Abstract
Objective
Anti-cyclic citrullinated peptide (CCP) antibodies are a serological marker for rheumatoid arthritis (RA); up to 10%–15% of patients with systemic lupus erythematosus (SLE) are also positive. While anti-CCP in RA is citrulline-dependent, anti-CCP in some other diseases is citrulline-independent and reacts with both CCP and the unmodified (arginine-containing) cyclic arginine peptide (CAP). We investigated the citrulline dependence of anti-CCP and its significance in the arthritis of SLE.
Methods
IgG anti-CCP was compared by ELISA to anti-CAP in sera from patients with SLE (n = 335) and RA (n = 47) and healthy controls (n = 35). SLE patients were divided into 5 groups based on their joint involvement: subset I: deforming/erosive arthritis (n = 20); II: arthritis fulfilling (or likely fulfilling) American College of Rheumatology criteria for RA but without erosions (n = 18); III: joint swelling but not fulfilling RA criteria (n = 39); IV: arthritis without documented joint swelling (n = 194); and V: no arthritis (n = 58).
Results
Anti-CCP (> 1.7 units) was found in 68% (32/47) of patients with RA and 17% (55/329) of those with SLE. It was more common in SLE patients with deforming/erosive arthritis (38%). High anti-CCP (> 10 units) was found in RA (26%) and deforming/erosive SLE (12%). High anti-CCP/CAP ratios (> 2, indicating a selectivity to CCP) were found in 91% of anti-CCP-positive RA and 50% of anti-CCP-positive SLE patients with deforming/erosive arthritis. Patients from subset II did not have high anti-CCP/CAP.
Conclusion
Citrulline dependence or high levels (> 10) of anti-CCP were common in SLE patients with deforming/erosive arthritis, while most anti-CCP in SLE patients was citrulline-independent. This may be useful in identifying a subset of SLE patients with high risk for development of deforming/erosive arthritis.
Key Indexing Terms: ANTI-CYCLIC CITRULLINATED PEPTIDE ANTIBODY, RHEUMATOID ARTHRITIS, SYSTEMIC LUPUS ERYTHEMATOSUS, DEFORMING ARTHRITIS, CITRULLINATION
Arthritis is one of the most common symptoms in systemic lupus erythematosus (SLE), seen in 60%–90% of patients1. In the majority of cases of SLE, arthritis is nondeforming and nonerosive and thus will not directly cause irreversible functional impairment. However, 4%–13% of patients with SLE develop a nonerosive but deforming arthritis known as Jaccoud’s-type arthritis2–6. Patients with severe erosive arthritis that is indistinguishable from that of rheumatoid arthritis (RA) have also been reported but this is less common1,7. These cases may be considered true SLE-RA overlap8, sometimes called “rhupus”9.
An ELISA-based test to detect autoantibodies to cyclic citrullinated peptide (CCP) using a peptide sequence derived from filaggrin has been used extensively as a new serological marker of RA10,11. Many studies have confirmed that the anti-CCP ELISA is as sensitive as rheumatoid factor (RF) and much more specific for RA when tested in various systemic rheumatic diseases11. In contrast to RF, which is positive in 20%–60% of cases of SLE and is not useful in differentiating arthritis patients with RA from those with SLE, anti-CCP is much less frequent in SLE11. Nevertheless, several studies have reported a 10%–15% prevalence of anti-CCP in patients with SLE12–15.
Early studies on anti-CCP emphasized the citrulline dependence of anti-CCP antibodies in RAsera10. That is, the autoantibodies reacted with the citrullinated peptide but were unreactive to the unmodified peptide containing arginine. However, virtually all studies that have reported positive anti-CCP in SLE simply used the commercial anti-CCP ELISA kit, without verifying the citrulline dependence of the anti-CCP antibodies. Anti-CCP in SLE may therefore be due to a citrulline-independent reactivity of anti-CCP, similar to the ones reported in autoimmune hepatitis16 and pulmonary tuberculosis17. One recent study16 partially addressed this issue, reporting that, in contrast to the citrulline independence of anti-CCP in autoimmune hepatitis, 67% of anti-CCP positivity in their SLE population was citrulline- dependent. However, a detailed description of the arthritis seen in these patients was not given16. Conversely, those studies that have described an association of deforming or erosive arthropathy in SLE with anti-CCP positivity did not verify the citrulline dependence of anti-CCP in these patients13,18–22. SLE in this subset may have a pathogenesis similar to RA and thus have citrulline-dependent anti-CCP antibodies, whereas anti-CCP in other subsets of SLE may be citrulline-independent.
In our study, patients with SLE were classified into subsets based on the clinical characteristics of the joint involvement. The citrulline dependence of their anti-CCP antibodies was examined by comparing the reactivity of antibodies to CCP to an unmodified peptide containing arginine (CAP, cyclic arginine peptide), and its association with different subsets of arthritis in SLE was analyzed.
MATERIALS AND METHODS
Patients
Sera were from patients enrolled in the University of Florida Center for Autoimmune Disease between February 2000 and July 2006. A total of 329 SLE and 47 RA patients were identified based on American College of Rheumatology (ACR) criteria23. Thirty-five healthy controls were also tested. An additional 6 Japanese patients with SLE, 3 with Jaccoud’s arthropathy and 3 with erosive arthritis typical of RA [one case as described24], were also studied. Jaccoud’s arthropathy was defined as described6. Ulnar deviation (> 20°), swan-neck deformity, boutonniere deformity, and Z-deformity were recorded for each patient; the score exceeding 5 points was considered Jaccoud’s arthropathy. The medical records were reviewed retrospectively, and patients with SLE were classified as described below based on their joint disease characteristics. The study protocol was approved by the institutional review board.
