Abstract
Tyrosine kinase receptors represent targets of great interest for cancer therapy. Here we demonstrate, for the first time, the importance of the orphan tyrosine kinase receptor, ROR2, in melanoma progression. Using melanoma tissue microarrays we show that ROR2 is expressed predominantly in metastatic melanoma. Because ROR2 has been shown to specifically interact with the non-canonical Wnt ligand, Wnt5A, this corroborates our previous data implicating Wnt5A as a mediator of melanoma metastasis. We show here that increases in Wnt5A cause increases in ROR2 expression, as well as the PKC-dependent, clathrin-mediated internalization of ROR2. WNT5A knockdown by siRNA decreases ROR2 expression, but silencing of ROR2 has no effect on WNT5A levels. ROR2 knockdown does, however, result in a decrease in signaling downstream of Wnt5A. Using in vitro and in vivo metastasis assays we demonstrate that ROR2 is necessary for the Wnt5A-mediated metastasis of melanoma cells. These data imply that ROR2 may represent a novel target for melanoma therapy.
Introduction
Wnt5A regulates melanoma progression and its pattern of expression can differentiate between melanomas of different invasive capacity (Bittner et al., 2000; Da Forno et al., 2008; Weeraratna et al., 2002). We have demonstrated that Wnt5A mediates its effects via PKC and the down-regulation of metastasis suppressors such as Kiss-1, the upregulation of metastasis-associated molecules such as CD44, and the initiation of an epithelial to mesenchymal transition (Dissanayake et al., 2007). Recently, we have shown that Wnt5A can suppress the expression of tumor-associated antigens such as MART1, and gp100, making melanoma cells less susceptible to T-cell mediated cytolysis (Dissanayake et al., 2008).
The orphan tyrosine kinase receptor, ROR2 mediates non-canonical Wnt signaling (Katoh, 2005; Saldanha et al., 1998, Oishi et al., 2003). ROR2 is a transmembrane receptor (Masiakowski & Carroll, 1992) that consists of a region of ten cysteines, previously identified as a Wnt binding domain (Saldanha et al., 1998). Mutations in ROR2 cause diseases such as brachydactyly type B (Oldridge et al., 2000) and autosomal recessive Robinow syndrome, a severe skeletal dysplasia (Afzal et al., 2000; van Bokhoven et al., 2000). Wnt5A requires ROR2 expression in order to mediate the migration of cells during mammalian palate development (He et al., 2008), and for the formation of filopodia in mouse embryonic fibroblasts, a process that requires filamin A expression (Nishita et al., 2006). We have recently shown that in melanoma cells, Wnt5A can increase the expression and calpain-mediated cleavage of filamin A, leading to increased melanoma cell motility (O'Connell et al., 2009). Here, we investigate the expression and role of ROR2 in melanoma and its relationship to Wnt5A expression.
Results
ROR2 expression correlates with levels of Wnt5A expression in human melanoma
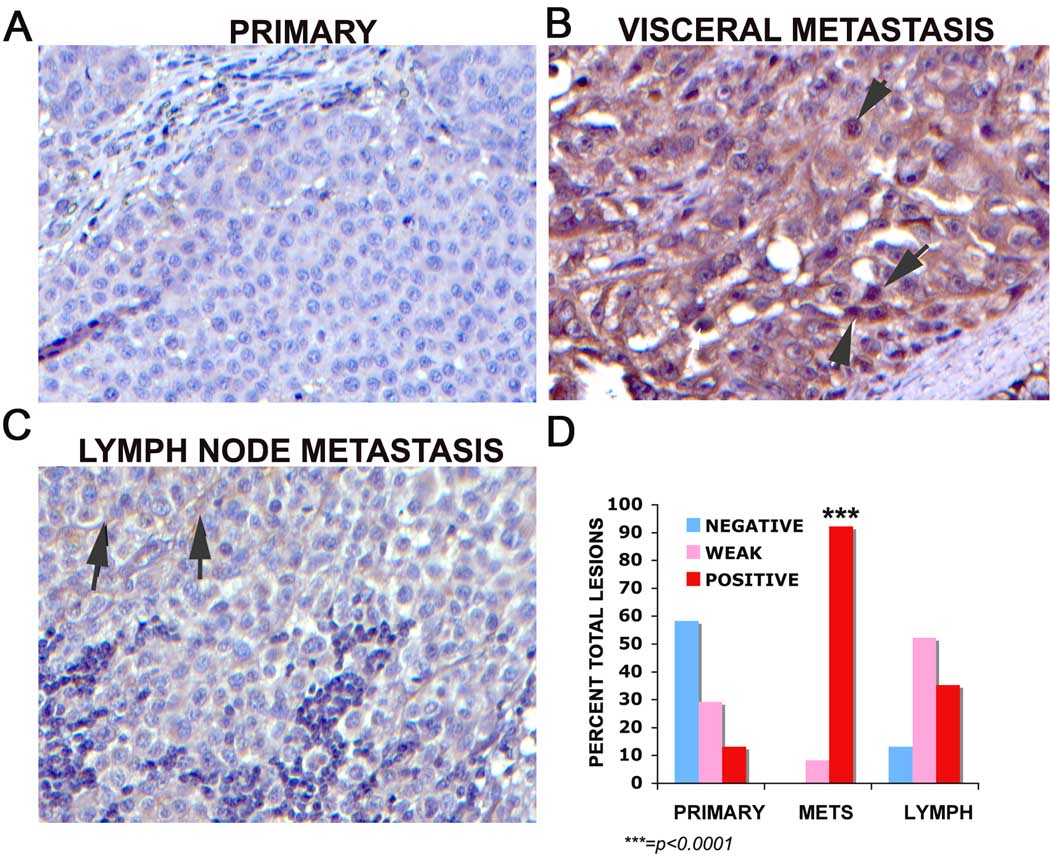
ROR2 expression was examined across a human melanoma tissue microarray (NCI Tissue Array Research Program, Bethesda, MD). Wnt5A expression is stronger in metastatic tissues, although it is also present in a heterogenous manner in primary tumors (Dissanayake et al., 2008). Our results demonstrate that ROR2 expression was absent in 28/48 primary melanomas (Figure 1A), weakly positive in the cytoplasm in 14 (1+) samples and strongly positive in 6 samples (2–3+). Four out of 31 lymph node metastases were negative for ROR2, 16 were weakly positive and 11 were positive. The pattern was most dramatic in other types of metastatic melanoma (comprised of both subcutaneous metastases and visceral metastases), as we have previously noted with Wnt5A (Dissanayake et al., 2008). In ROR2 positive visceral metastases (Figure 1B), staining appeared to be both cytoplasmic and could also be found in the nuclear or perinuclear region (Figure 1B, arrow). In the positive lymph node metastases, the location of ROR2 was largely at the membrane of the cells (Figure 1C, arrows). There were no visceral or subcutaneous metastases that were negative for ROR2, only 4 that were weakly positive, and the remaining 44 were strongly positive. These values are expressed as percentages in Figure 1D. χ2 analysis demonstrates that ROR2 expression is significantly increased in metastatic melanoma (p<0.001).
Figure 1. ROR2 expression in human melanoma.
Staining of a melanoma tissue microarray demonstrates that the majority of primary melanomas do not express ROR2 (A), whereas subcutaneous or visceral metastases of melanoma stained very strongly for ROR2 in the cytoplasm and nucleus (B, arrows demonstrate nuclear staining). Lymph node metastases for the most part had very light staining of ROR2, and in these specimens ROR2 appeared to be largely at the membrane of the cell (C, arrows). Overall staining results are represented as percentage of samples positive per group (D). The increase in ROR2 expression in metastatic melanoma as compared to primary melanoma is significant to p<0.0001.
