Abstract
Objective
To examine, in a community-based sample, the use of prescription drugs for lower urinary tract symptoms/benign prostatic hyperplasia (LUTS/BPH), overactive bladder, erectile dysfunction, urinary incontinence, and painful bladder syndrome; and to determine whether the use of recommended medications varied by sociodemographics, symptom severity, access to care, and other factors.
Subjects and methods
In a cross-sectional analysis of data obtained from 5503 men and women residents participating in the Boston Area Community Health Survey of Boston, MA, urological symptoms were ascertained by in-person interviews conducted during 2002–2005, using validated symptom scales. Medication use in the past 4 weeks was captured using a combination of drug-inventory methods and self-report.
Results
Compared to the prevalence of symptoms, the prevalence of use of medications for urological conditions was very low among men and women. The highest prevalence of use was among men with moderate-to-severe LUTS/BPH symptoms, where 9.6% used recommended drugs. Use of medications did not vary consistently by race/ethnicity or socioeconomic status, but was often associated with symptom severity. More frequent and more recent use of medical care was also associated with greater use of urological medications.
Conclusions
Only a small proportion of community-dwelling men and women with urological symptoms are receiving recommended effective drug treatments for urological conditions. While not all persons are candidates for drug treatment, our results suggest that there is a substantial unmet need in the general population.
Keywords: urological diseases, healthcare disparities, prescription drugs, clinical practice guideline, pharmacoepidemiology
Introduction
The burden of urological diseases in America is immense, both financially and personally [1]. While population-based administrative data might provide estimates of disease prevalence and patterns of care [2], poor access to care and shorter visits with overtaxed healthcare providers might result in an underestimation of certain medical conditions. The concept of the ‘illness iceberg’ describes the distribution of disease in the general population; only a small proportion is above the surface, presented to and diagnosed by physicians, while most remains below the surface [3]. Despite the high prevalence and adverse impact on quality of life of erectile dysfunction (ED), LUTS/BPH, overactive bladder (OAB), urinary incontinence (UI) and painful bladder syndrome (PBlS), those affected might not present to the healthcare system, and/or symptoms might not be elicited by healthcare providers. Thus, many do not receive symptom relief from proven pharmacotherapies. In addition, many urological conditions become more prevalent with age, adding to the comorbidity burden of the growing elderly population in the USA.
Our objectives were to explore the patterns of use of prescription drugs for urological conditions among community-dwelling men and women, considering drugs shown to be effective in randomized clinical trials and recommended in relevant clinical guidelines. We considered symptoms of LUTS/BPH and ED among men, and UI, OAB and PBlS among men and women. We first determined what medications were recommended for urological conditions at the time of our data collection. Subsequently, we determined whether the use of recommended medications varied by: (i) sociodemographics; (ii) symptom severity; (iii) inclination to seek care; (iv) health system factors, including access to care; and (v) the presence of comorbidities.
Subjects and methods
The Boston Area Community Health (BACH) Survey is supported by the USA National Institutes of Health (National Institute of Diabetes and Digestive and Kidney Diseases) and is a population-based, epidemiological cohort study conducted among 5503 men and women aged 30–79 years and residing in Boston, MA. A multistage, stratified-cluster sampling design was used to recruit similar numbers of persons in pre-specified groups defined according to age (30–39, 40–49, 50–59 and 60–79 years), race and ethnic groups (black, Hispanic, white) and gender. Baseline data were collected between April 2002 and June 2005 during a 2-h interview conducted by a trained, bilingual interviewer, after obtaining written informed consent. Interviews for 63.3% of eligible persons were completed, with a resulting study population of 2301 men and 3202 women, comprised of 1767 black, 1877 Hispanic and 1859 white participants. Information collected included comorbidities, medication use, anthropometrics, income, education and health-seeking behaviour. All protocols and procedures were approved by New England Research Institutes’ Institutional Review Board; further details were reported previously [4].
Because of the cross-sectional nature of the data used in this analysis, we estimated the prevalence of medication use: (i) among all BACH participants regardless of reported symptoms (presuming a curative treatment among those persons who were symptomatic before our data collection); and (ii) among those currently reporting symptoms. Participants were asked to gather all medications used in the past 4 weeks for the interviewer to record the label information. In addition, participants were asked whether they were taking drugs for specific indications, such as diabetes. Medication labels and/or responses were coded using the Slone Drug Dictionary [5], which classifies drugs using a modification of the American Hospital Formulary Service Drug Pharmacologic Therapeutic Classification System [6]. To define appropriate use of medications for urological conditions, we searched for medications recommended from published USA clinical guidelines available before or during our baseline data collection period (2002–2005), as presented in Table 1 [7–34], along with the pivotal trials supporting efficacy. Use of any urological medication in Table 1 defined a medication user under drug treatment for that urological condition. We combined treatments for UI and OAB because of the overlap in use of these medications for the two indications.
Table 1.
Pharmacological treatments, supportive clinical trials, and USA practice guidelines for lower urinary tract conditions and ED
| Condition | Pivotal trials supporting treatment efficacy* |
Relevant USA clinical practice guidelines that include drug treatments available during BACH baseline data collection (April 2002–June 2005)† |
|---|---|---|
| BPH | Doxazosin [7,8] | AUA guideline [10] ‘Alfuzosin, doxazosin, tamsulosin, and terazosin are appropriate treatment options for patients with LUTS secondary to BPH.’ |
| Terazosin [9] | AUA guideline [10] | |
| Alfuzosin [11,12] | AUA guideline [10] | |
| Tamsulosin [13] | AUA guideline [10] | |
| Prazosin [14,15] | AUA guideline [10] ‘Data are insufficient to support a recommendation for the use of prazosin…’ |
|
| Dutasteride [16,17] | AUA guideline [10] | |
| ‘The 5α-reductase inhibitors finasteride and dutasteride are appropriate and effective treatments for patients with LUTS associated with demonstrable prostatic enlargement.’ |
||
| Finasteride [18,19] | AUA guideline [10] | |
| ED | Sildenafil [20] | AUA guideline [21]. ‘Oral PDE 5 inhibitors, unless contraindicated, should be offered as a first-line of therapy for erectile dysfunction (based on review of data and Panel consensus).’ |
| Vardenafil [22] | AUA guideline [21] | |
| Tadalafil [23] | AUA guideline [21] | |
| Alprostadil [24–26], | ||
| papaverine | AUA guideline [21] ‘Recommendation: Patients who have failed a trial with PDE5 inhibitor therapy should be informed of the benefits and risks of other therapies, including the use of a different PDE5 inhibitor, alprostadil intraurethral suppositories, intracavernous drug injection, vacuum constriction devices, and penile prostheses (based on Panel consensus).’ |
|
| OAB | Oxybutynin [27,28] | No clinical guidelines found for data collection period. |
| Tolterodine tartrate [27,28] | No clinical guidelines found for data collection period. | |
| UI | Oxybutynin [29] | Agency for Health Care Policy and Research [30]‡ ‘Anticholinergic agents are the first-line pharmacologic therapy for patients with detrusor instability (DI)’ |
| Propantheline bromide [31] | Agency for Health Care Policy and Research [30]; ‘Propantheline is the second- line anticholinergic agent in the treatment of patients with DI who can tolerate the full dosage.’ |
|
| Hyoscyamine sulphate | Agency for Health Care Policy and Research [30]; ‘Hyoscyamine and other oral anticholinergics are known to be used in clinical practice in the treatment of DI; however, no scientific literature that met the panel’s criteria addressed the use of these agents for patients with UI. The panel therefore is not making recommendations regarding these drugs.’ |
|
| Flavoxate hydrochloride | Agency for Health Care Policy and Research [30]; Trials summarized; ‘Flavoxate is not recommended for the treatment of patients with DI.’ |
|
| PBlS/IC | Pentosan polysulphate [32] | No guidelines from medical organizations found. Drug reviewed in [33]§ |
| Amitriptyline [34] | Drug reviewed in [33] |
Trials listed are cited in review papers as pivotal and/or that the Food and Drug Administration (FDA) used for basis of approval. Trials were not found for all drugs. Some UI/OAB treatments used currently were FDA-approved during or after the baseline BACH data collection period and thus are not included above (solifenacin succinate; trospium chloride, darifenacin).
