Abstract
A series of reports in the past decade have ascribed pro-angiogenic activity to several thyroid hormone analogues, including L-thyroxine (T4), 3,5,3-triiodo-L-thyronine (T3) and diiodothyropropionic acid (DITPA). Model systems of angiogenesis have demonstrated that thyroid hormone-induced neovascularization is initiated at a cell surface receptor for the hormone on an integrin. The hormone signal is transduced within the cell by extracellular regulated kinase 1/2 (ERK1/2) into secretion of basic fibroblast growth factor (bFGF) and other vascular growth factors and consequent angiogenesis. Intact animal studies have shown that endogenous thyroid hormone supports blood vessel density in heart and brain and that thyroid hormone administration can induce angiogenesis in ischemic limbs.
INTRODUCTION
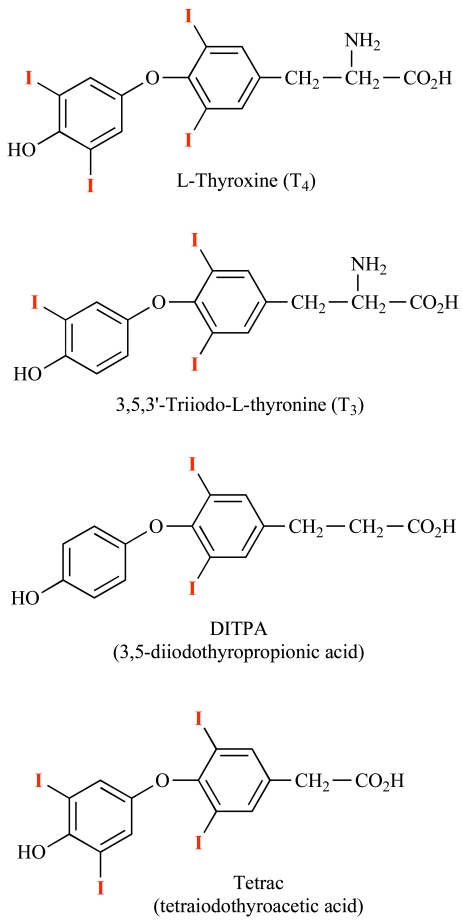
In studies of thyroid hormone-induced myocardial hypertrophy a decade ago, Tomanek and co-workers showed that administration of L-thyroxine (T4) to rats caused left ventricular capillary growth [1]. This was accompanied by accumulation of basic fibroblast growth factor (bFGF) mRNA and bFGF protein. Because these changes occurred within several days of animal exposure to T4, when the hypertrophic response was small, the authors concluded in this study and in a follow-up report [1, 2] that the vascular and myocyte actions of thyroid hormone in this model represented separate effects of T4. The same laboratory subsequently found that an inotropic iodothyronine analogue, diiodothyropropionic acid (DITPA), increased abundance of vascular endothelial growth factor (VEGF) protein and angiopoietin-1 (Ang-1) in rat coronary arterioles, as well as bFGF [3]. There was no increase in ventricular mass in the treated animals. The molecular basis of the thyroid hormone effect on bFGF and other growth factor gene expression was not investigated. An effect of the thyroid hormone analogue on Ang-1 was puzzling, since this factor stabilizes quiescent endothelium, whereas Ang-2 is a de-stabilizing agent that prepares previously quiescent endothelium for the action of growth factors such as VEGF [4]. The structures of pro-angiogenic thyroid hormone analogues are shown in Fig. (1).
Fig. (1).
Structures of thyroid hormone analogues. L-Thyroxine (T4), 3, 5, 3’-triiodo-L-thyronine (T3) and DITPA (diiodothyropropionic acid) are pro-angiogenic and initiate angiogenesis at the integrin αvβ3 receptor for thyroid hormone. The receptor is located at the Arg-gly-Asp (RGD) recognition site on the integrin. Tetrac (tetraiodothyroacetic acid) is a deaminated derivative of T4 and is anti-angiogenic, blocking the binding of agonist thyroid hormone analogues at the integrin receptor. Tetrac is also capable of inhibiting the angiogenic actions of vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) in the absence of thyroid hormone. Action of the growth factors requires crosstalk between the integrin RGD recognition domain and the specific receptors for the VEGF and bFGF.
The cardiomyopathic hamster was recently shown by Kuzman et al. to respond to chronic DITPA administration with an increase in myocardial blood flow [5] consistent with increased angiogenesis. Subsequently, this group reported that hypothyroidism decreased blood vessel density in rat brain [6] and heart [7] and that administration of DITPA or T4 to thyroidectomized animals prevented blood vessel loss. Several clinical studies have asserted that thyroid hormone as L-thyroxine may be effective in heart failure [8, 9] but the state of the coronary circulation before and during treatment is not known. Clinical trials of DITPA in the management of heart failure, conducted by the Department of Veterans Affairs and Titan Pharmaceuticals, Inc., have been terminated (ClinicalTrials.gov), but a hypolipidemic trial continues.
