Abstract
Background
The association of survival with characteristics of thrice-weekly hemodialysis (HD) treatment including dose or duration of treatment has not been completely elucidated, especially among different race and sex categories.
Study Design
We examined the associations of time-averaged and quarterly varying (time-dependent) delivered HD dose and treatment time and 5-year (7/2001–6/2006) survival.
Setting/Participants
88,153 thrice-weekly treated HD patients from DaVita dialysis clinics.
Predictors
HD treatment dose (single pool Kt/V) and treatment time.
Outcomes/Other Measurements
Five-year mortality.
Results
Thrice-weekly treatment time below 3 hrs (but >=2.5 hrs) per HD session compared to 3.5 hrs or longer (but <5 hrs) was associated with increased death risk independent of Kt/V dose. The greatest survival gain of higher HD dose was associated with a Kt/V approaching the 1.6 to 1.8 range, beyond which the survival gain was minimal, non-existent or even tended to reverse in African American men and those with 4 to 5 hrs of HD treatment. In non-Hispanic white women, a Kt/V above 1.8 continued to exhibit survival advantage trends esp. in time-dependent models.
Limitations
Our results may incorporate uncontrolled confounding. Achieved Kt/V may have different associations than targeted Kt/V.
Conclusions
HD treatment dose and time appear to have different associations with survival among different sex or race groups. Randomized controlled trials may be warranted to examine these associations across different racial and demographics groups.
Keywords: Hemodialysis dose, hemodialysis treatment time, malnutrition-inflammation-complex syndrome, racial disparities
Introduction
Currently over 90% of the 350,000 Americans with dialysis-dependent chronic kidney disease (CKD) undergo thrice-weekly hemodialysis (HD) treatment, usually between 3 to 4 hrs per treatment session in one of the 5,000 dialysis clinics in the United States.[1] Despite the recent popularity of more frequent or home-based therapy modalities such as short daily or nocturnal hemodialysis treatments,[2] it seems likely that the majority of HD outpatients will continue with the traditional thrice-weekly modality at least in the near future. Even though it was once believed that longer or higher doses of hemodialysis treatment would improve survival, a recent randomized controlled trial known as the HEMO Study [3] did not detect survival advantage for higher dialysis dose or the use of higher flux dialysis membranes. Subgroup analyses of the HEMO Study data, however, suggested that certain groups of HD patients such as women may benefit from higher hemodialysis doses.[3]
The dose of weekly hemodialysis treatment per patient is usually measured using the dimensionless term Kt/V.[4, 5] The prescribed Kt/V was traditionally developed as the product of the coefficient of dialysis membrane clearance (K) and treatment time (t) divided by the virtual volume of urea distribution (V), whereas the delivered Kt/V is measured using urea kinetic modeling.[4, 5] The National Kidney Foundation Kidney Disease Outcome Quality Initiative (KDOQI) guidelines on Dialysis Adequacy recommended target ranges for dialysis treatment including a Kt/V of at least 1.2 for HD patients.[6, 7] Some Large Dialysis Organizations (LDO) including DaVita strive to achieve Kt/V values greater than 1.4 or even higher minima. Higher Kt/V values can be the result of longer hemodialysis treatment sessions or higher flux membranes as well as smaller body sizes, i.e., smaller V. However, it is not clear whether longer hemodialysis sessions is independently associated with greater survival, irrespective of the magnitude of the Kt/V[8, 9] or vice versa. Furthermore, the cumulative effect of the dialysis dose over time on long-term survival is unclear. It is not known whether higher combined dose of Kt/V over time is associated with greater survival across different sex or racial subgroups of CKD patients, especially when African Americans are compared to other patients.
In the current study, we examine a contemporary 5-year cohort of over 88,000 HD patients, with special focus on sex and racial differences. We hypothesized that longer hemodialysis treatment sessions or higher doses of Kt/V over time are independently associated with lower mortality even after adjustment for potential confounders. We also hypothesized that these associations may exhibit clinically relevant differences across sexes and races.
Methods
Patients
We extracted, refined, and examined data from all individuals with CKD stage 5 who underwent HD treatment from July 2001 to June 2006, i.e., for 5 consecutive years, in one of the outpatient dialysis facilities of a United States based LDO, i.e., DaVita (prior to its acquisition of former Gambro dialysis facilities). The study was approved by the Institutional Review Committees of both Los Angeles Biomedical Research Institute at Harbor-UCLA and DaVita Clinical Research. Because of the large sample size studied, the anonymity of the patients studied, and the non-intrusive nature of the research, the requirement for a written consent form was exempted.
Clinical and Demographic Measures
The creation of the cohort has been described previously.[10–15] To minimize measurement variability, all repeated measures for each patient during any given calendar quarter, i.e., over a 13-week or 3-month interval, were averaged and the quarterly means in each of the 20 calendar quarters were used in time-dependent analyses. In addition to quarterly values, time-averaged values were calculated for up to 20 calendar quarters (q1 to q20) for the Kt/V, pre- and post-hemodialysis weight, and each other laboratory and clinical measure for each patient over the 5-year study period. Subsequently, all monthly to quarterly values were averaged over the 5-year observation period to create “time-averaged” values.
Dialysis vintage was defined as the duration of time between the first day of dialysis treatment and the first day that the patient entered the cohort. The first (baseline) study quarter for each patient was the calendar quarter in which patient's vintage was >45 days during at least half of the time of that quarter.
Laboratory Measures
Blood samples were drawn using uniform techniques in all of the DaVita dialysis clinics and were transported to the DaVita Laboratory in Deland, Florida, typically within 24 hrs. All laboratory values were measured by automated and standardized methods in the DaVita Laboratory. Most laboratory values were measured monthly, including serum urea nitrogen, creatinine, albumin, calcium, phosphorus, bicarbonate, and total iron-binding capacity (TIBC). Serum ferritin and intact PTH were measured at least quarterly. Hemoglobin was measured at least monthly in essentially all patients and weekly to bi-weekly in most patients. Most blood samples were collected pre-dialysis with the exception of the post-dialysis serum urea nitrogen (SUN) that was obtained to calculate urea kinetics.
Dialysis Dose
The Kt/V (single pool) was calculated using urea kinetic modeling equations that are derived from the following equation:[5, 16, 17]
where R is the ratio of post-dialysis to pre-dialysis SUN or Ct/C0, t the duration of HD treatment time in hours, UF is the amount of ultrafiltration (in liters) during the given HD session,[18] W is the post-dialysis weight (in kg), and C0 and Ct are the pre- and post-HD concentrations of SUN. The urea kinetic modeling equations used in DaVita laboratories to calculate Kt/V are more complex, and computational software programs are employed.
