Abstract
The H+-electrochemical gradient was originally considered as a driving force for solute transport only across cellular membranes of bacteria, plants and yeast. However, in the mammalian small intestine a H+electrochemical gradient is present at the epithelial brush-border membrane in the form of an acid microclimate. Over recent years a large number of H+-coupled cotransport mechanisms have been identified at the luminal membrane of the mammalian small intestine. These transporters are responsible for the initial stage in absorption of a remarkable variety of essential and non-essential nutrients and micronutrients including protein digestion products (di/tripeptides and amino acids), vitamins, short-chain fatty acids and divalent metal ions. Proton-coupled cotransporters expressed at the mammalian small intestinal brush-border membrane include: the di/tripeptide transporter PepT1 (SLC15A1); the proton-coupled amino-acid transporter PAT1 (SLC36A1); the divalent metal transporter DMT1 (SLC11A2); the organic anion transporting polypeptide OATP2B1 (SLC02B1); the monocarboxylate transporter MCT1 (SLC16A1); the proton-coupled folate transporter PCFT (SLC46A1); the sodium-glucose linked cotransporter SGLT1 (SLC5A1); and the excitatory amino acid carrier EAAC1 (SLC1A1). Emerging research demonstrates that the optimal intestinal absorptive capacity of certain H+-coupled cotransporters (PepT1 and PAT1) is dependent upon function of the brush-border Na+/H+ exchanger NHE3 (SLC9A3). The high oral bioavailability of a large number of pharmaceutical compounds is due, in part, to absorptive transport via these same H+-coupled cotransporters. Drugs undergoing H+-coupled cotransport across the intestinal brush-border membrane include those used to treat bacterial infections, hypercholesterolaemia, hypertension, hyperglycaemia, viral infections, allergies, epilepsy, schizophrenia, rheumatoid arthritis and cancer.
The role of the Na+-electrochemical gradient in solute transport
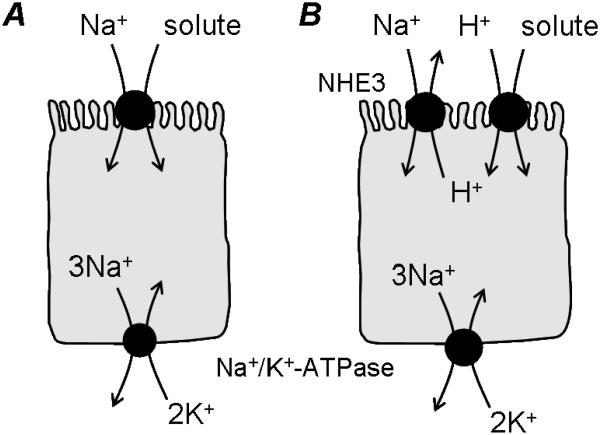
It is nearly 50 years since Halvor Christensen and colleagues proposed that movement of uncharged solutes across mammalian cell membranes might occur in the form of a complex between the carrier, the solute (the example being the amino acid glycine) and sodium (Riggs et al. 1958). They suggested that solute uptake could be driven by the energy stored in the transmembrane ionic gradients for cations present due to the asymmetric distribution of the alkali metals sodium and potassium (Riggs et al. 1958). Around the same time, Ricklis & Quastel (1958) and Csáky & Thale (1960) demonstrated that intestinal sugar transport was dependent upon the presence of mucosal sodium. These and other observations were unified by Crane and colleagues (Crane et al. 1961; Crane, 1962) under what became known as the “Na+ gradient hypothesis”. This hypothesis describes Na+ gradient dependent uphill accumulation of glucose across the intestinal brush-border membrane and has been adapted to describe the accumulative transport of many other solutes (both nutrients and drugs) (Fig. 1A). The energy for the whole process is in the form of ATP which is utilized by the basolateral Na+,K+-ATPase to drive three Na+ out of the cell in exchange for the inward movement of two K+, thus generating transmembrane and transepithelial concentration gradients for Na+ and K+. The resultant inside-negative membrane potential and high luminal Na+ concentration generate a large inwardly-directed Na+-electrochemical gradient across the intestinal mucosal surface. It is the energy stored within this transmembrane Na+-electrochemical gradient that can be used to drive the uphill (concentrative) accumulation of many solutes across the luminal surface of the small intestinal epithelium. A large body of evidence is now available to support the role of such secondary active Na+-coupled membrane transporters in the uphill transport of a wide variety of solutes across the intestinal brush-border membrane including: the excitatory amino acid carrier EAAC1 or EAAT3 (SLC1A1) which transports the anionic amino acids glutamate and aspartate (Kanai & Hediger, 2004); the sodium-glucose linked cotransporter SGLT1 (SLC5A1) (Wright & Turk, 2004); the multivitamin transporter SMVT (SLC5A6) which transports biotin, lipoate and pantothenate (Wright & Turk, 2004); the monocarboxylate transporters SMCT1 (SLC5A8) and SMCT2 (SLC5A12) which transport short-chain fatty acids, nicotinate and many other related compounds (Gopal et al. 2004; Coady et al. 2004; Srinivas et al. 2005); the serotonin transporter SERT (SLC6A4) (Martel et al. 2003); the taurine transporter TAUT (SLC6A6) (Chen et al. 2004); the neutral and dibasic amino acid transport system B0,+ (ATB0,+/SLC6A14) (Hatanaka et al. 2004); the neutral amino acid transport system B0 (B0AT1/SLC6A19) (Bröer et al. 2004); the IMINO transport system (SIT1/XT3s1/SLC6A20) (Kowalczuk et al. 2005; Takanaga et al. 2005); the bile salt transporter ASBT (SLC10A2) (Hagenbuch & Dawson, 2004); the dicarboxylate transporter NaDC-1 (SLC13A2) (Markovich & Murer, 2004); the ascorbic acid transporter SVCT1 (SLC23A1) (Takanaga et al. 2004); the nucleoside transporter CNT1 (SLC28A1) (Gray et al. 2004); the inorganic phosphate transporter NaPi-IIb (SLC34A2) (Murer et al. 2004).
Figure 1.
Schematic representation of Na+-coupled solute cotransport (the “Na+ gradient hypothesis”) (A) and the relationship (“functional cooperativity”) between H+-coupled cotransport (via PepT1 or PAT1) and the Na+/H+ exchanger NHE3 (B).
The role of the H+-electrochemical gradient in solute transport
Around the same time that the Na+ gradient hypothesis was formulated, Peter Mitchell proposed the “chemiosmotic hypothesis” (Mitchell, 1961). This hypothesis has been used (Mitchell, 1963; Mitchell, 1973) to propose that sugar transport across microbial cell membranes can be energized by a transmembrane H+-electrochemical gradient. This theory was confirmed with the demonstration of rheogenic 1:1 H+:lactose cotransport into Escherichia coli containing the lactose permease LacY (West, 1970; West & Mitchell, 1972; 1973). The H+-electrochemical gradient is now considered an essential driving force for transmembrane transport of many solutes in yeast, plants and bacteria (Henderson, 1990; Bush, 1993; Hediger, 1994; Wipf et al. 2002). Perhaps it was due to the origins of the various hypotheses regarding ion-driven solute transport that Na+-coupling became accepted generally as the primary means of solute movement in mammalian tissues whereas H+-coupling was considered to be specific for plants, yeast and bacteria. Unfortunately once such a doctrine becomes enshrined within the literature it is often difficult to dislodge from the scientific psyche. This paper reviews current evidence for the existence of a H+-electrochemical gradient at the brush-border surface of the mammalian small intestinal epithelium and the role this gradient plays in driving nutrient, micronutrient and drug absorption via numerous H+-coupled membrane transporters.
The acid microclimate
The H+-electrochemical gradient consists of both chemical and electrical gradient components (intestinal enterocytes have an inside-negative membrane potential). The first evidence for the existence of an area of low pH adjacent to the luminal surface of the small intestinal epithelium was produced almost 50 years ago (Hogben et al. 1959). It was suggested that a microclimate with a “virtual pH” of 5.3 existed at the luminal surface which was required to explain the differences between predicted rates and experimental measurements of absorption of various drugs (weak acids and bases) according to pH-partition theory (Schanker et al. 1958; Hogben et al. 1959). The acid microclimate has since been measured directly between pH 6.1-6.8 at the luminal surface of the mammalian small intestine both in vivo and in vitro by use of pH-sensitive microelectrodes (Lucas et al. 1975; Daniel et al. 1985; Shimada, 1987; McEwan et al. 1988; McKie et al. 1988; Shimada & Hoshi, 1988; Daniel et al. 1989). Surface pH measurements within the range of the “virtual pH” have only been observed using in vitro tissues in the presence of 10mM glucose (Lucas et al. 1980). These unusually low values have been attributed to a stimulatory effect of glucose on H+ secretion in vitro which may reflect an increase in H+/lactate efflux (Daniel & Rehner, 1986; Daniel et al. 1989). In addition, Hogben and colleagues (1959) may have simply overestimated the acidity of the microclimate because many of the weak acids used in their study (e.g. salicylic acid, benzoic acid) are now known to be substrates for H+-coupled transporters at the intestinal brush-border membrane (see subsection on OATP2B1 and MCT1 below). The apparent increase in passive non-ionic penetration of weak acids following a decrease in luminal pH may, at least partly, reflect an increase in uptake via pH gradient dependent carrier-mediated transport.
Exactly how the acid microclimate is generated is still a matter for debate. Many studies favour transmembrane H+ secretion into the negatively-charged mucus layer which will act as a diffusion barrier so that ion (H+) concentrations at the surface are different from those in the bulk lumen. In contrast, Shiau and colleagues (1985) suggest that the microclimate exists solely due to the presence of the mucus layer and is not due to H+ secretion although if this is correct it is not clear why the microclimate varies along the length of the small intestine and crypt-villus axis (Daniel et al. 1985; McEwan et al. 1988; Daniel et al. 1989). A role for membrane transport in maintenance of the acid microclimate seems certain as the microclimate is alkalinized by the removal of extracellular Na+, the presence of amiloride or by addition of various factors that increase intracellular cAMP or cGMP (Lucas et al. 1980; Shimada, 1987; McEwan et al. 1988; McKie et al. 1988; Shimada & Hoshi, 1988; Daniel et al. 1989). These characteristics all point towards a role for the apical Na+/H+ exchanger NHE3 (SLC9A3) (Murer et al. 1976; Brant et al. 1995; Hoogerwerf et al. 1996) in maintenance of the acid microclimate. Although NHE3 may play a role in acid microclimate maintenance it seems unlikely to be solely responsible for pH microclimate generation as NHE3 functions poorly at typical microclimate surface pH values (Orlowski, 1993; Thwaites et al. 2002; Anderson et al. 2004).
Here we review the current evidence for a diverse range of H+/solute cotransporters at the luminal membrane of the small intestinal epithelium. It should be noted that, by convention, these transporters are described here as H+/solute cotransporters rather than OH−/solute antiporters although there is little experimental evidence to distinguish between these two modes of transport.
SLC15A1: the di/tripeptide transporter PepT1
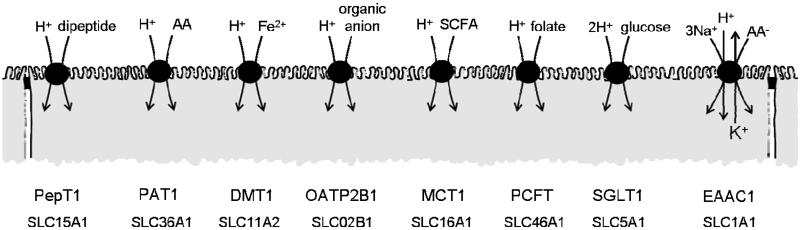
Protein is digested within the lumen of the mammalian small intestine to release small di- or tripeptides (2-3 amino acids in length) and individual amino acids. Protein is absorbed from diet in these two distinct forms. The first solid evidence for H+-coupled di/tripeptide transport (Fig. 2) came from a series of studies by Ganapathy & Leibach (Ganapathy & Leibach, 1983, 1985; Ganapathy et al. 1984) where dipeptide uptake into intestinal brush-border membrane vesicles (BBMV) was shown to be independent of Na+ but stimulated by a pH gradient (which was abolished in the presence of a protonophore). This pH-dependent stimulation of dipeptide uptake was associated with a depolarization of the membrane potential and was increased in the presence of a valinomycin-induced K+-diffusion potential. Boyd & Ward (1982) had been the first to suggest that dipeptide transport may be coupled to movement of a cation other than Na+. The identity of this cation and the H+-coupled, pH-dependent, rheogenic nature of dipeptide transport was eventually confirmed in 1993 in a series of studies using human intestinal epithelial Caco-2 cell monolayers in which H+/dipeptide cotransport was observed (Thwaites et al. 1993a; 1993b; 1993c; 1993f). Two earlier studies had demonstrated uptake and transepithelial transport of the aminocephalosporin antibiotics cephalexin (Dantzig & Bergin, 1990) and cephradine (Inui et al. 1992).
Figure 2.
Schematic representation of the H+-coupled nutrient, micronutrient and drug transporters at the brush-border membrane of the mammalian small intestine.