The existence of a discrete effect of thyroid hormone analogues on angiogenesis has been documented in the chick chorioallantoic membrane model (CAM) [10, 11] and in the human dermal microvascular endothelial cell (HDMEC) microtubular model [11, 12]. The molecular basis of this effect has been defined and shown to be initiated at a cell surface receptor for thyroid hormone on endothelial cells that we have recently described [11, 13, 14]. Thus, a nuclear thyroid hormone receptor (TR) isoform [15] is not primarily involved in the pro-angiogenic action of the hormone.
The concept of thyroid hormone as a pro-angiogenic agent is relevant to the clinical use of hormone analogues as inotropic [16, 17] or cholesterol-lowering agents [18, 19]. Pro-angiogenic agents may also be of interest in accelerating wound-healing. Thyroid hormone has been reported to accelerate wound-healing, but this has been attributed to an action of the hormone on keratinocytes [20]. In the setting of cancer, in contrast, anti-angiogenesis is desirable and anti-angiogenic clinical strategies are being extensively explored [21, 22].
THYROID HORMONE ACTION AT ITS CELL SURFACE RECEPTOR ON INTEGRIN αVβ3
In 2005, Bergh et al. demonstrated the existence of a high affinity thyroid hormone receptor on a structural plasma membrane protein, integrin αvβ3 [13]. A number of extracellular matrix (ECM) proteins are ligands of this heterodimeric protein [23] and the binding of each of these is a signal that is transduced by the integrin into an intracellular response (outside-in signaling). The protein can also transduce intracellular signals into extracellular events (inside-out signaling). The extracellular domain of this integrin includes an Asp-Gly-Asp (RGD) recognition site that is an important verification domain for the ligands of the protein; that is, each of the integrin ligands contain an RGD sequence that is required for the binding of each ECM protein ligand to its specific binding or receptor site on the integrin [23]. There may also be crosstalk between the RGD recognition site on αvβ3 and specific vascular growth factor receptors that may be clustered with the integrin [24, 25]. The initial description of the plasma membrane integrin receptor included evidence that the receptor mediated the effect of the hormone on angiogenesis [13], which had been previously reported [10].
The thyroid hormone receptor on integrin αvβ3 is located at the RGD recognition site and short RGD peptides interfere with the binding of thyroid hormone analogues to the integrin. T4 and 3,5,3’-triiodo-L-thyronine (T3) are agonist ligands at the hormone receptor site and are physiologically the most important thyroid hormone analogues. DITPA and GC-1 are iodothyronine analogues that also are agonist ligands at the integrin receptor [11, 14]. The thyroid hormone signal initiated at integrin αvβ3 is transduced by the mitogen-activated protein kinase (MAPK; extracellular regulated kinase, ERK1/2) cascade [10, 13]. The kinase activities upstream of MAPK for the thyroid hormone signal initiated at the cell surface have been identified [26], and several complex downstream events occur as a result of the activation of ERK1/2 by thyroid hormone. Such events may be local, e.g., regulation of the activity of the plasma membrane Na/H antiporter [27] that is important to myocardial function [28] or an increase in activity of cell membrane Na, K-ATPase (sodium pump) activity [29].
Other events initiated at the integrin involve several cellular compartments and include trafficking of proteins within the cell [30, 31] and complex cellular actions, such as angiogenesis [10]. Proteins in cytoplasm that translocate to the nucleus in response to the binding of thyroid hormone by the plasma membrane integrin receptor include activated ERK1/2 [26], and other signal transducing proteins [32, 33]. By this mechanism that does not primarily involve the nuclear receptor for thyroid hormone, the latter can amplify the intracellular responses to important systemic polypeptides that act at the plasma membrane. Examples of such polypeptides are interferon-γ [32] and epidermal growth factor-like ligands [33, 34].
The pro-angiogenic effect of thyroid hormone that is initiated at the integrin is induced by several thyroid hormone analogues, as noted above. The analogues are not interchangeable as angiogenic agents, however. For example, T4 acts on platelet integrin αvβ3 to cause agglutination [35], whereas T3, DITPA and GC-1 do not act on platelets. As a pro-angiogenic agent in the context of wound-healing, T4 would be more desirable than other analogues. In the setting of an ischemic organ or tissue, however, a pro- angiogenic agent should not promote aggregation of platelets. While T3 is more potent than T4 as an activator of cellular events that involve the intranuclear thyroid hormone receptor (TRβ1, TRα1), T4 and T3 are equipotent at the cell surface receptor and a case can be made at this receptor for the function of T4 as a hormone, rather than as a prohormone antecedent of T3 [36].