Treatment guidelines cited are specific to the USA and published before or during the baseline BACH data collection period, and might not reflect current treatment guidelines or practices. The recommendations without the supporting text have been excerpted from the guideline. For full context, please refer to the original guideline document.
Other medications listed in this guideline were considered nonspecific for UI and were not included in our treatment estimate (e.g. calcium-channel blockers, NSAIDS) or no BACH participants used them (dicyclomine hydrochloride).
Other medications listed in this guideline were considered nonspecific for PBlS (e.g. corticosteroids) and were not included in our treatment estimate.
ED was defined using the five-item short-form International Index of Erectile Function (IIEF-5), a self-administered, validated questionnaire [35]. Lower IIEF-5 scores indicate poorer erectile function (range 5–25). We defined no ED as scores of 22–25, mild ED as 17–21, and moderate/severe ED as 5–16. LUTS/BPH was defined using the IPSS, equivalent to the AUA Symptom Index that measures seven urological symptoms typically associated with BPH [36]. Higher scores indicate more symptoms (range 0–35). No LUTS/BPH was defined as 0, mild LUTS/BPH as 1–7, and moderate/severe LUTS/BPH as 8–35. UI was defined with two questions on involuntary urine leakage: (i) ‘Many people complain that they leak urine (wet themselves) or have accidents. In the last 12 months, have you leaked even a small amount of urine?’ If yes, respondents were asked how often, with possible responses of < 1/month, 1+/month, 1+/week, and daily. No UI was defined as no leakage in the last 12 months, mild UI was leakage < 1/month or 1+/month, and moderate/severe UI was leakage 1+/week or daily.
A respondent had no OAB if they reported none of the following symptoms: ‘During the last month, how often have you had: (i) to urinate again < 2 h after you finished urinating; (ii) difficulty postponing urination; (iii) frequent urination during the day; (iv) a strong urge or pressure to urinate immediately with no or little warning?’; (v) ‘In the last 7 days, how many times did you feel a strong urge or pressure that signalled the need to urinate immediately, whether or not you urinated or leaked urine?’; and (vi) ‘In the last 7 days, on average, how many times have you had to go to the bathroom to empty your bladder during the day?’ (a response of ≤7). Moderate/severe OAB was defined as a response of ‘fairly often’, ‘usually’, ‘almost always’ to question (i) or question (iii), or a response of ≥8 to question (vi) AND a response of ‘fairly often’, ‘usually’, ‘almost always’ to question (ii) or question (iv) or a response of ‘several times’, ‘many times’, ‘everyday’ to question (v). Otherwise, respondents had mild OAB.
Those responding ‘fairly often’, ‘usually’, or ‘almost always’ to one of the following: ‘In the last month, how often have you had (vii) pain increasing as the bladder fills or (viii) pain relieved by urination?’ with a reported duration of ≥3 months were defined as having moderate/severe PBlS. No PBlS was defined as a response of ‘I do not have the symptom’ to both questions. Otherwise, respondents had mild PBlS.
Symptom interference with activities of daily living was assessed using the validated Epstein scale [37]. An interference score of 0 was assigned for a reply of ‘none of the time’ while a score of 4 was assigned to ‘all of the time’ for seven questions on usual activities (resulting possible range, 0–28).
Race/ethnic groups were self-identified by participants and classified using the Office of Management and Budget categories, and were considered in order to measure health disparities [38]. A socioeconomic status (SES) variable was constructed as function of standardized income and education variables for the North-eastern USA and reclassified into lower, middle and high [39]. In addition, we asked participants four questions about economic hardship, as follows: ‘Are you having trouble paying for (health or medical care, medications)/ (transportation)/ (housing)/(food)?’ (yes vs no). Depressive symptoms were considered present among participants with at least five of eight symptoms on the abridged Center for Epidemiologic Studies Depression Scale [40]. Other comorbidities were based on self-report of healthcare provider diagnoses of diabetes (type 1 or type 2, hereinafter, ‘diabetes’), high blood pressure, heart attack, angina pectoris, congestive heart failure, or coronary artery bypass or angioplasty (any of these, hereinafter, ‘heart disease’). In addition to health insurance status (public only, private, none), variables related to healthcare access and care-seeking were created from the queries: ‘How many times in the last year did you go see a health care provider for any reason?’; ‘Do you go for regular care?’ (yes/no), and time elapsed since the last visit. We also asked ‘How important to you would be for you to seek medical care for…?’ considering symptoms of pain or burning in bladder, chronic pelvic pain, leaking urine, repeat urination within 2 h, and difficulty with erections. Responses indicating an inclination to seek care were recorded using a five-item scale, with higher scores indicating greater importance attributed to care-seeking.
