Abstract
Cardiovascular disease is the most common cause of death after kidney transplant. However, uncertainties regarding the optimal assessment of cardiovascular risk in potential transplant candidates have produced controversy and inconsistency in pretransplant cardiac evaluation practices. In this review, we consider the evidence supporting cardiac evaluation in kidney transplant candidates, generally focused on coronary artery disease, according to the World Health Organization principles for screening. The importance of pretransplant cardiac evaluation is supported by the high prevalence of coronary artery disease and by the incidence and adverse consequences of acute coronary syndromes in this population. Testing for coronary artery disease may be performed non-invasively by modalities including nuclear myocardial perfusion studies and dobutamine stress echocardiography. These tests have prognostic value for mortality but imperfect sensitivity and specificity for detecting angiographically-defined coronary artery disease in end-stage renal disease patients. Associations of angiographically-defined coronary artery disease with subsequent survival are also inconsistent, likely because plaque instability is more critical for infarction risk than angiographic stenosis. The efficacy and best methods of myocardial revascularization have not been examined in large, contemporary clinical trials among end-stage renal disease patients. Biomarkers such as cardiac troponin have prognostic value in end-stage renal disease but require further study to determine clinical applications in directing more expensive and invasive cardiac evaluation.
Keywords: Cardiovascular disease, Kidney transplant, Myocardial revascularization, Physician’s practice patterns, Risk assessment
Many advances have been made in the field of kidney transplantation since the first demonstration of this procedure as a viable form of renal replacement more than fifty years ago. However, questions remain regarding the optimal assessment of cardiovascular risk in renal transplant candidates. In 1968, the World Health Organization (WHO) articulated characteristics of diseases amenable to effective screening programs that hold substantial relevance for clinical evaluation policies today 1. In this review we consider the evidence supporting cardiac evaluation for coronary heart disease in kidney transplant candidates according to WHO principles (Box 1). Specifically, we summarize current knowledge from this population on: 1) the public health importance of coronary artery disease (CAD) and ischemic heart disease; 2) disease natural history in terms of the relationship of coronary artery stenoses to cardiac events and mortality; 3) the accuracy and use of testing for CAD; and 4) the efficacy and use of revascularization. We also briefly discuss cardiac biomarkers as emerging tools for cardiac evaluation and the importance of non-coronary heart disease in this population.
Box 1.
Evidence regarding pretransplant evaluation for coronary heart disease considered according to the World Health Organization principles for screening (1968).
| Public Health Impact |
| Natural History of Disease includes a Latent Stage for Detection and Intervention |
|
| Availability of Testing |
|
| Availability of Treatment |
|
| Cost-effectiveness |
|
Abbreviations: CAD, coronary artery disease; DSE, dobutamine stress echocardiography; ESRD, end-stage renal disease; MPS, myocardial perfusion studies
Public Health Importance of CAD in Kidney Transplant Candidates and Recipients
The main objectives of pretransplant cardiac evaluation are to identify existing cardiac conditions amenable to risk modification, and to exclude patients with such short expected near-term survival due to cardiac morbidity that transplantation would not yield adequate benefit from the allograft. It is known that patients on dialysis experience age-adjusted mortality substantially higher than that of the general population, and that the primary cause of death is heart disease. The challenge in conducting comprehensive, accurate and cost-effective pretransplant cardiac evaluation is exemplified by both the large size of the target population and the prevalent disease burden. The number of total listings for kidney and kidney-pancreas transplantation increased five-fold since 1991, such that per current Organ Procurement and Transplant Network (OPTN) records, more than 80,000 persons are awaiting these organs in 20092. Significant shifts in the age composition of the waitlist towards older adults aged >50 years (with marked increases in patients aged ≥65 years) is also increasing the comorbidity burden and medical complexity of the waitlist3.
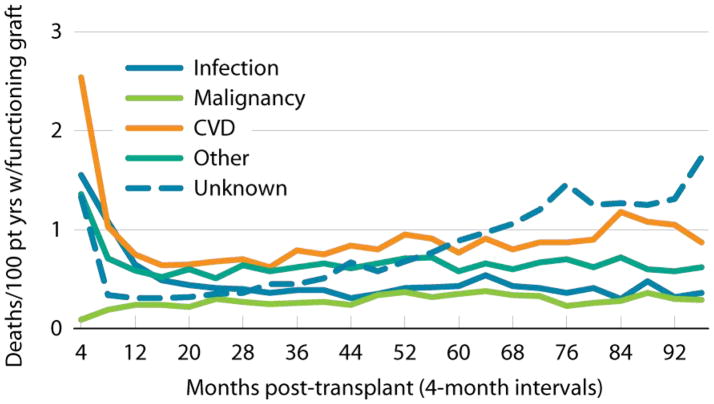
Current evaluation and selection procedures have not eliminated cardiovascular disease as a major public health problem in ESRD patients after candidate selection or transplantation. Estimates of three-year cumulative incidence of myocardial infarction based on billing claims algorithms have ranged from 8.7% to 16.7% after candidate listing, and from 4.7% to 11.1% after transplant4,5. Observational studies have shown particularly high frequencies of cardiovascular diagnoses in the first months after transplant4,6,7. Registry data identify cardiovascular diseases in aggregate as the most common cause of death with graft function at all time periods after transplant, accounting for 30% of graft loss from death overall, with the highest rates early after transplant (Figure 1)8.
Figure 1. Cardiovascular disease is the leading cause of death with graft function after kidney transplantation.
First-time, kidney-only transplant recipients, age 18 & older & transplanted 1997–2006, who died with a functioning graft (N=14,169). Cause of death obtained from OPTN when available, otherwise taken from ESRD Death Notification form. From the United States Renal Data System 2008 Annual Data Report8.
Natural History – Relationship of CAD to Subsequent Clinical Events in ESRD Patients
Angiographic studies from the 1970s to early 1990s detected CAD in high proportions of patients on long-term dialysis9–13. More recently, angiographically significant CAD was found in 53% of a sample of 30 incident ESRD patients without known cardiac history who consented to screening angiography, including 83% of the 12 participants with diabetes, although notably angiographic significance was liberally defined as lesions >50%14. Recent reports of angiography in patients undergoing transplant evaluation have documented CAD in 42%–81% of participants, with prevalence being higher in samples selected as facing “high-risk” by clinical criteria and with use of more liberal angiographic definitions of CAD15–22 (Table 1).
Table 1.
Recent descriptions of the outcome implications of angiographic coronary artery disease in ESRD patients including transplant candidates
| Reference | Participants and Design | Selection Criteria for Angiography | Angiographic Definition of CAD | Estimated CAD Prevalence | Associations of CAD with Clinical Events |
|---|---|---|---|---|---|
| De Lima et al, 200316 |
|
|
|
|
|
| Sharma, et al, 200515 |
|
|
|
|
|
| Charytan et al, 200717 |
|
|
|
|
|
| Gowdak, et al, 200718 |
|
|
|
|
|
| Gowdak, et al, 200719 |
|
|
|
|
|
| Hage et al, 200720 |
|
|
|
|
|
| Patel et al, 200821 |
|
|
|
|
|
| Hickson, et al, 200822 |
|
|
|
|
|
aHR, adjusted hazards ratio; CAD, coronary artery disease; CI, confidence interval; KT, kidney transplant; MACE, major adverse cardiovascular events; MI, myocardial infarction
Studies describing associations of angiographic coronary stenoses with subsequent clinical events in ESRD patients including those undergoing transplant evaluations have reached inconsistent conclusions (Table 2). De Lima et al prospectively studied 126 renal transplant candidates clinically classified as moderate (age ≥50 years) or high (diabetes, extracardiac vascular disease or known CAD) coronary risk with myocardial perfusion studies (MPS), dobutamine stress echocardiography (DSE) and coronary angiography16. Significant CAD, defined as >70% stenosis in ≥1 major epicardial artery on angiography, was found 42% of the sample. After median follow-up of 46 months, clinical risk stratification and coronary angiography predicted major cardiac events, but MPS and DSE did not. The probability of reaching the composite endpoint at 1,2 and 4 years in patients with angiographic CAD was 13%, 39%, 46% versus 2%, 6%, 6% in those without CAD (P<0.001).
