Abstract
Background
Chronic kidney disease (CKD) is associated with an increased risk for incident cardiovascular disease (CVD), but the role of statin for the primary prevention of acute cardiovascular events in CKD and the effect of statins on kidney function loss in persons without prevalent CVD have not been studied.
Study Design
Post-hoc analysis of the Air Force/Texas Coronary Atherosclerosis Prevention Study.
Setting and Participants
Multicenter, randomized, double-blind, placebo-controlled trial of 5608 men and 997 women without CVD randomized to lovastatin or placebo.
Intervention
Placebo or lovastatin 20 mg daily
Outcomes and Measurements
First major acute cardiovascular event in participants with mild CKD and kidney function loss among persons with or without CKD. Estimated glomerular filtration rate was calculated using the 4-variable Modification of Diet in Renal Disease Study equation.
Results
At baseline, the mean estimated glomerular filtration rate among CKD participants (n=304) was 53.3 ± 6.0 mL/min/1.73m2. After an average follow-up of 5.3 ± 0.8 years, the incidence of a fatal and non-fatal cardiovascular disease event was lower in CKD participants receiving lovastatin than in those on placebo (adjusted relative risk (RR): 0.31, 95% CI 0.13–0.72; p=0.01). Tests for interaction suggested that the benefit of lovastatin was independent of the presence of CKD. Lovastatin did not reduce the annualized mean decline in estimated glomerular filtration rate (−1.3 ± 0.07 vs. −1.4 ± 0.07 mL/min/1.73m2/year, respectively, p=0.1), the frequency of ≥ 25% decrease in kidney function [adjusted RR: 1.10, 95% CI 0.96 to 1.28, p=0.2] or incident CKD (adjusted RR 1.04, 95% CI 0.86 to 1.27, p=0.6).
Limitations
Unable to determine cause and duration of kidney disease and information regarding proteinuria was not available.
Conclusions
Lovastatin is effective for the primary prevention of CVD in CKD, but is not effective in decreasing kidney function loss in persons with no CVD.
INTRODUCTION
Chronic kidney disease (CKD) patients are at high risk for developing cardiovascular disease (CVD).1–3 In fact, epidemiological studies have established that cardiovascular events occur much more frequently than progression to end-stage renal disease in persons with CKD.4 CKD and CVD share analogous pathological mechanisms, suggesting a similarity of mesangial cell and vascular smooth muscle cell response to injury.5,6 This similarity of mechanisms raises the hypothesis that mediators to prevent CVD may also prevent the incidence and progression of CKD. However, there are few published data about the effect of statins on primary prevention of CVD and kidney function loss in persons without CVD.6–8
In the population with both CVD and CKD, statins have been shown to be effective for secondary prevention of cardiovascular events including myocardial infarction, coronary death, coronary revascularization and all-cause mortality.9,10 Furthermore, in persons with established CVD, statins appear to decrease the rate of kidney function loss.7,11,12 However, in persons without CVD, the effects of statins in CKD have not been thoroughly evaluated 6–8 either for their effects on primary cardiovascular prevention or progression of kidney disease.
In the current investigation from the primary prevention trial Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS) 13, we evaluated the effects of statins on a fairly unique kidney disease population, one without significant diabetes or CVD. We examined the effects of lovastatin on: 1) incidence of first major acute cardiovascular event in CKD patients, and 2) kidney function loss among persons with or without CKD.
METHODS
Study Database
We obtained a copy of the database of the AFCAPS/TexCAPS study by submitting a written proposal to Merck Research Laboratories. None of the authors were members of the AFCAPS/TexCAPS Investigators.
Study Design and Subjects
The design of AFCAPS/TexCAPS has been described previously.13,14 Briefly, the AFCAPS/TexCAPS study was a randomized, double-blind, placebo-controlled primary prevention trial that evaluated the effect of lovastatin versus placebo on the rate of first acute major coronary events in persons with normal to mildly elevated total cholesterol (TC) and low density lipoprotein cholesterol (LDL-C) levels, below average high density lipoprotein cholesterol (HDL-C) and no previous history of CVD.
