Abstract
Background and Purpose
Blood pressure (BP) is a predictor of concurrent and subsequently measured white matter hyperintensity (WMH), but longitudinal studies of WMH change and data in black participants are lacking. We hypothesized that WMH progression would be 1) strongly related to BP in blacks and whites, and 2) predicted more strongly by earlier (midlife) or cumulative BP measurements than by measures at older ages.
Methods
Participants were 983 individuals (49% African-American) from the Atherosclerosis Risk in Communities (ARIC) Study, who underwent cerebral MRIs in 1993–5 and 2004–6. Associations between BP (measured at each of five visits, in addition to a time-averaged cumulative BP) and progression of WMH were analyzed and compared.
Results
Cumulative SBP was the strongest BP predictor of WMH progression in adjusted models. Higher cumulative SBP by 20 mm Hg was associated with greater progression of WMH, and was similar in blacks (2.5 cc; p<0.0001) and whites (2.6 cc; p<0.0001). Higher cumulative SBP (per 20 mm Hg) was also associated with being in the top quintile of WMH progression (adjusted OR 2.0; 95% CI 1.6–2.6). Earlier SBP measurements were stronger predictors of WMH progression than later SBP, in blacks only.
Conclusions
In this population-based cohort, cumulative SBP was a stronger predictor of WMH progression than SBP from individual visits, in both blacks and whites. Earlier BPs were stronger predictors than BPs measured at later timepoints, in blacks only.
Keywords: leukoaraiosis, hypertension, epidemiology, MRI
Cerebrovascular injury from systemic disease takes the form not only of discrete strokes, but also of white matter injury. In past epidemiologic studies associations between vascular risk factors and resultant white matter disease1, 2 or cognitive impairment3–5 have been strongest when the risk factor was measured in midlife.
In general, blacks have more hypertension and more white matter disease than whites.6 Whether the excess of white matter disease in blacks is entirely due to differences in hypertension prevalence, severity or control, or whether hypertension differentially affects blacks, as compared to whites, is not fully explored. In addition, whether measurement of risk in midlife is as important in blacks, compared to whites, has not been evaluated. Finally, few prior studies have explored associations between vascular risk factors, such as BP, and progression of white matter disease. Progression of white matter disease has been identified as a more important predictor of cognitive decline than baseline white matter disease volume,7 and therefore warrants further study.
In the Atherosclerosis Risk in Communities (ARIC) cohort, we investigated associations between BP measurements over different time points over nearly 20 years, and progression of white matter hyperintensity (WMH) volume. We hypothesized that BP would be strongly associated with WMH progression and that this relationship would be: (1) at least as strong in black as in white participants; and (2) stronger when BP was measured earlier in life or summarized using a cumulative average compared to BP measured at later time points.
MATERIALS AND METHODS
Study Population
ARIC is a prospective study of middle-aged African-American and white men and women designed to investigate the natural history of atherosclerosis and its sequelae. At baseline (1987–89), 15,792 participants were sampled from four U.S communities: Forsyth County, NC; Jackson MS, suburbs of Minneapolis MN, and Washington County, MD. African-Americans were sampled exclusively in Jackson and over-sampled in Forsyth to facilitate race-specific analyses. The other two sites were predominantly white. Race and ethnicity were self-identified.
Study design and procedures have been published.8 After the baseline (visit 1), participants returned in 1990–1993 (visit 2), 1993–1995 (visit 3), 1996–1999 (visit 4), and, for a subset, 2004–2006 (Brain MRI visit, visit 5). At all visits concurrent medical, social, and medication history was collected, and BPs were measured.
Participants aged 55 and older at the Jackson and Forsyth County sites were invited for a brain MRI at visit 3 (1,949 individuals completed an MRI).9 All subjects without MRI contraindications who completed the visit 3 MRI were invited for a 2nd MRI scan between 2004–2006. These participants were more likely to be African-American than the remainder of the living ARIC sample, with comparable numbers of females.