Anti-CCP, CAP, and P peptide ELISA
Current anti-CCP ELISA kits are in their second or third generation25,26, and the sequence of the CCP peptides is proprietary and not available for synthesis. However, the sequence of the first-generation peptide is published10 and was used to examine the citrulline dependence of anti-CCP reactivity17. CCP (cfc1-cyc, amino acid 306–324 of filaggrin, where Arg312 is replaced with citrulline) and CAP (cf0-cyc, amino acid 306–324 of filaggrin) peptides were synthesized and cycled with Tl(CF3CO2)3 in DMF/anisole (19:1) at the ICBR Protein Core Facility of the University of Florida17. The carboxyl-terminal 22 amino-acid peptide of human ribosomal P0 protein, which carries a major human autoimmune epitope and has been used for screening of anti-ribosomal P antibodies, was also synthesized27. ELISA was performed as described17,28. Briefly, half the microtiter plate (Immobilizer Amino, Nunc, Naperville, IL, USA) wells were coated with 2 μg/ml CCP and the other half with 2 μg/ml CAP. Wells were then incubated with 1:500 diluted sera. A high-titer (up to 1:312,500) anti-CCP-positive serum was diluted 1:5 serially starting from 1:500 and run as a standard. Alkaline phosphatase-conjugated goat anti-human IgG (1:1000, γ-chain-specific; Southern Biotech, Birmingham, AB, USA) was used as a secondary antibody. The optical density (OD) 405 of each sample was converted into units using the SoftMax Pro 4.7 program (Molecular Devices, Sunnyvale, CA, USA) with 4-parameter analysis. The standard curve was established by defining the OD from a 1:312,500 dilution of the standard serum as 1 unit and applying units to each dilution as follows so that the units correlated with the amount of antibodies — 1:500 dilution, 625 units; 1:2500, 125 units; 1:12,500, 25 units; 1:62,500, 5 units; 1:312,500, 1 unit; 1:1,562,500, 0.2 units. Both anti-CCP and anti-CAP units were interpolated from the same standard curve. IgG anti-CCP units, anti- CAP units, and anti-CCP/anti-CAP ratios were analyzed. The cutoff values for anti-CCP positivity (1.7 units) and anti-CCP/anti-CAP ratio (2.0) were determined based on the mean and SD of controls and receiver-operating characteristic curves. For anti-CAP units, the same cutoff value as for anti-CCP was used.
Inhibition of ELISA reactivity using CCP or CAP peptide and effects of different concentrations of NaCl on antibody binding
Inhibition of ELISA reactivity with CCP or CAP was evaluated by incubating diluted serum with CCP or CAP peptide prior to applying to wells coated with CCP or CAP, as described17. To provide a sensitive measure of inhibition, each serum was diluted (1:125 to 2500, final concentration 1:250 to 1:5000) so that the reactivity was at the low linear range of the standard curve (10–50 units). Serially diluted CCP or CAP peptide (serial 1:10 dilutions from 5000 ng/ml to 0.5 ng/ml, final concentration after mixing with sera is 2500 ng/ml to 0.25 ng/ml) in 0.5% bovine serum albumin NET/NP40 or buffer alone was incubated with appropriately diluted sera prior to adding to the wells coated with CCP or CAP. OD 405 was converted into anti-CCP or anti-CAP units. The percentage inhibition of each sample was calculated as 100 × (units of the serum incubated with buffer – units of the serum incubated with an inhibitor)/units of the serum incubated with buffer.
In other experiments, the effects of different concentrations of NaCl on antibody binding to CCP or CAP were evaluated. Following incubation with serum samples and washing, wells were incubated 30 min with NET/NP40 that contained 0.15 M, 0.375 M, or 0.5 M NaCl, prior to an additional washing step. Wells were incubated with secondary antibodies and developed as before.
RESULTS
We hypothesized that anti-CCP in the majority of anti-CCP-positive cases of SLE would not be specific for CCP but would also react with CAP, in contrast to the citrullinated peptide-specific reactivity seen in sera from patients with RA10,16,17. We predicted that SLE patients with deforming/erosive arthritis would have citrulline-dependent anti-CCP similar to RA, and SLE with persistent arthritis fulfilling the RA criteria may also have similar reactivity.
Classification of SLE into subsets based on the characteristics of joint involvement
The medical records and research database were reviewed retrospectively. SLE patients were classified into 5 subsets, according to the characteristic of their joint involvement, and the data were compared between subsets (Figure 1).
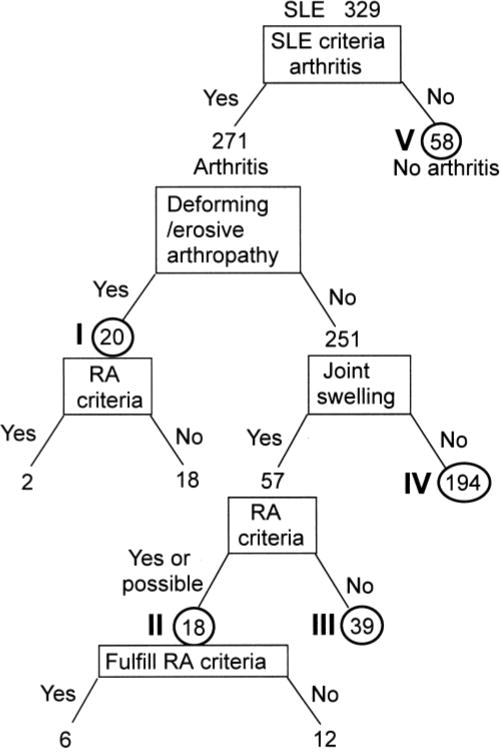
Figure 1.
