Abstract
The use of cochlear implants in patients with severe hearing losses but residual low-frequency hearing raises questions concerning the effects of chronic intracochlear electrical stimulation(ICES) on cortical responses to auditory and electrical stimuli. We investigated these questions by studying responses to tonal and electrical stimuli in primary auditory cortex (AI) of two groups of neonatally-deafened cats with residual high-threshold, low-frequency hearing. One group were implanted with a multi-channel intracochlear electrode at eight weeks of age, and received chronic ICES for up to nine months before cortical recording. Cats in the other group were implanted immediately prior to cortical recording as adults. In all cats in both groups, multi-neuron responses throughout the rostro-caudal extent of AI had low characteristic frequencies (CFs), in the frequency range of the residual hearing, and high-thresholds. Threshold and minimum latency at CF did not differ between the groups, but in the chronic ICES animals there was a higher proportion of electrically but not acoustically excited recording sites. Electrical response thresholds were higher and latencies shorter in the chronically stimulated animals. Thus, chronic implantation and ICES affected the extent of AI that could be activated by acoustic stimuli and resulted in changes in electrical response characteristics.
Keywords: Cochlear implant, auditory cortex, electrical stimulation, neural prosthesis, sensorineural hearing loss
INTRODUCTION
Intracochlear electrical stimulation (ICES) of the auditory nerve via a cochlear implant has provided over 120,000 individuals with a profound sensorineural hearing loss (SNHL) with auditory input. The speech perception ability of cochlear implantees improves over the post-implantation period (e.g. Blamey et al., 1996; McKay, 2005; Wilson & Dorman, 2008), and this improvement is likely to be at least partly attributable to post-implantation changes in auditory cortical responsiveness (for review see Green et al., 2005). In agreement with this view, there is a growing body of electrophysiological evidence from studies in profoundly deaf animals that chronic ICES can result in changes in central auditory system response characteristics (for review see Fallon et al., 2008), and that this plasticity can in part ameliorate the effects of deafness on these responses. Such changes have been described both in the midbrain nucleus, the inferior colliculus (IC, e.g., Snyder et al., 1990, 1995; Vollmer et al., 1999, 2005) and in the primary auditory cortex (AI, e.g., Dinse et al., 1997, 2003; Fallon et al., 2009; Klinke et al., 2001; Kral et al., 2002; Kral & Tillein, 2006)
In recent years implants have been used in patients with severe middle and high frequency hearing losses but with residual low-frequency hearing (for review see Turner et al., 2008). Clinical studies of the issues involved in combining acoustic and electrical hearing have focused on the preservation of acoustic hearing following electrode implantation, and on the extent to which listeners can combine the two modalities (e.g. Gantz & Turner, 2003; Kiefer et al., 2005; Simpson et al., 2009; Turner et al., 2008). These patients typically exhibit improved speech perception, particularly in the presence of background noise, than patients using electrical hearing alone. There is also clinical evidence of plasticity in cochlear implant patients with some residual hearing: after some years of device use, the pitch percept associated with stimulation of a given electrode comes to match the frequency range assigned to that electrode, rather than that expected from the normal cochlear pitch-place coding (McDermott et al., 2009; Reiss et al., 2007).
The perceptual experience of cochlear implant patients with residual low-frequency hearing is likely to depend on the survival of residual hair cells within the implanted cochlea, the nature of the responses evoked in the auditory cortex by acoustic and electrical stimuli, the behavioural relevance of this bimodal input, and on possible effects of chronic ICES on the responses to acoustic stimuli. Given the many forms of plasticity that have been demonstrated in the AI in both animal and human studies (for review see Irvine & Wright, 2005), it is possible, for example, that the strong input to AI neurons provided by ICES might result in a reduction in the efficacy of residual acoustic inputs. However, there appears to have been no previous electrophysiological study of the effects of chronic ICES in animals with residual low-frequency hearing.
We have therefore investigated possible central auditory system plasticity in an animal model of combined acoustic and electrical hearing by examining the responses evoked in the AI by the two modes of stimulation. Bilateral severe high-frequency hearing losses were produced by neonatal injections of ototoxic agents, resulting in animals with residual high-threshold hearing over a limited range of low frequencies. Cats in one group were implanted shortly after deafening and received chronic ICES throughout the post-deafening period; cats in the other group received no ICES and were implanted at the time of cortical recording.
MATERIALS AND METHODS
Neonatal deafening and assessment of residual hearing
Eight healthy kittens were neonatally deafened, using methods that have been described in detail previously (Coco et al., 2007). At 14 days of age, the animals were anaesthetized with a mixture of halothane and oxygen and deafened by the combination of a subcutaneous injection of Kanamycin (330 mg/kg; Kanamycin monosulphate, Sigma, USA) and an intravenous injection of Ethacrynic acid (27.5 mg/kg; Ethacrynate Sodium; Merck Research Laboratories, USA) (Shepherd & Martin, 1995). Hearing status was assessed by the measurement of click-evoked auditory brainstem responses (ABRs) at 1 month of age (2 weeks following the deafening procedure; Coco et al., 2007). All eight kittens had residual hearing and were randomly assigned to the either the chronic ICES group (DCS, n = 4) or the unstimulated group (DUS, n = 4). Kittens were housed in the institute’s animal facility for the period of up to 9 months until cortical recordings were carried out. The hearing-loss data presented in the Results indicates that their acoustic experience during this period would have been restricted to those components of the environmental sounds associated with the normal running of the facility and of vocalizations by themselves and other animals in the facility that were below approximately 2 kHz and above approximately 60 dB SPL.
In the DCS group, hearing was assessed periodically throughout the duration of the experiment by recording ABRs and acoustically-evoked auditory nerve compound action potentials (CAPs) for monaural acoustic stimulation of each ear to establish the frequency range of the hearing loss (Coco et al., 2007). The acoustic stimuli for the CAP recordings consisted of computer-generated tone-pips (1-ms rise/fall, 3-ms plateau) at frequencies from 0.5 to 16 kHz (no animal exhibited a response to stimuli above this frequency). CAPs were recorded differentially using the most apical intracochlear stimulating electrode (+ve) against a subcutaneous stainless steel electrode (neck −ve; thorax ground) and CAP thresholds were determined using a visual detection criterion (Rajan et al., 1991).
Cochlear electrodes and implantation
The electrode arrays consisted of 8 Platinum electrodes located on a silastic carrier (Shepherd et al., 1983). The electrodes were numbered 1–8, with electrode 1 the most apical and located in the upper basal turn of the cochlea. As described previously (Coco et al., 2007), the four DCS cats were bilaterally implanted at eight weeks of age: the left side with a intracochlear stimulating electrode array and leadwire assembly; the right side with a dummy electrode array. The dummy electrode was inserted as a control for insertion effects in the anatomical study to which data from these cats contributed (Coco et al., 2007). Under sterile conditions, the bulla cavity was opened and flushed with Amoxicillin (10 mg/ml; CSL, Australia), and the round window (RW) membrane was incised. The electrode array was then inserted via the RW into the scala tympani to a depth of 7 mm from the RW, resulting in the most apical electrode being located at the ~10-kHz place and the most basal electrode at the ~26-kHz place (Brown et al., 1992). The insertion point of the electrode at the RW was then sealed with crushed muscle or fascia to prevent perilymph leakage. The leadwire was fixed with Lars Mesh (Boston Scientific, USA) ties at the bulla and on the dorso-lateral part of the skull. The lead wire passed subcutaneously and then exited the body through an incision at the nape of the neck to allow for connection to a portable stimulator. In the four deafened unstimulated (DUS) cats, an electrode was implanted in the left cochlea immediately prior to the acute cortical recording session (see below) using the same insertion and fixing procedures.
Electrically evoked auditory brainstem responses
Electrically-evoked ABRs (EABRs) were periodically recorded from the DCS cats throughout the chronic ICES program, using the methods described by Coco et al. (2007). Briefly, optically isolated biphasic current pulses (100-µs per phase; 10-µs interphase gap) were generated under computer control and delivered to a pair of electrodes on the intracochlear electrode array. Responses were recorded using the same techniques as for the ABR except for the inclusion of a sample-and-hold circuit in order to remove electrical artifact (Black et al., 1983). Two recordings were made at each current level and current amplitude was reduced to levels below threshold. Threshold was defined as the smallest current level required to evoke a peak-trough response amplitude of at least 0.2 µV for wave IV of the EABR (within a latency window of 2.4–2.9 ms following stimulus onset) for both responses.
Chronic intracochlear electrical stimulation
A chronic ES program using custom-made programmable current source stimulators commenced fourteen days following implant surgery (Coco et al., 2007). The output of the stimulator delivered 100 µs/phase charge-balanced biphasic current pulses at a stimulus rate of 1200 pulses per second (pps). The stimulus waveform was amplitude-modulated to a depth of 50% at 30 Hz in order to mimic the temporally challenging stimuli used in contemporary cochlear implant speech processing strategies. Electrode shorting and capacitive coupling were used to ensure complete charge recovery (Huang et al., 1999). The amplitude of the stimulus waveform was set so that the minimum current level was equal to, and the maximum was 6 dB above, the post-operative EABR threshold. These stimulus levels were assessed by monitoring behavioral characteristics and were confirmed to be acceptable for each animal. The maximum stimulus current amplitudes used were in the range 0.25– 1.75 mA at 100 µs/phase, which produced charge densities within safe levels for use with Pt electrodes (Shepherd et al., 1983; Xu et al., 1997). Stimulators were carried in a harness worn by the animals to enable continuous stimulation without confining their daily activities. DCS animals received approximately 6 hours of ICES per day, 5 days per week for up to 9 months. In two animals (DCS 903 and 904) ICES was restricted to the upper basal turn (electrodes 1–3), while the remaining animals (DCS 911 and 912) received ICES to both the upper (electrodes 1–3) and lower (electrodes 6–8) basal turn. Both stimulus current and electrode voltage waveforms were monitored twice daily to ensure that the appropriate ICES levels were set for each animal and that electrode impedance was within normal range (2–8 kΩ)
Cortical recording
Cortical recordings were made in acute experiments, using techniques that have been described in detail previously (Brown et al., 2004; Rajan et al., 1993) and will be only briefly outlined here. Anesthesia was induced by sodium pentobarbitone (Nembutal; i.p., 45 mg/kg) and maintained with supplementary i.v. doses as required, and the cat’s core body temperature was maintained at around 37.5°C using a DC heating pad controlled by a rectal thermistor. Following tracheal cannulation the cat was placed in a head holding frame. The left bulla was exposed and opened in the cats in the DUS group to allow insertion of the stimulating electrode array, as described above. The left pinna was removed, and acoustic stimuli were delivered from a transducer in a sealed coupler, via a speculum that fitted tightly into the meatal stub. Calibrations were carried out in situ, using a probe microphone assembly (Brüel and Kjaer 0.5-inch microphone). All sounds pressure levels (SPLs) are expressed in dB re 20 µPa. Prior to cortical recording, the CAP audiogram for the left ear was obtained by measuring the N1 threshold for brief tone bursts (5-ms duration; 0.4/3.0-ms rise/fall times) at frequencies between 0.5 and 16 kHz, using signal averaging (10 ± 1 µV criterion).