Statistical Methods
Survival analyses included both conventional and time-dependent Cox proportional hazards regression modeling using both time-averaged (over 5-years) and quarterly averaged (over (each 13-week or 3-month calendar quarter or season) values, respectively. For each analysis, three models were examined based on the level of multivariate adjustment: (I) An unadjusted model that included mortality data, HD dose or HD treatment time (in either continuous or ordinal format) and entry calendar quarter (q1 through q20); (II) Case-mix adjusted models that included all of the above plus age, gender, race and ethnicity (African Americans and other self-categorized Blacks, Non-Hispanic Whites, Asians, Hispanics and others), diabetes mellitus, categories of dialysis vintage (<6 mos, 6 mos to 2 yrs, 2–5 yrs and ≥5 yrs), primary insurance (Medicare, Medicaid, private and others), marital status (married, single, divorced, widowed and other or unknown), the standardized mortality ratio of the dialysis clinic during entry quarter, and residual renal function during the entry quarter; and (III) Malnutrition-inflammation-cachexia syndrome (MICS) adjusted models which included all of the covariates in the case-mix model as well as 12 surrogates of nutritional status and inflammation, including BMI, and 11 laboratory variables as surrogates of the nutritional state or inflammation or minerals having known association with clinical outcomes in HD patients: (1) serum albumin, (2) serum TIBC, (3) serum ferritin; (4) serum creatinine, (5) serum phosphorus, (5) serum calcium, (7) intact PTH, (8) serum bicarbonate, (9) peripheral white blood cell count (WBC), (10) lymphocyte percentage, and (11) hemoglobin. The normalized protein nitrogen appearance (nPNA) also known as normalized protein catabiolic rate (nPCR) was not included due to its known correlation with Kt/V.[16, 18] Patients who were transplanted, switched to peritoneal dialysis or left DaVita clinics were censored at the time of the event. Plots of log [-log (survival rate)] against log (survival time) were performed to establish the validity of the proportionality assumption.
Additional sensitivity analyses were performed in the first 3-years (7/2001–6/2004) of this 5-year cohort, since comorbidy data as well as data on use of dialysis catheter were available in the 3-year cohort. Restricted cubic spline graphs were also utilized for time-dependent analyses as described previously [19–23] to illustrate systematic relations between Kt/V and death hazard across 4 groups of sex (women vs. men) and race (African Americans vs. non-Hispanic whites) in the 3-year (7/2001–6/2004) subcohort of the same patients with additional case-mix adjustments for comorbid states, smoking status and use of dialysis catheter. The cubic spline method also served to examine the non-linear associations of continuous Kt/V as an alternative to inappropriate linearity assumptions and to better examine interactions of sex and race with Kt/V.[24] Interaction terms based on sex and racial categories were included in most analyses. Missing covariate data (under 1% for most laboratory and demographic variables) were imputed by the mean or median of the existing values. Most analyses were carried out with the SAS, version 9.1, SAS Institute, Inc (www.sas.com). Time-dependent analyses with cubic spline graphs were carried out using Stata version 10.1 (Stata Corporation, www.stata.com).
Results
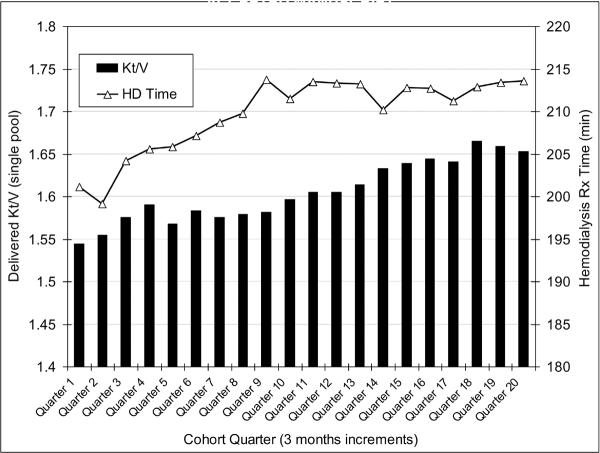
The original 5-year (7/2001–6/2006) national database of all DaVita HD patients included 152,058 adult subjects. After deleting those patients who did not maintain at least 45 days of hemodialysis treatment (9,151 patients from the first 19 calendar quarters and 3,579 patients from the last quarter), 139,328 HD patients remained. Out of the latter population, we identified 88,153 HD patients, in whom 90% or more of the monthly delivered Kt/V calculations were available, in whom the HD treatment times were recorded electronically and were stable (<0.5 hrs variability in >90% of the treatment sessions) over the observation period, and who had undergone thrice-weekly HD between 2.5 hrs and 5 hrs per treatment session. The latter range was chosen as an additional assurance to exclude short-daily or long nocturnal HD patients, although these modalities were present in <1% of HD patients between 2001 and 2006. These patients had a median cohort-time of 738 days. Demographic and survival data among the 51,175 HD patients not included in this study were similar to the main cohort (data not shown). Figure 1 shows the secular trends of the quarterly averaged Kt/V and HD treatment over the 5 years of the cohort. In the first (q01) vs. last (q20) calendar quarter, Kt/V and HD treatment time (mean±SD) were 1.54±0.33 and 201±61 min vs. 1.55±0.36 and 213±59 min, respectively.
Figure 1.
Secular trends of the quarterly averaged Kt/V and HD treatment over the 5 years of the cohort.
Footnote: Standard deviation (SD) are not includes to provide better visualization of the fine trends over time. In the first (q01) vs. last (q20) calendar quarter, Kt/V and HD treatment times (mean±SD) were 1.54±0.33 and 201±61 min vs. 1.55±0.36 and 213±59 min, respectively.
We divided the hemodialysis treatment time into four a priori selected categories of <3 hrs, 3 to <3.5 hrs, 3.5 to <4 hrs, and 4 hrs or longer (Table 1). As shown in Table 1 only 5.5% of patients (mostly women) had a HD treatment time shorter than 3 hrs per session. Patients who underwent longer HD treatment consisted of more men and more African Americans, and were younger and substantially heavier than the other three groups. The average Kt/V was between 1.58 and 1.64 with tendency towards lower values across longer HD treatment times.
Table 1.
Data in the base calendar quarter in 88,153 maintenance hemodialysis (HD) patients.
