Abstract
Traumatic brain injury caused by explosive or blast events is currently divided into four phases: primary, secondary, tertiary, and quaternary blast injury. These phases of blast-induced traumatic brain injury (bTBI) are biomechanically distinct, and can be modeled in both in-vivo and in-vitro systems. The purpose of this review is to consider the mechanical phases of bTBI, how these phases are reproduced with in-vitro models, and to review findings from these models to assess how each phase of bTBI can be examined in more detail. Highlighted are some important gaps in the literature that may be addressed in the future to better identify the exact contributing mechanisms for bTBI. These in-vitro models, viewed in combination with in-vivo models and clinical studies, can be used to assess both the mechanisms and possible treatments for this type of trauma.
Key words: biomechanics, blast, brain injury, in-vitro models
Introduction
Some of the first descriptions of blast effects on humans originated in World War I, where the term “shell shock” was first coined (Southborough, 1922). At the time, it was not known whether the basis of the associated symptoms was due to a psychological or biological condition. A series of reports by military medical officer Charles Myers (Myers, 1940; Myers, 1915), and the influence of neurologist Gordon Holmes (Macleod, 2004) led to the first descriptions of such symptoms to include a temporary altered mental state or confusion immediately following the blast. In more severe forms, unconsciousness would occur. Reports on this form of blast injury appeared periodically over the years, but not until very recently was the true extent of injury better elucidated (Pary et al., 1988; Macleod, 2004; Jones et al., 2007). The current Iraq conflict and the prominent role of improvised explosive devices (IED) dramatically increased the fraction of traumatic brain injuries (TBI). This perhaps led to the well publicized view that blast-induced traumatic brain injury (bTBI) is the signature brain injury for combat troops in today's military.
The Centers for Disease Control and Prevention (CDC) defines blast injury in four phases. However, the bulk of bTBI occurs in the first three phases: the primary injury phase is comprised of the response of brain tissue to the blast wave (an intense overpressurization impulse component of the blast). The secondary injury phase results from shrapnel penetration into the head. The tertiary injury phase results from head contact/acceleration forces as the body is moved by the “blast wind” (a forced super-heated air flow). The quaternary injury phase incorporates any injury not covered in the other three phases such as some of the extracranial injuries or “polytrauma” including hemorrhagic shock and chemical or thermal burn injuries (Lew, 2005; Scott et al., 2006) that can occur. This quaternary phase of bTBI can significantly alter the timing and consequences of the primary damage occurring in the first three phases, and therefore can be a major contributor to overall brain pathology. This may be particularly true in mild bTBI, where there are either minor or no complicating factors.
The purpose of this review is to consider the mechanical phases of bTBI, how these phases are reproduced with in-vitro models, and to review findings from these models to assess how each phase of bTBI can be examined in more detail. Also described, where relevant, is how the potential secondary mechanisms have been studied in these models of TBI. These in-vitro models, viewed in combination with in-vivo models and clinical studies, can be used to assess both the mechanisms and possible treatments for this type of trauma.
Defining Blast Injury
No one model can mimic the clinical and mechanical complexity resulting from a bTBI. For injuries in the civilian population, the mechanical factors are often grouped into either the focal/contact or acceleration/inertial forces that produce the different mixture of clinical injuries ranging from concussion to skull fracture, contusions, and diffuse axonal injury (DAI). However, bTBI are grouped by injuries resulting from the different physical aspects of the blast phenomenon; in short, primary bTBI is from the shockwave, secondary bTBI is from shrapnel, tertiary bTBI is from the blast wind, and quaternary covers the remaining mitigating factors. These groupings apply not only to studying brain injuries, but also to other body regions susceptible to injury from blast. This distinct method of grouping blast injuries may seem inconsistent with the classifications often used for civilian TBI resulting from falls/assaults or motor vehicle accidents; however, a fair amount of knowledge gained from civilian TBI studies can be used to study the military problem.
Primary blast injury
The primary blast injury phase is limited to injuries caused by the rapidly expanding blast wave (Scott et al., 2006). Past work identified typical pressure wave profiles generated during blast events (Wharton et al., 2000), and how this pressure wave propagates through air and biological tissues (Clemedson, 1956; Clemedson et al., 1956; Clemedson and Pettersson, 1956). Shock-waves from a blast are typically characterized as a rapid rise and fall of high pressures (Depalma et al., 2005). The shockwave passes on the order of milliseconds, although the exact profile and range is dependent on the size, type, and shape of the explosive. In air, the shockwave oscillates between over- and underpressure segments, but these waves dampen out quickly. In water, the shockwave maintains a normal pressure profile over a longer range than in air. The presence of structures and fluid interfaces complicate this pressure profile.
Most structural damage occurs when the shockwave travels from water to air. For this reason, a large number of studies focused on the fluid-air interfaces in the body, as they are highly vulnerable to the passing pressure waves. Studies in these areas have generated injury thresholds for the pulmonary system and the gut/bowels (Yang et al., 1996). As this pressure wave travels through the organs, there are stress concentrations that arise at tissue-air interfaces, reflections of stress waves at tissue interfaces, and possible constructive stress wave interference within internal points of tissue that can further complicate the pattern of injury (Depalma et al., 2005). Models to study the primary injury phase should account for both the blast pressure transmitted through the organ of interest and shock-wave reflection/transmission behavior at tissue interfaces.
Secondary blast injury
The secondary blast injury phase covers both the penetrating and nonpenetrating injuries that occur when high-velocity projectiles/fragments impact the head (Scott et al., 2006). Certainly, these injuries share some common ground with the ballistic wounds seen in civilians, but are considerably more complex because of the non-uniform size and number of the impacting fragments. This results in a myriad of injuries compared to traditional penetrating lesions. At the mechanistic level, these injuries are best modeled as a tissue laceration, with the obvious complicating mechanisms related to blood in the extracellular space, and the potential for secondary brain injuries such as hypoxia.
Tertiary blast injury
The tertiary blast injury includes the effects when the primary blast wind causes the person to collide with fixed or mobile objects. These types of injuries share the most in common with contact/acceleration injuries in civilians, where both the contact and acceleration forces can contribute to the different intracranial injuries that appear. Helmets worn by troops reduce the likelihood of injuries from direct contact for most of the head. However, current helmets are not designed to specifically reduce rotational acceleration following a helmeted impact (Richter et al., 2001; Pellman et al., 2003). There still is a high potential for inertially-based injuries that include subdural hematoma and DAI. As a result, the predominant mechanism for tertiary blast injuries in helmeted troops is the intracranial deformations caused by the head striking an object, or the head being struck by an object with sufficient mass to cause a significant inertial load. In non-helmeted victims, tertiary blast injuries can also include skull fracture or contusions from the focal contact forces when the unprotected head strikes an object or surface. Models to study this phase are best used to examine how tissue and cellular deformation change neuronal and glial functions in the CNS over time.
Quaternary blast injury
Quaternary blast injury includes conditions or the exacerbation of pre-existing conditions that develop from exposure to blast not covered in the other three phases. One such example would be the development of asthma and other breathing problems due to inhaling smoke and dust resulting from the blast. Any resulting burns would also be covered in this phase of injury. Other conditions could also include angina, hypertension, and crush injuries. Unconventional explosive devices such as nuclear weapons and dirty bombs will also emit harmful energy such as radiation that can further complicate sustained injuries. Traditionally, injury from toxic by-products of the explosion would be considered part of quaternary blast injury. However, it is now being considered a fifth emerging category. Injuries sustained in this phase can include the toxic residue of the explosives themselves (Steevens et al., 2002), their metabolites (Lachance et al., 2004), and the generation of toxic gases such as carbon monoxide (Deitchman et al., 1998; Bakke et al., 2001). This fifth emerging category of bTBI will not be discussed in this review because it needs more precise characterization (e.g., how these contaminants eventually surface in the brain parenchyma) before any systematic examination in an in-vitro setting.
Superimposed secondary insults
Briefly mentioned above, there are numerous complications that occur in blast injury not limited to the head that occur in each of the four phases. In the primary injury phase, the high pressure waves can cause damage and rupturing of air-filled cavities in the body such as the lungs, middle ear, and the gastrointestinal tract. In the second injury phase, the flying debris can cause penetration injuries to any part of the body, such as the eye, and may even cause loss of limbs. In the tertiary injury phase, the force at which the person is thrown can cause fracture and traumatic amputation type injuries. In the quaternary phase, complications such as burns or the development of asthma would make recovery or rehabilitation from bTBI more difficult. Much of these superimposed insults such as loss of limb or internal hemorrhage from damaged organs can cause secondary insults such as hypovolemic shock, hypoxia, and hypoxic-ischemia complications. While some of the secondary insults cannot be modeled in vitro, hypoxia and ischemia are secondary insults commonly modeled with an oxygen-glucose deprivation chamber or through chemical treatments. Where appropriate, the in-vitro studies that combined secondary insults with the mechanical effects are highlighted.
Blast-to-bench translation
Each of the first three categories of blast injury is biomechanically distinct, allowing each to be studied relatively independently. Primary blast injury, in a reduced form, consists of pressure wave transmission through tissue. However, the portion of this wave most injurious to the brain is unknown. Secondary blast damage is best reduced to a direct tearing or transection-type lesion. The mechanism of tertiary blast damage is modeled simply by stretching in-vitro preparations directly, but below the point of cellular structural failure. The nature of in-vitro models provides a tool to explore each mechanism, and can be made progressively more complex when adding in elements of secondary brain injury (such as excitotoxicity), or potentially damaging agents to the extracellular space.
The next sections are a review of current findings from the literature that fall within one of these three biomechanically distinct categories of blast injury. Most of the current literature for in-vitro models falls within tertiary blast injury, where the intracranial strains and stresses are of primary importance. For this reason, this area of the literature is reviewed first, followed by studies that provide some mechanistic insight into secondary blast injury. A summary of in-vitro work involving the mechanism of primary blast injury is next addressed, as this area has been the least studied. A brief discussion of possible factors that may induce quaternary bTBI is last, since this phase has the least biomechanical distinction in terms of injury mechanism.
Tertiary blast injury
Modeling considerations in tertiary bTBI
The acceleration/deceleration of the head that occurs in tertiary bTBI causes a shearing of the intracranial tissue. The membrane between the hemispheres separating the cerebrum from the cerebellum amplify these shearing strains in some brain regions, while limiting the strains in others (Meaney et al., 1995; Margulies et al., 1990). A number of increasingly sophisticated finite element (FE) models of the brain are excellent computational tools for relating head movement and brain structure to predicted areas of damage (Viano et al., 2005; Zhang et al., 2001; Kimpara et al., 2006). These models can estimate damage in regions for injuries with simple geometries. With more knowledge of the physical properties and measurements of the brain (Chu et al., 1994; Zhang et al., 2002), the exact levels of strain can be modeled. Injured and uninjured regions in a cell can also be distinguished. Although a number of critical issues remain, these computational models provide a “virtual window” into the brain for predicting tissue deformation that can occur during the tertiary bTBI. Investigators have used these estimates of intracranial motions and deformations to develop precise models to understand how cellular functions change in response to mechanical deformation resulting from sudden acceleration or deceleration. These models, termed cell-deformation or cell-stretching models, distinguish areas of damage from regions where no injury is expected.