Tetraiodothyroacetic acid (tetrac) is a deaminated form of T4 (Fig. 1) that is not an agonist at the receptor, but inhibits binding of agonist analogues to the integrin [13, 37]. Thus, tetrac is a useful probe of the participation of the receptor in actions of thyroid hormone analogues. Inside the cell, however, tetrac is a low-grade thyromimetic agent [38]. Thus, the most stringent application of tetrac as a marker of participation of the receptor in the actions of thyroid hormones is as re-formulations that cannot gain entry to the cell interior. One such re-formulation is nanoparticulate (poly[lactate-co-glycolic acid], PLGA) tetrac [39].
In addition to transduction of the iodothyronine signal vertically to the cell interior from the integrin receptor, the hormone-activated receptor on αvβ3 may alter the activities of specific polypeptide growth factor receptors that appear to be clustered with the integrin on the plasma membrane. For example, the epidermal growth factor (EGF)-like ligands bind to the cell surface EGF receptor (EGFR) and may have their signals amplified by action of thyroid hormone on the cell surface [34]. EGFR is found in heart cells [40] and EGFR signaling may be involved in myocardial hypertrophy [41]. In addition, EGFR signaling can result in VEGF and bFGF production [42], although this has been described in tumor cells and has not yet been looked for in myocardiocytes. These observations indicate the complexity of regulation of angiogenesis and imply that thyroid hormone can influence angiogenesis secondarily via the EGFR and an integrin receptor-EGFR interaction.
INDUCTION OF ANGIOGENESIS BY THYROID HORMONE
Several model systems have been exploited to demonstrate the pro-angiogenic activity of thyroid hormone. As noted above, these initially have been the CAM and the HDMEC models and now include a rabbit hind limb ischemia model. Translation of studies involving cultured cells or the CAM assay into intact animal organ or tissue ischemia is a critical step in establishing the utility of the pro-angiogenic activity of thyroid hormone analogues. A rabbit hind-limb ischemia paradigm has been recently used in testing the angiogenic effect of thyroid hormone in an intact animal [43]. In these initial intact animal studies, increase in angiogenesis has been quantitated by measuring blood vessel buds on limb angiography and by the ratio of blood vessels to muscle fiber on muscle biopsy.
What are the steps that follow activation of MAPK (ERK1/2) by thyroid hormone at the cell membrane receptor and that lead to neovascularization? The sequence of events is incompletely understood, but is known to require importation of activated (tyrosine-phosphorylated) ERK1/2 into the nucleus and its consequence, transcription of the basic fibroblast growth factor (bFGF) gene. In the CAM assay, the addition of anti-bFGF protein blocks the pro-angiogenic effect of thyroid hormone [10]. We know that the vascular endothelial growth factor (VEGF) gene may also be transcribed in response to the binding of thyroid hormone by the integrin receptor [44]. Finally, accumulation of Ang-2, but not Ang-1, has been shown to occur in endothelial cells stimulated by thyroid hormone analogues via the cell surface receptor [45]. This is what is expected as a step preparatory to the action on endothelium of VEGF [4], in contrast to another report cited above in which the hormone enhanced tissue accumulation of endothelium stabilizing Ang-1 [3].
What transcription factor activities are modulated by activated MAPK is not yet known, but thyroid hormone-directed MAPK is known to activate (phosphorylate) members of the nuclear superfamily of hormone receptors, such as TRβ1 and estrogen receptor-α (ERα) [46, 47]. These superfamily members are transcription factors whose activation involves phosphorylation and liganding, respectively, of T3 or estradiol. T3 has been shown to increase expression of the VEGF gene in a model of liver regeneration [44, 48] and thyroid hormone administration to intact animals, as noted above, can increase bFGF expression [1].
If TR isoforms are in fact involved in the pro-angiogenic action of iodothyronines that begin at the cell surface, then this is an example of the interface of membrane-initiated actions and downstream effects involving TR in which thyroid hormone need not be present in the cell nucleus. Nongenomic is a term applied to hormone actions that do not primarily require intranuclear complexing of hormone and relevant superfamily nuclear receptor family member [37, 39]. Nongenomic actions may involve the iodothyronine receptor on plasma membrane integrin αvβ3, or nuclear TR isoforms that are now appreciated to reside in cytoplasm [39, 49].