Classification of SLE patients into subsets based on characteristics of joint involvement. Subset I: Deforming/destructive arthropathy; II: Arthritis that fulfilled (n = 6) or possibly fulfilled (n = 12) criteria for RA; III: SLE with joint swelling, but did not appear to fulfill RA criteria; IV: SLE with joint tenderness as defined by ACR criteria for arthritis of SLE29, but with no joint swelling observed by a physician; V: No arthritis.
Out of a total of 329 SLE cases selected based on having 4 or more SLE criteria, 271 had arthritis that was consistent with the SLE classification criteria29. The remaining 58 patients, without arthritis but meeting the ACR criteria for the diagnosis of SLE, comprised subset V. Out of the 271 cases with arthritis, 19 had deforming (Jaccoud’s) arthritis, some with radiographic changes. One patient with SLE, who had radiographic changes consistent with RA but without typical Jaccoud’s-type deformity, was also included in this group (total of 20, subset I). In this group, 2 fulfilled the RA criteria and 18 did not. Among the 251 cases with arthritis but without deformity or documented radiographic changes, 57 had one or more swollen joints confirmed by a rheumatologist on at least one occasion. The medical records of these 57 cases were reviewed in detail by 2 rheumatologists to judge whether they had arthritis meeting the ACR criteria for RA. Six cases met the RA criteria, and an additional 12 cases were considered likely to fulfill the criteria. The latter included cases with multiple swollen joints consistent with RA, but the 6-week persistence required under the ACR criteria for RA was not directly confirmed, as the intervals of visits were either too short or too long. These 18 cases (subset II) were considered as a subset of patients who were likely to have chronic arthritis similar to RA. The remaining 39 cases (subset III) either never had joint swelling in a way that appeared likely to fulfill the RA criteria, even if the swelling persisted for 6 weeks (e.g., a single knee joint) or had transient joint swelling that was observed at only one visit. They were considered highly unlikely to have persistent arthritis consistent with RA. The remaining 194 cases (subset IV) had joint tenderness that was consistent with the ACR classification criteria for SLE but were without joint swelling confirmed at our institution.
Anti-CCP and anti-CAP antibodies and anti-CCP/CAP ratios
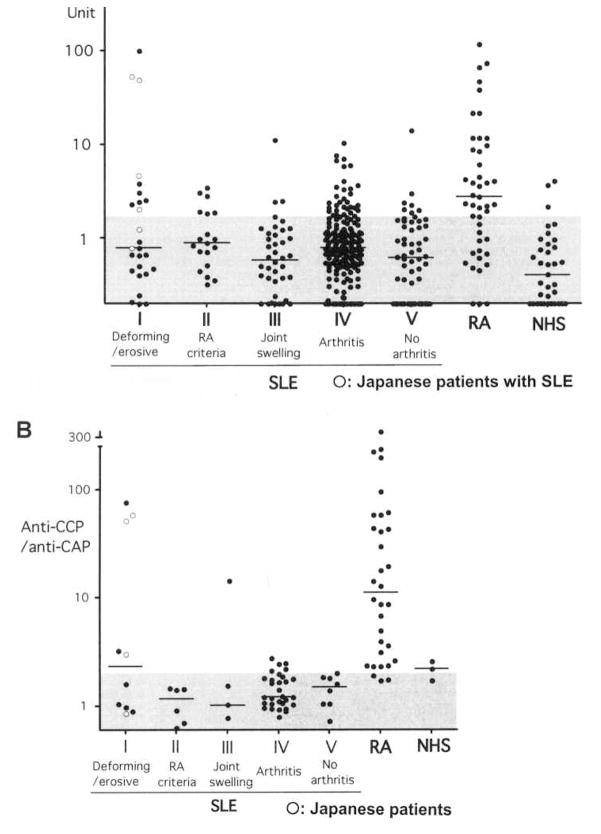
Anti-CCP and anti-CAP antibodies in sera from patients with SLE and RA and healthy controls were tested by ELISA. The OD were converted into units. Individual data (Figure 2A) and a summary (Table 1) are shown. Anti- CCP (> 1.7 units) were frequently positive in RA (68%) and very high levels (> 10 units) of anti-CCP were found almost exclusively in RA(26%, 12/47 cases) and a few SLE patients with deforming/erosive arthritis [1/20 in University of Florida patients (subset I) and 2/6 in the Japanese patients] but were rare in the rest of the SLE cohort (1%, 3/309; RA vs SLE, p < 0.001, Fisher exact test). One case of a high anti- CCP in subset III had typical SLE but also had persistent right elbow monoarthritis with joint swelling for years.
Figure 2.
A. Anti-CCP antibodies in sera from patients with SLE (total 329), RA (n = 49), and healthy controls (NHS; n = 35) were tested by ELISA. Six additional Japanese SLE patients with deforming/destructive arthropathy were included. OD were converted into units. Cutoff value of anti-CCP and anti-CAP is 1.7 units (shaded area). B. Anti-CCP/anti-CAP ratios in anti-CCP-positive sera (> 1.7 units) from SLE or RA cases or healthy controls (NHS). Data for 4 Japanese patients are shown. The ratios were high (> 2.0) in 91% of RA with anti-CCP, but was high only in SLE cases with deforming/destructive arthritis or a case with joint swelling not fulfilling RA criteria. Cutoff value of anti-CCP/anti-CAP ratio is 2.0 (shaded area).
Table 1.