AFCAPS/TexCAPS included 5608 men aged 45 to 73 years and 997 postmenopausal women aged 55 to 73 years who met the lipid entrance criteria at both 4 and 2 weeks prior to randomization with less than 15% difference in LDL-C values between visits. The lipid entry criteria included TC, 180–264 mg/dL; LDL-C, 130–190 mg/dL; HDL-C, ≤ 45 mg/dL for men or ≤47 mg/dL for women; and triglycerides, ≤400 mg/dL. Exclusion criteria included clinical evidence of atherosclerotic CVD, secondary hyperlipoproteinemia, nephrotic syndrome, uncontrolled hypertension, and type 1 or type 2 diabetes mellitus. Patients with decreased kidney function were not specifically excluded from the study.14
After a 12 week diet run-in, participants were randomized to treatment with lovastatin 20 mg daily or placebo and were followed for a median of 5.1 years. Lovastatin was titrated to 40 mg daily if the LDL-C level was greater than 110 mg/dL at the 3 month follow up visit. Lipids were measured from fresh fasting serum samples at a central laboratory at Wilford Hall Medical Center at Lackland Air Force Base in Texas. TC, HDL-C and triglycerides were measured enzymatically and LDL-C was calculated by the Friedewald formula. 15
Renal Function Assessments
Blood chemistry tests, including serum creatinine, were performed yearly at the central laboratory at Wilford Hall Medical Center. Methodology for the measurement of serum creatinine remained unchanged during the course of the study. AFCAPS/TexCAPS serum creatinine data were indirectly calibrated to The Cleveland Clinic [where serum creatinine was measured in the Modification of Diet in Renal Disease (MDRD) Study] in order to standardize the measurements and increase accuracy by using The Third National Health and Nutrition Examination Survey (NHANES III) data following a method previously reported.16–18 Since NHANES III share the demographic and baseline-lipid-level characteristics of AFCAPS/TexCAPS,19 and NHANES III data have been directly compared to the MDRD study, it was assumed that the mean serum creatinine for a given age, sex and race should be comparable in the two studies. A linear regression of data combining AFCAPS/TexCAPS and NHANES III showed that serum creatinine levels were 0.22 mg/dL higher in the AFCAPS/TexCAPS cohort during the baseline examination than among NHANES III participants after adjustment for age, sex and race. The analysis was limited to participants 45 to 73 and 55 to 73 years old for men and women, respectively who are white, African-American and Mexican American with a serum creatinine less than 2.0 mg/dL at baseline. The value of 0.22 mg/dL was then subtracted from measured creatinine before use in the current study. We estimated glomerular filtration rate (GFR) using the 4-variable MDRD Study equation.20 CKD was categorized as an estimated GFR less than 60 mL/min/1.73m2.20 Participants with an estimated GFR < 15 mL/min/1.73m2 (n=1) were excluded.
Study Outcomes
Lovastatin and Cardiovascular Outcomes
Our objective was to investigate whether treatment with lovastatin, compared with placebo, would decrease cardiovascular morbidity and mortality according to kidney function or CKD status across the spectrum of clinical events by measuring the rates of 7 endpoints: (1) unstable angina, (2) fatal or nonfatal myocardial infarction, (3) fatal or nonfatal coronary events, (4) fatal and nonfatal cardiovascular events, (5) fatal and nonfatal coronary revascularization procedures (6) coronary heart disease mortality, and (7) cardiovascular mortality. These 7 specific endpoints were examined separately in this analysis as each of these clinical events was pre-specified as the secondary objective in the original AFCAPS/TexCAPS study. We also evaluated the original primary endpoint from the AFCAPS/TexCAPS study, defined as time to a composite endpoint of first major cardiac events including unstable angina, fatal or nonfatal myocardial infarction and sudden cardiac death. An endpoint committee that was blinded to lipid levels and treatment assignment determined outcomes.13,14
Lovastatin and Renal Outcomes
Serum creatinine was measured at baseline and yearly until close out of the study. Our main objective in examining renal outcomes was to evaluate whether treatment with lovastatin, compared with placebo, had an effect on yearly change in kidney function decline. For this analysis we included only those participants [4994/6604 (76%)] from the original AFCAPS/TexCAPS who had at least four serum creatinine measurements with at least one of the follow up measurements at least 3 years after randomization. In addition, change in estimated GFR was dichotomized to analyze clinically relevant declines in kidney function: (1) ≥ 25% reduction in baseline estimated GFR and (2) occurrence of incident cases of CKD (defined as an estimated GFR < 60 mL/min/1.73m2) at the end of the follow-up. These two outcomes were chosen based on previous studies examining the effect of statins on kidney function loss.12
Statistical Analysis