The signal transduction and activator of transcription (STAT) family, such as STAT1α [32], and STAT3 [33], are also serine phosphorylated by thyroid hormone-activated ERK1/2. These cellular polyfunctional proteins are involved in the actions of a variety of cytokines [50], including the interferons, as noted above. While the STATs are activated primarily by tyrosine phosphorylation [51], specific serine phosphorylation of these proteins amplifies their transcriptional activity [26, 32]. The STAT proteins are involved in vascular growth factor biochemistry. For example, the VEGF signal is transduced by STAT1 [52] and STAT3 can be involved in VEGF gene expression [53] and either or both of these processes, based upon what we know about thyroid hormone, may be sites of hormone action that contribute to angiogenesis. The FGF receptor (FGFR) on the cell surface, when liganded, generates an intracellular signal that is STAT1-mediated [54]. Again, iodothyronines could amplify transduction of this signal and thus enhance angiogenesis. Finally, glycoprotein 130 (gp130) is an intracellular signaling protein activated in cardiac myocytes in the process of tissue hypertrophy. Via STAT3, gp130 can induce VEGF gene transcription [55] that is required for support of hypertrophy. This molecular signaling system is a candidate contributor to the studies mentioned above in which thyroid hormone induced myocardial hypertrophy [1] and one can speculate that amplification of STAT3 action by thyroid hormone-directed ERK1/2 might be the vehicle by which thyroid hormone can enhance angiogenesis whether or not cardiac hypertrophy is present. In summary, the STATs are attractive, but as yet unproved, candidate molecular mediators of thyroid hormone-induced neovascularization in the heart.
REFORMULATED THYROID HORMONE ANALOGUES THAT ACT AT THE INTEGRIN RECEPTOR
Unmodified T4 and T3 can act at the cell surface or can be taken up by cells via transport systems [56, 57]. Within the cell or at the plasma membrane, T4 must be converted to T3 in order to have nuclear or mitochondrial effects [57-59]. However, T4 can affect the state of the cytoskeleton without conversion to T3; further, rT3, but not T3, can also effect conversion of soluble actin to fibrous (F) actin in the cell [60]. Metabolism of T4 or T3 to T2 may be important to regulation of oxidative phosphorylation in mitochondria [61].
Thyroid hormone must be reformulated in order to obtain analogues that act exclusively at the integrin. Thyroid hormone (T4 or T3) with an agarose tail, although not taken up by the cell, stimulates angiogenesis [10]. A thyroid hormone nanoparticulate analogue we have developed is T4-poly (lactate-co-glycolic acid) (PLGA), mentioned above, and this is ether-bonded to the outer ring hydroxyl of T4. This agent is pro-angiogenic in the CAM and HDMEC microtubular models (Mousa SA, unpublished observations). Thus, the outer ring hydroxyl is not critical to the angiogenic action of the hormone.
POSSIBLE APPLICATIONS OF THYROID HORMONE ANALOGUES AS PRO-ANGIOGENIC AGENTS
As indicated above, short-term systemic administration of thyroid hormone can induce angiogenesis in an animal model of limb ischemia [43]. We have concluded from the CAM and HDMEC models of angiogenesis that neovascularization induced by iodothyronines is initiated at the integrin receptor for the hormone. Re-formulation of pro-angiogenic thyroid hormone analogues as agents that act exclusively at integrin αvβ3 and that do not enter the cell is desirable and has been achieved as iodothyronine nanoparticulates. It is proposed that these re-formulations may be administered locally for relief of blood vessel stenosis or occlusion by induction of new blood vessels. T3, DITPA and GC-1, but not T4, are suitable analogues for this use. Local short-term administration may be via temporary stents that are coated with thyroid hormone nanoparticles or by catheter proximal to stenoses in large vessels. Temporary stents are proposed because of the adverse events associated with permanent intravascular stents.
The anti-angiogenic property of tetrac, a thyroid hormone antagonist that acts at the integrin receptor for the hormone [13, 45], has been demonstrated in an intact animal model. Nude mouse recipients of a human cancer (medullary carcinoma of the thyroid gland) have been treated with parenteral tetrac or nanoparticulate tetrac for three weeks. The experimental cohort demonstrated a 60% decrease in tumor-associated vascularity, compared to control animals [62].
CONCLUSIONS
Thyroid hormone analogues are now recognized to be pro-angiogenic. These analogues include T4, T3, DITPA and GC-1. Studied in vitro, the pro-angiogenic activity of these analogues appears to be at a cell surface receptor for the hormone leading to transduction of the hormone signal by MAPK (ERK1/2). The proximate effectors of angiogenesis are bFGF and, very likely, VEGF. That is, an effect of the hormone initiated at the cell surface and that does not necessarily involve access of hormone to the cell interior, culminates in gene expression relevant to neovascularization. In intact animals, hypothyroidism has been shown to result in decreased blood vessel density in brain and heart and this is corrected by thyroid hormone administration. In studies of a hind limb ischemia model in intact rabbit, thyroid hormone administration induces new blood vessel formation. Thus, evidence from intact animals suggests that circulating thyroid hormone supports angiogenesis. It may be useful to test local, short-term administration of thyroid hormone analogues, for example, via temporary thyroid hormone-coated stents in management of ischemic tissues. T4 should not be used for pro-angiogenic intent in vessels because it causes platelet agglutination, whereas T3, DITPA and GC-1 do not have this platelet effect.