Anti-CCP, anti-CAP, and anti-CCP/anti-CAP ratios in patients with SLE, RA, and controls.
| Diagnosis/Subset | n | Anti-CCP > 1.7, % | Anti-CCP > 10, % | Anti-CAP > 1.7, % | Anti-CCP/anti-CAP > 2.0, % | Anti-CCP/anti-CAP > 2.0 (CCP > 1.7), % |
|---|---|---|---|---|---|---|
| Total SLE | 329 | 17 (55/329) | 1 (4/329) | 18 (59/329) | 6 (21/329) | 18 (10/55) |
| Arthritis (SLE criteria) | 271 | 17 (47/271) | 1 (3/271) | 18 (49/251) | 7 (19/271) | 19 (9/47) |
| I. Deforming arthropathy (Jaccoud’s) | 20 | 30 (6/20) | 5 (1/20) | 30 (6/20) | 10 (2/20) | 33 (2/6) |
| Nondeforming/nonerosive arthritis | 251 | 16 (41/251) | 1 (2/251) | 17 (43/251) | 7 (17/251) | 17 (7/41) |
| II. RA criteria | 18 | 33 (6/18) | 0 (0/18) | 39 (7/18) | 0 (0/18) | 0 (0/6) |
| III. Joint swelling (+), no RA criteria | 39 | 10 (4/39) | 3 (1/39) | 3 (1/39) | 3 (1/39) | 25 (1/4) |
| IV. Arthritis but no joint swelling | 194 | 16 (31/194) | 1 (1/194) | 18 (35/194) | 8 (16/194) | 19 (6/31) |
| V. No arthritis | 58 | 14 (8/58) | 2 (1/58) | 17 (10/58) | 3 (2/58) | 13 (1/8) |
| Japanese with SLE with deforming/erosive arthritis | 6 | 67 (4/6) | 33 (2/6) | 17 (1/6) | 50 (3/6) | 75 (3/4) |
| RA | 47 | 68 | 26 | 93 | 77 | 91 |
| Control | 35 | 9 | 0 | 11 | 17 | 67 |
CCP: citric citrullinated peptide; CAP: cyclic arginine peptide.
Anti-CCP antibodies in RA patients preferentially react with CCP and react poorly with unmodified CAP10. Thus, reactivity against CCP versus CAP was compared between RA and subsets of SLE to examine whether this measure could be used diagnostically to distinguish between the citrulline- dependent anti-CCP seen in RA and the citrulline-independent anti-CCP seen in SLE (Figure 2B, Table 1). Anti-CAP was found in 18% of SLE patients, a frequency similar to that of anti-CCP (17%). While 68% of RA patients were anti-CCP-positive, only 9% were anti-CAP-positive (p < 0.0001, Fisher test), consistent with the citrulline dependence of anti-CCP in RA. Anti-CCP/anti-CAP ratios were frequently high (> 2.0) in RA (77%) versus SLE (6%) (p < 0.0001, Fisher test). Among anti-CCP-positive (> 1.7) patients, anti-CCP/anti-CAP ratios were high in 91% of RA versus only 18% of SLE (p < 0.0001, Fisher test). However, 50% (5/10; 2/6 UF cases and 3/4 Japanese) of SLE patients with deforming arthropathy had high anti-CCP/anti-CAP ratios (p < 0.05 vs SLE with nondeforming arthropathy, Fisher test). Although SLE with arthritis (subset IV) that fulfilled RA criteria may have had a slightly higher frequency of anti-CCP, it was not associated with high anti-CCP/anti- CAP ratios (0%). Thus, a subset of SLE patients with deforming arthritis appeared to have high levels of anti-CCP and high anti-CCP/anti-CAP ratios, consistent with their citrulline dependence, and similar to RA. However, anti-CCP was negative in 70% of American patients and 33% of Japanese patients with deforming arthritis (subset I), indicating that the serological characteristics of this subset of SLE are more heterogeneous than the RA patients with joint deformities.
Reactivity of SLE sera with CCP, CAP, and ribosomal P peptide by ELISA
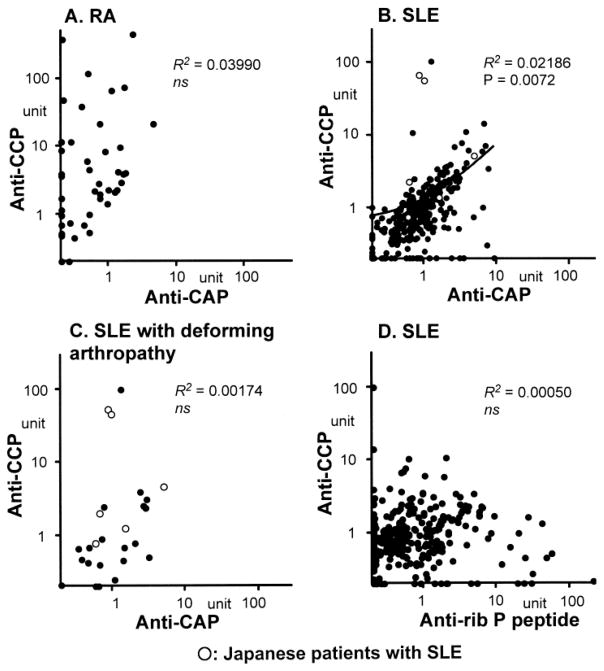
Data shown in Figure 2 suggest that anti- CCP in sera from RA is citrulline-dependent (preferentially or dominantly reactive with CCP vs CAP), whereas anti-CCP-positive SLE sera also react with CAP. The reactivity of each serum with CCP versus CAP is shown clearly in Figure 3. Consistent with previous data10,16,17, sera from RA patients predominantly reacted with CCP, as indicated by distribution of data points almost exclusively in the left upper area (Figure 3A). In contrast, the majority of SLE sera were along the diagonal line, indicating that these sera reacted with both CCP and CAP at similar levels (Figure 3B). Anti-CCP and anti-CAP had a significant correlation (R2 = 0.02186, p = 0.0072). The few sera with high anti-CCP and citrulline-dependent reactivity were mainly from patients with deforming arthropathy (subset I, Figure 3C; R2 = 0.00174, p not significant). Although the majority of sera from SLE patients reacted to CCP and CAP at similar levels, this was not simply the result of nonspecific binding as indicated by a lack of significant correlation of antibodies to CCP versus control ribosomal P peptide (Figure 3D; R2 = 0.00050, p not significant). The distribution pattern also was apparently quite different from that of anti-CCP versus anti- CAP (compare Figure 3B and 3D).