To assess whether there were differences in baseline characteristics and estimated GFR group, analysis of variance with treatment and estimated GFR categories was used for continuous variables and the Cochran-Mantel-Haenszel test was used for categorical variables. Values were reported as means ± SD or percentages; 95% CI were provided where appropriate. Within each estimated GFR group, the effect of lovastatin therapy on risks for the cardiovascular outcome was calculated using Cox proportional hazards regression models adjusted for age, sex, race, body mass index (BMI), smoking, family history of premature coronary artery disease (CAD), hypertension, diabetes, systolic and diastolic blood pressure, baseline glucose, total cholesterol, LDL-C, HDL-C, triglycerides, and use of angiotensin converting enzyme inhibitors (ACE inhibitors), beta blockers, calcium channel blockers, diuretics, alpha blockers and aspirin. Potential interactions of CKD and lovastatin were assessed qualitatively by comparing the stratified relative risk reductions in each group, and statistically by an interaction term in the multivariate model. For the yearly rate of change in kidney function, a regression model was fit for each treatment group, separately, using exact time of serum creatinine measurement and only those patients with 3 or more post-randomization measurements, one of which had to be at least 1095 days after randomization. To assess the difference between treatment groups in rate of change in kidney function, the same subset of patients was used. A categorical time variable (year of measurement) and a mixed model were used for the assessment. Cox proportional hazard analyses were used to assess treatment difference in time to ≥25% reduction in estimated GFR from baseline and time to incident CKD defined as an estimated GFR < 60 mL/min/1.73m2. The following baseline covariates were selected for inclusion: age, sex, race, BMI, smoking, family history of premature CAD, hypertension, diabetes, systolic and diastolic blood pressure, baseline glucose, total cholesterol, LDL-C, HDL-C, triglycerides, baseline kidney function and use of ACE inhibitors, beta blockers, calcium channel blockers, diuretics, alpha blockers, aspirin and lovastatin use. All tests were two-sided with significance defined as p<0.05.
RESULTS
Baseline Characteristics
All of the 6604 participants who fulfilled criteria for this analysis had sufficient data to estimate GFR at baseline. Of these 6604 participants, 304 (5%) had CKD at baseline, defined as an estimated GFR <60 mL/min/1.73m2. The mean duration of follow-up for the whole cohort was 5.3 ± 0.8 years (range: 0.2–7.2 years).
Baseline characteristics for the entire population by CKD status at baseline are summarized in Table 1. Participants with CKD were significantly older, more likely to be female, more likely to have hypertension, and had significantly higher total cholesterol and LDL-C levels than participants without CKD. Subjects with CKD also had a higher baseline use of ACE inhibitors, beta blockers, calcium channel blockers, and diuretics. The mean (±SD) values of estimated GFR among participants with and without CKD at baseline were 53 ± 6.0 mL/min/1.73m2 and 89 ± 18 mL/min/1.73m2, respectively.
Table 1.
Baseline Characteristics of Participants with and without CKD by Treatment Group
| eGFR ≥ 60 mL/min/1.73m2 | eGFR < 60 mL/min/1.73m2 | P-value | |||
|---|---|---|---|---|---|
| Characteristic | Lovastatin (n=3158) | Placebo (n=3142) | Lovastatin (n=145) | Placebo (n=159) | |
| Age (years) | 58 ± 7 | 58 ± 7 | 62 ± 8 | 62 ± 7.0 | <0.001 |
| Sex N (%) | |||||
| Men | 2686 (85) | 2683 (85) | 119 (82) | 120 (75) | |
| Women | 472 (15) | 459 (15) | 26 (18) | 39 (25) | 0.002 |
| Black N (%) | 103 (3) | 99 (3) | 2 (1) | 2 (1) | 0.06 |
| Hypertension N (%) | 663 (21) | 678 (22) | 56 (39) | 51 (32) | <0.001 |
| Diabetes N (%) | 82 (3) | 68 (2) | 2 (1) | 3 (2) | 0.4 |
| Smoker N (%) | 414 (13) | 380 (12) | 15 (10) | 9 (6) | 0.02 |
| Family History of CAD N (%) | 480 (15) | 516 (16) | 17 (12) | 22 (14) | 0.2 |
| BMI (kg/m2) | 27 ± 3 | 27 ± 3 | 27 ± 3 | 26 ± 3 | 0.03 |
| SBP (mmHg) | 138 ±17 | 138 ± 17 | 140 ± 18 | 143 ± 18 | 0.2 |
| DBP (mmHg) | 78 ± 10 | 78 ± 10 | 79 ± 10 | 79 ± 10 | 0.5 |
| TC (mg/dL) | 220 ± 23 | 220 ± 27 | 224 ± 23 | 220 ± 27 | 0.004 |
| LDL-C (mg/dL) | 147 ± 23 | 147 ± 23 | 151 ± 19 | 151 ± 23 | 0.03 |
| HDL-C (mg/dL) | 39 ± 8 | 39 ± 8 | 39 ± 8 | 39 ± 8 | 0.1 |
| Triglycerides (mg/dL) | 168 ± 80 | 168 ± 71 | 177 ± 71 | 168 ± 80 | 0.2 |
| Medications N (%) | |||||
| Aspirin | 548 (17) | 530 (17) | 22 (15) | 31 (19) | 0.9 |
| Beta Blocker | 130 (4) | 140 (4) | 11 (8) | 16 (10) | <0.001 |
| Calcium channel blocker | 154 (5) | 154 (5) | 17 (12) | 16 (10) | <0.001 |
| Alpha blocker | 66 (2) | 62 (2) | 2 (1) | 5 (3) | 0.7 |
| Diuretic | 182 (6) | 180 (6) | 21 (14) | 23 (14) | <0.001 |
| ACE Inhibitor | 227 (7) | 236 (8) | 17 (12) | 21 (13) | 0.001 |
| SCr (mg/dL) | 0.9 ± 0.2 | 0.9 ± 0.2 | 1.4 ± 0.3 | 1.4 ± 0.2 | <0.001 |
| eGFR (mL/min/1.73m2) | 89 ± 18 | 89 ± 18 | 53 ± 6 | 53 ± 6 | <0.001 |
Values are expressed as mean ± SD or Number (%). P values are comparing the two GFR groups.