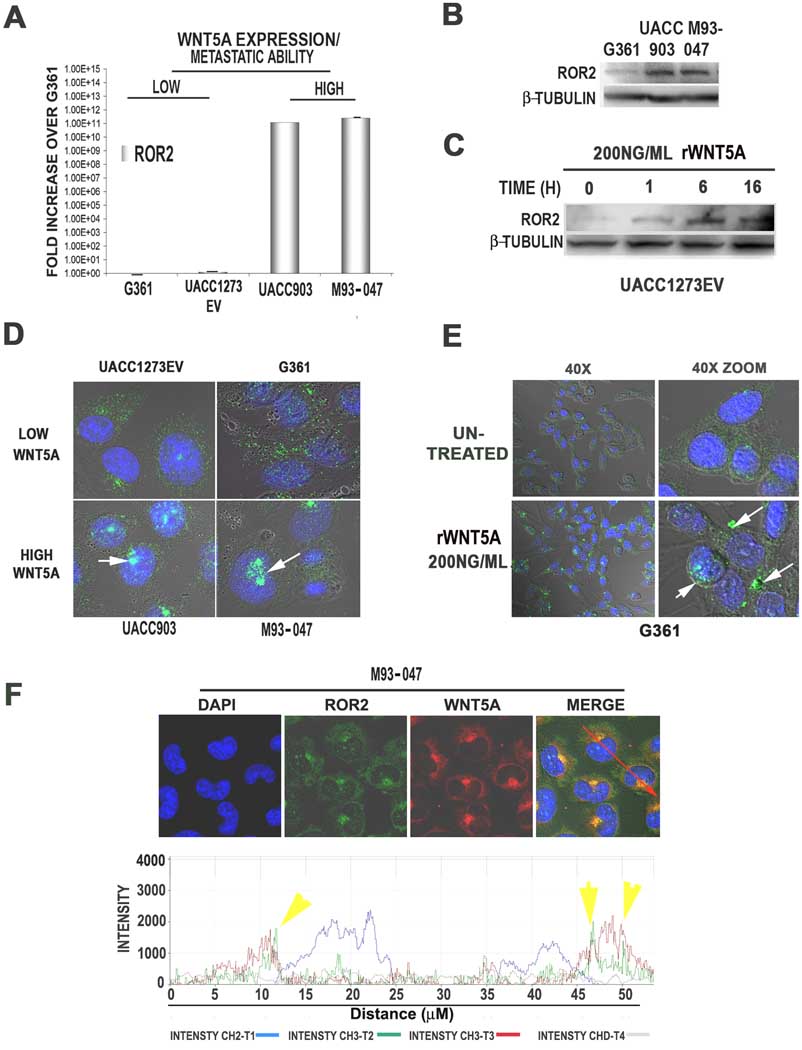
In order to study the effects of ROR2 in melanoma we selected 4 previously characterized melanoma cell lines (Dissanayake et al., 2008; Dissanayake et al., 2007). These include two less metastatic, Wnt5A-low cell lines, UACC1273EV and G361, and two more metastatic, Wnt5A-high cell lines, UACC903 and M93-047. ROR2 mRNA was highly expressed in the Wnt5A-high lines (UACC903 and M93-047) but not in the Wnt5A-low (G361 and UACC1273EV) melanoma lines (Figure 2A), unlike FZD2 and FZD5, the expression of which does not correlate to that of Wnt5A (Supplementary Figure 1). Western analysis also demonstrates that ROR2 protein expression is higher in Wnt5A high lines, consistent with the data from the tissue microarray (Figure 2B). Recombinant Wnt5A treatment resulted in an increase in ROR2 expression in the Wnt5A-low UACC1273EV cell line (Figure 2C), as well as the G361 cell line (see Figure 2E).
Figure 2. ROR2 expression and localization in human melanoma cell lines.
ROR2 mRNA is highly expressed in Wnt5A high lines (UACC903 and M93-047), but not Wnt5A low UACC1273EV and G361 cells (A). ROR2 protein levels are also higher in Wnt5A high lines than Wnt5A-low G361 cells, as shown by Western analysis (B). Treating ROR2-low UACC1273EV cells with rWnt5A increases ROR2 expression (C). Immunofluorescent analysis demonstrates that in Wnt5A-low lines, ROR2 is expressed in a diffuse cytoplasmic pattern in most cells. In Wnt5A-high lines, ROR2 is expressed in both a diffuse pattern as well as in perinuclear/ nuclear foci in the vast majority of the cells (D). Treatment of Wnt5A-low cells with rWnt5A increases the focal localization of ROR2 (E). Wnt5A and ROR2 expression are both expressed in the perinuclear foci, as shown by immunolocalization analysis (F).
In order to examine the localization of ROR2 in these cells we used immunofluorescent confocal microscopy. In the low-metastatic, Wnt5A-low lines, ROR2 staining was of low intensity, and was dispersed throughout the cytoplasm in a diffuse granular pattern, with perinuclear and nuclear foci in less than 20% of the cells (Figure 2D, UACC1273EV and G361). Wnt5A–high lines also expressed ROR2 in a light, granular cytoplasmic pattern, but also had very intense staining in perinuclear foci in the vast majority of cells (Figure 2D, UACC903 and M93-047), reflective of the tissue array staining. In order to ensure that this focal localization of ROR2 was Wnt5A-dependent, and not cell line specific, Wnt5A-low G361 and UACC1273EV cells were treated with recombinant Wnt5A (rWnt5A) for 16 hours. RWnt5A treatment resulted in an increase in the intensity of ROR2 along with the shift of ROR2 from a diffuse cytoplasmic distribution to perinuclear and nuclear foci (Figure 2E). We suspected that these results might be due to rapid internalization of ROR2 upon ligand binding, which would explain why cells that have high Wnt5A also have perinuclear and nuclear localization of ROR2. To determine if ROR2 and Wnt5A could both be found in these internal foci, Wnt5A-high cells were stained with ROR2 and Wnt5A. ROR2 and Wnt5A co-localize in the observed foci (Figure 2F), but it is unclear if this is a physical interaction.