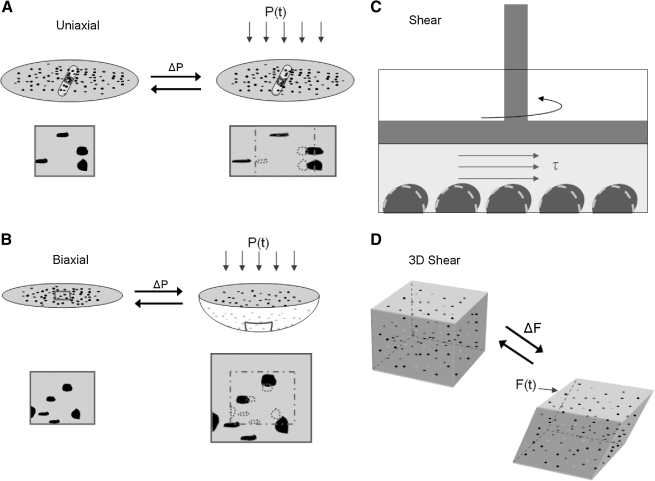
Reducing this complex pattern of deformation within the brain to an in-vitro model presents its own challenges. First, one must decide how to mimic the predominant strain within the brain (i.e., shearing of the tissue) in a two-dimensional system. Second, one must also balance the convenience of an experimental design against the similarity to the in-vivo environment. Converting an in-vivo (three-dimensional) shearing strain into an in-vitro (two-dimensional) equivalent strain field that can be applied to a cell monolayer is fairly straightforward. Namely, the monolayer can be extended in one direction, allowed to contract in a perpendicular direction, and remain unchanged in a third direction (Fig. 1A). This is the primary purpose of the uniaxial stretch system used in the past to mechanically injure dissociated cells and organotypic tissues (Lusardi et al., 2004a; Pfister et al., 2003). An alternative system used more widely is the biaxial system (Fig. 1B), which produces a stretch in two perpendicular directions across the culture surface. The in-vivo equivalent of the equibiaxial stretch system is relatively easy to construct, as it consists of a uniform expansion along a plane and a corresponding contraction out of plane. For non-equibiaxial stretch, the approximate in-vivo strain is broken into two components: a uniform expansion and an equivalent shear. The choice of which system to use is left to the investigator and likely depends upon past experience, simplicity, and cost. For tertiary bTBI, the uniaxial model offers an advantage because of its simple correlation to pure shear. However, the biaxial system has been more commonly used by past investigators, and therefore offers more past results for direct comparison.
FIG. 1.
Models of tertiary bTBI. (A and B) For two-dimensional monolayer cultures, the cells are typically plated on a transparent flexible membrane and the membrane is either deformed using an air pressure pule (P, ΔP) to strain the cells in both directions (biaxial), or primarily only in one direction (uniaxial). Shown are illustrations of both techniques, with highlights of how the cells in a region of the membrane (the dark objects) are deformed from their initial shape (dashed lines), and then returned rapidly to their initial shape. (C) A less commonly used technique on monolayer cultures is using hydrodynamic shear (λ), which is applied to the surface of the cell monolayer, to induce a deformation in the cells. (D) A new three-dimensional technique to study the response of cells embedded within a three-dimensional structure is also shown. The cells (the dark spots) are within a three-dimensional hydrogel that is deformed in shear by applying a force (F(t), ΔF) to the top of the gel.
A summary of the major findings from the work in this category appears in Table 1. In the following paragraphs, these studies are described more completely.
Table 1.
Summary of Injury Mechanisms from Tertiary bTBI
| Reference | In-vitro injury | RM | IM | IC | O | Major finding |
|---|---|---|---|---|---|---|
| Charles et al., 1991 | Astrocyte deformation | X | X | Ca2+ signaling after stretch | ||
| Floyd et al., 2004 | Astrocyte deformation | X | X | Uncoupling of IP3 signaling from Ca2+ | ||
| Ellis et al., 1995; Rzigalinski et al., 1997; Rzigalinski et al., 1998; Chen et al., 2004 | Astrocyte deformation | X | X | Ca2+ influx; cytoskeletal alteration; organelle disruption | ||
| Lamb et al., 1997 | Astrocyte deformation | X | X | Generation of hydroxyl and oxyradicals | ||
| Ahmed et al., 2000; Ahmed et al., 2002 | Astrocyte deformation | X | X | Decreased ATP and mitochondria membrane potential; increase in intracellular free [Ca2+] | ||
| Hoffman et al., 2000; Floyd et al., 2001 | Astrocyte deformation | X | X | Free radical generation; IP3 | ||
| Ellis et al., 2007 | Astrocyte deformation | X | Mechanical threshold for release of S100B, neuroprotective protein | |||
| Di et al., 2000 | Astrocyte deformation | X | Volume-activated ion channel changes | |||
| Floyd et al., 2005 | Astrocyte deformation | X | Intracellular [Na+] increases after injury | |||
| Ostrow et al., 2000 | Astrocyte deformation | X | X | X | Cytoplasmic Ca2+, IP3 increases, leading to increased endothelin-1 production | |
| Lea et al., 2002 | Astrocyte deformation | X | mGluR5 regulates NMDAR in mild, but not in severe stretch | |||
| Neary et al., 2003; Neary et al., 2005; Neary and Kang, 2006 | Astrocyte deformation | X | X | Purinergic receptor activation, signaling cascades | ||
| Zhang et al., 1996 | Neuronal stretch | X | Stretch-induced relief of Mg+ blockade of NMDAR | |||
| Geddes et al., 2003a; Serberst et al., 2005; Serberst et al., 2006 | Neuronal stretch | X | Increases in membrane permeability after injury | |||
| Goforth et al., 1999, 2004 | Neuronal stretch | X | X | Loss of AMPAR desensitization and potentiation of AMPA-evoked currents after injury | ||
| Kao et al., 2004 | Neuronal stretch | X | X | Potentiation of GABA currents | ||
| Tavalin et al., 1997 | Neuronal stretch | X | X | NMDAR delayed depolarization; mediated by electrogenic Na+ pump | ||
| Geddes et al., 2003b; Lusardi et al., 2004b | Neuronal stretch | X | Changes in cytosolic Ca2+ from NMDAR activation and mechanical pores | |||
| Chen et al., 2004 | Neuronal stretch | X | X | mGluR anatogonists prevent Ca2+ depletion of stores | ||
| Arundine et al., 2003 | Neuronal stretch | X | X | Stretch induces signaling cascade through NMDAR | ||
| Lea et al., 2003 | Neuronal stretch | X | Glia affects mGluR-1-mediated NMDAR modulation in neurons | |||
| Weber et al., 2001; Weber et al., 1999 | Neuronal stretch | X | X | Intercellular Ca2+ store mediated signaling changes | ||
| Pike et al., 2000 | Neuronal stretch | X | Calpain/caspase activation follows injury | |||
| Slemmer and Weber, 2005 | Repeated neuronal stretch | X | Cumulative damage with repeated injuries | |||
| Movsesyan and Faden, 2006 | In vivo controlled cortical impact | X | mGluR2 activation is neuroprotective | |||
| Wolf et al., 2001; Iwata et al., 2004 | Axonal stretch | X | NaCh dysregulation leads to influx of Ca2+ | |||
| Cargill and Thibault, 1996 | Neuronal stretch and hypoxia | X | Acute Ca2+ influx depends on rate and severity of mechanical injury | |||
| Glass et al., 2002 | Neuronal stretch and hypoxia | Combined stretch and hypoxia resulted in increased cell death over single stretch or hypoxia | ||||
| Engel et al., 2005 | Neuronal stretch and hypoxia | Increased cell death with combined treatment; neuroprotective agents not effective |
RM, receptor mediated; IM, ion mediated; IC, intracellular signaling; O, other.
In-vitro models of tertiary bTBI
In-vitro astrocyte deformation models
One of the earliest cell stretching systems studied was of primary astrocytes on both the single-cell and cell-monolayer level. For the single cell, injury was provoked by the mechanical poking of an astrocyte with the blunt end of a glass pipette tip (Charles et al., 1991). While this process can initiate a series of intercellular calcium waves that radiate from the stimulated cell, the exact level of mechanical stimulus used to create these waves was not well quantified. However, the use of astrocytic monolayers allowed for a correlation between levels of mechanical deformation and cellular injury. The cells in the monolayers showed cytoskeletal alterations, organelle disruption and derangement, and apparent changes in intracellular calcium ion influx (Ellis et al., 1995). These largely ultrastructural changes increased in severity as the level of mechanical deformation increased. At one point, significant cell death could be produced within 24 h of the initial mechanical insult. Unlike most in-vitro models used at the time, cell death did not propagate from an initial and immediate site of mechanical failure (i.e., the cells did not lyse and cause secondary death in neighboring cells). Rather, death was driven by the mechanically initiated intracellular events.
In a series of follow-up studies, this same group identified a potentially novel role for calcium in astrocytic injury (Rzigalinski et al., 1997), as well as the mechanisms responsible for calcium elevation (Rzigalinksi et al., 1998; Rzigalinksi et al., 1997; Chen et al., 2004). Lamb and associates (1997) studied the role of membrane repair over time in injured astrocytes. Ahmed and colleagues (2000) studied mitochondria and ATP regulation. Other researchers examined how the complex cascades regulating free radical generation and IP3 signaling contributed to the stretch injury response in astrocytes over time (Hoffman et al., 2000; Floyd et al., 2001). Recent work shows that mechanical stress leads to the release of proteins that can further exacerbate the initial injury (Ellis et al., 2007).
These works assembled a more complete picture on how disruption of potential physical linkages among intracellular structures can lead to transient calcium cascades, an important and unique step in the mechanoactivation cascade in astrocytes (Floyd et al., 2004). Other efforts have shown that mechanical injury leads to changes in volume-activated ion channels within astrocytes (Di et al., 2000), alterations in intracellular sodium (Floyd et al., 2005), production of endothelin-1 to influence vascular tone (Ostrow et al., 2000), and a potential crossover influence on the N-methyl-d-aspartate (NMDA) receptor (NMDAR) in strain-injured neurons (Lea et al., 2002). In addition, several studies now link the initial mechanical insult to the activation of purinergic receptors and the subsequent activation of intracellular signaling cascades (Neary et al., 2003; Neary et al., 2005; Neary and Kang, 2006).
In-vitro neuron deformation models
Although several studies discuss astrocyte response to a controlled mechanical stretch, the neuron is by far the most studied cell type in this area. A number of different devices are available for studying neuronal response to biaxial stretch (Ellis et al., 1995; Morrison et al., 1998; Geddes and Cargill, 2001) (Fig. 1B), uniaxial stretch (Lusardi et al., 2004a; Pfister et al., 2003) (Fig. 1A), and general deformations in neurons or axons by using a fluid shear pulse (Laplaca and Thibault, 1997; Edwards et al., 2001; Nakayama et al., 2001) (Fig. 1C). Early studies showed that calcium signaling initiated from the site of primary mechanical injury was predominantly mediated by electrophysiological changes in the NMDAR (Zhang et al., 1996). An alternative mechanism of injury suggests that nonspecific increases in plasma membrane permeability contribute to neuronal fate (Geddes et al., 2003a; Serbest et al., 2005; Serbest et al., 2006). Activating the NMDAR is key for the desensitization of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPAR) after stretch (Goforth et al., 1999, 2004), potentiation of γ-aminobutyric acidA currents (Kao et al., 2004), and changes in the mitochondria membrane potential (Ahmed et al., 2000, 2002). All this may explain the potential vulnerability of hippocampal neurons (Geddes et al., 2003b) to mechanical trauma. However, the NMDAR is critical only for the induction of a stretch-induced delayed depolarization that can often accompany stretch injury (Tavalin et al., 1997); the maintenance of this modestly increasing depolarization occurring hours following injury is mainly mediated by the electrogenic sodium pump in the membrane. Acute and long-term changes in cytosolic calcium are not only related to NMDAR activation (Zhang et al., 1996; Geddes et al., 2003b; Lusardi et al., 2004b), but are also influenced by other glutamate receptors (Chen et al., 2004). It is important to note that changes in the NMDAR after stretch are not universally observed across different laboratories (Arundine et al., 2003). Changes in neuronal NMDARs are also sensitive to the presence of glia in the culture (Lea et al., 2003; Lea et al., 2002).