Abbreviations: ACE Inhibitor= angiotensin converting enzyme inhibitor; CAD= coronary artery disease; eGFR= estimaged glomerular filtration rate; HDL= high density lipoprotein cholesterol; LDL= low density lipoprotein cholesterol.
Conversion factors for units: total cholesterol in mg/dL to mmol/L, x0.02586; LDL cholesterol in mg/dL to mmol/L, x0.02586; HDL cho mmol/L, x0.02586; triglycerides in mg/dL to mmol/L, x0.01129; creatinine in mg/dL to μmol/L, 88.4.
During the double-blind study period, 449 participants had an identifying clinical event; 420/6300 (6.6%) of participants with baseline estimated GFR ≥60 mL/min/1.73m2 and 29/304 (9.5%) of participants with an estimated GFR < 60 mL/min/1.73m2 (relative risk [RR]: 1.52, 95% CI 1.04–2.21;p=0.03). Participants with an estimated GFR < 60 mL/min/1.73m2 receiving lovastatin had mean changes from baseline in total cholesterol, LDL-C, HDL-C and serum triglycerides, of −20%, −27 %, +7.4 % and −15 % respectively. The corresponding values in the placebo group were +1.5%, +1.9 %, +1.1 % and +4.2 %, respectively. Similar changes in lipids values were observed in participants with an estimated GFR ≥60 mL/min/1.73m2 receiving lovastatin and placebo (not shown).
Effect of Lovastatin on Cardiovascular Endpoints in Participants with CKD
Complete baseline and follow-up data were available in all 304 participants with CKD. The mean duration of follow-up was 5.1 ± 0.8 years for CKD patients evaluated for the 7 clinical cardiovascular endpoints. Lovastatin use was associated with a lower rate of cardiovascular events in CKD, even after adjustment for potential confounders (Table 2). The rates of fatal and non-fatal coronary events [adjusted relative risk (RR), 0.35, 95% Confidence Interval (CI) 0.13 to 0.93; p=0.03], fatal and non-fatal cardiovascular events (adjusted RR, 0.39 [95% CI 0.16 to 0.93; p=0.03], and coronary revascularization procedures (adjusted RR, 0.23 [95% CI 0.07 to 0.77; p=0.01] were significantly lower. However, lovastatin use did not significantly reduce the incidence of unstable angina and fatal and non-fatal myocardial infarction (adjusted RR, 0.49 [95% CI 0.11 to 2.28; p=0.4] and 0.10 [95% CI 0.01 to 1.32; p=0.08], respectively). Participants with mild CKD treated with lovastatin had an adjusted RR of 0.32 [CI, 0.10 to 1.11; p=0.06] for the primary endpoint defined as a composite of unstable angina, myocardial infarction and sudden death. The adjusted and unadjusted RRs were similar for all models. Of note, no significant reduction in the incidence of coronary heart disease and cardiovascular mortality was seen in the lovastatin group in participants with and without CKD. For all of these clinical events, the effects of lovastatin did not differ significantly among subgroups with and without CKD (p >0.1 for all interaction tests).
Table 2.