Recordings were made from the right auditory cortex, contralateral to the ear to which acoustic and electrical stimuli were presented. A calibrated digital photograph of the cortex was obtained after removal of the dura, and the position of each electrode penetration was subsequently marked on the photograph, relative to the cortical vasculature. A modified Davies chamber with its top parallel to the surface of the middle ectosylvian gyrus (MEG) was positioned over the exposure, secured to the skull, filled with sterile saline, and sealed with a glass plate. Electrode penetrations were made approximately orthogonal to the gyral surface, using a hydraulic micromanipulator mounted in the glass plate on top of the Davies chamber.
All recordings were made with the cat in a sound-attenuated, electrically shielded chamber. Cortical recordings were made using 1 – 2 MΩ parylene-insulated tungsten microelectrodes. In all cats, electrode penetrations were made into the rostral bank of the posterior ectosylvian sulcus (PES), in an attempt to record from neurons with pre-lesion CFs in the range of the residual hearing, and along a number of lines covering the entire rostro-caudal extent of the MEG, in order to allow recordings across the entire tonotopic axis of putative AI. At each gyral recording site, the electrode was advanced 500 – 700 µm into the cortex before searching for tone- and/or electrically-evoked multi-unit (neuronal “cluster”) activity. In successful penetrations into the rostral bank of PES, an initial recording was made at a depth in the range 1000–2000 µm, and subsequent recordings were made at approximately 500-µm intervals below 2000 µm, until responsiveness was lost. Once a cluster containing clearly-defined action potentials was obtained, its responsiveness to acoustic and electrical stimuli was determined audiovisually. For the subsequent collection of quantitative data, a Schmitt trigger was set at a level well above the noise floor and exceeded only by clearly defined action potentials. The Schmitt trigger output pulses were timed by the computer with an accuracy of 10 µs.
If the cluster was acoustically responsive, the CF was estimated audiovisually, and a response area was obtained by presenting, under computer control, a frequency-SPL matrix that was centered about the putative CF and varied over an SPL range from below threshold to 100 or 110 dB. Stimuli were 50-ms pure tone bursts with 5-ms rise/fall times, presented at a rate of 2 Hz. Five stimuli were presented at each frequency–SPL combination, and the combinations were presented in a pseudorandom order across the matrix. The software returned values for the number of spikes within a specified count window and the mean first-spike latency at each frequency-SPL combination. All CF determinations and measurements of threshold and minimum latency at CF were based on the quantitative responses area data. CF and threshold were defined as the frequency-SPL combination with the lowest SPL that resulted in a measurable increase in firing above spontaneous activity. Latency at a given SPL – frequency combination was measured as the mean first-spike latency across the 5 stimulus presentations, and minimum latency at CF was identified as the shortest mean latency recorded at any SPL at the CF.
If the cluster was responsive to ICES, an input-output (IO) function was obtained for bipolar stimulation of an apical (1–3) and a basal pair of electrodes (6–8). Stimuli consisted of 100-µs/phase charge-balanced biphasic current pulses presented at a rate of 2 Hz. For each bipolar pair, stimulus current was increased from below threshold to saturation level in approximate 10 linear steps. The software returned values for the number of spikes within a specified count window and the mean first-spike latency at each electrode pair–current combination. Each IO function was fitted with a saturating Gaussian function (Sachs & Abbas, 1974), from which the threshold (defined as the current required to achieve a half maximal response), was determined. Cortical thresholds were expressed relative to EABR threshold for that intracochlear stimulating electrode, as hearing status and stimulating electrode location can both alter threshold (Fallon et al., 2009).
Histology
All cats were sacrificed with an overdose of sodium pentobarbitone (150 mg/kg, i.v.) on completion of the acute recording session. Methods used for perfusion and histological examination of the cochleae have been described in detail in (Coco et al., 2007).
This study was conducted under the approval of the Animal Research Ethics Committee, Royal Victorian Eye and Ear Hospital, Melbourne, Australia.
RESULTS
Detailed physiological data were obtained from all eight cats (132 recording sites in the DUS cats and 122 in the DCS cats) and are presented below.
The histological findings in both groups were as described in Coco et al. (2007), in which detailed cochlear histology are presented for one of the chronic ICES cats (DCS 911; there designated I7p; their Fig. 5). In brief, there was no evidence of hair cell survival in the basal turn of any cochlea and there was no significant difference in hair cell survival between the two groups in either the middle (Mann-Whitney Rank Sum test; p = 0.22) or apical (p = 0.13) turns, with approximately 30% and 90% survival respectively, despite the fact that one cohort had been chronically implanted and received ICES. There was also no significant difference in spiral ganglion neuron survival between the two groups (two-way ANOVA, Group × Turn; p = 0.75), with approximately 40%, 60% and 95% survival in the lower, middle and apical turns, respectively.
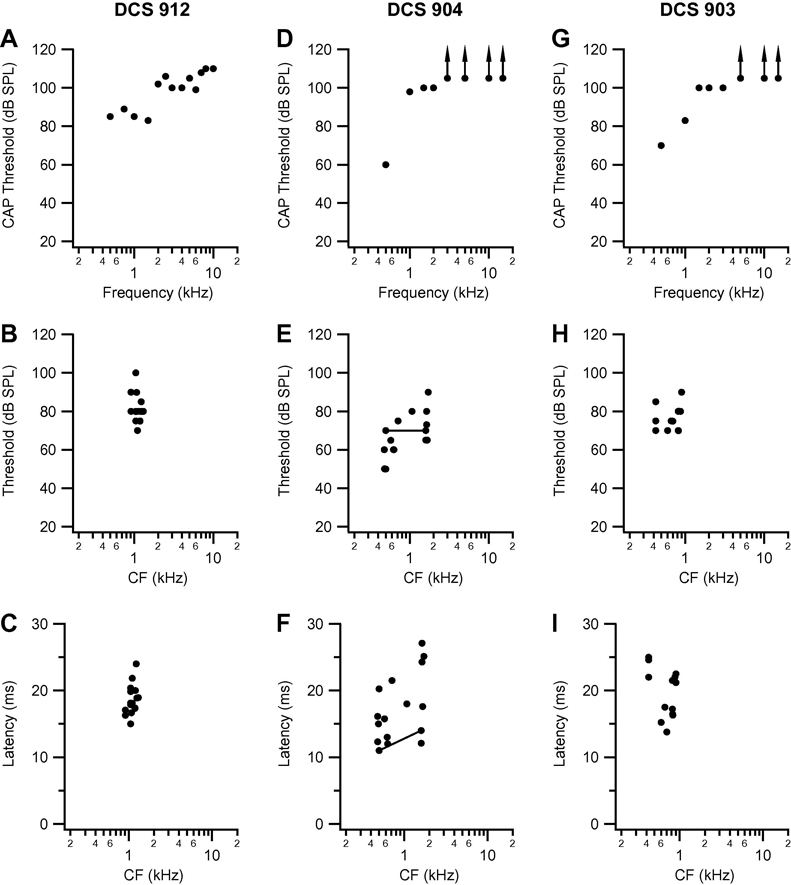
Fig. 5.
Compound action potential audiogram, and threshold, and minimum latency at CF, as a function of CF in the three other DCS cats: DCS 912 (A, B, C), DCS 904 (D, E, F) and DCS 903 (G, H, I). Conventions as in Fig. 2.
Frequency organization and auditory response characteristics in control cats
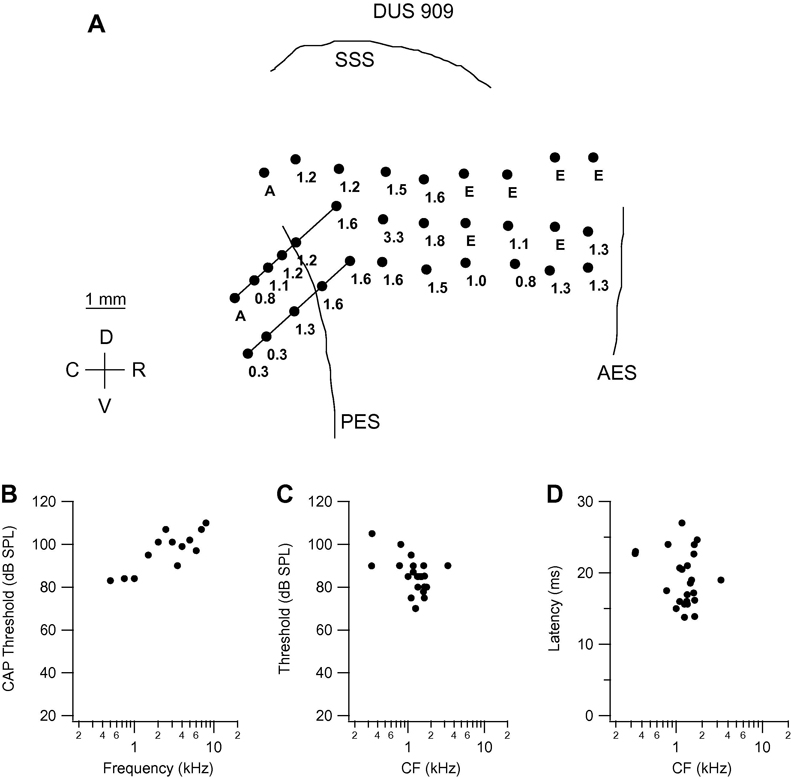
Data for a representative individual cat in the DUS group are presented in Fig. 1. The CAP thresholds for cat DUS 909 (Fig. 1B) reveal low-frequency, high-threshold residual hearing, with lowest thresholds, in the range 80–85 dB, at frequencies from 0.5 to 1 kHz. Threshold increases steeply to 95–105 dB at frequencies from 1.5 to 6 kHz, except for the lower (90 dB) value at 3.5 kHz. The cortical mapping data for this animal (Fig. 1A) reveal that neurons with low CF (0.3 – 0.8 kHz) were encountered deep in the rostral bank of the PES, but that CFs at almost all other recording sites more superficially in the sulcus and across the MEG had CFs in the range 0.8 – 1.8 kHz.
