Abstract
Major histocompatibility complex (MHC) class I heavy chain/β2m heterodimers assemble with antigenic peptides via interactions with peptide-loading complex proteins, including tapasin and ERp57. In human cells, a cysteine residue within tapasin (C95) has been shown to form a covalent bond with ERp57. In this study, we focused on the effect of this tapasin amino acid residue in mouse cells expressing the MHC class I molecule H2-Kd. We demonstrated that a large disulfide-bonded complex was present in the mouse cells that included ERp57, tapasin, and Kd. Furthermore, in mouse cells, unlike human cells, we found that tapasin mutated at C95 can participate in a non-covalent complex with ERp57. Comparison of our findings to earlier findings with a human molecule (HLA-B*4402) also revealed that a tapasin C95 mutation has a stronger effect on the maturation and stability of Kd than HLA-B*4402. Overall, our results characterize the influence of this tapasin cysteine residue on the stable surface expression of a mouse MHC class I molecule, and reveal differences in tapasin C95 interactions and effects between mouse and human systems.
Keywords: antigen presentation, cysteine, ERp57, H2-Kd, major histocompatibility complex, tapasin
INTRODUCTION
Within the endoplasmic reticulum, a complex composed of several proteins assists in the provision of MHC class I heavy chain/β2m heterodimers with peptides. Various studies have shown that this complex includes beta 2-microglobulin (β2m), tapasin, the transporter associated with antigen processing (TAP), calreticulin, ERp57, Bap 29/31, and protein disulfide isomerase.1-5 Tapasin is a necessary component of the peptide-loading complex,6-14 and it interacts directly with TAP,5,8,13,15-20 the MHC class I heavy chain,8 and ERp57.21 The interaction of tapasin and ERp57 in a stable dimeric complex has been visualized by X-ray crystallographic analysis.22
A disulfide bond is formed between the cysteine at position 95 in tapasin (conserved in human, mouse, and rat) and the cysteine at position 57 in ERp57.5,15-16,18,21,23-25 This disulfide bond is evident when N-ethylmaleimide or methyl methanethiosulfonate (MMTS) is used to treat cells prior to lysis, since these reagents preserve the covalent linkage between ERp57 and tapasin.21,23 For a tapasin-dependent MHC class I allotype, tapasin disulfide linkage to ERp57 was shown to enable tapasin to prevent reduction of the α2 domain disulfide bond in the peptide-binding groove.26 Trimeric disulfide-linked complexes that include tapasin, ERp57, and the MHC class I heavy chain have also been identified.27-28 A human tapasin mutant with a substitution at position 95 (C95A) could not be detected in non-covalent association with ERp57, suggesting that formation of the disulfide bond is a necessary part of human tapasin interaction with ERp57.21 Using purified proteins, direct binding of tapasin to peptide-deficient MHC class I molecules has been demonstrated,29 and soluble tapasin modified to bind to the MHC class I heavy chain via a carboxy-terminal leucine zipper was shown to be capable of stabilizing open forms of MHC class I molecules.30 In another study, recombinant human tapasin alone failed to facilitate peptide binding by the MHC class I heavy chain, though conjugates of recombinant ERp57 and tapasin were active.31
MHC class I heavy chains in the peptide-loading complex of cells expressing a tapasin C95A mutant fail to reach their normal oxidation state.21,26 The thermostability of B44 molecules is also lower in such cells.21,26 Furthermore, ~20% fewer B44 molecules pass through the Golgi and the quantity of B44 molecules is ~20% lower on cells expressing human tapasin C95A instead of wild type tapasin.21
In this study, we examined the effect of the cysteine at position 95 in mouse tapasin on the murine MHC class I molecule Kd. Mouse tapasin C95 mutants were found to associate non-covalently with mouse ERp57, demonstrating that tapasin and ERp57 are able to interact in the absence of the disulfide bond in a mouse system. The majority of Kd molecules assembled in cells expressing mouse tapasin C95S or C95A did not pass through the Golgi, which indicates a more severe phenotype for Kd than was noted previously for B44 in human cells expressing tapasin C95A. Expression of mouse tapasin C95S or C95A (compared to wild type tapasin) lowered the cell surface expression of folded Kd, and the Kd molecules that reached the surface were unstable. Overall, these studies contribute to the understanding of the similarities and differences in the impact of the tapasin cysteine 95 residue in the mouse and human.
RESULTS
A subset of mouse ERp57 molecules and virtually all mouse tapasin molecules were found within a ~145 kD complex in MMTS-treated cells
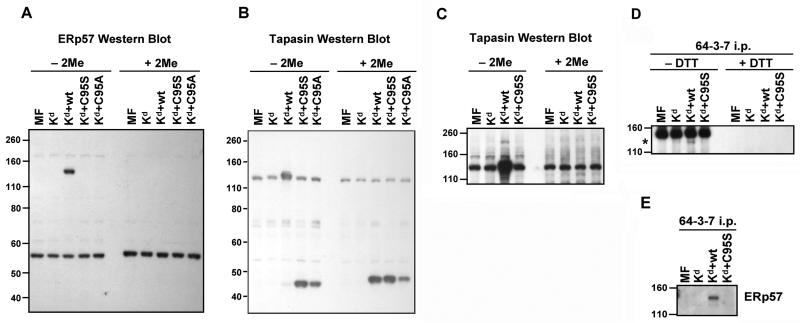
To analyze mouse tapasin/ERp57 interactions, we immunoblotted for ERp57 and tapasin on lysates of MMTS-treated MF-Kd cells not transfected with tapasin, or transfected with mouse wild type tapasin, tapasin C95S, or tapasin C95A. Prior to electrophoresis, the samples were either treated or mock treated with the reducing agent 2-mercaptoethanol. By probing with antibodies for mouse ERp57 or mouse tapasin, we found that a subset of mouse ERp57 molecules were covalently linked in a large complex with wild type mouse tapasin. The molecular weight of the large complex on the ERp57 blot and the tapasin blot was ~145 kD (Figure 1A, B, C), which is larger than the expected molecular weight of a complex containing only ERp57 and tapasin (57+48=105 kD). In some experiments in which we immunoprecipitated open Kd from lysates of MMTS-treated cells expressing wild type tapasin, we also observed a ~145 kD complex, suggesting that ERp57, tapasin, and Kd form a trimer in the MF cells (Figure 1D). Probing the Kd immunoprecipitates with anti-ERp57 also revealed the presence of the ~145 kD complex in cells expressing wild type tapasin, further confirming the presence of ERp57 in this complex (Figure 1E). In the longest exposures of the tapasin blots (Figure 1C) and ERp57 blots (data not shown), an even larger complex (~200 kD) could be seen in the lane corresponding to the cells transfected with Kd plus wild type tapasin. The ~200 kD band shown in Figure 1C likely corresponds to a complex of ERp57, tapasin, MHC class I heavy chain, and protein disulfide isomerase, as identified in human cells by Santos et al.27
Figure 1.