A cDNA was isolated from rabbit small intestine which, when expressed in Xenopus laevis oocytes was able to induce H+-coupled, Na+-independent, di and tripeptide uptake (Fei et al. 1994; Boll et al. 1994). This transporter was named PepT1 (Fei et al. 1994) and was later isolated from human ileum (Liang et al. 1995) and human intestinal Caco-2 cells (Walker et al. 1998). PepT1 mRNA is found along the length of the small intestine and is localized to the absorptive enterocytes in the duodenum, jejunum and ileum (Freeman et al. 1995). PepT1 protein is immunolocalized to the brush-border membrane of human small intestine and human intestinal Caco-2 cell monolayers (Walker et al. 1998). PepT1 represents the first member (SLC15A1) of solute carrier family 15 which also includes three other related sequences (Daniel & Kottra, 2004). PepT1 is a low-affinity, high-capacity, transport system which has a very broad substrate specificity and plays a unique role in nutrient absorption, transporting most of the potential 400 dipeptides and 8000 tripeptides which result from enzymatic breakdown of dietary protein. PepT1 can also transport a large number of hydrophilic drugs and is, therefore, responsible for the high levels of oral bioavailability of many pharmaceutical compounds. Examples of PepT1 substrates include (Table 1) penicillin and cephalosporin antibiotics, angiotensin-converting enzyme (ACE) inhibitors, anti-cancer drugs, and pro-drugs of levodopa or 3,4-dihydroxy-l-phenylalanine (l-DOPA) and the anti-viral agents acyclovir, ganciclovir and azidothymidine or zidovudine (ZT) (Rubio-Aliaga & Daniel, 2002).
Table 1.
Intestinal H+-coupled nutrient, micronutrient and drug transporters
| Gene | Protein | Aliases | Examples of natural substrates, drugs & related compounds |
|---|---|---|---|
| SLC15A1 | PepT1 | di/tripeptides, β-lactam antibiotics (cephalosporins, penicillins), angiotensin-converting enzyme inhibitors, δ-aminolevulinic acid, bestatin, pro-drugs for l-DOPA and anti-virals (e.g. acyclovir, ganciclovir, AZT) | |
| SLC36A1 | PAT1 | imino acid carrier LYAAT-1 tramdorin 3 |
d- and l-amino and imino acids (e.g. proline, alanine, hydroxyproline), d-serine, GABA, MeAIB, taurine, betaine, d- and l-cycloserine, vigabatrin, short-chain fatty acids (e.g. butyrate, acetate, propionate) |
| SLC11A2 | DMT1 | NRAMP2 DCT1 |
Fe2+, Mn2+, Ni2+, Co2+ |
| SLC02B1 | OATP2B1 | OATP-B SLC21A9 |
oestrone-3-sulphate, DHEAS, benzoate, nicotinate, taurocholate*, BSP, salicylate, valproate, pravastatin*, atorvastatin, fexofenadine, glibenclamide |
| SLC16A1 | MCT1 | short-chain fatty acids (e.g. l- and d-lactate, pyruvate, acetate, butyrate, propionate), ketone bodies (acetoacetate, β-hydroxybutyrate), nicotinate, salicylate, benzoate, valproate, atorvastatin, pravastatin | |
| SLC46A1 | PCFT | HCP1 | folate, methotrexate, permetrexed, 5-methyltetrahydrofolate, 5-formyltetrahydrofolate, heme |
| SLC5A1 | SGLT1 | d-glucose, d-galactose | |
| SLC1A1 | EAAC1 | System XAG- EAAT3 |
l-glutamate, l- and d-aspartate, l-cysteine |
OATP2B1 transports taurocholate and pravastatin only at acidic values of pH.
SLC36A1: the proton-coupled amino-acid transporter PAT1
Protein is also absorbed across the intestinal brush-border membrane in the form of individual amino acids. Transepithelial transport of amino acids across the intestinal epithelium is mediated via a number of amino acid transport systems arranged in parallel and series at the apical and basolateral membranes of the intestinal enterocyte. For many years it was considered that intestinal amino acid transport was solely a function of Na+-dependent transport systems although there was never much evidence against the involvement of other transmembrane ionic gradients (see Thwaites & Anderson, 2007).
A H+-coupled, pH gradient dependent, Na+-independent, rheogenic amino acid transporter was identified at the brush-border membrane of the human intestinal epithelial cell line Caco-2 (Fig. 2) (Thwaites et al. 1993d; 1993e; 1994b; 1995a; 1995b; 1995c; Thwaites & Stevens, 1999; Thwaites et al. 2000). This transporter was named system PAT (Thwaites et al. 1994b; Thwaites & Anderson, 2007). The related cDNA has recently been isolated from rat (Sagné et al. 2001), mouse (Boll et al. 2002), human (Chen et al. 2003a) and rabbit (Miyauchi et al. 2005). The cloned transporter is known as PAT1. PAT1 mRNA is expressed in all regions of the small intestine and PAT1 protein is immunolocalised to the brush-border membrane of the human and rat small intestine, and human intestinal Caco-2 cell monolayers (Chen et al. 2003a; Anderson et al. 2004). PAT1-like transport has been detected at the brush-border membrane of rat jejunum (Anderson et al. 2004; Iñigo et al. 2006). Like, PepT1, PAT1 is also a low-affinity, high-capacity, transporter which plays a role in both nutrient and drug absorption (Thwaites & Anderson, 2007). PAT1 has a broad substrate specificity (Table 1) transporting both d- and l-imino and amino acids, ß- and γ-amino acids, and a large number of neuromodulatory and anti-bacterial agents (Thwaites et al. 1993d; 1993e; 1994b; 1995a; 1995b; 1995c; Thwaites & Stevens, 1999; Thwaites et al. 2000; Boll et al. 2002; Chen et al. 2003a; Boll et al. 2003; Anderson et al. 2004; Metzner et al. 2004; 2005; Abbot et al. 2006; Thwaites & Anderson, 2007). PAT1 can also function in two different modes either as an electrogenic H+/amino acid cotransporter or as an electroneutral H+/anion cotransporter for short-chain fatty acids (Foltz et al. 2004a) (Table 1).
PAT1 is the first member (SLC36A1) of solute carrier family 36 which includes three other related sequences in all mammalian genomes (Chen et al. 2003b; Bermingham & Pennington, 2004; Boll et al. 2004). PAT2 (SLC36A2) is also a H+-coupled amino acid transporter but has a distinct substrate specificity and tissue distribution from PAT1 and is not expressed in the small intestine (Boll et al. 2002; Chen et al. 2003b; Foltz et al. 2004b; Rubio-Aliaga et al. 2004; Kennedy et al. 2005a). Iminoglycinuria is a disorder characterised by defective renal tubular reabsorption of amino acids (glycine, proline and hydroxyproline) and in some cases there is also defective transport in the small intestine (Online Mendelian Inheritance in Man (OMIM) database, OMIM 242600, www.ncbi.nlm.nih.gov). Bröer has recently suggested (Bröer, 2006) that iminoglycinuria is likely to be a multigene disorder which may include several transporters such as PAT1, PAT2 and IMINO. The ancient nature of the H+-electrochemical gradient is emphasised, by the PAT-related transporters, as there are more than four related sequences in the genomes of lower eukaryotes (Sagné et al. 2001). For example, there are eight PAT-related sequences in Drosophila melanogaster. The D. melanogaster transporter CG1139 has similar functional characteristics to PAT1 (following heterologous expression in X. laevis oocytes). Overexpression of CG1139 is associated with modulation of eye and wing growth suggesting that PAT transporters might regulate growth in vivo (Goberdhan et al. 2005).
SLC11A2: the divalent metal transporter DMT1
The H+-coupled divalent metal transporter DMT1 (Fig. 2) was originally cloned and named NRAMP2 (Vidal et al. 1995) because of its sequence similarity to the natural resistance-associated macrophage protein NRAMP1. The function of the protein remained unknown until the mRNA was isolated by expression cloning and shown to transport divalent metals, for example Fe2+, when expressed in X. laevis oocytes (Gunshin et al. 1997). The transporter was renamed DCT1 (divalent cation transporter). This transporter is the major route for intestinal absorption of non-haem iron and transports iron in the ferrous Fe2+ form following reduction of the ferric Fe3+ form by an apical ferrireductase (Mackenzie & Garrick, 2005). The role of this transporter in intestinal Fe2+ absorption was confirmed by the identification of a G185R mutation in the Nramp2 gene in both the microcytic (mk) mouse and Belgrade (b) rat that have abnormal intestinal iron absorption (Fleming et al. 1997; 1998). Inclusion of the G185R mutation in the NRAMP2/DCT1 clone leads to a loss of function (Su et al. 1998). The transporter is now known as DMT1 or SLC11A2, the second member of solute carrier family 11 (Mackenzie & Hediger, 2004). DMT1 has been immunolocalised to the brush-border membrane of the proximal small intestine and human intestinal Caco-2 cells (Canonne-Hergaux et al. 1999; Tandy et al. 2000). Knockdown of DMT1 in Caco-2 cells leads to a reduction in Fe2+ uptake (Bannon et al. 2003). Targeted deletion of the Slc11a2 gene in mouse intestine is associated with anaemia once the animals become dependent upon intestinal nutrition (Gunshin et al. 2005). As well as H+/Fe2+ cotransport (Gunshin et al. 1997), DMT1 can also mediate transport of a range of divalent metals (e.g. Mn2+, Ni2+, Co2+)(Table 1).
SLC02B1: the organic anion transporting polypeptide OATP2B1
The pH-partition theory (see subsection on acid microclimate above) was proposed to account for the increased absorption of organic anions across the intestinal wall. However, it is now evident that many organic anions are substrates for pH-dependent carrier-mediated transport systems expressed at the intestinal brush-border membrane. The organic anion transporting polypeptide OATP2B1 (Fig. 2) is a member (SLC02B1) of the solute carrier family SLC0 (Hagenbuch & Meier, 2004). This SLC family is relatively unusual as different transport proteins are expressed in different mammalian species and the relationship of human OATPs to rat and mouse oatps is not clear. OATP2B1 was originally isolated from human brain and named OATP-B (Tamai et al. 2000; Kullak-Ublick et al. 2001) or SLC21A9 (Hagenbuch & Meier, 2003). OATP2B1 mRNA is expressed in the human small intestine (Tamai et al. 2000; Kullak-Ublick et al. 2001; Sai et al. 2006) and OATP2B1 protein is immunolocalised at the brush-border surface of both human small intestine (Kobayashi et al. 2003) and human intestinal Caco-2 cell monolayers (Sai et al. 2006). Heterologous expression of OATP2B1 produces a Na+-independent, pH-gradient dependent transporter (Nozawa et al. 2004) with a relatively narrow substrate specificity compared to other OATPs. OATP2B1 transports the physiological sulfate-conjugated steroids oestrone-3-sulfate and dehydroepiandrosterone sulfate (DHEAS) (Tamai et al. 2000; Kullak-Ublick et al. 2001) and at lower pH (consistent with pH within the acid microclimate) broadens its specificity to include taurocholate (Nozawa et al. 2004) suggesting that OATP2B1 may play a role in the enterohepatic circulation of both bile acids and oestrogen (Sai et al. 2006). OATP2B1 may also play an important role in oral drug delivery as it can transport bromosulphothalein (BSP), the 3-hydroxy-3-methylglutaryl-Coenzyme A (HMG-CoA) reductase inhibitors pravastatin and atorvastatin (used clinically to reduce hypercholesterolaemia), the anti-histamine fexofenadine and the anti-diabetic glibenclamide (Kobayashi et al. 2003; Nozawa et al. 2004; Satoh et al. 2005; Grube et al. 2006) (Table 1). However, there is some overlap in the apparent substrate specificity between OATP2B1 and the monocarboxylate transporter MCT1 (SLC16A1, see subsection on MCT1 below). Results from studies using intact tissues where these transporters may be coexpressed should be interpreted carefully, in particular in tissues from species where it is not clear which SLC0 members are expressed at the intestinal luminal membrane. Compounds that are potential substrates for both OATP2B1 and MCT1 include nicotinate, benzoate, salicylate, valproate, pravastatin and atorvastatin (Table 1).
OATP2B1-like function is consistent with transport characteristics at the apical surface of human intestinal Caco-2 cell monolayers and BBMV prepared from rabbit jejunum (Tamai et al. 1995a; Sai et al. 2006) although it is not clear which SLC0 members are expressed in the rabbit small intestine. The ability of grapefruit (and other citrus) juice and constituents to reduce the oral bioavailability of fexofenadine (Dresser et al. 2002) seems likely to be mediated by inhibition of intestinal absorption via OATP2B1 (Satoh et al. 2005), identifying OATP2B1 as a potential site for diet-drug interactions. The physiological and pharmacological role played by OATP2B1 in intestinal absorption may also vary between individuals. For example, a single nucleotide polymorphism (found in 31% of the Japanese population investigated within the study) leads to an amino acid change in the OATP2B1 protein (S486F) which is associated with a greater than 50% reduction in transport capacity (Nozawa et al. 2002).
SLC16A1: the monocarboxylate transporter MCT1
The monocarboxylate transporter MCT1 is the first member (SLC16A1) of solute carrier family 16 (Halestrap & Meredith, 2004). The first SLC16 transporter to be identified at the molecular level was originally isolated from a Chinese hamster ovary (CHO) cell line and named MEV because it caused an increase in mevalonate uptake in transiently transfected cells (Kim et al. 1992). MEV includes a single base change in the sequence compared to the wild-type cDNA (MCT1). The hamster wild-type cDNA was identified as a transporter of lactate and pyruvate and named MCT1 (Garcia et al. 1994) and later isolated from rat small intestine (Takanaga et al. 1995). MCT1 requires coexpression in situ of the ancillary protein CD147 for proper cell surface expression and function (Halestrap & Meredith, 2004).