ROR2 is internalized via clathrin, in a PKC dependent manner
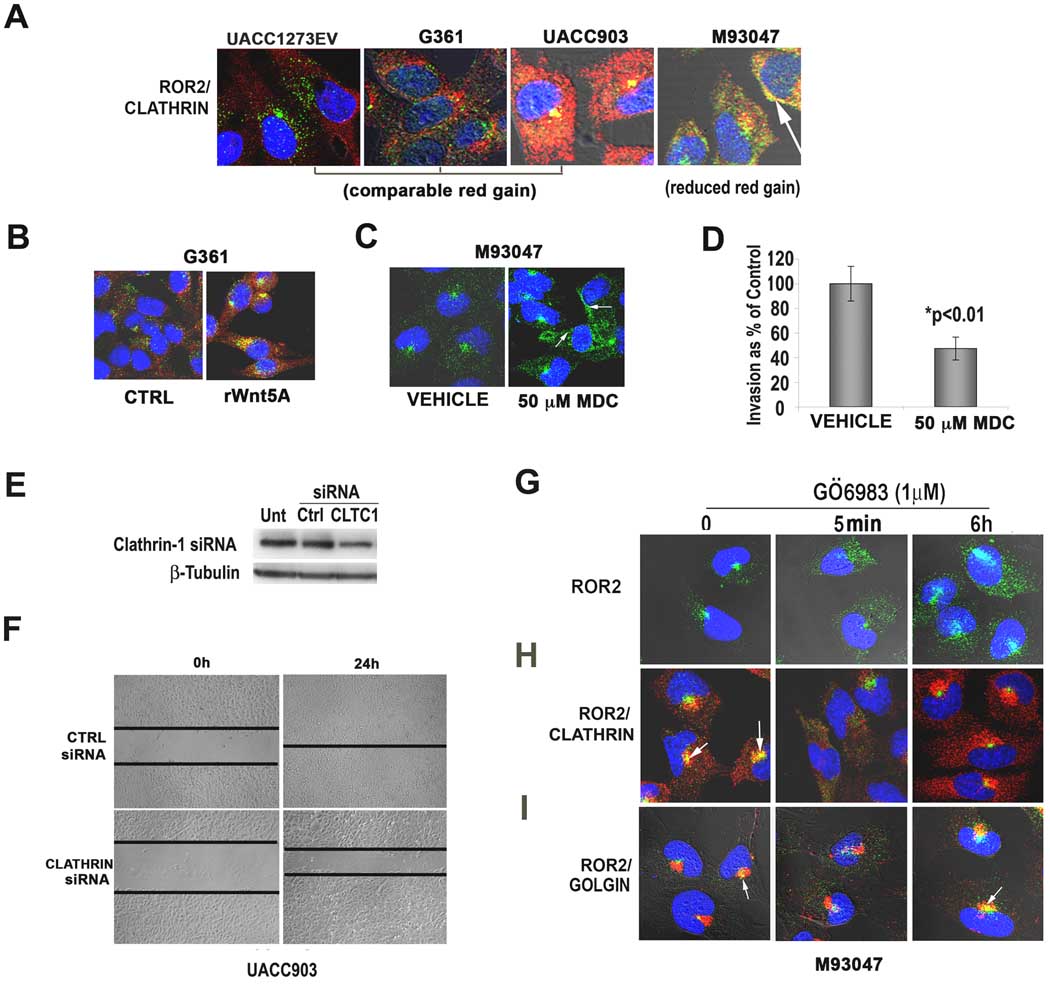
The perinuclear localization of Wnt5A/ROR2 was consistent with reports that Wnt signaling can occur from within endosomal compartments (Blitzer & Nusse, 2006). Neurotrophic tyrosine kinase receptors, to which ROR2 bears significant homology, have also been shown to signal from within endosomes upon ligand binding (Howe et al., 2001). This requires internalization via clathrin, and ligand-bound Trk receptors are often found in clathrin-coated endosomes in neuronal cells. Immunofluorescent analyses demonstrated that ROR2 (green) co-localized with clathrin (red) in Wnt5A high cell lines (Figure 3A), but not caveolin (Supplementary Figure 2A). In Wnt5A high cells, ROR2 also co-stains with both EEA1, a marker of the early endosome (Supplementary Figure 2B), supporting the theory that ROR2 is being internalized into endosomes. To determine if Wnt5A causes clathrin-mediated internalization of ROR2, cells were treated with rWnt5A. Indeed, after only 5 minutes of treatment with Wnt5A, ROR2 is internalized into foci, and colocalizes with clathrin (Figure 3B). To determine whether clathrin was required for ROR2 internalization, we inhibited clathrin using mono-dansyl cadaverine (MDC). Upon treatment of cells with 50µM of MDC for 1 hour, ROR2 internalization was inhibited in the majority of cells (Figure 3C, arrows). To demonstrate that clathrin-mediated receptor internalization is important for melanoma cell invasion, we treated cells with MDC (50µM for 1 hour), and subjected them to an invasion assay. Cells treated with MDC were unable to invade through Matrigel at the same rate as the untreated cells (Figure 3D). For an even more specific approach, we transfected cells with Clathrin 1 siRNA, which knocked down a large portion of the clathrin in the cell (Figure 3E), after which a wound-healing assay was performed. Cells treated with Clathrin 1 siRNA were also unable to close a wound as fast those transfected with a control siRNA (Figure 3F), indicating the importance of Clathrin in melanoma motility.
Figure 3. ROR2 is internalized via clathrin in a PKC-dependent manner.
Immunofluorescent analysis indicates that ROR2 (green) co-localizes with clathrin (red, A, arrows). Wnt5A treatment causes an immediate internalization of ROR2 and co-localization of ROR2 and clathrin (B). Mono-dansyl cadaverine (MDC) inhibits the internalization of ROR2 via clathrin and causes its redistribution from foci to the periphery of the cell (C, arrows). MDC inhibition also decreases the invasion of melanoma cells through Matrigel (D). Clathrin siRNA decreases clathrin expression as demonstrated by Western blot analysis (E) and decreased clathrin inhibits the motility of melanoma cells as shown in a wound-healing assay (F). In Wnt5A-high cells, PKC inhibition results in the movement of ROR2 (green) from foci to a diffuse cytoplasmic distribution in cells, upon PKC inhibition (G). Foci reappear at 6h after treatment. Co-staining with clathrin (red) indicates that PKC inhibition may affect ROR2 internalization, as in the presence of PKC inhibitor, ROR2 does not co-localize with clathrin (H). Re-emergence of ROR2-positive foci at 6h of PKC inhibitor treatment corresponds to increased co-localization with the golgi (I, arrows).
Wnt5A acts via the release of intracellular calcium and the activation of PKC, and resulting in a positive feedback loop, where increased PKC can increase Wnt5A expression and signaling. ROR2 can be phosphorylated at serine and threonine residues upon Wnt5A treatment (Yamamoto et al., 2007), but it is unclear if PKC can phosphorylate ROR2, or affect its expression or subcellular localization. We have previously demonstrated that the PKC inhibitor GÖ6983 can inhibit Wnt5A signaling and melanoma cell motility (Dissanayake et al., 2007, O’Connell et al, 2008). To ascertain whether ROR2 internalization could be affected by PKC inhibition in Wnt5A-high cells, cells were treated with GÖ6983, resulting in a redistribution of ROR2 from its perinuclear foci to the cytoplasm, beginning as early as 5 minutes (Figure 3G). By 6 hours of GÖ6983 treatment, foci reappeared, and there was also an increase in ROR2 expression. To determine if PKC activity was necessary for the internalization of ROR2 via clathrin, Wnt5A-high cells were treated with GÖ6983 and then stained for the co-expression of ROR2 and clathrin. (Figure 3H). In the presence of GÖ6983, ROR2 did not co-localize with clathrin, and this separation could be observed after 5 minutes of PKC inhibition. ROR2 and clathrin remained separate at 6 hours of treatment, despite the re-emergence of ROR2 positive foci. We hypothesized that the increased expression and focal redistribution of ROR2 might correspond to an increase in ROR2 synthesis and secretion, in response to PKC inhibition, as part of an initial signaling feedback loop. To confirm this, we stained cells for the co-expression of ROR2 and golgin (Figure 3I). At 5 minutes of PKC inhibition, there was no association between the cytoplasmic ROR2 and the golgi. However, unlike clathrin, at 6 hours there was a re-association of ROR2 with the golgi (Figure 3I, arrows), indicative of increased processing and secretion of the protein. These data imply that the activation of PKC by Wnt5A/ROR2 results in a phosphorylation of the ROR2 receptor, or some other intermediate, which in turn results in the clathrin-mediated internalization of ROR2.