ERp57, wild type tapasin, and open Kd molecules were found associate covalently in a ~145 kD complex in mouse cells. MF cells transfected with Kd alone or Kd plus wild type tapasin, tapasin C95S, or tapasin C95A were treated with MMTS and lysed. Samples of the lysates were not treated or treated with 2-mercaptoethanol (indicated as 2Me), electrophoresed on a 10% acrylamide Tris-glycine gel and probed on a Western blot with (A) an antiserum against mouse ERp57 or (B) a monoclonal antibody against mouse tapasin. In (C), a longer film exposure of a tapasin blot including Kd alone, Kd plus wild type tapasin, and Kd plus tapasin C95S is shown. Bands of similar intensity that appear in all lanes in A, B, and C are presumably non-specific background bands. (D) Kd molecules were immunoprecipitated with antibody 64-3-7 from lysates of MMTS-treated MF cells expressing Kd alone or with mouse wild type or C95S tapasin. The immunoprecipitates were not treated or treated with DTT, electrophoresed on a 10% acrylamide Tris-glycine gel, transferred to a membrane, and probed with 64-3-7 to identify Kd heavy chains. A dark band is present at the very top of the panel in all lanes that is due simply to recognition of the immunoprecipitating 64-3-7 antibody (added to all samples) by the goat anti-mouse secondary antibody. An asterisk indicates the specific band (a high molecular weight complex containing Kd). Similar results were also obtained when 2-mercaptoethanol treatment of the lysates, instead of DTT treatment of the immunoprecipitates, was performed. (E) Kd molecules were immunoprecipitated with antibody 64-3-7 from lysates of MMTS-treated MF cells expressing Kd alone or with mouse wild type or C95S tapasin. The immunoprecipitates were electrophoresed on a 10% acrylamide Tris-glycine gel, transferred to a membrane, and probed with anti-ERp57 antiserum.
Almost all the wild type mouse tapasin molecules were found in the complex containing disulfide-bonded tapasin/ERp57 (Figure 1B), but only a subset of the ERp57 molecules were in the high molecular weight complex (Figure 1A). Some ERp57 molecules unconjugated to wild type mouse tapasin were detected (Figure 1A), as would be expected since ERp57 is known to bind to many other proteins in the endoplasmic reticulum besides tapasin. In their studies with human cells, Peaper et al. also found free ERp57, ranging from 20-85% of the total ERp57 (varying with the level of cellular tapasin).23 As anticipated, based on results in previous studies of human tapasin C95A,21 the mouse tapasin C95S and C95A mutants were unable to form a high molecular weight conjugate with ERp57 (Figure 1A, B, E).
Although mouse tapasin and ERp57 could form a covalent linkage, the disulfide bond was found not to be required for their association
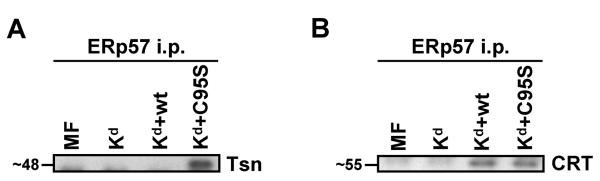
Previous analyses with human tapasin suggested that the disulfide bond between ERp57 and tapasin was necessary for human tapasin/ERp57 association.21 To determine whether this was also the case with mouse tapasin and mouse ERp57, we immunoprecipitated ERp57 from lysates of MMTS-treated MF cells expressing Kd plus no tapasin, wild type tapasin, or tapasin C95S, and assessed by Western blot whether there was free (non-conjugated) tapasin associated with ERp57. Non-conjugated tapasin C95S (~48 kD) did co-immunoprecipitate with ERp57 from the lysates of MF-Kd cells transfected with this mutant (Figure 2A). Non-conjugated mouse tapasin C95A also co-immunoprecipitated with ERp57, though somewhat more weakly than mouse tapasin C95S (data not shown). Note that, in the cells expressing wild type tapasin, the tapasin proteins are virtually all are in high molecular weight conjugates (Figure 1B), and so wild type tapasin would not be visible within the molecular weight range displayed in Figure 2A. Thus, in mouse cells, ERp57 and tapasin C95 mutants can associate in the absence of intermolecular disulfide bonding interactions (although ERp57 and wild type tapasin preferentially associate covalently). However, our data do not define whether the interaction in the absence of disulfide bonding is direct or via another component of the peptide-loading complex.
Figure 2.
Mouse tapasin C95S was discovered to bind non-covalently with ERp57, and both wild type and C95S tapasin were found to bind only non-covalently with calreticulin. (A) Immunoprecipitations with an antiserum against mouse ERp57 were performed on lysates of MMTS-treated MF cells transfected with Kd alone or Kd plus wild type tapasin or tapasin C95S. The immunoprecipitates were electrophoresed on a 10% acrylamide Tris-glycine gel and probed on a Western blot with a monoclonal antibody against mouse tapasin. (B) In MF cells, calreticulin was discovered to interact with ERp57 in the presence of wild type or C95S tapasin, but not in the absence of tapasin. ERp57 was immunoprecipitated from the indicated MMTS-treated cell lysates with anti-ERp57 antiserum, electrophoresed on a 10% acrylamide Tris-glycine gel, and associated calreticulin was detected by Western blotting with an anti-calreticulin antibody.