ACKNOWLEDGEMENTS
Portions of the observations from the laboratories of the authors described in this review were supported by an endowment established by M. Frank Rudy and Margaret C. Rudy.
REFERENCES
- 1.Tomanek RJ, Doty MK, Sandra A. Early coronary angiogenesis in response to thyroxine. Growth characteristics and upregulation of basic fibroblast growth factor. Circ Res. 1998;82:587–593. doi: 10.1161/01.res.82.5.587. [DOI] [PubMed] [Google Scholar]
- 2.Tomanek RJ, Busch TL. Coordinated capillary and myocardial growth in response to thyroxine treatment. Anat Rec. 1998;251:44–49. doi: 10.1002/(SICI)1097-0185(199805)251:1<44::AID-AR8>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 3.Wang X, Zheng W, Christensen LP, et al. DITPA stimulates bFGF, VEGF, angiopoietin, and Tie-2 and facilitates coronary arteriolar growth. Am J Physiol. 2003;284:H613–618. doi: 10.1152/ajpheart.00449.2002. [DOI] [PubMed] [Google Scholar]
- 4.Fiedler U, Augustin HG. Angiopoietins: a link between angiogenesis and inflammation. Trends Immunol. 2006;27:552–558. doi: 10.1016/j.it.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Kuzman JA, Tang Y, Vogelsang KA, et al. Thyroid hormone analog, diiodothyropropionic acid (DITPA), exerts beneficial effects on chamber and cellular remodeling in cardiomyopathic hamsters. Can J Physiol Pharmacol. 2007;85:3121–318. doi: 10.1139/y07-011. [DOI] [PubMed] [Google Scholar]
- 6.Schlenker EH, Hora M, Liu Y, et al. Effects of thyroidectomy, T4, and DITPA replacement on brain blood vessel density in adult rats. Am J Physiol. 2008;294:R1504–1509. doi: 10.1152/ajpregu.00027.2008. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y, Redetzke RA, Said S, et al. Serum thyroid hormone levels may not accurately reflect thyroid tissue levels and cardiac function in mild hypothyroidism. Am J Physiol. 2008;294:H2137–2142. doi: 10.1152/ajpheart.01379.2007. [DOI] [PubMed] [Google Scholar]
- 8.Fazio S, Palmieri EA, Lombardi G, et al. Effects of thyroid hormone on the cardiovascular system. Recent Prog Horm Res. 2004;59:31–50. doi: 10.1210/rp.59.1.31. [DOI] [PubMed] [Google Scholar]
- 9.Pingitore A, Galli E, Barison A, et al. Acute effects of triiodothyronine (T3) replacement therapy in patients with chronic heart failure and low-T3 syndrome: a randomized, placebo-controlled study. J Clin Endocrinol Metab. 2008;93:1351–1358. doi: 10.1210/jc.2007-2210. [DOI] [PubMed] [Google Scholar]
- 10.Davis FB, Mousa SA, O’Connor L, et al. Proangiogenic action of thyroid hormone is fibroblast growth factor-dependent and is initiated at the cell surface. Circ Res. 2004;94:1500–1506. doi: 10.1161/01.RES.0000130784.90237.4a. [DOI] [PubMed] [Google Scholar]
- 11.Mousa SA, O’Connor L, Davis FB, et al. Proangiogenesis action of the thyroid hormone analog 3,5-diiodothyropropionic acid (DITPA) is initiated at the cell surface and is integrin mediated. Endocrinology. 2006;147:1602–1607. doi: 10.1210/en.2005-1390. [DOI] [PubMed] [Google Scholar]
- 12.Mousa SA, Davis FB, Mohamed S, et al. Pro-angiogenesis action of thyroid hormone and analogs in a three-dimensional in vitro microvascular endothelial sprouting model. Int Angiol. 2006;25:407–413. [PubMed] [Google Scholar]
- 13.Bergh JJ, Lin H-Y, Lansing L, et al. Integrin αvβ3 contains a cell surface receptor site for thyroid hormone that is linked to activation of mitogen-activated protein kinase and induction of angiogenesis. Endocrinology. 2005;146:2864–2871. doi: 10.1210/en.2005-0102. [DOI] [PubMed] [Google Scholar]
- 14.Mousa SA, O’Connor LJ, Bergh JJ, et al. The proangiogenic action of thyroid hormone analogue GC-1 is initiated at an integrin. J Cardiovasc Pharmacol. 2005;46:356–360. doi: 10.1097/01.fjc.0000175438.94906.a0. [DOI] [PubMed] [Google Scholar]