Table 2.
Advantages and disadvantages of available methods for detecting coronary artery disease.
| Method | Advantages | Disadvantages |
|---|---|---|
Myocardial Perfusion Study (MPS)
|
|
|
Stress Echocardiography
|
|
|
Cardiac Computed Tomography (CT)
|
|
|
| Invasive Coronary Angiography |
|
|
Additional observational studies have also reported increased unadjusted risk of all-cause mortality and major cardiovascular events in patients with angiographic CAD15,18 while other investigations identified risk only certain patient sub-groups, such as those with proximal CAD17 or with non-diabetic renal failure19. Several recent studies have found no associations of CAD with subsequent patient survival, although it is difficult to disentangle the impact of revascularization from that of CAD itself in these observational designs20–22. Notably, investigations in the general population have demonstrated that most myocardial infarctions result from plaques that rupture or erode, resulting in thrombus formation and either partial or total occlusion of arteries that did not previously contain significant stenoses23. Infarction may be most likely with “vulnerable” or “unstable” plaques that have thinner epithelial layering (“thin wall atheromoa”) than surrounding plaque but are more vulnerable to rupture and subsequent thrombosis24.
Accuracy of Non-invasive Testing for CAD in Potential Kidney Transplant Candidates
Non-invasive testing for CAD is available as myocardial perfusion studies (MPS), stress echocardiography and most recently cardiac computed tomographic angiography (Table 2). These tests have imperfect sensitivity and specificity in patients with renal failure, or in the case of tomographic angiography, have not been evaluated in this population. Abnormalities on MPS correlate well with the presence of CAD in the general population, with mean weighted sensitivity 88% and mean weighted specificity 74%25. The performance MPS in identifying CAD among ESRD patients is more variable, with reported sensitivities and specificities ranging from 37–90% and 40–90%, respectively26–29. Nonetheless, MPS results do have prognostic value for cardiac events and mortality in the ESRD population30,31. In a meta-analysis of twelve studies involving thallium-201 scintigraphy and dobutamine stress echocardiography (DSE), ESRD patients with inducible ischemia had approximately six-times the risk of myocardial infarction and four-times the risk of cardiac death as patients without inducible defects32. Moreover, patients with fixed defects also had nearly five-times the risk of cardiac death. The prognostic value of MPS has been demonstrated with other perfusion tracers. For example, in a study of 126 ESRD patients who underwent 99m-technetium MPS as part of their pretransplant assessment, presence of a reversible defect was associated with three-times the risk of post-transplant cardiac events (HR 3.1, 95% CI 1.1–18.2) and nearly twice the risk of death (HR 1.92, 95% CI 1.1–4.4) compared to normal test results33.
DSE is a commonly used, safe method of non-invasive CAD risk assessment. Among patients without advanced kidney disease, stress echocardiography has mean weighted sensitivity of 86% and specificity of 81% for detecting angiographically significant CAD (variably defined across studies as ≥50–75% stenosis)34. As with MPS and other non-invasive tests, the accuracy of DSE increases for higher degree stenoses (≥70%) and multivessel obstructive CAD. Similar to MPS, the accuracy of DSE for detecting CAD in ESRD patients including transplant candidates has been variable, with reported sensitivities of 37–95% and specificities of 71–95%15,35–39. However, abnormal test results have been associated with increased risk of adverse clinical outcomes15,35–41. Among 485 patients with advanced kidney disease (on dialysis or with serum creatinine >3 mg/dl) the percentage of ischemic segments by DSE was an independent predictor of mortality and offered prognostic information incremental to clinical data42. Nonetheless, inconsistent results in some studies have led some to question the routine use of DSE for pre-transplant cardiac evaluation. In an aforementioned investigation of 126 renal transplant candidates studied with MPS, the accuracy of non-invasive testing to detect CAD was limited: MPS sensitivity 64%, specificity 53%; DSE sensitivity 44%, specificity 87%16. Clinical risk stratification and coronary angiography predicted the freedom from cardiac events, but non-invasive test results did not.
The incorporation of clinical risk scores may better identify which patients will benefit from pretransplant testing with either DSE or MPS43, 44. In a study of 244 patients with chronic kidney disease (mean age 54 years; 169 dialysis-dependent), participants were classified dichotomously as either low or high-risk based on Framingham, Portland and Brisbane risk scores, then further stratified according to DSE results and followed 20±14 months for major cardiac events (defined as cardiovascular death, myocardial infarction, acute coronary syndrome)41. Based on the different clinical scoring systems, the prevalence of high-risk clinical classification varied from 34%–79% and the proportion of high-risk patients with an abnormal DSE ranged from 39%–50%. Depending on the clinical score chosen, 25%–44% of high-risk patients with an abnormal DSE had a cardiac event, compared with 8%–22% of high-risk patients with a normal DSE. Cardiac events occurred in 2.0%–9.7% of the low-risk patients and DSE results did not improve event prediction in the low-clinical risk subgroups. It is also notable that while low-risk clinical status was associated with better outcomes, it did not predict freedom from subsequent cardiac events.
Recently, the development of electron beam and multi-detector cardiac computed tomography for detection and quantification of coronary artery calcification (CAC) has been shown to improve cardiovascular risk prediction as compared to the Framingham score in asymptomatic patients without kidney disease45. Among 205 maintenance hemodialysis patients aged >18 years, Raggi et al detected evidence of CAC in >83% of the participants46. These results were concordant with prior studies documenting significantly greater intracoronary calcification in ESRD compared with non-ESRD patients, with particular disparities in young cohorts47–49. Although one study found CAC to be an independent predictor of death in maintenance hemodialysis patients50, the role of CAC as a prognostic marker in the ESRD population is yet to be adequately defined51. Other studies demonstrate a poor correlation between CAC score and angiographic CAD in patients with advanced kidney disease52–54. This has been hypothesized to reflect a high burden of medial vascular calcification in ESRD compared to the intimal calcification seen in the non-ESRD population55. For these reasons, CAC quantification is not currently recommended for assessment of pretransplant cardiovascular risk.
Cardiac computed tomography angiography (64–320 slice and dual-source) is a highly sensitive tool for evaluating symptomatic patients with low-intermediate pre-test probability of obstructive CAD56,57. However, this modality has not been studied in patients with significant kidney disease, and its accuracy may be limited in this population due to a high burden of calcified coronary atherosclerosis. Further, safety may be limited by the attendant exposure to iodinated contrast.