Calreticulin associated with ERp57 in the presence of either wild type or C95S mouse tapasin
ERp57 normally also interacts with another component of the peptide-loading complex, calreticulin. We assessed the effect of the mutation at C95 in mouse tapasin on the association of calreticulin with ERp57, using lysates of MMTS-treated MF cells transfected with Kd alone or Kd plus either wild type tapasin or tapasin C95S. Immunoprecipitating mouse ERp57 and immunoblotting with anti-calreticulin antibody revealed that free calreticulin association with ERp57 was unchanged by the presence of mouse tapasin C95S, relative to the presence of wild type mouse tapasin (Figure 2B). This finding suggests that the ~200 kD complex (Figure 1C) does not include calreticulin. This observation is consistent with previous findings of a noncovalent association between human ERp57 and calreticulin that is unaffected by a tapasin C95A mutation.21 In the absence of any tapasin, there was less mouse ERp57/calreticulin association detected (Figure 2B), although ERp57/calreticulin association could still be perceived in tapasin's absence, particularly on long film exposures (data not shown).
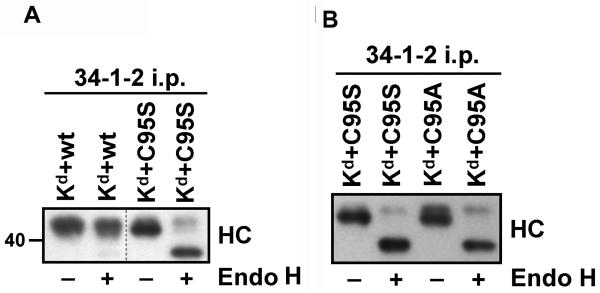
Mutation of C95 in mouse tapasin inhibited the migration of Kd through the Golgi
To assess the impact of the C95S mutation in mouse tapasin on Kd maturation, folded Kd molecules were immunoprecipitated with antibody 34-1-2 from lysates of the MF cells transduced with Kd and either mouse wild type tapasin, C95S tapasin, or C95A tapasin. The immunoprecipitates were either mock treated or treated with endoglycosidase H (Endo H), and then electrophoresed on a 4→20% acrylamide gel and Western blotted to reveal the Kd heavy chain. Previous studies have shown that conversion from bearing Endo H-sensitive, high mannose oligosaccharides to Endo H-resistant, complex oligosaccharides is indicative of MHC class I molecule trafficking through the medial Golgi.32-33 Most of the folded Kd molecules assembled in the presence of mouse tapasin C95S were sensitive to Endo H digestion, and only a minority (29%) of folded Kd molecules assembled in MFs expressing tapasin C95S had traversed the Golgi, as indicated by acquisition of Endo H resistance (Figure 3A). Compared to Kd in cells with tapasin C95S, the limited acquisition of Endo H resistance (25%) was also similar for Kd expressed in the presence of mouse tapasin C95A (Figure 3B). In contrast, the percentage of Endo H-resistant Kd molecules in the MFs expressing wild type tapasin was 97% (Figure 3A).
Figure 3.
Normal maturation of Kd was shown to be less facilitated by mouse tapasin C95S or mouse tapasin C95A compared to wild type tapasin, as most Kd molecules assembled in the presence of these tapasin mutants were found not to exhibit the mature glycosylation pattern that is characteristic of MHC class I molecules that have passed through the Golgi. Kd molecules were immunoprecipitated with antibody 34-1-2 from lysates of MF cells expressing Kd along with mouse wild type, C95S tapasin, or C95A tapasin. Samples were untreated (−) or treated (+) with Endo H as indicated, and all samples were treated with 2-mercaptoethanol before loading on the gel. The immunoprecipitates were electrophoresed on a 4→20% acrylamide Tris-glycine gel, transferred to a membrane, and probed with 64-3-7 to identify Kd heavy chains. (A) Wild type and C95S tapasin, (B) C95S tapasin and C95A tapasin.
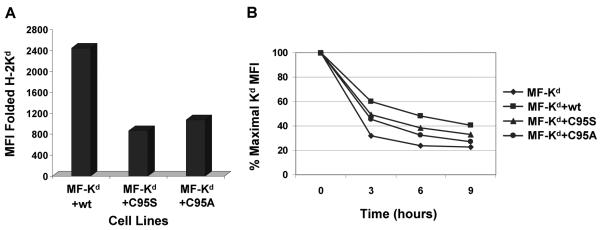
Mutation of C95 in mouse tapasin lowered the expression and stability of Kd at the cell surface
To determine the impact of tapasin C95S and C95A on the level of Kd at the cell surface, we analyzed the relative amount of folded Kd at the surface of MF cells transfected with wild type mouse tapasin, C95S tapasin, or C95A tapasin. These assays showed that the level of folded Kd at the plasma membrane declined by 65% in the presence of tapasin C95S and 56% in the presence of tapasin C95A (Figure 4A). Thus, compared to wild type mouse tapasin, the mouse tapasin C95S and tapasin C95A mutants both decreased the expression of folded Kd at the cell surface.
Figure 4.
Stable surface expression of Kd was impaired by mouse tapasin C95S and tapasin C95A compared to wild type tapasin. (A) Relative to cells expressing wild type mouse tapasin, cells expressing mouse C95S tapasin or C95A tapasin had a reduced level of cell surface Kd. Cells were incubated with an antibody against the folded form of the Kd molecule (34-1-2) or with secondary antibody only. Results obtained with the secondary antibody only were less than 4.0. Values on the y axis are relative mean fluorescence intensity (MFI) units obtained with antibody 34-1-2. (B) Surface Kd molecules on cells expressing mouse tapasin C95S or tapasin C95A had a faster turnover rate than those assembled in the presence of wild type mouse tapasin. MF cells expressing Kd with no tapasin, wild type tapasin, tapasin C95S, or tapasin C95A were incubated with 2 μg/ml brefeldin A in complete medium for 0, 3, 6, or 9 hours. After the incubation with brefeldin A, the cells were washed, stained with anti-Kd antibody 34-1-2, and analyzed by flow cytometry.
The stability of the folded, cell-surface Kd molecules was monitored by treating MF cells expressing no tapasin, mouse wild type tapasin, mouse C95S tapasin, or mouse C95A tapasin with brefeldin A for various time periods and then measuring cell surface Kd expression by flow cytometry with the 34-1-2 antibody. Brefeldin A inhibits protein transport in the Golgi, and thereby prevents the arrival of new MHC class I molecules at the cell surface.34-35 The quantity of Kd molecules on cells expressing mouse tapasin C95S or mouse tapasin C95A was observed to decline more rapidly than the number of Kd molecules on cells expressing wild type mouse tapasin, indicating decreased stability of the Kd molecules on tapasin C95S or tapasin C95A transfectants (Figure 4B).