There were 2301 men and 3202 women who participated in the BACH Survey. Due to the complex two-stage cluster sampling design, all analyses were weighted inversely proportionally to the probability of selection, and were conducted using 9.0.1 of SUDAAN [41,42]. Weighting ensures that estimates can be interpreted as representative of the community-dwelling Boston population. To test differences in stratified analyses, a chi-square test was used for categorical covariates and a Wald-type F-test from linear regression for continuous variables. Missing data were replaced by plausible values using multiple imputation [43]; < 1% of data were missing for most variables. For income (used to construct SES) 3%, 4% and 11% were missing for white, black and Hispanic subjects, respectively. Medication variables were not imputed.
Results
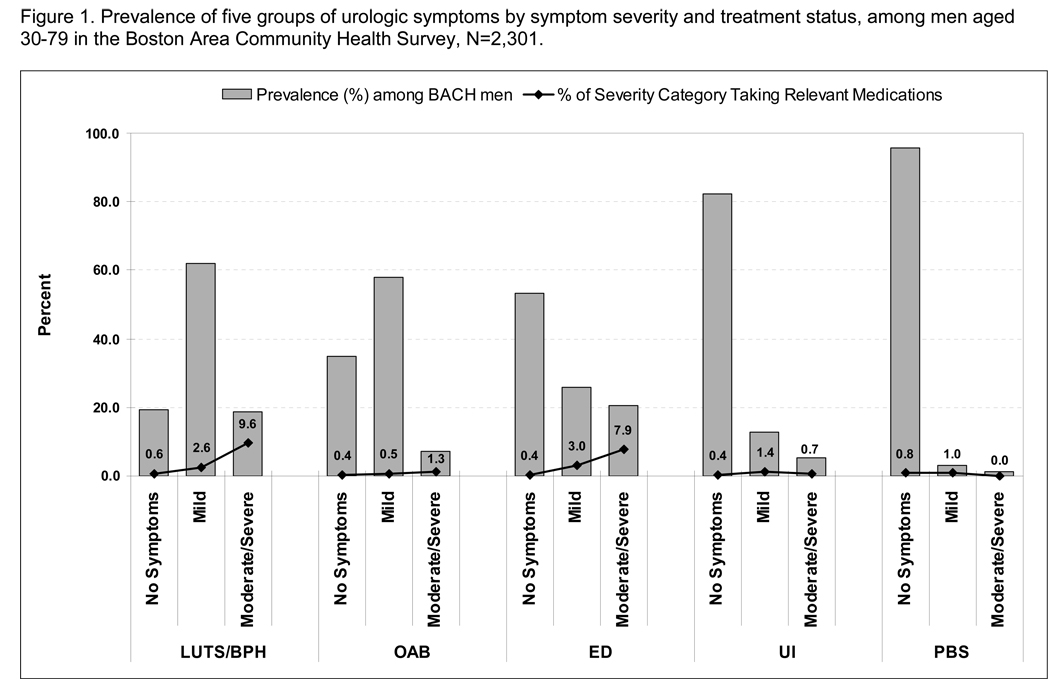
The overall prevalence of conditions and drug treatment by gender and severity are depicted in Fig 1a,b. For men and women, the proportion using medications (shown in the dotted line) was low for all conditions and severity categories, even moderate to severe. For example, while 18.7% of BACH men had moderate to severe LUTS/BPH symptoms, only 9.6% of this symptom group were taking relevant medications (doxazosin, terazosin, alfuzosin, tamsulosin, prazosin, or finasteride) while 90.4% were untreated with drugs (Fig. 1a). Of the moderate/severe ED group, ≈8% were taking relevant medications, and for both ED and LUTS/BPH there was a trend towards increasing medication use with increasing severity. For OAB, UI and PBlS, the proportion taking relevant medications was < 1% for most symptom severity groups.
Fig. 1.
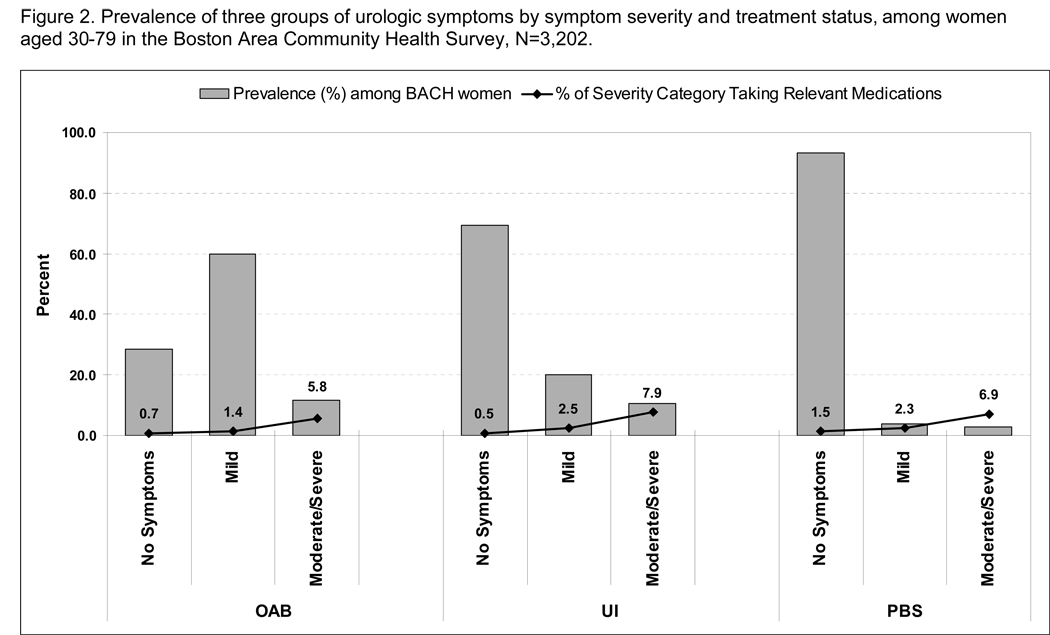
Prevalence of five (a) and three (b) groups of urological symptoms by symptom severity and treatment status, among: a, 2301 men aged 30–79 years; and b, 3202 women aged 30–79 years in the BACH Survey.
Similarly, among women, the prevalence of use of medications was also quite low, in contrast to the prevalence of symptoms, but increased with severity (Fig. 1b). For moderate to severe OAB and moderate to severe PBlS, ≈6% and ≈7% of women, respectively, who had such symptoms were using relevant medications, while 8% of those with moderate to severe UI were. The prevalence of drug treatment for mild symptoms was much lower for UI, OAB and PBlS.