MCT1 functions as a H+-coupled transporter (Bröer et al. 1998) of a wide variety of anions (Table 1, Fig. 2) including the vitamin B3 nicotinate, the monocarboxylates l- and d-lactate (with a preference for the l-form), pyruvate, acetate, propionate and butyrate, and the ketone bodies acetoacetate and ß-hydroxybutyrate (Tamai et al. 1995b, 1999). MCT1 also transports benzoate (a food preservative) and salicylate (which have been used to estimate the acid microclimate and which are also substrates for OATP2B1, see above), d,l-2-hydroxy-(4-methylthio)butanoic acid (a feed supplement), and a number of other pharmaceutical compounds including the anti-convulsant valproate, and the HMG-CoA reductase inhibitors pravastatin and atorvastatin (also OATP2B1 substrates) (Tamai et al. 1995b, 1999; Wu et al. 2000; Martín-Venegas et al. 2007). A key functional characteristic of MCT1 activity is its sensitivity to inhibition by α-cyano-4-hydroxycinnamate (Bröer et al. 1998).
The short-chain fatty acids butyrate, propionate and acetate are the major organic anions in the lumen of the large intestine where they are produced by bacterial fermentation of dietary fibre and undigested carbohydrates. Butyrate is a major energy source for colonocytes. There is a physiological role for a butyrate uptake mechanism at the luminal membrane of the colon and there is evidence for both MCT1 (SLC16A1) and SMCT1 (SLC6A8) in the literature (Ritzhaupt et al. 1998; Ganapathy et al. 2005). We will not discuss the evidence for colonic transport here in any further detail as the focus of this review is the role of the H+-electrochemical gradient in absorption across the small intestine where there is substantial evidence for an inward H+ gradient (see subsection on acid microclimate above). The physiological role for a monocarboxylate transporter in the small intestine must be to mediate monocarboxylate absorption from diet (it is possible that a greater requirement for small intestinal uptake might be needed in animals practicing coprophagy). MCT1-like activity has been demonstrated as pH-gradient dependent lactate uptake and lactate-sensitive nicotinate uptake in rabbit and rat small intestinal BBMV (Tiruppathi et al. 1988; Simanjuntak et al. 1990; Takanaga et al. 1996).
MCT1 mRNA has been detected in the small intestine of human, rabbit, rat, hamster and Caco-2 cells (Kim et al. 1992; Tamai et al. 1995b; Price et al. 1998; Hadjiagapiou et al. 2000; Englund et al. 2006; Seithel et al. 2006). An in situ hybridisation study of mouse and rat gastrointestinal tract identified MCT1 mRNA throughout from the stomach to the distal colon with greatest intensity in the caecum (Iwanaga et al. 2006). In the small intestine the strongest signal was in the rat ileum (Iwanaga et al. 2006). Immunolocalization studies of MCT1 within the small intestinal epithelium have provided conflicting observations (Garcia et al. 1994; 1995; Tamai et al. 1999; Gill et al. 2005; Iwanaga et al. 2006). MCT1 was immunolocalized to the basolateral membrane in the epithelial cells along the length of the hamster gastrointestinal tract although only images of basolateral staining in stomach and caecum were presented (Garcia et al. 1994; 1995). In a more recent study, MCT1-immunoreactivty was absent from the villus epithelium in the mouse ileum although basolateral immunoreactivity was observed in the crypts (Iwanaga et al. 2006). Similarly no immunoreactivity was observed in human terminal ileum (Iwanaga et al. 2006). In contrast, MCT1 was found along the length of the rat gastrointestinal tract which in the small intestine showed a greater intensity in the duodenum than the ileum and also in the crypts than the villus (Tamai et al. 1999). Staining in the crypts was mainly basolateral but both lateral and brush-border staining was observed in the villus epithelium (Tamai et al. 1999). Immunoblotting of apical and basolateral membranes purified from human jejunum and ileum identified MCT1 expression in both membranes with stronger expression in the brush-border (Gill et al. 2005).
The human intestinal epithelial cell line Caco-2 was derived from a human colonic adenocarcinoma but expresses a small intestinal enterocyte-like phenotype when grown as confluent polarised monolayers on permeable filters (Delie & Rubas, 1997). A number of investigators (for examples see, Stein et al. 2000; Hadjiagapiou et al. 2000; Buyse et al. 2002) have measured butyrate transport in Caco-2 cell monolayers and identified an MCT1-like transporter at the brush-border membrane. In addition, MCT1 and the ancillary protein CD147 have both been immunolocalised to the brush-border membrane of Caco-2 cells (Buyse et al. 2002). Thus, it seems likely, that MCT1 mediates butyrate transport across the brush-border membrane of Caco-2 cells. However, it is not clear (and the purpose for choosing Caco-2 cells is not apparent in some of the studies) whether the MCT1-like transport is representative of butyrate transport across the small intestinal epithelium, large intestinal epithelium or both.
Although it is evident that monocarboxylate transport in the intestine is mediated by a number of transport systems that can be coupled to either H+ or Na+ movement, the relative expression of each transporter may vary between the apical and basolateral membranes of epithelial cells in the small and large intestines and may differ between species. Many fascinating questions remain to be answered, for example, what is the relative contribution of SMCT1, SMCT2, PAT1 and MCT1 (and other members of the SLC16 family of MCTs) to monocarboxylate transport and does the contribution of each transporter vary along the crypt-villus and longitudinal axes? The availability of functional clones and transporter specific antisera should provide evidence to elucidate the roles of these transporters in the near future.
SLC46A1: the proton-coupled folate transporter PCFT
Folic acid (vitamin B9) is an essential nutrient and folate deficiency is the most common vitamin deficiency in Western societies. In mammals the sole source of folate is through absorption from diet. A pH-gradient dependent, Na+-independent, folate transporter has been characterised in a number of studies using BBMV (mainly jejunal) from human, rabbit and rat small intestine (Selhub & Rosenberg, 1981; Schron et al. 1985; Said et al. 1987; Mason et al. 1990). This high-affinity transporter also transports the antineoplastic and immunosuppressive agent methotrexate, 5-methyltetrahydrofolate and 5-formyltetrahydrofolate. A transporter with similar characteristics has been identified at the apical membrane of human intestinal Caco-2 cell monolayers (Vincent et al. 1985; Mason et al. 1990; Kneuer & Honscha, 2004) and rat intestinal epithelial IEC-6 cells (Said et al. 1996; Wang et al. 2005). The molecular identity of this pH-gradient dependent, high-affinity, folate transporter has been revealed recently with the isolation of the proton-coupled folate transporter PCFT (Qiu et al. 2006) (see Fig. 2, Table 1). PCFT is the first member (SLC46A1) of solute carrier family 46 and this human cDNA was identified using a data mining approach to search for sequences with weak homology to the reduced folate transporter RFT (SLC19A1, see below). In either Xenopus oocytes or mammalian cell lines, PCFT has identical characteristics to those previously characterised in intestinal tissues and it functions as an electrogenic, H+-coupled, Na+-independent transporter with a particularly high affinity for the thymidylate synthase inhibitor permetrexed (Wang et al. 2004; Qiu et al. 2006; Zhao & Goldman, 2007). PCFT mRNA is found throughout the human intestine with greatest abundance in the duodenum and Caco-2 cell line (Qiu et al. 2006). PCFT was originally isolated from mouse small intestine as a haem transporter and named heme carrier protein 1 or HCP1 (Shayeghi et al. 2005) although the affinity for haem is much lower than for folates (Qiu et al. 2006). PCFT/HCP1 protein was immunolocalised to the mucosal surface of mouse duodenum (Shayeghi et al. 2005) consistent with a role of this transport protein in absorption of essential components of diet.
For a number of years the reduced folate transporter RFT was considered the prime candidate for proton-coupled folate transport across the intestinal brush-border. RFT (SLC19A1) is the first member of solute carrier family 19 (Ganapathy et al. 2004) and is also known as the reduced folate carrier RFC (Dixon et al. 1994), folate transporter FOLT (Prasad et al. 1995) or intestinal folate carrier 1 (IFC1) (Said et al. 1996). RFT was originally cloned from a mouse cDNA library (Dixon et al. 1994) and was later cloned from many tissues including the human small intestine (Nguyen et al. 1997). The tissue distribution of RFT is consistent with a role in folate absorption as: mRNA is found in human intestinal Caco-2 cells and human and rat small intestine (Prasad et al. 1995; Nguyen et al. 1997; Said et al. 2000); in situ hybridisation in human jejunum localises RFT mRNA to epithelial cells particularly in the upper half of the villus (Nguyen et al. 1997); RFT protein is immunolocalised to the brush-border membrane of the duodenum, jejunum and ileum (Wang et al. 2001); Western blot analysis identifies RFT in jejunal brush-border membranes (Said et al. 2000).
The relative contribution of both PCFT and RFT to intestinal folate absorption will require further investigation. In contrast to PCFT, RFT has a strong preference for reduced rather than oxidised folates and RFT functions probably as a folate anion/OH− antiport transporting folate, methotrexate, and thiamine monophosphate (Zhao et al. 2002; Ganapathy et al. 2004). RFT is unlikely to be responsible for the pH-gradient dependent (low pH stimulated) uptake observed in intestinal cell lines and BBMV (Selhub & Rosenberg, 1981; Schron et al. 1985; Vincent et al. 1985; Said et al. 1987; Mason et al. 1990; Said et al. 1996; Kneuer & Honscha, 2004; Wang et al. 2005) since RFT expression in oocytes is associated with a decrease in uptake as pHo is reduced (Nguyen et al. 1997; Kumar et al. 1998) rather than the increase in uptake observed in PCFT-expressing oocytes (Qiu et al. 2006). Goldman and colleagues (Qiu et al. 2006) have identified that a loss-of-function mutation in PCFT is the molecular basis of hereditary folate malabsorption (OMIM 229050, www.ncbi.nlm.nih.gov) in a single family. High doses of oral 5-formyltetrahydrofolate relieved the symptoms of the disorder in the two members of the family affected suggesting that a second, lower affinity, folate transporter is also involved in intestinal absorption. Identification of this second transporter as RFT requires further investigation. It will be interesting to identify the nature of the defect in other patients suffering from hereditary folate malabsorption and also if any compensatory changes in RFT expression are observed.
SLC5A1: the sodium-glucose linked cotransporter SGLT1
Thus far we have described a series of intestinal transport systems that function as H+/solute symporters. There is no evidence to suggest that solute movement through these transporters is linked directly to Na+ cotransport (although some at least are linked indirectly to Na+ transport, see subsection on NHE3 below). There are, however, some sugar transporters that can function either as Na+ or H+ cotransporters, for example, the E. coli melibiose transporter (Tsuchiya & Wilson, 1978). Here we describe an example of a mammalian transporter that can use either the Na+ or H+ electrochemical gradients to drive solute transport.
The sodium-coupled glucose cotransporter SGLT1 is the first member (SLC5A1) of solute carrier family 5 (Wright et al. 2004). Although SGLT1 is often considered the archetypal Na+/solute cotransporter, the Na+-gradient hypothesis (Crane et al. 1961) was after all proposed to account for the Na+-dependency of sugar absorption, SGLT1 can also function as a H+/sugar cotransporter (Hirayama et al. 1994) (Fig. 2). The evidence for Na+-glucose cotransport is compelling: the interrelationship between NaCl, water and glucose absorption was demonstrated using rat ileum in vitro (Curran, 1960); coupled influx of Na+ and glucose was demonstrated using flat sheets of rabbit ileum (Schultz & Zalusky, 1964); the brush-border location of the cotransport mechanism was identified unequivocally using rat intestinal BBMV (Hopfer et al. 1973); a cDNA (SGLT1) was isolated from rabbit small intestinal mucosa which, when expressed in X. laevis oocytes, produced a transport system with all of the characteristics of the intestinal Na+ driven glucose transporter (Hediger et al. 1987). SGLT1 transport is characterised as Na+-dependent uptake of D-glucose and D-galactose (Table 1) that is inhibitable by phlorizin. However, pH-dependent, Na+-independent glucose transport has also been observed. Hoshi and colleagues (Hoshi et al. 1986) demonstrated that D-glucose uptake into rabbit intestinal BBMV in Na+-free conditions was stimulated by decreasing extravesicular pH in the presence of an inside-negative vesicular membrane potential. Similarly glucose-stimulated phlorizin-sensitive current in X. laevis oocytes expressing rabbit SGLT1 was enhanced by decreasing extracellular pH in the absence of extracellular Na+ suggesting that SGLT1 can also function in a H+-coupled mode (Hirayama et al. 1994). SGLT1 has an affinity for H+ that is approximately 500 times greater than Na+ (K0.5 0.007 and 4mM, respectively) whereas the affinity for glucose is approximately 25 times lower in the H+-coupled compared to Na+-coupled mode (Hirayama et al. 1994; Quick et al. 2001; Wright et al. 2004).