Effect of Lovastatin Use on Acute Cardiovascular Events in Persons with CKD*
| Event | Placebo n(%) | Lovastatin, n(%)¶ | Unadjusted RR (95% CI) | Parsimonious model RR (95% CI)† | Fully adjusted model RR (95% CI) ‡ | P value |
|---|---|---|---|---|---|---|
| Unstable angina | 6 (3.8) | 3 (2.1) | 0.56 (0.14–2.23) | 0.58 (0.14–2.31) | 0.49 (0.11–2.28) | 0.4 |
| Fatal and non-fatal MI | 6 (3.8) | 2 (1.4) | 0.37 (0.07–1.81) | 0.19 (0.04–1.01) | 0.10 (0.01–1.32) | 0.08 |
| Fatal and non-fatal coronary events | 18 (11.3) | 7 (4.8) | 0.42 (0.17–0.99) | 0.29 (0.12–0.72) | 0.35 (0.13–0.93) | 0.03 |
| Fatal and non-fatal CV events | 21 (13.2) | 8 (5.5) | 0.40 (0.18–0.91) | 0.31 (0.13–0.72) | 0.39 (0.16–0.93) | 0.03 |
| Coronary revascularizations procedures | 16 (10.1) | 4(2.8) | 0.26 (0.09–0.79) | 0.18 (0.06–0.57) | 0.23(0.07–0.77) | 0.01 |
| CHD mortality | 0 (0.0) | 0 (0.0) | – – – | – – – | – – – | – – – |
| CV mortality | 1(0.6%) | 0 (0.0) | – – – | – – – | – – – | – – – |
All models were based on complete data for 304 participants with chronic kidney disease (159 in the placebo group and 145in the lovastatin group). Relative risk (RR) derived from Cox proportional hazards models.
Percentages are cumulative incidences
Parsimonious model: age, sex, smoking, hypertension, systolic blood pressure and diabetes
Fully adjusted model: age, sex, race, body mass index, smoking, family history of premature coronary artery disease, hypertension, diabetes, systolic and diastolic blood pressure, baseline glucose, total cholesterol, low density lipoprotein-cholesterol, high density lipoprotein-cholesterol, triglycerides, and use of angiotensin converting enzyme inhibitors, beta blockers, calcium channel blockers, diuretics, alpha blockers and aspirin.
p value only for fully adjusted model
Effect of Lovastatin on Kidney Function Loss
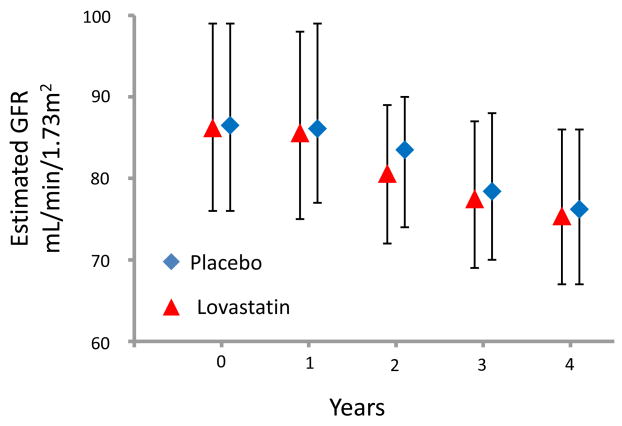
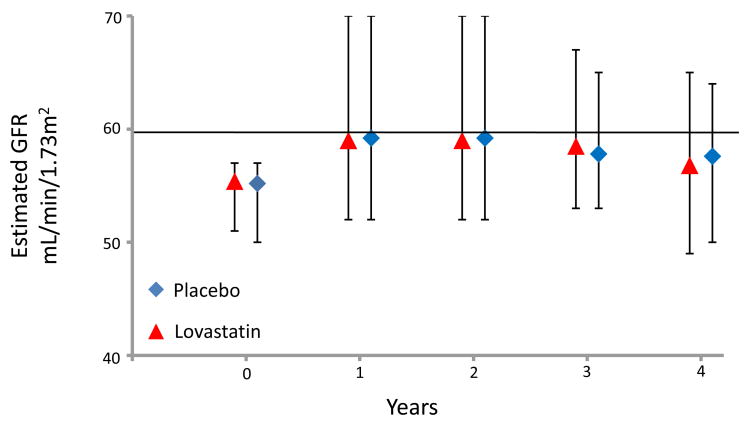
Of the 6604 participants who fulfilled criteria in the original AFCAPS/TexCAPS trial, 4994 subjects (76% of total) had data available to calculate the yearly change in estimated GFR (mL/min/1.73m2 per year) as well as to examine other renal endpoints. Overall, there was no difference in the annualized mean rate of decline in estimated GFR in those who received lovastatin compared with those individuals on placebo (−1.3 ± 0.07 vs. −1.4 ± 0.07 mL/min/1.73m2/year, respectively, p=0.1) A similar magnitude in kidney function loss in those treated with lovastatin and placebo was observed when only participants with an estimated GFR ≥60 mL/min/1.73 m2 (p=0.1) or CKD (p=0.9) at baseline were evaluated. The annual median (IQR) estimated GFR values for participants with normal kidney function and individuals with mild CKD stratified by treatment are depicted in Figure 1 and 2.