Among all men, the use of medications for LUTS/BPH and ED increased significantly with increasing age, while older groups also used more UI/OAB medications (Table 2). Men of upper SES were significantly more likely to be receiving drug treatment for ED, but there were no significant differences in medication use by race/ethnicity for any condition (although there was a pattern of greater use of drugs among whites compared to minorities for LUTS/BPH and ED). Among all women, those in older groups were more likely to be under drug treatment for UI/OAB, while for PBlS, women aged 50–59 years were most likely to be taking relevant medications. There were significant variations in medication use by race and SES for PBlS; Hispanic women and those of low SES were most likely to receive relevant medications. Of the three race/ethnicity groups, Hispanic women were least likely to use drugs for UI/OAB.
Table 2.
The prevalence of use (%) of medications for urological conditions by demographics and SES among 5503 men and women in the BACH Survey
| Men using | Women using | |||||
|---|---|---|---|---|---|---|
| Factor | UI/OAB druga | PBlS drugb | LUTS/BPH drugc | ED drugd | UI/OAB druga | PBlS drugb |
| Overall | 0.54 | 0.79 | 3.52 | 2.61 | 1.70 | 1.65 |
| Age, years | ||||||
| 30–39 | 0.49 | 0.26 | 0.70 | 1.62 | 0.05 | 0.44 |
| 40–49 | 0.00 | 0.58 | 1.12 | 0.94 | 1.62 | 1.35 |
| 50–59 | 0.05 | 1.79 | 5.27 | 5.69 | 2.80 | 4.04 |
| 60–69 | 1.29 | 0.61 | 9.62 | 3.94 | 3.26 | 1.97 |
| 70–79 | 2.55 | 2.01 | 12.07 | 3.92 | 3.26 | 1.55 |
| P | 0.078 | 0.275 | < 0.001 | 0.027 | < 0.001 | 0.004 |
| Race/ethnicity | ||||||
| Black | 0.68 | 0.35 | 2.84 | 1.95 | 1.39 | 2.01 |
| Hispanic | 0.39 | 1.20 | 2.28 | 1.87 | 0.61 | 3.08 |
| White | 0.51 | 0.88 | 4.06 | 3.04 | 2.12 | 1.13 |
| P | 0.834 | 0.214 | 0.140 | 0.559 | 0.028 | 0.040 |
| SES | ||||||
| Lower | 0.30 | 1.73 | 4.72 | 0.79 | 2.05 | 3.14 |
| Middle | 0.36 | 0.30 | 3.25 | 2.09 | 1.68 | 1.08 |
| Upper | 1.07 | 0.84 | 2.94 | 5.24 | 1.28 | 0.81 |
| P | 0.533 | 0.065 | 0.279 | 0.008 | 0.625 | 0.035 |
P values from overall chi-square test. Medications were:
oxybutynin, tolterodine tartrate, propantheline bromide, hyoscyamine sulphate, and flavoxate hydrochloride
pentosan polysulphate and amitriptyline
alfuzosin, doxazosin, tamsulosin, terazosin, and finasteride (no participant took dutasteride)
sildenafil, tadalafil, alprostadil, vardenafil, papaverine.
There was variation among men and women in the proportion using medications for urological conditions by health-insurance status; those with public insurance generally had a higher prevalence of drug treatment than those with no insurance or private insurance, except for ED, where drug treatment was highest among those with private insurance (Table 3). For men and women, the use of medications was usually higher among those seeking regular care, who saw their healthcare provider more than five times in the past year, and who saw their provider within the last 6 months. There was not a significantly lower prevalence of medication use among those reporting trouble paying for healthcare and/or medications.
Table 3.
The prevalence of use (%) of medications for urological conditions by healthcare system factors among 5503 men and women in the BACH Survey
| Men using | Women using | |||||
|---|---|---|---|---|---|---|
| Factor | UI/OAB druga | PBlS drugb | LUTS/BPH drugc | ED drugd | UI/OAB druga | PBlS drugb |
| Healthcare system factors | ||||||
| Health insurance | ||||||
| Private | 0.53 | 0.40 | 3.53 | 3.33 | 1.69 | 1.08 |
| Public | 0.31 | 2.49 | 5.57 | 1.47 | 2.03 | 2.96 |
| None | 0.84 | 0.51 | 0.98 | 0.74 | 0.67 | 1.27 |
| P | 0.624 | 0.070 | < 0.001 | 0.062 | 0.125 | 0.106 |
| Regular care | ||||||
| yes | 0.70 | 0.94 | 4.14 | 2.57 | 1.75 | 1.76 |
| no | 0.00 | 0.30 | 1.46 | 2.75 | 0.96 | 0.00 |
| P | 0.012 | 0.083 | 0.008 | 0.918 | 0.436 | < 0.001 |
| N healthcare provider visits in last year | ||||||
| 0 | 0.00 | 0.32 | 0.00 | 0.00 | 0.00 | 0.00 |
| 1–4 | 0.12 | 0.34 | 2.78 | 2.11 | 0.76 | 0.66 |
| 5–9 | 0.34 | 1.43 | 5.08 | 3.80 | 2.88 | 1.59 |
| ≥10 | 1.81 | 1.46 | 5.39 | 3.86 | 2.36 | 3.41 |
| P | 0.040 | 0.263 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| When did you last see a healthcare provider? | ||||||
| < 6 months | 0.75 | 1.08 | 4.82 | 3.46 | 1.96 | 1.98 |
| 6–12 months | 0.07 | 0.00 | 0.95 | 1.30 | 0.53 | 0.00 |
| 1–2 years | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 2–5 years | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| ≥5 years | 0.00 | 1.55 | 0.00 | 0.00 | 0.00 | 0.00 |
| P | 0.154 | 0.025 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| Reported trouble paying for healthcare/medications | ||||||
| Yes | 0.77 | 0.37 | 2.74 | 2.83 | 1.68 | 2.99 |
| No | 0.49 | 0.88 | 3.68 | 2.57 | 1.71 | 1.35 |
| P | 0.560 | 0.178 | 0.306 | 0.844 | 0.970 | 0.072 |
P values from overall chi-square test. Medications were:
oxybutynin, tolterodine tartrate, propantheline bromide, hyoscyamine sulphate, and flavoxate hydrochloride
pentosan polysulphate and amitriptyline
alfuzosin, doxazosin, tamsulosin, terazosin, and finasteride (no participant took dutasteride)
sildenafil, tadalafil, alprostadil, vardenafil, papaverine.
Men with heart disease and high blood pressure were significantly more likely to be treated with drugs for ED than were those without these comorbidities, and the prevalence of ED treatment was also higher among men with diabetes (Table 4). There was a general pattern of more comorbid conditions among men using medications for UI/OAB or PBlS, while the prevalence of use of medications for LUTS/BPH was significantly higher among those with diabetes, heart disease or high blood pressure than in those without these conditions. Among women, the prevalence of use of medications for UI/OAB or PBlS was higher among those with diabetes, heart disease and high blood pressure (although differences were not always statistically significant), while women using medications for PBlS were significantly more likely to have depressive symptoms than non-users.