The progression of damage to injured neurons in vitro continues to reveal important new insights into how the traumatically injured brain may respond to mechanical injury over the ensuing days. This is important when considering short- and long-term treatment options. Post-injury changes in intracellular calcium signaling can lead to altered neuronal function under agonist stimulation (Weber et al., 2001, 2002; Weber et al., 1999), the consequences of which are yet to be fully understood. Activations of cysteine proteases, calpain, and caspase-3 also occur hours following injury, suggesting that the injury includes both apoptotic and necrotic forms of cell death (Pike et al., 2000). Furthermore, recent evidence showed that different mechanoactivation cascades occur when neurons are subjected to different mechanical strain fields (uniaxial or biaxial) (Geddes-Klein et al., 2006), indicating that moving in-vitro findings into in-vivo models, with their associated complex strain fields, will be important to consider when interpreting findings from the in-vitro work. Mild TBI in vitro appears to increase cellular susceptibility to secondary excitotoxic insults. For a single mild injury, the interplay between receptors and molecules in the synapse also extends to receptor cross-talk. This is mediated in part by the coupling between the NMDAR and molecules within the post-synaptic density (Arundine et al., 2003; Arundine et al., 2004).
Undoubtedly, issues involving long-term changes in neuronal function will become more complex as the field moves towards using systems designed for three-dimensional strain/stress fields along with improved tissue culture methods. This new paradigm can increase understanding of how the orientation of neurons within the tissue can affect the injury response (Cullen and Laplaca, 2006; Laplaca et al., 2005) (Fig. 1D). New information on neuronal susceptibility will likely show new interactions among receptor subtypes that might influence therapy development.
Another factor that can affect therapeutic development is the frequency of injury. Repeated mild injuries have an effect on neuronal viability and the release of neuronal proteins that is distinct from the effects of a single mild TBI. Repeated mild TBI are modeled as multiple mechanical simulations and may have particular relevance to bTBI, where there is the possibility for repeated blast exposures. The length of time between repeated insults to mechanical stress can change the timing of protein release and nuclear dye uptake (Slemmer and Weber, 2005). Information on treatment strategies gleaned from these in-vitro models of repeated injury will certainly make these strategies more sophisticated.
In-vitro axon deformation models
DAI is one of the most important common pathologies of civilian TBI and may also be a common pathology in bTBI. Accordingly, Smith and associates (1999) developed an in-vitro model of dynamic axon deformation to mimic the mechanical loading conditions of in-vivo DAI. This model induces uniaxial stretch only to axons spanning two populations of neurons. In an initial study, it was found that rapid axonal stretch injury induced a loss of elasticity, demonstrating direct mechanical damage to the axonal cytoskeleton. Subsequent studies demonstrated that traumatic axonal injury induces a deleterious feed-forward cycle linked to calcium influx into the axoplasm and causes dysregulation of the voltage-gated sodium channel (NaCh) (Wolf et al., 2001). Although the sodium channel is not a direct pathway for calcium entry following axon stretch injury, its sensitivity to traumatic mechanical stimulation is the upstream mechanism. Specifically, pathologic sodium entry through a dysregulated NaCh induces activation of voltage-gated calcium channels and reversal of sodium/calcium exchangers. The resultant increase in intra-axonal calcium levels triggers proteolysis of the inactivation loop of the NaCh. As such, sodium influx through the NaCh is unmitigated, resulting in increasing levels of calcium. This in turn fuels additional pathologic changes that may result in axon degeneration or may impair the ability of the axon to maintain normal function (Iwata et al., 2004), similar to other channelopathies reported in the literature. In-vivo evidence also shows that sodium channel proteolysis is a component of axonal damage after TBI, but concludes further that myelinated axons respond differently to stretch injury than unmyelinated axons in the same brain region (Reeves et al., 2005). Future in-vitro studies may begin exploring some of the potential mechanisms that regulate NaCh proteolysis, as well as the differences between myelinated and unmyelinated axons, using more sophisticated culturing techniques to myelinate axons in vitro. Parallel in-vivo DAI studies using TBI models such as a weight drop impaction to the head (Kallakuri et al., 2003) can further determine the effects of laceration in a more complicated system.
In vitro cell combination, brain slice, and organotypic deformation models
In recent years, the continued refinement of knowledge for mechanotransduction in both astrocytes and neurons has been complemented with emerging information on microglia, past work on endothelial cells, and a continuing series of investigations on cross-talk among cell types (McKinney et al., 1996; Ellis et al., 2007). The organotypic brain slice culture—literally a living slice of brain cultured over time—maintains the approximate architecture and proportion of cell types in the brain. These models are a reasonable extension of studies using either pure neuronal or astrocytic populations, or even studies using mixed neuronal glial cultures that are not designed to mimic the in-situ architecture of the brain. Data from these organotypic slice culture models have already established that neuronal death in the cortex has a mechanical threshold distinct from that seen in hippocampal slices (Cater et al., 2006; Morrison et al., 2003). Moreover, the genes expressed by injured organotypic tissue have some level of correspondence with activated genes reported for in-vivo TBI (Morrison et al., 2000a, 2000b), therefore this preparation can also be used to study gene expression changes over time. These models now also extend to isolated segments of white matter to study DAI (Shi and Pryor, 2002; McBride et al., 2006; Shi and Whitebone, 2006). Ultimately, treatment strategies must consider injury severity (Cater et al., 2007) and protect other cell type populations such as astrocytes and oligodendrocytes that also die during injury (Deridder et al., 2006).
Modeling secondary insults with tertiary bTBI in vitro
While not all types of complications can be modeled in vitro, hypoxic-ischemic conditions can be mimicked in slices and cell cultures with the use of an oxygen-glucose deprivation chamber or through chemical means; the application of sodium azide or potassium cyanide with iodoacetic acid both deplete cellular ATP. Several labs have performed combined mechanical stretch and hypoxia treatments to determine how the combined injuries affect neuronal survivability. Cargill and Thibault (1996) showed that at high rates of mechanical stretch, chemical hypoxia did not affect acute response. At low rates of deformation, calcium influx was independent of stretch but dependent on hypoxic-ischemic conditions. Glass and colleagues (2002) and Engel and associates (2005) each found that the combination of moderate stretch and hypoxic treatments resulted in increased cell death over a stretch or hypoxia alone. Furthermore, agents that have shown neuroprotection for either hypoxia or mechanical injury are not effective in treating combined insults (Engel et al., 2005). This suggests that the combined insult needs careful consideration when developing effective treatments (Glass et al., 2002), especially for those suffering from polytrauma.
Secondary blast injury
Increasing effort is being made to study secondary bTBI since there is a higher mortality rate associated with penetrating brain injuries. According to the CDC, firearm use is the leading cause of TBI-associated death. Since penetrating injuries from complex skull fracture are not uncommon in motor vehicle crashes, this also adds to the sequelae of more diffuse types of injuries. Although the penetrating injury involves both vascular and neural tissue in the brain parenchyma, little is known about how the two factors interact in vitro to create a picture similar to what is observed clinically. At the in-vitro level, penetrating injuries are best reproduced using a direct laceration or scratching of cell monolayers. Although relatively simple technology is needed, this type of model has received less attention in the past decade compared to the cell-stretch models previously described. Similar to the summary of tertiary bTBI models, the past in-vitro work relevant to secondary bTBI is summarized in tabular form in Table 2, and in the following paragraphs.
Table 2.
Summary of Injury Mechanisms from Secondary bTBI
| Reference | In-vitro injury | RM | IM | IC | O | Summary |
|---|---|---|---|---|---|---|
| Regan and Choi, 1994 | Stylus | X | X | Injury mediated by excessive NMDAR activation and dihydropyridine-sensitive mechanisms | ||
| Regan and Panter, 1995 | Stylus | X | X | Injury was reduced with MK801 and free radical scavengers | ||
| Mukhin et al., 1997a, 1998 | Stylus | X | Glutamate receptor antagonists were neuroprotective | |||
| Mukhin et al., 1997b; Mukhin et al., 1996 | Stylus | X | Activation of mGluR1 exacerbates injury | |||
| Faden et al., 1997 | Punch laceration (stylus) | X | Group III mGluR agonists are neuroprotective | |||
| Wang et al., 2005 | Injured tissue from humans/rodents | X | Performed proteomics on injured tissue to determine biomarkers of injury | |||
| Lucas et al., 1985; Emery et al., 1987; Kirkpatrick et al., 1985 | Laser transection | X | Damage to cytoskeleton, neurofilaments microtubules, organelles (especially mitochondria) | |||
| Lucas et al., 1991 | Laser transection | X | At low [Ca2+], Na+ mediates injury | |||
| Rosenberg et al., 1996a | Laser transection | X | MPSS helps stabilized damaged membranes | |||
| Rosenberg et al., 1996b; Rosenberg et al., 2001 | Laser transection | X | Na+ and Cl– ions may mediate injury | |||
| Balentine et al., 1988 | Weight drop | X | In vitro injury is similar to in vivo spinal cord trauma | |||
| Epstein, 1971 | Spinner bottle | X | Determined viability and oxidative metabolism of rat cortices after stir-bar-induced trauma |
RM, receptor mediated; IM, ion mediated; IC, intracellular signaling; O, other.
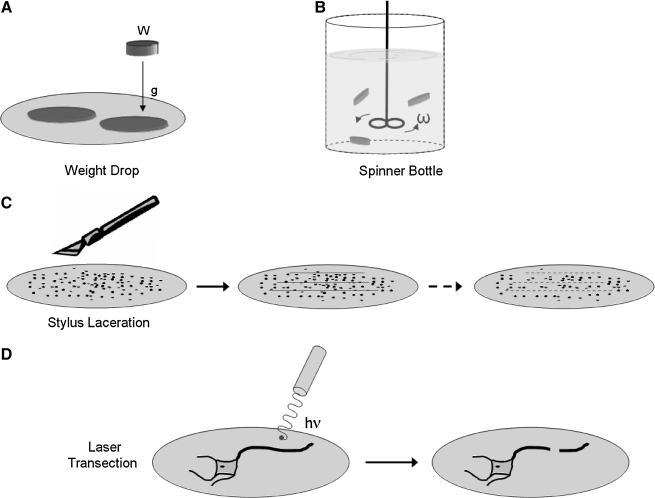
Some of the earliest in-vitro models used to study TBI fall under this category. Slices of the mouse spinal cord were subject to a direct weight drop to approximate the mechanical forces seen in the cortex of a depressed skull fracture (Balentine et al., 1988) (Fig. 2A). Similarly, small chunks of the cortex were placed in a spinner bottle to detect potential mechanism(s) of damage that were mediated by the forces during transection (Epstein 1971) (Fig. 2B). Even though these studies were among the first to use laceration on more complex tissue, there were no further reports comparing in-vitro laceration models on dissociated cultures to these tissue structures.
FIG. 2.
Models of secondary bTBI. (A and B) The most simple models used on tissue slices and aggregates involve using either a dropped weight (W) or rotating spinner (ω) bottle to induce the primary injury. (C) More recent techniques use a stylus to induce a primary injury, which can be designed to injure several regions of the culture simultaneously. (D) A less commonly used approach is to use a laser (energy hv) to precisely injure local regions of the cell to study the regenerative or repair response.
Several years later, Tecoma and colleagues (1989) used a stylus to scratch a region of cells containing a mixture of neurons and glia, tearing the cell layer to study cell death in the surrounding regions of the micro-laceration (Fig. 2C). Interestingly, mostly neurons died while glial cells were spared. Cell death could be controlled by administering two different NMDAR antagonists, supporting concurrent in-vivo controlled cortical impact studies used to develop NMDA-based treatments for TBI (Raghavendra Rao et al., 2001; Dempsey et al., 2000). Subsequent scratch studies showed that dihydropyridines could augment the protection offered by NMDAR antagonists (Regan and Choi, 1994); 21-aminosteroids showed a similar effect (Regan and Panter, 1995). In a later series of studies, Mukhin and associates (1997a, 1998) used this laceration model as a method to simultaneously test different pharmacological approaches, with the goal of achieving a model for high-throughput screening. Notable improvements in this field included a precise dose-response for the laceration technique in producing cell death, and an expansion in the number of therapeutic targets studied. Beginning with NMDAR antagonists, work from this new model soon showed that different glutamate receptors (mGluR1) work both alone and in combination with traditional NMDAR antagonists (Mukhin et al., 1997b, 1996). Intriguingly, new approaches using mGluR3 receptor agonists would also prove neuroprotective in this model (Faden et al., 1997), suggesting a more complex interplay between neuronal glutamate receptors during recovery from a laceration injury. This in-vitro model also has the potential for testing injury biomarkers (Wang et al., 2005). A possible in-vivo model of the in-vitro laceration model could be a simple cortical ablation.