Figure 3.
Correlations among antibodies to CCP, CAP, and ribosomal P peptide by ELISA. A. RA sera (n = 47) were tested for anti-CCP versus anti-CAP. B. Anti-CCP versus anti-CAP in SLE sera (329 Americans, 6 Japanese). C. Anti-CCP versus anti-CAP in SLE cases with deforming/destructive arthropathy (subset I: 20 Americans, 6 Japanese). D. Anti-CCP versus anti-ribosomal P peptide antibodies in SLE sera (n = 329 Americans).
Specificity of anti-CCP and anti-CAP by inhibition assay and sensitivity to NaCl
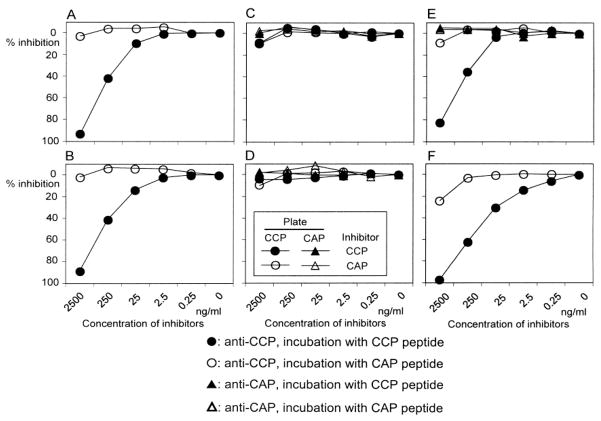
The question whether soluble CCP or CAP peptides could inhibit antibody binding to solid-phase CCP or CAP coating the wells was examined using sera from 3 SLE cases with deforming arthritis (2 cases are illustrated in Figure 4A, 4B), 4 SLE cases without deforming/erosive arthritis (3 cases shown in Figure 4C, 4D, 4E), and 7 RA sera (one case; Figure 4F). In the 3 sera from SLE with deforming arthritis (all were positive for anti-CCP and negative for anti-CAP), antibody binding to CCP was nearly completely inhibited by CCP in a dose-dependent manner (80.4%–97.4% at 2500 ng/ml CCP; Figure 4A, 4B). CAP had no clear effects on anti-CCP binding in these cases, similarly to many RA sera. In 3 SLE cases without deforming arthritis and with anti-CCP and anti-CAP positivity, inhibition of anti-CCP or anti-CAP by either CCP or CAP was less than 10% (Figure 4C, 4D). A case of SLE with persistent synovitis only in the right elbow had high levels of anti-CCP and weak anti-CAP reactivity, and here soluble CCP inhibited binding to plate-bound CCP by 83% (Figure 4E). Inhibition of anti-CAP by CCP or CAP was not clear in all cases tested (Figure 4C–4E). CAP had no clear effects on anti-CCP binding in some cases, whereas it appeared to inhibit anti-CCP by up to ~25% in others (Figure 4C–4F).
Figure 4.
Inhibition of antibodies to CCP or CAP by preincubation with CCP or CAP peptide. Sera from anti-CCP-positive SLE (A, B: subset I) and anti-CCP and anti-CAP-positive SLE (C to E; C and D: subset IV, E: subset III) or RA (F) were incubated with serially diluted CCP or CAP peptide, before addition to wells coated with CCP or CAP. The OD 405 of each sample was converted into units. % Inhibition = 100 × (units of serum incubated with buffer – units of serum incubated with inhibitor)/units of serum incubated with buffer.
Unlike certain low affinity antibodies found in other conditions30, neither anti-CCP nor anti-CAP reactivity was affected significantly (< 5%) by incubation of wells with higher concentrations of NaCl (buffer containing 0.375Mor 0.5 M NaCl, data not shown), suggesting that these interactions are of relatively high affinity.
DISCUSSION
The anti-CCP antibody ELISA has quickly become a standard serological test for the diagnosis of RA. Although early studies emphasized the citrulline dependence of anti-CCP seen in RA10, most clinical studies have used a commercial anti-CCP ELISA kit and thus have not established the citrulline dependence of the antibody reactivity. Only a few studies examined the citrulline dependence of anti-CCP in SLE or its relationship with a subset of SLE16.
One recent study examined the citrulline dependence of anti-CCP antibodies in autoimmune hepatitis, other liver diseases, RA, and various rheumatic diseases16. The authors reported that anti-CCP in autoimmune hepatitis and other liver diseases is non-citrulline-dependent and needs to be interpreted with care. In contrast, all 41 anti-CCP-positive RA sera contained citrulline-preferred anti-CCP reactivity, consistent with the previous study10 and our present report (Figure 2B, Figure 3A). In addition, a majority of anti-CCP-positives in non-RA rheumatic diseases, including 6 out 9 patients with SLE, were citrulline-dependent16. However, the clinical characteristics of these patients were not described in detail. In one report, a high prevalence of anti- CCP antibodies was seen in patients with pulmonary tuberculosis31. Our recent study17 examining the citrulline dependence of anti-CCP in patients with tuberculosis suggested that they are citrulline-independent, similar to findings in liver diseases16.
