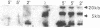Abstract
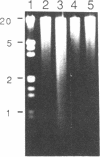
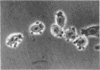
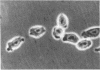
Previous studies demonstrated that in the transformed CHO (Chinese hamster ovary) cell a substantial part of the genome behaves as though its genes are sequestered from effective contact with soluble constituents of the intracellular fluid. The reverse transformation reaction, initiated by cAMP derivatives, causes this cell to regain the morphology, growth regulation, surface characteristics, and sensitivity of its DNA to digestion by DNase I that are characteristic of normal fibroblasts. In this paper we show that this action of cAMP is gene specific. In examination of 47 different genetic loci, some, like ribosomal RNA genes, are found to be sensitive to DNase I hydrolysis both in the absence and in the presence of cAMP; some are resistant under both conditions; and some are resistant in the untreated cell but become sensitive after cAMP treatment. Unlike other gene exposure reactions, which are irreversible and connected with differentiation phenomena, that produced by cAMP is readily reversed when the reagent is removed. A sequence of events is observed after cAMP treatment, the first of which is reorganization of the cytoskeleton. Afterwards, metabolic changes occur over periods as long as 72 hr. The cAMP-induced cytoskeleton-mediated gene exposure reaction appears to be an important genetic regulatory mechanism in mammalian cells and to have special implications for cancer.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. N., Vanderbilt J. N., Lawson G. M., Tsai M. J., O'Malley B. W. Chromatin structure of the ovalbumin gene family in the chicken oviduct. Biochemistry. 1983 Jan 4;22(1):21–30. doi: 10.1021/bi00270a004. [DOI] [PubMed] [Google Scholar]
- Ashall F., Puck T. T. Cytoskeletal involvement in cAMP-induced sensitization of chromatin to nuclease digestion in transformed Chinese hamster ovary K1 cells. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5145–5149. doi: 10.1073/pnas.81.16.5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch J. B., Weintraub H. Temporal order of chromatin structural changes associated with activation of the major chicken vitellogenin gene. Cell. 1983 May;33(1):65–76. doi: 10.1016/0092-8674(83)90335-5. [DOI] [PubMed] [Google Scholar]
- Cho-Chung Y. S., Redler B. H. Dibutyryl cyclic AMP mimics ovariectomy: nuclear protein phosphorylation in mammary tumor regression. Science. 1977 Jul 15;197(4300):272–275. doi: 10.1126/science.195337. [DOI] [PubMed] [Google Scholar]
- Garel A., Axel R. Selective digestion of transcriptionally active ovalbumin genes from oviduct nuclei. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3966–3970. doi: 10.1073/pnas.73.11.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin G. H., Sanders C., Johns E. W. A new group of chromatin-associated proteins with a high content of acidic and basic amino acids. Eur J Biochem. 1973 Sep 21;38(1):14–19. doi: 10.1111/j.1432-1033.1973.tb03026.x. [DOI] [PubMed] [Google Scholar]
- Groudine M., Weintraub H. Propagation of globin DNAase I-hypersensitive sites in absence of factors required for induction: a possible mechanism for determination. Cell. 1982 Aug;30(1):131–139. doi: 10.1016/0092-8674(82)90019-8. [DOI] [PubMed] [Google Scholar]
- Hamilton W. G., Ham R. G. Clonal growth of chinese hamster cell lines in protein-free media. In Vitro. 1977 Sep;13(9):537–547. doi: 10.1007/BF02627849. [DOI] [PubMed] [Google Scholar]
- Hamilton W. G., Ham R. G. Clonal growth of chinese hamster cell lines in protein-free media. In Vitro. 1977 Sep;13(9):537–547. doi: 10.1007/BF02627849. [DOI] [PubMed] [Google Scholar]
- Hsie A. W., Puck T. T. Morphological transformation of Chinese hamster cells by dibutyryl adenosine cyclic 3':5'-monophosphate and testosterone. Proc Natl Acad Sci U S A. 1971 Feb;68(2):358–361. doi: 10.1073/pnas.68.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakunaga T., Leavitt J., Hamada H. A mutation in actin associated with neoplastic transformation. Fed Proc. 1984 May 15;43(8):2275–2279. [PubMed] [Google Scholar]