Figure 1.
Annual Median (IQR) Estimated Glomerular Filtration Rate (GFR) in Participants with Normal Kidney Function Stratified by Treatment
Figure 2.
Annual Median (IQR) Estimated Glomerular Filtration Rate (GFR) in Participants with Chronic Kidney Disease Stratified by Treatment
Lovastatin did not significantly reduce the rate of kidney function loss, defined as ≥25% decrease in estimated GFR from baseline (16% vs 14%; p=0.06). After multivariate adjustment the RR for kidney function loss was 1.10 (95% CI 0.96 to 1.28; p=0.2). Similar results were observed when the analysis was limited to participants with baseline CKD (3.9% vs 3.0%; p=0.8). Among participants without CKD at baseline, 408 (8%) had developed incident CKD at the end of follow-up. Lovastatin did not reduce the risk of incident CKD compared to placebo (8.8% vs 8.3%, p=0.6; adjusted RR 1.04, 95% CI 0.86 to 1.27, p=0.6).
Safety of Lovastatin
Overall, treatment with lovastatin was well tolerated in CKD patients. An increase of creatinine kinase to more than ten times the upper limit of normal occurred in only 1 participant with CKD in the placebo group. None of the individuals receiving lovastatin had a significant increase in creatinine kinase. No cases of rhabdomyolysis occurred in a participant with CKD in the lovastatin group. In CKD participants, elevations of more than 3 times the upper limit of normal in liver function tests were rare, and the incidence was similar in both treatment groups.
DISCUSSION
Our results show that lovastatin reduces first major cardiovascular events in persons with CKD defined as an estimated GFR <60mL/min/1.73m2. The beneficial effect of lovastatin on patients with CKD persisted after adjustment for important covariates known to be associated with CVD. These findings are of significance as patients with CKD experience a high rate of cardiovascular events.
Lovastatin did not result in a decrease in cardiovascular or coronary heart disease (CHD) mortality. However, the number of deaths in AFCAPS/TexCAPS was low (157 total deaths; 42 cardiovascular deaths, of which 26 were CHD deaths) and the study was not powered to detect treatment differences in these low frequency endpoints. Lovastatin also did not result in a decrease in myocardial infarctions or unstable angina. Since only a small number of these events (8 myocardial infarctions and 9 cases of unstable angina) occurred in patients with CKD, it is difficult to detect differences between the two treatment groups. Although results were not statistically significant, non-significant incidence of unstable angina, fatal and non-fatal myocardial infarction and sudden death events was quantitatively lower (3.4% vs. 7.5%) with the use of lovastatin when compare to placebo in those participants with a decrease in kidney function. Furthermore, lovastatin did not result in any renoprotective effects as it did not decrease: (1) annualized mean rate of decline in estimated GFR; (2) the frequency of a ≥25% decline from baseline in kidney function in those with and without CKD at the initiation of the study and (3) the rate of incident cases of CKD in those with normal or near normal kidney function at baseline.
To our knowledge, this is the first primary prevention study to demonstrate a significant reduction in cardiovascular events from lovastatin therapy in persons with CKD. Statins have also been shown to be effective for secondary prevention of CVD in participants with moderate or more advanced CKD. Tonelli et al9 performed a post-hoc analysis of the Pravastatin Pooling Project (PPP), a database containing results from 3 randomized (1 primary and 2 secondary prevention) controlled trials of pravastatin verses placebo. Among the 4,491 participants with moderate CKD (defined as an estimated GFR between 30 and 59 mL/min/1.73m2) and with or at risk of CHD, pravastatin showed a 23% reduction in the incidence of the composite outcome of time to myocardial infarction, coronary death, or coronary revascularization. Of note, the effects of pravastatin on primary prevention were not thoroughly examined in this post-hoc analysis.