Table 4.
The prevalence of the use (%) of medications for urological conditions by the presence of comorbidity among 5503 men and women in the BACH Survey
| Men using | Women using | |||||
|---|---|---|---|---|---|---|
| Factor | UI/OAB druga | PBlS drugb | LUTS/BPH drugc | ED drugd | UI/OAB druga | PBlS drugb |
| Diabetes | ||||||
| Yes | 0.57 | 2.09 | 7.38 | 8.01 | 3.43 | 3.11 |
| No | 0.53 | 0.66 | 3.13 | 2.06 | 1.52 | 1.50 |
| P | 0.942 | 0.178 | 0.025 | 0.107 | 0.122 | 0.114 |
| Heart disease | ||||||
| Yes | 0.28 | 1.26 | 7.70 | 7.65 | 4.19 | 1.93 |
| No | 0.56 | 0.74 | 3.05 | 2.04 | 1.49 | 1.63 |
| P | 0.318 | 0.506 | 0.011 | 0.026 | 0.130 | 0.741 |
| Depressive symptoms | ||||||
| Yes | 1.05 | 1.86 | 4.22 | 3.18 | 1.96 | 2.97 |
| No | 0.45 | 0.62 | 3.41 | 2.52 | 1.63 | 1.32 |
| P | 0.370 | 0.166 | 0.572 | 0.767 | 0.627 | 0.042 |
| High blood pressure | ||||||
| Yes | 0.93 | 1.34 | 6.57 | 5.65 | 2.69 | 3.65 |
| No | 0.39 | 0.60 | 2.45 | 1.54 | 1.31 | 0.86 |
| P | 0.312 | 0.192 | < 0.001 | 0.014 | 0.053 | 0.002 |
P values from overall chi-square test. Medications were:
oxybutynin, tolterodine tartrate, propantheline bromide, hyoscyamine sulphate, and flavoxate hydrochloride
pentosan polysulphate and amitriptyline
alfuzosin, doxazosin, tamsulosin, terazosin, and finasteride (no participant took dutasteride)
sildenafil, tadalafil, alprostadil, vardenafil, papaverine.
We examined whether or persons taking medications had different scores indicating a different inclination to seek care for five types of urological symptoms, and for differences in symptom interference scores, our measure of symptom bother. There were no differences in mean scores for care-seeking by prevalence of medication use among men or women for any symptom except urinary frequency, where scores were significantly higher among women who were treated for UI or OAB (Table 5). However, symptom interference scores were related to the prevalence of medication use. Except for PBlS and ED among men, there was a higher prevalence of urological drug use among those who reported that they were bothered by their symptoms. Differences were generally more pronounced among women than men in this comparison.
Table 5.
The mean scores for inclination to seek care and symptom interference by medication treatment status for urological conditions among 5503 men and women in the BACH Survey
| Men, drug for | Women, drug for | |||||||
|---|---|---|---|---|---|---|---|---|
| Factor | UIa | OABa | PBlSb | BPHc | EDd | UIe | OABa | PBlSb |
| Inclination to seek caree | ||||||||
| Pain or burning in your bladder | ||||||||
| Taking PBlS drug | 4.60 | 4.70 | ||||||
| Not taking PBlS drug | 4.54 | 4.60 | ||||||
| P | 0.685 | 0.186 | ||||||
| Chronic pain in your pelvic area | ||||||||
| Taking PBlS drug | 4.28 | 4.71 | ||||||
| Not taking PBlS drug | 4.51 | 4.66 | ||||||
| P | 0.311 | 0.506 | ||||||
| Needing to wear a pad or dealing with wetness from leaking urine | ||||||||
| Taking UI/OAB drug | 4.16 | 4.47 | ||||||
| Not taking UI/OAB drug | 4.47 | 4.49 | ||||||
| P | 0.420 | 0.830 | ||||||
| Finding you need to urinate < 2 h after you finished urinating | ||||||||
| Taking UI/OAB drug | 3.42 | 3.42 | 3.58 | 4.17 | 4.17 | |||
| Not taking UI/OAB drug | 3.48 | 3.48 | 3.47 | 3.56 | 3.56 | |||
| P | 0.761 | 0.761 | 0.405 | < 0.001 | < 0.001 | |||
| Difficulty obtaining or maintaining an erection | ||||||||
| Taking ED med | 4.28 | |||||||
| Not taking ED med | 4.19 | |||||||
| P | 0.470 | |||||||
| Symptom interferencef | ||||||||
| Taking med | 4.60 | 4.60 | 2.12 | 3.50 | 1.35 | 7.40 | 7.40 | 7.88 |
| Not taking med | 1.32 | 1.32 | 1.33 | 1.26 | 1.34 | 2.14 | 2.14 | 2.13 |
| P | 0.098 | 0.098 | 0.477 | < 0.001 | 0.992 | 0.001 | 0.001 | < 0.001 |
P values are from an ANOVA testing the null hypothesis that the average inclination to seek care/symptom interference is the same regardless of use of a medication for a urological condition.
P values from overall chi-square test. Medications were:
oxybutynin, tolterodine tartrate, propantheline bromide, hyoscyamine sulphate, and flavoxate hydrochloride
pentosan polysulphate and amitriptyline
alfuzosin, doxazosin, tamsulosin, terazosin, and finasteride (no participant took dutasteride)
sildenafil, tadalafil, alprostadil, vardenafil, papaverine.
5 point Likert scale where 1 = very unimportant to 5 = very important. Higher average scores indicate more importance to seek care.
Interference with activities. Higher average scores indicate more interference, range 0–28.
Discussion
In a population-based study of community-dwelling men and women, the prevalence of use of medications for urological conditions, including ED, was lower than the prevalence of symptoms. Use did not consistently vary or appear to be explained by a lack of health insurance, SES, race/ethnicity, or economic hardship. We consistently found that persons who visited their healthcare provider more often and more recently were more likely to be on drug treatments, as were those who had major comorbidities and those who were most bothered by their symptoms, as measured by the interference score. Our findings suggest that symptom severity was also a factor in receiving drug treatment. We estimated the use of urological drugs among those with and without symptoms (presuming cure among those previously symptomatic). A greater proportion of persons with more severe symptoms were on treatments and this might suggest that those persons receiving drug treatments were still experiencing symptoms, or were reporting symptoms that occurred before their use of these medications. Regardless, because the proportion treated with drugs is so small, our results also suggest that there is a substantial pool of symptomatic persons using the healthcare system who are not receiving those drug treatments recommended by clinical guidelines.