Brain MRI
Visit 3
Visit 3 brain MRI’s were performed on 1.5T scanners, and 5-mm thick contiguous axial images were obtained9, 10 and interpreted at the ARIC MRI Reading Center. Proton-density-weighted images were viewed to grade the relative severity of WMH using a 0 to 9 scale developed for the Cardiovascular Health Study (CHS)11 (referred to in this manuscript as “White matter grade”). Subcortical and periventricular WMH were visually evaluated together. The reproducibility of visual scoring of WMH in CHS was good, with inter- and intra-reader agreement within 1 grade of 92% and 94.5%, and relaxed kappas of 0.81 and 0.93, respectively.11 White matter grade was evaluated on all visit 3 brain MRI’s.
Visit 5
In 2004–2006, as part of the ARIC Brain MRI ancillary study (visit 5), 1,134 participants underwent a second 1.5T brain MRI. These scans were rated using both the qualitative white matter grade, and a semiautomated volumetric analysis. Brain and leukoaraiosis volumes were determined from axial fluid-attenuated inversion recovery (FLAIR) images. An automated algorithm was used to segment each of the FLAIR images into voxels assigned to one of three categories based on signal intensity — normal brain, CSF, or leukoaraiosis. The leukoaraiosis maps were manually edited to exclude infarcts and other lesions. The mean absolute error and test-retest coefficient of variation for this method are 6.6% and 1.4%, respectively, for leukoaraiosis volume. Total intracranial volume was manually measured from T1-weighted sagittal images. Volumetric measurements of WMH were standardized to an intracranial volume of 1500 cc.
Two individuals with severe WMH at visit 3 were excluded (white matter grade 7), because these individuals would be unlikely to show progression. Nine subjects were excluded because of prevalent stroke at visit 3. The final sample included 983 individuals with interpretable MRI scans from both visits.
Calculation of WMH change
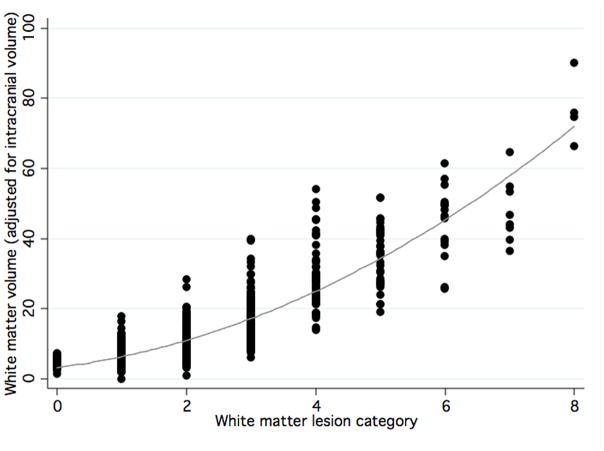
Fully quantitative WMH volumes were not possible from all visit 3 scans. Therefore, WMH volume at visit 3 was estimated using a prediction equation (R2=0.80) relating volume from visual grades (Figure 1). The prediction equation was created using actual data from visit 5 (visual grades and quantitative volumes). Visit 3 visual grades were then entered into the equation to calculate estimated visit 3 volumes. Change in WMH volume was calculated by subtracting estimated WMH volume at visit 3 from WMH volume at visit 5. One value with an apparent decrease of > 20 cc was excluded, as this was likely due to measurement error.
Figure 1.
Scatterplot of semiautomated white matter volume for each qualitative white matter grade.11 Both measurements are made from the same follow-up Brain MRI (visit 5). The line shows a quadratic fit of the data.
Blood Pressure
Sitting brachial BP was measured using standardized protocols by trained technicians using a random zero sphygmomanometer, with 5 minutes’ rest between measurements. At all visits, blood pressure was measured 3 times, and our analyses use the average of the last 2 values for that visit.
Antihypertensive medications were recorded at each visit. A cumulative, time-averaged value was calculated, yielding a value between 0 and 1 for each individual’s proportion of time they had spent over the duration of the study on the medication.