In our study, lovastatin did not have any renoprotective effects. Studies investigating the renoprotective effects of statins have mainly included patients with prevalent CVD and have yielded contradictory results. In the Heart Protection Study, the overall decline of estimated GFR was smaller in type 2 diabetics receiving simvastatin 40 mg per day compared with placebo.21 In another post-hoc analysis of the PPP,12 participants with moderate CKD treated with pravastatin had a 34% reduction in the rate of kidney function loss, but again the absolute reduction in the rate of loss was small. Moreover, similar to our findings with lovastatin, pravastatin did not reduce the frequency of a ≥25% decline in kidney function in patients with and without moderate CKD or the risk of developing incident CKD. In this analysis, the effects of pravastatin on kidney function loss in the sub-group of participants without prevalent CVD was not examined.
A recent meta-analysis of 27 placebo-controlled trials with 39,704 participants suggested that, compared with placebo, statins reduce the rate of kidney function loss by 1.22 mL/min/1.73m2/year.7 The effect of statins on kidney function loss was more pronounced in participants with prevalent CVD but was non significant for studies of participants with diabetic, or hypertensive kidney disease. More recently, Strippoli et al 8 performed a meta-analysis of 50 randomized and quasi-randomized trials of statins in patients with all stages of kidney disease (n=30,144). The authors found that statins had uncertain renoprotective effects. Thus, statins may decrease the rate of kidney function decline mainly in patients with prevalent CVD but the clinical significance of this effect is unclear.
Many of the risk factors for the development of CKD are also risk factors for the development of CVD such as age, diabetes and hypertension. Given the shared risk factors it is plausible that the pathogenesis of CVD and CKD are very similar and modification of these risk factors could result in prevention. However, as seen in our study, treatment of dyslipidemia resulted in primary prevention of CVD but did not result in primary prevention of CKD or kidney function loss suggesting that the pathogenesis of the two diseases are indeed different.
Dyslipidemia is a significant risk factor for the development of CVD, but its role in CKD is not as clear. In animal studies, dietary cholesterol loading induces mesangial proliferation, glomerulosclerosis and modest proteinuria in animals with normal renal function.22 The Physician’s Health Study23 evaluated the development of renal dysfunction in 4483 men without baseline CKD over 13 years and found that elevated LDL-C levels, total cholesterol levels, and lower HDL-C levels were significantly associated with the development of new kidney dysfunction. However, data on proteinuria was not included in the study, which is an important potential confounder.
Our study has several limitations and strengths that should be considered. First, we were unable to determine the cause and duration of kidney disease and did not have information regarding microalbuminuria or proteinuria. Second, we defined CKD based upon estimated GFR rather than direct measurements of GFR; but direct measurements are rarely used in clinical practice. Third, there were very few patients with advanced kidney disease (stage 4). Hence, the results of this study should not be extrapolated to advance stages of CKD. Fourth, hemodynamic changes that could have occurred from changes in dosage of antihypertensive medications that could potentially affect kidney function were not measured. Fifth, females and minorities were under-represented in the AFCAPS/TexCAPS trial. Finally, because this is a post-hoc analysis, our results should be interpreted with some degree of caution as evaluation of the potential renoprotective effect of lovastatin was not the primary end-point of the AFCAPS/TexCAPS.
Notwithstanding these limitations, our study also has several strengths. First, this is the first study to demonstrate the effectiveness of statins for the primary prevention of cardiovascular events in patients with CKD. Other strengths include the large cohort size, comprehensive dataset, and ability to link demographic and clinical factors with patient outcomes. In addition, all laboratory measurements, including serum creatinine, were performed at a central laboratory. Finally, the AFCAPS/TexCAPS cohort has been shown to have similar demographic and baseline-lipid-level characteristics on average as NHANES III, and therefore of the non-institutionalized US adult patient population19.
In conclusion, the benefit of lovastatin for primary cardiovascular prevention and the low rate of adverse events seen in the present study suggest that physicians should consider increasing the use of statins in the CKD population to reduce the burden of CVD. At this time, the use of statins solely for primary renoprotection appears to be ineffective. However, randomized controlled trials are required to definitely resolve this issue and are currently ongoing.
Acknowledgments
Support: This study was funded by an Amgen fellowship grant.