Fig. 1.
Frequency organization and response characteristics in cat DUS 909. A. Drawing of middle ectosylvian gyrus, based on photograph taken at the time of cortical recording. Filled circles on the gyral surface indicate the locations of microelectrode penetrations, and the number next to each symbol indicates the characteristic frequency (CF; in kHz) of the cluster of neurons recorded from in that penetration. Angled lines indicate long penetrations into the rostral bank of the posterior ectosylvian sulcus (PES), and the filled circles on these lines indicate the locations of recording sites along the penetrations. Points marked “E” are those at which responses to intracochlear electrical stimulation but no response to acoustic stimulation could be recorded. Points marked “A” are those at which there was a weak response to acoustic stimulation (for which a CF could not be determined) and no response to intracochlear electrical stimulation. Other abbreviations: AES: anterior ectosylvian sulcus; C: caudal; D: dorsal; SSS: suprasylvian sulcus; R: rostral; V: ventral. B. Compound action potential (CAP) thresholds as a function of frequency in the stimulated ear (viz., contralateral to cortex from which recordings were made). In this and the CAP audiograms presented in later figures, no response could be recorded at the highest SPL available at frequencies above the highest frequency for which a threshold is plotted. C, D. Threshold at CF and minimum latency at CF, respectively, as a function of CF.
The severe hearing loss in this and all of the other animals in the study (see details below), and the associated fact that responses to acoustic stimuli could only be recorded at very low frequencies, had the consequence that the boundaries of AI could not be determined physiologically. The fact that very low frequencies were encountered deep in the most ventral sulcal bank penetrations (e.g., Fig. 1 and Fig. 4), and that the most rostral recording sites in all animals were caudal to the AES, makes it unlikely that the data include recordings from either the posterior or anterior auditory fields. The fact that most ventral gyral recordings were made in a line running rostrally from a PES penetration in which very low-frequency neurons were recorded, also makes it unlikely that recordings were made in AII, which in any case tend to be poorly responsive in barbiturate anesthetized animals. It is possible that one or two of the most caudal gyral points (e.g., those dorsal of the top of PES in Fig. 1 and Fig. 4) might have been made in the dorsal posterior field, but the overwhelming majority of recordings were undoubtedly made in AI. The density and distributions of recording sites in all cats were similar to those shown on Fig. 1 and Fig. 4, except that in one of the control cats (DUS 917; data for which are presented in Fig. 2D–F) sulcal bank penetrations failed to yield data (presumably because the electrode orientation, determined by the orientation of the Davies chamber, was not aligned with the sulcal bank).
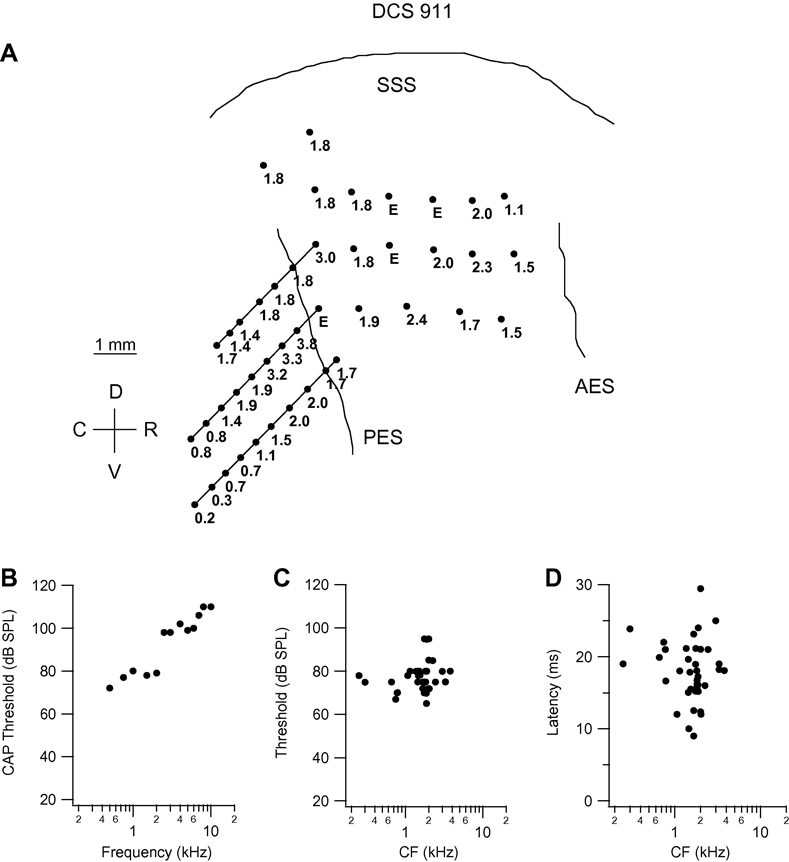
Fig. 4.
Frequency organization and response characteristics in cat DCS 911. Conventions as in Fig. 1.
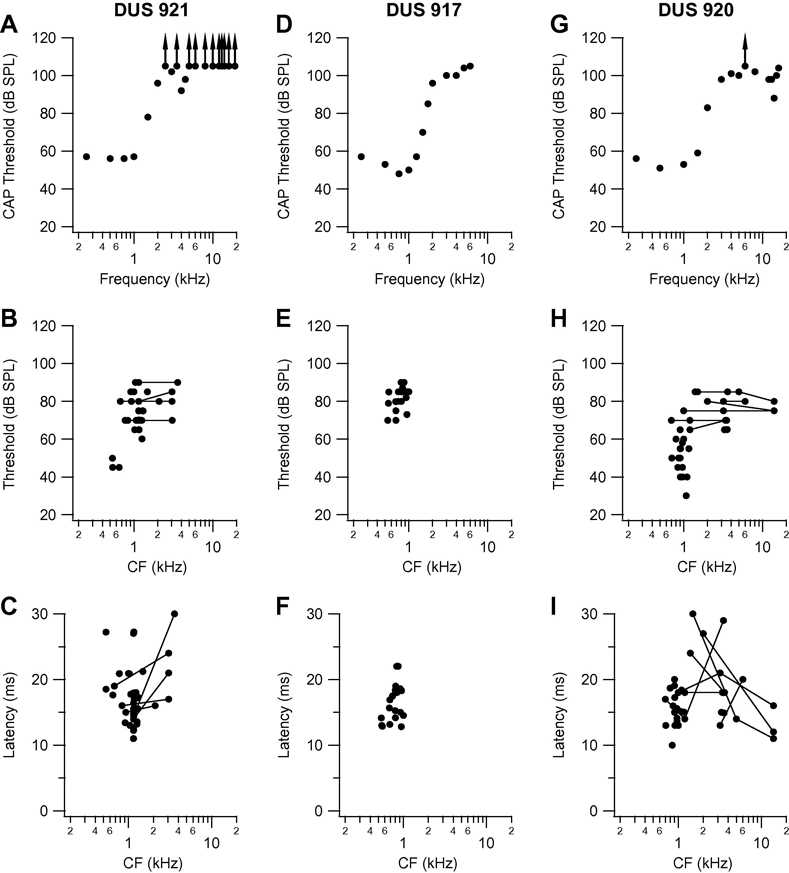
Fig. 2.
Compound action potential audiogram and variation in threshold, and minimum latency at characteristic frequency (CF) as a function of CF for the three other DUS cats: 921 (A, B, C). 917 (D, E, F) and 920 (G, H, I). Points joined by lines in threshold and latency plots are those for clusters that had dual CF at the frequencies joined by the lines. Other conventions as in analogous panels in Fig. 1.
The region mapped in cat DUS 909 is that in which the AI representation of frequencies ranging from approximately 0.1 to 40 kHz or more would normally be represented (Merzenich et al., 1975; Reale & Imig, 1980). The fact that most neuron clusters in this region have CFs in a limited low-frequency range reflects a dramatic change in frequency organization such that the neurons at most recording locations have CFs very different from those they would have had if the cat had not been deafened.
Although low-CF sites were seen up to the high-frequency border of putative AI adjacent to the anterior ectosylvian sulcus (AES), there were a number of points in the rostral half of the gyrus at which no response to acoustic stimulation could be recorded. These points are marked E in the map, because at each site multi-unit clusters responsive to ICES were encountered at one or more depths, indicating that the failure to record acoustic responses did not reflect a general lack of responsiveness at these sites. The only point on the gyrus with CF outside the 0.8 – 1.8 kHz range is that located approximately 2.5 mm rostral of the dorsal tip of the PES in the middle row of penetrations, which had a CF of 3.3 kHz (corresponding to the dip in the CAP audiogram). Threshold and minimum latency at CF are plotted as a function of CF in Figs. 1C and D. As would be expected from the elevated peripheral thresholds shown in the CAP audiogram, the thresholds at CF at most recording sites were high, in the range 75–105 dB. Minimum latency at CF varied in the range 13–27 ms.