Statistical analysis
Stata version 8.2 for Macintosh was used.12 White matter disease progression was analyzed continuously and as quintiles of WMH volume change. Cross-sectional measures of SBP at each of the ARIC visits were considered as well as cumulative SBP measures. The cumulative values, determined as the area-under-the-curve of exam-specific values divided by time between first and last exam, can be interpreted as the estimated mean daily SBP over the entire period. Other covariates included age, sex, race, body mass index from visit 3 (BMI), visit 3 smoking status, diabetes (fasting glucose≥ 126 mg/dL at visit 3), education (categorized <=8, 9–11, 12–13, 14–16, 17–20, or >=21 years), and prevalent coronary heart disease (visit 3). Linear regression was used to determine the association between SBP and WMH volume change (with larger values indicating a larger increase in WMH volume from visit 3 to 5), and logistic regression was used to estimate its effect on the risk of being in the highest quintile (vs. the lower 4 quintiles) of WMH volume change. Models were analyzed for the entire sample and separately by race.
A separate analysis was performed to compare the effect of “early” (visits 1 and 2) versus “late” (visits 4 and 5) SBP on WMH volume change, using averages of visit 1 and 2 SBP (“early”) and of visit 4 and 5 SBP (“late”). Both of these averaged SBPs were entered into the same race, sex, and age-adjusted model, to determine the association of early versus late SBP with WMH volume change independent of SBP at the other time point.
We also analyzed the role of antihypertensive medications in the association between SBP and white matter disease. Based on methods reported previously,13 a constant of 10 mm Hg was added to the SBP value from a given visit for any individual on antihypertensive treatment at the time of that visit. For cumulative SBP modeling, if the individual had been on an antihypertensive medication at least 50% of the time, the same constant was added to the cumulative SBP value. Additional analyses were conducted adjusting for cumulative antihypertensive use (between 0 and 1) as a covariate. Secondary analyses were also performed adjusting for baseline (visit 3) WMH. Finally, we performed a secondary analysis using change in WMH visual grade from visit 3 to visit 5 as the dependent variable. These models were estimated using ordinal logistic regression. Change in WMH visual grade was calculated by subtracting grade at visit 3 from grade at visit 5.
RESULTS
Patient characteristics
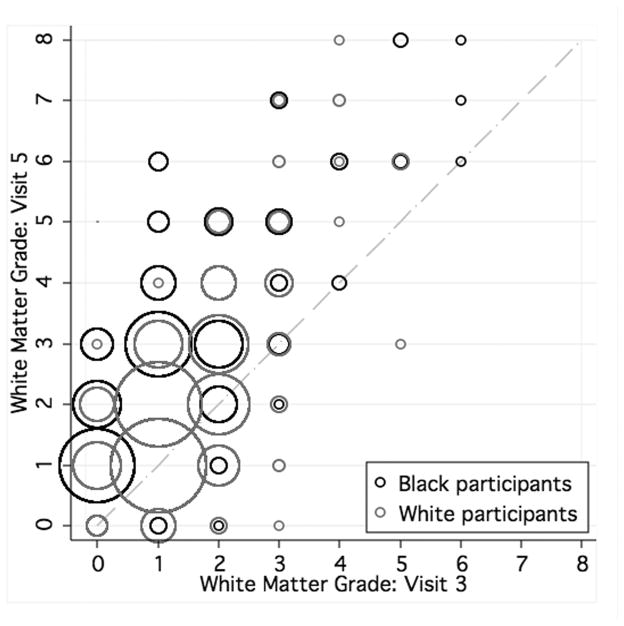
As seen in Table 1, approximately half of the participants were black, and SBP was generally higher at later than at earlier visits. Distribution of white matter grade among individuals who underwent MRI at both visits (3 and 5) is displayed in Figure 2. Fewer than 4% of whites, compared with 10% of blacks, progressed by ≥2 grades (p<0.0001). WMH volume (standardized to 1500 cc total intracranial volume) at visit 5 averaged 13 cc (median 9.1 cc), SD was 11 cc, maximum value 90 cc. The average WMH change (from visit 3 to 5) was 5.2 cc (median 2.7 cc, SD 8.6).