In situ hybridisation studies localised SGLT1 to the absorptive villus epithelial cells in duodenum, jejunum and ileum (Hwang et al. 1991; Smith et al. 1992; Freeman et al. 1993). Immunocytochemical studies have identified SGLT1 at the brush-border membrane of the rabbit and rat small intestinal epithelium, human jejunum and Caco-2 cells (Hwang et al. 1991; Yoshida et al. 1995; Khoursandi et al. 2004). The physiological importance of SGLT1 is emphasised in patients with glucose-galactose malabsorption syndrome (OMIM 606824, www.ncbi.nlm.nih.gov) where mutations in SGLT1 are associated with defective intestinal absorption of D-glucose and D-galactose (Wright et al. 1991). Thus, SGLT1 is an example of a transport protein that may be able to adapt to maximise sugar absorption by utilising local transmembrane ionic gradients for cations (Na+ and H+) along the length of the small intestine which may vary in magnitude both in time and space.
SLC1A1: the excitatory amino acid carrier EAAC1
The excitatory amino acid carrier EAAC1, also known as EAAT3, is the first member (SLC1A1) of solute carrier family 1 (Kanai & Hediger, 2004). EAAC1 differs from the other transport systems described above as inward solute flux is coupled to the inward movement of both H+ and Na+. EAAC1 was originally isolated from a rabbit jejunal cDNA library (Kanai & Hediger, 1992) and is a high affinity transport system for the anionic amino acids L-glutamate, L- and D-aspartate and the dipolar amino acid L-cysteine (Kanai et al. 1994; Zerangue & Kavanaugh, 1996a; 1996b). EAAC1 has also been isolated from human ileum and kidney (Kanai et al. 1994).
Transport of, for example, glutamate by EAAC1 is accompanied by the inward movement of 3 Na+, 1 H+ and the countertransport of 1 K+ (Fig. 2). Two models have been proposed to account for the ionic dependency of EAAC1. Zerangue & Kavanaugh (1996a) suggested that the H+ is cotransported with the anionic amino acid glutamate resulting in an intracellular acidification upon release of H+ and solute inside the cell. This model is supported by their observation that the zwitterion L-cysteine is transported by the carrier but does not lead to an intracellular acidification because after release cysteine (pK 8.3) will remain protonated. In contrast, Auger & Attwell (2000) suggest that inward H+ movement occurs during the K+-countertransport stage of the transport cycle where glutamate is not bound to the carrier.
EAAC1 corresponds to the amino acid transport system XAG− (Christensen, 1984). The H+, Na+ and K+ dependency of system XAG− has been demonstrated in the human and rabbit intestinal brush-border membrane using jejunal BBMV (Berteloot, 1984; Harig et al. 1987; Maenz et al. 1992). Northern blot analysis identifies high levels of expression of EAAC1 in the duodenum, jejunum and ileum and in situ hybridisation localises EAAC1 mRNA to the small intestinal epithelium (Kanai & Hediger, 1992). EAAC1 protein has been immunolocalised to the intestinal brush-border membrane in both rat and mouse small intestine although in these studies EAAC1 protein showed greater expression in the lower part of the villus (Rome et al. 2002; Iwanaga et al. 2005).
The role of the Na+/H+ exchanger NHE3 (SLC9A3) in maintenance of the H+-electrochemical gradient and optimal H+/solute cotransport
The investigation of intestinal di/tripeptide transport was key to challenging the belief that the Na+ gradient hypothesis could account for all ion-driven solute absorption in the mammalian small intestine. Until the early 1980s most studies of intestinal absorption used intact tissue preparations (either in vivo or in vitro) in which it was difficult to control experimental conditions at the mucosal surface. Early studies of intestinal dipeptide uptake using intact tissue preparations identified a Na+-dependent mechanism (Ganapathy & Leibach, 1985; Ganapathy et al. 2006). However, Ganapathy & colleagues (Ganapathy & Leibach, 1983; 1985; Ganapathy et al.1984) were able to demonstrate Na+-independent, pH-gradient dependent uptake of dipeptides using intestinal BBMV. They suggested that the apparent Na+-tissues was due to a requirement for an apical Na+/H+ exchanger, in conjunction with basolateral Na+,K+-ATPase, to generate and maintain the inward H+ gradient (Fig. 1B). The role of NHE3 in maintaining intracellular pH and, therefore, the H+ gradient was demonstrated using Caco-2 cells loaded with the pH-sensitive dye BCECF. Recovery from dipeptide-induced intracellular acidification was via selective activation of apical Na+/H+ exchange without any activation of basolateral Na+/H+ exchange (Thwaites et al. 1993c; 1994a). The Na+/H+ exchanger activated following PepT1-mediated H+/dipeptide cotransport was identified as NHE3 (SLC9A3) by use of selective NHE inhibitors (Thwaites et al. 1999; 2002; Kennedy et al. 2002; Anderson et al. 2003; Kennedy et al. 2005b).
The functional relationship between H+/solute cotransport and NHE3 is not limited to PepT1 as PAT1-mediated H+/amino acid uptake leads to a similar selective activation of NHE3 (Thwaites et al. 1994b; 1995a; 1995b; 1995c; 1999; 2000; Anderson et al. 2004; Anderson & Thwaites, 2005). The relationship between PAT1 and NHE3 (Fig. 1B) explains the apparent Na+-dependence of amino acid transport via PAT1 (also known as the imino acid carrier) in intact epithelia (Anderson et al. 2004; Anderson & Thwaites, 2005; Thwaites & Anderson, 2007). The extent of the “functional cooperativity” between NHE3 and the other H+/solute cotransporters reviewed here is not known but may be limited to either the high-capacity, low-affinity, cotransporters or those cotransporters that demonstrate pH-dependence within the range of pH values measured within the extracellular microclimate. Inhibition of NHE3 activity by activators of the protein kinase A pathway (e.g. forskolin, 8-Br-cAMP, vasoactive intestinal peptide, pituitary-adenylate cyclase-activating peptide, VPAC1 receptor agonists, phosphodiesterase inhibitors) reduces the absorptive capacity of the H+-coupled cotransporters PepT1 and PAT1 at the intestinal brush-border membrane (Thwaites et al. 2002; Anderson et al. 2003; 2004; Anderson & Thwaites, 2005; Kennedy et al. 2005b; Anderson & Thwaites, 2007) and would presumably reduce the absorptive capacity of any transporter dependent upon maintenance of the H+-electrochemical gradient.
Conclusion
Absorption across the wall of the small intestine is mediated by a multitude of membrane proteins that allow nutrient, micronutrient and drug transport to proceed against a concentration gradient by coupling uphill movement of solute to downhill movement of ions. There is substantial evidence for the presence of both Na+ and H+-coupled solute cotransporters at the brush-border membrane of the mammalian small intestine. The lists presented here are not exhaustive and additional H+-coupled solute cotransporters are likely to be expressed at the brush-border of the mammalian small intestine. For example, the copper (Cu1+) transporter Ctr1, which is the first member (SLC31A1) of solute carrier family 31 (Petris, 2004), has not yet been demonstrated to be H+-coupled but is pH-dependent with 64Cu uptake in Ctr1-expressing HEK293 cells increasing as extracellular pH is reduced from pH 7.5 to 5.5 (Lee et al. 2002). Ctr1 protein has been immunolocalised to the apical membrane of human, rat and mouse small intestine and human intestinal Caco-2 cells (Klomp et al. 2002; Bauerly et al. 2004; Kuo et al. 2006; Nose et al. 2006).
Many of the mammalian H+-coupled cotransporters described here are also localised in lysosomes in a variety of non-intestinal cell types where they mediate lysosomal efflux using the outward H+-gradient generated by H+-ATPase activity. In contrast, the driving forces (Na+ and H+-electrochemical gradients) for the intestinal ion-coupled cotransporters are generated ultimately by activity of the basolateral Na+,K+-ATPase. Some H+-coupled cotransporters (perhaps those high-affinity, low-capacity carriers) may be driven solely via the inside-negative membrane potential. In contrast, some of the high-capacity cotransporters (e.g. PepT1 and PAT1) require an additional transporter (NHE3) (see Fig. 1B), which utilises the inward Na+ gradient, to maintain the H+ gradient across the luminal membrane during absorption. Na+/H+ exchangers play key roles throughout evolution (Skulachev, 1994) as “gradient converters” allowing utilisation of both Na+ and H+ gradients. These antiport proteins may allow adaptation (at the level of individual transport proteins, cells or tissues) to changes in local ionic microenvironments both in time and space. The primary means by which NHE3 maintains the H+-electrochemical gradient during solute absorption is through H+ efflux and regulation of intracellular pH. How the extracellular microclimate is established remains unclear. Overall, the ability to generate and maintain transmembrane ionic gradients during absorption controls the absorptive capacity of the human small intestinal epithelium and, by extension, drug transport via the oral route.
Acknowledgements
This work was supported by the Wellcome Trust (grant number: 078640/Z/05/Z) and BBSRC (grant number: 13/D17277).
Footnotes
Publisher's Disclaimer: The definitive version is available at www.blackwell-synergy.com and www.expphysiol.org
References
- Abbot EL, Grenade DS, Kennedy DJ, Gatfield KM, Thwaites DT. Vigabatrin transport across the human intestinal epithelial (Caco-2) brush-border membrane is via the H+-coupled amino acid transporter hPAT1. Br J Pharmacol. 2006;147:298–306. doi: 10.1038/sj.bjp.0706557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CMH, Grenade DS, Boll M, Foltz M, Wake KA, Kennedy DJ, Munck LK, Miyauchi S, Taylor PM, Campbell FC, Munck BG, Daniel H, Ganapathy V, Thwaites DT. H+/amino acid transporter 1 (PAT1) is the imino acid carrier: an intestinal nutrient/drug transporter in human and rat. Gastroenterology. 2004;127:1410–1422. doi: 10.1053/j.gastro.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Anderson CMH, Mendoza ME, Kennedy DJ, Raldua D, Thwaites DT. Inhibition of intestinal dipeptide transport by the neuropeptide VIP is an anti-absorptive effect via the VPAC1 receptor in a human enterocyte-like cell line (Caco-2) Br J Pharmacol. 2003;138:564–573. doi: 10.1038/sj.bjp.0705049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CMH, Thwaites DT. Indirect regulation of the intestinal H+-coupled amino acid transporter hPAT1 (SLC36A1) J Cell Physiol. 2005;204:604–613. doi: 10.1002/jcp.20337. [DOI] [PubMed] [Google Scholar]
- Anderson CMH, Thwaites DT. Regulation of intestinal hPepT1 (SLC15A1) activity by phosphodiesterase inhibitors is via inhibition of NHE3 (SLC9A3) Biochim Biophys Acta. 2007;1768:1822–1829. doi: 10.1016/j.bbamem.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger C, Attwell D. Fast removal of synaptic glutamate by postsynaptic transporters. Neuron. 2000;28:547–558. doi: 10.1016/s0896-6273(00)00132-x. [DOI] [PubMed] [Google Scholar]
- Bannon DI, Abounader R, Lees PS, Bressler JP. Effect of DMT1 knockdown on iron, cadmium, and lead uptake in Caco-2 cells. Am J Physiol. 2003;284:C44–C50. doi: 10.1152/ajpcell.00184.2002. [DOI] [PubMed] [Google Scholar]
- Bauerly KA, Kelleher SL, Lönnerdal B. Functional and molecular responses of suckling rat pups and human intestinal Caco-2 cells to copper treatment. J Nutr Biochem. 2004;15:155–162. doi: 10.1016/j.jnutbio.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Bermingham JR, Pennington J. Organization and expression of the SLC36 cluster of amino acid transporter genes. Mamm Genome. 2004;14:114–125. doi: 10.1007/s00335-003-2319-3. [DOI] [PubMed] [Google Scholar]