Summary data for the other three cats in the DUS group are presented in Fig. 2. The CAP audiogram for cat DUS 921 (Fig. 2A) reveals lowest thresholds, in the range 55–60 dB, at frequencies from 0.25 to 1.0 kHz, increasing steeply to more than 100 dB at frequencies above 2.5 kHz, except for the small dip at 4 kHz. In the cortical frequency map in this animal three points deep in PES had CF ≤ 0.7 kHz but the majority of points in PES and across the MEG had CF in the range 0.8 to 1.5 kHz. A small number of points had double-peaked tuning curves, i.e., thresholds within a 5-dB range at two frequencies separated by other frequencies with higher thresholds; the second CF in each case was in the range 2.1 – 3.6 kHz. In the plots of threshold and latency as a function of CF (Figs. 2B and C), the values for such points are joined by lines. Thresholds varied in the range 60–90 dB, except for three points (the low-CF points deep in PES mentioned above) with threshold in the range 44–49 dB. Minimum latency at CF varied widely, from 11 to 30 ms. As in cat DUS 909, there were a few recording sites on the rostral MEG at which no acoustic response could be recorded but at which there were responses to ICES, indicating that the lack of auditory drive did not reflect a general lack of responsiveness.
In cat DUS 917, the CAP audiogram (Fig. 2D) indicates a loss with a steep slope from a threshold of ~50 dB at 1 kHz to thresholds of 100 dB and higher at frequencies above 2 kHz. As in the cases previously described, acoustically responsive points across the entire rostro-caudal extent of putative AI had low CF, in the range 0.5 to 1.1 kHz. Thresholds at CF (Fig. 2E) ranged from 70 to 90 dB, and minimum latencies (Fig. 2F) from 12 to 22 ms. In the fourth cat (DUS 920) the hearing loss was unusual (Fig. 2G). There was normal low-frequency residual hearing, with thresholds from 50–60 dB at frequencies below 1.5 kHz, increasing to more than 100 dB at 4 kHz, but the CAP audiogram revealed a small area of high-frequency sensitivity around 14 kHz, with a threshold of 88 dB. The majority of points across putative AI had CF in the range 0.9 to 1.5 kHz, but a few had CF in the region of 3.5 kHz, and a number of points had multiple CFs (as defined earlier by thresholds within 5 dB) in one or more of these two ranges, and in some cases in the region of 14 kHz. The clusters with second or third CF in the region of 14 kHz are indicated in Figs. 2 H and I, by the filled circles joined by lines. The threshold data for this cat (Fig. 2H) indicate that the majority of points had thresholds in the range 50 to 80 dB, but that a small number of points (those deep in PES) had thresholds as low as 30 dB. Minimum latency at CF (Fig. 2I) varied in the range 10 to 30 ms.
Responses to intracochlear electrical stimulation in control cats
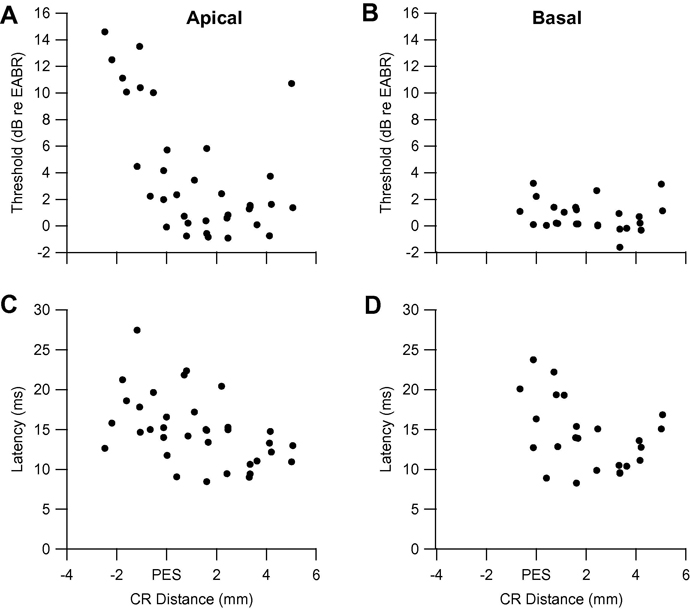
Electrical response data for cat DUS 921 (for which acoustic data were presented in Fig. 2A–C) are presented in Fig. 3. All clusters in this cat, and all cats in both groups, exhibited monotonic increases in the probably of firing, and decreases in mean first spike latency, with increasing stimulus currents (cf. Fallon et al., 2009). Thresholds for ICES on an apical bipolar pair of electrodes are plotted as a function of caudo-rostral distance across the cortex in Fig. 3A. With the exception of a small number of points deep in the PES that could be driven by apical but not by basal ICES, the overwhelming majority of points had thresholds in the range −1 to 7 dB re EABR threshold and there was no evidence of a circumscribed region of low response thresholds. Minimum latencies for apical ICES are shown in Fig. 3C, and varied in the range 8 to 28 ms. Data for ICES on a basal pair of electrodes for this animal are plotted in a similar manner in Fig. 3B and D. Basal ICES resulted in significantly lower thresholds (in the range −2 to 4 dB re EABR threshold) than apical ICES (T-test; P = 0.03), while minimum latencies were similar, in the range 8 to 24 ms (P = 0.85). Similar patterns of responses were seen for the other three DUS cats.
Fig. 3.
Threshold and minimum latency to apical (A, C) and basal (B, D) intracochlear electrical stimulation as a function of caudo-rostral distance across AI in cat DUS 921; distances were measured across the gyral surface and along sulcal bank penetrations from the entry point on the surface, and are plotted relative to the dorsal tip of PES.
Frequency organization and auditory response characteristics in chronically electrically stimulated cats
Data for the four cats in the DCS group are presented in Fig. 4–Fig. 5, in the same format as the DUS cat data. In Fig. 4, the cortical map and associated plots are presented for cat DCS 911, the CAP audiogram (Fig. 4B) for which reveals thresholds in the range 70–80 dB up to 2 kHz, rising steeply to 100 dB and more at frequencies of 2.5 kHz and higher. In the cortical frequency map (Fig. 4A), all acoustically responsive points on the gyrus, and most in the sulcal bank, had CF in the range 1.1–2.4 kHz, with a few in the range 3–4 kHz. Deep in the more ventral sulcal bank penetrations, CFs in the range 0.2 – 0.8 kHz were encountered, and the fact that the lowest CFs were encountered deepest in the sulcus suggests that they represented the low frequency edge of AI. Thresholds at CF varied in the range from 65 to 95 dB (Fig. 4C), and minimum latency at CF in the range 9–30 ms (Fig. 4D).
Summary data for the other three DCS cats are presented in Fig. 5. The CAP audiogram for cat DCS 912 (Fig. 5A) shows residual low-frequency hearing, with thresholds in the range 83–90 dB at frequencies up to 1.5 kHz, with a sharp jump to thresholds above 100 dB at 2 kHz and higher frequencies. All points at which responses to acoustic stimulation could be recorded had CFs in a restricted range from 0.9 to 1.3 kHz. Thresholds at CF varied in the range 68–100 dB (Fig 5B), and minimum latency at CF in the range 15–24 ms (Fig. 5C). In a large proportion of the recording sites in this cat, no response to acoustic stimulation could be recorded, but responses to ICES were recorded at one or more depths (E points).
The CAP audiograms for cats DCS 904 and 903 (Figs. 5D and G, respectively) are unfortunately based on a small number of points at low frequencies, because the RW recordings in these animals were dominated by cochlear microphonic potentials, and determination of CAP thresholds at some frequencies was therefore compromised. The CAP audiogram for cat DCS 904 (Fig. 5D) reveals a threshold of ~60 dB at 0.5 kHz, and thresholds of 100 dB or more at 1 kHz and higher frequencies. Points across the caudo-rostral extent of putative AI had CFs in the range 0.5 – 1.8 kHz, and the plots of threshold and minimum latency at CF (Fig. 5 E and F) indicate that threshold varied in the range 50–90 dB, and latency in the range 11 to 27 ms. A large proportion of recording sites in this cat were unresponsive to acoustic stimulation but responded securely to ICES (4 of 19; 16%). The CAP audiogram for cat DCS 903 (Fig. 5G) was similar to that for cat DCS 904, with a threshold of 70 dB at 0.5 kHz, increasing to 83 dB at 1.0 kHz and to 100 dB or more at 1.5 kHz and higher frequencies. Points across the rostro-caudal extent of putative AI had CF in the range 0.4 – 1.0 kHz (Fig. 5 H and I), but as in cat DCS 904, there were a large number of recording sites (9 of 26; 34.6%), mainly in the middle to rostral portion of the MEG, at which neurons were electrically but not acoustically responsive. Thresholds varied in the range 70–90 dB (Fig. 5 H) and minimum latency in the range 13–25 ms (Fig. 5 I).
Comparisons between auditory response characteristics in the two groups
As indicated by the CAP audiograms in Fig. 1, Fig. 2, Fig. 4, and Fig. 5, the hearing losses in individual animals were idiosyncratic. In order to compare the losses between the groups, two quantitative measures were used: the residual threshold (i.e., the minimum threshold in the region of residual hearing) and the cut-off frequency (i.e., the frequency at which the CAP threshold increased to 50% of the difference between the residual threshold and the maximum SPL at which a response could be recorded). The mean residual threshold in the DCS group (mean ± standard error of mean: 71 ± 5 dB) was higher than that in the DUS group (60 ± 8 dB), but this difference was not statistically significant (P = 0.25; T-test was used for this and all other comparisons of group means unless otherwise stated). The mean cut-off frequencies were very similar (DUS: 1.7 ± 0.1 kHz; DCS: 1.5 ± 0.1) and also not significantly different (P = 0.6).
