation electrophoresis showed IgM (kappa-type) monoclonal gammopathy. An electrophysiologic study revealed a moderate degree of right median motor neuropathy at the wrist level, compatible with carpal tunnel syndrome, but there was no evidence of peripheral nervous system involvement, typical of WM.
The patient received chemotherapy and intravenous steroid injections as follows : one cycle of chlorambucil (2 mg orally tid per day on days 1-12) and methylprednisolone sodium (60 mg intravenously per day on days 1-5). However, his symptoms slowly worsened, and he died 1 month later. A postmortem pathologic study was not performed at the request of the family.
DISCUSSION
WM is a rare condition, and accounts for only 2% of monoclonal gammopathies, with an age-adjusted incidence of 3.4 per million among males and 1.7 per million among females7). Neurologic complications may occur in up to 25% of patients with WM11). The most frequent neurologic complication is sensorimotor polyneuropathy11). CNS manifestations are usually caused by serum hyperviscosity8). Only extremely rare cases of Bing-Neel syndrome, involving direct CNS infiltration by malignant cells, have been reported1,3,4,9-11).
Because of the rarity of Bing-Neel syndrome, the radiologic features have yet to be elucidated. Some findings have been reported in presumed cases or histopathologically-demonstrated cases1,5). Bing-Neel syndrome can be subdivided into diffuse and tumoral forms11). In the diffuse infiltrative form, the cerebral lymphoid infiltration predominates in the pons, medulla, periventricular white matter, and leptomeningeal spaces. In the tumoral form, the lesion may be unifocal or multifocal, and is usually located in the deep subcortical hemispheric regions. Nervous system infiltrations appear as contrast-enhancement and/or thickening of meningeal sheaths on computerized tomography or MRI, and may involve the brain parenchyma and cauda equina7,11). In our case, MRI displayed edematous infiltrative lesions in the periventricular white matter, basal ganglia, thalamus, hypothalamus, midbrain, pons, cerebellar peduncle, and cerebellum. Enhancement was observed in lesions of the right middle cerebellar peduncle on the T1-weighted gadolinium image (Fig. 1). Based on the presence of IgM kappa-type paraprotein on the CSF immunofixation electrophoresis, multiple involvement of the brain parenchyma, and a history of WM, the patient was diagnosed with Bing-Neel syndrome. The conventional MRI findings corresponded to the diffuse form of Bing-Neel syndrome. Arias et al.1) reported DWI findings in one case of Bing-Neel syndrome, in which numerous areas of increased signal intensity were observed in different arterial territories, and which were prominent in T2, FLAIR, and DWI sequences with a low ADC value. Arias et al.1) suggested multiple infarcts due to a neoplastic vascular obstruction. In our case, the lesions showed high signal intensity on DWI and a normal ADC value. We believe that the lesion on MRI in our case represented vasogenic edema caused by direct malignant cell infiltration into the brain parenchyma rather than being due to vascular obstruction. The lesion may have developed due to vascular endothelial cell injury caused by malignant cell perivascular accumulation. Previous histopathologic studies have revealed considerable lymphoid cell infiltration in the meninges and along vessels with perivascular plasma cell accumulation in Virchow-Robin spaces3,6,13).
The patient presented with various behavioral symptoms, including memory disturbance, which were related to a lesion in the anterior nucleus of the right thalamus. The lesion may involve disconnected fibers between the amygdalo-hippocampal areas of the medial temporal region and the thalamus. Despite diffuse high signal intensities in the cerebral peduncle and basis pontis on MRI, motor examination of our patient revealed normal strength and tone; however, cerebellar symptoms were prominent. The discrepancy between MRI lesions and symptom severity may relate to vasogenic edema. Malignant cell infiltration in the perivascular spaces cause damage to the blood-brain barrier (BBB), resulting in extravasation of plasma, which may present as abnormal signal intensities on MRI. However, neurologic symptoms may not have developed during this stage. The extravasated plasma can damage white matter, inducing a cascade of pathologic events, including cellular proliferation, demyelination, and petechial hemorrhage, leading to delayed cellular degeneration14). The enhancing lesion on MRI may be a more significant lesion which directly correlates with clinical symptoms.
Serum hyperviscosity, which is observed in 15% of patients with WM, has been suggested to be the most common pathogenesis of CNS symptoms in WM8). The serum hyperviscosity is related to increasing concentrations of IgM pentameters in serum, which result in red blood cell aggregation and an increased viscosity12). The resultant microcirculatory impairment cause clinical symptoms, generally the triad of mucosal bleeding, visual changes, and neurologic symptoms8). Neurologic complications vary from mild symptoms (headache, lightheadedness, and fatigue) to severe symptoms (mental confusion, stroke, and focal neurologic deficits)2). Symptoms usually develop when IgM increases beyond 3 g/L7,8,11). In the case herein, a low IgM level (1.49 g/L) on admission and vasogenic edema on DWI were findings incompatible with the hyperviscosity syndrome in WM.