- Berteloot A. Characteristics of glutamic acid transport by rabbit intestinal brush-border membrane vesicles. Effects of Na+-, K+- and H+-gradients. Biochim Biophys Acta. 1984;775:129–140. doi: 10.1016/0005-2736(84)90163-9. [DOI] [PubMed] [Google Scholar]
- Boll M, Daniel H, Gasnier B. The SLC36 family: proton-coupled transporters for absorption of selected amino acids from extracellular and intracellular proteolysis. Pflügers Archiv. 2004;447:776–779. doi: 10.1007/s00424-003-1073-4. [DOI] [PubMed] [Google Scholar]
- Boll M, Foltz M, Anderson CMH, Oechsler C, Kottra G, Thwaites DT, Daniel H. Substrate recognition by the mammalian proton-dependent amino acid transporter PAT1. Mol Membr Biol. 2003;20:261–269. doi: 10.1080/0968768031000100759. [DOI] [PubMed] [Google Scholar]
- Boll M, Foltz M, Rubio-Aliaga I, Kottra G, Daniel H. Functional characterization of two novel mammalian electrogenic proton-dependent amino acid cotransporters. J Biol Chem. 2002;277:22966–22973. doi: 10.1074/jbc.M200374200. [DOI] [PubMed] [Google Scholar]
- Boll M, Markovich D, Weber WM, Korte H, Daniel H, Murer H. Expression cloning of a cDNA from rabbit small intestine related to proton-coupled transport of peptides, β-lactam antibiotics and ACE-inhibitors. Pflügers Archiv. 1994;429:146–149. doi: 10.1007/BF02584043. [DOI] [PubMed] [Google Scholar]
- Boyd CAR, Ward MR. A micro-electrode study of oligopeptide absorption by the small intestinal epithelium of Necturus maculosus. J Physiol. 1982;324:411–428. doi: 10.1113/jphysiol.1982.sp014121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brant SR, Yun CHC, Donowitz M, Tse CM. Cloning, tissue distribution, and functional analysis of the human Na+/H+ exchanger isoform, NHE3. Am J Physiol. 1995;269:C198–C206. doi: 10.1152/ajpcell.1995.269.1.C198. [DOI] [PubMed] [Google Scholar]
- Bröer S. The molecular basis of neutral amino acidurias. Acta Biomed. 2006;77(suppl. 3):6–8. [Google Scholar]
- Bröer A, Klingel K, Kowalczuk S, Rasko JE, Cavanaugh J, Bröer S. Molecular cloning of mouse amino acid transport system B0, a neutral amino acid transporter related to Hartnup disorder. J Biol Chem. 2004;279:24467–24476. doi: 10.1074/jbc.M400904200. [DOI] [PubMed] [Google Scholar]
- Bröer S, Schneider HP, Bröer A, Rahman B, Hamprecht B, Deitmer JW. Characterization of the monocarboxylate transporter 1 expressed in Xenopus laevis oocytes by changes in cytosolic pH. Biochem J. 1998;333:167–174. doi: 10.1042/bj3330167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush DR. Proton-coupled sugar and amino-acid transporters in plants. Annu Rev Plant Physiol Plant Mol Biol. 1993;44:513–542. [Google Scholar]
- Buyse M, Sitaraman SV, Liu X, Bado A, Merlin D. Luminal leptin enhances CD147/MCT-1-mediated uptake of butyrate in the human intestinal cell line Caco2-BBE. J Biol Chem. 2002;277:28182–28190. doi: 10.1074/jbc.M203281200. [DOI] [PubMed] [Google Scholar]
- Canonne-Hergaux F, Gruenheid S, Ponka P, Gros P. Cellular and subcellular localization of the Nramp2 iron transporter in the intestinal brush border and regulation by dietary iron. Blood. 1999;93:4406–4417. [PubMed] [Google Scholar]
- Chen NH, Reith ME, Quick MW. Synaptic uptake and beyond: the sodium- and chloride-dependent neurotransmitter transporter family SLC6. Pflügers Archiv. 2004;447:519–31. doi: 10.1007/s00424-003-1064-5. [DOI] [PubMed] [Google Scholar]
- Chen Z, Fei YJ, Anderson CMH, Wake KA, Miyauchi S, Huang W, Thwaites DT, Ganapathy V. Structure, function and immunolocalization of a proton-coupled amino acid transporter (hPAT1) in the human intestinal cell line Caco-2. J Physiol. 2003a;546:349–361. doi: 10.1113/jphysiol.2002.026500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Kennedy DJ, Wake KA, Zhuang L, Ganapathy V, Thwaites DT. Structure, tissue expression pattern, and function of the amino acid transporter rat PAT2. Biochem Biophys Res Commun. 2003b;304:747–754. doi: 10.1016/s0006-291x(03)00648-x. [DOI] [PubMed] [Google Scholar]
- Christensen HN. Naming plan for membrane transport systems for amino acids. Neurochem Res. 1984;9:1757–1758. doi: 10.1007/BF00968086. [DOI] [PubMed] [Google Scholar]
- Coady MJ, Chang MH, Charron FM, Plata C, Wallendorff B, Sah JF, Markowitz SD, Romero MF, Lapointe JY. The human tumour suppressor gene SLC5A8 expresses a Na+-monocarboxylate cotransporter. J Physiol. 2004;557:719–731. doi: 10.1113/jphysiol.2004.063859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane RK. Hypothesis for mechanism of intestinal active transport of sugars. Fed Proc. 1962;21:891–895. [PubMed] [Google Scholar]
- Crane RK, Miller D, Bihler I. The restrictions on possible mechanism of intestinal active transport of sugars. In: Kleinzeller A, Kotyk A, editors. Membrane Transport and Metabolism. Academic Press; London: 1961. pp. 439–449. [Google Scholar]
- Csáky TZ, Thale M. Effect of ionic environment on intestinal sugar transport. J Physiol. 1960;151:59–65. [PMC free article] [PubMed] [Google Scholar]
- Curran PF. Na, Cl, and water transport by rat ileum in vitro. J Gen Physiol. 1960;43:1137–1148. doi: 10.1085/jgp.43.6.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel H, Fett C, Kratz A. Demonstration and modification of intervillous pH profiles in rat small intestine in vitro. Am J Physiol. 1989;257:G489–G495. doi: 10.1152/ajpgi.1989.257.4.G489. [DOI] [PubMed] [Google Scholar]
- Daniel H, Kottra G. The proton oligopeptide cotransporter family SLC15 in physiology and pharmacology. Pflügers Archiv. 2004;447:610–618. doi: 10.1007/s00424-003-1101-4. [DOI] [PubMed] [Google Scholar]
- Daniel H, Neugebauer B, Kratz A, Rehner G. Localization of acid microclimate along intestinal villi of rat jejunum. Am J Physiol. 1985;248:G293–G298. doi: 10.1152/ajpgi.1985.248.3.G293. [DOI] [PubMed] [Google Scholar]
- Daniel H, Rehner G. Effect of metabolizable sugars on the mucosal surface pH of rat intestine. J Nutr. 1986;116:768–777. doi: 10.1093/jn/116.5.768. [DOI] [PubMed] [Google Scholar]
- Dantzig AH, Bergin L. Uptake of the cephalosporin, cephalexin, by a dipeptide transport carrier in the human intestinal cell line, Caco-2. Biochim Biophys Acta. 1990;1027:211–217. doi: 10.1016/0005-2736(90)90309-c. [DOI] [PubMed] [Google Scholar]
- Delie F, Rubas W. A human colonic cell line sharing similarities with enterocytes as a model to examine oral absorption: advantages and limitations of the Caco-2 model. Crit Rev Ther Drug Carrier Syst. 1997;14:221–286. [PubMed] [Google Scholar]
- Dixon KH, Lanpher BC, Chiu J, Kelley K, Cowan KH. A novel cDNA restores reduced folate carrier activity and methotrexate sensitivity to transport deficient cells. J Biol Chem. 1994;269:17–20. [PubMed] [Google Scholar]
- Dresser GK, Bailey DG, Leake BF, Schwarz UI, Dawson PA, Freeman DJ, Kim RB. Fruit juices inhibit organic anion transporting polypeptide-mediated drug uptake to decrease the oral availability of fexofenadine. Clin Pharmacol Ther. 2002;71:11–20. doi: 10.1067/mcp.2002.121152. [DOI] [PubMed] [Google Scholar]
- Englund G, Rorsman F, Ronnblom A, Karlbom U, Lazorova L, Grasjo J, Kindmark A, Artursson P. Regional levels of drug transporters along the human intestinal tract: co-expression of ABC and SLC transporters and comparison with Caco-2 cells. Eur J Pharm Sci. 2006;29:269–277. doi: 10.1016/j.ejps.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Fei YJ, Kanai Y, Nussberger S, Ganapathy V, Leibach FH, Romero MF, Singh SK, Boron WF, Hediger MA. Expression cloning of a mammalian proton-coupled oligopeptide transporter. Nature. 1994;368:563–566. doi: 10.1038/368563a0. [DOI] [PubMed] [Google Scholar]
- Fleming MD, Romano MA, Su MA, Garrick LM, Garrick MD, Andrews NC. Nramp2 is mutated in the anemic Belgrade (b) rat: evidence of a role for Nramp2 in endosomal iron transport. Proc Natl Acad Sci USA. 1998;95:1148–1153. doi: 10.1073/pnas.95.3.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming MD, Trenor CC, Su MA, Foernzler D, Beier DR, Dietrich WF, Andrews NC. Microcytic anaemia mice have a mutation in Nramp2, a candidate iron transporter gene. Nat Genet. 1997;16:383–386. doi: 10.1038/ng0897-383. [DOI] [PubMed] [Google Scholar]
- Foltz M, Boll M, Raschka L, Kottra G, Daniel H. A novel bifunctionality: PAT1 and PAT2 mediate electrogenic proton/amino acid and electroneutral proton/fatty acid symport. FASEB J. 2004a;18:1758–1760. doi: 10.1096/fj.03-1387fje. [DOI] [PubMed] [Google Scholar]
- Foltz M, Oechsler C, Boll M, Kottra G, Daniel H. Substrate specificity and transport mode of the proton-dependent amino acid transporter mPAT2. Eur J Biochem. 2004b;271:3340–3347. doi: 10.1111/j.1432-1033.2004.04268.x. [DOI] [PubMed] [Google Scholar]
- Freeman TC, Bentsen BS, Thwaites DT, Simmons NL. H+/di-tripeptide transporter (PepT1) expression in the rabbit intestine. Pflügers Archiv. 1995;430:394–400. doi: 10.1007/BF00373915. [DOI] [PubMed] [Google Scholar]
- Freeman TC, Collins AJ, Heavens RP, Tivey DR. Genetic regulation of enterocyte function: a quantitative in situ hybridisation study of lactase-phlorizin hydrolase and Na+-glucose cotransporter mRNAs in rabbit small intestine. Pflügers Archiv. 1993;422:570–576. doi: 10.1007/BF00374004. [DOI] [PubMed] [Google Scholar]
- Ganapathy V, Burckhardt G, Leibach FH. Characteristics of glycylsarcosine transport in rabbit intestinal brush-border membrane vesicles. J Biol Chem. 1984;259:8954–8959. [PubMed] [Google Scholar]
- Ganapathy V, Gopal E, Miyauchi S, Prasad PD. Biological functions of SLC5A8, a candidate tumour suppressor. Biochem Soc Trans. 2005;33:237–240. doi: 10.1042/BST0330237. [DOI] [PubMed] [Google Scholar]
- Ganapathy V, Gupta N, Martindale RG. Protein digestion and absorption. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. Elsevier; San Diego: 2006. pp. 1667–1692. [Google Scholar]
- Ganapathy V, Leibach FH. Role of pH gradient and membrane potential in dipeptide transport in intestinal and renal brush-border membrane vesicles from the rabbit. J Biol Chem. 1983;258:14189–14192. [PubMed] [Google Scholar]
- Ganapathy V, Leibach FH. Is intestinal peptide transport energized by a proton gradient? Am J Physiol. 1985;249:G153–G160. doi: 10.1152/ajpgi.1985.249.2.G153. [DOI] [PubMed] [Google Scholar]
- Ganapathy V, Smith SB, Prasad PD. SLC19: the folate/thiamine transporter family. Pflügers Archiv. 2004;447:641–646. doi: 10.1007/s00424-003-1068-1. [DOI] [PubMed] [Google Scholar]
- Garcia CK, Brown MS, Pathak RK, Goldstein JL. cDNA cloning of MCT2, a second monocarboxylate transporter expressed in different cells than MCT1. J Biol Chem. 1995;270:1843–1849. doi: 10.1074/jbc.270.4.1843. [DOI] [PubMed] [Google Scholar]
- Garcia CK, Goldstein JL, Pathak RK, Anderson RG, Brown MS. Molecular characterization of a membrane transporter for lactate, pyruvate, and other monocarboxylates: implications for the Cori cycle. Cell. 1994;76:865–873. doi: 10.1016/0092-8674(94)90361-1. [DOI] [PubMed] [Google Scholar]
- Gill RK, Saksena S, Alrefai WA, Sarwar Z, Goldstein JL, Carroll RE, Ramaswamy K, Dudeja PK. Expression and membrane localization of MCT isoforms along the length of the human intestine. Am J Physiol. 2005;289:C846–C852. doi: 10.1152/ajpcell.00112.2005. [DOI] [PubMed] [Google Scholar]
- Goberdhan DCI, Meredith D, Boyd CAR, Wilson C. PAT-related amino acid transporters regulate growth via a novel mechanism that does not require bulk transport of amino acids. Development. 2005;132:2365–2375. doi: 10.1242/dev.01821. [DOI] [PubMed] [Google Scholar]
- Gopal E, Fei YJ, Sugawara M, Miyauchi S, Zhuang L, Martin P, Smith SB, Prasad PD, Ganapathy V. Expression of slc5a8 in kidney and its role in Na+-coupled transport of lactate. J Biol Chem. 2004;279:44522–44532. doi: 10.1074/jbc.M405365200. [DOI] [PubMed] [Google Scholar]
- Gray JH, Owen RP, Giacomini KM. The concentrative nucleoside transporter family, SLC28. Pflügers Archiv. 2004;447:728–734. doi: 10.1007/s00424-003-1107-y. [DOI] [PubMed] [Google Scholar]