The auditory response characteristics (viz. threshold and minimum latency at CF) of AI multi-unit responses in the two groups of cats are compared in Table 1. In calculating group means, the lower threshold and shorter latency were entered into the analysis for those points which had multiple CFs. The threshold and minimum latency data indicate that the mean values for the two groups were very similar and were not significantly different. Another way in which the presence of electrical input from the high-frequency region of the cochlea in the cats that received chronic ICES might affect acoustic responsiveness is in terms of the number of cortical sites at which neurons are electrically but not acoustically excited. Comparison of the proportions of points classified as “E” (no response to acoustic stimulation but secure response to ICES) was based only on recording sites on the gyrus, because, as noted earlier, a lack of acoustic responsiveness at a site in a sulcal bank penetration could be attributable to the recording site being located in the white matter or in unresponsive cortical layers. The proportion of gyral points classified as “E” was substantially higher in the DCS than in the DUS cats (30.6% vs 13.6%; Table 1). This difference was significant (P < 0.05; Chi-square test), suggesting that the presence of input to neurons at these sites from ICES of the high-frequency region of the cochlea reduced the probability of their exhibiting responses to low-frequency acoustic stimuli. The proportions of recording sites at which neurons were acoustically but not electrically excited was very small in both groups (8.8% and 5.2% in DCS and DUS groups, respectively), and the difference was not significant (P = 0.42).
Table 1.
Cortical response summary
| Group | Acoustic Stimulation | Electric Stimulation | |||||
|---|---|---|---|---|---|---|---|
| Apical | Basal | ||||||
| Threshold ± SEM (dB) |
Latency ± SEM (ms) |
“E Points” (%) |
Threshold ± SEM (dB re EABR) |
Latency ± SEM (ms) |
Threshold ± SEM (dB re EABR) |
Latency ± SEM (ms) |
|
| DUS | 73.5 ± 1.5 | 17.4 ± 0.4 | 13.6 | 4.8 ± 0.6 | 12.8 ± 0.4 | 1.0 ± 0.4 # | 12.6 ± 0.4 |
| DCS | 76.2 ± 1.1 | 18.2 ± 0.4 | 30.6** | 6.1 ± 0.5* | 12.1 ± 0.5† | 3.1 ± 0.4 #* | 11.6 ± 0.5† |
Significant difference from corresponding apical group (T-test; P < 0.05)
Significant difference from corresponding DUS group (T-test; P < 0.05)
Significant difference from corresponding DUS group (Chi-squared test; P < 0.05)
Significant difference in latency between groups (ANOVA; P <0.05), and no significant interaction
"E Points": recording sites on the gyrus that are responsive to ICES but not acoustic stimulation
Responses to intracochlear electrical stimulation in chronically electrically stimulated cats and comparisons between response characteristics in the two groups
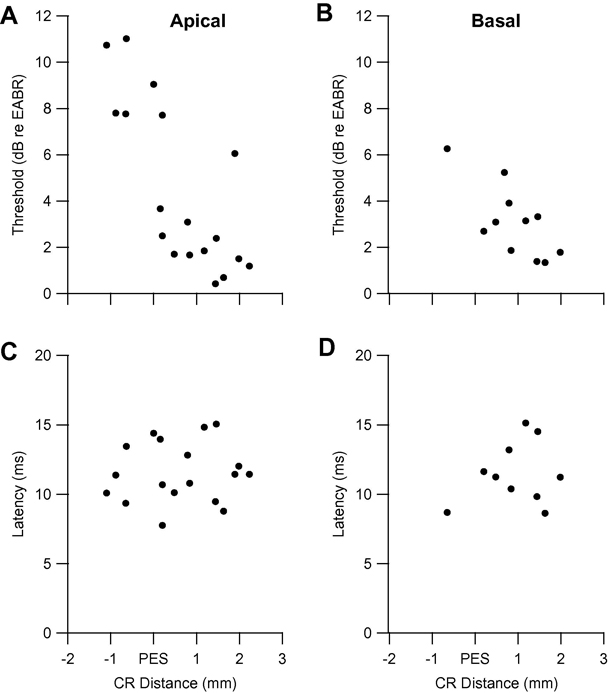
Electrical response data for cat DCS 903 (for which acoustic data were presented in Fig. 5 G–I) are presented in Fig. 6. Thresholds for apical and basal ICES were similar (varying in the ranges 0 to 11 dB and 1 to 6 dB, respectively), and were not significantly different (P = 0.32). Minimum latencies were also similar for apical (range 7–15 ms) and basal (range 8 – 15 ms) ICES, and were not significantly different (P = 0.28). Again there was no evidence of a circumscribed region of low response thresholds or minimum latencies for this, or any other DCS, animal.
Fig. 6.
Threshold and minimum latency to apical (A, C) and basal (B, D) intracochlear electrical stimulation as a function of caudo-rostral distance across AI in cat DCS 903. Conventions as in Fig. 3.
The electrical response characteristics of multi-unit responses in the two groups of cats are compared in Table 1. The effects of the two variables (group and electrode location) were evaluated by two-way analysis of variance (ANOVA): there was a significant effect for group in the threshold analysis (P < 0.001), and post-hoc tests indicated that the mean normalized threshold for the DCS group was higher than that for the DUS group for both electrode locations. The normalized thresholds for basal ICES were significantly lower (P< 0.001) than for apical ICES, and there was no significant interaction term (P = 0.30). The minimum latency for the DCS group was significantly shorter than for the DUS group (P = 0.04). There was no difference between apical and basal ICES (P = 0.26), and again, there was no significant interaction term (P = 0.89). Finally, the thresholds at points that were unresponsive to acoustic stimulation did not differ significantly from those at points that could be driven by both acoustic and electric stimuli in either the DUS (P = 0.35) or DCS (P = 0.98) groups.
DISCUSSION
We have previously shown that hair cells apical to an electrode array can survive chronic implantation and ICES in the absence of electrode insertion trauma or severe inflammation (Coco et al., 2007; Shepherd et al., 1983; Xu et al., 1997), and the histological evidence from the chronically implanted cats in this study confirms this finding. The major aim of the present study was to extend this work functionally, by determining the effects of chronic ICES via a cochlear implant on the auditory response characteristics in the AI of cats with residual high-threshold low-frequency hearing. The deafening procedure resulted in severe hearing losses in all of the cats in the two groups, with residual hearing at frequencies below ~ 1.5 kHz, and with mean minimum CAP thresholds in this frequency range of 60–71 dB.
Although the severe hearing losses in all of the cats in the study prevented the definition of AI by physiological criteria, the considerations examined in the Results support the view that the overwhelming majority of recordings were made in AI. In comparison to previous studies of the effects of ICES in congenitally deaf cats (Kral et al., 2002, 2009) or in adult cats deafened immediately prior to recording (Raggio and Schreiner, 1994, 1999), in which recordings were restricted to the gyral surface, we sampled more of the rostro-caudal extent of AI (because of our sulcal bank penetrations), but less of the dorso-ventral extent of the gyral region AI. Our more extensive sampling of the rostro-caudal extent of AI was driven by the need to obtain recordings across the entire tonotopic axis of putative AI. In all of the cats in both groups, the frequency organization of the AI was dramatically different from that in normal cats. The nature of this change in frequency organization will be considered in the following section, followed by an evaluation of the effects of chronic implantation and ICES on neuronal auditory response characteristics and the possible clinical implications of these effects.
Nature of Changes in Frequency Organization
In all of the cats in both experimental groups, most of the recording sites in the region (rostral bank of PES and the MEG) that in normal cats would contain the AI tonotopic representation of the frequency range from ~0.1 to ~ 40 kHz (Merzenich et al., 1975; Reale and Imig, 1980) had CFs in a limited low-frequency range over which the CAP audiograms of these cats rose steeply from a region of moderate-to-severe loss to a region of profound loss. That is, AI was largely occupied by a massively larger than normal region in which neurons had CF in a limited low-frequency range. This change in frequency organization is similar to that described previously as a consequence of restricted cochlear lesions in adults (e.g. Noreña et al., 2003; Rajan et al., 1993; Robertson & Irvine, 1989) and after neonatal lesions produced by either ototoxic agents (e.g. Harrison et al., 1991, 1993) or by loud-sound exposure (e.g. Eggermont & Komiya, 2000; Seki & Eggermont, 2002).
An enlarged representation of frequencies at the steeply-sloping edge of a profound hearing loss could be a manifestation of neural plasticity, in which neurons deprived of their normal input by the cochlear lesion develop new responses at frequencies represented at the edge of the cochlear lesion. Plasticity of this sort is directly analogous to that seen in the somatosensory and visual systems after analogous lesions to restricted regions of the receptor surface (for reviews see Irvine & Wright, 2005; Kaas & Florence, 2001). As argued elsewhere, however, a change in frequency organization of this sort is not in itself evidence of neural plasticity (e.g. Rajan et al., 1993; Robertson & Irvine, 1989). Auditory cortical neurons respond over a relatively broad frequency range at higher SPLs, and the responses at these levels reflect convergence of input across frequency channels (e.g. Snyder & Sinex, 2002). A given cluster’s new CF at a frequency lower than its original CF (at which input has been eliminated by the cochlear lesion) could simply reflect a pre-lesion response at that lower frequency - what has been termed the “residue” of the pre-lesion response area (e.g. Rajan & Irvine, 1998b; Rajan et al., 1993). If this were the case, the change in frequency organization would simply be a passive consequence of the removal of peripheral input and would provide no evidence of a dynamic process of plasticity.