Table 1.
Characteristics of ARIC participants who completed 2 interpretable MRI scans (N=983)
| Variable | |
|---|---|
| % Black (N (%)) | 485 (49.3%) |
| % Female (N (%)) | 605 (61.6%) |
| Age at visit 5 (mean (SD)) | 72 (4) |
| Visit 1 SBP (mm Hg) (mean (SD)) | 121 (17) |
| Visit 2 SBP (mm Hg) (mean (SD)) | 120 (18) |
| Visit 3 SBP (mm Hg) (mean (SD))* | 126 (19) |
| Visit 4 SBP (mm Hg) (mean (SD)) | 129 (19) |
| Visit 5 SBP (mm Hg) (mean (SD))* | 133 (19) |
| Cumulative mean SBP (mm Hg) (mean (SD)) | 127 (14) |
| % Hypertension** (visit 1) (N (%)) | 358 (37%) |
| % History of myocardial infarction (visit 1) (N (%)) | 10 (1%) |
| % Diabetes Mellitus*** (visit 1) (N (%)) | 78 (8%) |
| % Current smoking (visit 1) (N (%)) | 189 (19%) |
| % Prevalent Coronary Heart Disease (visit 1) | 13 (1%) |
| Body Mass Index (visit 1) (kg/m2) (mean (SD)) | 27 (4) |
Brain MRI was also done at these visits.
Defined as SBP≥140/90 mm Hg or hypertension medication use in preceding two weeks.
Defined as fasting glucose≥126 mg/dL
Figure 2.
Distribution of white matter grade categories at visits 3 and 5, by race. Diagonal line demonstrates no change in grade. Larger circles indicate more individuals.
Analysis of WMH volume change as continuous variable
Analyzed as a continuous variable, SBP was strongly associated with WMH volume change (Table 2). Cumulative mean SBP was a stronger predictor than SBP measured at the different examinations. Earlier SBP values had stronger associations with change in WMH than SBP values measured later, but in stratified analysis, this was seen only for blacks. In adjusted models, each 20 mm Hg higher cumulative mean SBP was associated with a 2.4 cc greater increase in WMH volume between visits 3 and 5 with a similar association in blacks and whites (2.5 cc and 2.6 cc respectively, Table 2).
Table 2.
Adjusted beta coefficients for effect of SBP (per 20 mm Hg) on white matter hyperintensity (WMH) volume change (in cc) between cerebral MRI visits (from visit 3 to visit 5) in the ARIC study.
| Systolic blood pressure measurement | Median years from baseline MRI | Beta coefficient* (95% CI): All participants | Beta coefficient** (95% CI): Whites only | Beta coefficient** (95% CI): Blacks only |
|---|---|---|---|---|
| Visit 1 | −5.8 years | 1.44 (0.80–2.09) | 1.07 (0.19–1.97) | 1.98 (1.04–2.92) |
| Visit 2 | −3.0 years | 1.47 (0.85–2.10) | 1.26 (0.38–2.13) | 1.89 (0.99–2.79) |
| Visit 3: Baseline MRI | 0 years | 1.10 (0.54–1.67) | 1.18 (0.39–1.96) | 1.14 (0.32–1.96) |
| Visit 4 | +3.0 years | 1.31 (0.73–1.88) | 1.93 (1.18–2.68) | 0.78 (−0.09–1.66) |
| Visit 5: Follow-up MRI | +10.6 years | 1.28 (0.70–1.85) | 1.39 (0.61–2.16) | 1.36 (0.51–2.22) |
| Cumulative mean SBP | 2.35 (1.58–3.12) | 2.56 (1.56–3.56) | 2.48 (1.30–3.67) | |
Adjusted for age, sex, education, prevalent CHD at visit 3, diabetes at visit 3, BMI at visit 3, race, and smoking status at visit 3.
Same as above but without adjustment for race
A race X SBP interaction term was significant for visits 1 and 2, indicating that the association between WMH volume and SBP measured at these early visits was stronger in blacks. The race × SBP interaction was not significant in the models for visits 3, 4, or 5, or cumulative SBP.