DISCUSSION
The molecular weight of a large complex on ERp57 and tapasin blots from MMTS-treated cells was ~145 kD (Figure 1A and B). This complex could also be seen in immunoprecipitates of open Kd molecules probed on Western blots with 64-3-7 or anti-ERp57 serum (Figure 1D, E). A tapasin/ERp57 conjugate that was previously demonstrated in MMTS-treated human cells was ~100 kD, at the approximate molecular weight of a complex containing only ERp57 and tapasin.23 Trimeric complexes of ~150 kD consisting of ERp57, tapasin, and MHC class I heavy chain have also been reported in human cells, but in the studies in which they were noted the tapasin/ERp57 dimer was also observed and was quantitatively dominant over the trimer.27,28 Thus, our finding of such a large covalently linked complex containing ERp57 and tapasin in the absence (or virtual absence) of conjugated tapasin/ERp57 dimers was surprising.
For B35, site-directed mutagenesis of a cysteine residue in the transmembrane/cytoplasmic tail region (C308) abrogated disulfide formation with tapasin, and it was therefore proposed that the C308 residue in B35 formed a disulfide bond with a cysteine in the transmembrane/cytoplasmic region of tapasin.28 No cysteine is present in the transmembrane/cytoplasmic regions of either Kd or mouse tapasin,16,36 indicating that cysteine residues in the lumenal domains of Kd and mouse tapasin are evidently involved in their disulfide bonding. Notably, Santos et al. have proposed a model of a trimeric complex containing the human MHC class I molecule, tapasin, and ERp57 in which the linkage of ERp57 to the MHC class I heavy chain is via the cysteine in the heavy chain peptide-binding groove.27
Previous studies by Dick et al.21 did not detect a non-covalent association between human tapasin C95A and ERp57 in 721.220 cells. In contrast, we have shown that tapasin C95 mutants in MFs maintain a non-covalent association with ERp57 (Figure 2A and data not shown). Thus, in the mouse, disulfide bonding between ERp57 and tapasin occurs, but is not required for incorporation of ERp57 into a tapasin/ERp57 complex. As noted by Wearsch and Cresswell,31 fish and birds do not have an equivalent cysteine in tapasin, indicating that formation of a covalent tapasin/ERp57 complex cannot occur in these species. The ability of ERp57 and tapasin in the mouse to interact either covalently or non-covalently may therefore be an intermediate evolutionary step.
In the presence of wild type tapasin, Endo H resistance was increased as expected; in contrast, in the presence of mouse tapasin C95S or tapasin C95A, the majority of Kd molecules were found to be susceptible to Endo H digestion (Figure 3). Based on these results from our Endo H experiments, a functional implication of our finding that mouse tapasin C95S can interact non-covalently with ERp57 is that tapasin/ERp57 association in the absence of disulfide bond formation is not sufficient to permit normal assembly complex function. These Endo H results for Kd can be compared to previous data with B*4402 molecules expressed in 721.220.37 Kd maintains a partial level of cell surface expression on human cells in the absence of tapasin,38 but it is highly tapasin-dependent in mouse cells (no surface expression in the absence of tapasin).39 B*4402 has been characterized as a tapasin-dependent MHC class I allotype (with virtually no expression detected above background staining on tapasin-deficient B*4402-transfected 721.220 cells).40 B*4402 molecules in the presence of a human tapasin C95A mutant have only a slightly reduced rate of maturation and are fully Endo H resistant within two hours.37 Only ~20% fewer B*4402 molecules were found to be Endo H resistant and surface expressed on cells expressing human tapasin C95A compared to wild type tapasin.21 In contrast, only 25-29% of Kd molecules became Endo H resistant in cells with mouse tapasin C95A or C95S (Figure 3), and Kd surface expression on these cells was only 35-44% of the Kd level on cells expressing wild type tapasin (Figure 4A). Thus, the impact of a tapasin C95 mutation is more severe on the maturation of Kd than B*4402. Although tapasin C95 has been shown to be an ERp57 interaction site,21 the ability of mouse tapasin C95S or mouse tapasin C95A to cause the retention of the majority of Kd molecules in MF cells (Figure 3) may involve factors other than absence of tapasin/ERp57 interaction. In a related study, Garbi et al.41 found that Kb molecules in mouse cells lacking ERp57 were able to leave the endoplasmic reticulum and had an increased rate of maturation. This observation suggests that egress of MHC class I molecules is not dependent on tapasin-ERp57 interaction.
Our finding that the stability of Kd on MFs expressing tapasin C95S or tapasin C95A was reduced (Fig. 4B) differed from previous findings that the surface turnover of a different mouse MHC class I molecule, Kb, is similar in the presence of either C95A tapasin or wild type tapasin.42 This difference could be due to allotypic differences in the Kd versus Kb heavy chain structure. Alternatively, species-specific factors may play a role, since a mouse tapasin C95S mutant and mouse cells were used in our study but a human tapasin C95A mutant and human tapasin-deficient (721.220) cells were used in the study with Kb.42 In 721.220 cells, the high affinity of human β2m for mouse MHC class I molecules may contribute to greater overall stability for the transfected Kb molecules. In contrast to Kb, HLA-B*4402 expressed with C95A tapasin in 721.220 cells showed reduced thermostability.21,42 Our findings with Kd, together with the findings with HLA-B*4402, suggest that expression of tapasin C95 mutants can result in the loading of sub-optimal peptides.
Collectively, our experiments have revealed several interesting aspects of tapasin in the mouse system. In the mouse, tapasin interaction with ERp57 is not entirely dependent on covalent bonding to ERp57 via the C95 amino acid residue. The ability of folded forms of Kd to reach the cell surface is strongly impaired by tapasin C95S or tapasin C95A, relative to wild type tapasin. Overall, our findings show that extension of tapasin analysis to additional types of MHC class I molecules reveals complexity and inter-species variability inherent in tapasin's ability to regulate antigen presentation by MHC class I molecules.