Wnt5A affects ROR2 expression and requires ROR2 for signaling
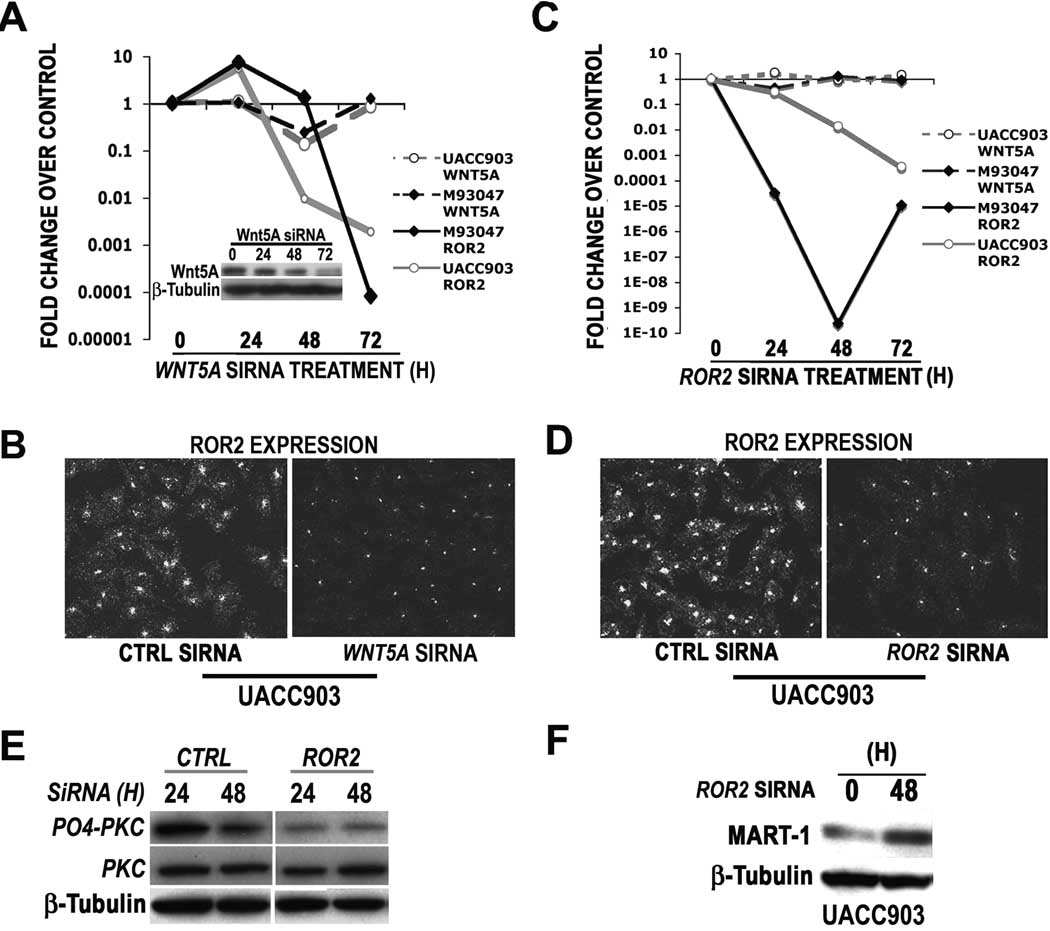
To further explore the relationship between Wnt5A and ROR2, we assessed whether knockdown of WNT5A affected levels of ROR2 expression. Cells were treated with siRNA against WNT5A for 24, 48 and 72 hours, after which time ROR2 mRNA was analyzed by real time PCR (Figure 4A). 72 hours after the knockdown of WNT5A mRNA, ROR2 mRNA expression was dramatically decreased (Figure 4A). This was concomitant with the maximal decrease in Wnt5A protein at 72h (Figure 4A, inset). Immunofluorescent analysis demonstrated that ROR2 protein levels were also reduced after WNT5A siRNA treatment (Figure 4B). To determine if ROR2 modulation could affect WNT5A expression, Wnt5A-high cells were treated with ROR2 siRNA, which was maximally reduced in UACC903 cells by 72 hours and M93-047 cells by 48 hours (Figure 4C). ROR2 protein is also reduced post siRNA treatment (Figure 4D). However, in these Wnt5A-high cells, WNT5A expression is not greatly affected by ROR2 knockdown (Figure 4C, dashed lines). While WNT5A expression was not regulated by knockdown of ROR2, Wnt5A signaling was markedly attenuated upon the lowering of ROR2 levels. This was demonstrated by decreases in the levels of PO4-PKC (Figure 4E), and by increases in levels of proteins that we have shown to be suppressed by Wnt5A (Dissanayake et al., 2008) such as MART1 (Figure 4F). These data demonstrate that WNT5A expression governs that of ROR2, and while ROR2 downregulation cannot affect WNT5A expression, it can affect the downstream signal transduction from this ligand.
Figure 4. Wnt5A expression modulates ROR2 expression, and requires ROR2 for signaling.
WNT5A knockdown results in a decrease in ROR2 mRNA (A, solid lines) in both UACC903 and M93-047 cells as analyzed by real time PCR (A) and in ROR2 protein as analyzed by immunofluorescence (B). Wnt5A protein is maximally decreased at 72hours, coincident with the greatest levels of ROR2 knockdown (A, inset). ROR2 knockdown results in a decrease in ROR2 mRNA (C) and protein (D), but not WNT5A 28 mRNA (C, dashed lines). However ROR2 knockdown does inhibit Wnt5A signaling as demonstrated by a decrease in PO4-PKC (E) and an increase in MART-1 expression by Western analysis (F).
Reduction of ROR2 results in a decrease in the invasion and metastasis of melanoma cells
We have previously shown that rWnt5A can increase the metastasis of melanoma cells in an in vivo assay (Dissanayake et al., 2008). In that experiment, we used the well-accepted melanoma metastasis model of B16F10 melanoma cells, which form pulmonary metastases upon tail vein injection. These cells have low levels of Wnt5A, but treatment with rWnt5A can further increase the levels of Wnt5A that they produce (Supplementary Figure 4), and activate Wnt5A-mediated signaling (Dissanayake et al., 2008). In a previous study, in order to see the metastasis-promoting effects of Wnt5A, we reduced both the number of cells injected into the mice (2 × 105), and the time for development of metastases (~2 weeks). This resulted in very few control mice presenting with pulmonary metastases. However, rWnt5A treatment dramatically increased both the incidence and number of metastases (Dissanayake et al., 2008).