There are few previous estimates of treatment patterns for urological symptoms using community-based samples to which to compare our results. A recent survey of subjects aged 57–85 years showed that 37% of men had ED, but only 14% reported using medications or supplements to improve sexual function [44]. A large survey of > 12 000 men aged 50–80 years in the USA and six European countries showed that while 90% of men had LUTS, only 19% had sought medical help for urinary problems and only 11% were medically treated [45]. Swedish surveys showed that 25–46% of men with UI seek medical attention, and only 27% of those who sought medical attention received pharmacological therapy [46,47]. Finally, another survey of six European countries indicated that 19% of the men and women surveyed had OAB symptoms, 60% of those affected had seen a physician, and 27% were receiving pharmacotherapy [48].
While we emphasize the potential for unmet need in this study, we are by no means suggesting that all persons who have symptoms are necessarily candidates for drug treatment. Persons might not be substantially bothered by their symptoms, or instead of pharmacotherapy, might choose to pursue lifestyle and/or behavioural modifications, the latter of which were not captured in this analysis. In addition, we do not know in our study whether it was medically appropriate to prescribe these drugs, as contraindications for each study participant are unknown. For instance, some urological medications (oxybutynin, amitryptiline and hyoscyamine) are recommended to be avoided in persons aged ≥65 years in the 2002 update of the Beers criteria, due to an unacceptable risk of adverse drug events [49]. Our results also do not capture those who might have been prescribed urological drug treatments in the past but stopped taking them for any reason more than a month before the interview, or persons who received a prescription but it was not completed due to cost or other factors. Thus, our results might not necessarily indicate poor prescribing practices.
Our study has several strengths. First, the population basis of our study included a variety of age groups, both genders and a diversity of race/ethnicity and SES, and allowed us to capture information among persons in poverty, among the uninsured and among those who do not otherwise present to medical care. Second, we had comprehensive ascertainment of medication use in our study, as well as information about symptom interference with daily activities and typical use of the healthcare system. Finally, the general applicability of the BACH study population to the USA population is known. Medicare coverage and health insurance coverage in BACH were similar to the National Health and Nutrition Examination Survey, National Health Interview Survey and Behavioural Risk Factor Surveillance Survey, as were the distributions of common comorbidities (except asthma, more common in BACH) [4,50]. We measured disease states using symptom proxies while drugs are prescribed for clinical diagnoses, and not all relevant clinical information for a drug treatment decision was captured in this analysis. However, this aspect of our study design also might be viewed as a unique strength, by allowing us to capture the true population-based burden of symptoms in the community. Our methods might have overestimated medication use, because we used a relatively inclusive definition of treatment (including older drugs such as papaverine and propantheline bromide) and made an assumption that any urological drug use was for urological conditions. However, we still found a very low prevalence of drug treatment. This might also reflect a delay in dissemination of newer clinical guidelines for ED and LUTS/BPH to the ‘bedside’.
Our results suggest that there is a potentially unmet need for drug treatment of urological symptoms when considering a community-based sample. Given the established association of urological symptoms with health-related quality-of-life [51–54], our results have both clinical and public health relevance, and suggest that clinicians should be aware of urological symptoms and work with patients on optimal strategies for alleviation.
Acknowledgements
The authors gratefully acknowledge Gretchen R. Chiu and Varant Kupelian for assistance with this manuscript. Funding for the BACH Survey was provided by National Institute of Diabetes and Digestive and Kidney Diseases DK 56842. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health. The corresponding author retains the right to provide a copy of the final manuscript to the NIH upon acceptance for publication, for public archiving in PubMed Central as soon as possible, but no later than 12 months after publication.
Abbreviations
- UI
urinary incontinence
- OAB
overactive bladder
- PBlS
painful bladder syndrome
- ED
erectile dysfunction
- BACH
Boston Area Community Health (Survey)
- IIEF
International Index of Erectile Function
- SES
socioeconomic status
Footnotes
Conflict of Interest
Susan A. Hall is a former employee of and former consultant to GlaxoSmithKline.
Contributor Information
Susan A. Hall, New England Research Institutes, Watertown, Boston, MA
Carol L. Link, New England Research Institutes, Watertown, Boston, MA
Jim C. Hu, Division of Urologic Surgery, Brigham and Women’s Hospital, Boston, MA
Paul W. Eggers, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, Maryland, USA
John B. McKinlay, New England Research Institutes, Watertown, Boston, MA
References
- 1.Williams RD. Urologic Diseases in America project. J Urol. 2005;173:679. doi: 10.1097/01.ju.0000154031.18562.0c. [DOI] [PubMed] [Google Scholar]
- 2.Litwin MS, Saigal CS, Yano EM, et al. Urologic diseases in America Project: analytical methods and principal findings. J Urol. 2005;173:933–937. doi: 10.1097/01.ju.0000152365.43125.3b. [DOI] [PubMed] [Google Scholar]
- 3.Last J. The clinical iceberg. Lancet. 1963;2:28–30. [Google Scholar]