The cell laceration model can be further refined to study the response of a single neuron. Laser microsurgery, as a technique, has evolved to where an investigator can harness the power of a laser to cut out regions of an individual cell (Gross et al., 1983) (Fig. 2D), or even transiently permeabilize cell regions to deliver genetic material for subsequent study (Barrett et al., 2006). The location of transection in a neuron is crucial. Nuclear damage occurs readily if the transection point is close to the soma, and decays to nearly zero if the lesion is far from the cell body (>300 μm) (Lucas et al., 1985). Injury from this very controlled and focal disruption of the neuronal structure consists of cytoskeletal damage to the neurofilaments and microtubules, as well as damage to the organelles, most notably the mitochondria (Lucas et al., 1985; Emery et al., 1987; Rosenberg et al., 1996a; Kirkpatrick et al., 1985). Extracellular calcium does little to control the damage observed in the soma from this type of laceration, but significant changes were observed when different extracellular calcium conditions were used to study the changes in dendritic morphology and structure (Lucas et al., 1990). In one of the earliest reports that expanded on the calcium hypothesis, it was suggested that other ions (potassium and sodium) could play a key role in dendritic integrity after dendritic transection (Rosenberg and Lucas, 1996b; Rosenberg et al., 2001). Laser ablation in vivo would be a comparable model (Nishimura et al., 2006a; Davalos et al., 2005).
Despite the relatively simple technology needed to mimic secondary bTBI in vitro, there is little published work that examines the role of secondary insults on either the exacerbation of initial injury cascades, or the promotion of new, more complex cascades of damage. Given the likelihood that the secondary injury associated with hemorrhagic shock, hypoxic-ischemic injury, or free radical generation are high in the moderate and severely brain-injured patients, this area remains ripe for new exploration in understanding not only the mechanisms of cell death that may be activated, but also the potential pro-survival signals that are possibly activated with these injuries.
Primary blast injury
What is currently known of primary bTBI
As noted in the introduction, the initial phase of blast injury consists of the pressure wave transmitting through the body, as well as the associated wind force that can cause direct damage to external tissues. Remarkably little is known about the response of CNS cultured cells from either form of mechanical input. The cellular response to shockwaves is often mentioned only in passing as a potential complication when interpreting data from the effects of ultrasound on tissue structure or function. The transmission of shockwaves through the scalp and skull is also considerably complex, so proper representation of shock input to tissue or cell culture preparations remains to be defined. One open question is how the shockwave affects tissue as it travels through. Nevertheless, the limited reports on such effects suggest that the shockwave is capable of primary tissue damage in vivo that resembles, but is not exactly similar to, the direct laceration damage that can appear with penetrating fragments and secondary brain injury. Certainly, the laser-induced shock-wave pulse can be controlled to create nanosurgical procedures capable of in-vivo transection of individual nerve fibers within the roundworm Caenorhabditis elegans (Yanik et al., 2004). Less clear is whether this shock-wave pulse can also produce regions of transient permeability changes in the plasma membrane without long-term transection damage. The shock-wave transmission at the interface between different structures (e.g., the white/gray matter interface and the solid/fluid interface at the blood-brain barrier) also presents an opportunity for additional damage.
Aside from the complex shock-wave patterns that can emerge in blast, what about the general influence of pressure on biological functions? Few studies have looked at pressure-mediated effects in vitro despite past concussive-based in-vivo insult studies that showed the ill effects of pressure on the nervous system (Denny-Brown and Russell, 1940; Gurdjian et al., 1962). Many also consider the peak intracranial pressure as a reasonable predictor of tissue injury (Stalhammar and Olsson, 1975). High negative transient pressures seen during a blast event can cause cavitation-based damage, but achieving similar pressure levels at the in-vitro level is difficult. Quasistatic hydrostatic pressures on cells causes cellular function and potential membrane-based changes in neurons and astrocytes (Murphy and Horrocks, 1993; Salvador-Silva et al., 2004), but the time-scales in these experiments are considerably longer than any blast event.
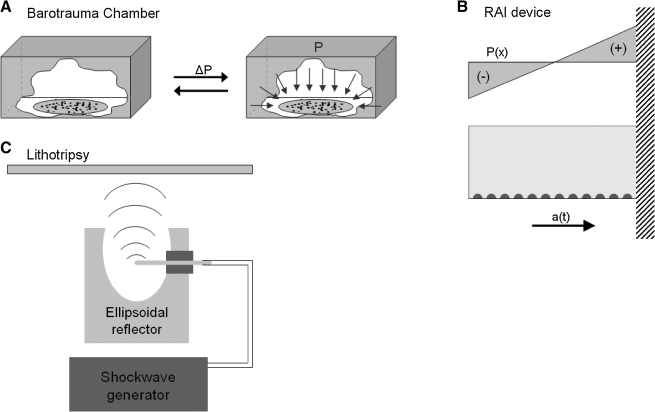
By far the most commonly used device to study the effect of pressure on the CNS is a barotrauma chamber, often built from existing or slightly modified fluid percussion devices used in TBI studies (Shepard et al., 1991) (Fig. 3A). Transient pressures from this type of chamber are sufficient to cause production of cytokines, specifically interleukin-6, and may contribute to cerebrovascular dysfunction (Hariri et al., 1994). This pressure chamber is readily amenable to test treatments for reversing pressure-mediated injury; successful treatments include a peptide to control Bcl-2 activation, and inhibitors for leukotrienes, nitric oxide, ADP-ribosylation, and the anticonvulsant felbamate (Wallis et al., 1995, 1996; Girard et al., 1996; Panizzon et al., 1996, 1998). Another type of transient pressure model is the rapid acceleration injury (RAI) model (Fig. 3B). This model can be used to study multiple impacts, creating a brief pressure gradient during the impact. Lucas and Wolf (1991) showed that neurons exposed to multiple acceleration/pressure forces caused soma swelling, nuclear prominence, and sometimes neuronal death, depending on the severity. Even though in-vitro models do not yet study changes in cell viability after transient pressurization, they strongly suggest that the biological effects of the pressure transient need to be explored more thoroughly in the future.
FIG. 3.
Models of primary bTBI. (A) In the barotrauma chamber, even pressure (ΔP) is applied to all cells and slices to induce pressure-mediated injury. In a hydrostatic chamber, the pressure is quasistatic; in a fluid percussion chamber, the pressure is transient. (B) In the rapid acceleration injury (RAI) model, a flask containing the cells is swung on a pendulum from specified heights. A pressure transient (P(x)) is induced by the fluid upon impact of the pendulum/flask against a stationary object at the lowest point of the trajectory. The resulting injury is from a combination of acceleration (a(t)) and pressure forces. (C) In lithotripsy, shockwaves are generated and reflected off the ellipsoidal reflector.
A current method that can be used to generate high-amplitude, transient pressure pulses is extracorporeal shock-wave lithotripsy (ESWL) (Fig. 3C). ESWL is currently used to treat kidney stones; the high-pressure shockwaves physically break up the stones. However, some also use this technology to study shock-wave damage on tissue. Two aspects of shock-wave pressure that have been shown to cause damage is the high transient pressure and the generation of cavitation bubbles in fluids subjected to high tensile stress. Howard and Sturtevant (1997) used ESWL in vitro on thin membranes immersed in tissue-mimicking fluids to study the mechanism of failure. The contributing factors that caused membrane failure were: shock compression causing the membrane to fail in shear, bubble collapse also caused membrane failure, and inhomogeneity of the tissue. These mechanisms of damage are not cavitation-based, as they can occur in fluids where cavitation does not occur. The threshold for the damage depends on the magnitude of the shock, the number of exposures, and physical properties of the medium. There is also a pressure-induced compression and lateral extension that can induce shear damage to structures that include the plasma membrane, organelles, and intracellular membranes (Howard and Sturtevant, 1997). The impedance properties of the medium focus and defocus the entrant shockwave, leading to a complex pattern of damage. This membrane-based damage or “nanoshearing” is currently unexplored in the blast literature.
A second phenomenon associated with shockwaves is the generation of cavitation/bubble formation, which seems to be dependent on fluid properties. The higher the presence of cavitation nuclei in the fluid, the greater the number of cavitation bubbles that will generate with a passing shockwave. Degassing the fluid reduces the amount of cavitation nuclei and subsequent bubble formation (Pa et al., 2005). Other labs have shown that the formation and collapse of cavitation bubbles from the transmission of a shockwave through tissue can lead to minute local injury. Laser pulses can produce controlled cavitation in vivo to create damage limited to a single microvessel in the cortex (Nishimura et al., 2006b), or cut off individual nerve fibers in simple neural networks in vivo (Yanik et al., 2004). These in-vitro experiments can be analyzed along with in-vivo blast studies, such as using a bio-shock tube to expose the whole body to blast, to further elucidate how cells respond to fast and extreme changes in pressure (Wang et al., 1998; Cernak et al., 2001a, 2001b) and to the presence of cavitation. Possible future endeavors to study primary bTBI could include FE simulations to model injury from a shockwave. FE tools used to model tertiary bTBI could possibly be coupled to Eulerian based hydrocodes designed to simulate shock situations. Current hydrocodes have been coupled to Lagrangian-based codes to model blast damage to structures (Cullis, 2001; Clutter and Stahl, 2004). Such programs could possibly be expanded upon with the aid of experimental data (Chavko et al., 2007; Clemedson, 1956; Clemedson et al., 1956; Clemedson and Pettersson, 1956) to determine shock-wave transmission through bone and tissue.
A final important area that is not yet explored is the role of secondary factors in the survival and function of cells following primary bTBI. It is not surprising that this particular area is unexplored, as the development and use of the in-vitro techniques to study this part of the force spectrum are not yet available.
The results of the in-vitro models used to study primary bTBI have been summarized in Table 3.
Table 3.
Summary of Injury Mechanisms from Primary bTBI
| Reference | In-vitro injury | RM | IM | IC | O | Major findings |
|---|---|---|---|---|---|---|
| Murphy and Horrocks, 1993 | Hydrostatic pressures | X | Pressure induces cellular membrane damage | |||
| Salvador-Silva et al., 2004 | Hydrostatic pressures | X | Pressure induces changes in human optic nerve head astrocyte migration | |||
| Hariri et al., 1994 | Fluid percussion | X | Production of IL-6 | |||
| Wallis et al., 1995; Wallis et al., 1996; Girard et al., 1996; Panizzon et al., 1996; Panizzon et al., 1998 | Fluid percussion | X | X | X | Potential treatments: Bcl-2 activation; inhibition of leukotrienes, nitric oxide, ADP-ribosylation, NMDAR; felbamate; metalloporphyrins | |
| Nishimura et al., 2006b | Laser pulse | Produced three forms of vascular insult: rupture, extravasation, blockage | ||||
| Howard and Sturtevant 1997 | Lithotripsy | X | Cellular membranes fail in fatigue by shear |
RM, receptor mediated; IM, ion mediated; IC, intracellular signaling; O, other.
Quaternary blast injury
Simply put, the quaternary injury phase comprises any conditions not covered in the previous three phases. For conventional explosives, types of quaternary injury would involve burns or the onset of asthma due to dust inhalation. However, there are other types of weapons that may cause further TBI. Radiological dispersal devices, often called a “dirty nuke” or “dirty bomb,” are used to spread sub-lethal levels of radioactive materials over a large area. Nuclear weapons also emit electromagnetic pulses (EMP) in addition to spreading thermal radiation, radioactive material, and emitting an intense blinding light.