Use and Efficacy of Angiography and Revascularization in ESRD Patients
Coronary angiography remains the gold standard modality for detecting CAD. Despite the imperfect performance of non-invasive testing described above, commonly suggested algorithms for cardiac evaluation of asymptomatic kidney transplant candidates reserve coronary angiography for patients with abnormal non-invasive testing43,44,58. The rationale for non-invasive testing prior to angiography relates to concerns for procedure-related risks and costs. Contrast-induced nephropathy has been reported to complicate angiography in 2%–50% of samples depending on case definition and patient mix, with increased risk associated with chronic kidney disease, congestive heart failure, diabetes, advanced age, and intravascular volume depletion59–62.
Two recent randomized trials failed to support benefit of revascularization over contemporary medical management in stable general population samples, including patients awaiting major vascular surgery63, 64, although the relevance of these findings to ESRD patients is not known. There are limited direct data on the efficacy of coronary revascularization in ESRD patients. In 1992, Manske et al randomly assigned 31 insulin-dependent diabetic transplant candidates with CAD (>75% stenosis) to revascularization or medical therapy with a calcium channel blocker and aspirin65. Ultimately, 10 of 13 medically managed and 2 of 13 revascularized patients reached the primary endpoint of unstable angina, myocardial infarction, or cardiac death. Contemporary relevance of these findings is limited by the small study sample size, high event rate among the medically managed group, and subsequent advances in “standard” medical management of CAD including angiotensin-converting enzyme inhibitors and statins.
Several recent observational studies have reported outcomes after revascularization in selected samples of potential transplant candidates. In a study of 300 patients who underwent multimodality non-invasive testing as part of the candidate evaluation at one center, crude survival was not different in patients who underwent revascularization compared to those who underwent angiography without revascularization or no angiography, although there was suggestion of a benefit of revascularization in the subset of 34 patients found to have obstructive CAD (15% versus 52% mortality)21. Hage et al described 3,698 patients evaluated for kidney transplant at a single center in 2001–2004. MPS was performed in 60% and 7% of the sample subsequently underwent coronary angiography. The presence and severity of CAD on angiography was not predictive of survival, and coronary revascularization was only associated with survival in patients with three-vessel CAD20. The relatively low use of coronary interventions after pre-transplant cardiac evaluation is also motivating scrutiny of the clinical and cost effectiveness of pre-transplant cardiac evaluation as currently applied. Several single center observational and a registry study have found that only 2.9%–9.5% of patients who receive pretransplant cardiac testing proceed to angioplasty or surgical bypass21,31,43,66,67.
The best method of revascularization in patients with advanced kidney disease is controversial. A retrospective study of dialysis patients captured in the United States Renal Data System (USRDS) prior to the wide-spread use of drug-eluting stents (DES) suggested a slight long-term benefit of surgical bypass over percutaneous intervention. However, these data are limited by the retrospective design and the inherent risk for procedure referral bias based on coronary anatomy and patient characteristics68. An updated analysis of USRDS data from 2003–2005 by the same authors including patients treated with DES found superior 12-month, unadjusted post-procedure survival in dialysis patients who received DES (69.7%) compared to bypass (66.6%) or non-DES (63.6%)69. However, unadjusted 36-month survival favored bypass over DES (42.0% versus 38.1%), especially among patients who received an internal mammary artery bypass conduit. In multivariable regression, there was no significant difference in overall adjusted mortality with DES versus bypass, although non-DES was associated with higher adjusted mortality compared to surgery. These data highlight the relatively grave prognosis faced by hemodialysis patients who undergo cardiac bypass surgery compared to mean five-year survival estimates after bypass in the general population of 85%–90%70. Current guidelines do not consider the degree of kidney disease in recommendations for angioplasty and bypass except that the presence of significant kidney disease is a factor in risk prediction models for perioperative mortality with bypass surgery70.
Current Practice Variations and Consensus-Based Guidelines
Uncertainties regarding the clinical implications of test results and the impact of revascularization have lead to practice variation in pretransplant cardiac evaluation. In a 1993 survey of directors at OPTN-participating centers, noninvasive stress testing was reported as the most common first approach to cardiac evaluation of asymptomatic patients, prompted by diabetes at 86% of responding centers, age (mean threshold 52 years) at 67%, and risk factor burden at 68%71. Notable minorities of centers advocated first-line angiography for patients with diabetes (15%), older age (7%; mean threshold 57 yrs) or multiple risk factors (8%). A subsequent survey of OPTN centers found that 8% of programs reported use of cardiac testing for all deceased-donor transplant candidates whereas 18% did not routinely order cardiac evaluation for any asymptomatic patient group72. Cardiac re-evaluation policies among listed candidates appear equally variable. In a survey of 68 centers in 2005, 51% of program representatives indicated reliance on the initial cardiac evaluation and cardiac history, 7% used American College of Cardiology/American Heart Association (ACC/AHA) criteria for non-cardiac surgery in the general population to guide cardiac revaluation, and 32% applied a combination of ACC/AHA criteria, the initial cardiac evaluation and cardiac history73.
Complementary to survey-based studies, a retrospective study of the USRDS registry used billing claims as measures of cardiac evaluation services in Medicare beneficiaries transplanted in 1991–200467. Forty-six percent of the sample received non-invasive stress testing or angiography at some time before transplant (65% of high risk - defined as diabetes, prior ischemic heart disease, or ≥2 other coronary risk factors, and 20% of “lower risk”). There was substantial heterogeneity in cardiac evaluation frequency according to patient-level factors even within risk groups. After adjustment for patient traits and consistent within risk profile-stratified samples, transplantation without cardiac evaluation was also more likely for African American persons, women, and patients in certain geographic regions.
Several national organizations have sponsored consensus-based guidelines in efforts to standardize cardiac evaluation practices in the pretransplant and general surgical patient (Table 3)74–76. However, differences in recommendations can lead to disparate conclusions on the appropriateness of cardiac evaluation for the individual patient. A recent study considered the recommended frequencies of cardiac evaluation that would result from application of these guidelines to 328 patients referred for transplant evaluation at one center in 2004–200777. Recommended cardiac evaluation based on the clinical characteristics of the sample ranged from 19% with application of ACC/AHA guidelines for noncardiac surgery in the general population to 94% with use of American Society of Transplantation (AST) guidelines for the evaluation of kidney transplant candidates.
Table 3.
Summary of current consensus-based guidelines for preoperative cardiac evaluation.
| Organization and Target Population | Recommendations |
|---|---|
| American Society of Transplantation, Kidney Transplant Candidates74 |
Clinical Practice Guidelines for the Evaluation of Renal Transplantation Candidates
|
| Kidney Disease Outcomes Quality Initiative (K/DOQI), Dialysis Patients on the Transplant Waitlist75 |
Clinical Practice Guidelines for Cardiovascular Disease in Dialysis Patients
|
| American College of Cardiology/American Heart Association (ACC/AHA), General Patients Preparing for Noncardiac Surgery76 |
Guidelines for Perioperative Cardiovascular Evaluation for Noncardiac Surgery
|
An argument that “periodic cardiac surveillance testing after waitlist may be unnecessary” is offered by a prospective, observational study of 604 patients on the kidney transplant waitlist in British Columbia in 1998–2001. The reference cardiac surveillance guideline was specified as: a) among patients with normal cardiac evaluation at listing – annual testing in those with diabetes, every two years in those with ischemic heart disease or peripheral vascular disease, or every three years in others; b) among patient revascularized as part of listing process – annual testing after percutaneous revascularization and every three years after coronary artery bypass grafting. Surveillance based on ongoing clinical assessment resulted in fewer investigations (n=171) than suggested by guidelines (n=503) over a mean period of mean follow-up of 3.7 ± 1.8 years78. There was no difference in total cardiovascular event rates after listing among subsets who did receive the recommended frequency of investigations (99 per 1000-person years) and those who did not (67 per 1000-person years).