- 4.McKinlay JB, Link CL. Measuring the Urologic Iceberg. Design and Implementation of the Boston Area Community Health (BACH) Survey. Eur Urol. 2007;52:389–396. doi: 10.1016/j.eururo.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelley KE, Kelley TP, Kaufman DW, Mitchell AA. The Slone Drug Dictionary: are search driven pharmacoepidemiology tool. Pharmacoepidemiol Drug Safety. 2003;12:168–169. [Google Scholar]
- 6.American Society of Health-System Pharmacists. Bethesda: American Society of Health-System Pharmacists, Inc; AHFS Drug Information. 2007
- 7.Fawzy A, Braun K, Lewis GP, Gaffney M, Ice K, Dias N. Doxazosin in the treatment of benign prostatic hyperplasia in normotensive patients: a multicenter study. J Urol. 1995;154:105–109. [PubMed] [Google Scholar]
- 8.Gillenwater JY, Conn RL, Chrysant SG, et al. Doxazosin for the treatment of benign prostatic hyperplasia in patients with mild to moderate essential hypertension: a double-blind, placebo-controlled, dose–response multicenter study. J Urol. 1995;154:110–115. [PubMed] [Google Scholar]
- 9.Wilt TJ, Howe RW, Rutks IR, MacDonald R. Terazosin for benign prostatic hyperplasia. Cochrane Database Syst Rev. 2002 doi: 10.1002/14651858.CD003851. CD003851. [DOI] [PubMed] [Google Scholar]
- 10.AUA. AUA guideline on management of benign prostatic hyperplasia (2003). Chapter 1: Diagnosis and treatment recommendations. J Urol. 2003;170:530–547. doi: 10.1097/01.ju.0000078083.38675.79. [DOI] [PubMed] [Google Scholar]
- 11.Roehrborn CG. Efficacy and safety of once-daily alfuzosin in the treatment of lower urinary tract symptoms and clinical benign prostatic hyperplasia: a randomized, placebo-controlled trial. Urology. 2001;58:953–959. doi: 10.1016/s0090-4295(01)01448-0. [DOI] [PubMed] [Google Scholar]
- 12.van Kerrebroeck P, Jardin A, Laval KU, van Cangh P. Efficacy and safety of a new prolonged release formulation of alfuzosin 10 mg once daily versus alfuzosin 2.5 mg thrice daily and placebo in patients with symptomatic benign prostatic hyperplasia. ALFORTI Study Group. Eur Urol. 2000;37:306–313. doi: 10.1159/000052361. [DOI] [PubMed] [Google Scholar]
- 13.Wilt TJ, MacDonald R, Rutks I. Tamsulosin for benign prostatic hyperplasia. Cochrane Database Syst Rev. 2003 doi: 10.1002/14651858.CD002081.pub2. CD002081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirby RS, Coppinger SW, Corcoran MO, Chapple CR, Flannigan M, Milroy EJ. Prazosin in the treatment of prostatic obstruction. A placebo-controlled study. Br J Urol. 1987;60:136–142. doi: 10.1111/j.1464-410x.1987.tb04950.x. [DOI] [PubMed] [Google Scholar]
- 15.Martorana G, Giberti C, P D, et al. The effect of prazosin in benign prostatic hypertrophy, a placebo controlled double-blind study. IRCS Med Sci. 1984;12:11–12. [Google Scholar]
- 16.Roehrborn CG, Boyle P, Nickel JC, Hoefner K, Andriole G. Efficacy and safety of a dual inhibitor of 5-alpha-reductase types 1 and 2 (dutasteride) in men with benign prostatic hyperplasia. Urology. 2002;60:434–441. doi: 10.1016/s0090-4295(02)01905-2. [DOI] [PubMed] [Google Scholar]
- 17.Roehrborn CG, Marks LS, Fenter T, et al. Efficacy and safety of dutasteride in the four-year treatment of men with benign prostatic hyperplasia. Urology. 2004;63:709–715. doi: 10.1016/j.urology.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Gormley GJ, Stoner E, Bruskewitz RC, et al. The Finasteride Study Group. The effect of finasteride in men with benign prostatic hyperplasia. N Engl J Med. 1992;327:1185–1191. doi: 10.1056/NEJM199210223271701. [DOI] [PubMed] [Google Scholar]
- 19.McConnell JD, Bruskewitz R, Walsh P, et al. Finasteride Long-Term Efficacy and Safety Study Group. The effect of finasteride on the risk of acute urinary retention and the need for surgical treatment among men with benign prostatic hyperplasia. N Engl J Med. 1998;338:557–563. doi: 10.1056/NEJM199802263380901. [DOI] [PubMed] [Google Scholar]
- 20.Padma-Nathan H, Steers WD, Wicker PA Sildenafil Study Group. Efficacy and safety of oral sildenafil in the treatment of erectile dysfunction: a double-blind, placebo-controlled study of 329 patients. Int J Clin Pract. 1998;52:375–379. [PubMed] [Google Scholar]
- 21.Montague DK, Jarow JP, Broderick GA, et al. Chapter 1. The management of erectile dysfunction: an AUA update. J Urol. 2005;174:230–239. doi: 10.1097/01.ju.0000164463.19239.19. [DOI] [PubMed] [Google Scholar]
- 22.Hellstrom WJ, Gittelman M, Karlin G, et al. Sustained efficacy and tolerability of vardenafil, a highly potent selective phosphodiesterase type 5 inhibitor, in men with erectile dysfunction: results of a randomized, double-blind, 26-week placebo-controlled pivotal trial. Urology. 2003;61:8–14. doi: 10.1016/s0090-4295(03)00115-8. [DOI] [PubMed] [Google Scholar]
- 23.Carson CC, Rajfer J, Eardley I, et al. The efficacy and safety of tadalafil: an update. BJU Int. 2004;93:1276–1281. doi: 10.1111/j.1464-410X.2004.04819.x. [DOI] [PubMed] [Google Scholar]
- 24.Godschalk MF, Chen J, Katz PG, Mulligan T. Treatment of erectile failure with prostaglandin E1: a double-blind, placebo-controlled, dose–response study. J Urol. 1994;151:1530–1532. doi: 10.1016/s0022-5347(17)35293-x. [DOI] [PubMed] [Google Scholar]
- 25.Linet OI, Ogrinc FG The Alprostadil Study Group. Efficacy and safety of intracavernosal alprostadil in men with erectile dysfunction. N Engl J Med. 1996;334:873–877. doi: 10.1056/NEJM199604043341401. [DOI] [PubMed] [Google Scholar]
- 26.Padma-Nathan H, Hellstrom WJ, Kaiser FE, et al. Medical Urethral System for Erection (MUSE) Study Group. Treatment of men with erectile dysfunction with transurethral alprostadil. N Engl J Med. 1997;336:1–7. doi: 10.1056/NEJM199701023360101. [DOI] [PubMed] [Google Scholar]
- 27.Diokno AC, Brown MB, Goldstein N, Herzog AR. Epidemiology of bladder emptying symptoms in elderly men. J Urol. 1992;148:1817–1821. doi: 10.1016/s0022-5347(17)37038-6. [DOI] [PubMed] [Google Scholar]
- 28.Hay-Smith J, Herbison P, Ellis G, Morris A. Which anticholinergic drug for overactive bladder symptoms in adults. Cochrane Database Syst Rev. 2005 doi: 10.1002/14651858.CD005429. CD005429. [DOI] [PubMed] [Google Scholar]
- 29.Holmes DM, Montz FJ, Stanton SL. Oxybutinin versus propantheline in the management of detrusor instability. A patient-regulated variable dose trial. Br J Obstet Gynaecol. 1989;96:607–612. doi: 10.1111/j.1471-0528.1989.tb03263.x. [DOI] [PubMed] [Google Scholar]