Analysis of ”significant progression”
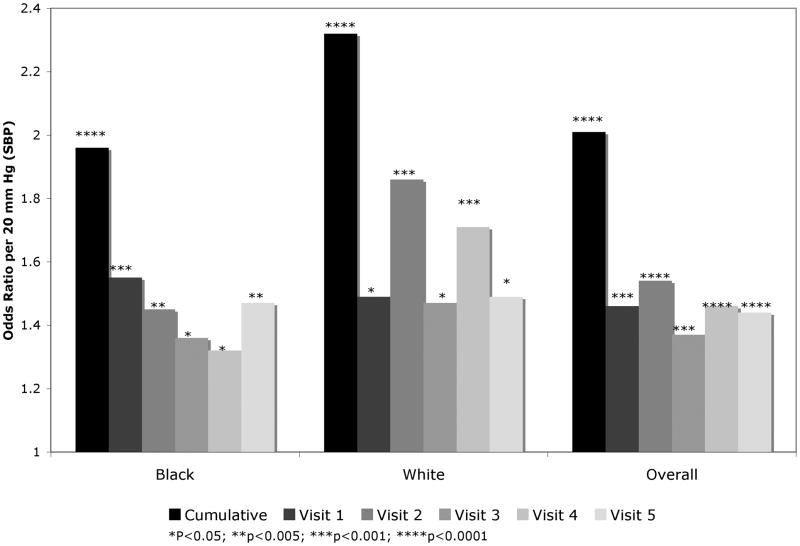
Figure 3 shows the adjusted odds ratio (per 20 mm Hg (approximately 1 SD) increase in SBP) of being in the highest quintile of change in WMH volume in the range of 1.3 to 2.3 for SBP measured at the different visits (or using a cumulative value).
Figure 3.
Adjusted odds ratios (OR) of top quintile of change in white matter hyperintensity volume, per 20 mm Hg higher SBP at varying time points and shown by race. Cumulative time-averaged SBP and SBP measurements from each visit are displayed.
Analysis of effect of timing of SBP measurement in prediction of WMH volume change
Inclusion of both early SBP (an average of SBP from the first two visits) and late SBP (average of values from visits 4 and 5) in the same age- and sex-adjusted model permitted assessment of the importance of the timing of BP in predicting WMH progression. In a model including all participants, beta coefficients were similar (0.91 and 1.51 cc of progression per 20 mm Hg) for early versus late SBP values (respectively). Findings were similar in white participants. However, in black participants, the coefficient associated with an equivalent difference (20 mm Hg) in SBP from early visits was 2.17 cc (95% CI 0.96–3.38), after adjustment for later SBP, compared to only 0.68 cc additional WMH progression associated with a difference of 20 mm Hg in later visits (after adjustment for earlier SBP).
Secondary analyses
When use of antihypertensive medications is accounted for by adding a 10 mm Hg constant, as described in the methods section, the association with SBP was partially attenuated but remained significant. Complete results when cumulative use of antihypertensive medication is included as a covariate, in the prediction of worst amount of WMH change (top quintile), are included in the Supplementary table. Cumulative SBP remains the most important predictor.
When the continuous analyses were repeated including adjustment for WMH grade at visit 3, results remained statistically significant, but point estimates were moderately decreased.
When the primary analyses were repeated but using change in WMH grade (without using the estimated volumes), similar results were found, with the strongest effect from the cumulative SBP value. The adjusted OR for advancing one category in WMH visual grade, per 20 mm Hg of SBP, was 1.27 when SBP was measured from visit 1 (95% CI 1.10–1.48), 1.24 (95% CI 1.07–1.44) from visit 2, 1.14 (1.00–1.30) from visit 3, 1.30 (95% CI 1.13–1.48) from visit 4, 1.18 (95% CI 1.04–1.35) from visit 5, and 1.46 for cumulative SBP (95% CI 1.22–1.75).