The radioactive, electromagnetic, and thermal energy components need consideration for their long-term consequences on the brain. Much of the work on radiation toxicity is in vivo and studies the radiation-induced injury to the CNS due to tumor treatments. In-vitro studies that have looked at x-ray exposure to neuronal cultures show that free radical scavengers can be neuroprotective (Noel and Tofilon, 1998). In-vitro studies have shown that ionizing radiation induces apoptosis in endothelial cells in vitro (Haimovitz-Friedman et al., 1994) and CNS endothelial cells in vivo, thereby damaging vasculature (Peña et al., 2000). Further radiation studies in culture show that astrocytes provide protection to neurons through the release of proteins (Noel and Tofilon, 1998). Information on electromagnetic radiation effects on the brain generally stem from studies on the effects of mobile phones on people (Munshi and Jalali, 2002; Christ and Kuster, 2005). Mobile phones generate a relatively weak, but constant electromagnetic field, whereas the EMP generated from an explosion is considerably higher, but passes quickly. Mechanisms for injury based on weak electromagnetic fields are not often well described, and it would be difficult to scale these mechanisms to the much stronger level of an EMP generated by a nuclear explosion. One potential effect of the EMP to consider is how cortical activity can be modulated by a single, brief EMP. Early studies on transcranial magnetic stimulation (TMS) were met with some concern on how the technology would affect cortical function. Laser studies show that this potential side effect is not a problem with TMS. However, a much larger EMP has the potential to alter the existing neural network in some manner and warrants at least some consideration in the future. Thermal effects from the blast are not well studied either, although the transient nature of the thermal exposure would make significant transfer of energy into the brain unlikely. More severe circumstances would result in burns. The results of the in-vitro models used to study quaternary bTBI have been summarized in Table 4.
Table 4.
Summary of Injury Mechanisms from Quaternary bTBI
| Reference | In-vitro injury | RM | IM | IC | O | Summary |
|---|---|---|---|---|---|---|
| Noel and Tofilon, 1998 | Radiation (x-ray) | X | X-ray induces neuronal apoptosis; astrocytes secrete neuroprotective proteins | |||
| Haimovitz-Friedman et al., 1994 | Radiation (ionizing) | X | Radiation interacts with the cellular membrane and induces apoptotic signals | |||
| Munshi and Jalali, 2002 | – | Review of cell phone hazards | ||||
| Christ and Kuster, 2005 | – | Factors that affect energy absorption |
RM, receptor mediated; IM, ion mediated; IC, intracellular signaling; O, other.
Conclusions and Future Perspectives
Just as there is no single in-vitro or in-vivo model that can be favored as the model to study civilian TBI, it is equally difficult to pick a particular model as the signature in-vitro model for bTBI. The blast phenomenon will change depending on the type of IED used, the presence of surrounding structures, atmospheric conditions (i.e., wind), and the direction of the blast with respect to the person. There is a high degree of variation in each blast situation. Certainly the advantage with the in-vitro models over in-vivo models is the ability to control the mechanical injury and reduce other potentially complicating factors. On the whole, current in-vitro TBI models capture the scope of the response at both the molecular and cellular scale, but further examination is needed. For injuries related to the blast environment, past in-vitro studies are strongly biased towards injuries sustained in the tertiary blast phase. Eventually, studies related to the mechanism of tertiary bTBI will likely include more functional measures of injury, test therapeutics over a longer treatment window, and begin evaluating how different brain regions may be vulnerable to different secondary brain injury mechanisms. Undoubtedly, this work will impact the treatment of injuries not only related to the blast environment, but also cross over into treating similar injuries from the traditional TBI environment (e.g., motor vehicle accidents, falls, and assaults). When considering in-vitro approaches for studying secondary blast phase related injuries, there remain large areas yet to be examined. For example, while early laceration studies in vitro provided some insight into the mechanisms and treatments for neuronal death, a considerable amount of work is needed to identify more effective treatments with a wider therapeutic window. Further studies will also determine whether increased neuroprotection corresponds to better cellular function. A significant issue that needs resolution is how the presence of factors in the extracellular space (e.g., blood, serum, and activated macrophages) can complicate recovery from transection injury. Moreover, the time of death for neuronal and glial cells following transection needs better definition, as the transection location has been shown to affect the window for cellular survival.
The most unexplored area are the mechanisms causing the primary blast injury phase. Even though in-vivo experiments show that significant intracranial pressures can transmit through the brain during a blast, there is very limited information on how these pressures can affect biological function, and how this shockwave is transmitted/reflected through the brain, as well as how this wave can cause damage at the cellular and molecular scale. Moreover, the influence of repeated exposures to a blast event, and their long-term effect on neuronal function, are not known.
Quaternary blast injury is likely the most difficult to study simply because it encompasses so much. Multiple factors contribute to injury in this phase. Because there is not a single physical force that can be directly tied to the injury mechanism, it lacks a distinct biomechanical form of study. Therefore in-vitro models from the three phases—primary, secondary, and tertiary—should be used to examine how the toxic products can contribute to the injuries observed in this phase.
In closing, it is worth noting that the relative wealth of new research directions in understanding bTBI with in-vitro approaches presents a very simple challenge: which ones should be studied first? It is natural to believe that as the biomechanical environment within the brain during a blast event is better characterized, the precise in-vitro model best suited to study primary bTBI will become clear. This may be one of the most important next questions, as relatively little is known about the cellular and molecular transduction events in primary bTBI. In addition, the effects of secondary insults on the primary, secondary, and tertiary phases of injury are also critically important and need clarification, as these can help better identify the potential therapies for the broader picture of bTBI. Similarly, it could also be interesting to combine two or more in-vitro models to study the multiple effects of blast. Regardless of the specific questions addressed by an investigator, there is opportunity for quick and meaningful progress in the field of bTBI that will have an immediate impact on both military and civilian suffering.
Acknowledgements
Funds for this work were provided by the National Institutes of Health grants NS 35172 and HD 41699.
Author Disclosure Statement
No conflicting financial interests exist.
References
- Ahmed S.M. Rzigalinski B.A. Willoughby K.A. Sitterding H.A. Ellis E.F. Stretch-induced injury alters mitochondrial membrane potential and cellular ATP in cultured astrocytes and neurons. J. Neurochem. 2000;74:1951–1960. [PubMed] [Google Scholar]
- Ahmed S.M. Weber J.T. Liang S. Willoughby K.A. Sitterding H.A. Rzigalinski B.A. Ellis E.F. NMDA receptor activation contributes to a portion of the decreased mitochondrial membrane potential and elevated intracellular free calcium in strain-injured neurons. J. Neurotrauma. 2002;19:1619–1629. doi: 10.1089/089771502762300274. [DOI] [PubMed] [Google Scholar]
- Arundine M. Aarts M. Lau A. Tymianski M. Vulnerability of central neurons to secondary insults after in vitro mechanical stretch. J. Neurosci. 2004;24:8106–8123. doi: 10.1523/JNEUROSCI.1362-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arundine M. Chopra G.K. Wrong A. Lei S. Aarts M.M. MacDonald J.F. Tymianski M. Enhanced vulnerability to NMDA toxicity in sublethal traumatic neuronal injury in vitro. J. Neurotrauma. 2003;20:1377–1395. doi: 10.1089/089771503322686166. [DOI] [PubMed] [Google Scholar]
- Bakke B. Ulvestad B. Stewart P. Lund M.B. Eduard W. Effects of blasting fumes on exposure and short-term lung function changes in tunnel construction workers. Scand. J. Work Environ. Health. 2001;27:250–257. doi: 10.5271/sjweh.612. [DOI] [PubMed] [Google Scholar]
- Balentine J.D. Greene W.B. Bornstein M. In vitro spinal cord trauma. Lab. Invest. 1988;58:93–99. [PubMed] [Google Scholar]
- Barrett L.E. Sul J.Y. Takano H. van Bockstaele E.J. Haydon P.G. Eberwine J.H. Region-directed phototransfection reveals the functional significance of a dendritically synthesized transcription factor. Nat. Methods. 2006;3:455–460. doi: 10.1038/nmeth885. [DOI] [PubMed] [Google Scholar]
- Cargill R.S., 2nd Thibault L.E. Acute alterations in [Ca2+]i in NG108-15 cells subjected to high strain rate deformation and chemical hypoxia: an in vitro model for neural trauma. J. Neurotrauma. 1996;13:395–407. doi: 10.1089/neu.1996.13.395. [DOI] [PubMed] [Google Scholar]
- Cater H.L. Gitterman D. Davis S.M. Benham C.D. Morrison B., 3rd Sundstrom L.E. Stretch-induced injury in organotypic hippocampal slice cultures reproduces in vivo post-traumatic neurodegeneration: role of glutamate receptors and voltage dependent calcium channels. J. Neurochem. 2007;101:434–447. doi: 10.1111/j.1471-4159.2006.04379.x. [DOI] [PubMed] [Google Scholar]
- Cater H.L. Sundstrom L.E. Morrison B., 3rd Temporal development of hippocampal cell death is dependent on tissue strain but not strain rate. J. Biomech. 2006;39:2810–2818. doi: 10.1016/j.jbiomech.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Cernak I. Wang Z. Jiang J. Bian X. Savic J. Cognitive deficits following blast injury-induced neurotrauma: possible involvement of nitric oxide. Brain Inj. 2001;15:593–612. doi: 10.1080/02699050010009559. [DOI] [PubMed] [Google Scholar]
- Cernak I. Wang Z. Jiang J. Bian X. Savic J. Ultrastructural and functional characteristics of blast injury-induced neurotrauma. J. Trauma. 2001;50:695–706. doi: 10.1097/00005373-200104000-00017. [DOI] [PubMed] [Google Scholar]