- Grube M, Kock K, Oswald S, Draber K, Meissner K, Eckel L, Bohm M, Felix SB, Vogelgesang S, Jedlitschky G, Siegmund W, Warzok R, Kroemer HK. Organic anion transporting polypeptide 2B1 is a high-affinity transporter for atorvastatin and is expressed in the human heart. Clin Pharmacol Ther. 2006;80:607–620. doi: 10.1016/j.clpt.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Gunshin H, Fujiwara Y, Custodio AO, Direnzo C, Robine S, Andrews NC. Slc11a2 is required for intestinal iron absorption and erythropoiesis but dispensable in placenta and liver. J Clin Invest. 2005;115:1258–1266. doi: 10.1172/JCI24356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollan JL, Hediger MA. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997;388:482–488. doi: 10.1038/41343. [DOI] [PubMed] [Google Scholar]
- Hadjiagapiou C, Schmidt L, Dudeja PK, Layden TJ, Ramaswamy K. Mechanism(s) of butyrate transport in Caco-2 cells: role of monocarboxylate transporter 1. Am J Physiol. 2000;279:G775–G780. doi: 10.1152/ajpgi.2000.279.4.G775. [DOI] [PubMed] [Google Scholar]
- Hagenbuch B, Dawson P. The sodium bile salt cotransport family SLC10. Pflügers Archiv. 2004;447:566–70. doi: 10.1007/s00424-003-1130-z. [DOI] [PubMed] [Google Scholar]
- Hagenbuch B, Meier PJ. The superfamily of organic anion transporting polypeptides. Biochim Biophys Acta. 2003;1609:1–18. doi: 10.1016/s0005-2736(02)00633-8. [DOI] [PubMed] [Google Scholar]
- Hagenbuch B, Meier PJ. Organic anion transporting polypeptides of the OATP/ SLC21 family: phylogenetic classification as OATP/ SLCO superfamily, new nomenclature and molecular/functional properties. Pflügers Archiv. 2004;447:653–665. doi: 10.1007/s00424-003-1168-y. [DOI] [PubMed] [Google Scholar]
- Halestrap AP, Meredith D. The SLC16 gene family-from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflügers Archiv. 2004;447:619–628. doi: 10.1007/s00424-003-1067-2. [DOI] [PubMed] [Google Scholar]
- Harig JM, Rajendran VM, Barry JA, Ramaswamy K. Transport characteristics of L-glutamate in human jejunal brush-border membrane vesicles. Biochim Biophys Acta. 1987;903:358–364. doi: 10.1016/0005-2736(87)90226-4. [DOI] [PubMed] [Google Scholar]
- Hatanaka T, Haramura M, Fei YJ, Miyauchi S, Bridges CC, Ganapathy PS, Smith SB, Ganapathy V, Ganapathy ME. Transport of amino acid-based prodrugs by the Na+- and Cl+-coupled amino acid transporter ATB0,+ and expression of the transporter in tissues amenable for drug delivery. J Pharmacol Exp Ther. 2004;308:1138–1147. doi: 10.1124/jpet.103.057109. [DOI] [PubMed] [Google Scholar]
- Hediger MA. Structure, function and evolution of solute transporters in prokaryotes and eukaryotes. J Exp Biol. 1994;196:15–49. doi: 10.1242/jeb.196.1.15. [DOI] [PubMed] [Google Scholar]
- Hediger MA, Coady MJ, Ikeda TS, Wright EM. Expression cloning and cDNA sequencing of the Na+/glucose co-transporter. Nature. 1987;330:379–381. doi: 10.1038/330379a0. [DOI] [PubMed] [Google Scholar]
- Henderson PJF. Proton-linked sugar transport systems in bacteria. J Bioenerg Biomembr. 1990;22:525–569. doi: 10.1007/BF00762961. [DOI] [PubMed] [Google Scholar]
- Hirayama BA, Loo DD, Wright EM. Protons drive sugar transport through the Na+/glucose cotransporter (SGLT1) J Biol Chem. 1994;269:21407–21410. [PubMed] [Google Scholar]
- Hogben CAM, Tocco DJ, Brodie BB, Schanker LS. On the mechanism of intestinal absorption of drugs. J Pharmacol Exp Ther. 1959;125:275–282. [PubMed] [Google Scholar]
- Hoogerwerf WA, Tsao SC, Devuyst O, Levine SA, Yun CH, Yip JW, Cohen ME, Wilson PD, Lazenby AJ, Tse CM, Donowitz M. NHE2 and NHE3 are human and rabbit intestinal brush-border proteins. Am J Physiol. 1996;270:G29–G41. doi: 10.1152/ajpgi.1996.270.1.G29. [DOI] [PubMed] [Google Scholar]
- Hopfer U, Nelson K, Perrotto J, Isselbacher KJ. Glucose transport in isolated brush border membrane from rat small intestine. J Biol Chem. 1973;248:25–32. [PubMed] [Google Scholar]
- Hoshi T, Takuwa N, Abe M, Tajima A. Hydrogen ion-coupled transport of D-glucose by phlorizin-sensitive sugar carrier in intestinal brush-border membranes. Biochim Biophys Acta. 1986;861:483–488. doi: 10.1016/0005-2736(86)90458-x. [DOI] [PubMed] [Google Scholar]
- Hwang ES, Hirayama BA, Wright EM. Distribution of the SGLT1 Na+/glucose cotransporter and mRNA along the crypt-villus axis of rabbit small intestine. Biochem Biophys Res Commun. 1991;181:1208–1217. doi: 10.1016/0006-291x(91)92067-t. [DOI] [PubMed] [Google Scholar]
- Iñigo C, Barber A, Lostao MP. Na+ and pH dependence of proline and β-alanine absorption in rat small intestine. Acta Physiol. 2006;186:271–278. doi: 10.1111/j.1748-1716.2006.01538.x. [DOI] [PubMed] [Google Scholar]
- Inui K, Yamamoto M, Saito H. Transepithelial transport of oral cephalosporins by monolayers of intestinal epithelial cell line Caco-2: specific transport systems in apical and basolateral membranes. J Pharmacol Exp Ther. 1992;261:195–201. [PubMed] [Google Scholar]
- Iwanaga T, Goto M, Watanabe M. Cellular distribution of glutamate transporters in the gastrointestinal tract of mice: an immunohistochemical and in situ hybridization approach. Biomed Res. 2005;26:271–278. doi: 10.2220/biomedres.26.271. [DOI] [PubMed] [Google Scholar]
- Iwanaga T, Takebe K, Kato I, Karaki S, Kuwahara A. Cellular expression of monocarboxylate transporters (MCT) in the digestive tract of the mouse, rat, and humans, with special reference to slc5a8. Biomed Res. 2006;27:243–254. doi: 10.2220/biomedres.27.243. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Hediger MA. Primary structure and functional characterization of a high-affinity glutamate transporter. Nature. 1992;360:467–471. doi: 10.1038/360467a0. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Hediger MA. The glutamate/neutral amino acid transporter family SLC1: molecular, physiological and pharmacological aspects. Pflügers Archiv. 2004;447:469–479. doi: 10.1007/s00424-003-1146-4. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Stelzner M, Nussberger S, Khawaja S, Hebert SC, Smith CP, Hediger MA. The neuronal and epithelial human high affinity glutamate transporter. Insights into structure and mechanism of transport. J Biol Chem. 1994;269:20599–20606. [PubMed] [Google Scholar]
- Kennedy DJ, Gatfield KM, Winpenny JP, Ganapathy V, Thwaites DT. Substrate specificity and functional characterisation of the H+/amino acid transporter rat PAT2 (Slc36a2) Br J Pharmacol. 2005a;144:28–41. doi: 10.1038/sj.bjp.0706029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DJ, Leibach FH, Ganapathy V, Thwaites DT. Optimal absorptive transport of the dipeptide glycylsarcosine is dependent on functional Na+/H+ exchange activity. Pflügers Archiv. 2002;445:139–146. doi: 10.1007/s00424-002-0910-1. [DOI] [PubMed] [Google Scholar]
- Kennedy DJ, Raldua D, Thwaites DT. Dual modes of 5-(N-ethyl-N-isopropyl)amiloride modulation of apical dipeptide uptake in the human small intestinal epithelial cell line Caco-2. Cell Mol Life Sci. 2005b;62:1621–1631. doi: 10.1007/s00018-005-5078-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoursandi S, Scharlau D, Herter P, Kuhnen C, Martin D, Kinne RK, Kipp H. Different modes of sodium-D-glucose cotransporter-mediated D-glucose uptake regulation in Caco-2 cells. Am J Physiol. 2004;287:C1041–C1047. doi: 10.1152/ajpcell.00197.2004. [DOI] [PubMed] [Google Scholar]
- Kim CM, Goldstein JL, Brown MS. cDNA cloning of MEV, a mutant protein that facilitates cellular uptake of mevalonate, and identification of the point mutation responsible for its gain of function. J Biol Chem. 1992;267:23113–23121. [PubMed] [Google Scholar]
- Klomp AE, Tops BB, Van Denberg IE, Berger R, Klomp LW. Biochemical characterization and subcellular localization of human copper transporter 1 (hCTR1) Biochem J. 2002;364:497–505. doi: 10.1042/BJ20011803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneuer C, Honscha W. The H+-dependent reduced folate carrier 1 of humans and the sodium-dependent methotrexate carrier-1 of the rat are orthologs. FEBS Lett. 2004;566:83–86. doi: 10.1016/j.febslet.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Kobayashi D, Nozawa T, Imai K, Nezu J, Tsuji A, Tamai I. Involvement of human organic anion transporting polypeptide OATP-B (SLC21A9) in pH-dependent transport across intestinal apical membrane. J Pharmacol Exp Ther. 2003;306:703–708. doi: 10.1124/jpet.103.051300. [DOI] [PubMed] [Google Scholar]
- Kowalczuk S, Bröer A, Munzinger M, Tietze N, Klingel K, Bröer S. Molecular cloning of the mouse IMINO system: an Na+- and Cl−-dependent proline transporter. Biochem J. 2005;386:417–22. doi: 10.1042/BJ20050100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullak-Ublick GA, Ismair MG, Stieger B, Landmann L, Huber R, Pizzagalli F, Fattinger K, Meier PJ, Hagenbuch B. Organic anion-transporting polypeptide B (OATP-B) and its functional comparison with three other OATPs of human liver. Gastroenterology. 2001;120:525–533. doi: 10.1053/gast.2001.21176. [DOI] [PubMed] [Google Scholar]
- Kumar CK, Nguyen TT, Gonzales FB, Said HM. Comparison of intestinal folate carrier clone in IEC-6 cells and in Xenopus oocytes. Am J Physiol. 1998;274:C289–C294. doi: 10.1152/ajpcell.1998.274.1.C289. [DOI] [PubMed] [Google Scholar]
- Kuo YM, Gybina AA, Pyatskowit JW, Gitschier J, Prohaska JR. Copper transport protein (Ctr1) levels in mice are tissue specific and dependent on copper status. J Nutr. 2006;136:21–26. doi: 10.1093/jn/136.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Pena MM, Nose Y, Thiele DJ. Biochemical characterization of the human copper transporter Ctr1. J Biol Chem. 2002;277:4380–4387. doi: 10.1074/jbc.M104728200. [DOI] [PubMed] [Google Scholar]
- Liang R, Fei YJ, Prasad PD, Ramamoorthy S, Han H, Yang-Feng TL, Hediger MA, Ganapathy V, Leibach FH. Human intestinal H+/peptide cotransporter. Cloning, functional expression, and chromosomal localization. J Biol Chem. 1995;270:6456–6463. doi: 10.1074/jbc.270.12.6456. [DOI] [PubMed] [Google Scholar]
- Lucas ML, Lei FH, Blair JA. The influence of buffer pH, glucose and sodium ion concentration on the acid microclimate in rat proximal jejunum in vitro. Pflügers Archiv. 1980;385:137–142. doi: 10.1007/BF00588693. [DOI] [PubMed] [Google Scholar]
- Lucas ML, Schneider W, Haberich FJ, Blair JA. Direct measurement by pH-microelectrode of the pH microclimate in rat proximal jejunum. Proc R Soc Lond B. 1975;192:39–48. doi: 10.1098/rspb.1975.0150. [DOI] [PubMed] [Google Scholar]
- Mackenzie B, Hediger MA. SLC11 family of H+-coupled metal-ion transporters NRAMP1 and DMT1. Pflügers Archiv. 2004;447:571–579. doi: 10.1007/s00424-003-1141-9. [DOI] [PubMed] [Google Scholar]
- Mackenzie B, Garrick MD. Iron Imports. II. Iron uptake at the apical membrane in the intestine. Am J Physiol. 2005;289:G981–G986. doi: 10.1152/ajpgi.00363.2005. [DOI] [PubMed] [Google Scholar]
- Maenz DD, Chenu C, Breton S, Berteloot A. pH-dependent heterogeneity of acidic amino acid transport in rabbit jejunal brush border membrane vesicles. J Biol Chem. 1992;267:1510–1516. [PubMed] [Google Scholar]
- Markovich D, Murer H. The SLC13 gene family of sodium sulphate/carboxylate cotransporters. Pflügers Archiv. 2004;447:594–602. doi: 10.1007/s00424-003-1128-6. [DOI] [PubMed] [Google Scholar]
- Martel F, Monteiro R, Lemos C. Uptake of serotonin at the apical and basolateral membranes of human intestinal epithelial (Caco-2) cells occurs through the neuronal serotonin transporter (SERT) J Pharmacol Exp Ther. 2003;306:355–62. doi: 10.1124/jpet.103.049668. [DOI] [PubMed] [Google Scholar]
- Martin-Venegas R, Rodriguez-Lagunas MJ, Geraert PA, Ferrer R. Monocarboxylate transporter 1 mediates dl-2-Hydroxy-(4-methylthio)butanoic acid transport across the apical membrane of Caco-2 cell monolayers. J Nutr. 2007;137:49–54. doi: 10.1093/jn/137.1.49. [DOI] [PubMed] [Google Scholar]