METHODS
Cell lines
MF is a fibroblast cell line generated from H-2b tapasin−/− mice6 that was made by Drs. A. Grandea and L. Van Kaer and colleagues (Vanderbilt University, Nashville, TN). A Kd cDNA and a mouse wild type tapasin cDNA16 (a kind gift from Dr. P. Wang, Barts and London School of Medicine) were cloned separately into the pMIN vector, packaged using 293E cells, and transduced into mouse tapasin MFs. Transduced MF cell lines were also created that expressed Kd in pMIN along with no tapasin or with mouse tapasin C95S or mouse tapasin C95A in pMIN. The Kd heavy chain had an epitope tag for the 64-3-7 antibody, so that open, peptide-free Kd could be recognized by 64-3-7 in immunoprecipitations and flow cytometry, and so that total Kd could be recognized by 64-3-7 on Western blots. This epitope tag has been shown not to affect peptide binding and trafficking of MHC class I molecules.38,43-45 Mouse tapasin C95S and mouse tapasin C95A were made by site-directed mutagenesis using the QuikChange kit (Stratagene) with the wild type mouse tapasin cDNA16 as a template. All cells were grown at 37°C in 5% CO2 in DMEM containing 10% fetal bovine serum, 4 mM HEPES, 2 mM L-glutamine, penicillin (100 U/ml), streptomycin (100 μg/ml), and 3 × 10−6 (vol/vol) 2-mercaptoethanol. The media reagents were purchased from Invitrogen with the exception of the fetal bovine serum, which was from Atlanta Biologicals.
Antibodies
The 64-3-7 monoclonal antibody binds to the α1 domain of open, peptide-free Ld,46-47 and also to the α1 domains of open forms of MHC class I molecules other than Ld to which the 64-3-7 epitope has been incorporated by site-directed mutagenesis. 38,43-45 In the present study, the 64-3-7 antibody was used to recognize open, epitope-tagged Kd. The 34-1-2 antibody binds to folded Kd on the α1 domain.48 Additional information supporting that the 34-1-2 antibody recognizes the peptide-binding region is that weak cross-reactive binding of 34-1-2 to Ld is strongly increased by Ld association with human β2m or particular peptide ligands, or by mutation of Ld at positions in the peptide-binding region.49-50 The 64-3-7 and 34-1-2 monoclonal antibodies, a hamster anti-mouse tapasin monoclonal antibody, and a rabbit anti-mouse ERp57 serum49 used in this study were all provided by Dr. T. Hansen. A rabbit anti-calreticulin serum used for some experiments was purchased from Stressgen.
Immunoprecipitations and Western blots
In preparation for immunoprecipitations, cells were washed twice in cold PBS and then incubated on ice for 10 min in 10 mM MMTS/PBS. Cells were then treated with 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS) lysis buffer.51 The CHAPS buffer contained 1% CHAPS (Roche Applied Science) in Tris-buffered saline (pH 7.4), plus freshly added 0.2 mM phenylmethanesulphonylfluoride and 10 mM MMTS and excess antibody. After the lyates were incubated for 1 h on ice, they were centrifuged to pellet the nuclei and the supernatants were incubated with excess Protein A-Sepharose beads (Amersham Biosciences) on ice for 45 min. The beads were washed 4 times in 0.1% CHAPS in TBS (pH 7.4) and boiled in 0.125 M Tris (pH 6.8)/2% SDS/12% glycerol/0.02% bromophenol blue for 5 min to remove the proteins from the beads. Where indicated, a 10X glycoprotein denaturing buffer (New England Biolabs) consisting of 0.4 M DTT and 5% SDS was added to a final concentration of 1X, and the sample was boiled for 10 min.
The eluted immunoprecipitates were electrophoresed on pre-cast SDS-PAGE gels purchased from Invitrogen and the proteins were transferred from the gels to Immobilon-P membranes (Millipore). After overnight blocking in milk, the membranes were incubated in diluted Ab for 2 h, washed with 0.05% Tween 20/PBS 3 times, and incubated in diluted peroxidase-conjugated goat anti-mouse or anti-rabbit IgG (Jackson ImmunoResearch) or peroxidase-conjugated goat anti-hamster IgG (Jackson ImmunoResearch) for 1 h. Blots were then washed 3 times in 0.3% Tween 20/PBS, incubated with enhanced chemiluminescence Western blot reagents (Pierce Chemical Co.), and exposed to Kodak BioMax film (Eastman Kodak).
For the endoglycosidase (Endo) H assay, the immunoprecipitations were performed as described above, with the following exceptions. The cells were washed in 20 mM iodoacetamide (Sigma-Aldrich) in PBS three times prior to lysis with CHAPS buffer, and the CHAPS buffer contained 20 mM iodoacetamide instead of MMTS. The proteins were eluted from Protein A-Sepharose by boiling for 5 min in 25 mM Tris (pH 8.3)/0.2 M glycine/0.1% SDS. After boiling, the samples were centrifuged and the supernatants were removed and placed in fresh tubes. A 10X glycoprotein denaturing buffer (New England Biolabs) was added to 9 μl of each of the supernatants (making a final concentration of 1X), and the samples were boiled for 10 min. After boiling, the volume of each sample was increased by addition of 2 μl of 10X G5 reaction buffer (New England Biolabs) plus either 2 μl of Endo H (New England Biolabs) or 2 μl of water (for the mock digestion), along with water sufficient to raise the final volume to 20 μl. The tubes were incubated for 1 h at 37°C, and 5 μl of 0.5 M Tris (pH 6.8)/8% SDS/48% glycerol/0.08% bromophenol blue/8% 2-mercaptoethanol were added. The samples were electrophoresed on precast SDS-PAGE gels (Invitrogen) and Western blots were performed as described above.
For Western blots on proteins from cell lysates without an intervening immunoprecipitation step, the cells were washed twice with ice-cold PBS and then incubated on ice for 10 min in 10 mM MMTS/PBS. The cells were then lysed in 150 mM NaCl/20 mM Tris (pH 7.5)/5 mM EDTA/0.5% Triton X-100/0.2 mM phenylmethanesulphonylfluoride/10 mM MMTS. The lysates were incubated on ice for 1 h and then centrifuged to pellet cell nuclei. SDS-PAGE loading buffer (with or without 2-mercaptoethanol) was added to the supernatants to achieve a final concentration of 0.125 M Tris (pH 6.8)/2% SDS/12% glycerol/0.02% bromophenol blue (and 2% 2-mercaptoethanol, in the cases in which 2-mercaptoethanol was used). Supernatants were boiled for 5 min followed by addition of iodoacetamide (15 mM final concentration) to each sample before loading onto SDS-PAGE gels.22 Transfer of the proteins onto blotting membranes and the processing of the membranes was done as described above.