- Charles A.C. Merrill J.E. Dirksen E.R. Sanderson M.J. Intercellular signaling in glial cells: Calcium waves and oscillations in response to mechanical stimulation and glutamate. Neuron. 1991;6:983–992. doi: 10.1016/0896-6273(91)90238-u. [DOI] [PubMed] [Google Scholar]
- Chavko M. Koller W.A. Prusaczyk W.K. McCarron R.M. Measurement of blast wave by a miniature fiber optic pressure transducer in the rat brain. J. Neurosci. Methods. 2007;159:277–281. doi: 10.1016/j.jneumeth.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Chen T. Willoughby K.A. Ellis E.F. Group I metabotropic receptor antagonism blocks depletion of calcium stores and reduces potentiated capacitative calcium entry in strain-injured neurons and astrocytes. J. Neurotrauma. 2004;21:271–281. doi: 10.1089/089771504322972068. [DOI] [PubMed] [Google Scholar]
- Christ A. Kuster N. Differences in RF energy absorption in the heads of adults and children. Bioelectromagnetics Suppl. 2005;7:S31–S44. doi: 10.1002/bem.20136. [DOI] [PubMed] [Google Scholar]
- Chu C.S. Lin M.S. Huang H.M. Lee M.C. Finite element analysis of cerebral contusion. J Biomech. 1994;27:187–194. doi: 10.1016/0021-9290(94)90208-9. [DOI] [PubMed] [Google Scholar]
- Clemedson C.J. Jonsson A. Pettersson H. Propagation of an air transmitted shock wave in muscular tissue. Nature. 1956;177:380–381. doi: 10.1038/177380a0. [DOI] [PubMed] [Google Scholar]
- Clemedson C.J. Pettersson H. Propagation of a high explosive air shock wave through different parts of an animal body. Am. J. Physiol. 1956;184:119–126. doi: 10.1152/ajplegacy.1955.184.1.119. [DOI] [PubMed] [Google Scholar]
- Clemedson C.J. Shock wave transmission to the central nervous system. Acta Physiol. Scand. 1956;37:204–214. doi: 10.1111/j.1748-1716.1956.tb01356.x. [DOI] [PubMed] [Google Scholar]
- Clutter J.K. Stahl M. Hydrocode simulations of air and water shocks for facility vulnerability assessments. J. Hazard. Mater. 2004;106:7–16. doi: 10.1016/j.jhazmat.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Cullen D.K. Laplaca M.C. Neuronal response to high rate shear deformation depends on heterogeneity of the local strain field. J. Neurotrauma. 2006;23:1304–1319. doi: 10.1089/neu.2006.23.1304. [DOI] [PubMed] [Google Scholar]
- Cullis I.G. Blast waves and how they interact with structures. J. R. Army Med. Corps. 2001;147:16–26. doi: 10.1136/jramc-147-01-02. [DOI] [PubMed] [Google Scholar]
- Davalos D. Grutzendler J. Yang G. Kim J.V. Zuo Y. Jung S. Littman D.R. Dustin M.L. Gan W. ATP mediates rapid microglial response to local brain injury in vivo. Nature Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- Deitchman S. Decker J. Santis L. A novel source of carbon monoxide poisoning: explosives used in construction. Ann. Emerg. Med. 1998;32:381–384. doi: 10.1016/s0196-0644(98)70019-8. [DOI] [PubMed] [Google Scholar]
- Dempsey R.J. Baskaya M.K. Dogan A. Fehlings M. Sekhon L.H.S. Muizelaar J.P. Young W. Bullock M.R. Ny-Brown D. Russell W.R. Attenuation of brain edema, blood-brain barrier breakdown, and injury volume by ifenprodil, a polyamine-site N-methyl-D-aspartate receptor antagonist, after experimental traumatic brain injury in rates. Neurosurgery. 2000;47:399–406. doi: 10.1097/00006123-200008000-00024. [DOI] [PubMed] [Google Scholar]
- Denny-Brown D. Russell W.R. Experimental cerebral concussion. J. Physiol. 99, 1940:153. doi: 10.1113/jphysiol.1940.sp003887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depalma R.G. Burris D.G. Champion H.R. Hodgson M.J. Blast injuries. N. Engl. J. Med. 2005;352:1335–1342. doi: 10.1056/NEJMra042083. [DOI] [PubMed] [Google Scholar]
- Deridder M.N. Simon M.J. Siman R. Auberson Y.P. Raghupathi R. Meaney D.F. Traumatic mechanical injury to the hippocampus in vitro causes regional caspase-3 and calpain activation that is influenced by NMDA receptor subunit composition. Neurobiol. Dis. 2006;22:165–176. doi: 10.1016/j.nbd.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Di X. Goforth P.B. Bullock R. Ellis E. Satin L. Mechanical injury alters volume activated ion channels in cortical astrocytes. Acta Neurochir. Suppl. 2000;76:379–383. doi: 10.1007/978-3-7091-6346-7_79. [DOI] [PubMed] [Google Scholar]
- Edwards M.E. Wang S.S. Good T.A. Role of viscoelastic properties of differentiated SH-SY5Y human neuroblastoma cells in cyclic shear stress injury. Biotechnol. Prog. 2001;17:760–767. doi: 10.1021/bp010040m. [DOI] [PubMed] [Google Scholar]
- Ellis E.F. McKinney J.S. Willoughby K.A. Liang S. Povlishock J.T. A new model for rapid stretch-induced injury of cells in culture: characterization of the model using astrocytes. J. Neurotrauma. 1995;12:325–339. doi: 10.1089/neu.1995.12.325. [DOI] [PubMed] [Google Scholar]
- Ellis E.F. Willoughby K.A. Sparks S.A. Chen T. S100B protein is released from rat neonatal neurons, astrocytes, and microglia by in vitro trauma and anti-S100 increases trauma-induced delayed neuronal injury and negates the protective effect of exogenous S100B on neurons. J. Neurochem. 2007;101:1463–1470. doi: 10.1111/j.1471-4159.2007.04515.x. [DOI] [PubMed] [Google Scholar]
- Emery D.G. Lucas J.H. Gross G.W. The sequence of ultrastructural changes in cultured neurons after dendrite transection. Exp. Brain Res. 1987;67:41–51. doi: 10.1007/BF00269451. [DOI] [PubMed] [Google Scholar]
- Engel D.C. Slemmer J.E. Vlug A.S. Maas A.I. Weber J.T. Combined effects of mechanical and ischemic injury to cortical cells: secondary ischemia increases damage and decreases effects of neuroprotective agents. Neuropharmacology. 2005;49:985–995. doi: 10.1016/j.neuropharm.2005.05.021. [DOI] [PubMed] [Google Scholar]
- Epstein M.H. Relative susceptibility of elements of the cerebral cortex to mechanical trauma in the rat. J. Neurosurg. 1971;35:517–522. doi: 10.3171/jns.1971.35.5.0517. [DOI] [PubMed] [Google Scholar]
- Faden A.I. Ivanova S.A. Yakovlev A.G. Mukhin A.G. Neuroprotective effects of group III mGluR in traumatic neuronal injury. J. Neurotrauma. 1997;14:885–895. doi: 10.1089/neu.1997.14.885. [DOI] [PubMed] [Google Scholar]
- Floyd C.L. Gorin F.A. Lyeth B.G. Mechanical strain injury increases intracellular sodium and reverses Na+/Ca2+ exchange in cortical astrocytes. Glia. 2005;51:35–46. doi: 10.1002/glia.20183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd C.L. Rzigalinski B.A. Sitterding H.A. Willoughby K.A. Ellis E.F. Antagonism of group I metabotropic glutamate receptors and PLC attenuates increases in inositol trisphosphate and reduces reactive gliosis in strain-injured astrocytes. J. Neurotrauma. 2004;21:205–216. doi: 10.1089/089771504322778668. [DOI] [PubMed] [Google Scholar]
- Floyd C.L. Rzigalinski B.A. Weber J.T. Sitterding H.A. Willoughby K.A. Ellis E.F. Traumatic injury of cultured astrocytes alters inositol (1,4,5)-trisphosphate-mediated signaling. Glia. 2001;33:12–23. doi: 10.1002/1098-1136(20010101)33:1<12::aid-glia1002>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Geddes D.M. Cargill R.S., 2nd An in vitro model of neural trauma: device characterization and calcium response to mechanical stretch. J. Biomech. Eng. 2001;123:247–255. doi: 10.1115/1.1374201. [DOI] [PubMed] [Google Scholar]
- Geddes D.M. Cargill R.S., 2nd Laplaca M.C. Mechanical stretch to neurons results in a strain rate and magnitude-dependent increase in plasma membrane permeability. J. Neurotrauma. 2003a;20:1039–1049. doi: 10.1089/089771503770195885. [DOI] [PubMed] [Google Scholar]
- Geddes D.M. Laplaca M.C. Cargill R.S., 2nd Susceptibility of hippocampal neurons to mechanically induced injury. Exp. Neurol. 2003b;184:420–427. doi: 10.1016/s0014-4886(03)00254-1. [DOI] [PubMed] [Google Scholar]
- Geddes-Klein D.M. Schiffman K.B. Meaney D.F. Mechanisms and consequences of neuronal stretch injury in vitro differ with the model of trauma. J. Neurotrauma. 2006;23:193–204. doi: 10.1089/neu.2006.23.193. [DOI] [PubMed] [Google Scholar]
- Girard J. Panizzon K. Wallis R.A. Azelastine protects against CA1 traumatic neuronal injury in the hippocampal slice. Eur. J. Pharmacol. 1996;300:43–49. doi: 10.1016/0014-2999(95)00804-7. [DOI] [PubMed] [Google Scholar]
- Glass T.F. Reeves B. Sharp F.R. Modeling both the mechanical and hypoxic features of traumatic brain injury in vitro in rats. Neurosci. Lett. 2002;328:133–136. doi: 10.1016/s0304-3940(02)00510-4. [DOI] [PubMed] [Google Scholar]
- Goforth P.B. Ellis E.F. Satin L.S. Enhancement of AMPA-mediated current after traumatic injury in cortical neurons. J. Neurosci. 1999;19:7367–7374. doi: 10.1523/JNEUROSCI.19-17-07367.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goforth P.B. Ellis E.F. Satin L.S. Mechanical injury modulates AMPA receptor kinetics via an NMDA receptor-dependent pathway. J. Neurotrauma. 2004;21:719–732. doi: 10.1089/0897715041269704. [DOI] [PubMed] [Google Scholar]
- Gross G.W. Lucas J.H. Higgins M.L. Laser microbeam surgery: ultrastructural changes associated with neurite transection in culture. J. Neurosci. 1983;3:1979–1993. doi: 10.1523/JNEUROSCI.03-10-01979.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurdjian E.S. Lissner H.R. Patrick L.M. Protection of the head and neck in sports. JAMA. 1962;182:509–512. doi: 10.1001/jama.1962.03050440001001. [DOI] [PubMed] [Google Scholar]
- Haimovitz-Friedman A. Kan C. Ehleiter D. Persaud R.S. Mcloughlin M. Fuks Z. Kolesnick R.N. Ionizing radiation acts on cellular membranes to generate ceramide and initiate apoptosis. J. Exp. Med. 1994;180:525–535. doi: 10.1084/jem.180.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri R.J. Chang V.A. Barie P.S. Wang R.S. Sharif S.F. Ghajar J.B. Traumatic injury induces interleukin-6 production by human astrocytes. Brain Res. 1994;636:139–142. doi: 10.1016/0006-8993(94)90188-0. [DOI] [PubMed] [Google Scholar]
- Hoffman S.W. Rzigalinski B.A. Willoughby K.A. Ellis E.F. Astrocytes generate isoprostanes in response to trauma or oxygen radicals. J. Neurotrauma. 2000;17:415–420. doi: 10.1089/neu.2000.17.415. [DOI] [PubMed] [Google Scholar]
- Howard D. Sturtevant B. In vitro study of the mechanical effects of shockwave lithotripsy. Ultrasound Med. Biol. 1997;23:1107–1122. doi: 10.1016/s0301-5629(97)00081-1. [DOI] [PubMed] [Google Scholar]
- Iwata A. Stys P.K. Wolf J.A. Chen X.H. Taylor A.G. Meaney D.F. Smith D.H. Traumatic axonal injury induces proteolytic cleavage of the voltage-gated sodium channels modulated by tetrodotoxin and protease inhibitors. J. Neurosci. 2004;24:4605–4613. doi: 10.1523/JNEUROSCI.0515-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones E. Thomas A. Ironside S. Shell shock: an outcome study of a First World War “PIE” unit. Psychol. Med. 2007;37:215–223. doi: 10.1017/S0033291706009329. [DOI] [PubMed] [Google Scholar]