- Mason JB, Shoda R, Haskell M, Selhub J, Rosenberg IH. Carrier affinity as a mechanism for the pH-dependence of folate transport in the small intestine. Biochim Biophys Acta. 1990;1024:331–335. doi: 10.1016/0005-2736(90)90362-r. [DOI] [PubMed] [Google Scholar]
- McEwan GTA, Daniel H, Fett C, Burgess MN, Lucas ML. The effect of Escherichia coli STa enterotoxin and other secretagogues on mucosal surface pH of rat small intestine in vivo. Proc R Soc Lond B. 1988;234:219–237. doi: 10.1098/rspb.1988.0045. [DOI] [PubMed] [Google Scholar]
- McKie AT, Kusel M, McEwan GTA, Lucas ML. The effect of heat stable Escherichia coli enterotoxin, theophylline and forskolin on cyclic nucleotide levels and mucosal surface (acid microclimate) pH in rat proximal jejunum in vivo. Biochim Biophys Acta. 1988;971:325–331. doi: 10.1016/0167-4889(88)90148-6. [DOI] [PubMed] [Google Scholar]
- Metzner L, Kalbitz J, Brandsch M. Transport of pharmacologically active proline derivatives by the human proton-coupled amino acid transporter hPAT1. J Pharmacol Exp Ther. 2004;309:28–35. doi: 10.1124/jpet.103.059014. [DOI] [PubMed] [Google Scholar]
- Metzner L, Kottra G, Neubert K, Daniel H, Brandsch M. Serotonin, l-tryptophan, and tryptamine are effective inhibitors of the amino acid transport system PAT1. FASEB J. 2005;19:1468–1473. doi: 10.1096/fj.05-3683com. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961;191:144–8. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Molecule, group and electron translocation through natural membranes. Biochem Soc Symp. 1963;22:142–169. [Google Scholar]
- Mitchell P. Performance and conservation of osmotic work by proton-coupled solute porter systems. Bioenergetics. 1973;4:63–91. doi: 10.1007/BF01516051. [DOI] [PubMed] [Google Scholar]
- Miyauchi S, Abbot EL, Zhuang L, Subramanian R, Ganapathy V, Thwaites DT. Isolation and function of the amino acid transporter PAT1 (slc36a1) from rabbit and discrimination between transport via PAT1 and system IMINO in renal brush-border membrane vesicles. Mol Membr Biol. 2005;22:549–559. doi: 10.1080/09687860500421779. [DOI] [PubMed] [Google Scholar]
- Murer H, Forster I, Biber J. The sodium phosphate cotransporter family SLC34. Pflügers Archiv. 2004;447:763–767. doi: 10.1007/s00424-003-1072-5. [DOI] [PubMed] [Google Scholar]
- Murer H, Hopfer U, Kinne R. Sodium/proton antiport in brush-border membrane vesicles isolated from rat small intestine and kidney. Biochem J. 1976;154:597–604. [PMC free article] [PubMed] [Google Scholar]
- Nguyen TT, Dyer DL, Dunning DD, Rubin SA, Grant KE, Said HM. Human intestinal folate transport: cloning, expression, and distribution of complementary RNA. Gastroenterology. 1997;112:783–791. doi: 10.1053/gast.1997.v112.pm9041240. [DOI] [PubMed] [Google Scholar]
- Nose Y, Kim BE, Thiele DJ. Ctr1 drives intestinal copper absorption and is essential for growth, iron metabolism, and neonatal cardiac function. Cell Metab. 2006;4:235–244. doi: 10.1016/j.cmet.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Nozawa T, Imai K, Nezu J, Tsuji A, Tamai I. Functional characterization of pH-sensitive organic anion transporting polypeptide OATP-B in human. J Pharmacol Exp Ther. 2004;308:438–445. doi: 10.1124/jpet.103.060194. [DOI] [PubMed] [Google Scholar]
- Nozawa T, Nakajima M, Tamai I, Noda K, Nezu JI, Sai Y, Tsuji A, Yokoi T. Genetic polymorphisms of human organic anion transporters OATP-C (SLC21A6) and OATP-B (SLC21A9): allele frequencies in the Japanese population and functional analysis. J Pharmacol Exp Ther. 2002;302:804–813. doi: 10.1124/jpet.302.2.804. [DOI] [PubMed] [Google Scholar]
- Orlowski J. Heterologous expression and functional properties of amiloride high affinity (NHE-1) and low affinity (NHE-3) isoforms of the rat Na/H exchanger. J Biol Chem. 1993;268:16369–16377. [PubMed] [Google Scholar]
- Petris MJ. The SLC31 (Ctr) copper transporter family. Pflügers Archiv. 2004;447:752–755. doi: 10.1007/s00424-003-1092-1. [DOI] [PubMed] [Google Scholar]
- Prasad PD, Ramamoorthy S, Leibach FH, Ganapathy V. Molecular cloning of the human placental folate transporter. Biochem Biophys Res Commun. 1995;206:681–687. doi: 10.1006/bbrc.1995.1096. [DOI] [PubMed] [Google Scholar]
- Price NT, Jackson VN, Halestrap AP. Cloning and sequencing of four new mammalian monocarboxylate transporter (MCT) homologues confirms the existence of a transporter family with an ancient past. Biochem J. 1998;329:321–328. doi: 10.1042/bj3290321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu A, Jansen M, Sakaris A, Min SH, Chattopadhyay S, Tsai E, Sandoval C, Zhao R, Akabas MH, Goldman ID. Identification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption. Cell. 2006;127:917–928. doi: 10.1016/j.cell.2006.09.041. [DOI] [PubMed] [Google Scholar]
- Quick M, Loo DD, Wright EM. Neutralization of a conserved amino acid residue in the human Na+/glucose transporter (hSGLT1) generates a glucose-gated H+ channel. J Biol Chem. 2001;276:1728–1734. doi: 10.1074/jbc.M005521200. [DOI] [PubMed] [Google Scholar]
- Ricklis E, Quastel JH. Effects of cations on sugar absorption by isolated surviving guinea pig intestine. Can J Biochem Physiol. 1958;36:347–362. [PubMed] [Google Scholar]
- Riggs TR, Walker LM, Christensen HN. Potassium migration and amino acid transport. J Biol Chem. 1958;233:1479–1484. [PubMed] [Google Scholar]
- Ritzhaupt A, Wood IS, Ellis A, Hosie KB, Shirazi-Beechey SP. Identification and characterization of a monocarboxylate transporter (MCT1) in pig and human colon: its potential to transport L-lactate as well as butyrate. J Physiol. 1998;513:719–732. doi: 10.1111/j.1469-7793.1998.719ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rome S, Barbot L, Windsor E, Kapel N, Tricottet V, Huneau JF, Reynes M, Gobert JG, Tome D. The regionalization of PepT1, NBAT and EAAC1 transporters in the small intestine of rats are unchanged from birth to adulthood. J Nutr. 2002;132:1009–1011. doi: 10.1093/jn/132.5.1009. [DOI] [PubMed] [Google Scholar]
- Rubio-Aliaga I, Boll M, Vogt-Weisenhorn DM, Foltz M, Kottra G, Daniel H. The proton/amino acid cotransporter PAT2 is expressed in neurons with a different subcellular localization than its paralog PAT1. J Biol Chem. 2004;279:2754–2760. doi: 10.1074/jbc.M305556200. [DOI] [PubMed] [Google Scholar]
- Rubio-Aliaga I, Daniel H. Mammalian peptide transporters as targets for drug delivery. Trends Pharmacol Sci. 2002;23:434–440. doi: 10.1016/s0165-6147(02)02072-2. [DOI] [PubMed] [Google Scholar]
- Sagné C, Agulhon C, Ravassard P, Darmon M, Hamon M, El Mestikawy S, Gasnier B, Giros B. Identification and characterization of a lysosomal transporter for small neutral amino acids. Proc Natl Acad Sci USA. 2001;98:7206–7211. doi: 10.1073/pnas.121183498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sai Y, Kaneko Y, Ito S, Mitsuoka K, Kato Y, Tamai I, Artursson P, Tsuji A. Predominant contribution of organic anion transporting polypeptide OATP-B (OATP2B1) to apical uptake of estrone-3-sulfate by human intestinal Caco-2 cells. Drug Metab Dispos. 2006;34:1423–1431. doi: 10.1124/dmd.106.009530. [DOI] [PubMed] [Google Scholar]
- Said HM, Chatterjee N, Haq RU, Subramanian VS, Ortiz A, Matherly LH, Sirotnak FM, Halsted C, Rubin SA. Adaptive regulation of intestinal folate uptake: effect of dietary folate deficiency. Am J Physiol. 2000;279:C1889–C1895. doi: 10.1152/ajpcell.2000.279.6.C1889. [DOI] [PubMed] [Google Scholar]
- Said HM, Ghishan FK, Redha R. Folate transport by human intestinal brush-border membrane vesicles. Am J Physiol. 1987;252:G229–G236. doi: 10.1152/ajpgi.1987.252.2.G229. [DOI] [PubMed] [Google Scholar]
- Said HM, Nguyen TT, Dyer DL, Cowan KH, Rubin SA. Intestinal folate transport: identification of a cDNA involved in folate transport and the functional expression and distribution of its mRNA. Biochim Biophys Acta. 1996;1281:164–172. doi: 10.1016/0005-2736(96)00005-3. [DOI] [PubMed] [Google Scholar]
- Satoh H, Yamashita F, Tsujimoto M, Murakami H, Koyabu N, Ohtani H, Sawada Y. Citrus juices inhibit the function of human organic anion-transporting polypeptide OATP-B. Drug Metab Dispos. 2005;33:518–523. doi: 10.1124/dmd.104.002337. [DOI] [PubMed] [Google Scholar]
- Schanker LS, Tocco DJ, Brodie BB, Hogben CAM. Absorption of drugs from the rat small intestine. J Pharmacol Exp Ther. 1958;123:81–88. [PubMed] [Google Scholar]
- Schron CM, Washington C, Blitzer BL. The transmembrane pH gradient drives uphill folate transport in rabbit jejunum. Direct evidence for folate/hydroxyl exchange in brush border membrane vesicles. J Clin Invest. 1985;76:2030–2033. doi: 10.1172/JCI112205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz SG, Zalusky R. Ion transport in isolated rabbit ileum. II. The interaction between active sodium and active sugar transport. J Gen Physiol. 1964;47:1043–1059. doi: 10.1085/jgp.47.6.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seithel A, Karlsson J, Hilgendorf C, Bjorquist A, Ungell AL. Variability in mRNA expression of ABC- and SLC-transporters in human intestinal cells: comparison between human segments and Caco-2 cells. Eur J Pharm Sci. 2006;28:291–299. doi: 10.1016/j.ejps.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Selhub J, Rosenberg IH. Folate transport in isolated brush border membrane vesicles from rat intestine. J Biol Chem. 1981;256:4489–4493. [PubMed] [Google Scholar]
- Shayeghi M, Latunde-Dada GO, Oakhill JS, Laftah AH, Takeuchi K, Halliday N, Khan Y, Warley A, McCann FE, Hider RC, Frazer DM, Anderson GJ, Vulpe CD, Simpson RJ, McKie AT. Identification of an intestinal heme transporter. Cell. 2005;122:789–801. doi: 10.1016/j.cell.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Shiau YF, Fernandez P, Jackson MJ, McMonagle S. Mechanisms maintaining a low-pH microclimate in the intestine. Am J Physiol. 1985;248:G608–G617. doi: 10.1152/ajpgi.1985.248.6.G608. [DOI] [PubMed] [Google Scholar]
- Shimada T. Factors effecting the microclimate pH in rat jejunum. J Physiol. 1987;392:113–127. doi: 10.1113/jphysiol.1987.sp016772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T, Hoshi T. Na+-dependent elevation of the acidic cell surface pH (microclimate pH) of rat jejunal villus cells induced by cyclic nucleotides and phorbol ester: possible mediators of the regulation of the Na+/H+ antiporter. Biochim Biophys Acta. 1988;937:328–334. doi: 10.1016/0005-2736(88)90255-6. [DOI] [PubMed] [Google Scholar]
- Simanjuntak MT, Tamai I, Terasaki T, Tsuji A. Carrier-mediated uptake of nicotinic acid by rat intestinal brush-border membrane vesicles and relation to monocarboxylic acid transport. J Pharmacobiodyn. 1990;13:301–309. doi: 10.1248/bpb1978.13.301. [DOI] [PubMed] [Google Scholar]
- Skulachev VP. Chemiosmotic concept of the membrane bioenergetics: what is already clear and what is still waiting for elucidation? J Bioenerg Biomembr. 1994;26:589–598. doi: 10.1007/BF00831533. [DOI] [PubMed] [Google Scholar]
- Smith MW, Turvey A, Freeman TC. Appearance of phloridzin-sensitive glucose transport is not controlled at mRNA level in rabbit jejunal enterocytes. Exp Physiol. 1992;77:525–528. doi: 10.1113/expphysiol.1992.sp003616. [DOI] [PubMed] [Google Scholar]
- Srinivas SR, Gopal E, Zhuang L, Itagaki S, Martin PM, Fei YJ, Ganapathy V, Prasad PD. Cloning and functional identification of slc5a12 as a sodium-coupled low-affinity transporter for monocarboxylates (SMCT2) Biochem J. 2005;392:655–664. doi: 10.1042/BJ20050927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein J, Zores M, Schroder O. Short-chain fatty acid (SCFA) uptake into Caco-2 cells by a pH-dependent and carrier mediated transport mechanism. Eur J Nutr. 2000;39:121–125. doi: 10.1007/s003940070028. [DOI] [PubMed] [Google Scholar]