DISCUSSION
In this large cohort with serial BP measurements and two MRI studies per subject, cumulative mean SBP was a much stronger predictor of white matter disease progression than SBPs from individual time points. In blacks, earlier (midlife) measurements of SBP had slightly stronger associations with WMH change than did later measurements. More blacks than whites had substantial progression of WMH, although an equivalent increase in cumulative mean SBP was associated with similar increases in WMH volume change in blacks and whites. Substantially more blacks had a known diagnosis of hypertension (41.6%) at the start of the ARIC study than did whites (16.6%). Perhaps the ethnic differences in progression may reflect longer duration of hypertension in blacks, even earlier than measured in this study.
Although others have hypothesized that WMH may result from degenerative or demyelinating injury,14 our results support a significant vascular component to the progression of WMH. Our findings are also consistent with previous studies suggesting the importance of measurement of vascular risk factors in midlife in predicting subsequent cognitive impairment and white matter disease; 1–5, 15 Other studies have supported a relatively weak cross-sectional association between measurement of BP and white matter hyperintensity. In the Cardiovascular Health Study, in participants over 65, higher SBP was associated with higher white matter grade (graded categorically).16 Our focus was on progression of WMH; we did find evidence for an association between SBP measured at the time of the first MRI and WMH progression, but found that in blacks (who have not been as well represented in other epidemiologic studies) it was not as strong as associations with SBP measured prior to the first MRI. We cannot explain the relative lack of influence of earlier SBP values in whites in this study. Whites had less baseline WMH, so had lower volumes of WMH in general. The effect of SBP on WMH progression in whites was quite variable from visit to visit. This variability remained even after adjustment for antihypertensive medication use. Blood pressure variability in general warrants further study, as wide fluctuations in blood pressure may be problematic for the integrity of the white matter.
To our knowledge, a cumulative variable, taking into account SBP levels over approximately 15 years, has not been explored previously to describe relationships with white matter disease. Its effect size portends the potential utility of this measure in future studies on the long-term effects of hypertension. The association between WMH progression and a 20 mm Hg higher cumulative SBP was equivalent to the increase in progression associated with a 7–8 year difference in baseline age.
In the Rotterdam study, higher SBP and DBP both predicted the progression of periventricular WM disease, but this was using a categorical scale, their effect sizes tended to be smaller, for comparable differences in WMH progression, and their results were not significant consistently.17,18 This may be because the Rotterdam study recruited primarily white participants with access to excellent health care, who, as our results suggest, may have weaker associations between early BP measurements and white matter disease. We also believe that the additional information provided by volumetric measurement of white matter disease helps to better define the extent of disease progression.
Mechanisms underlying WMH are important to explore given the known relationships between WMH and subsequent dementia or cognitive impairment.19,20 Progression may be particularly important; in one recent study, individuals who became cognitively impaired had an average increase of WMH of 2.4 cc/year, compared to those who were cognitively intact (mean WMH increase 0.8 cc/year).
A limitation of this study is the lack of direct volumetric imaging at the 3rd visit. However, the validity of our approach for estimating visit 3 WMH volumes based on the relationship between categorical and volumetric ratings at visit 5 is supported by the excellent fit by the equation we used for making those estimates. Another limitation is the lack of information about ambulatory BP. Given that cumulative SBP was a strong predictor of white matter disease, detailed ambulatory BP measurements might provide even stronger prediction but are cost prohibitive and impractical over any lengthy time frame. Associations were still present, though attenuated, when antihypertensive use was included in models, suggesting that some injury from hypertension might be eliminated by the use of antihypertensives. However, the degree to which an individual’s SBP was lowered by medications could not be well examined in this study, using our methods, since some individuals might have better control on antihypertensive medications, others might require 3 or 4 medications, and still others might be on antihypertensive medications, but for reasons other than blood pressure control (for instance, beta-blockers for rate control in atrial fibrillation).
Persons who completed both cerebral MRIs may represent a biased sample of the population. Individuals with more advanced leukoaraiosis or with dementia might be underrepresented. This potential bias might be conservative-- leading us to underestimate the strength of the associations between blood pressure and white matter disease progression.