| All patients (n=88,153) | Time on HD <3 hrs (n=4,889) | Time on HD 3 to <3.5 hrs (n=26,603) | Time on HD 3.5 to <4 hrs (n=29,744) | Time on HD >=4 hrs (n=26,917) | |
|---|---|---|---|---|---|
| Percentage of patients (%) | 100 | 6 | 30 | 34 | 30 |
| Age (years) | 61.8 ± 15.5 | 64.2 ± 16.3 | 64.4 ± 15.9 | 62.1 ± 15.3 | 58.7 ± 14.7 |
| Gender (% women) | 45 | 61 | 55 | 45 | 34 |
| Diabetes mellitus (%) | 44 | 35 | 41 | 45 | 47 |
| Race and ethnicity: | |||||
| Caucasians (%) | 41 | 47 | 43 | 40 | 38 |
| Blacks (%) | 32 | 24 | 25 | 33 | 38 |
| Asians (%) | 2.9 | 5 | 4.7 | 2.5 | 1.2 |
| Hispanics (%) | 15 | 12 | 17 | 16 | 13 |
| Dialysis vintage: | |||||
| 3–6 mo (%) | 11 | 13 | 13 | 11 | 8 |
| 6–24 mo (%) | 30 | 31 | 32 | 31 | 29 |
| 2–5 years (%) | 35 | 34 | 34 | 36 | 35 |
| > 5 years (%) | 23 | 22 | 20 | 22 | 27 |
| Primary Insurance: | |||||
| Medicaid (%) | 5 | 5 | 6 | 6 | 5 |
| Marital status: | |||||
| Divorced (%) | 6 | 6 | 5 | 6 | 6 |
| Single (%) | 21 | 19 | 18 | 21 | 24 |
| Widowed (%) | 12 | 16 | 15 | 12 | 9 |
| Weight* (kg) | 75.1 ± 21.1 | 66.8 ± 19.1 | 68.8 ± 18.2 | 74.4 ± 18.9 | 83.6 ± 23.3 |
| Height (m) | 1.67 ± 0.11 | 1.63 ± 0.11 | 1.64 ± 0.11 | 1.67 ± 0.11 | 1.71 ± 0.11 |
| BMI (kg/m2) | 26.8 ± 6.9 | 24.9 ± 6.3 | 25.4 ± 6.2 | 26.5 ± 6.5 | 28.6 ± 7.6 |
| Obesity** (%) | 25 | 16 | 18 | 23 | 34 |
| Delivered Kt/Vsp | 1.61 ± 0.32 | 1.64 ± 0.39 | 1.63 ± 0.32 | 1.61 ± 0.30 | 1.58 ± 0.31 |
| QB (ml/min) | 377 ± 73 | 363 ± 82 | 370 ± 72 | 378 ± 73 | 386 ± 71 |
| nPCR (nPNA) | 0.95 ± 0.26 | 0.94 ± 0.29 | 0.96 ± 0.27 | 0.95 ± 0.25 | 0.95 ± 0.25 |
| Kru | 0.45 ± 1.48 | 0.85 ± 1.89 | 0.65 ± 1.72 | 0.37 ± 1.34 | 0.27 ± 1.22 |
| Serum albumin (g/dL) | 3.68 ± 0.46 | 3.66 ± 0.48 | 3.65 ± 0.46 | 3.67 ± 0.46 | 3.71 ± 0.45 |
| creatinine (mg/dL) | 8.1 ± 3.3 | 7.5 ± 3.2 | 7.6 ± 3.1 | 8.1 ± 3.3 | 8.7 ± 3.5 |
| ferritin (ng/mL) | 525 ± 499 | 543 ± 495 | 534 ± 507 | 524 ± 513 | 514 ± 478 |
| phosphorus (mg/dL) | 5.6 ± 1.5 | 5.6 ± 1.5 | 5.5 ± 1.5 | 5.6 ± 1.5 | 5.7 ± 1.5 |
| calcium (mg/dL) | 9.2 ± 0.7 | 9.2 ± 0.8 | 9.2 ± 0.7 | 9.2 ± 0.7 | 9.2 ± 0.7 |
| bicarbonate (mEq/L) | 22.1 ± 2.9 | 21.8 ± 3.1 | 22.0 ± 3.0 | 22.1 ± 2.9 | 22.1 ± 2.9 |
| TIBC (mg/dL) | 207 ± 46 | 206 ± 48 | 206 ± 47 | 206 ± 46 | 208 ± 45 |
| Blood hemoglobin (g/dL) | 12.0 ± 1.4 | 11.9 ± 1.4 | 12.0 ± 1.3 | 12.0 ± 1.3 | 12.0 ± 1.4 |
| WBC (per fl) | 7.5 ± 2.6 | 7.6 ± 2.6 | 7.6 ± 2.6 | 7.5 ± 2.6 | 7.4 ± 2.4 |
| Lymphocyte (% WBC) | 20.5 ± 7.9 | 19.7 ± 7.7 | 20.1 ± 7.8 | 20.6 ± 7.9 | 21.0 ± 8.0 |
Values are in percentage or mean ± SD, as appropriate. Note that patients with treatment time <2.5 hrs or >5 hrs per session were excluded to assure exclusion of short daily or nocturnal HD patients. p-value <0.001 unless otherwise specified. Abbreviations and definitions: Kt/Vsp, single-pool Kt/V; QB, blood flow; nPNA, normalized protein nitrogen appearance; Kru, residual renal clearance.
Post-dialysis.
BMI>30 kg/m2
a0.001 < p < 0.01, b 0.01 < p < 0.05,c p >0.05, ¥ median (IQR)
Table 2 shows which exact HD treatment time (in minutes) was used by at least 1% of the HD patients during a representative calendar quarter (Quarter 12, or April to June 2004). As shown in Table 2, 75% of the HD patients of this calendar quarter received HD treatments in 15 minute increment time. Of note, 63% of patients in the 4th group (>=4 hr treatment) received exactly 4 hrs of HD treatment.
Table 2.
Hemodialysis treatment time from a representative calendar quarter
| HD Treatment Time Groups | Sample size | Proportion (%) | Kt/Vsp (mean ± SD) |
|---|---|---|---|
| Group 1: <3hr (n=3,139 [6.7%]) | |||
| 2 hrs & 30 min (150 min) | 606 | 1.3 | 1.69 ± 0.47 |
| 2 hrs & 45 min (165 min) | 596 | 1.3 | 1.67 ± 0.32 |
| Group 2: 3 to <3.5 hrs (n=12,683 [26.9%)) | |||
| 3 hrs (180 min) | 7,379 | 15.7 | 1.63 ± 0.34 |
| 3hrs & 15 min (195 min) | 2,574 | 5.5 | 1.63 ± 0.28 |
| Group 3: 3.5 to <4 hrs (n=15,083 [32.1%]) | |||
| 3 hrs & 30 min (210 min) | 9,188 | 19.5 | 1.62 ± 0.31 |
| 3 hrs & 45 min (225 min) | 2,491 | 5.3 | 1.59 ± 0.28 |
| Group 4: >= 4hrs (n=16,111 [34.25%]) | |||
| 4hrs (240 min) | 10,246 | 21.8 | 1.60 ± 0.32 |
| 4 hrs & 15 min (255 min) | 1,162 | 2.5 | 1.52 ± 0.25 |
| 4 hrs & 30 min (270 min) | 1,195 | 2.5 | 1.51 ± 0.29 |
| Other HD patients with <1% per group | 11,579 | 24.6 | |
| All HD patients in quarter | 47,016 | 100 | 1.61 ± 0.32 |
Data are from the 12th calendar quarter (out of 20 quarters analyzed for the study), comprising records from May, June and July 2004. Only treatment time groups (in minutes of treatment) that include more than 1% of the patients in quarter 12 are listed. Note that patients with treatment time <2.5 hrs or >5 hrs per session were excluded. Abbreviations and definitions: Kt/Vsp, single-pool Kt/V; Hd, hemodialysis.
We divided the delivered Kt/V into eight a priori selected groups of <1.0, ≥2.2 and 6 groups of 0.2 increments in-between. Table 3 shows the eight a priori selected groups of delivered Kt/V. Crude morality was the lowest in the highest Kt/V group, even though patients in the latter group had the smallest body size and lowest proportion of African Americans, both usually associated with higher mortality.[25] We also examined bivariate and multivariate adjusted correlations between Kt/V and relevant variables in the entire cohort. The Kt/V was negatively correlated with measures of body size, and positively with nPNA (nPCR). It exhibited a weak positive association with HD time and a weak negative association with serum phosphorus (data not shown).
Table 3.