- 15.Yen PM, Ando S, Feng X, et al. Thyroid hormone action at the cellular, genomic and target gene levels. Mol Cell Endocrinol. 2006;246:121–127. doi: 10.1016/j.mce.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 16.Tomanek RJ, Butters CA, Zimmerman MB. Initiation of cardiac hypertrophy in response to thyroxine is not limited by age. Am J Physiol (Heart Circ Physiol) 1993;264:H1041–H1047. doi: 10.1152/ajpheart.1993.264.4.H1041. [DOI] [PubMed] [Google Scholar]
- 17.Pennock GD, Raya TE, Bahl JJ. Cardiac effects of 3,5-diiodothyropropionic acid, a thyroid hormone analog with inotropic selectivity. J Pharmacol Exp Ther. 1992;263:163–169. [PubMed] [Google Scholar]
- 18.Morkin E, Ladenson P, Goldman S, et al. Thyroid hormone analogs for treatment of hypercholesterolemia and heart failure: past, present and future prospects. J Mol Cell Cardiol. 2004;37:1137–1146. doi: 10.1016/j.yjmcc.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 19.Trost SU, Swanson E, Gloss B, et al. The thyroid hormone receptor-beta-selective agonist GC-1 differentially affects plasma lipids and cardiac activity. Endocrinology. 2000;141:3057–3064. doi: 10.1210/endo.141.9.7681. [DOI] [PubMed] [Google Scholar]
- 20.Safer JD, Crawford TM, Holick MF. Topical thyroid hormone accelerates wound healing in mice. Endocrinology. 2005;146:4425–4430. doi: 10.1210/en.2005-0192. [DOI] [PubMed] [Google Scholar]
- 21.Fukumura D, Jain RK. Tumor microenvironment abnormalities: causes, consequences, and strategies to normalize. J Cell Biochem. 2007;101:937–949. doi: 10.1002/jcb.21187. [DOI] [PubMed] [Google Scholar]
- 22.Mayer EL, Lin NU, Burstein HJ. Novel approaches to advanced breast cancer: bevacizumab and lapatinib. J Natl Compr Canc Netw. 2007;5:314–323. doi: 10.6004/jnccn.2007.0026. [DOI] [PubMed] [Google Scholar]
- 23.Plow EF, Haas TA, Zhang L, et al. Ligand binding to integrins. J Biol Chem. 2000;275:21785–21788. doi: 10.1074/jbc.R000003200. [DOI] [PubMed] [Google Scholar]
- 24.Van Lonkhuyzen DR, Hollier BG, Shooter GK, et al. Chimeric vitronectin:insulin-like growth factor proteins enhance cell growth and migration through co-activation of receptors. Growth Factors. 2007;25:295–308. doi: 10.1080/08977190701803752. [DOI] [PubMed] [Google Scholar]
- 25.Chen CP, Lee MY, Huang JP, et al. Trafficking of multipotent mesenchymal stromal cells from maternal circulation through the placenta involves vascular endothelial growth factor receptor-1 and integrins. Stem Cells. 2008;26:550–561. doi: 10.1634/stemcells.2007-0406. [DOI] [PubMed] [Google Scholar]
- 26.Lin H-Y, Davis FB, Gordinier JK, et al. Thyroid hormone induces activation of mitogen-activated protein kinase in cultured cells. Am J Physiol. 1999;276:C1014–1024. doi: 10.1152/ajpcell.1999.276.5.C1014. [DOI] [PubMed] [Google Scholar]
- 27.D’Arezzo S, Incerpi S, Davis FB, et al. Rapid nongenomic effects of 3,5,3’-triiodo-L-thyronine on the intracellular pH of L-6 myoblasts are mediated by intracellular calcium mobilization and kinase pathways. Endocrinology. 2004;145:5694–5703. doi: 10.1210/en.2004-0890. [DOI] [PubMed] [Google Scholar]
- 28.Fliegel L. Molecular biology of the myocardial Na+/H+ exchanger. J Mol Cell Cardiol. 2008;44:228–237. doi: 10.1016/j.yjmcc.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 29.Lei J, Mariash CN, Bhargava M, et al. T3 increases Na-K-ATPase activity via a MAPK/ERK1/2-dependent pathway in rat adult alveolar epithelial cells. Am J Physiol. 2008;294:L749–L754. doi: 10.1152/ajplung.00335.2007. [DOI] [PubMed] [Google Scholar]
- 30.Davis PJ, Davis FB, Lin H-Y. Promotion by thyroid hormone of cytoplasm-to-nucleus shuttling of thyroid hormone receptors. Steroids. 2008;73:1013–1017. doi: 10.1016/j.steroids.2007.12.030. [DOI] [PubMed] [Google Scholar]