The strengths of this study include the large sample size with excellent follow-up, standardized measurement of blood pressure and other vascular risk factors, and the availability of serial MRI scans in a largely asymptomatic population. We believe that our use of the volumetrics provides an advantage over the use of categorical ratings, as has been typically used in other studies. We have shown the importance of BP in subclinical vascular disease. Further studies may focus on clinical sequelae. Because white matter disease has been associated with cognitive dysfunction, reduction in white matter disease progression may be a means by which long-term antihypertensive treatment might prevent cognitive impairment and dementia. In our study, the relationship between SBP and white matter disease was linear, suggesting that changes in SBP are critical across a wide range of values. The relative health of the sample, however, limits our ability to make conclusions about individuals with very high or very low BP values, as there were very few individuals at these extremes.
SUMMARY
Cumulative SBP provides valuable information in estimating risk of progression of white matter disease, beyond that of BPs taken at individual time points. In blacks, earlier BPs may be more important than later life blood pressures in predicting white matter disease progression. These findings further emphasize the importance of persistently elevated BP in the development of subclinical cerebral vascular disease.
Supplementary Material
Acknowledgments
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, and N01-HC-55022. This work was also supported by grant R01-HL70825. The authors thank the staff and participants of the ARIC study for their important contributions.
Footnotes
CONFLICT OF INTEREST: Dr. Knopman has served on a Data Safety Monitoring Board for Sanofi-Aventis Pharmaceuticals, will serve on a Data Safety Monitoring Board for Lilly, and is an investigator for clinical trials sponsored by Baxter Pharmaceuticals, Elan Pharmaceuticals and Forest Pharmaceuticals. He has served as a one-time consultant to GlaxoSmithKline regarding an anti-Alzheimer drug. He is an Associate Editor of Neurology for which he receives compensation from the American Academy of Neurology. Dr. Jack has received research support from and has been a consultant for Pfizer. The other authors have neither conflicts of interest nor other financial disclosures.
References
- 1.Havlik RJ, Foley DJ, Sayer B, Masaki K, White L, Launer LJ. Variability in midlife systolic blood pressure is related to late-life brain white matter lesions. Stroke. 2002;33:26–30. doi: 10.1161/hs0102.101890. [DOI] [PubMed] [Google Scholar]
- 2.Jeerakathil T, Wolf PA, Beiser A, Massaro J, Seshadri S, D’Agostino RB, DeCarli C. Stroke risk profile predicts white matter hyperintensity volume. The Framingham study. Stroke. 2004;35:1857–1861. doi: 10.1161/01.STR.0000135226.53499.85. [DOI] [PubMed] [Google Scholar]
- 3.Swan GE, DeCarli C, Miller BL, Reed T, Wolf PA, Jack LM, Carmelli D. Association of midlife blood pressure to late-life cognitive decline and brain morphology. Neurology. 1998;51:986–993. doi: 10.1212/wnl.51.4.986. [DOI] [PubMed] [Google Scholar]
- 4.Launer LJ, Masaki K, Petrovitch H, Foley D, Havlik RJ. The association between midlife blood pressure levels and late-life cognitive function. The Honolulu-Asia Aging Study. JAMA. 1995;274:1846–1851. [PubMed] [Google Scholar]
- 5.Freitag MH, Peila R, Masaki K, Petrovitch H, Ross GW, White LR, Launer LJ. Midlife pulse pressure and incidence of dementia: The Honolulu-Asia Aging Study. Stroke. 2006;37:33–37. doi: 10.1161/01.STR.0000196941.58869.2d. [DOI] [PubMed] [Google Scholar]