Baseline Kt/V categories in 88,153 maintenance hemodialysis (HD) patients
| Kt/Vsp | Sample size | Deaths | Women (%) | Black (%) | HD time (minutes) M ± SD | Weight (post-HD, kg) | BMI (kg/m2) | BMI>30 (%) | QB (ml/min) |
|---|---|---|---|---|---|---|---|---|---|
| < 1.0 | 3,473 (4%) | 1,358 [39%] | 25 | 42 | 207 ± 34 | 93 ± 28 | 30 ± 9 | 44 | 344 ± 75 |
| 1.0 to <1.2 | 9,201 (10%) | 3,367 [37%] | 28 | 40 | 210 ± 31 | 86 ± 24 | 29 ± 8 | 35 | 357 ± 73 |
| 1.2 to <1.4 | 19,790 (22%) | 7,382 [37%] | 34 | 36 | 212 ± 30 | 80 ± 20 | 27 ± 7 | 29 | 372 ± 74 |
| 1.4 to <1.6 | 24,071 (27%) | 8,954 [37%] | 44 | 33 | 212 ± 29 | 75 ± 20 | 27 ± 7 | 24 | 383 ± 71 |
| 1.6 to <1.8 | 16,696 (19%) | 6,311 [38%] | 56 | 29 | 212 ± 29 | 69 ± 17 | 26 ± 6 | 19 | 387 ± 70 |
| 1.8 to <2.0 | 8,226 (9%) | 3,118 [38%] | 65 | 24 | 212 ± 29 | 65 ± 16 | 25 ± 6 | 16 | 386 ± 74 |
| 2.0 to <2.2 | 3,590 (4%) | 1,303 [36%] | 68 | 21 | 213 ± 28 | 64 ± 17 | 24 ± 6 | 14 | 384 ± 71 |
| >=2.2 | 3,106(4%) | 1,034 [33%] | 63 | 19 | 209 ± 31 | 66 ± 19 | 25 ± 6 | 15 | 375 ± 73 |
Values for sample size are shown as no. (proportion of the HD patients in each Kt/V category); values for deaths are shown as no. [death rate in the indicated group during the 5 years of observation].
Abbreviations and definitions: Kt/Vsp, single-pool Kt/V; QB, blood flow; BMI, body mass index; HD, hemodialysis.
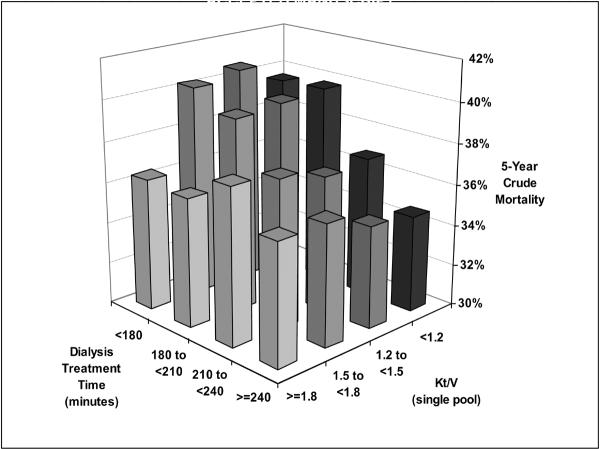
The original eight Kt/V groups were collapsed into 4 groups of <1.2, 1.2 to <1.5, 1.5 to <1.8 and ≥1.8 to be comparable with the 4 groups of hemodialysis time. Figure 2 shows the crude mortality of the four groups of HD treatment time across 4 commensurate groups of Kt/V. Within each of the four Kt/V groups the longest HD treatment time (4 hrs or more but <5 hrs) appeared associated with the lowest crude mortality, but this apparent survival advantage was more prominent in lower Kt/V ranges than the higher ones.
Figure 2.
Five-year crude mortality of 88,153 HD patients across four groups of HD treatment times and 4 strata of Kt/V (7/2001–6/2006).
Footnote: Crude rate calculations are based on patient numbers in each group.
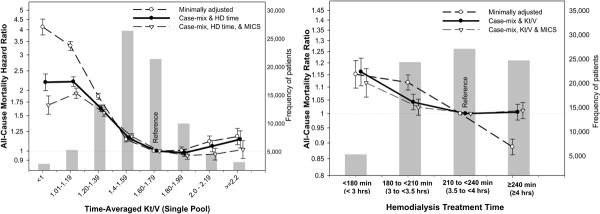
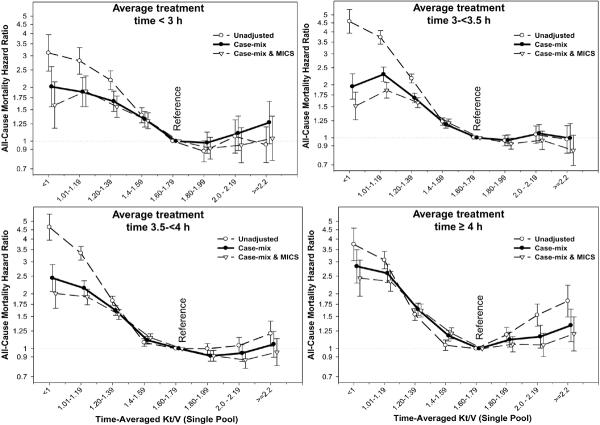
In order to examine and compare the associations of time-averaged Kt/V and HD time with mortality, Cox regression was used to obtain hazard ratios of each of these measures while adjusted for the other measure as well as interaction terms for sex and race. As shown in the Left Panel of Figure 3 the greatest survival was associated with a Kt/V above 1.6, especially 1.6 to 2.0, whereas Kt/V below 1.4 was associated with 50% to 100% increased death risk compared to the Kt/V in the 1.6 to 1.8 range (reference). As shown in the right Panel of Figure 3, a longer HD treatment was associated with the greater survival, although in multivariate adjusted models including for Kt/V, no obvious survival difference between 3.5 to <4 hrs and ≥4 hrs of was observed. Figure 4 shows the Kt/V-mortality associations within each of the 4 HD time increments. The survival advantage of Kt/V in 1.6 to 2.0 range appeared somewhat consistent across the different HD time groups although subtle differences were observed with Kt/V above 2.0 (see Figure 4).
Figure 3.
Death hazard ratios of HD treatment measures in 88,153 HD patients over 5 years (7/2001–6/2006). Left panel: Death hazard ratios of time-averaged Kt/V (single-pool). Right panel: Death hazard ratios of HD treatment time.
Footnote: Underlying bars indicate number of HD patients in each group. Note that the survival analyses include interaction terms for sex and race.
Figure 4.
Death hazard ratios of time-averaged Kt/V (single-pool) across 4 mutually exclusive groups of HD time in 88,153 HD patients (7/2001–6/2006)
Footnote: Note that the survival analyses include interaction terms for sex and race.