- 31.Hiroi Y, Kim H-H, Ying H, et al. Rapid nongenomic actions of thyroid hormone. Proc Natl Acad Sci USA. 2006;103:14104–14109. doi: 10.1073/pnas.0601600103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin H-Y, Martino LJ, Wilcox BD, et al. Potentiation by thyroid hormone of human IFN-γ-induced HLA-DR expression. J Immunol. 1998;161:843–849. [PubMed] [Google Scholar]
- 33.Lin H-Y, Shih A, Davis FB, et al. Thyroid hormone promotes the phosphorylation of STAT3 and potentiates the action of epidermal growth factor in cultured cells. Biochem J. 1999;338:427–432. [PMC free article] [PubMed] [Google Scholar]
- 34.Shih A, Zhang S, Cao HJ, et al. Disparate effects of thyroid hormone on actions of epidermal growth factor and transforming growth factor-α are mediated by 3’,5’-cyclic adenosine 5’-monophosphate-dependent protein kinase II. Endocrinology. 2004;145:1708–1717. doi: 10.1210/en.2003-0742. [DOI] [PubMed] [Google Scholar]
- 35.Mousa SA, O’Connor LJ, Davis FB, et al. Human platelet aggregation and degranulation is enhanced in vitro by L-thyroxine (T4), but not by 3,5,3’-triiodo-L-thyronine (T3), GC-1, or diiodothyropropionic acid (DITPA) Thyroid. 2007;17(Suppl 1):S-99. doi: 10.1177/1076029609348315. [DOI] [PubMed] [Google Scholar]
- 36.Davis PJ, Davis FB, Lin H-Y. L-Thyroxine acts as a hormone as well as a prohormone at the cell membrane. Immun Endocrinol Metab Agents Med Chem. 2006;6:235–240. [Google Scholar]
- 37.Davis PJ, Davis FB, Cody V. Membrane receptors mediating thyroid hormone action. Trends Endocrinol Metab. 2005;16:429–435. doi: 10.1016/j.tem.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 38.Lameloise N, Siegrist-Kaiser C, O’Connell M, et al. Differences between the effects of thyroxine and tetraiodothyroacetic acid on TSH suppression and cardiac hypertrophy. Eur J Endocrinol. 2001;144:145–154. doi: 10.1530/eje.0.1440145. [DOI] [PubMed] [Google Scholar]
- 39.Davis PJ, Leonard JL, Davis FB. Mechanisms of nongenomic actions of thyroid hormone. Front Neuroendocrinol. 2008;29:211–218. doi: 10.1016/j.yfrne.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 40.Browe DM, Baumgarten CM. EGFR kinase regulates volume-sensitive chloride current elicited by integrin stretch via PI-3K and NADPH oxidase in ventricular myocytes. J Gen Physiol. 2006;127:237–251. doi: 10.1085/jgp.200509366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liao Y, Asakura M, Takashima S, et al. Amlodipine ameliorates myocardial hypertrophy by inhibiting EGFR phosphorylation. Biochem Biophys Res Commun. 2005;327:1083–1087. doi: 10.1016/j.bbrc.2004.12.112. [DOI] [PubMed] [Google Scholar]
- 42.De Luca A, Carotenuto A, Rachiglio A, et al. The role of the EGFR signaling in tumor microenvironment. J Cell Physiol. 2008;214:559–567. doi: 10.1002/jcp.21260. [DOI] [PubMed] [Google Scholar]
- 43.El Eter E, Rebbaa H, Alkayali A, Mousa SA. Role of thyroid hormone analogues in angiogenesis and the development of collaterals in rabbit hind limb ischemia model. J Thrombosis Thrombolysis. 2007;5(suppl 1):375. [Google Scholar]
- 44.Bockhorn M, Frilling A, Benko T, et al. Tri-iodothyronine as a stimulator of liver regeneration after partial and subtotal hepatectomy. Eur Surg Res. 2007;39:58–63. doi: 10.1159/000098443. [DOI] [PubMed] [Google Scholar]
- 45.Mousa SA, Bergh JJ, Dier E, et al. Tetraiodothyroacetic acid, a small molecule integrin ligand, blocks angiogenesis induced by vascular endothelial growth factor and basic fibroblast growth factor. Angiogenesis. 2008;11:183–190. doi: 10.1007/s10456-007-9088-7. [DOI] [PubMed] [Google Scholar]
- 46.Lin H-Y, Zhang S, West BL, et al. Identification of the putative MAP kinase docking site in the thyroid hormone receptor-β1 DNA-binding domain: functional consequences of mutations at the docking site. Biochemistry. 2003;42:7571–7579. doi: 10.1021/bi0273967. [DOI] [PubMed] [Google Scholar]