Publications on anti-CCP and deforming/erosive arthropathy in SLE are summarized in Table 2. The criteria used to select subjects were quite different among the studies; deforming or erosive arthropathy was used in some18,20–22, while others were based on ACR criteria for RA13,19, suggesting that variability in patient selection criteria may be partly responsible for the inconsistent results. Two studies that used deformity or erosion as selection criteria18,22 showed low frequencies (13% and 7%, respectively) of anti-CCP, similar to our present study, whereas others that primarily used erosion20,21 reported a high prevalence (80% and 50%, respectively; Table 1). However, erosion itself does not appear to be a significant factor because the majority of cases in the former studies also had erosions (10/16 and 11/14, respectively)18,22. Anti-CCP among erosive cases was 20% in one study18. Cases of SLE selected based on also fulfilling the RA criteria appear to have a high prevalence of anti-CCP in general (44%–100%)13,19,22,32. However, this was not the case in our present study (Table 1). The published reports are inconsistent, even considering the difference in selection criteria. Some have suggested that anti-CCP is not common even in cases of SLE with erosive arthropathy18,22, and that anti-CCP may be used to differentiate SLE and RA. In contrast, other studies reported that erosive arthropathy20 or “rhupus”19 that has overlapping features of SLE and RA9 is associated with positive anti-CCP.
Table 2.
Deforming/erosive arthropathy and anti-CCP antibodies in SLE.
| Report | Selection | Anti-CCP in Deforming/Erosive Arthropathy, % | Anti-CCP in Nonerosive Arthropathy, % | Deforming/Erosive Arthropathy in Anti-CCP, % |
|---|---|---|---|---|
| Mediwake18 2001 | Deformity or erosion | 13 (2/16) Erosive 20 (2/10) Nonerosive 0 (0/6) |
2 (1/50) | 67 (2/3) |
| Takasaki13 2004 (MCTD, 7/8 had SLE) | RA criteria or anti-CCP | 44 (4/9) | NA | 50 (4/8) deformity 63 (5/8) erosion |
| Amezcua-Guerra19 2006 | RA criteria | 57 (4/7) | 0 | 100 (4/4) |
| Rothfield32 2007 | RA criteria? | 67 (2/3) | NA | NA |
| Martinez20 2007 | Erosion | 80 (4/5) | NA | NA |
| Chan21 2008 | Erosion | 50 (6/12) | 3 (2/59) | 88 (7/8) |
| Damian-Abrego22 2008 | Deformity (11/14 had erosion) | 7 (1/14) | 5 (1/20) | 90 (10/11) |
| RA criteria | 100 (9/9) | |||
| Kakumanu 2009 | American, deformity or erosion | 30 (6/20) | 16 (41/251) | 13 (6/47) |
| 2009 | Japanese, deformity or erosion | 67 (4/6) | NA | NA |
NA: not applicable.
Our data showed very good correlation of anti-CCP and anti-CAP in SLE in general (Figure 3B), in striking contrast to the CCP-dominant reactivity in RA (Figure 3A). Only a few exceptional cases of SLE with deforming arthropathy had citrulline-dependent reactivity similar to that of RA. This is also shown by the low anti-CCP/anti-CAP ratios in SLE (Figure 2B). A different cutoff leading to different selection of anti-CCP-positive patients in SLE is likely to be a main reason for the differences from some studies16. In our study, if SLE patients with anti-CCP > 10 units were analyzed for citrulline dependence, 67% (4/6) of them preferentially reacted with CCP (see data points with anti-CCP > 10, Figure 3B), similar to the citrulline-preferred reactivity in 67% (6/9) of anti-CCP-positive SLE cases in the study by Vannini, et al16. Also, 3 of 5 high anti-CCP-positive patients had deforming/erosive arthropathy, consistent with other studies. However, it is apparent that the majority of SLE sera react with CCP and CAP at similar levels, and some even react preferentially with CAP (Figure 3B, data points on the x-axis). This is in striking contrast to the pattern of RA patients (Figure 3A vs 3B). It was surprising that none (0/4) of the SLE patients with severe erosive arthropathy typical of RA had very high (> 10) anti-CCP. The 3 sera with the highest anti-CCP were all patients with typical Jaccoud’s-type arthropathy (Figure 2B, 3B) rather than typical erosive RA. It has been emphasized that the pathogenesis of deformity in SLE cases with Jaccoud’s-type arthropathy is different from that of RA — loosened ligaments and joint capsule in the former compared to synovitis causing bone and cartilage destruction in the latter2. However, the presence of high levels of citrulline-dependent anti-CCP suggests a common pathogenic mechanism between RA-type synovitis and certain Jaccoud’s-type arthropathy in SLE. Increased C-reactive protein in SLE patients with Jaccoud’s arthritis5 may be consistent with this. The pathogenic mechanisms and degree of inflammation in Jaccoud’s arthropathy may be heterogeneous, and only a subset of Jaccoud’s may have factors similar to RA and develop anti- CCP. It has been suggested that citrullination occurs regardless of the diagnosis or location of inflammation33. Thus, what determines the production of citrulline-dependent anti- CCP antibodies may be the degree of citrullination, along with additional unknown genetic and environmental factors.
The characteristics of the antibody reactivity with CAP peptide remain to be clarified, because it was not clearly inhibited with either CAP or CCP peptide, in contrast to nearly complete inhibition of anti-CCP reactivity by CCP peptide (Figure 4). One possible explanation for this is a difference in anti-CAP recognition of solid-phase peptide epitope on the plate compared to the liquid-phase peptide used for the inhibition assay. Resistance of anti-CCP and anti- CAP reactivity to 0.5 M NaCl buffer suggests that antibody reactivity is of relatively high affinity; however, it is possible that the affinity of CCP-specific reactivity is higher than that of CAP reactivity, thus the anti-CCP is more readily inhibited. The concentration of the peptide used appeared to be high enough to significantly inhibit anti-CCP reactivity, but might not be enough to inhibit anti-CAP reactivity.
It has been proposed that the definition of “SLE-RA overlap” should be based on typical radiographic changes of RA, rather than merely fulfilling RA criteria, because radiographic change is the unique characteristic separating RA from other forms of arthritis, and certain patients with other diagnoses can fulfill RA criteria8. This idea has been supported by others20,34,35. Although the number is small, none of the 4 patients in our study with features consistent with typical SLE-RA overlap had high levels (> 10) of anti-CCP comparable to that of RA. This was unexpected, but similar to a report showing that only 1 of 8 SLE patients who had severe erosive arthropathy was anti-CCP-positive18.