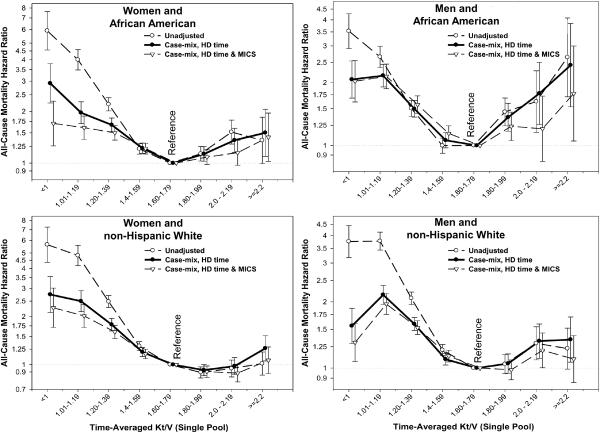
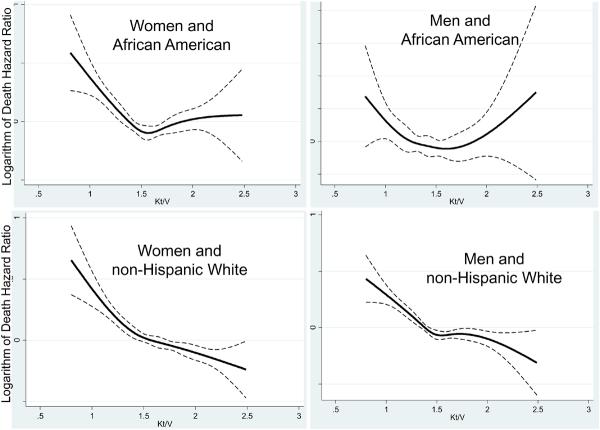
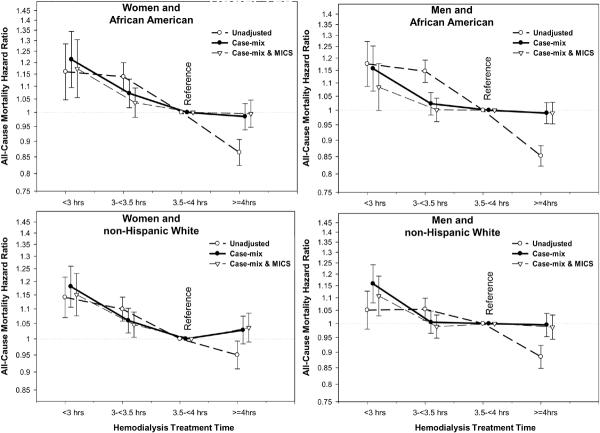
We also examined the Kt/V-mortality associations separately within 4 categories of sex and race (Figures 5 and 6). As shown in Figure 5, a more prominent reverse J-shape or U-shape trend towards worsening survival with higher Kt/V values were observed in African Americans and in men, in that Kt/V above 2.2 was associated with increased mortality (compared to the reference group of 1.6–<1.8). Time-dependent survival analyses using quarterly varying Kt/V values in the 3-year subcohort of 38,919 HD patients (with an averaged Kt/V of 1.54±0.31), after adjustment for case-mix, body size measures, comorbid conditions and dialysis catheter, showed similar cubic spline trends with a U-shape association in African American men, whereas non-Hispanic white women showed an almost linear survival advantage of higher Kt/V doses (see Figure 6). Figure 7 shows similar race and gender subgroup analyses for the HD time increments indicating that women tend to show more prominent survival advantages of longer HD treatments. Additional sensitivity analyses in subgroups of patients with or without a dialysis catheter or with blood flow rates (QB) below 350 ml/min or 300 ml/min (as additional surrogates of catheter use) showed similar associations (data not shown).
Figure 5.
Death hazard ratios of time-averaged Kt/V (single-pool) groups across 4 mutually exclusive sex and race categories in 88,153 HD patients (7/2001–6/2006)
Figure 6.
Cubic splines (solid lines) and 95% confidence levels (dashed line) of the case-mix adjusted death hazard ratios of time-dependent quarterly varying Kt/V (single-pool) across 4 mutually exclusive sex and race categories in 38,919 HD patients (7/2001–6/2004).
Footnote: Note that that the case-mix adjustments include all demographic variables in the time-averaged analyses, plus comorbid states, smoking status and use of catheter.
Figure 7.
Death hazard ratios of the 4 HD treatment time groups across 4 mutually exclusive sex and race categories in 88,153 HD patients (7/2001–6/2006)
Discussion
We studied 88,156 thrice-weekly treated HD patients in a US based LDO for up to 5 years from July 2001 through June 2006. HD time below 3 hrs per treatment session, compared to 3.5 hrs or longer, was associated with increased death risk independent of Kt/V dose. The greatest survival gain of higher HD doses was seen with a Kt/V approaching 1.6 to 1.8 range, beyond which the survival gain was minimal or non-existent. Indeed in African American men, a Kt/V above 1.8 (compared to 1.6 to <1.8) appeared associated with increased mortality. Similar U-shape or reverse J-shape Kt/V-survival trends were also noted among those receiving 4 hrs or more (but <5 hrs) HD per treatment session. However, in time-dependent models non-Hispanic white women showed an almost linearly continued survival advantage of Kt/V above 1.8. Because the time-averaged Kt/V findings are based on the average of all monthly delivered Kt/V values over the entire study period, if causal the associations are indicative of a cumulative effect of the dialysis dose over time, rather than one point in time. These findings may have important implications for the provision of adequate dialysis dose and treatment time across different sex and race subgroups of HD patients.
The 5-year survival of only 35% seen among dialysis patients in the United States [1] is worse than that for most types of cancer in the 21st century.[26] Although almost half of the deaths in dialysis patients are attributed to cardiovascular disease, traditional cardiovascular risk factors such as hypercholesterolemia, hypertension, diabetes mellitus or obesity have not been shown to explain this high mortality.[25–27] Several non-traditional risk factors, however, are uniquely associated with mortality in HD patients including – but not limited to – dialysis adequacy,[7] nutritional and inflammatory status,[28–30] bone and mineral disorders,[20, 31, 32] and anemia.[10, 33–35] Whereas nutritional status, esp. lower levels of albumin,[36] prealbumin[21] or TIBC values,[19] continues to remain the strongest survival predictor in dialysis patients, it is generally expected that better, longer or more frequent dialysis treatment should improve nutritional status and, hence, survival.[37]
Even though most [38–44] but not all [45] previous observational studies indicated survival advantages of higher HD dose, the randomized controlled HEMO Study [3] did not confirm such observational findings when comparing higher HD dose (Kt/Vsp of 1.71±0.11; where Vsp is single-pool V) compared to the control group (Kt/Vsp of 1.32±0.09) in 1,846 HD patients. African Americans, however, composed over 60% of the HEMO Study subjects,[3] as compared to 30% to 35% of HD patients across the nation and in our study. Additional analyses by Greene et al advanced the hypotheses that the “dose-targeting bias” may explain these discrepancies.[46, 47] Nevertheless, the sustained expectation about the role of dialysis adequacy has led to heightened efforts to revive and to popularize frequent (daily or nocturnal) dialysis in order to improve clinical outcomes.[48, 49]
Two HD treatment-related factors implicated in the poor outcome of HD patients are the delivered HD dose, usually measured as Kt/V, and the size of molecules removed.[49] It has been postulated that, independent of Kt/V, longer HD treatment sessions (e.g.,>3.5 hrs) could more effectively remove larger molecules, such as beta-2 microglobulin, the accumulation of which is implicated as a cause of adverse outcomes in dialysis patients.[50] In the National Cooperative Dialysis Study, HD time was an independent predictor of greater survival with p value of 0.06.[51] We found that HD time shorter than 3.5 hrs were associated with increased mortality relative to 3.5 to 4 hrs independent of case-mix. This association was reduced after further adjustment for laboratory surrogates of nutritional and inflammatory status, although the latter adjustment may be incorrect if longer HD time exerts a survival benefit by improving the nutritional status of dialysis patients.