- 47.Tang H-Y, Lin H-Y, Zhang S, et al. Thyroid hormone causes mitogen-activated protein kinase-dependent phosphorylation of the nuclear estrogen receptor. Endocrinology. 2004;145:3265–3272. doi: 10.1210/en.2004-0308. [DOI] [PubMed] [Google Scholar]
- 48.Moro L, Marra E, Capuano F, et al. Thyroid hormone treatment of hypothyroid rats restores the regenerative capacity and the mitochondrial membrane permeability properties of the liver after partial hepatectomy. Endocrinology. 2004;145:5121–5128. doi: 10.1210/en.2004-0909. [DOI] [PubMed] [Google Scholar]
- 49.Moeller LC, Cao X, Dumitrescu AM, et al. Thyroid hormone mediated changes in gene expression can be initiated by cytosolic action of the thyroid hormone receptor beta through the phosphatidylinositol 3-kinase pathway. Nucl Recept Signal. 2006;4:1–4. doi: 10.1621/nrs.04020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Darnell JE Jr. Interferon research: impact on understanding transcriptional control. Curr Top Microbiol Immunol. 2007;316:155–163. doi: 10.1007/978-3-540-71329-6_8. [DOI] [PubMed] [Google Scholar]
- 51.Schindler C, Levy DE, Decker T, et al. JAK-STAT signaling: from interferons to cytokines. J Biol Chem. 2007;282:20059–20063. doi: 10.1074/jbc.R700016200. [DOI] [PubMed] [Google Scholar]
- 52.Bartoli M, Gu X, Tsai NT, et al. Vascular endothelial growth factor activates STAT proteins in aortic endothelial cells. J Biol Chem. 2000;275:33189–33192. doi: 10.1074/jbc.C000318200. [DOI] [PubMed] [Google Scholar]
- 53.Xu Q, Briggs J, Park S, et al. Targeting Stat3 blocks both HIF-1 and VEGF expression induced by multiple oncogenic growth signaling pathways. Oncogene. 2005;24:5552–5560. doi: 10.1038/sj.onc.1208719. [DOI] [PubMed] [Google Scholar]
- 54.Citores L, Bai L, Sørensen V, et al. Fibroblast growth factor receptor-induced phosphorylation of STAT1 at the Golgi apparatus without translocation to the nucleus. J Cell Physiol. 2007;212:148–156. doi: 10.1002/jcp.21014. [DOI] [PubMed] [Google Scholar]
- 55.Funamoto M, Fujio Y, Kunisada K, et al. Signal transducer and activator of transcription 3 is required for glycoprotein 130-mediated induction of vascular endothelial growth factor in cardiac myocytes. J Biol Chem. 2000;275:10561–10566. doi: 10.1074/jbc.275.14.10561. [DOI] [PubMed] [Google Scholar]
- 56.Friesema EC, Jansen J, Visser TJ. Thyroid hormone transporters. Biochem Soc Trans. 2005;33:228–232. doi: 10.1042/BST0330228. [DOI] [PubMed] [Google Scholar]
- 57.Refetoff S, Dumitrescu AM. Syndromes of reduced sensitivity to thyroid hormone: genetic defects in hormone receptors, cell transporters and deiodination. Best Pract Res Clin Endocrinol Metab. 2007;21:277–305. doi: 10.1016/j.beem.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 58.Bianco AC, Kim BW. Deiodinases: implications of the local control of thyroid hormone action. J Clin Invest. 2006;116:2571–2579. doi: 10.1172/JCI29812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Köhrle J. Thyroid hormone transporters in health and disease. Best Pract Res Clin Endocrinol Metab. 2007;21:173–191. doi: 10.1016/j.beem.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 60.Farwell AP, Dubord-Tomasetti SA, Pietrzykowski AZ, et al. Dynamic nongenomic actions of thyroid hormone in the developing rat brain. Endocrinology. 2006;147:2567–2574. doi: 10.1210/en.2005-1272. [DOI] [PubMed] [Google Scholar]
- 61.Lombardi A, Lanni A, de Lange P, et al. Acute administration of 3,5-diiodo-L-thyronine to hypothyroid rats affects bioenergetic parameters in rat skeletal muscle mitochondria. FEBS Lett. 2007;581:5911–5916. doi: 10.1016/j.febslet.2007.11.073. [DOI] [PubMed] [Google Scholar]
- 62.Yulcin M, Lansing L, Bharali DJ, et al. Tetrac and nanoparticulate tetrac arrest growth and inhibit tumor angiogenesis in xenografts of human medullary carcinoma of the thyroid. Abstract, 79th Annual Meeting of the American Thyroid Association, Chicago, IL. 2008. (Thyroid 2008; 18: in press)