Biomarkers for Cardiac Risk Assessment in Transplant Candidates
Several biomarkers, namely the cardiac troponins (cTn), have been proposed as tools in the cardiac evaluation of ESRD patients. The kidneys participate in clearance of cTnT but the source of elevations, even in dialysis patients, appears to be cardiac. While a dynamic rise and fall in cTn with appropriate clinical signs or symptoms is suggestive of acute coronary syndromes, persistent elevations in cTn may reflect other forms of cardiac injury such as strain from hypertension, volume overload or left ventricular hypertrophy that portend worse prognosis79. Risk stratification of asymptomatic patients with biomarkers is distinct from, but complementary to, the task of diagnosing acute coronary syndromes. A number of studies have shown consistent associations of elevated levels of cTnT isoforms with all-cause and cardiac death risk in asymptomatic ESRD patients. In a recent meta-analysis of 28 studies in this patient population, cTnT >0.10 ng/ml was associated with more than doubling of the mortality experienced by patients with lower cTnT levels (pooled RR 2.62, 95% CI 2.17–3.20)80. Risk in relation to cTnI has been more heterogeneous, and may reflect lack of assay standardization and/or use of a broader range of cut-points.
The Food and Drug Administration approved the measurement of cTnT for mortality prediction in persons with chronic renal failure in 2004, but use of this biomarker is not yet adopted in the clinical practice guidelines of the Kidney Disease Outcomes Quality Initiative (KDOQI). Putative applications of cardiac biomarkers in potential kidney transplant candidates include risk stratification within protocols for initial disease screening and surveillance after listing. Two recent studies examined cTnT among patients referred for kidney transplant candidates in relation to subsequent death (Table 4). In a cohort study of 144 patients evaluated for transplant candidacy and followed for vital status over an average of 2.3 years, Sharma et al found that concomitant elevation in cTnT >0.06 ng/ml and ischemia-modified albumin >95 KU/L was associated with seven times the odds of death after adjustment for multiple factors including severe CAD and positive DSE, although the individual markers were not independently associated with mortality81. Hickson et al. studied cTnT at evaluation in relation to transplant-censored mortality among 644 potential candidates, and observed a 64% increase in the adjusted relative risk of death with each increment in cTnT level according to the cutpoints: <0.01, 0.01–0.03, 0.04–0.09, and ≥0.10 ng/ml22. A recent prospective cohort study found correlations of cTnT with death among stable transplant recipients, estimating 2.7-times the mortality over an average of 3.8 years follow-up with cTnT ≥0.03 versus <0.0182. Although intriguing, it is currently not known how cTn may be rationally applied to direct use of more expensive or invasive diagnostic testing such as MPS, DSE or angiography in practice.
Table 4.
Summary of recent studies associations of cardiac biomarkers with clinical outcomes in kidney transplant candidates and recipients.
| Authors, Year | Design & Data Source | Participants and Selection | Study Measures & Distributions | Clinical Outcomes | Associations/Effect Sizes |
|---|---|---|---|---|---|
| Sharma et al., 200681 | Prospective cohort, Medical records and phone calls for follow-up |
|
|
|
|
| Connolly et al. 200882 | Prospective cohort, Registry mortality data and phone calls for follow-up | Convenience sample of 379 with functioning KT at two Irish hospitals, ≥3 mo post-KT and “well” at enrollment (June 2000–December 2002) |
|
|
|
| Hickson et al, 200822 | Retrospective cohort, Clinical records of one center | 644 evaluated for KT candidacy at one Midwestern center (September 2004–December 2006) |
|
|
|
aHR, adjusted hazards ratio; aOR, adjusted odds ratio; CAD, coronary artery disease; CI, confidence interval; cTnT, cardiac troponin T; DSE, dobutamine stress echocardiography; KT, kidney transplant
Other Forms of Heart Disease in ESRD Patients
In addition to CAD, other forms of cardiovascular disease are common among kidney transplant candidates and bear important relationships with mortality. Perhaps the best studied of these is cardiomyopathy with or without clinical heart failure. Two reports from one large center using stress single photon emission computed tomography (SPECT) in potential candidates meeting AST criteria for pretransplant ischemia evaluation found left ventricular systolic dysfunction (LVSD), defined as left ventricular ejection fraction (LVEF) ≤40%–45%, in 16%–18%83,84. The majority (61–63%) of these patients did not have evidence of ischemia by perfusion imaging, suggesting nonischemic etiologies. Of note, these studies were retrospective and included patients with incidentally detected LVSD. Since an unspecified number of patients with prior diagnoses of heart failure were excluded and SPECT was performed in a selected 50–60% of patients meeting AST criteria, the prevalence of LVSD in the full cohort of potential candidates is not known. Based on Medicare billing claims as measures of clinical diagnoses in a recent USRDS cohort, the adjusted incidence of new-onsetheart failure was estimated as 7%, 12%, and 32% at 6, 12, and 36 months after listing, respectively6.
The presence of LVSD has prognostic implications after renal transplantation, independent of CAD and ischemia. In a single center study, median survival in patients with LVEF <40% was 49 months compared with 72 months in patients with higher LVEF; after adjustment for ischemia and other risk factors, the relative risk of mortality increased by 2.5% for each percent decline in LVEF84. Cumulative mortality for patients with LVSD awaiting transplantation was almost 6-fold higher than the reported mortality for patients with similar degrees of LVSD in the general population85. In a study of transplant recipients from the same center, LVSD was associated with 4.8-times the risk of cardiac death, 2-times the risk of all-cause mortality, and 1.8-times the risk of cardiac complications compared to patients with normal cardiac function83. A registry-based study also found that new-onset heart failure after transplant is a potent predictor of subsequent death (adjusted HR 2.6, 95% CI 2.4–2.9)6.
Because of the serious prognostic implications of heart failure, many patients with LVSD are not considered candidates for renal transplantation. However, reversal of some cases of cardiac dysfunction after transplant has been documented in case reports and a small but growing body of prospective, serial echocardiographic studies86–89. In the largest study, which included 103 recipients at a single center, mean LVEF improved from 32% pretransplant to 52% one year after transplant89. While these data are impressive, it is important to note that 50% of these patients were also found to have CAD prior to transplantation and 90% of these patients underwent subsequent revascularization. In addition, most of the patients in this study were taking cardioprotective medications (beta-blockers, angiotensin-converting enzyme inhibitors, Angiotensin-2 receptor blockers), whereas other studies have reported less use of these medications in transplant candidates with LVSD. Use of devices such as implantable cardioverter-defibrillators have not been studied in this population, which is important since LVSD may contribute to the high rate of sudden cardiac death afflicting ESRD populations90.