- Kallakuri S. Cavanaugh J.M. Özaktay A.C. Takebayashi T. The effect of varying impact energy on diffuse axonal injury in the rat brain: a preliminary study. Exper. Brain Res. 2003;148:419–424. doi: 10.1007/s00221-002-1307-2. [DOI] [PubMed] [Google Scholar]
- Kao C.Q. Goforth P.B. Ellis E.F. Satin L.S. Potentiation of GABA(A) currents after mechanical injury of cortical neurons. J. Neurotrauma. 2004;21:259–270. doi: 10.1089/089771504322972059. [DOI] [PubMed] [Google Scholar]
- Kimpara H. Nakahira Y. Iwamoto M. Miki K. Ichihara K. Kawano S. Taguchi T. Investigation of anteroposterior head-neck responses during severe frontal impacts using a brain-spinal cord complex FE model. Stapp Car Crash J. 2006;50:509–544. doi: 10.4271/2006-22-0019. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick J.B. Higgins M.L. Lucas J.H. Gross G.W. In vitro simulation of neural trauma by laser. J. Neuropathol. Exp. Neurol. 1985;44:268–284. doi: 10.1097/00005072-198505000-00005. [DOI] [PubMed] [Google Scholar]
- Lachance B. Renoux A.Y. Sarrazin M. Hawari J. Sunahara G. Toxicity and bioaccumulation of reduced TNT metabolites in the earthworm Eisenia andrei exposed to amended forest soil. Chemosphere. 2004;55:1339–1348. doi: 10.1016/j.chemosphere.2003.11.049. [DOI] [PubMed] [Google Scholar]
- Lamb R.G. Harper C.C. McKinney J.S. Rzigalinski B.A. Ellis E.F. Alterations in phosphatidylcholine metabolism of stretch-injured cultured rat astrocytes. J. Neurochem. 1997;68:1904–1910. doi: 10.1046/j.1471-4159.1997.68051904.x. [DOI] [PubMed] [Google Scholar]
- Laplaca M.C. Thibault L.E. An in vitro traumatic injury model to examine the response of neurons to a hydrodynamically-induced deformation. Ann. Biomed. Eng. 1997;25:665–677. doi: 10.1007/BF02684844. [DOI] [PubMed] [Google Scholar]
- Laplaca M.C. Cullen D.K. McLoughlin J.J. Cargill R.S., 2nd High rate shear strain of three-dimensional neural cell cultures: a new in vitro traumatic brain injury model. J. Biomech. 2005;38:1093–1105. doi: 10.1016/j.jbiomech.2004.05.032. [DOI] [PubMed] [Google Scholar]
- Lea P.M. Custer S.J. Vicini S. Faden A.I. Neuronal and glial mGluR5 modulation prevents stretch-induced enhancement of NMDA receptor current. Pharmacol. Biochem. Behav. 2002;73:287–298. doi: 10.1016/s0091-3057(02)00825-0. [DOI] [PubMed] [Google Scholar]
- Lea P.M.T. Custer S.J. Stoica B.A. Faden A.I. Modulation of stretch induced enhancement of neuronal NMDA receptor current by mGluR1 depends upon presence of glia. J. Neurotrauma. 2003;20:1233–1249. doi: 10.1089/089771503770802907. [DOI] [PubMed] [Google Scholar]
- Lew H.L. Guest Editorial: Rehabilitation needs of an increasing population of patients: Traumatic brain injury, polytrauma, and blast-related injuries. J. Rehabil. Res. Dev. 2005;42:13–16. [PubMed] [Google Scholar]
- Lucas J.H. Emery D.G. Higgins M.L. Gross G.W. Neuronal survival and dynamics of ultrastructural damage after dendrotomy in low calcium. J. Neurotrauma. 1990;7:169–192. doi: 10.1089/neu.1990.7.169. [DOI] [PubMed] [Google Scholar]
- Lucas J.H. Gross G.W. Emery D.G. Gardner C.R. Neuronal survival or death after dendrite transection close to the perikaryon: correlation with electrophysiologic, morphologic, and ultrastructural changes. Cent. Nerv. Syst. Trauma. 1985;2:231–255. doi: 10.1089/cns.1985.2.231. [DOI] [PubMed] [Google Scholar]
- Lucas J.H. Wolf A. In vitro studies of multiple impact injury to mammalian CNS neurons: prevention of perikaryal damage and death by ketamine. Brain Res. 1991;543:181–193. doi: 10.1016/0006-8993(91)90027-s. [DOI] [PubMed] [Google Scholar]
- Lusardi T.A. Rangan J. Sun D. Smith D.H. Meaney D.F. A device to study the initiation and propagation of calcium transients in cultured neurons after mechanical stretch. Ann. Biomed. Eng. 2004a;32:1546–1558. doi: 10.1114/b:abme.0000049038.75368.75. [DOI] [PubMed] [Google Scholar]
- Lusardi T.A. Wolf J.A. Putt M.E. Smith D.H. Meaney D.F. Effect of acute calcium influx after mechanical stretch injury in vitro on the viability of hippocampal neurons. J. Neurotrauma. 2004b;21:61–72. doi: 10.1089/089771504772695959. [DOI] [PubMed] [Google Scholar]
- Macleod A.D. Shell shock, Gordon Holmes and the Great War. J. R. Soc. Med. 2004;97:86–89. doi: 10.1258/jrsm.97.2.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies S.S. Thibault L.E. Gennarelli T.A. Physical model simulations of brain injury in the primate. J. Biomech. 1990;23:823–836. doi: 10.1016/0021-9290(90)90029-3. [DOI] [PubMed] [Google Scholar]
- McBride J.M. Smith D.T. Byrn S.R. Borgens R.B. Shi R. Dose responses of three 4-aminopyridine derivatives on axonal conduction in spinal cord trauma. Eur. J. Pharm. Sci. 2006;27:237–242. doi: 10.1016/j.ejps.2005.10.003. [DOI] [PubMed] [Google Scholar]
- McKinney J.S. Willoughby K.A. Liang S. Ellis E.F. Stretch-induced injury of cultured neuronal, glial, and endothelial cells. Effect of polyethylene glycol-conjugated superoxide dismutase. Stroke. 1996;27:934–940. doi: 10.1161/01.str.27.5.934. [DOI] [PubMed] [Google Scholar]
- Meaney D.F. Smith D.H. Shreiber D.I. Bain A.C. Miller R.T. Ross D.T. Gennarelli T.A. Biomechanical analysis of experimental diffuse axonal injury. J. Neurotrauma. 1995;12:689–694. doi: 10.1089/neu.1995.12.689. [DOI] [PubMed] [Google Scholar]
- Morrison B., 3rd Cater H.L. Wang C.C. Thomas F.C. Hung C.T. Ateshian G.A. Sundstrom L.E. A tissue level tolerance criterion for living brain developed with an in vitro model of traumatic mechanical loading. Stapp Car Crash J. 2003;47:93–105. doi: 10.4271/2003-22-0006. [DOI] [PubMed] [Google Scholar]
- Morrison B., 3rd Eberwine J.H. Meaney D.F. Mcintosh T.K. Traumatic injury induces differential expression of cell death genes in organotypic brain slice cultures determined by complementary DNA array hybridization. Neuroscience. 2000a;96:131–139. doi: 10.1016/s0306-4522(99)00537-0. [DOI] [PubMed] [Google Scholar]
- Morrison B., 3rd Meaney D.F. Mcintosh T.K. Mechanical characterization of an in vitro device designed to quantitatively injure living brain tissue. Ann. Biomed. Eng. 1998;26:381–390. doi: 10.1114/1.61. [DOI] [PubMed] [Google Scholar]
- Morrison B., 3rd Meaney D.F. Margulies S.S. Mcintosh T.K. Dynamic mechanical stretch of organotypic brain slice cultures induces differential genomic expression: relationship to mechanical parameters. J. Biomech. Eng. 2000b;122:224–230. doi: 10.1115/1.429650. [DOI] [PubMed] [Google Scholar]
- Movsesyan V.A. Faden A.I. Neuroprotective effects of selective group II mGluR activation in brain trauma and traumatic neuronal injury. J. Neurotrauma. 2006;23:117–127. doi: 10.1089/neu.2006.23.117. [DOI] [PubMed] [Google Scholar]
- Mukhin A. Fan L. Faden A.I. Activation of metabotropic glutamate receptor subtype mGluR1 contributes to post-traumatic neuronal injury. J. Neurosci. 1996;16:6012–6020. doi: 10.1523/JNEUROSCI.16-19-06012.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhin A.G. Ivanova S.A. Allen J.W. Faden A.I. Mechanical injury to neuronal/glial cultures in microplates: role of NMDA receptors and pH in secondary neuronal cell death. J. Neurosci. Res. 1998;51:748–758. doi: 10.1002/(SICI)1097-4547(19980315)51:6<748::AID-JNR8>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Mukhin A.G. Ivanova S.A. Faden A.I. mGluR modulation of posttraumatic neuronal death: role of NMDA receptors. Neuroreport. 1997b;8:2561–2566. doi: 10.1097/00001756-199707280-00028. [DOI] [PubMed] [Google Scholar]
- Mukhin A.G. Ivanova S.A. Knoblach S.M. Faden A.I. New in vitro model of traumatic neuronal injury: evaluation of secondary injury and glutamate receptor mediated neurotoxicity. J. Neurotrauma. 1997a;14:651–663. doi: 10.1089/neu.1997.14.651. [DOI] [PubMed] [Google Scholar]
- Munshi A. Jalali R. Cellular phones and their hazards: the current evidence. Natl. Med. J. India. 2002;15:275–277. [PubMed] [Google Scholar]
- Murphy E.J. Horrocks L.A. A model for compression trauma: pressure induced injury in cell cultures. J. Neurotrauma. 1993;10:431–444. doi: 10.1089/neu.1993.10.431. [DOI] [PubMed] [Google Scholar]
- Myers C.S. Shellshock in France 1914–1918. Cambridge: Cambridge University. 1940:Press. [Google Scholar]
- Myers C.S. A contribution to the study of shellshock. Being an account of the cases of loss of memory, vision, smell and taste admitted to the Duchess of Westminster's War Hospital, Le Touquet. Lancet. 1915;i:316–320. [Google Scholar]
- Nakayama Y. Aoki Y. Niitsu H. Studies on the mechanisms responsible for the formation of focal swellings on neuronal processes using a novel in vitro model of axonal injury. J. Neurotrauma. 2001;18:545–554. doi: 10.1089/089771501300227341. [DOI] [PubMed] [Google Scholar]
- Neary J.T. Kang Y. P2 purinergic receptors signal to glycogen synthase kinase-3beta in astrocytes. J. Neurosci. Res. 2006;84:515–524. doi: 10.1002/jnr.20969. [DOI] [PubMed] [Google Scholar]
- Neary J.T. Kang Y. Tran M. Feld J. Traumatic injury activates protein kinase B/Akt in cultured astrocytes: role of extracellular ATP and P2 purinergic receptors. J. Neurotrauma. 2005;22:491–500. doi: 10.1089/neu.2005.22.491. [DOI] [PubMed] [Google Scholar]
- Neary J.T. Kang Y. Willoughby K.A. Ellis E.F. Activation of extracellular signal-regulated kinase by stretch-induced injury in astrocytes involves extracellular ATP and P2 purinergic receptors. J. Neurosci. 2003;23:2348–2356. doi: 10.1523/JNEUROSCI.23-06-02348.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura N. Schaffer C.B. Kleinfeld D. In vivo manipulation of biological systems with femtosecond laser pulses. Proceedings of SPIE, the International Society for Optical Engineering. 2006a:6261. [Google Scholar]
- Nishimura N. Schaffer C.B. Friedman B. Tsai P.S. Lyden P.D. Kleinfeld D. Targeted insult to subsurface cortical blood vessels using ultrashort laser pulses: three models of stroke. Nat. Methods. 2006b;3:99–108. doi: 10.1038/nmeth844. [DOI] [PubMed] [Google Scholar]
- Noel F. Tofilon P.J. Astrocytes protect against x-ray-induced neuronal toxicity in vitro. Neuroreport. 1998;9:1133–1137. doi: 10.1097/00001756-199804200-00032. [DOI] [PubMed] [Google Scholar]
- Ostrow L.W. Langan T.J. Sachs F. Stretch-induced endothelin-1 production by astrocytes. J. Cardiovasc. Pharmacol. 2000;36:S274–S277. doi: 10.1097/00005344-200036051-00081. [DOI] [PubMed] [Google Scholar]
- Panizzon K.L. Dwyer B.E. Nishimura R.N. Wallis R.A. Neuroprotection against CA1 injury with metalloporphyrins. Neuroreport. 1996;7:662–666. doi: 10.1097/00001756-199601310-00067. [DOI] [PubMed] [Google Scholar]
- Panizzon K.L. Shin D. Frautschy S. Wallis R.A. Neuroprotection with Bcl-2(20–34) peptide against trauma. Neuroreport. 1998;9:4131–4136. doi: 10.1097/00001756-199812210-00024. [DOI] [PubMed] [Google Scholar]