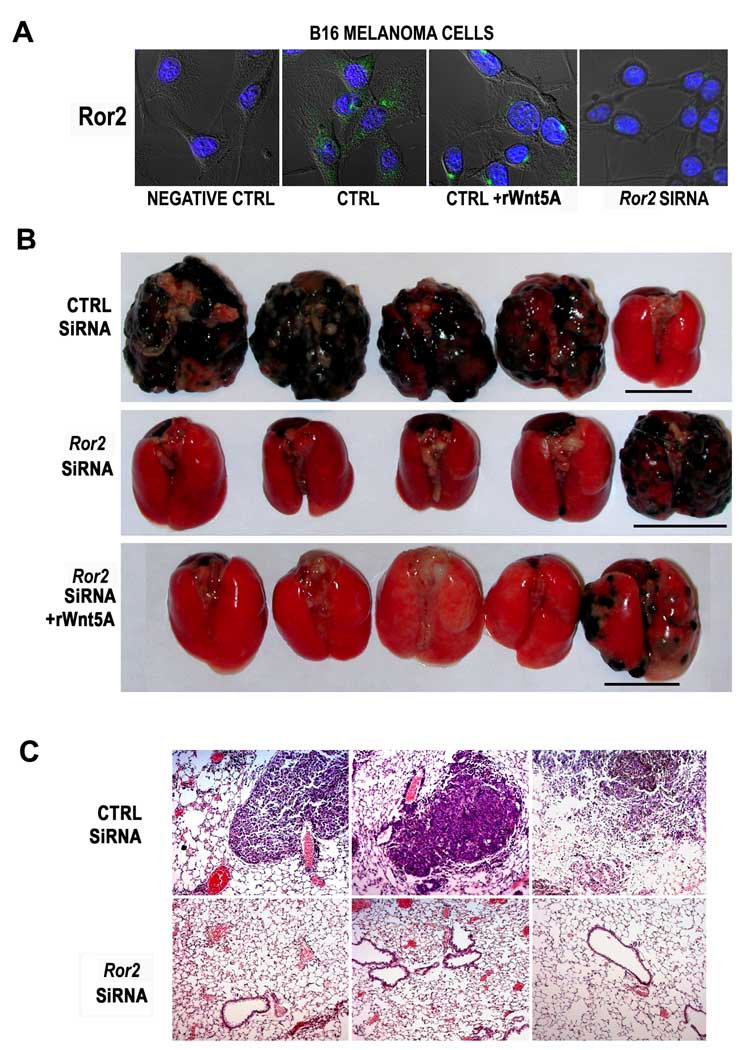
In B16 cells, Ror2 is largely cytoplasmic, but treatment of these cells with rWnt5A dramatically increased the number of foci (Figure 5A, +rWnt5A). We used mouse Ror2 siRNA to knockdown Ror2 expression, and confirmed its effectiveness (Figure 5A, Ror2 siRNA). Previous in vivo studies have indicated the effective use of naked duplex siRNAs in vivo. Lewis et al have shown that siRNA duplexes can be injected i.v. into animals and detected within 24 hours of injection in organs such as liver, lung kidney and spleen (Lewis et al., 2002). Other experiments have shown that even single-dose i.v. injections of siRNA duplexes can be effective over a period of as long as ten days, in diseases such as autoimmune hepatitis (Song et al., 2003). Liang et al (Liang et al., 2005) have shown that it is possible to use naked siRNA duplexes in vivo to inhibit cancer metastasis, and that this is most effective when siRNA is systemically- re-administered every 48–72 hours. Following this protocol, we treated B16 cells with control or Ror2 siRNA, and Ror2 siRNA treated cells were then treated with either rWnt5A or vehicle controls. To determine if Ror2 knockdown could decrease pulmonary metastases, we attempted to maximize the amount of metastases, by injecting the mice with 1 × 106 cells, which were injected via the tail vein, into 10 mice per group. Twice a week, rWnt5A treated mice received booster injections of rWnt5A. In addition, mice injected with siRNA transfected cells received booster injections of siRNA (i.e., all mice received either control siRNA or Ror2 siRNA injections) according to the protocol of Liang et al (Liang et al., 2005). Eight out of nine mice injected with control siRNA developed pulmonary metastases. In contrast, only 3 out of 10 mice treated with Ror2 siRNA developed metastases (p<0.02), and rWnt5A treatment could not significantly increase pulmonary metastases in these mice (4/10 mice developed metastases). Representative lung metastases are shown in Figure 5B. Immunohistochemistry revealed that Ror2 siRNA treated mice had very low levels of microscopic metastases. These data imply that fewer Ror2 negative cells either reach or survive at the sites of extravasation, 12 and only a subset of those have the propensity to establish tumorigenic colonies (Figure 5C).
Figure 5. ROR2 knockdown inhibits the in vivo invasion of melanoma cells in a murine model.
B16 melanoma cells express the Ror2 receptor in a pattern, and at levels similar to that of UACC1273EV cells (A, CTRL). Treatment of B16 cells with rWnt5A causes redistribution of Ror2 to perinuclear foci, as seen with human cells (A, CTRL+rWnt5A). Treating B16 cells with Ror2 siRNA results in a decrease in Ror2 expression (A, Ror2 siRNA). In vivo tail vein metastasis assays demonstrate that Ror2 knockdown can significantly inhibit the pulmonary metastasis of melanoma cells, an effect that cannot be recovered by the addition of rWnt5A (B). Outliers in each group are shown and underlined. Immunohistochemical analysis of these tumors indicates that there are very few micrometastases in the ROR2 siRNA treated mice (C).
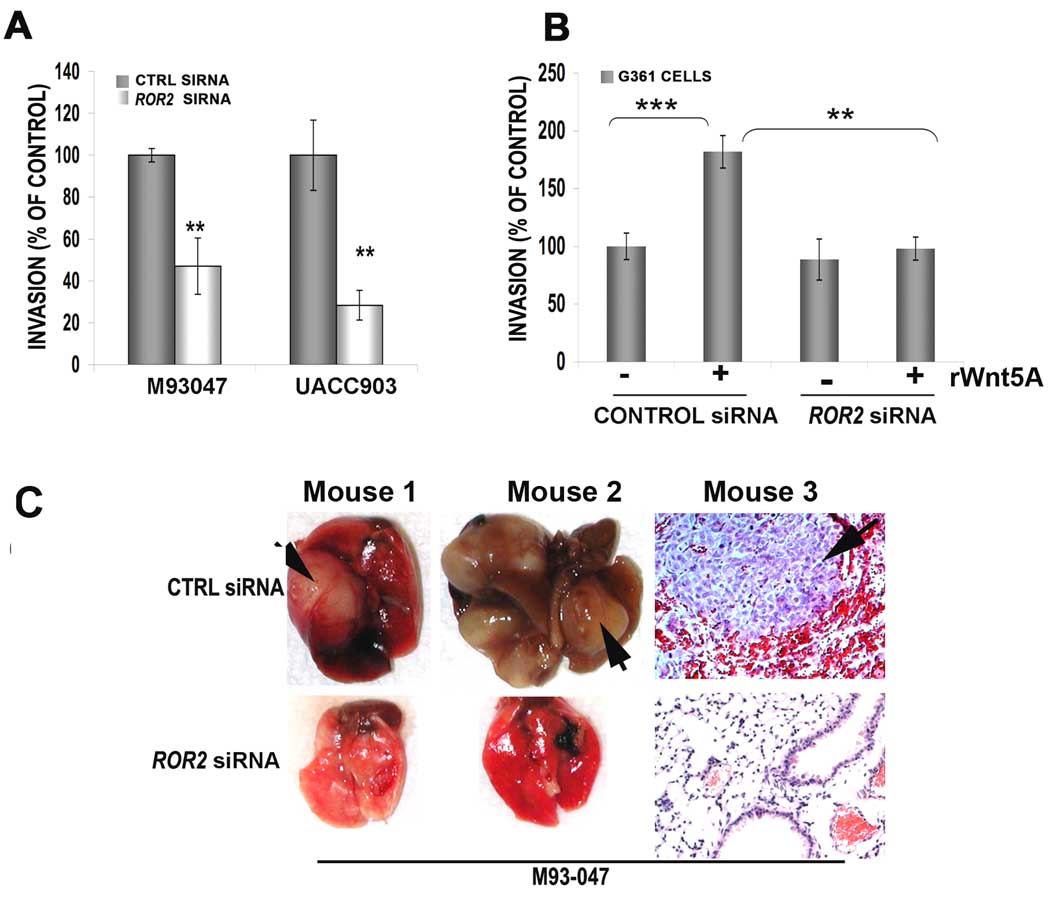
In order to determine if ROR2 is important in Wnt5A-mediated invasion in human melanoma cells as well, a Matrigel invasion assay was performed after 48 hours of ROR2 siRNA treatment in Wnt5A-high cell lines (UACC903 and M93-047). ROR2 siRNA inhibits migration of these highly metastatic melanoma cells through Matrigel by over 50% (Figure 6A). Further, treatment of G361 melanoma cells with rWnt5A in the presence of control siRNA increased their invasion through Matrigel, but in the presence of ROR2 siRNA, rWnt5A was unable to increase migration (Figure 6B). ROR2 siRNA appears to have little effect by itself, but this is likely due to the fact that G361 cells are so non-metastatic, there is already minimal invasion through Matrigel (Supplementary Figure 3A). We also performed wound-healing assays that supported these observations (Supplementary Figure 3B–D).