Conclusions
Defining best practices for pretransplant cardiac evaluation based on current evidence is challenging.. DSE, a non-invasive, relatively inexpensive tool with minimal risk for nephrotoxicity, is an attractive method for cardiac evaluation in renal transplant candidates. Although the accuracy of DSE for detection of angiographic CAD is imperfect in this population, with specificity (71–95%) appearing better than sensitivity (37–95%), both DSE and MPS offer some prognostic value for the risk of future cardiac events and mortality. Incorporation of clinical risk profiles and possibly biomarkers may guide more selective testing and hence may improve clinical and cost effectiveness, but further study is required for broad implementation. As many plaque ruptures producing myocardial infarction are not localized to sites of angiographic stenosis and angiography poses risks such as contrast nephropathy, the role and best methods of pretransplant revascularization of CAD in ESRD patients are also controversial. Further, the extent of revascularization and the subsequent impact of revascularization on short and long term cardiovascular risk are not well-defined, leading to uncertainty about the timing and frequency of diagnostic testing and interventions. Nevertheless, given the prevalence of CAD and its contribution to morbidity and mortality before and after kidney transplantation, focused screening among patients at highest risk (e.g. known multi-vessel disease, multiple risk factors, or findings suggestive of prior infarction) should be pursued. Other forms of heart disease such as cardiomyopathy with and without heart failure also have important prognostic implications in this population and warrant consideration as potential targets of evaluation protocols. In all cases, risk factor reduction for primary and secondary prevention of ischemic heart disease, is indicated. Broader prospective data, ideally from clinical trials, is urgently needed to strengthen the evidence base for pretransplant cardiac evaluation practices.
Acknowledgments
The views expressed in this manuscript are those of the authors and in no way should be seen as an official policy or interpretation of the National Institutes of Health, the Department of Army, the Department of Defense, or the United States government.
Support: Dr. Lentine is supported by a grant from the National Institute of Diabetes Digestive and Kidney Diseases (NIDDK), K08DK073036.
Footnotes
Financial Disclosure: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wilson JM, Jungner YG. Principles and practice of mass screening for disease. Bol Oficina Sanit Panam. 1968 Oct;65(4):281–393. [PubMed] [Google Scholar]
- 2. [access date January 21, 2009];Organ Procurement and Transplant Network Database. http://www.optn.org./data/
- 3.U.S. Renal Data System: USRDS. 2008 Annual Data Report. Bethesda: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2008. Altas of ESRD, Transplantation, Figure 7.4. http://www.usrds.org/2008/view/esrd_07.asp. [Google Scholar]
- 4.Lentine KL, Brennan DC, Schnitzler MA. Incidence and predictors of myocardial infarction after kidney transplantation. Journal of the American Society of Nephrology. 2005;16(2):496–506. doi: 10.1681/ASN.2004070580. [DOI] [PubMed] [Google Scholar]
- 5.Kasiske BL, Maclean JR, Snyder JJ. Acute myocardial infarction and kidney transplantation. J Am Soc Nephrol. 2006 Mar;17(3):900–907. doi: 10.1681/ASN.2005090984. [DOI] [PubMed] [Google Scholar]
- 6.Lentine KL, Schnitzler MA, Abbott KC, et al. De novo congestive heart failure after kidney transplantation: a common condition with poor prognostic implications. Am J Kidney Dis. 2005 Oct;46(4):720–733. doi: 10.1053/j.ajkd.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 7.Lentine KL, Schnitzler MA, Abbott KC, et al. Incidence, predictors, and associated outcomes of atrial fibrillation after kidney transplantation. Clin J Am Soc Nephrol. 2006;1:288–296. doi: 10.2215/CJN.00920805. [DOI] [PubMed] [Google Scholar]
- 8.U.S. Renal Data System: USRDS. 2008 Annual Data Report. Bethesda: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2008. Altas of ESRD, Transplantation, Figure 7.31. http://www.usrds.org/2008/view/esrd_07.asp. [Google Scholar]
- 9.Bennett WM, Kloster F, Rosch J, Barry J, Porter GA. Natural history of asymptomatic coronary arteriographic lesions in diabetic patients with end-stage renal disease. Am J Med. 1978 Nov;65(5):779–784. doi: 10.1016/0002-9343(78)90796-9. [DOI] [PubMed] [Google Scholar]
- 10.Weinrauch L, D’Elia JA, Healy RW, Gleason RE, Christleib AR, Leland OS., Jr Asymptomatic coronary artery disease: angiographic assessment of diabetics evaluated for renal transplantation. Circulation. 1978 Dec;58(6):1184–1190. doi: 10.1161/01.cir.58.6.1184. [DOI] [PubMed] [Google Scholar]
- 11.Braun WE, Phillips DF, Vidt DG, et al. Coronary artery disease in 100 diabetics with end-stage renal failure. Transplant Proc. 1984 Jun;16(3):603–607. [PubMed] [Google Scholar]
- 12.Lorber MI, Van Buren CT, Flechner SM, et al. Pretransplant coronary arteriography for diabetic renal transplant recipients. Transplant Proc. 1987 Feb;19(1 Pt 2):1539–1541. [PubMed] [Google Scholar]
- 13.Manske CL, Wilson RF, Wang Y, Thomas W. Prevalence of, and risk factors for, angiographically determined coronary artery disease in type I-diabetic patients with nephropathy. Arch Intern Med. 1992 Dec;152(12):2450–2455. [PubMed] [Google Scholar]
- 14.Ohtake T, Kobayashi S, Moriya H, et al. High prevalence of occult coronary artery stenosis in patients with chronic kidney disease at the initiation of renal replacement therapy: an angiographic examination. J Am Soc Nephrol. 2005 Apr;16(4):1141–1148. doi: 10.1681/ASN.2004090765. [DOI] [PubMed] [Google Scholar]
- 15.Sharma R, Pellerin D, Gaze DC, et al. Dobutamine stress echocardiography and the resting but not exercise electrocardiograph predict severe coronary artery disease in renal transplant candidates. Nephrol Dial Transplant. 2005 Oct;20(10):2207–2214. doi: 10.1093/ndt/gfi005. [DOI] [PubMed] [Google Scholar]
- 16.De Lima JJ, Sabbaga E, Vieira ML, et al. Coronary angiography is the best predictor of events in renal transplant candidates compared with noninvasive testing. Hypertension. 2003 Sep;42(3):263–268. doi: 10.1161/01.HYP.0000087889.60760.87. [DOI] [PubMed] [Google Scholar]