Arthritis is one of the most common symptoms in SLE, seen in 60%–90% of patients6,18. In the majority of cases, the arthritis in SLE is nondeforming and nonerosive. Thus, it will not directly cause irreversible functional impairment. However, 4%–13% of SLE patients develop nonerosive deforming arthritis known as Jaccoud’s arthritis2–6, in which the deformity is characterized by reversible ulnar deviation, and the extent of erosions is minimal compared with the degree of deformity. The development of severe chronic erosive arthritis that is indistinguishable from that of RA may indicate a true coexistence of SLE and RA8. It appears to be less common and is seen in 1%–3% of patients1,6,9,18.
It would be clinically useful to identify a subset of patients at an early stage of the disease process who have a high risk of developing deforming/erosive arthropathy. If these individuals could be identified prior to the onset of irreversible damage, they could be monitored more carefully for arthritis activity, and if necessary, receive aggressive treatment. High levels of anti-CCP appear to be one important marker, and combined with elevated anti-CCP/CAP ratios, may have additional specificity. Anti-CCP/anti-CAP ratios > 2.0 identified 38% (5/13) of those with deforming arthritis, and if anti-CCP/anti-CAP > 3.0 was used as the cutoff, 83% (5/6) had deforming arthropathy. This certainly appears to be a more promising marker to predict deforming/erosive arthropathy than merely fulfilling the RA criteria. However, the sensitivity is rather low. Additional biomarkers to identify a subset of SLE patients who are likely to develop deforming/erosive arthropathy would likely provide for better management and thus a better quality of life.
Acknowledgments
Supported by National Institutes of Health grants R01-AR40391 and M01R00082. Dr. Satoh was supported in part by a grant from Lupus Foundation of America, Inc.
We thank Marlene Sarmiento, Annie Chan, and Frances Reeves for clinical assistance.
References
- 1.Wallace DJ. Differential diagnosis and disease associations. In: Wallace DJ, Hahn BH, editors. Dubois’ lupus erythematosus. Philadelphia: Lea & Febiger; 1993. pp. 473–84. [Google Scholar]
- 2.Bywaters EGL. Jaccoud’s syndrome. Clin Rheum Dis. 1975;1:125–48. [Google Scholar]
- 3.Esdaile JM, Danoff D, Rosenthall L, Gutkowski A. Deforming arthritis in systemic lupus erythematosus. Ann Rheum Dis. 1981;40:124–6. doi: 10.1136/ard.40.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alarcon-Segovia D, Abud-Mendoza C, Diaz-Jouanen E, Iglesias A, De los Reyes V, Hernandez-Ortiz J. Deforming arthropathy of the hands in systemic lupus erythematosus. J Rheumatol. 1988;15:65–9. [PubMed] [Google Scholar]
- 5.Spronk PE, ter Borg EJ, Kallenberg CG. Patients with systemic lupus erythematosus and Jaccoud’s arthropathy: a clinical subset with an increased C reactive protein response? Ann Rheum Dis. 1992;51:358–61. doi: 10.1136/ard.51.3.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Vugt RM, Derksen RH, Kater L, Bijlsma JW. Deforming arthropathy or lupus and rhupus hands in systemic lupus erythematosus. Ann Rheum Dis. 1998;57:540–4. doi: 10.1136/ard.57.9.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amezcua-Guerra LM. Overlap between systemic lupus erythematosus and rheumatoid arthritis: Is it real or just an illusion? J Rheumatol. 2009;36:4–6. doi: 10.3899/jrheum.081067. [DOI] [PubMed] [Google Scholar]
- 8.Satoh M, Ajmani AK, Akizuki M. What is the definition for coexistent rheumatoid arthritis and systemic lupus erythematosus? Lupus. 1994;3:137–8. doi: 10.1177/096120339400300215. [DOI] [PubMed] [Google Scholar]
- 9.Panush RS, Edwards NL, Longley S, Webster E. ‘Rhupus’ syndrome. Arch Intern Med. 1988;148:1633–6. [PubMed] [Google Scholar]
- 10.Schellekens GA, de Jong BA, van den Hoogen FH, van de Putte LB, van Venrooij WJ. Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies. J Clin Invest. 1998;101:273–81. doi: 10.1172/JCI1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Avouac J, Gossec L, Dougados M. Diagnostic and predictive value of anti-cyclic citrullinated protein antibodies in rheumatoid arthritis: a systematic literature review. Ann Rheum Dis. 2006;65:845–51. doi: 10.1136/ard.2006.051391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vasishta A. Diagnosing early-onset rheumatoid arthritis: the role of anti-CCP antibodies. Am Clin Lab. 2002;21:34–6. [PubMed] [Google Scholar]
- 13.Takasaki Y, Yamanaka K, Takasaki C, Matsushita M, Yamada H, Nawata M, et al. Anticyclic citrullinated peptide antibodies in patients with mixed connective tissue disease. Mod Rheumatol. 2004;14:367–75. doi: 10.1007/s10165-004-0325-2. [DOI] [PubMed] [Google Scholar]
- 14.Sauerland U, Becker H, Seidel M, Schotte H, Willeke P, Schorat A, et al. Clinical utility of the anti-CCP assay: experiences with 700 patients. Ann NY Acad Sci. 2005;1050:314–8. doi: 10.1196/annals.1313.033. [DOI] [PubMed] [Google Scholar]
- 15.Matsui T, Shimada K, Ozawa N, Hayakawa H, Hagiwara F, Nakayama H, et al. Diagnostic utility of anti-cyclic citrullinated peptide antibodies for very early rheumatoid arthritis. J Rheumatol. 2006;33:2369–71. [PubMed] [Google Scholar]