Figure 6. ROR2 decreases motility and invasiveness in metastatic in human melanoma cell lines in vitro and in vivo.
Using a Matrigel invasion assay, ROR2 knockdown inhibits the invasion of UACC903 and M93-047 cells by over 50% (A). Two different siRNAs were used to demonstrate the requirement for ROR2, see also Supplementary Figure 3. G361 cells are similarly affected in a matrigel invasion assay, where Wnt5A treatment can significantly (**=p<0.01, ***=p<0.001) increase their migration through Matrigel, but not in the presence of ROR2 siRNA (B). When M93-047 29 cells are injected via the tail vein into nude mice, ROR2 knockdown results in a decreased ability of M93-047 cells to form pulmonary metastases (C). Two lungs with evidence of macroscopic metastases are shown and a histology section from a third mouse (C, arrow) is shown in order to demonstrate the hemorrhaging seen in these tumors.
Finally, to determine if ROR2 inhibition could regulate the metastasis of human melanoma cells in vivo, M93-047 melanoma cells were injected via the tail vein of nude mice. Cells were either transfected with a control siRNA or with ROR2 siRNA for 48 hours prior to injection. As in the previously described B16 model, mice were injected with control or ROR2 siRNA bi-weekly. Of the five mice injected with M93-047 cells treated with control siRNA, 4 had tumors that were macroscopically visible (Figure 6C). All had several microscopically visible tumors, usually surrounded by areas of massive hemorrhage (Figure 6C, Mouse 3, CTRL SIRNA arrows). Only one of the ROR2 siRNA treated mice had macroscopically visible tumors. The rest of the ROR2 siRNA treated mice did not have any evidence of tumor spread, and very few micrometastases. Three representative mouse lungs from each group are shown in Figure 6C, two macroscopically, one microscopically. These data support our B16 mouse model, and confirm that ROR2 may indeed mediate melanoma metastasis.
Discussion
In the present study we have characterized, for the first time, the expression and role of ROR2 in melanoma, which bears significant homology to neurotrophic tyrosine kinase receptors (specifically NTRK2). The increased expression of neurotrophic tyrosine kinase receptors has previously been shown to correlate to increased aggression in many types of cancer (Desmet & Peeper, 2006; Weeraratna et al., 2001) and may provide specific targets for cancer therapy (Weeraratna et al., 2000). Our study demonstrates that ROR2 is a key mediator of Wnt5A-induced invasion in melanoma. Increases in Wnt5A increased ROR2 expression, and internalization, and reduction of WNT5A decreased ROR2 expression. However, reduction of ROR2 did not affect WNT5A levels, although it did inhibit Wnt5A signaling and both in vivo and in vitro metastasis. ROR2 is upregulated predominantly in metastatic tumors, and correlates to Wnt5A expression. A recent study confirmed that the expression of Wnt5A is strongly correlated to poorer survival in melanoma patients (Da Forno et al., 2008). Our data suggest the same might be true for ROR2.
Wnt5A can act via the Frizzled family of receptors, and in humans Wnt5A binds to Fzd2 or Fzd5 to activate non-canonical signaling. We have previously demonstrated that inhibition of Fzd5 can inhibit melanoma cell motility, implicating this receptor in melanoma invasion (Weeraratna et al., 2002). In rheumatoid arthritis, the inhibition of Fzd5 can also inhibit the Wnt5A-mediated migration of synovial fibroblasts (Sen et al., 2001). Elegant work from the Andersson lab has shown that when using the hexapeptide Foxy-5, which is a Wnt5A derivative, Wnt5A effects can be mimicked in the presence of a Fzd2 antibody, but not a Fzd5 antibody, further highlighting the requirement for Fzd5 (Safholm et al., 2006, 2008). As we have shown here, ROR2 is also critically required for Wnt5A-mediated melanoma metastasis, just as it is for Wnt5A-induced, but not Wnt3A-induced, migration of osteoblasts (Nishita et al., 2006). There are two key advantages of pursuing ROR2 as a molecular target over Fzd5 in melanoma. First, ROR2 is a Wnt5A-specific receptor (Nishita et al., 2006, Liu et al., 2008) that is not known to activate canonical Wnt signaling, unlike Fzd5, which has been shown to activate axis induction (β-catenin mediated) upon Wnt5A binding (He et al., 1997). Second, unlike Fzd5, which is expressed in the granular layer of normal skin (Romanowska et al., 2009), and across all stages of melanoma (Supplementary Figure 1), ROR2 is expressed increasingly during malignancy, allowing us to target the most aggressive cells, potentially without disrupting normal skin function.
We have also explored the significance of the subcellular localization of ROR2 in highly metastatic cells. It has been shown that canonical Wnt proteins such as Wingless are first secreted to the cell surface via the golgi, then compartmentalized into endosomes and recycled to the surface (Pfeiffer et al., 2002). The localization of ROR2 is dependent on the amount of Wnt5A present: in the presence of high amounts of Wnt5A, there is more internalized ROR2. Our observations support data from other groups that show that ROR2 localizes to rab35 positive endosomes upon phosphorylation (Akbarzadeh et al., 2008). A recent study also shows that Wnt5A treatment can result in the phosphorylation of ROR2 on its serine and threonine residues (Yamamoto et al., 2007). This is of particular interest as we have shown that, in melanoma, Wnt5A can act via PKC, a serine/threonine kinase. In the presence of PKC inhibitors, Wnt5A cannot mediate many of its effects nor modulate the invasive capacity of melanoma (Dissanayake et al., 2007). Inhibiting PKC can inhibit the clathrin-mediated internalization of ROR2, suggesting that PKC-phosphorylation of ROR2 is necessary for its internalization and potentially, its downstream signaling. Since receptors that are internalized via clathrin are often packaged into endosomes and recycled to the surface for increased signaling, our data indicate that ROR2 phosphorylation may be a pivotal step in the previously described positive feedback loop between PKC and Wnt5A (Jonsson et al., 1998).
Thus far, data from our laboratory indicates that Wnt5A expression is increased in more aggressive melanoma cells, and secreted by them (Dissanayake et al., 2007). However, Wnt5A remains bound tightly to the surface of the melanoma cells by heparan sulfate proteoglycans (O’Connell et al, in submission), which we believe can present Wnt5A to its receptors, thereby increasing autocrine signaling. We have also shown that via PKC and STAT3, Wnt5A suppresses the expression of melanoma differentiation antigens, decreasing the immunogenicity of melanoma cells (Dissanayake et al., 2008). Wnt5A causes an epithelial to mesenchymal transition, decreases the expression of metastasis suppressors such as kiss-1 (Dissanayake et al., 2007) and affects cytoskeletal proteins (O’Connell et al., 2009). In this study we show that many of these effects likely depend on the expression of ROR2, as in its absence, melanoma differentiation antigen expression and PKC signaling are decreased (Figure 4), as is melanoma metastasis. In conclusion, our findings suggest that Wnt5A and ROR2 act synergistically to increase metastatic potential in melanoma, making ROR2 an attractive molecular target for melanoma therapy.