Flow cytometry
Cells in PBS with 0.5% BSA and 2 mM EDTA were adjusted to 5 × 106/ml, and 0.1 ml aliquots were added to a 96-well plate. The plate was centrifuged to pellet the cells and the supernatant was removed, and excess mAb or BSA/EDTA/PBS (as a control) was added and the cells were incubated for 30 min on ice. After the cells were washed twice with BSA/EDTA/PBS, they were incubated on ice for 30 min with PE-conjugated, Fc-specific F(ab')2 goat anti-mouse IgG (Jackson ImmunoResearch). Cells were washed 3 times in BSA/EDTA/PBS, resuspended in BSA/EDTA/PBS, and assayed on a FACSCalibur flow cytometer (BD Biosciences), with Cell Quest software (BD Biosciences) used for statistical analysis. For brefeldin A assays, the brefeldin A (Sigma-Aldrich) was added to the medium (2 μg/ml) for varied amounts of time prior to harvest of the cells.
ACKNOWLEDGEMENTS
We thank Haley Capek for helpful comments on the manuscript, Dr. Ping Wang for the gift of the mouse tapasin cDNA, and Drs. T. Hansen, A. Grandea, and L. Van Kaer for gifts of cell lines. We also gratefully acknowledge the assistance of the University of Nebraska Medical Center Cell Analysis Facility, Monoclonal Antibody Facility, and DNA Sequencing Core Facility. This work was supported by NIH Grant GM57428 (to J.C.S.), NIH/NCI Training Grant T32 CA009476 Fellowship (to L.C.S.), and UNMC Graduate Studies Fellowships (to L.C.S. and A.T.). Core facilities at the University of Nebraska Medical center receive support from the NIH/NCI Cancer Center Support Grant P30 CA036727 (to the Eppley Cancer Center) and from the Nebraska Research Initiative. The DNA Sequencing Core Facility also receives partial support from the NIH/NCRR INBRE Program P20 RR016469 Grant.
Footnotes
The authors have no financial conflict of interest.
REFERENCES
- 1.Pamer E, Cresswell P. Mechanisms of MHC class I-restricted antigen processing. Annu. Rev. Immunol. 1998;16:323–358. doi: 10.1146/annurev.immunol.16.1.323. [DOI] [PubMed] [Google Scholar]
- 2.Paquet ME, Cohen-Doyle M, Shore GC, Williams DB. Bap29/31 influences the intracellular traffic of MHC class I molecules. J. Immunol. 2004;172:7548–7555. doi: 10.4049/jimmunol.172.12.7548. [DOI] [PubMed] [Google Scholar]
- 3.Park B, Lee S, Kim E, Cho K, Riddell SR, Cho S, Ahn K. Redox regulation facilitates optimal peptide selection by MHC class I during antigen processing. Cell. 2006;127:369–382. doi: 10.1016/j.cell.2006.08.041. [DOI] [PubMed] [Google Scholar]
- 4.Farmery MR, Allen S, Allen AJ, Bulleid NJ. The role of ERp57 in disulfide bond formation during the assembly of major histocompatibility complex class I in a synchronized semipermeabilized cell translation system. J. Biol. Chem. 2000;275:14933–14938. doi: 10.1074/jbc.275.20.14933. [DOI] [PubMed] [Google Scholar]
- 5.Ortmann B, Copeman J, Lehner PJ, Sadasivan B, Herberg JA, Grandea AG, Riddell SR, Tampé R, Spies T, Trowsdale J, Cresswell P. A critical role for tapasin in the assembly and function of multimeric MHC class I-TAP complexes. Science. 1997;277:1306–1309. doi: 10.1126/science.277.5330.1306. [DOI] [PubMed] [Google Scholar]
- 6.Grandea AG, III, Golovina TN, Hamilton SE, Sriram V, Spies T, Brutkiewicz RR, Harty JT, Eisenlohr LC, Van Kaer L. Impaired assembly yet normal trafficking of MHC class I molecules in tapasin mutant mice. Immunity. 2000;13:213–222. doi: 10.1016/s1074-7613(00)00021-2. [DOI] [PubMed] [Google Scholar]
- 7.Garbi N, Tan P, Diehl AD, Chambers BJ, Ljunggren H-G, Momburg F, Hämmerling GJ. Impaired immune responses and altered peptide repertoire in tapasin-deficient mice. Nature Immunol. 2000;3:234–238. doi: 10.1038/79775. [DOI] [PubMed] [Google Scholar]
- 8.Sadasivan B, Lehner PJ, Ortmann B, Spies T, Cresswell P. Roles for calreticulin and a novel glycoprotein, tapasin, in the interaction of MHC class I molecules with TAP. Immunity. 1996;5:103–114. doi: 10.1016/s1074-7613(00)80487-2. [DOI] [PubMed] [Google Scholar]
- 9.Grandea AG, III, Androlewicz MJ, Athwal RS, Geraghty DE, Spies T. Dependence of peptide binding by MHC class I molecules on their interaction with TAP. Science. 1995;270:105–108. doi: 10.1126/science.270.5233.105. [DOI] [PubMed] [Google Scholar]
- 10.Copeman J, Bangia N, Cross JC, Cresswell P. Elucidation of the genetic basis of the antigen presentation defects in the mutant cell line .220 reveals polymorphism and alternative splicing of the tapasin gene. Eur. J. Immunol. 1998;28:3788–3791. doi: 10.1002/(SICI)1521-4141(199811)28:11<3783::AID-IMMU3783>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 11.Li S, Paulsson KM, Chen S, Sjögren H-O, Wang P. Tapasin is required for efficient peptide binding to transporter associated with antigen processing. J. Biol. Chem. 2000;275:1581–1586. doi: 10.1074/jbc.275.3.1581. [DOI] [PubMed] [Google Scholar]
- 12.Solheim JC, Harris MR, Kindle CS, Hansen TH. Prominence of β2-microglobulin, class I heavy chain conformation, and tapasin in the interactions of class I heavy chain with calreticulin and the transporter associated with antigen processing. J. Immunol. 1997;158:2236–2241. [PubMed] [Google Scholar]