- 30.Agency for Health Care Policy and Research. Archived Clinical Practice Guidelines: Urinary Incontinence in Adults: Acute and Chronic Management; Clinical Practice Guideline Number 2 (Update) 1996 [Google Scholar]
- 31.Blaivas JG, Labib KB, Michalik SJ, Zayed AA. Cystometric response to propantheline in detrusor hyperreflexia: therapeutic implications. J Urol. 1980;124:259–262. doi: 10.1016/s0022-5347(17)55398-7. [DOI] [PubMed] [Google Scholar]
- 32.Hwang P, Auclair B, Beechinor D, Diment M, Einarson TR. Efficacy of pentosan polysulfate in the treatment of interstitial cystitis: a meta-analysis. Urology. 1997;50:39–43. doi: 10.1016/S0090-4295(97)00110-6. [DOI] [PubMed] [Google Scholar]
- 33.Peeker R, Fall M. Treatment guidelines for classic and nonulcer interstitial cystitis. Int Urogynecol J Pelvic Floor Dysfunct. 2000;11:23–32. doi: 10.1007/s001920050006. [DOI] [PubMed] [Google Scholar]
- 34.van Ophoven A, Pokupic S, Heinecke A, Hertle L. A prospective, randomized, placebo controlled, double-blind study of amitriptyline for the treatment of interstitial cystitis. J Urol. 2004;172:533–536. doi: 10.1097/01.ju.0000132388.54703.4d. [DOI] [PubMed] [Google Scholar]
- 35.Rosen RC, Cappelleri JC, Smith MD, Lipsky J, Pena BM. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res. 1999;11:319–326. doi: 10.1038/sj.ijir.3900472. [DOI] [PubMed] [Google Scholar]
- 36.The Measurement Committee of the American Urological Association. Barry MJ, Fowler FJ, Jr, O’Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. J Urol. 1992;148:1549–1557. doi: 10.1016/s0022-5347(17)36966-5. [DOI] [PubMed] [Google Scholar]
- 37.Epstein RS, Deverka PA, Chute CG, et al. Validation of a new quality of life questionnaire for benign prostatic hyperplasia. J Clin Epidemiol. 1992;45:1431–1445. doi: 10.1016/0895-4356(92)90205-2. [DOI] [PubMed] [Google Scholar]
- 38.Executive Office of the President of the United States. Office of Management and Budget. [Accessed January 2008];Federal Register Notice: Revisions to the Standards for the Classification of Federal Data on Race and Ethnicity. 1997 Available at http://www.whitehouse.gov/omb/fedreg/1997standards.html. [Google Scholar]
- 39.Green LW. Manual for scoring socioeconomic status for research on health behavior. Public Health Report. 1970;85:815–827. [PMC free article] [PubMed] [Google Scholar]
- 40.Turvey CL, Wallace RB, Herzog R. A revised CES-D measure of depressive symptoms and a DSM-based measure of major depressive episodes in the elderly. Int Psychogeriatr. 1999;11:139–148. doi: 10.1017/s1041610299005694. [DOI] [PubMed] [Google Scholar]
- 41.Research Triangle Institute. SUDAAN Language Manual Release 9.0. Research Triangle Park, NC: 2004. [Google Scholar]
- 42.Cochran W. Sampling Techniques. 3rd edn. New York, NY: John Wiley and Sons; 1977. [Google Scholar]
- 43.Schafer JL. Analysis of Incomplete Multivariate Data, London. New York: Chapman & Hall; 1997. [Google Scholar]
- 44.Lindau ST, Schumm LP, Laumann EO, Levinson W, O’Muircheartaigh CA, Waite LJ. A study of sexuality and health among older adults in the United States. N Engl J Med. 2007;357:762–774. doi: 10.1056/NEJMoa067423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosen R, Altwein J, Boyle P, et al. Lower urinary tract symptoms and male sexual dysfunction: the multinational survey of the aging male (MSAM-7) Eur Urol. 2003;44:637–649. doi: 10.1016/j.eururo.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 46.Fall M, Frankenberg S, Frisen M, Larsson B, Petren M. [456,000 Swedes may have urinary incontinence. Only every fourth person seeks help for the disorder] Lakartidningen. 1985;82:2054–2056. [PubMed] [Google Scholar]
- 47.Malmsten UG, Milsom I, Molander U, Norlen LJ. Urinary incontinence and lower urinary tract symptoms: an epidemiological study of men aged 45–99 years. J Urol. 1997;158:1733–1737. doi: 10.1016/s0022-5347(01)64113-2. [DOI] [PubMed] [Google Scholar]
- 48.Milsom I, Abrams P, Cardozo L, Roberts RG, Thuroff J, Wein AJ. How widespread are the symptoms of an overactive bladder and how are they managed? A population-based prevalence study. BJU Int. 2001;87:760–766. doi: 10.1046/j.1464-410x.2001.02228.x. [DOI] [PubMed] [Google Scholar]
- 49.Fick DM, Cooper JW, Wade WE, Waller JL, Maclean JR, Beers MH. Updating the Beers criteria for potentially inappropriate medication use in older adults. results of a US consensus panel of experts. Arch Intern Med. 2003;163:2716–2724. doi: 10.1001/archinte.163.22.2716. [DOI] [PubMed] [Google Scholar]
- 50.New England Research Institutes. Can the BACH data be generalized to the 2006, U.S. population? Comparison of BACH batch 1–4 data with data from three national surveys (NHIS, NHANES, and BRFSS) Technical report. Available at http://www.neriscience.com/web/documents/v2Combined%20Comparison%20of%20BACH%20Batch%201-5%20to%20NHIS%20NHANES%20BRFSS.pdf.
- 51.Litman HJ, McKinlay JB. The future magnitude of urological symptoms in the USA: projections using the Boston Area Community Health survey. BJU Int. 2007;100:820–825. doi: 10.1111/j.1464-410X.2007.07018.x. [DOI] [PubMed] [Google Scholar]
- 52.Coyne KS, Sexton CC, Irwin DE, Kopp ZS, Kelleher CJ, Milsom I. The impact of overactive bladder, incontinence and other lower urinary tract symptoms on quality of life, work productivity, sexuality and emotional well-being in men and women: results from the EPIC study. BJU Int. 2008;101:1388–1395. doi: 10.1111/j.1464-410X.2008.07601.x. [DOI] [PubMed] [Google Scholar]
- 53.Scarpero HM, Fiske J, Xue X, Nitti VW. American Urological Association Symptom Index for lower urinary tract symptoms in women. correlation with degree of bother and impact on quality of life. Urology. 2003;61:1118–1122. doi: 10.1016/s0090-4295(03)00037-2. [DOI] [PubMed] [Google Scholar]
- 54.Kupelian V, Wei JT, O’Leary MP, et al. Prevalence of lower urinary tract symptoms and effect on quality of life in a racially and ethnically diverse random sample: the Boston Area Community Health (BACH) Survey. Arch Intern Med. 2006;166:2381–2387. doi: 10.1001/archinte.166.21.2381. [DOI] [PubMed] [Google Scholar]