- Su MA, Trenor CC, Fleming JC, Fleming MD, Andrews NC. The G185R mutation disrupts function of the iron transporter Nramp2. Blood. 1998;92:2157–63. [PubMed] [Google Scholar]
- Takanaga H, Mackenzie B, Hediger MA. Sodium-dependent ascorbic acid transporter family SLC23. Pflügers Archiv. 2004;447:677–682. doi: 10.1007/s00424-003-1104-1. [DOI] [PubMed] [Google Scholar]
- Takanaga H, Mackenzie B, Suzuki Y, Hediger MA. Identification of mammalian proline transporter SIT1 (SLC6A20) with characteristics of classical system imino. J Biol Chem. 2005;280:8974–8984. doi: 10.1074/jbc.M413027200. [DOI] [PubMed] [Google Scholar]
- Takanaga H, Maeda H, Yabuuchi H, Tamai I, Higashida H, Tsuji A. Nicotinic acid transport mediated by pH-dependent anion antiporter and proton cotransporter in rabbit intestinal brush-border membrane. J Pharm Pharmacol. 1996;48:1073–1077. doi: 10.1111/j.2042-7158.1996.tb05902.x. [DOI] [PubMed] [Google Scholar]
- Takanaga H, Tamai I, Inaba S, Sai Y, Higashida H, Yamamoto H, Tsuji A. cDNA cloning and functional characterization of rat intestinal monocarboxylate transporter. Biochem Biophys Res Commun. 1995;217:370–377. doi: 10.1006/bbrc.1995.2786. [DOI] [PubMed] [Google Scholar]
- Tamai I, Nezu J, Uchino H, Sai Y, Oku A, Shimane M, Tsuji A. Molecular identification and characterization of novel members of the human organic anion transporter (OATP) family. Biochem Biophys Res Commun. 2000;273:251–260. doi: 10.1006/bbrc.2000.2922. [DOI] [PubMed] [Google Scholar]
- Tamai I, Sai Y, Ono A, Kido Y, Yabuuchi H, Takanaga H, Satoh E, Ogihara T, Amano O, Izeki S, Tsuji A. Immunohistochemical and functional characterization of pH-dependent intestinal absorption of weak organic acids by the monocarboxylic acid transporter MCT1. J Pharm Pharmacol. 1999;51:1113–1121. doi: 10.1211/0022357991776804. [DOI] [PubMed] [Google Scholar]
- Tamai I, Takanaga H, Maeda H, Ogihara T, Yoneda M, Tsuji A. Proton-cotransport of pravastatin across intestinal brush-border membrane. Pharm Res. 1995a;12:1727–1732. doi: 10.1023/a:1016269806840. [DOI] [PubMed] [Google Scholar]
- Tamai I, Takanaga H, Maeda H, Sai Y, Ogihara T, Higashida H, Tsuji A. Participation of a proton-cotransporter, MCT1, in the intestinal transport of monocarboxylic acids. Biochem Biophys Res Commun. 1995b;214:482–489. doi: 10.1006/bbrc.1995.2312. [DOI] [PubMed] [Google Scholar]
- Tandy S, Williams M, Leggett A, Lopez-Jimenez M, Dedes M, Ramesh B, Srai SK, Sharp P. Nramp2 expression is associated with pH-dependent iron uptake across the apical membrane of human intestinal Caco-2 cells. J Biol Chem. 2000;275:1023–1029. doi: 10.1074/jbc.275.2.1023. [DOI] [PubMed] [Google Scholar]
- Thwaites DT, Anderson CMH. Deciphering the mechanisms of intestinal imino (and amino) acid transport: the redemption of SLC36A1. Biochim Biophys Acta. 2007;1768:179–197. doi: 10.1016/j.bbamem.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Thwaites DT, Armstrong G, Hirst BH, Simmons NL. d-Cycloserine transport in human intestinal epithelial (Caco-2) cells: mediation by a H+-coupled amino acid transporter. Br J Pharmacol. 1995a;115:761–766. doi: 10.1111/j.1476-5381.1995.tb14998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thwaites DT, Basterfield L, McCleave PMJ, Carter SM, Simmons NL. Gamma-aminobutyric acid (GABA) transport across human intestinal epithelial (Caco-2) cell monolayers. Br J Pharmacol. 2000;129:457–464. doi: 10.1038/sj.bjp.0703069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thwaites DT, Brown CDA, Hirst BH, Simmons NL. Transepithelial glycylsarcosine transport in intestinal Caco-2 cells mediated by expression of H+-coupled carriers at both apical and basal membranes. J Biol Chem. 1993a;268:7640–7642. [PubMed] [Google Scholar]
- Thwaites DT, Brown CDA, Hirst BH, Simmons NL. H+-coupled dipeptide (glycylsarcosine) transport across apical and basal borders of human intestinal Caco-2 cell monolayers display distinctive characteristics. Biochim Biophys Acta. 1993b;1151:237–245. doi: 10.1016/0005-2736(93)90108-c. [DOI] [PubMed] [Google Scholar]
- Thwaites DT, Ford D, Glanville M, Simmons NL. H+/solute-induced intracellular acidification leads to selective activation of apical Na+/H+ exchange in human intestinal epithelial cells. J Clin Invest. 1999;104:629–635. doi: 10.1172/JCI7192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thwaites DT, Hirst BH, Simmons NL. Direct assessment of dipeptide/H+ symport in intact human intestinal (Caco-2) epithelium: a novel method utilising continuous intracellular pH measurement. Biochem Biophys Res Commun. 1993c;194:432–438. doi: 10.1006/bbrc.1993.1838. [DOI] [PubMed] [Google Scholar]
- Thwaites DT, Hirst BH, Simmons NL. Substrate specificity of the di/tripeptide transporter in human intestinal epithelia (Caco-2): identification of substrates that undergo H+-coupled absorption. Br J Pharmacol. 1994a;113:1050–1056. doi: 10.1111/j.1476-5381.1994.tb17099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thwaites DT, Kennedy DJ, Raldua D, Anderson CMH, Mendoza ME, Bladen CL, Simmons NL. H+/dipeptide absorption across the human intestinal epithelium is controlled indirectly via a functional Na+/H+ exchanger. Gastroenterology. 2002;122:1322–1333. doi: 10.1053/gast.2002.32992. [DOI] [PubMed] [Google Scholar]
- Thwaites DT, McEwan GTA, Brown CDA, Hirst BH, Simmons NL. Na+-independent, H+-coupled transepithelial β-alanine absorption by human intestinal Caco-2 cell monolayers. J Biol Chem. 1993d;268:18438–18441. [PubMed] [Google Scholar]
- Thwaites DT, McEwan GTA, Brown CDA, Hirst BH, Simmons NL. l-Alanine absorption in human intestinal Caco-2 cells driven by the proton electrochemical gradient. J Membr Biol. 1994b;140:143–151. doi: 10.1007/BF00232902. [DOI] [PubMed] [Google Scholar]
- Thwaites DT, McEwan GTA, Cook MJ, Hirst BH, Simmons NL. H+-coupled (Na+-independent) proline transport in human intestinal (Caco-2) epithelial cell monolayers. FEBS Lett. 1993e;333:78–82. doi: 10.1016/0014-5793(93)80378-8. [DOI] [PubMed] [Google Scholar]
- Thwaites DT, McEwan GTA, Hirst BH, Simmons NL. Transepithelial dipeptide (glycylsarcosine) transport across epithelial monolayers of human Caco-2 cells is rheogenic. Pflügers Archiv. 1993f;425:178–180. doi: 10.1007/BF00374520. [DOI] [PubMed] [Google Scholar]
- Thwaites DT, McEwan GTA, Hirst BH, Simmons NL. H+-coupled α-methylaminoisobutyric acid transport in human intestinal Caco-2 cells. Biochim Biophys Acta. 1995b;1234:111–118. doi: 10.1016/0005-2736(94)00268-t. [DOI] [PubMed] [Google Scholar]
- Thwaites DT, McEwan GTA, Simmons NL. The role of the proton electrochemical gradient in the transepithelial absorption of amino acids by human intestinal Caco-2 cell monolayers. J Membr Biol. 1995c;145:245–256. doi: 10.1007/BF00232716. [DOI] [PubMed] [Google Scholar]
- Thwaites DT, Stevens BC. H+/zwitterionic amino acid symport at the brush-border membrane of human intestinal epithelial (Caco-2) cells. Exp Physiol. 1999;84:275–284. [PubMed] [Google Scholar]
- Tiruppathi C, Balkovetz DF, Ganapathy V, Miyamoto Y, Leibach FH. A proton gradient, not a sodium gradient, is the driving force for active transport of lactate in rabbit intestinal brush-border membrane vesicles. Biochem J. 1988;256:219–223. doi: 10.1042/bj2560219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya T, Wilson TH. Cation-sugar cotransport in the melibiose transport system of Escherichia coli. Membr Biochem. 1978;2:63–79. doi: 10.3109/09687687809063858. [DOI] [PubMed] [Google Scholar]
- Vidal S, Belouchi AM, Cellier M, Beatty B, Gros P. Cloning and characterization of a second human NRAMP gene on chromosome 12q13. Mamm Genome. 1995;6:224–230. doi: 10.1007/BF00352405. [DOI] [PubMed] [Google Scholar]
- Vincent ML, Russell RM, Sasak V. Folic acid uptake characteristics of a human colon carcinoma cell line, Caco-2. A newly-described cellular model for small intestinal epithelium. Hum Nutr Clin Nutr. 1985;39:355–360. [PubMed] [Google Scholar]
- Walker D, Thwaites DT, Simmons NL, Gilbert HJ, Hirst BH. Substrate upregulation of the human small intestinal peptide transporter, hPepT1. J Physiol. 1998;507:697–706. doi: 10.1111/j.1469-7793.1998.697bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Rajgopal A, Goldman ID, Zhao R. Preservation of folate transport activity with a low-pH optimum in rat IEC-6 intestinal epithelial cell lines that lack reduced folate carrier function. Am J Physiol. 2005;288:C65–C71. doi: 10.1152/ajpcell.00307.2004. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhao R, Goldman ID. Characterization of a folate transporter in HeLa cells with a low pH optimum and high affinity for permetrexed distinct from the reduced folate carrier. Clin Cancer Res. 2004;10:6256–6264. doi: 10.1158/1078-0432.CCR-04-0645. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhao R, Russell RG, Goldman ID. Localization of the murine reduced folate carrier as assessed by immunohistochemical analysis. Biochim Biophys Acta. 2001;1513:49–54. doi: 10.1016/s0005-2736(01)00340-6. [DOI] [PubMed] [Google Scholar]
- West I, Mitchell P. Proton-coupled β-galactoside translocation in non-metabolizing Escherichia coli. Bioenergetics. 1972;3:445–462. doi: 10.1007/BF01516082. [DOI] [PubMed] [Google Scholar]
- West IC. Lactose transport coupled to proton movements in Escherichia coli. Biochem Biophys Res Commun. 1970;41:655–661. doi: 10.1016/0006-291x(70)90063-x. [DOI] [PubMed] [Google Scholar]
- West IC, Mitchell P. Stoicheiometry of lactose-protein symport across the plasma membrane of Escherichia coli. Biochem J. 1973;132:587–592. doi: 10.1042/bj1320587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wipf D, Ludewig U, Tegeder M, Rentsch D, Koch W, Frommer WB. Conservation of amino acid transporters in fungi, plants and animals. Trends Biochem Sci. 2002;27:139–147. doi: 10.1016/s0968-0004(01)02054-0. [DOI] [PubMed] [Google Scholar]
- Wright EM, Loo DD, Hirayama BA, Turk E. Surprising versatility of Na+-glucose cotransporters: SLC5. Physiology. 2004;19:370–376. doi: 10.1152/physiol.00026.2004. [DOI] [PubMed] [Google Scholar]
- Wright EM, Turk E. The sodium/glucose cotransport family SLC5. Pflügers Archiv. 2004;447:510–518. doi: 10.1007/s00424-003-1063-6. [DOI] [PubMed] [Google Scholar]
- Wright EM, Turk E, Zabel B, Mundlos S, Dyer J. Molecular genetics of intestinal glucose transport. J Clin Invest. 1991;88:1435–1440. doi: 10.1172/JCI115451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Whitfield LR, Stewart BH. Atorvastatin transport in the Caco-2 cell model: contributions of P-glycoprotein and the proton-monocarboxylic acid co-transporter. Pharm Res. 2000;17:209–215. doi: 10.1023/a:1007525616017. [DOI] [PubMed] [Google Scholar]
- Yoshida A, Takata K, Kasahara T, Aoyagi T, Saito S, Hirano H. Immunohistochemical localization of Na+-dependent glucose transporter in the rat digestive tract. Histochem J. 1995;27:420–426. [PubMed] [Google Scholar]
- Zerangue N, Kavanaugh MP. Flux coupling in a neuronal glutamate transporter. Nature. 1996a;383:634–637. doi: 10.1038/383634a0. [DOI] [PubMed] [Google Scholar]
- Zerangue N, Kavanaugh MP. Interaction of l-cysteine with a human excitatory amino acid transporter. J Physiol. 1996b;493:419–423. doi: 10.1113/jphysiol.1996.sp021393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R, Gao F, Goldman ID. Reduced folate carrier transports thiamine monophosphate: an alternative route for thiamine delivery into mammalian cells. Am J Physiol. 2002;282:C1512–C1517. doi: 10.1152/ajpcell.00547.2001. [DOI] [PubMed] [Google Scholar]
- Zhao R, Goldman ID. The molecular identity and characterization of a proton-coupled folate transporter PCFT; biological ramifications and impact on the activity of permetrexed. Cancer Metastasis Rev. 2007;26:129–139. doi: 10.1007/s10555-007-9047-1. [DOI] [PubMed] [Google Scholar]