- 6.Prabhakaran S, Wright CB, Yoshita M, Delapaz R, Brown T, DeCarli C, Sacco RL. Prevalence and determinants of subclinical brain infarction. Neurology. 2008;70:425–430. doi: 10.1212/01.wnl.0000277521.66947.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silbert LC, Howieson DB, Dodge H, Kaye JA. Cognitive impairment risk: White matter hyperintensity progression matters. Neurology. 2009;73:120–125. doi: 10.1212/WNL.0b013e3181ad53fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) study: Design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 9.Mosley TH, Knopman DS, Catellier DJ, Bryan N, Hutchinson RG, Grothues CA, Folsom AR, Cooper LS, Burke GL, Liao D, Szklo M. Cerebral MRI findings and cognitive functioning. The Atherosclerosis Risk in Communities study. Neurology. 2005;64:2056–2062. doi: 10.1212/01.WNL.0000165985.97397.88. [DOI] [PubMed] [Google Scholar]
- 10.Liao D, Cooper L, Cai J, Toole JF, Bryan NR, Hutchinson RG, Tyroler HA. Presence and severity of cerebral white matter lesions and hypertension, its treatment, and its control. The ARIC study. Atherosclerosis Risk in Communities study. Stroke. 1996;27:2262–2270. doi: 10.1161/01.str.27.12.2262. [DOI] [PubMed] [Google Scholar]
- 11.Manolio TA, Kronmal RA, Burke GL, Poirier V, O’Leary DH, Gardin JM, Fried LP, Steinberg EP, Bryan RN. Magnetic resonance abnormalities and cardiovascular disease in older adults. The Cardiovascular Health Study. Stroke. 1994;25:318–327. doi: 10.1161/01.str.25.2.318. [DOI] [PubMed] [Google Scholar]
- 12.Stata Corp. Stata statistical software: Release 8.0. 2002 [Google Scholar]
- 13.Tobin MD, Sheehan NA, Scurrah KJ, Burton PR. Adjusting for treatment effects in studies of quantitative traits: Antihypertensive therapy and systolic blood pressure. Stat Med. 2005;24:2911–2935. doi: 10.1002/sim.2165. [DOI] [PubMed] [Google Scholar]
- 14.Rosenberg GA. Inflammation and white matter damage in vascular cognitive impairment. Stroke. 2009;40 (3 Suppl):S20–23. doi: 10.1161/STROKEAHA.108.533133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stewart R, Xue QL, Masaki K, Petrovitch H, Ross GW, White LR, Launer LJ. Change in blood pressure and incident dementia: A 32-year prospective study. Hypertension. 2009;54:233–240. doi: 10.1161/HYPERTENSIONAHA.109.128744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Longstreth WT, Jr, Manolio TA, Arnold A, Burke GL, Bryan N, Jungreis CA, Enright PL, O’Leary DH, Fried L. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study. Stroke. 1996;27:1274–1282. doi: 10.1161/01.str.27.8.1274. [DOI] [PubMed] [Google Scholar]
- 17.van Dijk EJ, Prins ND, Vrooman HA, Hofman A, Koudstaal PJ, Breteler MM. Progression of cerebral small vessel disease in relation to risk factors and cognitive consequences. Stroke. 2008;39:2712–2719. doi: 10.1161/STROKEAHA.107.513176. [DOI] [PubMed] [Google Scholar]
- 18.de Leeuw FE, de Groot JC, Oudkerk M, Witteman JC, Hofman A, van Gijn J, Breteler MM. A follow-up study of blood pressure and cerebral white matter lesions. Ann Neurol. 1999;46:827–833. doi: 10.1002/1531-8249(199912)46:6<827::aid-ana4>3.3.co;2-8. [DOI] [PubMed] [Google Scholar]
- 19.Yoshita M, Fletcher E, Harvey D, Ortega M, Martinez O, Mungas DM, Reed BR, DeCarli CS. Extent and distribution of white matter hyperintensities in normal aging, MCI, and AD. Neurology. 2006;67:2192–2198. doi: 10.1212/01.wnl.0000249119.95747.1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Flier WM, van Straaten EC, Barkhof F, Verdelho A, Madureira S, Pantoni L, Inzitari D, Erkinjuntti T, Crisby M, Waldemar G, Schmidt R, Fazekas F, Scheltens P. Small vessel disease and general cognitive function in nondisabled elderly: The LADIS study. Stroke. 2005;36:2116–2120. doi: 10.1161/01.STR.0000179092.59909.42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.