- Kao F. T., Faik P., Puck T. T. Extension of branching processes from hybrids of brain and Chinese hamster ovary cells. Exp Cell Res. 1979 Aug;122(1):83–91. doi: 10.1016/0014-4827(79)90563-9. [DOI] [PubMed] [Google Scholar]
- Mathis D., Oudet P., Chambon P. Structure of transcribing chromatin. Prog Nucleic Acid Res Mol Biol. 1980;24:1–55. doi: 10.1016/s0079-6603(08)60670-4. [DOI] [PubMed] [Google Scholar]
- Meek W. D., Puck T. T. Role of the microfibrillar system in knob action of transformed cells. J Supramol Struct. 1979;12(3):335–354. doi: 10.1002/jss.400120306. [DOI] [PubMed] [Google Scholar]
- Moore E. E., Moritz E. A., Mitra N. S. A variant F9 embryonal carcinoma cell line which undergoes incomplete differentiation in retinoic acid. Cancer Res. 1985 Sep;45(9):4387–4396. [PubMed] [Google Scholar]
- Nielson S. E., Puck T. T. Deposition of fibronectin in the course of reverse transformation of Chinese hamster ovary cells by cyclic AMP. Proc Natl Acad Sci U S A. 1980 Feb;77(2):985–989. doi: 10.1073/pnas.77.2.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puck T. T. Biochemical and genetic studies on mammalian cells. In Vitro. 1971 Nov-Dec;7(3):115–119. doi: 10.1007/BF02617954. [DOI] [PubMed] [Google Scholar]
- Puck T. T. Cyclic AMP, the microtubule-microfilament system, and cancer. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4491–4495. doi: 10.1073/pnas.74.10.4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puck T. T., Erikson R. L., Meek W. D., Nielson S. E. Reverse transformation of vole cells transformed by avian sarcoma virus containing the src gene. J Cell Physiol. 1981 Jun;107(3):399–412. doi: 10.1002/jcp.1041070312. [DOI] [PubMed] [Google Scholar]
- Puck T. T. Studies on cell transformation. Somatic Cell Genet. 1979 Nov;5(6):973–990. doi: 10.1007/BF01542655. [DOI] [PubMed] [Google Scholar]
- Puck T. T., Waldren C. A., Hsie A. W. Membrane dynamics in the action of dibutyryl adenosine 3':5'-cyclic monophosphate and testosterone on mammalian cells. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1943–1947. doi: 10.1073/pnas.69.7.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed K. C., Mann D. A. Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acids Res. 1985 Oct 25;13(20):7207–7221. doi: 10.1093/nar/13.20.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumsby G., Puck T. T. Ornithine decarboxylase induction and the cytoskeleton in normal and transformed cells. J Cell Physiol. 1982 May;111(2):133–139. doi: 10.1002/jcp.1041110203. [DOI] [PubMed] [Google Scholar]
- Schonberg S., Patterson D., Puck T. T. Resistance of Chinese hamster ovary cell chromatin to endonuclease digestion. I. Reversal by cAMP. Exp Cell Res. 1983 Apr 15;145(1):57–62. doi: 10.1016/s0014-4827(83)80007-x. [DOI] [PubMed] [Google Scholar]
- Seale R. L., Annunziato A. T., Smith R. D. High mobility group proteins: abundance, turnover, and relationship to transcriptionally active chromatin. Biochemistry. 1983 Oct 11;22(21):5008–5015. doi: 10.1021/bi00290a020. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- TJIO J. H., PUCK T. T. Genetics of somatic mammalian cells. II. Chromosomal constitution of cells in tissue culture. J Exp Med. 1958 Aug 1;108(2):259–268. doi: 10.1084/jem.108.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeponteau B., Lundell M., Martinson H. Torsional stress promotes the DNAase I sensitivity of active genes. Cell. 1984 Dec;39(3 Pt 2):469–478. doi: 10.1016/0092-8674(84)90454-9. [DOI] [PubMed] [Google Scholar]
- Volpe J. J. Microtubules and the regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase. J Biol Chem. 1979 Apr 25;254(8):2568–2571. [PubMed] [Google Scholar]
- Volpe J. J., Obert K. A. Cytoskeletal structures and 3-hydroxy-3-methylglutaryl coenzyme A reductase in C-6 glial cells. A role for microfilaments. J Biol Chem. 1981 Feb 25;256(4):2016–2021. [PubMed] [Google Scholar]
- Weintraub H., Groudine M. Chromosomal subunits in active genes have an altered conformation. Science. 1976 Sep 3;193(4256):848–856. doi: 10.1126/science.948749. [DOI] [PubMed] [Google Scholar]
- Weisbrod S., Weintraub H. Isolation of a subclass of nuclear proteins responsible for conferring a DNase I-sensitive structure on globin chromatin. Proc Natl Acad Sci U S A. 1979 Feb;76(2):630–634. doi: 10.1073/pnas.76.2.630. [DOI] [PMC free article] [PubMed] [Google Scholar]