Materials and Methods
Cell Culture
UACC903, M93-047, and UACC1273EV were maintained in RPMI (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS, Invitrogen), 100 units/ml penicillin and streptomycin and 4mM L-glutamine. G-361 cells were maintained in McCoy’s (Invitrogen) supplemented with 10% FBS, 100 units/ml penicillin and streptomycin and 4mM L-glutamine. All cell lines were cultured at 37°C in 5% CO2 and the medium was replaced every two to three days.
siRNA Transfection
HP-validated CTRL and ROR2 siRNA (200nmoles, Qiagen, Valencia, CA) were transfected into cells using Lipofectamine (Invitrogen) for 24, 48 and 72 hours as previously described (O'Connell et al., 2008). Sequences for siRNA were: CTRL: sense – 5’-UUCUCCGAACGUGUCGUCACGU-3’, antisense – 5’-ACGUGACACGUUCGGAGAA-3’; ROR2: sense – 5’-GGUUUGGGAAAGUAUACAA-3’, antisense – 5’-UUGUAGACUUUCCCAAACC-3’. A second siRNA to ROR2 was used to confirm specificity of the effects of ROR2 knockdown on migration (Supplementary Figure 3E,F), and the sequence for this siRNA was: ROR2 siRNA 2: Sense 5'-r(GGUGCUUUACGCAGAAUAATT)dTdT-3'Antisense 5'-r(UUAUUCUGCGUAAAGCACCAG)dGdG-3'.
Treatments
For recombinant Wnt5A treatment, 200ng/ml of rWnt5A (R & D Systems, Minneapolis MN) was added for 16 hours. For PKC inhibition experiments, GO6983 (Sigma, St. Louis, MO) was added to cells at a concentration of 1µM for the indicated time. These concentrations and treatment times have been previously established as optimal for these cells (Dissanayake et al., 2007). For monodansylcadaverine (MDC) treatments, cells were treated with 50µM MDC (Sigma) for 1 hour.
Immunofluorescence
Cells were seeded into 1 well chamber slides (Nunc, Rochester, NY) at 2 × 105 cells / slide and incubated overnight. Cells were then fixed and blocked as previously described (O'Connell et al., 2008). Primary antibodies used were: ROR2 (1:100, Cell Signaling, Danvers, MA), caveolin (1:100) and clathrin (1:100, BD Bioscience, Bedford, MA), EEA1 (1:100, Abcam, Cambridge, MA), and golgin-97 (1:100, Invitrogen). Primary antibodies were added in blocking buffer and cells were incubated overnight at 4°C. Cells were then washed in PBS and stained with the appropriate secondary antibody (1:2000, Alexa fluor-488 or Alexa fluor-555, Invitrogen). Cells were incubated for 1 hour at room temperature and then washed in PBS and mounted in Prolong Gold anti fade reagent containing DAPI (Invitrogen).
Immunohistochemistry (IHC)
Paraffin tissue sections were rehydrated through a xylene / alcohol series, and antigen retrieval was performed as previously described (Dissanayake et al., 2008). Slides were then placed in primary antibody (ROR2, 1:10, Abgent, San Diego, CA) and left at 4°C overnight. Slides were washed in PBS (3× 5 minutes) and treated with anti-rabbit biotinylated HRP (1: 500, Amersham Biosciences, Piscataway, NJ) for 1 hour. Slides were washed, developed using diamino-benzidine (DAB) chromagen (Lab Vision, Fremont, CA) and counterstained using hematoxylin, and then scored by a pathologist (SMH).
Real Time PCR
RNA was extracted using Trizol (Invitrogen) and an RNeasy Mini kit (Qiagen) as previously described (O'Connell et al., 2008). Gene expression was quantified using the SYBR green method of real time PCR and mRNA levels were compared to standard curves. Primers were designed to cross intron exon junctions where possible: ROR2: forward – GGCAGAACCCATCCTCGTG, reverse – CGACTGCGAATCCAGGACC; WNT5A: forward – TAAGCCCAGGAGTTGCTTTG, reverse – GCAGAGAGGCTGTGCTCCTA; FZD2: forward – ACATCGCCTACAACCAGACC, reverse – CCTTCACCAGCGGATAGAAC; FZD5: forward – GTGACCCAGGGACGGAG, reverse – GAGAGACGGTTAGGGCTCG. Real time PCR was performed on an ABI Prism 7300 sequence detection system using standard conditions. Samples were normalized against the 18S gene, using Universal 18S primers (Ambion, Austin, TX), and expression was calculated using the standard curve method according to the manufacturer’s protocol (Perkin Elmer, Waltham, MA).
SDS-PAGE and Western Blotting
50µg of protein was isolated and subjected to SDS-PAGE and transferred onto PVDF as previously described (O'Connell et al., 2008). Primary antibodies were added at appropriate dilutions in 5% milk/TBST and incubated overnight at 4°C with rotation. Primary antibodies used were clathrin (1:1000, BD Bioscience, Bedford, MA), biotinylated Wnt5A (1:100, R&D Systems), PO4-PKC (1;1000, Cell Signaling), MART-1 (1:200, Lab Vision, Freemont, CA), and β-tubulin (1:1000, Cell Signaling). Membranes were washed 3X in PBS, then the appropriate secondary antibodies (e.g., anti-mouse IgG, streptavidin, or anti-rabbit IgG) conjugated to horseradish peroxidase were added at 1:2000 for 1 hour at room temperature. Bands were visualized using ECL Plus (Amersham, Uppsala, Sweden).
Matrigel Invasion assays
Invasion assays were performed using transwell migration chambers (Corning life Sciences, Lowell, MA). 8µM filters were coated with 150 µL of 80µg/mL reconstituted basement membrane (Matrigel) (Becton Dickinson, Franklin Lakes, NJ). Cells were serum starved for 16 hours, during which time rWnt5A (200ng/ mL) was added to the cells as indicated, counted and 2 × 105 cells were seeded onto the filters. 20% fetal calf serum was placed in the lower well to act as a chemoattractant. Cells were allowed to invade and adhere to the lower chamber, after which they were stained using crystal violet, and counted. All cell lines were assayed in triplicate.
In vivo pulmonary metastasis assays
In vivo pulmonary metastasis assays were performed as previously described under ACUC protocol number LI-286-2010 (Dissanayake et al., 2008). Briefly, B16F10 mouse melanoma cells were grown in RPMI containing 10% serum. Cells were seeded in 6-well plates and grown until approximately 70% confluent. B16 cells were then transfected with either a CTRL or Ror2 siRNA for 48 hours. Sequence for siRNA were: CTRL: forward – r(UUCUCCGAACGUGUCACGU)dTdT, reverse – r(ACGUGACACGUUCGGAGAA)dTdT; Ror2: forward - r(GGGAGUGCCUUGUAUUAAA)dTdT, reverse – r(UUUAAUACAAGGCACUCCC)dTdT. After 36 hours of transfection, cells requiring treatment with Wnt5A were treated with 100ng/ml (optimal dose for B16 cells) for 16 hours. Next, transfected, treated B16 cells were injected into 8-week old C57BL/6 mice at 1×106 cells/mouse. Mice were left for 17 days and were injected i.p. with 100ng/ml Wnt5A (75ng/mouse) and 200nmols siRNA occurring on days 4, 7, and 10 via subcutaneous injection. At day 17, the mice were sacrificed and the lungs harvested. For nude mice experiments, M93-047 cells were transfected with either control or ROR2 siRNA. Cells were injected into the tail vein of athymic nude mice (BalbCNu/Nu), as described above, with the exception that metastases were given four weeks to develop, as preliminary assays indicated that this was the necessary amount of time for formation of pulmonary metastases. C57BL/6 and BalbCNu/Nu mice (Charles River Laboratories, Frederick, MD) were housed at the National Institute on Aging animal facility, Baltimore, MD. Animal care was provided according to the Guide for the Care and Use of Laboratory Animals (ref: National Research Council. Guide for the Care and Use of 22 Laboratory Animals. Washington, DC: National Academy Press; 1985; NIH publication number 86–23).