Financial Disclosure: Mr. Cook is an employee of Merck & Co., Inc, which markets lovastatin. The remaining authors report no relevant financial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 2.Menon V, Sarnak MJ. The epidemiology of chronic kidney disease stages 1 to 4 and cardiovascular disease: a high-risk combination. Am J Kidney Dis. 2005;45:223–232. doi: 10.1053/j.ajkd.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 3.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 4.Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med. 2004;164:659–663. doi: 10.1001/archinte.164.6.659. [DOI] [PubMed] [Google Scholar]
- 5.Diamond JR. Analogous pathobiologic mechanisms in glomerulosclerosis and atherosclerosis. Kidney Int Suppl. 1991;31:S29–S34. [PubMed] [Google Scholar]
- 6.Fried LF. Effects of HMG-CoA reductase inhibitors (statins) on progression of kidney disease. Kidney Int. 2008;74:571–576. doi: 10.1038/ki.2008.231. [DOI] [PubMed] [Google Scholar]
- 7.Sandhu S, Wiebe N, Fried LF, Tonelli M. Statins for improving renal outcomes: a meta-analysis. J Am Soc Nephrol. 2006;17:2006–2016. doi: 10.1681/ASN.2006010012. [DOI] [PubMed] [Google Scholar]
- 8.Strippoli GF, Navaneethan SD, Johnson DW, et al. Effects of statins in patients with chronic kidney disease: meta-analysis and meta-regression of randomised controlled trials. BMJ. 2008;336:645–651. doi: 10.1136/bmj.39472.580984.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tonelli M, Isles C, Curhan GC, et al. Effect of pravastatin on cardiovascular events in people with chronic kidney disease. Circulation. 2004;110:1557–1563. doi: 10.1161/01.CIR.0000143892.84582.60. [DOI] [PubMed] [Google Scholar]
- 10.Tonelli M, Moyé L, Sacks FM, Kiberd B, Curhan G Cholesterol and Recurrent Events (CARE) Trial Investigators. Pravastatin for secondary prevention of cardiovascular events in persons with mild chronic renal insufficiency. Ann Intern Med. 2003;138:98–104. doi: 10.7326/0003-4819-138-2-200301210-00010. [DOI] [PubMed] [Google Scholar]
- 11.Tonelli M, Moyé L, Sacks FM, Cole T, Curhan GC Cholesterol and Recurrent Events Trial Investigators. Effect of pravastatin on loss of renal function in people with m oderate chronic renal insufficiency and cardiovascular disease. J Am Soc Nephrol. 2003;14:1605–1613. doi: 10.1097/01.asn.0000068461.45784.2f. [DOI] [PubMed] [Google Scholar]
- 12.Tonelli M, Isles C, Craven T, et al. Effect of pravastatin on rate of kidney function loss in people with or at risk for coronary disease. Circulation. 2005;112:171–178. doi: 10.1161/CIRCULATIONAHA.104.517565. [DOI] [PubMed] [Google Scholar]
- 13.Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;279:1615–1622. doi: 10.1001/jama.279.20.1615. [DOI] [PubMed] [Google Scholar]
- 14.Downs JR, Beere PA, Whitney E, et al. Design & rationale of the Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS) Am J Cardiol. 1997;80:287–293. doi: 10.1016/s0002-9149(97)00347-0. [DOI] [PubMed] [Google Scholar]
- 15.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 16.Manjunath G, Tighiouart H, Coresh J, et al. Level of kidney function as a risk factor for cardiovascular outcomes in the elderly. Kidney Int. 2003;63:1121–1129. doi: 10.1046/j.1523-1755.2003.00838.x. [DOI] [PubMed] [Google Scholar]
- 17.Muntner P, He J, Astor BC, Folsom AR, Coresh J. Traditional and nontraditional risk factors predict coronary heart disease in chronic kidney disease: results from the atherosclerosis risk in communities study. J Am Soc Nephrol. 2005;16:529–538. doi: 10.1681/ASN.2004080656. [DOI] [PubMed] [Google Scholar]
- 18.Weiner DE, Tighiouart H, Amin MG, et al. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol. 2004;15:1307–1315. doi: 10.1097/01.asn.0000123691.46138.e2. [DOI] [PubMed] [Google Scholar]
- 19.Clearfield M, Whitney EJ, Weis S, et al. Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS): baseline characteristics and comparison with USA population. J Cardiovasc Risk. 2000;7:125–133. doi: 10.1177/204748730000700207. [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, Coresh J, Balk E, et al. National Kidney Foundation. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 21.Collins R, Armitage J, Parish S, Sleigh P, Peto R. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003;361:2005–2016. doi: 10.1016/s0140-6736(03)13636-7. [DOI] [PubMed] [Google Scholar]
- 22.Kasiske BL, O’Donnell MP, Schmitz PG, Kim Y, Keane WF. Renal injury of diet-induced hypercholesterolemia in rats. Kidney Int. 1990;37:880–891. doi: 10.1038/ki.1990.62. [DOI] [PubMed] [Google Scholar]
- 23.Schaeffner ES, Kurth T, Curhan GC, et al. Cholesterol and the risk of renal dysfunction in apparently healthy men. J Am Soc Nephrol. 2003;14:2084–2091. doi: 10.1681/ASN.V1482084. [DOI] [PubMed] [Google Scholar]