In previous studies of changes in cortical and subcortical frequency organization after cochlear lesions that left a low-frequency region with normal thresholds, we have argued that the signature of such a passive change in the frequency map would be a progressive increase in threshold at the new CF as a function of position across the tonotopic axis of the structure (e.g. Rajan & Irvine, 1998b; Rajan et al., 1993). This increase would be expected because the new CFs at recording sites with progressively higher pre-lesion CF would be progressively further up the low-frequency slope of the pre-lesion tuning curve. As a consequence of this progressive increase in threshold, the thresholds at their new CF of neurons in the region of the expanded representation of lesion-edge frequencies are significantly higher than those of neurons whose pre-lesion CF was at that frequency. In studies of adult plasticity after restricted cochlear lesions, progressive increases in threshold of precisely this sort, and significant elevations of mean threshold, were seen in the dorsal cochlear nucleus (Rajan & Irvine, 1998a) and in the majority of penetrations through the central nucleus of the inferior colliculus (Irvine et al., 2003), The changes in frequency organization in those structures were therefore identified as passive consequences of the cochlear lesions. In contrast, thresholds across the reorganized region do not change systematically, and are not significantly elevated, in the AI (Rajan et al., 1993) or in the ventral nucleus of the medial geniculate nucleus (Kamke et al., 2003) after such lesions, and the changes in frequency organization in these structures have therefore been attributed to plasticity.
The evidence on thresholds in reorganized AI in studies of the effects of neonatal lesion is less clear. The ototoxic cochlear lesions produced by Harrison and his colleagues (1991; 1993) resulted in severe high-frequency hearing losses and an enlarged representation of lesion-edge frequencies, but these authors did not present evidence on thresholds in the reorganized area of cortex. Eggermont and Komiya (2000) reported that thresholds in the reorganized area of cortex after exposure of kittens to a loud tone did not differ from those of neurons with the same CF in control animals, indicating that the changed frequency organization reflected plasticity rather than residual responses. In Seki and Eggermont’s (2002) study of loud-tone induced cochlear damage, most animals suffered only mild to moderate hearing losses; only a small number of animals showed changes in tonotopy, and thresholds were elevated in some but not in others
As these considerations indicate, the changes in frequency organization seen in the present study could be attributable to either cortical plasticity or residual responses. Unfortunately, the limited low-frequency range of the residual hearing in the cats in both groups, and the very high thresholds at those frequencies, have the consequence that information on thresholds does not allow a decision to be made between these alternatives. First, although some points deep in PES had very low CFs (e.g., those at the bottom of the more ventral sulcal bank penetrations in cats DUS 909 (Fig 1A) and DCS 911 (Fig. 4A)), in most cases the CFs at recording sites in the sulcal bank were in the same range as those on the gyrus (e.g., those in DCS 912; Fig. 5A). It is therefore not possible to identify with certainty recording sites at which the CF is the same as the pre-lesion CF, and it is consequently impossible to compare thresholds at such points with those at points with new CF. Second, although threshold in most cases did not appear to change systematically across putative AI, the substantial peripheral loss in sensitivity in the frequency range of the expanded representation in all of the cats imposes severe limits on the range over which threshold can vary, and the absence of any systematic variation in threshold might simply reflect this limited range. In summary, there are no grounds on which it can be determined with certainty whether the changed frequency organization reflects plasticity or residual responses.
Regardless of the processes underlying the changes in frequency organization, however, the fact that the changes were of exactly the same sort in the two groups indicates that chronic implantation and ICES had no effect on this aspect of the responsiveness of AI neurons to acoustic stimulation.
Effects of Chronic Implantation and ICES on Other Neuronal Response Characteristics
Although the results do not allow the nature of the observed changes in frequency organization to be determined, they do provide unique evidence on the effects of chronic implantation and ICES on basic characteristics of the cortical responses to both auditory and electrical stimulation. Table 1 indicates that the mean auditory response thresholds and minimum latencies in the two groups were not significantly different, so chronic implantation and ICES had no effect on these response characteristics. Of greater importance is the fact that the proportion of electrically but not acoustically activated sites was significantly higher in the stimulated than the unstimulated cats (Table 1). This finding suggests that chronic implantation and ICES might have partially limited the extent to which either new auditory responses could develop or residual auditory responses could be retained in the regions of cortex deprived of their normal input by the cochlear lesion. Although our data provide no direct evidence on the mechanisms underlying this effect, it is tempting to speculate that it might reflect use-based changes in the efficacy of the synapses by which electrical and acoustic input activated the cortical neurons, or neurons earlier in the ascending pathways to the cortical neurons. If the electrical input activated those neurons more strongly and more frequently than the acoustic input, then those synapses conveying electrical input would be expected to be strengthened and those conveying acoustic input to be weakened in accordance with established principles for the effects of correlated activity on synaptic strength (e.g., Bi & Poo, 2001).
Another issue is whether the period of chronic implantation and ICES might modify the responses of cortical neurons to electrical stimulation and whether residual low-frequency hearing affects these changes. Our finding that all clusters in both groups had monotonic electrical IO functions and showed monotonic decreases in latency with increasing stimulus current is in agreement with our previous results in profoundly deaf cats (Fallon et al., 2009). The monotonic decrease in latency is in agreement with Raggio and Schreiner’s (1994) description of electrical response latencies in AI of adult cats deafened immediately prior to recording. However, our finding that all IO functions were monotonic is in contrast to their finding that IO functions at almost half of their recording sites were non-monotonic (see discussion of this difference by Fallon et al. (2009)).
As summarized in Table 1 there was a significant 2 – 3 dB increase in normalized threshold for ICES in the chronically stimulated animals. This increase in threshold with chronic ICES is consistent with previous reports using chronic ICES in profoundly deaf cats (Fallon et al., 2009). There was also a small (0.9 ms) but significant reduction in electrical response latency of the chronically stimulated animals compared to the unstimulated controls. There have been no previous reports of the effects of chronic ICES on multi-unit response latencies in AI, but the decrease is consistent with Kral et al.’s (2002) report of a decrease in the latency of the first positive waves of the cortical field potentials in two congenitally deaf cats (implanted at 2.5 and 5 months, respectively) that received 5 months of ICES. This result is also in accord with previous reports of decreased response latencies in the IC of profoundly deaf cats receiving chronic ICES (Snyder et al., 1995; Vollmer et al., 2005; Vollmer et al., 1999). Taken together, the changes in responses to electrical stimuli in our animals are similar to those seen with such stimulation in profoundly deaf animals, and indicate that the residual low-frequency hearing in our animals did not substantially alter the effects of chronic implantation and ICES.
These conclusions concerning the effects of chronic implantation and ICES must be qualified by two considerations. The first is that the chronic ICES regime used in this study involved stimulation for only six hours/day for five days/week, whereas the acoustic stimulation was present 24 hours/day. Although there is an obvious imbalance in the duration of the electrical and acoustical input experienced by the stimulated cats, it should also be noted that the acoustic input was limited to a very restricted frequency – SPL domain (viz., components of sounds in the animal facility environment below ~ 2 kHz and above ~60 dB), so AI neurons would have been much more weakly driven by acoustic than electrical stimulation. It is nevertheless possible that stimulation strategies and durations that more closely approximate those of cochlear implant users (viz., of the order of 16 hours/day for 7 days a week) might have had greater effects on frequency organization and/or on the auditory or electrical response characteristics of cortical neurons. The second qualification relates to the fact that the chronic ICES did not provide the animal with any behaviourally relevant information about its acoustic environment. It has been shown that continuous exposure to un-informative broad-band acoustic stimulation during the critical period may arrest cortical development (Chang and Merzenich, 2003). The arrested development is characterised by an expanded cortical area that remains responsive to pure-tone stimuli and a decrease in tuning of individual cortical locations, when compared to age-matched controls. However, in the present study, we saw a reduction in the proportion of sites that were acoustically responsive and no change to the basic response characteristics of AI neurons. Nevertheless, if the electrical stimulation conveyed environmental information to the cats, as would be the case if it were derived from a clinical speech processor worn by the animal (see, e.g., Fallon et al., 2009), or if its salience were enhanced by behavioral conditioning procedures (e.g., Vollmer et al., 1999; Kral et al., 2002) it might well have a greater impact on cortical response characteristics.
In comparing the two groups of cats in this study, it should also be noted that they differed in the fact that the DCS cats were both chronically implanted and stimulated. Where there is no difference between the groups (viz., in frequency organization and acoustic response characteristics) it is clear that neither implantation per se nor the presentation of chronic ICES had an effect. Where there is a difference between the groups, that difference could in principle be attributable to implantation alone or to the combination of implantation and chronic ICES. It seems unlikely that the substantial and significant difference between the groups in the proportion of electrically but not acoustically activated sites could be attributable to implantation alone, but the small differences in threshold might well be attributable to one or both factors.
Clinical Implications
The major result of this study is that that chronic implantation and ICES had no effect on the changes in the frequency organization of AI consequent on a severe hearing loss with residual high-threshold low-frequency hearing, or on the basic auditory response characteristics of AI neurons. However, chronic implantation and ICES was associated with a significantly higher proportion of locations in the AI at which neurons were electrically but not acoustically excited. The former results suggests that chronic implantation and ICES does not have any deleterious effects on basic aspects of auditory cortical processing, and thus would not affect aspects of residual hearing that depend on these characteristics. However, the clinical significance of the increased proportion of recording locations that are electrically but not acoustically responsive is more difficult to determine. Most of this increase was in the region of cortex in which the middle-to-high frequencies, at which there was a profound hearing loss, would normally have been represented. Neurons in this region that were acoustically responsive exhibited responses (whether new or residual) at the very low frequencies spared by the cochlear lesion. The effect of a reduction in acoustic responsiveness in these regions will depend on the extent to which such responses contribute to auditory perceptual experience at those low frequencies, but there is only limited and indirect evidence bearing on this issue.