We found that low values of both time-averaged and time-dependent Kt/V over the observation period was consistently associated with higher mortality, in that compared to a Kt/V in 1.6 to 1.8 range, a Kt/V <1.6 was associated with incrementally higher increased mortality (Figures 2 through 6). However, our analyses showed that the survival advantage of increasing Kt/V above 1.8 is either negligible or non-existent. Indeed, in the case-mix adjusted model, a Kt/V above 2.0 was associated with slight increase in mortality. Subgroup analyses across combined sex and race categories found that this U-shaped association was most prominent in African American men (Figures 5 and 6) and those whose HD treatment time was less than 3 hrs or 4 hrs or longer (Figure 4). Consistent with our findings, several observational studies have proposed that an increased HD dose is associated with greater survival in whites as compared with blacks,[41] in white women as compared with black men,[42] in smaller patients,[42] and possibly in patients with diabetes as compared with nondiabetic patients.[43] Subgroup analysis in the HEMO study suggests that the high Kt/V had a greater benefit in women but not in whites or patients with diabetes.[3] Because of the strong association between sex and body size, the issue of possible dependence of the dose effect on size is complex.[46].Although the observational nature of these associations must caution causal interpretation, the U-shape or reverse J-shaped associations for the thrice-weekly HD regimen may indicate that unusually high dialysis doses (such as weekly Kt/V above 2.0) may not be beneficial and might even be harmful, especially among African Americans and/or men. The latter observation might reflect the sensitivity of African Americans to larger swings in serum potassium (leading to disequilibrium and/or arrhythmias) [52] or to higher ultrafiltration volumes.[53]
A limitation of our study is that we could not examine some factors associated with different levels of delivered Kt/V such as patient compliance or missed treatments, dialysis membrane type and type of practice patterns.[54] It is possible that factors that lead to higher delivered Kt/V dose such as better treatment compliance or superior patient care are independently associated with greater survival, although we observed only weak associations of Kt/V with available clinical and laboratory values. These limitations reflect the observational nature of our study. We also lacked detailed and updated data of comorbid states and explicit laboratory markers of inflammation such as C-reactive protein. However, our 3-year subcohort included comorbid conditions and dialysis catheter status, which were adjusted for. Even our MICS adjusted models included many laboratory values, because higher HD dose may lead to greater survival by virtue of improving the nutritional status, MICS adjusted models may be misleading due to adjustment for these intermediates. If so, the case-mix adjusted models are more appropriate. The strengths of our study include: (1) Its contemporary nature, since all patient data were obtained from the 21st century (2001–2006); (2) uniform laboratory measurements, with all laboratory data obtained from one single facility, (3) large sample size; (4) time averaged Kt/V and laboratory data, with most values representing means of up to 60 monthly measurements; and (5) examining a 5-year cohort rather than shorter (1 to 3 year) periods of time as in previous studies.[39, 40]
In conclusion, in a large cohort of HD outpatients, who were observed for up to 5 years during the first decade of the 21st century, we found that both time-averaged and time-dependent delivered Kt/V below 1.6 or HD time shorter than 3 hrs per session was associated with increased mortality. We observed no consistent survival advantage with Kt/V above 2.0 or HD time greater than 4 hrs (but <5 hrs). Treatment dose and time appeared to have different associations with mortality among different sex or race groups. These associations, although observational in nature, are consistent with some but not all findings of the HEMO Study. [3] The latter study was performed almost a decade ago and represents a different patient population.[55] Given the high mortality in dialysis patients, the major policy implications of dialysis treatment regimens, and the recent heightened enthusiasm about frequent or longer HD treatment strategies, studies of dialysis adequacy need to continue.[48] Subgroup analyses remain important and may warrant new controlled trials by comparing racial and gender groups.
Acknowledgement
The abstracts of this paper were presented orally during the American Society of Nephrology (ASN) annual conferences, November 1–6, 2007, in San Francisco, CA; and November 2–7, 2008, Philadelphia, PA. We thank Mr. Robert Lehn at DaVita Laboratories in Deland, FL, Mr. Joe Weldon, from DaVita Informatics, for proving the national database, and Mr. Chris Rucker and Ms. Beth Bennett from DaVita Clinical Research for their continued support.
Support: The study was supported by research grants to Dr. Kalantar-Zadeh from the National Institute of Diabetes, Digestive and Kidney Disease of the National Institute of Health (R01 DK078106), an American Heart Association grant (0655776Y), a research grant from DaVita Clinical Research, and a philanthropic grant from Mr. Harold Simmons.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.United States Renal Data System United States Renal Data System 2006 Annual Data Report Atlas of Chronic Kidney Disease & End-Stage Renal Disease in the United States. Am J Kidney Dis. 2007;49:1–296. doi: 10.1053/j.ajkd.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 2.Suri RS, Garg AX, Chertow GM, Levin NW, Rocco MV, Greene T, Beck GJ, Gassman JJ, Eggers PW, Star RA, Ornt DB, Kliger AS. Frequent Hemodialysis Network (FHN) randomized trials: study design. Kidney Int. 2007;71:349–359. doi: 10.1038/sj.ki.5002032. [DOI] [PubMed] [Google Scholar]
- 3.Eknoyan G, Beck GJ, Cheung AK, Daugirdas JT, Greene T, Kusek JW, Allon M, Bailey J, Delmez JA, Depner TA, Dwyer JT, Levey AS, Levin NW, Milford E, et al. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med. 2002;347:2010–2019. doi: 10.1056/NEJMoa021583. [DOI] [PubMed] [Google Scholar]
- 4.Gotch FA, Sargent JA. A mechanistic analysis of the National Cooperative Dialysis Study (NCDS) Kidney Int. 1985;28:526–534. doi: 10.1038/ki.1985.160. [DOI] [PubMed] [Google Scholar]
- 5.Daugirdas JT. The post: pre dialysis plasma urea nitrogen ratio to estimate K.t/V and NPCR: validation. Int J Artif Organs. 1989;12:420–427. [PubMed] [Google Scholar]