- 17.Charytan D, Kuntz RE, Mauri L, DeFilippi C. Distribution of coronary artery disease and relation to mortality in asymptomatic hemodialysis patients. Am J Kidney Dis. 2007 Mar;49(3):409–416. doi: 10.1053/j.ajkd.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 18.Gowdak LH, de Paula FJ, Cesar LA, et al. Screening for significant coronary artery disease in high-risk renal transplant candidates. Coron Artery Dis. 2007 Nov;18(7):553–558. doi: 10.1097/MCA.0b013e3282f08e99. [DOI] [PubMed] [Google Scholar]
- 19.Gowdak LH, de Paula FJ, Cesar LA, et al. Diabetes and coronary artery disease impose similar cardiovascular morbidity and mortality on renal transplant candidates. Nephrol Dial Transplant. 2007 May;22(5):1456–1461. doi: 10.1093/ndt/gfl781. [DOI] [PubMed] [Google Scholar]
- 20.Hage FG, Smalheiser S, Zoghbi GJ, et al. Predictors of survival in patients with end-stage renal disease evaluated for kidney transplantation. Am J Cardiol. 2007 Sep 15;100(6):1020–1025. doi: 10.1016/j.amjcard.2007.04.045. [DOI] [PubMed] [Google Scholar]
- 21.Patel RK, Mark PB, Johnston N, et al. Prognostic value of cardiovascular screening in potential renal transplant recipients: a single-center prospective observational study. Am J Transplant. 2008 Aug;8(8):1673–1683. doi: 10.1111/j.1600-6143.2008.02281.x. [DOI] [PubMed] [Google Scholar]
- 22.Hickson LJ, Cosio FG, El-Zoghby ZM, et al. Survival of patients on the kidney transplant wait list: relationship to cardiac troponin T. Am J Transplant. 2008 Nov;8(11):2352–2359. doi: 10.1111/j.1600-6143.2008.02395.x. [DOI] [PubMed] [Google Scholar]
- 23.Little WC, Applegate RJ. The shadows leave a doubt--the angiographic recognition of vulnerable coronary artery plaques. J Am Coll Cardiol. 1999 Apr;33(5):1362–1364. doi: 10.1016/s0735-1097(99)00020-0. [DOI] [PubMed] [Google Scholar]
- 24.Slevin M, Wang Q, Font MA, et al. Atherothrombosis and plaque heterology: different location or a unique disease? Pathobiology. 2008;75(4):209–225. doi: 10.1159/000132382. [DOI] [PubMed] [Google Scholar]
- 25.Klocke FJ, Baird MG, Lorell BH, et al. ACC/AHA/ASNC guidelines for the clinical use of cardiac radionuclide imaging--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASNC Committee to Revise the 1995 Guidelines for the Clinical Use of Cardiac Radionuclide Imaging) J Am Coll Cardiol. 2003 Oct 1;42(7):1318–1333. doi: 10.1016/j.jacc.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 26.Koistinen MJ, Huikuri HV, Pirttiaho H, Linnaluoto MK, Takkunen JT. Evaluation of exercise electrocardiography and thallium tomographic imaging in detecting asymptomatic coronary artery disease in diabetic patients. Br Heart J. 1990 Jan;63(1):7–11. doi: 10.1136/hrt.63.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marwick TH, Steinmuller DR, Underwood DA, et al. Ineffectiveness of dipyridamole SPECT thallium imaging as a screening technique for coronary artery disease in patients with end-stage renal failure. Transplantation. 1990 Jan;49(1):100–103. doi: 10.1097/00007890-199001000-00022. [DOI] [PubMed] [Google Scholar]
- 28.Dahan M, Viron BM, Faraggi M, et al. Diagnostic accuracy and prognostic value of combined dipyridamole-exercise thallium imaging in hemodialysis patients. Kidney Int. 1998 Jul;54(1):255–262. doi: 10.1046/j.1523-1755.1998.00988.x. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt A, Stefenelli T, Schuster E, Mayer G. Informational contribution of noninvasive screening tests for coronary artery disease in patients on chronic renal replacement therapy. Am J Kidney Dis. 2001 Jan;37(1):56–63. doi: 10.1053/ajkd.2001.20584. [DOI] [PubMed] [Google Scholar]
- 30.Morrow CE, Schwartz JS, Sutherland DE, et al. Predictive value of thallium stress testing for coronary and cardiovascular events in uremic diabetic patients before renal transplantation. Am J Surg. 1983 Sep;146(3):331–335. doi: 10.1016/0002-9610(83)90409-9. [DOI] [PubMed] [Google Scholar]
- 31.Patel AD, Abo-Auda WS, Davis JM, et al. Prognostic value of myocardial perfusion imaging in predicting outcome after renal transplantation. Am J Cardiol. 2003 Jul 15;92(2):146–151. doi: 10.1016/s0002-9149(03)00529-0. [DOI] [PubMed] [Google Scholar]
- 32.Rabbat CG, Treleaven DJ, Russell JD, Ludwin D, Cook DJ. Prognostic value of myocardial perfusion studies in patients with end-stage renal disease assessed for kidney or kidney-pancreas transplantation: a meta-analysis. J Am Soc Nephrol. 2003 Feb;14(2):431–439. doi: 10.1097/01.asn.0000047560.51444.3a. [DOI] [PubMed] [Google Scholar]
- 33.Wong CF, Little MA, Vinjamuri S, Hammad A, Harper JM. Technetium myocardial perfusion scanning in prerenal transplant evaluation in the United kingdom. Transplant Proc. 2008 Jun;40(5):1324–1328. doi: 10.1016/j.transproceed.2008.03.143. [DOI] [PubMed] [Google Scholar]
- 34.Cheitlin MD, Armstrong WF, Aurigemma GP, et al. ACC/AHA/ASE 2003 Guideline Update for the Clinical Application of Echocardiography: summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography) J Am Soc Echocardiogr. 2003 Oct;16(10):1091–1110. doi: 10.1016/S0894-7317(03)00685-0. [DOI] [PubMed] [Google Scholar]
- 35.Reis G, Marcovitz PA, Leichtman AB, et al. Usefulness of dobutamine stress echocardiography in detecting coronary artery disease in end-stage renal disease. Am J Cardiol. 1995 Apr 1;75(10):707–710. doi: 10.1016/S0002-9149(99)80658-4. [DOI] [PubMed] [Google Scholar]
- 36.Bates JR, Sawada SG, Segar DS, et al. Evaluation using dobutamine stress echocardiography in patients with insulin-dependent diabetes mellitus before kidney and/or pancreas transplantation. Am J Cardiol. 1996 Jan 15;77(2):175–179. doi: 10.1016/s0002-9149(96)90591-3. [DOI] [PubMed] [Google Scholar]
- 37.Herzog CA, Marwick TH, Pheley AM, White CW, Rao VK, Dick CD. Dobutamine stress echocardiography for the detection of significant coronary artery disease in renal transplant candidates. Am J Kidney Dis. 1999 Jun;33(6):1080–1090. doi: 10.1016/S0272-6386(99)70145-9. [DOI] [PubMed] [Google Scholar]
- 38.Ferreira PA, de Lima VC, Campos Filho O, et al. Feasibility, safety and accuracy of dobutamine/atropine stress echocardiography for the detection of coronary artery disease in renal transplant candidates. Arq Bras Cardiol. 2007 Jan;88(1):45–51. doi: 10.1590/s0066-782x2007000100008. [DOI] [PubMed] [Google Scholar]
- 39.Tita C, Karthikeyan V, Stroe A, Jacobsen G, Ananthasubramaniam K. Stress echocardiography for risk stratification in patients with end-stage renal disease undergoing renal transplantation. J Am Soc Echocardiogr. 2008 Apr;21(4):321–326. doi: 10.1016/j.echo.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 40.Marwick TH, Lauer MS, Lobo A, Nally J, Braun W. Use of dobutamine echocardiography for cardiac risk stratification of patients with chronic renal failure. J Intern Med. 1998 Aug;244(2):155–161. doi: 10.1046/j.1365-2796.1998.00354.x. [DOI] [PubMed] [Google Scholar]