- Pary R. Lippmann S.B. Turns D.M. Tobias C.R. Post-traumatic stress disorder in Vietnam veterans. Am. Fam. Physician. 1988;37:145–150. [PubMed] [Google Scholar]
- Pellman E.J. Viano D.C. Tucker A.M. Casson I.R. Waeckerle J.F. Concussion in professional football: reconstruction of game impacts and injuries. Neurosurgery. 2003;53:799–812. doi: 10.1093/neurosurgery/53.3.799. ; discussion 812–794. [DOI] [PubMed] [Google Scholar]
- Peña L.A. Zvi F. Kolesnick R.N. Radiation-induced apoptosis of endothelia cells in the murine central nervous system: protection by fibroblast growth factor and sphingomyelinase deficiency. Cancer Res. 2000;60:321–327. [PubMed] [Google Scholar]
- Pfister B.J. Weihs T.P. Betenbaugh M. Bao G. An in vitro uniaxial stretch model for axonal injury. Ann. Biomed. Eng. 2003;31:589–598. doi: 10.1114/1.1566445. [DOI] [PubMed] [Google Scholar]
- Pike B.R. Zhao X. Newcomb J.K. Glenn C.C. Anderson D.K. Hayes R.L. Stretch injury causes calpain and caspase-3 activation and necrotic and apoptotic cell death in septo-hippocampal cell cultures. J. Neurotrauma. 2000;17:283–298. doi: 10.1089/neu.2000.17.283. [DOI] [PubMed] [Google Scholar]
- Raghavendra Rao V.L. Dogan A. Todd K.G. Bowen K.K. Dempsey R.J. Neuroprotection by memantine, a non-competitive NMDA receptor antagonist after traumatic brain injury in rats. Brain Res. 2001;991:96–100. doi: 10.1016/s0006-8993(01)02617-8. [DOI] [PubMed] [Google Scholar]
- Reeves T.M. Phillips L.L. Povlishock J.T. Myelinated and unmyelinated axons of the corpus callosum differ in vulnerability and functional recovery following traumatic brain injury. Exp. Neurol. 2005;196:126–137. doi: 10.1016/j.expneurol.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Regan R.F. Choi D.W. The effect of NMDA, AMPA/kainate, and calcium channel antagonists on traumatic cortical neuronal injury in culture. Brain Res. 1994;633:236–242. doi: 10.1016/0006-8993(94)91544-x. [DOI] [PubMed] [Google Scholar]
- Regan R.F. Panter S.S. Traumatic neuronal injury in cortical cell culture is attenuated by 21-aminosteroids. Brain Res. 1995;682:144–150. doi: 10.1016/0006-8993(95)00330-s. [DOI] [PubMed] [Google Scholar]
- Richter M. Otte D. Lehmann U. Chinn B. Schuller E. Doyle D. Sturrock K. Krettek C. Head injury mechanisms in helmet-protected motorcyclists: prospective multicenter study. J. Trauma. 2001;51:949–958. doi: 10.1097/00005373-200111000-00021. [DOI] [PubMed] [Google Scholar]
- Rosenberg L.J. Lucas J.H. Reduction of NaCl increases survival of mammalian spinal neurons subjected to dendrite transection injury. Brain Res. 1996b;734:349–353. [PubMed] [Google Scholar]
- Rosenberg L.J. Emery D.G. Lucas J.H. Effects of sodium and chloride on neuronal survival after neurite transection. J. Neuropathol. Exp. Neurol. 2001;60:33–48. doi: 10.1093/jnen/60.1.33. [DOI] [PubMed] [Google Scholar]
- Rosenberg L.J. Jordan R.S. Gross G.W. Emery D.G. Lucas J.H. Effects of methylprednisolone on lesioned and uninjured mammalian spinal neurons: viability, ultrastructure, and network electrophysiology. J. Neurotrauma. 1996a;13:417–437. doi: 10.1089/neu.1996.13.417. [DOI] [PubMed] [Google Scholar]
- Rzigalinski B.A. Liang S. Mckinney J.S. Willoughby K.A. Ellis E.F. Effect of Ca2+ on in vitro astrocyte injury. J. Neurochem. 1997;68:289–296. doi: 10.1046/j.1471-4159.1997.68010289.x. [DOI] [PubMed] [Google Scholar]
- Rzigalinski B.A. Weber J.T. Willoughby K.A. Ellis E.F. Intracellular free calcium dynamics in stretch-injured astrocytes. J. Neurochem. 1998;70:2377–2385. doi: 10.1046/j.1471-4159.1998.70062377.x. [DOI] [PubMed] [Google Scholar]
- Salvador-Silva M. Aoi S. Parker A. Yang P. Pecen P. Hernandez M.R. Responses and signaling pathways in human optic nerve head astrocytes exposed to hydrostatic pressure in vitro. Glia. 2004;45:364–377. doi: 10.1002/glia.10342. [DOI] [PubMed] [Google Scholar]
- Scott S.G. Belanger H.G. Vanderploeg R.D. Massengale J. Scholten J. Mechanism-of-injury approach to evaluating patients with blast-related polytrauma. J. Am. Osteopath. Assoc. 2006;106:265–270. [PubMed] [Google Scholar]
- Serbest G. Horwitz J. Barbee K. The effect of poloxamer-188 on neuronal cell recovery from mechanical injury. J. Neurotrauma. 2005;22:119–132. doi: 10.1089/neu.2005.22.119. [DOI] [PubMed] [Google Scholar]
- Serbest G. Horwitz J. Jost M. Barbee K. Mechanisms of cell death and neuroprotection by poloxamer 188 after mechanical trauma. FASEB J. 2006;20:308–310. doi: 10.1096/fj.05-4024fje. [DOI] [PubMed] [Google Scholar]
- Shepard S.R. Ghajar J.B. Giannuzzu R. Kupferman S. Harir R.J. Fluid percussion barotrauma chamber: a new in vitro model for traumatic brain injury. J. Surg. Res. 1991;51:417–424. doi: 10.1016/0022-4804(91)90144-b. [DOI] [PubMed] [Google Scholar]
- Shi R. Pryor J.D. Pathological changes of isolated spinal cord axons in response to mechanical stretch. Neuroscience. 2002;110:765–777. doi: 10.1016/s0306-4522(01)00596-6. [DOI] [PubMed] [Google Scholar]
- Shi R. Whitebone J. Conduction deficits and membrane disruption of spinal cord axons as a function of magnitude and rate of strain. J. Neurophysiol. 2006;95:3384–3390. doi: 10.1152/jn.00350.2005. [DOI] [PubMed] [Google Scholar]
- Slemmer J.E. Weber J.T. The extent of damage following repeated injury to cultured hippocampal cells is dependent on the severity of insult and inter-injury interval. Neurobiol. Dis. 2005;18:421–431. doi: 10.1016/j.nbd.2004.09.022. [DOI] [PubMed] [Google Scholar]
- Smith D.H. Wolf J.A. Lusardi T.A. Lee V.M. Meaney D.F. High tolerance and delayed elastic response of cultured axons to dynamic stretch injury. J. Neurosci. 1999;19:4263–4269. doi: 10.1523/JNEUROSCI.19-11-04263.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southborough L. Report of the war office committee of inquiry into “shell-shock”. London: 1922:HMSO. [Google Scholar]
- Stalhammar L. Olsson Y. Experimental brain damage from fluid pressures due to impact acceleration. IV. Comparative studies with acceleration-concussion. Acta Neurol. Scand. 1975;52:94–110. doi: 10.1111/j.1600-0404.1975.tb05764.x. [DOI] [PubMed] [Google Scholar]
- Steevens J.A. Duke B.M. Lotufo G.R. Bridges T.S. Toxicity of the explosives 2,4,6-trinitrotoluene, hexahydro-1,3,5-trinitro-1,3,5-triazine, and octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine in sediments to Chironomus tentans and Hyalella azteca: Low-dose hormesis and high-dose mortality. Environ. Toxicol. Chem. 2002;21:1475–1482. [PubMed] [Google Scholar]
- Committee of Enquiry into “Shell-shock.”. London: Printed & pub. by H.M. Stationery off.; 1922. Southborough, Francis John Stephens Hopwood Great Britain. War Office. [Google Scholar]
- Tavalin S.J. Ellis E.F. Satin L.S. Inhibition of the electrogenic Na pump underlies delayed depolarization of cortical neurons after mechanical injury or glutamate. J. Neurophysiol. 1997;77:632–638. doi: 10.1152/jn.1997.77.2.632. [DOI] [PubMed] [Google Scholar]
- Tecoma E.S. Monyer H. Goldberg M.P. Choi D.W. Traumatic neuronal injury in vitro is attenuated by NMDA antagonists. Neuron. 1989;2:1541–1545. doi: 10.1016/0896-6273(89)90042-1. [DOI] [PubMed] [Google Scholar]
- Viano D.C. Casson I.R. Pellman E.J. Zhang L. King A.I. Yang K.H. Concussion in professional football: brain responses by finite element analysis. Neurosurgery. 2005;57:891–916. doi: 10.1227/01.neu.0000186950.54075.3b. [DOI] [PubMed] [Google Scholar]
- Wallis R.A. Panizzon K.L. Felbamate neuroprotection against CA1 traumatic neuronal injury. Eur J. Pharmacol. 1995;294:475–482. doi: 10.1016/0014-2999(95)00568-4. [DOI] [PubMed] [Google Scholar]
- Wallis R.A. Panizzon K.L. Girard J.M. Traumatic neuroprotection with inhibitors of nitric oxide and ADP-ribosylation. Brain Res. 1996;710:169–177. doi: 10.1016/0006-8993(95)01278-8. [DOI] [PubMed] [Google Scholar]
- Wang K.K. Ottens A.K. Liu M.C. Lewis S.B. Meegan C. Oli M.W. Tortella F.C. Hayes R.L. Proteomic identification of biomarkers of traumatic brain injury. Expert Rev. Proteomics. 2005;2:603–614. doi: 10.1586/14789450.2.4.603. [DOI] [PubMed] [Google Scholar]
- Wang Z. Sun L. Yang Z. Leng H. Jiang J. Yu H. Gu J. Li Z. Development of serial bioshock tubes and their application. Chin. Med. J. (Engl). 1998;111:109–113. [PubMed] [Google Scholar]
- Weber J.T. Rzigalinski B.A. Ellis E.F. Traumatic injury of cortical neurons causes changes in intracellular calcium stores and capacitative calcium influx. J. Biol. Chem. 2001;276:1800–1807. doi: 10.1074/jbc.M009209200. [DOI] [PubMed] [Google Scholar]
- Weber J.T. Rzigalinski B.A. Willoughby K.A. Moore S.F. Ellis E.F. Alterations in calcium-mediated signal transduction after traumatic injury of cortical neurons. Cell Calcium. 1999;26:289–299. doi: 10.1054/ceca.1999.0082. [DOI] [PubMed] [Google Scholar]
- Weber J.T. Rzigalinski B.A. Ellis E.F. Calcium responses to caffeine and muscarinic receptor agonists are altered in traumatically injured neurons. J. Neurotrauma. 2002;19:1433–1443. doi: 10.1089/089771502320914660. [DOI] [PubMed] [Google Scholar]
- Wharton R.K. Formby S.A. Merrifield R. Airblast TNT equivalence for a range of commercial blasting explosives. J. Hazard Mater. 2000;79:31–39. doi: 10.1016/s0304-3894(00)00168-0. [DOI] [PubMed] [Google Scholar]
- Wolf J.A. Stys P.K. Lusardi T. Meaney D. Smith D.H. Traumatic axonal injury induces calcium influx modulated by tetrodotoxin-sensitive sodium channels. J. Neurosci. 2001;21:1923–1930. doi: 10.1523/JNEUROSCI.21-06-01923.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z. Wang Z. Tang C. Ying Y. Biological effects of weak blast waves and safety limits for internal organ injury in the human body. J. Trauma. 1996;40:S81–S84. doi: 10.1097/00005373-199603001-00019. [DOI] [PubMed] [Google Scholar]
- Yanik M.F. Cinar H. Cinar H.N. Chisholm A.D. Jin Y. Ben-Yakar A. Neurosurgery: functional regeneration after laser axotomy. Nature 432, 2004:822. doi: 10.1038/432822a. [DOI] [PubMed] [Google Scholar]
- Zhang L. Bae J. Hardy W.N. Monson K.L. Manley G.T. Goldsmith W. Yang K.H. King A.I. Computational study of the contribution of the vasculature on the dynamic response of the brain. Stapp Car Crash J. 2002;46:145–164. doi: 10.4271/2002-22-0008. [DOI] [PubMed] [Google Scholar]
- Zhang L. Rzigalinski B.A. Ellis E.F. Satin L.S. Reduction of voltage-dependent Mg2+ blockade of NMDA current in mechanically injured neurons. Science. 1996;274:1921–1923. doi: 10.1126/science.274.5294.1921. [DOI] [PubMed] [Google Scholar]
- Zhang L. Yang K.H. Dwarampudi R. Omori K. Li T. Chang K. Hardy W.N. Khalil T.B. King A.I. Recent advances in brain injury research: a new human head model development and validation. Stapp Car Crash J. 2001;45:369–394. doi: 10.4271/2001-22-0017. [DOI] [PubMed] [Google Scholar]