A magnetoencephalographic study of humans with steeply-sloping hearing losses of the sort that produce cortical reorganization in animals has provided evidence for an expanded representation of lesion-edge frequencies analogous to that seen in the animal studies (Dietrich et al., 2001). A consistent finding in humans with such hearing losses, which in some cases have been shown to be associated with high-frequency “dead regions” in the cochlea (for discussion see Kluk & Moore, 2006; Moore & Vinay, 2009), is a small but statistically-significant improvement in frequency discrimination at lesion-edge frequencies (Kluk & Moore, 2006; McDermott et al., 1998; Moore & Vinay, 2009; Thai-Van et al., 2003; Thai-Van et al., 2007). Performance on a number of other auditory tasks (viz. loudness perception, intensity discrimination, frequency sweep detection, gap detection and discrimination) in such listeners has been found not to exhibit unusual characteristics that might reflect the changed frequency organization (see Buss et al., 1998; Irvine et al., 2000; McDermott et al., 1998). However, Moore & Vinay (2009) have recently reported that subjects with acquired high-frequency dead regions showed improved amplitude modulation detection and consonant identification at low frequencies. It is not known if cortical reorganization occurred in these subjects, or if the improved performance on these tasks and on frequency discrimination at lesion edge frequencies depends on the characteristics of AI neurons in the region of changed frequency organization. Nevertheless, these data suggest that a larger than normal representation of lesion-edge frequencies might have perceptual consequences, and it is therefore possible that the reduction in the proportion of recording sites that were acoustically responsive to frequencies in the range of residual hearing that we observed in animals receiving chronic ICES might also have perceptual consequences.
Consideration of the possible clinical implications of the present data with respect to cochlear implantation in humans with residual hearing must be qualified by at least three considerations. Two of these, discussed in detail in the previous section, relate to the facts that electrical stimulation was presented for a shorter period (six hours/day for five days/week) than is the case in cochlear implant users (of the order of 16 hours/day for 7 days a week) and that it did not provide behaviourally relevant information. The third is that implantation in humans with residual hearing is most common in post-lingually deaf adults in which there has been a progressive hearing loss over time. In contrast, our chronically implanted animals were neonatally deafened, by a procedure that produced a relatively abrupt hearing loss, and were implanted at eight weeks of age, prior to the critical period of greatest plasticity in the developing auditory system (Kral et al., 2006). It would be expected that chronic ICES during this developmental period would be more likely to result in plastic changes in cortex than such stimulation later in life. The fact that changes in the chronically stimulated cats in our study were restricted to the proportion of acoustically responsive neurons therefore suggests that substantial changes in adult implantees would be extremely unlikely.
ACKNOWLEDGEMENTS
This work was funded by NIDCD (NO1-DC-3–1005 & HHS-N-263-2007-00053-C) and The Bionic Ear Institute. The Bionic Ear Institute acknowledges the support it receives from the Victorian Government through its Operational Infrastructure Support Program. The authors are grateful to Jin Xu and Helen Feng for electrode manufacture and surgical assistance; Anne Coco and Stephanie Epp for research assistance; Sue Pierce for veterinary advice; Elisa Borg for animal maintenance; Rodney Millard for engineering support and helpful discussions on experimental design, and Hugh McDermott, Peter Blamey and two anonymous reviewers for comments on an earlier version of the manuscript.
ABBREVIATIONS
- ICES
Intracochlear electrical stimulation
- SNHL
sensorineural hearing loss
- IC
inferior colliculus
- AI
primary auditory cortex
- ABR
auditory brainstem response
- CAP
compound action potential
- RW
round window
- EABR
Electrically-evoked auditory brainstem response
- SPL
sounds pressure level
- MEG
middle ectosylvian gyrus
- PES
posterior ectosylvian sulcus
- IO
input-output
- AES
anterior ectosylvian sulcus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bi G, Poo M. Synaptic modification by correlated activity: Hebb's postulate revisited. Annu Rev Neurosci. 2001;24:139–166. doi: 10.1146/annurev.neuro.24.1.139. [DOI] [PubMed] [Google Scholar]
- Black RC, Clark GM, O'Leary SJ, Walters C. Intracochlear electrical stimulation of normal and deaf cats investigated using brainstem response audiometry. Acta Otolaryngol Suppl. 1983;399:5–17. doi: 10.3109/00016488309105588. [DOI] [PubMed] [Google Scholar]
- Blamey P, Arndt P, Bergeron F, Bredberg G, Brimacombe J, Facer G, Larky J, Lindstrom B, Nedzelski J, Peterson A, Shipp D, Staller S, Whitford L. Factors affecting auditory performance of postlinguistically deaf adults using cochlear implants. Audiology and Neuro-Otology. 1996;1:293–306. doi: 10.1159/000259212. [DOI] [PubMed] [Google Scholar]
- Brown M, Irvine DRF, Park VN. Perceptual learning on an auditory frequency discrimination task by cats: association with changes in primary auditory cortex. Cereb Cortex. 2004;14:952–965. doi: 10.1093/cercor/bhh056. [DOI] [PubMed] [Google Scholar]
- Brown M, Shepherd RK, Webster WR, Martin RL, Clark GM. Cochleotopic selectivity of a multichannel scala tympani electrode array using the 2-deoxyglucose technique. Hear Res. 1992;59:224–240. doi: 10.1016/0378-5955(92)90119-8. [DOI] [PubMed] [Google Scholar]
- Buss E, Hall JW, 3rd, Grose JH, Hatch DR. Perceptual consequences of peripheral hearing loss: do edge effects exist for abrupt cochlear lesions? Hear Res. 1998;125:98–108. doi: 10.1016/s0378-5955(98)00131-2. [DOI] [PubMed] [Google Scholar]
- Chang EF, Merzenich MM. Environmental noise retards auditory cortical development. Science. 2003;300:498–502. doi: 10.1126/science.1082163. [DOI] [PubMed] [Google Scholar]
- Coco A, Epp SB, Fallon JB, Xu J, Millard RE, Shepherd RK. Does cochlear implantation and electrical stimulation affect residual hair cells and spiral ganglion neurons? Hear Res. 2007;225:60–70. doi: 10.1016/j.heares.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich V, Nieschalk M, Stoll W, Rajan R, Pantev C. Cortical reorganization in patients with high frequency cochlear hearing loss. Hear Res. 2001;158:95–101. doi: 10.1016/s0378-5955(01)00282-9. [DOI] [PubMed] [Google Scholar]
- Dinse HR, Godde B, Reuter G, Cords SM, Hilger T. Auditory cortical plasticity under operation: reorganization of auditory cortex induced by electric cochlear stimulation reveals adaptation to altered sensory input statistics. Speech Communication. 2003;41:201–219. [Google Scholar]
- Dinse HR, Reuter G, Cords SM, Godde B, Hilger T, Lenarz T. Optical imaging of cat auditory cortical organization after electrical stimulation of a multichannel cochlear implant: differential effects of acute and chronic stimulation. Am J Otol. 1997;18:S17–S18. [PubMed] [Google Scholar]
- Eggermont JJ, Komiya H. Moderate noise trauma in juvenile cats results in profound cortical topographic map changes in adulthood. Hear Res. 2000;142:89–101. doi: 10.1016/s0378-5955(00)00024-1. [DOI] [PubMed] [Google Scholar]
- Fallon JB, Irvine DRF, Shepherd RK. Cochlear implants and brain plasticity. Hear Res. 2008;238:110–117. doi: 10.1016/j.heares.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon JB, Irvine DRF, Shepherd RK. Cochlear implant use following neonatal deafness influences the cochleotopic organization of the primary auditory cortex in cats. J Comp Neurol. 2009;512:101–114. doi: 10.1002/cne.21886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantz BJ, Turner CW. Combining acoustic and electrical hearing. Laryngoscope. 2003;113:1726–1730. doi: 10.1097/00005537-200310000-00012. [DOI] [PubMed] [Google Scholar]
- Green KM, Julyan PJ, Hastings DL, Ramsden RT. Auditory cortical activation and speech perception in cochlear implant users: effects of implant experience and duration of deafness. Hear Res. 2005;205:184–192. doi: 10.1016/j.heares.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Harrison RV, Nagasawa A, Smith DW, Stanton S, Mount RJ. Reorganization of auditory cortex after neonatal high frequency cochlear hearing loss. Hear Res. 1991;54:11–19. doi: 10.1016/0378-5955(91)90131-r. [DOI] [PubMed] [Google Scholar]
- Harrison RV, Stanton SG, Nagasawa A, Ibrahim D, Mount RJ. The effects of long-term cochlear hearing loss on the functional organization of central auditory pathways. J Otolaryngol. 1993;22:4–11. [PubMed] [Google Scholar]
- Huang CQ, Shepherd RK, Carter PM, Seligman PM, Tabor B. Electrical stimulation of the auditory nerve: direct current measurement in vivo. IEEE Trans Biomed Eng. 1999;46:461–470. doi: 10.1109/10.752943. [DOI] [PubMed] [Google Scholar]
- Irvine DRF, Wright BA. Plasticity in Spectral Processing. In: Malmierca M, Irvine DRF, editors. Auditory Spectral Processing. San Diego: Elsevier Academic; 2005. pp. 435–472. [Google Scholar]
- Irvine DRF, Rajan R, McDermott HJ. Injury-induced reorganization in adult auditory cortex and its perceptual consequences. Hear Res. 2000;147:188–199. doi: 10.1016/s0378-5955(00)00131-3. [DOI] [PubMed] [Google Scholar]