- 41.Rakhit DJ, Armstrong KA, Beller E, Isbel NM, Marwick TH. Risk stratification of patients with chronic kidney disease: results of screening strategies incorporating clinical risk scoring and dobutamine stress echocardiography. Am Heart J. 2006 Aug;152(2):363–370. doi: 10.1016/j.ahj.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 42.Bergeron S, Hillis GS, Haugen EN, Oh JK, Bailey KR, Pellikka PA. Prognostic value of dobutamine stress echocardiography in patients with chronic kidney disease. Am Heart J. 2007 Mar;153(3):385–391. doi: 10.1016/j.ahj.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 43.Kasiske BL, Malik MA, Herzog CA. Risk-stratified screening for ischemic heart disease in kidney transplant candidates. Transplantation. 2005 Sep 27;80(6):815–820. doi: 10.1097/01.tp.0000173652.87417.ca. [DOI] [PubMed] [Google Scholar]
- 44.Lewis MS, Wilson RA, Walker KW, et al. Validation of an algorithm for predicting cardiac events in renal transplant candidates. Am J Cardiol. 2002 Apr 1;89(7):847–850. doi: 10.1016/s0002-9149(02)02197-5. [DOI] [PubMed] [Google Scholar]
- 45.Budoff MJ, Shaw LJ, Liu ST, et al. Long-term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. J Am Coll Cardiol. 2007 May 8;49(18):1860–1870. doi: 10.1016/j.jacc.2006.10.079. [DOI] [PubMed] [Google Scholar]
- 46.Raggi P, Boulay A, Chasan-Taber S, et al. Cardiac calcification in adult hemodialysis patients. A link between end-stage renal disease and cardiovascular disease? J Am Coll Cardiol. 2002 Feb 20;39(4):695–701. doi: 10.1016/s0735-1097(01)01781-8. [DOI] [PubMed] [Google Scholar]
- 47.Braun J, Oldendorf M, Moshage W, Heidler R, Zeitler E, Luft FC. Electron beam computed tomography in the evaluation of cardiac calcification in chronic dialysis patients. Am J Kidney Dis. 1996 Mar;27(3):394–401. doi: 10.1016/s0272-6386(96)90363-7. [DOI] [PubMed] [Google Scholar]
- 48.Goodman WG, Goldin J, Kuizon BD, et al. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med. 2000 May 18;342(20):1478–1483. doi: 10.1056/NEJM200005183422003. [DOI] [PubMed] [Google Scholar]
- 49.Oh J, Wunsch R, Turzer M, et al. Advanced coronary and carotid arteriopathy in young adults with childhood-onset chronic renal failure. Circulation. 2002 Jul 2;106(1):100–105. doi: 10.1161/01.cir.0000020222.63035.c0. [DOI] [PubMed] [Google Scholar]
- 50.Matsuoka M, Iseki K, Tamashiro M, et al. Impact of high coronary artery calcification score (CACS) on survival in patients on chronic hemodialysis. Clin Exp Nephrol. 2004 Mar;8(1):54–58. doi: 10.1007/s10157-003-0260-0. [DOI] [PubMed] [Google Scholar]
- 51.Greenland P, Bonow RO, Brundage BH, et al. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography) developed in collaboration with the Society of Atherosclerosis Imaging and Prevention and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol. 2007 Jan 23;49(3):378–402. doi: 10.1016/j.jacc.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 52.Haydar AA, Hujairi NM, Covic AA, Pereira D, Rubens M, Goldsmith DJ. Coronary artery calcification is related to coronary atherosclerosis in chronic renal disease patients: a study comparing EBCT-generated coronary artery calcium scores and coronary angiography. Nephrol Dial Transplant. 2004 Sep;19(9):2307–2312. doi: 10.1093/ndt/gfh120. [DOI] [PubMed] [Google Scholar]
- 53.Sharples EJ, Pereira D, Summers S, et al. Coronary artery calcification measured with electron-beam computerized tomography correlates poorly with coronary artery angiography in dialysis patients. Am J Kidney Dis. 2004 Feb;43(2):313–319. doi: 10.1053/j.ajkd.2003.10.035. [DOI] [PubMed] [Google Scholar]
- 54.Tong LL, Mehrotra R, Shavelle DM, Budoff M, Adler S. Poor correlation between coronary artery calcification and obstructive coronary artery disease in an end-stage renal disease patient. Hemodial Int. 2008 Jan;12(1):16–22. doi: 10.1111/j.1542-4758.2008.00234.x. [DOI] [PubMed] [Google Scholar]
- 55.Shroff RC, McNair R, Figg N, et al. Dialysis accelerates medial vascular calcification in part by triggering smooth muscle cell apoptosis. Circulation. 2008 Oct 21;118(17):1748–1757. doi: 10.1161/CIRCULATIONAHA.108.783738. [DOI] [PubMed] [Google Scholar]
- 56.Vanhoenacker PK, Heijenbrok-Kal MH, Van Heste R, et al. Diagnostic performance of multidetector CT angiography for assessment of coronary artery disease: meta-analysis. Radiology. 2007 Aug;244(2):419–428. doi: 10.1148/radiol.2442061218. [DOI] [PubMed] [Google Scholar]
- 57.Meijboom WB, Weustink AC, Pugliese F, et al. Comparison of diagnostic accuracy of 64-slice computed tomography coronary angiography in women versus men with angina pectoris. Am J Cardiol. 2007 Nov 15;100(10):1532–1537. doi: 10.1016/j.amjcard.2007.06.061. [DOI] [PubMed] [Google Scholar]
- 58.Le A, Wilson R, Douek K, et al. Prospective risk stratification in renal transplant candidates for cardiac death. Am J Kidney Dis. 1994 Jul;24(1):65–71. doi: 10.1016/s0272-6386(12)80161-2. [DOI] [PubMed] [Google Scholar]
- 59.Parfrey PS, Griffiths SM, Barrett BJ, et al. Contrast material-induced renal failure in patients with diabetes mellitus, renal insufficiency, or both. A prospective controlled study. N Engl J Med. 1989 Jan 19;320(3):143–149. doi: 10.1056/NEJM198901193200303. [DOI] [PubMed] [Google Scholar]
- 60.Nikolsky E, Aymong ED, Dangas G, Mehran R. Radiocontrast nephropathy: identifying the high-risk patient and the implications of exacerbating renal function. Rev Cardiovasc Med. 2003;4 (Suppl 1):S7–S14. [PubMed] [Google Scholar]
- 61.Mehran R, Aymong ED, Nikolsky E, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004 Oct 6;44(7):1393–1399. doi: 10.1016/j.jacc.2004.06.068. [DOI] [PubMed] [Google Scholar]
- 62.Dangas G, Iakovou I, Nikolsky E, et al. Contrast-induced nephropathy after percutaneous coronary interventions in relation to chronic kidney disease and hemodynamic variables. Am J Cardiol. 2005 Jan 1;95(1):13–19. doi: 10.1016/j.amjcard.2004.08.056. [DOI] [PubMed] [Google Scholar]
- 63.McFalls EO, Ward HB, Moritz TE, et al. Coronary-artery revascularization before elective major vascular surgery. N Engl J Med. 2004 Dec 30;351(27):2795–2804. doi: 10.1056/NEJMoa041905. [DOI] [PubMed] [Google Scholar]
- 64.Boden WE, O’Rourke RA, Teo KK, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007 Apr 12;356(15):1503–1516. doi: 10.1056/NEJMoa070829. [DOI] [PubMed] [Google Scholar]