- 13.Lehner PJ, Surman MJ, Cresswell P. Soluble tapasin restores MHC class I expression and function in the tapasin-negative cell line .220. Immunity. 1998;8:221–231. doi: 10.1016/s1074-7613(00)80474-4. [DOI] [PubMed] [Google Scholar]
- 14.Bangia N, Lehner PJ, Hughes EA, Surman M, Cresswell P. The N-terminal region of tapasin is required to stabilize the MHC class I loading complex. Eur. J. Immunol. 1999;29:1858–1870. doi: 10.1002/(SICI)1521-4141(199906)29:06<1858::AID-IMMU1858>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 15.Li S, Sjögren H-O, Hellman U, Pettersson RF, Wang P. Cloning and functional characterization of a subunit of the transporter associated with antigen processing. Proc. Natl. Acad. Sci. USA. 1997;94:8708–8713. doi: 10.1073/pnas.94.16.8708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li S, Paulsson KM, Sjogren H-O, Wang P. Peptide-bound major histocompatibility complex class I molecules associate with tapasin before dissociation from transporter associated with antigen processing. J. Biol. Chem. 1999;274:8649–8654. doi: 10.1074/jbc.274.13.8649. [DOI] [PubMed] [Google Scholar]
- 17.Grandea AG, III, Comber PG, Wenderfer SE, Schoenhals G, Fruh K, Monaco JJ, Spies T. Sequence, linkage to H2-K, and function of mouse tapasin in MHC class I assembly. Immunogenetics. 1998;48:260–265. doi: 10.1007/s002510050430. [DOI] [PubMed] [Google Scholar]
- 18.Deverson EV, Powis SJ, Morrice NA, Herberg JA, Trowsdale J, Butcher GW. Rat tapasin: cDNA cloning and identification as a component of the class I MHC assembly complex. Genes and Immunity. 2001;2:48–51. doi: 10.1038/sj.gene.6363727. [DOI] [PubMed] [Google Scholar]
- 19.Tan P, Kropshofer H, Mandelboim O, Bulbuc N, Hämmerling GJ, Momburg F. Recruitment of MHC class I molecules by tapasin into the transporter associated with antigen processing-associated complex is essential for optimal peptide loading. J. Immunol. 2002;168:1950–1960. doi: 10.4049/jimmunol.168.4.1950. [DOI] [PubMed] [Google Scholar]
- 20.Petersen JL, Hickman-Miller HD, McIlhaney MM, Vargas SE, Purcell AW, Hildebrand WH, Solheim JC. A charged amino acid residue in the transmembrane/cytoplasmic region of tapasin influences MHC class I assembly and maturation. J. Immunol. 2005;174:962–969. doi: 10.4049/jimmunol.174.2.962. [DOI] [PubMed] [Google Scholar]
- 21.Dick TP, Bangia N, Peaper DR, Cresswell P. Disulfide bond isomerization and the assembly of MHC class I-peptide complexes. Immunity. 2002;16:87–98. doi: 10.1016/s1074-7613(02)00263-7. [DOI] [PubMed] [Google Scholar]
- 22.Dong G, Wearsch PA, Peaper DR, Cresswell P, Reinisch KM. Insights into MHC class I peptide loading from the structure of the tapasin-ERp57 thiol oxidoreductase heterodimer. Immunity. 2009;30:21–32. doi: 10.1016/j.immuni.2008.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peaper DR, Wearsch PA, Cresswell P. Tapasin and ERp57 form a stable disulfide-linked dimer within the MHC class I peptide-loading complex. EMBO J. 2005;24:3613–3623. doi: 10.1038/sj.emboj.7600814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garbi N, Hammerling G, Tanaka S. Interaction of ERp57 and tapasin in the generation of MHC class I-peptide complexes. Curr. Opin. Immunol. 2007;19:1–7. doi: 10.1016/j.coi.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 25.Antoniou AN, Powis SJ. Characterization of the ERp57-Tapasin complex by rapid cellular acidification and thiol modification. Antioxid. Redox Signal. 2003;5:375–9. doi: 10.1089/152308603768295104. [DOI] [PubMed] [Google Scholar]
- 26.Kienast A, Preuss M, Winkler M, Dick TP. Redox regulation of peptide receptivity of major histocompatibility complex class I molecules by ERp57 and tapasin. Nat. Immunol. 2007;8:864–872. doi: 10.1038/ni1483. [DOI] [PubMed] [Google Scholar]
- 27.Santos SG, Campbell EC, Lynch S, Wong V, Antoniou AN, Powis SJ. Major histocompatibility complex class I-ERp57-tapasin interactions within the peptide-loading complex. J. Biol. Chem. 2007;282:17587–17593. doi: 10.1074/jbc.M702212200. [DOI] [PubMed] [Google Scholar]
- 28.Chambers JE, Jessop CE, Bulleid NJ. Formation of a major histocompatibility complex class I tapasin disulfide indicates a change in spatial organization of the peptide-loading complex during assembly. J. Biol. Chem. 2008;283:1862–1869. doi: 10.1074/jbc.M708196200. [DOI] [PubMed] [Google Scholar]
- 29.Rizvi SM, Raghavan M. Direct peptide-regulatable interactions between MHC class I molecules and tapasin. Proc. Natl. Acad. Sci. USA. 2006;103:18220–18225. doi: 10.1073/pnas.0605131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen M, Bouvier M. Analysis of interactions in a tapasin/class I complex provides a mechanism for peptide selection. EMBO J. 2007;26:1681–1690. doi: 10.1038/sj.emboj.7601624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wearsch PA, Cresswell P. Selective loading of high-affinity peptides onto major histocompatibility complex class I molecules by the tapasin-ERp57 heterodimer. Nat. Immunol. 2007;8:873–881. doi: 10.1038/ni1485. [DOI] [PubMed] [Google Scholar]
- 32.Krangel MS, Orr HT, Strominger JL. Assembly and maturation of HLA-A and HLA-B antigens. Cell. 1979;18:979–991. doi: 10.1016/0092-8674(79)90210-1. [DOI] [PubMed] [Google Scholar]