In addition, Bing-Neel syndrome must be distinguished from other cerebral diseases, such as glioblastoma multiforme (a thick irregular ring of tissue around a necrotic core and marked, but inhomogeneous contrast enhancement in most MRIs), multifocal leukoencephalopathy (multifocal oval or round subcortical white matter hyperintensities in the parieto-occipital area by MRI), and primary central nervous system lymphoma (PCL; three-fourths of all PCLs in immunologically-normal patients enhance strongly and homogenously, while AIDS-related PCLs may appear considerably more heterogeneous, with hemorrhagic and necrotic foci by MRI). Clinical findings, including radiologic studies, may help differentiating those CNS diseases.
CONCLUSION
Herein, we report the diffuse form of Bing-Neel syndrome based on accentuated DWI, suggesting vasogenic edema due to malignant cell infiltration in the perivascular space. Although histologic analysis is a confirmative study to verify direct cell infiltration into the brain, a key finding of Bing-Neel syndrome, the DWI findings representing vasogenic edema and infiltrative findings in routine MRI may suggest direct malignant cell infiltration into the CNS as a pathogenic mechanism of CNS symptoms in patients with WM. A brain MRI with DWI may be a good supportive tool to diagnose Bing-Neel syndrome.
References
- 1.Arias M, Pereiro Zabala I, Requena Caballero I, Sesar Ignacio A, Arias Rivas S, Villamayor Blanco B. [Rapidly progressing dementia as the presenting symptom of Waldenström's macroglobulinemia : findings from magnetic resonance imaging of the brain in Bing Neel syndrome] Rev Neurol. 2004;38:640–642. [PubMed] [Google Scholar]
- 2.Bang SM, Park SR, Park SH, Cho EK, Yoon SS, Shin DB, et al. Clinical features of Waldenstrom macroglobulinemia in Korea. Korean J Intern Med. 2004;19:137–140. doi: 10.3904/kjim.2004.19.3.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bing J, Neel A. Two cases of hyperglobulinemia with affection of the central nervous system on a toxi-infectious basis. Acta Med Scand. 1936;88:492–506. [Google Scholar]
- 4.Delgado J, Canales MA, Garcia B, Alvarez-Ferreira J, Garcia-Grande A, Hernandez-Navarro F. Radiation therapy and combination of cladribine, cyclophosphamide, and prednisone as treatment of Bing-Neel syndrome : case report and review of the literature. Am J Hematol. 2002;69:127–131. doi: 10.1002/ajh.10023. [DOI] [PubMed] [Google Scholar]
- 5.Drappatz J, Akar S, Fisher DC, Samuels MA, Kesari S. Imaging of Bing-Neel syndrome. Neurology. 2008;70:1364. doi: 10.1212/01.wnl.0000309212.22661.84. [DOI] [PubMed] [Google Scholar]
- 6.Edgar R, Dutcher TF. Histopathology of the Bing-Neel syndrome. Neurology. 1961;11:239–245. doi: 10.1212/wnl.11.3.239. [DOI] [PubMed] [Google Scholar]
- 7.Gertz MA, Fonseca R, Rajkumar SV. Waldenstrom's macroglobulinemia. Oncologist. 2000;5:63–67. doi: 10.1634/theoncologist.5-1-63. [DOI] [PubMed] [Google Scholar]
- 8.Ghobrial IM, Gertz MA, Fonseca R. Waldenstrom macroglobulinaemia. Lancet Oncol. 2003;4:679–685. doi: 10.1016/s1470-2045(03)01246-4. [DOI] [PubMed] [Google Scholar]
- 9.Imai F, Fujisawa K, Kiya N, Ninomiya T, Ogura Y, Mizoguchi Y, et al. Intracerebral infiltration by monoclonal plasmacytoid cells in Waldenstrom's macroglobulinemia--case report. Neurol Med Chir (Tokyo) 1995;35:575–579. doi: 10.2176/nmc.35.575. [DOI] [PubMed] [Google Scholar]
- 10.Kim HD, Shin KC, Cho HS, Kim MK, Lee KH, Hyun MS. Therapeutic experience of Bing-Neel Syndrome associated with Waldenstrom's macroglobulinemia. J Korean Med Sci. 2007;22:1079–1081. doi: 10.3346/jkms.2007.22.6.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Logothetis J, Silverstein P, Coe J. Neurologic aspects of Waldenstrom's macroglobulinemia; report of a case. Arch Neurol. 1960;3:564–573. doi: 10.1001/archneur.1960.00450050084010. [DOI] [PubMed] [Google Scholar]
- 12.Mehta J, Singhal S. Hyperviscosity syndrome in plasma cell dyscrasias. Semin Thromb Hemost. 2003;29:467–471. doi: 10.1055/s-2003-44554. [DOI] [PubMed] [Google Scholar]
- 13.Shimizu K, Fujisawa K, Yamamoto H, Mizoguchi Y, Hara K. Importance of central nervous system involvement by neoplastic cells in a patient with Waldenström's macroglobulinemia developing neurologic abnormalities. Acta Haematol. 1993;90:206–208. doi: 10.1159/000204461. [DOI] [PubMed] [Google Scholar]
- 14.Wagner KR, Dean C, Beiler S, Bryan DW, Packard BA, Smulian AG, et al. Plasma infusions into porcine cerebral white matter induce early edema, oxidative stress, pro-inflammatory cytokine gene expression and DNA fragmentation : implications for white matter injury with increased blood-brain-barrier permeability. Curr Neurovasc Res. 2005;2:149–155. doi: 10.2174/1567202053586785. [DOI] [PubMed] [Google Scholar]