- 65.Manske CL, Wang Y, Rector T, Wilson RF, White CW. Coronary revascularisation in insulin-dependent diabetic patients with chronic renal failure. Lancet. 1992 Oct 24;340(8826):998–1002. doi: 10.1016/0140-6736(92)93010-k. [DOI] [PubMed] [Google Scholar]
- 66.Lewis MS, Wilson RA, Walker K, et al. Factors in cardiac risk stratification of candidates for renal transplant. J Cardiovasc Risk. 1999 Aug;6(4):251–255. doi: 10.1177/204748739900600410. [DOI] [PubMed] [Google Scholar]
- 67.Lentine KL, Schnitzler MA, Brennan DC, et al. Cardiac evaluation before kidney transplantation: a practice patterns analysis in Medicare-insured dialysis patients. Clin J Am Soc Nephrol. 2008 Jul;3(4):1115–1124. doi: 10.2215/CJN.05351107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Herzog CA, Ma JZ, Collins AJ. Long-term outcome of renal transplant recipients in the United States after coronary revascularization procedures. Circulation. 2004 Jun 15;109(23):2866–2871. doi: 10.1161/01.CIR.0000129317.12580.68. [DOI] [PubMed] [Google Scholar]
- 69.Herzog CA, Solid CA. Long-term survival of U.S. dialysis patients after surgical bypass or percutaneous coronary stent placement in the drug-eluting stent era. J Am Soc Nephrol. 2008;19 (Suppl):11A. [Google Scholar]
- 70.Eagle KA, Guyton RA, Davidoff R, et al. ACC/AHA 2004 guideline update for coronary artery bypass graft surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1999 Guidelines for Coronary Artery Bypass Graft Surgery) Circulation. 2004 Oct 5;110(14):e340–437. [PubMed] [Google Scholar]
- 71.Ramos EL, Kasiske BL, Alexander SR, et al. The evaluation of candidates for renal transplantation. The current practice of U.S. transplant centers. Transplantation. 1994 Feb 27;57(4):490–497. [PubMed] [Google Scholar]
- 72.Danovitch GM, Hariharan S, Pirsch JD, et al. Management of the waiting list for cadaveric kidney transplants: report of a survey and recommendations by the Clinical Practice Guidelines Committee of the American Society of Transplantation. J Am Soc Nephrol. 2002 Feb;13(2):528–535. doi: 10.1681/ASN.V132528. [DOI] [PubMed] [Google Scholar]
- 73.Zarifian A, O’Rourke M. Managing the kidney waiting list. Prog Transplant. 2006 Sep;16(3):242–246. doi: 10.1177/152692480601600310. [DOI] [PubMed] [Google Scholar]
- 74.Kasiske BL, Cangro CB, Hariharan S, et al. The evaluation of renal transplantation candidates: clinical practice guidelines. Am J Transplant. 2001;1 (Suppl 2):3–95. [PubMed] [Google Scholar]
- 75.National Kidney Foundation. K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis. 2005 Apr;45(4 Suppl 3):S1–153. [PubMed] [Google Scholar]
- 76.Eagle KA, Berger PB, Calkins H, et al. ACC/AHA Guideline Update for Perioperative Cardiovascular Evaluation for Noncardiac Surgery--Executive Summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1996 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery) Anesth Analg. 2002 May;94(5):1052–1064. doi: 10.1097/00000539-200205000-00002. [DOI] [PubMed] [Google Scholar]
- 77.Friedman S, Palac R, Zlotnick D, Costa S. A Call to Action: Rates of Noninvasive Stress Testing are Dependent on which Set of National Guidelines are Applied. J Am Coll Cardiol. 2009;53 (supplement A):377. [Google Scholar]
- 78.Gill JS, Ma I, Landsberg D, Johnson N, Levin A. Cardiovascular events and investigation in patients who are awaiting cadaveric kidney transplantation. J Am Soc Nephrol. 2005 Mar;16(3):808–816. doi: 10.1681/ASN.2004090810. [DOI] [PubMed] [Google Scholar]
- 79.Jaffe AS, Babuin L, Apple FS. Biomarkers in acute cardiac disease: the present and the future. J Am Coll Cardiol. 2006 Jul 4;48(1):1–11. doi: 10.1016/j.jacc.2006.02.056. [DOI] [PubMed] [Google Scholar]
- 80.Khan NA, Hemmelgarn BR, Tonelli M, Thompson CR, Levin A. Prognostic value of troponin T and I among asymptomatic patients with end-stage renal disease: a meta-analysis. Circulation. 2005 Nov 15;112(20):3088–3096. doi: 10.1161/CIRCULATIONAHA.105.560128. [DOI] [PubMed] [Google Scholar]
- 81.Sharma R, Gaze DC, Pellerin D, et al. Ischemia-modified albumin predicts mortality in ESRD. Am J Kidney Dis. 2006 Mar;47(3):493–502. doi: 10.1053/j.ajkd.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 82.Connolly GM, Cunningham R, McNamee PT, Young IS, Maxwell AP. Troponin T is an independent predictor of mortality in renal transplant recipients. Nephrol Dial Transplant. 2008 Mar;23(3):1019–1025. doi: 10.1093/ndt/gfm738. [DOI] [PubMed] [Google Scholar]
- 83.Siedlecki A, Foushee M, Curtis JJ, et al. The impact of left ventricular systolic dysfunction on survival after renal transplantation. Transplantation. 2007 Dec 27;84(12):1610–1617. doi: 10.1097/01.tp.0000295748.42884.97. [DOI] [PubMed] [Google Scholar]
- 84.de Mattos AM, Siedlecki A, Gaston RS, et al. Systolic dysfunction portends increased mortality among those waiting for renal transplant. J Am Soc Nephrol. 2008 Jun;19(6):1191–1196. doi: 10.1681/ASN.2007040503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McDonagh TA, Cunningham AD, Morrison CE, et al. Left ventricular dysfunction, natriuretic peptides, and mortality in an urban population. Heart. 2001 Jul;86(1):21–26. doi: 10.1136/heart.86.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Burt RK, Gupta-Burt S, Suki WN, Barcenas CG, Ferguson JJ, Van Buren CT. Reversal of left ventricular dysfunction after renal transplantation. Ann Intern Med. 1989 Oct 15;111(8):635–640. doi: 10.7326/0003-4819-111-8-635. [DOI] [PubMed] [Google Scholar]
- 87.Melchor JL, Espinoza R, Gracida C. Kidney transplantation in patients with ventricular ejection fraction less than 50 percent: features and posttransplant outcome. Transplant Proc. 2002 Nov;34(7):2539–2540. doi: 10.1016/s0041-1345(02)03478-4. [DOI] [PubMed] [Google Scholar]
- 88.Oppert M, Schneider U, Bocksch W, et al. Improvement of left ventricular function and arterial blood pressure 1 year after simultaneous pancreas kidney transplantation. Transplant Proc. 2002 Sep;34(6):2251–2252. doi: 10.1016/s0041-1345(02)03223-2. [DOI] [PubMed] [Google Scholar]
- 89.Wali RK, Wang GS, Gottlieb SS, et al. Effect of kidney transplantation on left ventricular systolic dysfunction and congestive heart failure in patients with end-stage renal disease. J Am Coll Cardiol. 2005 Apr 5;45(7):1051–1060. doi: 10.1016/j.jacc.2004.11.061. [DOI] [PubMed] [Google Scholar]
- 90.Cheung AK, Sarnak MJ, Yan G, et al. Cardiac diseases in maintenance hemodialysis patients: results of the HEMO Study. Kidney Int. 2004 Jun;65(6):2380–2389. doi: 10.1111/j.1523-1755.2004.00657.x. [DOI] [PubMed] [Google Scholar]