- 33.Owen MJ, Kissonerghis A-M, Lodish HF. Biosynthesis of HLA-A and HLA-B antigens in vivo. J Biol Chem. 1980;255:9678–9684. [PubMed] [Google Scholar]
- 34.Yewdell JW, Bennink JR. Brefeldin A specifically inhibits presentation of protein antigens to cytotoxic T lymphocytes. Science. 1989;244:1072–1075. doi: 10.1126/science.2471266. [DOI] [PubMed] [Google Scholar]
- 35.Doms RW, Russ G, Yewdell JW. Brefeldin A redistributes resident and itinerant Golgi proteins to the endoplasmic reticulum. J. Cell Biol. 1989;109:61–72. doi: 10.1083/jcb.109.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watts S, Wheeler C, Morse R, Goodenow RS. Amino acid comparison of the class I antigens of the mouse major histocompatibility complex. Immunogenetics. 1989:390–392. doi: 10.1007/BF02425281. [DOI] [PubMed] [Google Scholar]
- 37.Peaper DR, Cresswell P. The redox activity of ERp57 is not essential for its functions in MHC class I peptide loading. Proc. Natl. Acad. Sci. USA. 2008;105:10477–10482. doi: 10.1073/pnas.0805044105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Myers NB, Harris MR, Connolly JM, Lybarger L, Yu YY, Hansen TH. Kb, Kd, and Ld molecules share common tapasin dependencies as determined using a novel epitope tag. J. Immunol. 2000;165:5656–5663. doi: 10.4049/jimmunol.165.10.5656. [DOI] [PubMed] [Google Scholar]
- 39.Simone LC, Wang X, Tuli A, McIlhaney MM, Solheim JC. Influence of the tapasin C-terminus on the assembly of MHC class I allotypes. Immunogenetics. 2009;61:43–54. doi: 10.1007/s00251-008-0335-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zernich D, Purcell AW, Macdonald WA, Kjer-Nielsen L, Ely LK, Laham N, Crockford T, Mifsud NA, Bharadwaj M, Chang L, Tait BD, Holdsworth R, Brooks AG, Bottomley SP, Beddoe T, Peh CA, Rossjohn J, McCluskey J. Natural HLA class I polymorphism controls the pathway of antigen presentation and susceptibility to viral evasion. J. Exp. Med. 2004;200:13–24. doi: 10.1084/jem.20031680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garbi N, Tanaka S, Momburg F, Hammerling GJ. Impaired assembly of the major histocompatibility complex class I peptide-loading complex in mice deficient in the oxidoreductase ERp57. Nat. Immunol. 2006;7:93–102. doi: 10.1038/ni1288. [DOI] [PubMed] [Google Scholar]
- 42.Howarth M, Williams A, Tolstrup AB, Elliot T. Tapasin enhances MHC class I peptide presentation according to peptide half-life. Proc. Natl. Acad. Sci. USA. 2004;101:11737–11742. doi: 10.1073/pnas.0306294101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu YYL, Myers NB, Hilbert CH, Harris MR, Balendiran GK, Hansen TH. Definition and transfer of a serological epitope specific for peptide-empty forms of MHC class I. Int. Immunol. 1999;11:1897–1906. doi: 10.1093/intimm/11.12.1897. [DOI] [PubMed] [Google Scholar]
- 44.Harris MR, Lybarger L, Myers NB, Hilbert C, Solheim JC, Hansen TH, Yu YY. Interactions of HLA-B27 with the peptide loading complex as revealed by heavy chain mutations. Int. Immunol. 2001;13:1275–1282. doi: 10.1093/intimm/13.10.1275. [DOI] [PubMed] [Google Scholar]
- 45.Lybarger L, Yu YYL, Chun T, Wang C-R, Grandea AG, III, Van Kaer L, Hansen TH. Tapasin enhances peptide-induced expression of H2-M3 molecules, but is not required for the retention of open conformers. J. Immunol. 2001;167:2097–2105. doi: 10.4049/jimmunol.167.4.2097. [DOI] [PubMed] [Google Scholar]
- 46.Solheim JC, Carreno BM, Smith JD, Gorka J, Myers NB, Wen Z, Martinko JM, Lee DR, Hansen TH. Binding of peptides lacking consensus anchor residue alters H-2Ld serologic recognition. J. Immunol. 1993;151:5387–5397. [PubMed] [Google Scholar]
- 47.Smith JD, Myers NB, Gorka J, Hansen TH. Model for the in vivo assembly of nascent Ld class I molecules and for the expression of unfolded Ld molecules at the cell surface. J. Exp. Med. 1993;178:2035–2046. doi: 10.1084/jem.178.6.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ozato K, Evans GA, Shykind B, Margulies D, Seidman JG. Hybrid H-2 histocompatibility gene products assign domains recognized by alloreactive T cells. Proc. Natl. Acad. Sci. USA. 1983;80:2040–2043. doi: 10.1073/pnas.80.7.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nieto MC, Song ES, McKinney D, McMillan M, Goodenow RS. The association of H-2Ld with human β-2 microglobulin induces localized conformational changes in the α-1 and -2 superdomain. Immunogenetics. 1989;30:361–369. doi: 10.1007/BF02425276. [DOI] [PubMed] [Google Scholar]
- 50.Solheim JC, Carreno BM, Myers NB, Lee DR, Hansen TH. Peptide-induced rescue of serologic epitopes on class I MHC molecules. J. Immunol. 1995;154:1188–1197. [PubMed] [Google Scholar]
- 51.Harris MR, Lybarger L, Yu YYL, Myers NB, Hansen TH. Association of ERp57 with mouse MHC class I molecules is tapasin dependent and mimics that of calreticulin and not calnexin. J. Immunol. 2001;166:6686–6692. doi: 10.4049/jimmunol.166.11.6686. [DOI] [PubMed] [Google Scholar]
- 52.Turnquist HR, Solheim JC. Analysis of MHC class I interactions with endoplasmic reticulum proteins. Methods Mol. Biol. 2001;156:165–173. doi: 10.1385/1-59259-062-4:165. [DOI] [PubMed] [Google Scholar]