- 6.National Kidney Foundation Kidney Disease Dialysis Outcome Quality Initiative,: K/DOQI clinical practice guidelines for hemodialysis adequacy. Am J Kidney Dis. 1998 doi: 10.1016/s0272-6386(97)70027-1. [DOI] [PubMed] [Google Scholar]
- 7.National Kidney Foundation Clinical practice guidelines for hemodialysis adequacy, update 2006. Am J Kidney Dis. 2006;48(Suppl 1):S2–90. doi: 10.1053/j.ajkd.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 8.Kurella M, Chertow GM. Dialysis session length (“t”) as a determinant of the adequacy of dialysis. Semin Nephrol. 2005;25:90–95. doi: 10.1016/j.semnephrol.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 9.Basile C, Lomonte C. Dialysis time is the crucial factor in the adequacy of hemodialysis. Kidney Int. 2008;74:965–966. doi: 10.1038/ki.2008.367. [DOI] [PubMed] [Google Scholar]
- 10.Kalantar-Zadeh K, Lee GH, Miller JE, Streja E, Jing J, Robertson JA, Kovesdy CP. Predictors of hyporesponsiveness to erythropoiesis-stimulating agents in hemodialysis patients. Am J Kidney Dis. 2009;53:823–834. doi: 10.1053/j.ajkd.2008.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalantar-Zadeh K, Regidor DL, Kovesdy CP, Van Wyck D, Bunnapradist S, Horwich TB, Fonarow GC. Fluid retention is associated with cardiovascular mortality in patients undergoing long-term hemodialysis. Circulation. 2009;119:671–679. doi: 10.1161/CIRCULATIONAHA.108.807362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Regidor DL, Kovesdy CP, Mehrotra R, Rambod M, Jing J, McAllister CJ, Van Wyck D, Kopple JD, Kalantar-Zadeh K. Serum alkaline phosphatase predicts mortality among maintenance hemodialysis patients. J Am Soc Nephrol. 2008;19:2193–2203. doi: 10.1681/ASN.2008010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shinaberger CS, Greenland S, Kopple JD, Van Wyck D, Mehrotra R, Kovesdy CP, Kalantar-Zadeh K. Is controlling phosphorus by decreasing dietary protein intake beneficial or harmful in persons with chronic kidney disease? Am J Clin Nutr. 2008;88:1511–1518. doi: 10.3945/ajcn.2008.26665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shinaberger CS, Kopple JD, Kovesdy CP, McAllister CJ, van Wyck D, Greenland S, Kalantar-Zadeh K. Ratio of paricalcitol dosage to serum parathyroid hormone level and survival in maintenance hemodialysis patients. Clin J Am Soc Nephrol. 2008;3:1769–1776. doi: 10.2215/CJN.01760408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Streja E, Kovesdy CP, Greenland S, Kopple JD, McAllister CJ, Nissenson AR, Kalantar-Zadeh K. Erythropoietin, iron depletion, and relative thrombocytosis: a possible explanation for hemoglobin-survival paradox in hemodialysis. Am J Kidney Dis. 2008;52:727–736. doi: 10.1053/j.ajkd.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalantar-Zadeh K, Supasyndh O, Lehn RS, McAllister CJ, Kopple JD. Normalized protein nitrogen appearance is correlated with hospitalization and mortality in hemodialysis patients with Kt/V greater than 1.20. J Ren Nutr. 2003;13:15–25. doi: 10.1053/jren.2003.50005. [DOI] [PubMed] [Google Scholar]
- 17.Depner TA. Hemodialysis adequacy: basic essentials and practical points for the nephrologist in training. Hemodial Int. 2005;9:241–254. doi: 10.1111/j.1492-7535.2005.01138.x. [DOI] [PubMed] [Google Scholar]
- 18.Shinaberger CS, Kilpatrick RD, Regidor DL, McAllister CJ, Greenland S, Kopple JD, Kalantar-Zadeh K. Longitudinal associations between dietary protein intake and survival in hemodialysis patients. Am J Kid Dis. 2006;48:37–49. doi: 10.1053/j.ajkd.2006.03.049. [DOI] [PubMed] [Google Scholar]
- 19.Bross R, Zitterkoph J, Pithia J, Benner D, Rambod M, Kovesdy CP, Kopple JD, Kalantar-Zadeh K. Association of serum total iron-binding capacity and its changes over time with nutritional and clinical outcomes in hemodialysis patients. Am J Nephrol. 2009;29:571–581. doi: 10.1159/000191470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shantouf R, Kovesdy CP, Kim Y, Ahmadi N, Luna A, Luna C, Rambod M, Nissenson AR, Budoff MJ, Kalantar-Zadeh K. Association of serum alkaline phosphatase with coronary artery calcification in maintenance hemodialysis patients. Clin J Am Soc Nephrol. 2009;4:1106–1114. doi: 10.2215/CJN.06091108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rambod M, Kovesdy CP, Bross R, Kopple JD, Kalantar-Zadeh K. Association of serum prealbumin and its changes over time with clinical outcomes and survival in patients receiving hemodialysis. Am J Clin Nutr. 2008;88:1485–1494. doi: 10.3945/ajcn.2008.25906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rambod M, Kovesdy CP, Kalantar-Zadeh K. Malnutrition-Inflammation Score for risk stratification of patients with CKD: is it the promised gold standard? Nat Clin Pract Nephrol. 2008;4:354–355. doi: 10.1038/ncpneph0834. [DOI] [PubMed] [Google Scholar]
- 23.Rambod M, Kovesdy CP, Kalantar-Zadeh K. Combined high serum ferritin and low iron saturation in hemodialysis patients: the role of inflammation. Clin J Am Soc Nephrol. 2008;3:1691–1701. doi: 10.2215/CJN.01070308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–561. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 25.Kalantar-Zadeh K, Kovesdy CP, Derose SF, Horwich TB, Fonarow GC. Racial and survival paradoxes in chronic kidney disease. Nat Clin Pract Nephrol. 2007;3:493–506. doi: 10.1038/ncpneph0570. [DOI] [PubMed] [Google Scholar]
- 26.Kalantar-Zadeh K, Abbott KC, Kronenberg F, Anker SD, Horwich TB, Fonarow GC. Epidemiology of dialysis patients and heart failure patients. Semin Nephrol. 2006;26:118–133. doi: 10.1016/j.semnephrol.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 27.Kalantar-Zadeh K, Derose SF, Nicholas S, Benner D, Sharma K, Kovesdy CP. Burnt-out diabetes: impact of chronic kidney disease progression on the natural course of diabetes mellitus. J Ren Nutr. 2009;19:33–37. doi: 10.1053/j.jrn.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rambod M, Bross R, Zitterkoph J, Benner D, Pithia J, Colman S, Kovesdy CP, Kopple JD, Kalantar-Zadeh K. Association of Malnutrition-Inflammation Score with quality of life and mortality in hemodialysis patients: a 5-year prospective cohort study. Am J Kidney Dis. 2009;53:298–309. doi: 10.1053/j.ajkd.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kovesdy CP, Kalantar-Zadeh K. Why is protein-energy wasting associated with mortality in chronic kidney disease? Semin Nephrol. 2009;29:3–14. doi: 10.1016/j.semnephrol.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalantar-Zadeh K, Kopple JD, Kamranpour N, Fogelman AM, Navab M. HDL-inflammatory index correlates with poor outcome in hemodialysis patients. Kidney Int. 2007;72:1149–1156. doi: 10.1038/sj.ki.5002491. [DOI] [PubMed] [Google Scholar]