- 16.Vannini A, Cheung K, Fusconi M, Stammen-Vogelzangs J, Drenth JP, Dall’aglio AC, et al. Anti-cyclic citrullinated peptide positivity in non-rheumatoid arthritis disease samples: citrulline-dependent or not? Ann Rheum Dis. 2007;66:511–6. doi: 10.1136/ard.2006.058933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kakumanu P, Yamagata H, Sobel ES, Reeves WH, Chan EKL, Satoh M. Patients with pulmonary tuberculosis are frequently positive for anti-cyclic citrullinated peptide antibodies but also react with unmodified arginine-containing peptide. Arthritis Rheum. 2008;58:1576–81. doi: 10.1002/art.23514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mediwake R, Isenberg DA, Schellekens GA, van Venrooij WJ. Use of anti-citrullinated peptide and anti-RA33 antibodies in distinguishing erosive arthritis in patients with systemic lupus erythematosus and rheumatoid arthritis. Ann Rheum Dis. 2001;60:67–8. doi: 10.1136/ard.60.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amezcua-Guerra LM, Springall R, Marquez-Velasco R, Gomez-Garcia L, Vargas A, Bojalil R. Presence of antibodies against cyclic citrullinated peptides (anti-CCP) in patients with rhupus: a cross-sectional study. Arthritis Res Ther. 2006;8:R144. doi: 10.1186/ar2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez JB, Valero JS, Bautista AJ, Restrepo JF, Matteson EL, Rondon F, et al. Erosive arthropathy: clinical variance in lupus erythematosus and association with anti-CCP case series and review of the literature. Clin Exp Rheumatol. 2007;25:47–53. [PubMed] [Google Scholar]
- 21.Chan MT, Owen P, Dunphy J, Cox B, Carmichael C, Korendowych E, et al. Associations of erosive arthritis with anti-cyclic citrullinated peptide antibodies and MHC Class II alleles in systemic lupus erythematosus. J Rheumatol. 2008;35:77–83. [PubMed] [Google Scholar]
- 22.Damian-Abrego G, Cabiedes J, Cabral A. Anti-citrullinated peptide antibodies in lupus patients with or without deforming arthropathy. Lupus. 2008;17:300–4. doi: 10.1177/0961203307087613. [DOI] [PubMed] [Google Scholar]
- 23.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 24.Satoh M, Yamagata H, Watanabe F, Matsushita Y, Sakamoto K, Yoshida K, et al. A case of long-standing classical rheumatoid arthritis complicated by serological and clinical characteristics of systemic lupus erythematosus. Scand J Rheumatol. 1993;22:138–40. doi: 10.3109/03009749309099259. [DOI] [PubMed] [Google Scholar]
- 25.Vander Cruyssen B, Nogueira L, Van Praet J, Deforce D, Elewaut D, Serre G, et al. Do all anti-citrullinated protein/peptide antibody (ACPA) tests measure the same? Evaluation of discrepancy between ACPA tests in RA and non-RA patients. Ann Rheum Dis. 2007;67:542–6. doi: 10.1136/ard.2007.071654. [DOI] [PubMed] [Google Scholar]
- 26.Coenen D, Verschueren P, Westhovens R, Bossuyt X. Technical and diagnostic performance of 6 assays for the measurement of citrullinated protein/peptide antibodies in the diagnosis of rheumatoid arthritis. Clin Chem. 2007;53:498–504. doi: 10.1373/clinchem.2006.078063. [DOI] [PubMed] [Google Scholar]
- 27.Elkon K, Bonfa E, Llovet R, Danho W, Weissbach H. Properties of the ribosomal P2 protein autoantigen are similar to those of foreign protein antigens. Proc Natl Acad Sci USA. 1988;85:5186–9. doi: 10.1073/pnas.85.14.5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Satoh M, Hamilton KJ, Ajmani AK, Dong X, Wang J, Kanwar Y, et al. Induction of anti-ribosomal P antibodies and immune complex glomerulonephritis in SJL mice by pristane. J Immunol. 1996;157:3200–6. [PubMed] [Google Scholar]
- 29.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 30.Cucnik S, Kveder T, Rozman B, Bozic B. Binding of high-avidity anti-beta 2-glycoprotein I antibodies. Rheumatology. 2004;43:1353–6. doi: 10.1093/rheumatology/keh349. [DOI] [PubMed] [Google Scholar]
- 31.Elkayam O, Segal R, Lidgi M, Caspi D. Positive anti-cyclic citrullinated proteins and rheumatoid factor during active lung tuberculosis. Ann Rheum Dis. 2006;65:1110–2. doi: 10.1136/ard.2005.045229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rothfield NF, Lim AA. Systemic lupus erythematosus evolving into rheumatoid arthritis. J Rheumatol. 2006;33:188–90. [PubMed] [Google Scholar]
- 33.Makrygiannakis D, af Klint E, Lundberg IE, Lofberg R, Ulfgren AK, Klareskog L, et al. Citrullination is an inflammation-dependent process. Ann Rheum Dis. 2006;65:1219–22. doi: 10.1136/ard.2005.049403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jawad AS, Habib S. The definition for coexistent rheumatoid arthritis and systemic lupus erythematosus. Lupus. 1995;4:166–8. doi: 10.1177/096120339500400221. [DOI] [PubMed] [Google Scholar]
- 35.Ostendorf B, Scherer A, Specker C, Modder U, Schneider M. Jaccoud’s arthropathy in systemic lupus erythematosus: differentiation of deforming and erosive patterns by magnetic resonance imaging. Arthritis Rheum. 2003;48:157–65. doi: 10.1002/art.10753. [DOI] [PubMed] [Google Scholar]