- Irvine DRF, Rajan R, Smith S. Effects of restricted cochlear lesions in adult cats on the frequency organization of the inferior colliculus. J Comp Neurol. 2003;467:354–374. doi: 10.1002/cne.10921. [DOI] [PubMed] [Google Scholar]
- Kaas JH, Florence SL. Reorganization of sensory and motor systems in adults mammals after injury. In: Kaas JH, editor. The Mutable Brain. Amsterdam: Harwood Academic Publishers; 2001. pp. 165–242. [Google Scholar]
- Kamke MR, Brown M, Irvine DRF. Plasticity in the tonotopic organization of the medial geniculate body in adult cats following restricted unilateral cochlear lesions. J Comp Neurol. 2003;459:355–367. doi: 10.1002/cne.10586. [DOI] [PubMed] [Google Scholar]
- Kiefer J, Pok M, Adunka O, Sturzebecher E, Baumgartner W, Schmidt M, Tillein J, Ye Q, Gstoettner W. Combined electric and acoustic stimulation of the auditory system: results of a clinical study. Audiology and Neuro-Otology. 2005;10:134–144. doi: 10.1159/000084023. [DOI] [PubMed] [Google Scholar]
- Klinke R, Hartmann R, Heid S, Tillein J, Kral A. Plastic changes in the auditory cortex of congenitally deaf cats following cochlear implantation. Audiol Neurootol. 2001;6:203–206. doi: 10.1159/000046833. [DOI] [PubMed] [Google Scholar]
- Kluk K, Moore BC. Dead regions in the cochlea and enhancement of frequency discrimination: Effects of audiogram slope, unilateral versus bilateral loss, and hearing-aid use. Hear Res. 2006;222:1–15. doi: 10.1016/j.heares.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Kral A, Hartmann R, Tillein J, Heid S, Klinke R. Hearing after congenital deafness: Central auditory plasticity and sensory deprivation. Cereb Cortex. 2002;12:797–807. doi: 10.1093/cercor/12.8.797. [DOI] [PubMed] [Google Scholar]
- Kral A, Tillein J. Brain plasticity under cochlear implant stimulation. Adv Otorhinolaryngol. 2006;64:89–108. doi: 10.1159/000094647. [DOI] [PubMed] [Google Scholar]
- Kral A, Tillein J, Heid S, Klinke R, Hartmann R. Cochlear implants: cortical plasticity in congenital deprivation. Prog Brain Res. 2006;157:283–313. doi: 10.1016/s0079-6123(06)57018-9. [DOI] [PubMed] [Google Scholar]
- Kral A, Tillein J, Hubka P, Schiemann D, Heid S, Hartmann R, Engel AK. Spatiotemporal patterns of cortical activity with bilateral cochlear implants in congenital deafness. J Neurosci. 2009;29:811–827. doi: 10.1523/JNEUROSCI.2424-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott HJ, Sucher C, Simpson A. Electro-Acoustic Stimulation. Audiology and Neuro-Otology. 2009 doi: 10.1159/000206489. in press. [DOI] [PubMed] [Google Scholar]
- McDermott HJ, Lech M, Kornblum MS, Irvine DRF. Loudness perception and frequency discrimination in subjects with steeply sloping hearing loss: possible correlates of neural plasticity. J. Acoust. Soc. Am. 1998;104:2314–2325. doi: 10.1121/1.423744. [DOI] [PubMed] [Google Scholar]
- McKay CM. Spectral processing in cochlear implants. Int Rev Neurobiol. 2005;70:473–509. doi: 10.1016/S0074-7742(05)70014-3. [DOI] [PubMed] [Google Scholar]
- Merzenich MM, Knight PL, Roth GL. Representation of cochlea within primary auditory cortex in the cat. J Neurophysiol. 1975;38:231–249. doi: 10.1152/jn.1975.38.2.231. [DOI] [PubMed] [Google Scholar]
- Moore BC, Vinay SN. Enhanced discrimination of low-frequency sounds for subjects with high-frequency dead regions. Brain. 2009;132:524–536. doi: 10.1093/brain/awn308. [DOI] [PubMed] [Google Scholar]
- Noreña AJ, Tomita M, Eggermont JJ. Neural changes in cat auditory cortex after a transient pure-tone trauma. J Neurophysiol. 2003;90:2387–2401. doi: 10.1152/jn.00139.2003. [DOI] [PubMed] [Google Scholar]
- Raggio MW, Schreiner CE. Neuronal responses in cat primary auditory cortex to electrical cochlear stimulation. I. Intensity dependence of firing rate and response latency. J Neurophysiol. 1994;72:2334–2359. doi: 10.1152/jn.1994.72.5.2334. [DOI] [PubMed] [Google Scholar]
- Raggio MW, Schreiner CE. Neuronal responses in cat primary auditory cortex to electrical cochlear stimulation. III. Activation patterns in short- and long-term deafness. J Neurophysiol. 1999;82:3506–3526. doi: 10.1152/jn.1999.82.6.3506. [DOI] [PubMed] [Google Scholar]
- Rajan R, Irvine DRF. Absence of plasticity of the frequency map in dorsal cochlear nucleus of adult cats after unilateral partial cochlear lesions. J Comp Neurol. 1998a;399:35–46. [PubMed] [Google Scholar]
- Rajan R, Irvine DRF. Neuronal responses across cortical field A1 in plasticity induced by peripheral auditory organ damage. Audiology and NeuroOtology. 1998b;3:123–144. doi: 10.1159/000013786. [DOI] [PubMed] [Google Scholar]
- Rajan R, Irvine DRF, Cassell JF. Normative N1 audiogram data for the barbiturate-anaesthetised domestic cat. Hear Res. 1991;53:153–158. doi: 10.1016/0378-5955(91)90222-u. [DOI] [PubMed] [Google Scholar]
- Rajan R, Irvine DRF, Wise LZ, Heil P. Effect of unilateral partial cochlear lesions in adult cats on the representation of lesioned and unlesioned cochleas in primary auditory cortex. J Comp Neurol. 1993;338:17–49. doi: 10.1002/cne.903380104. [DOI] [PubMed] [Google Scholar]
- Reale RA, Imig TJ. Tonotopic organization in auditory cortex of the cat. J Comp Neurol. 1980;192:265–291. doi: 10.1002/cne.901920207. [DOI] [PubMed] [Google Scholar]
- Reiss LA, Turner CW, Erenberg SR, Gantz BJ. Changes in pitch with a cochlear implant over time. JARO. 2007;8:241–257. doi: 10.1007/s10162-007-0077-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D, Irvine DRF. Plasticity of frequency organization in auditory cortex of guinea pigs with partial unilateral deafness. J Comp Neurol. 1989;282:456–471. doi: 10.1002/cne.902820311. [DOI] [PubMed] [Google Scholar]
- Sachs MB, Abbas PJ. Rate versus level functions for auditory-nerve fibers in cats: tone-burst stimuli. J. Acoust. Soc. Am. 1974;56:1835–1847. doi: 10.1121/1.1903521. [DOI] [PubMed] [Google Scholar]
- Seki S, Eggermont JJ. Changes in cat primary auditory cortex after minor-to-moderate pure-tone induced hearing loss. Hear Res. 2002;173:172–186. doi: 10.1016/s0378-5955(02)00518-x. [DOI] [PubMed] [Google Scholar]
- Shepherd RK, Martin RL. Onset of ototoxicity in the cat is related to onset of auditory function. Hear Res. 1995;92:131–142. doi: 10.1016/0378-5955(95)00211-1. [DOI] [PubMed] [Google Scholar]
- Shepherd RK, Clark GM, Black RC. Chronic electrical stimulation of the auditory nerve in cats. Physiological and histopathological results. Acta Otolaryngol Suppl. 1983;399:19–31. doi: 10.3109/00016488309105589. [DOI] [PubMed] [Google Scholar]
- Simpson A, McDermott HJ, Dowell RC, Sucher C, Briggs RJ. Comparison of two frequency-to-electrode maps for acoustic-electric stimulation. Int J Audiol. 2009;48:63–73. doi: 10.1080/14992020802452184. [DOI] [PubMed] [Google Scholar]
- Snyder R, Leake P, Rebscher S, Beitel R. Temporal resolution of neurons in cat inferior colliculus to intracochlear electrical stimulation: effects of neonatal deafening and chronic stimulation. J Neurophysiol. 1995;73:449–467. doi: 10.1152/jn.1995.73.2.449. [DOI] [PubMed] [Google Scholar]
- Snyder RL, Sinex DG. Immediate changes in tuning of inferior colliculus neurons following acute lesions of cat spiral ganglion. J Neurophysiol. 2002;87:434–452. doi: 10.1152/jn.00937.2000. [DOI] [PubMed] [Google Scholar]
- Snyder RL, Rebscher SJ, Cao KL, Leake PA, Kelly K. Chronic intracochlear electrical stimulation in the neonatally deafened cat. I: Expansion of central representation. Hear Res. 1990;50:7–33. doi: 10.1016/0378-5955(90)90030-s. [DOI] [PubMed] [Google Scholar]
- Thai-Van H, Micheyl C, Moore BC, Collet L. Enhanced frequency discrimination near the hearing loss cut-off: a consequence of central auditory plasticity induced by cochlear damage? Brain. 2003;126:2235–2245. doi: 10.1093/brain/awg228. [DOI] [PubMed] [Google Scholar]
- Thai-Van H, Micheyl C, Noreña AJ, Veuillet E, Gabriel D, Collet L. Enhanced frequency discrimination in hearing-impaired individuals: a review of perceptual correlates of central neural plasticity induced by cochlear damage. Hear Res. 2007;233:14–22. doi: 10.1016/j.heares.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Turner CW, Reiss LA, Gantz BJ. Combined acoustic and electric hearing: preserving residual acoustic hearing. Hear Res. 2008;242:164–171. doi: 10.1016/j.heares.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmer M, Leake PA, Beitel RE, Rebscher SJ, Snyder RL. Degradation of temporal resolution in the auditory midbrain after prolonged deafness is reversed by electrical stimulation of the cochlea. J Neurophysiol. 2005;93:3339–3355. doi: 10.1152/jn.00900.2004. [DOI] [PubMed] [Google Scholar]
- Vollmer M, Snyder RL, Leake PA, Beitel RE, Moore CM, Rebscher SJ. Temporal properties of chronic cochlear electrical stimulation determine temporal resolution of neurons in cat inferior colliculus. J Neurophysiol. 1999;82:2883–2902. doi: 10.1152/jn.1999.82.6.2883. [DOI] [PubMed] [Google Scholar]
- Wilson BS, Dorman MF. Cochlear implants: a remarkable past and a brilliant future. Hear Res. 2008;242:3–21. doi: 10.1016/j.heares.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Shepherd RK, Millard RE, Clark GM. Chronic electrical stimulation of the auditory nerve at high stimulus rates: a physiological and histopathological study. Hear Res. 1997;105:1–29. doi: 10.1016/s0378-5955(96)00193-1. [DOI] [PubMed] [Google Scholar]