- 31.Kalantar-Zadeh K, Duong U, Dukkipati R, Patel T, Dezfuli A, Kovesdy CP. Kidney Bone Disease and Mortality in CKD: The Role of Vitamin D, Alkaline Phosphatase and Minerals. Kidney Int Suppl. 2009 doi: 10.1038/ki.2010.189. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalantar-Zadeh K, Kuwae N, Regidor DL, Kovesdy CP, Kilpatrick RD, Shinaberger CS, McAllister CJ, Budoff MJ, Salusky IB, Kopple JD. Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int. 2006;70:771–780. doi: 10.1038/sj.ki.5001514. [DOI] [PubMed] [Google Scholar]
- 33.Regidor DL, Kopple JD, Kovesdy CP, Kilpatrick RD, McAllister CJ, Aronovitz J, Greenland S, Kalantar-Zadeh K. Associations between changes in hemoglobin and administered erythropoiesis-stimulating agent and survival in hemodialysis patients. J Am Soc Nephrol. 2006;17:1181–1191. doi: 10.1681/ASN.2005090997. [DOI] [PubMed] [Google Scholar]
- 34.Kalantar-Zadeh K. Aronoff GR: Hemoglobin variability in anemia of chronic kidney disease. J Am Soc Nephrol. 2009;20:479–487. doi: 10.1681/ASN.2007070728. [DOI] [PubMed] [Google Scholar]
- 35.Kalantar-Zadeh K, Streja E, Miller JE, Nissenson AR. Intravenous iron versus erythropoiesis-stimulating agents: friends or foes in treating chronic kidney disease anemia? Adv Chronic Kidney Dis. 2009;16:143–151. doi: 10.1053/j.ackd.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 36.Kalantar-Zadeh K, Kilpatrick RD, Kuwae N, McAllister CJ, Alcorn H, Jr., Kopple JD, Greenland S. Revisiting mortality predictability of serum albumin in the dialysis population: time dependency, longitudinal changes and population-attributable fraction. Nephrol Dial Transplant. 2005;20:1880–1888. doi: 10.1093/ndt/gfh941. [DOI] [PubMed] [Google Scholar]
- 37.Combe C, Chauveau P, Laville M, Fouque D, Azar R, Cano N, Canaud B, Roth H, Leverve X, Aparicio M. Influence of nutritional factors and hemodialysis adequacy on the survival of 1,610 French patients. Am J Kidney Dis. 2001;37:S81–88. doi: 10.1053/ajkd.2001.20756. [DOI] [PubMed] [Google Scholar]
- 38.Shinzato T, Nakai S, Akiba T, Yamazaki C, Sasaki R, Kitaoka T, Kubo K, Shinoda T, Kurokawa K, Marumo F, Sato T, Maeda K. Survival in long-term haemodialysis patients: results from the annual survey of the Japanese Society for Dialysis Therapy. Nephrol Dial Transplant. 1997;12:884–888. doi: 10.1093/ndt/12.5.884. [DOI] [PubMed] [Google Scholar]
- 39.Wolfe RA, Ashby VB, Daugirdas JT, Agodoa LY, Jones CA, Port FK. Body size, dose of hemodialysis, and mortality. Am J Kidney Dis. 2000;35:80–88. doi: 10.1016/S0272-6386(00)70305-2. [DOI] [PubMed] [Google Scholar]
- 40.Port FK, Ashby VB, Dhingra RK, Roys EC, Wolfe RA. Dialysis dose and body mass index are strongly associated with survival in hemodialysis patients. J Am Soc Nephrol. 2002;13:1061–1066. doi: 10.1681/ASN.V1341061. [DOI] [PubMed] [Google Scholar]
- 41.Owen WF, Jr., Chertow GM, Lazarus JM, Lowrie EG. Dose of hemodialysis and survival: differences by race and sex. Jama. 1998;280:1764–1768. doi: 10.1001/jama.280.20.1764. [DOI] [PubMed] [Google Scholar]
- 42.Lowrie EG, Chertow GM, Lew NL, Lazarus JM, Owen WF. The urea [clearance x dialysis time] product (Kt) as an outcome-based measure of hemodialysis dose. Kidney Int. 1999;56:729–737. doi: 10.1046/j.1523-1755.1999.00584.x. [DOI] [PubMed] [Google Scholar]
- 43.Collins AJ, Ma JZ, Umen A, Keshaviah P. Urea index and other predictors of hemodialysis patient survival. Am J Kidney Dis. 1994;23:272–282. doi: 10.1016/s0272-6386(12)80984-x. [DOI] [PubMed] [Google Scholar]
- 44.Saran R, Bragg-Gresham JL, Levin NW, Twardowski ZJ, Wizemann V, Saito A, Kimata N, Gillespie BW, Combe C, Bommer J, Akiba T, Mapes DL, Young EW, Port FK. Longer treatment time and slower ultrafiltration in hemodialysis: associations with reduced mortality in the DOPPS. Kidney Int. 2006;69:1222–1228. doi: 10.1038/sj.ki.5000186. [DOI] [PubMed] [Google Scholar]
- 45.Held PJ, Port FK, Wolfe RA, Stannard DC, Carroll CE, Daugirdas JT, Bloembergen WE, Greer JW, Hakim RM. The dose of hemodialysis and patient mortality. Kidney Int. 1996;50:550–556. doi: 10.1038/ki.1996.348. [DOI] [PubMed] [Google Scholar]
- 46.Greene T, Daugirdas J, Depner T, Allon M, Beck G, Chumlea C, Delmez J, Gotch F, Kusek JW, Levin N, Owen W, Schulman G, Star R, Toto R, et al. Association of achieved dialysis dose with mortality in the hemodialysis study: an example of “dose-targeting bias”. J Am Soc Nephrol. 2005;16:3371–3380. doi: 10.1681/ASN.2005030321. [DOI] [PubMed] [Google Scholar]
- 47.Greene T. Randomized and observational studies in nephrology: how strong is the evidence? Am J Kidney Dis. 2009;53:377–388. doi: 10.1053/j.ajkd.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 48.Lee CP, Zenios SA, Chertow GM. Cost-effectiveness of frequent in-center hemodialysis. J Am Soc Nephrol. 2008;19:1792–1797. doi: 10.1681/ASN.2008010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pierratos A. Does frequent nocturnal hemodialysis result in better outcomes than conventional thrice-weekly hemodialysis? Nat Clin Pract Nephrol. 2008;4:132–133. doi: 10.1038/ncpneph0699. [DOI] [PubMed] [Google Scholar]
- 50.Cheung AK, Rocco MV, Yan G, Leypoldt JK, Levin NW, Greene T, Agodoa L, Bailey J, Beck GJ, Clark W, Levey AS, Ornt DB, Schulman G, Schwab S, et al. Serum beta-2 microglobulin levels predict mortality in dialysis patients: results of the HEMO study. J Am Soc Nephrol. 2006;17:546–555. doi: 10.1681/ASN.2005020132. [DOI] [PubMed] [Google Scholar]
- 51.Lowrie EG, Laird NM, Parker TF, Sargent JA. Effect of the hemodialysis prescription of patient morbidity: report from the National Cooperative Dialysis Study. N Engl J Med. 1981;305:1176–1181. doi: 10.1056/NEJM198111123052003. [DOI] [PubMed] [Google Scholar]
- 52.Kovesdy CP, Regidor DL, Mehrotra R, Jing J, McAllister CJ, Greenland S, Kopple JD, Kalantar-Zadeh K. Serum and dialysate potassium concentrations and survival in hemodialysis patients. Clin J Am Soc Nephrol. 2007;2:999–1007. doi: 10.2215/CJN.04451206. [DOI] [PubMed] [Google Scholar]
- 53.Kalantar-Zadeh K, Regidor DL, Kovesdy CP, Van Wyck D, Bunnapradist S, Horwich TB, Fonarow GC. Fluid Retention is Associated with Increased Cardiovascular Mortality in Chronic Hemodialysis Patients. Circulation. 2009 doi: 10.1161/CIRCULATIONAHA.108.807362. e-published Jan 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Greene T. What did we learn from the HEMO Study? Implications of secondary analyses. Contrib Nephrol. 2005;149:69–82. doi: 10.1159/000085459. [DOI] [PubMed] [Google Scholar]
- 55.Rocco MV, Cheung AK, Greene T, Eknoyan G. The HEMO Study: applicability and generalizability. Nephrol Dial Transplant. 2005;20:278–284. doi: 10.1093/ndt/gfh304. [DOI] [PubMed] [Google Scholar]