Supplementary Material
Acknowledgements
We thank Ms. Ana Lustig for assistance with animal experiments. This work was supported by the Intramural Research Program of the National Institute on Aging, Baltimore MD 21224.
References
- Afzal AR, Rajab A, Fenske CD, Oldridge M, Elanko N, Ternes-Pereira E, et al. Recessive Robinow syndrome, allelic to dominant brachydactyly type B, is caused by mutation of ROR2. Nat Genet. 2000;25:419–422. doi: 10.1038/78107. [DOI] [PubMed] [Google Scholar]
- Akbarzadeh S, Wheldon LM, Sweet SM, Talma S, Mardakheh FK, Heath JK. The deleted in brachydactyly B domain of ROR2 is required for receptor activation by recruitment of Src. PLoS ONE. 2008;3:e1873. doi: 10.1371/journal.pone.0001873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittner M, Meltzer P, Chen Y, Jiang Y, Seftor E, Hendrix M, et al. Molecular classification of cutaneous malignant melanoma by gene expression profiling. Nature. 2000;406:536–540. doi: 10.1038/35020115. [DOI] [PubMed] [Google Scholar]
- Blitzer JT, Nusse R. A critical role for endocytosis in Wnt signaling. BMC Cell Biol. 2006;7:28. doi: 10.1186/1471-2121-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Forno PD, Pringle JH, Hutchinson P, Osborn J, Huang Q, Potter L, et al. WNT5A expression increases during melanoma progression and correlates with outcome. Clin Cancer Res. 2008;14:5825–5832. doi: 10.1158/1078-0432.CCR-07-5104. [DOI] [PubMed] [Google Scholar]
- Desmet CJ, Peeper DS. The neurotrophic receptor TrkB: a drug target in anti-cancer therapy? Cell Mol Life Sci. 2006;63:755–759. doi: 10.1007/s00018-005-5490-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dissanayake SK, Olkhanud PB, O’Connell MP, Carter A, French AD, Camilli TC, et al. Wnt5A Regulates Expression of Tumor Associated Antigens in Melanoma Via Changes in STAT3 Phosphorylation. Cancer Res. 2008;68 doi: 10.1158/0008-5472.CAN-08-2149. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dissanayake SK, Wade M, Johnson CE, O'Connell MP, Leotlela PD, French AD, et al. The Wnt5A/protein kinase C pathway mediates motility in melanoma cells via the inhibition of metastasis suppressors and initiation of an epithelial to mesenchymal transition. J Biol Chem. 2007;282:17259–17271. doi: 10.1074/jbc.M700075200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F, Xiong W, Yu X, Espinoza-Lewis R, Liu C, Gu S, et al. Wnt5a regulates directional cell migration and cell proliferation via Ror2-mediated noncanonical pathway in mammalian palate development. Development. 2008;135:3871–3879. doi: 10.1242/dev.025767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Saint-Jeannet JP, Wang Y, Nathans J, Dawid I, Varmus H. A member of the Frizzled protein family mediating axis induction by Wnt-5A. Science. 1997;275:1652–1654. doi: 10.1126/science.275.5306.1652. [DOI] [PubMed] [Google Scholar]
- Hikasa H, Shibata M, Hiratani I, Taira M. The Xenopus receptor tyrosine kinase Xror2 modulates morphogenetic movements of the axial mesoderm and neuroectoderm via Wnt signaling. Development. 2002;129:5227–5239. doi: 10.1242/dev.129.22.5227. [DOI] [PubMed] [Google Scholar]
- Howe CL, Valletta JS, Rusnak AS, Mobley WC. NGF signaling from clathrin-coated vesicles: evidence that signaling endosomes serve as a platform for the Ras-MAPK pathway. Neuron. 2001;32:801–814. doi: 10.1016/s0896-6273(01)00526-8. [DOI] [PubMed] [Google Scholar]
- Jonsson M, Smith K, Harris AL. Regulation of Wnt5a expression in human mammary cells by protein kinase C activity and the cytoskeleton. Br J Cancer. 1998;78:430–438. doi: 10.1038/bjc.1998.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh M. WNT/PCP signaling pathway and human cancer (review) Oncol Rep. 2005;14:1583–1588. [PubMed] [Google Scholar]
- Lewis DL, Hagstrom JE, Loomis AG, Wolff JA, Herweijer H. Efficient delivery of siRNA for inhibition of gene expression in postnatal mice. Nat Genet. 2002;32:107–108. doi: 10.1038/ng944. [DOI] [PubMed] [Google Scholar]
- Liang Z, Yoon Y, Votaw J, Goodman MM, Williams L, Shim H. Silencing of CXCR4 blocks breast cancer metastasis. Cancer Res. 2005;65:967–971. [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Rubin B, Bodine PV, Billiard J. Wnt5a induces homodimerization and activation of Ror2 receptor tyrosine kinase. J Cell Biochem. 2008;105:497–502. doi: 10.1002/jcb.21848. [DOI] [PubMed] [Google Scholar]
- Masiakowski P, Carroll RD. A novel family of cell surface receptors with tyrosine kinase-like domain. J Biol Chem. 1992;267:26181–26190. [PubMed] [Google Scholar]
- Nishita M, Yoo SK, Nomachi A, Kani S, Sougawa N, Ohta Y, et al. Filopodia formation mediated by receptor tyrosine kinase Ror2 is required for Wnt5a-induced cell migration. J Cell Biol. 2006;175:555–562. doi: 10.1083/jcb.200607127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell MP, Fiori JL, Baugher KM, Indig FE, French AD, Camilli TC, et al. Wnt5A Activates the Calpain-Mediated Cleavage of Filamin A. J Invest Dermatol. 2009 doi: 10.1038/jid.2008.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell MP, French AD, Leotlela PD, Weeraratna AT. Assaying Wnt5A-mediated invasion in melanoma cells. Methods Mol Biol. 2008;468:243–253. doi: 10.1007/978-1-59745-249-6_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi I, Suzuki H, Onishi N, Takada R, Kani S, Ohkawara B, et al. The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes Cells. 2003;8:645–654. doi: 10.1046/j.1365-2443.2003.00662.x. [DOI] [PubMed] [Google Scholar]
- Oldridge M, Fortuna AM, Maringa M, Propping P, Mansour S, Pollitt C, et al. Dominant mutations in ROR2, encoding an orphan receptor tyrosine kinase, cause brachydactyly type B. Nat Genet. 2000;24:275–278. doi: 10.1038/73495. [DOI] [PubMed] [Google Scholar]
- Pfeiffer S, Ricardo S, Manneville JB, Alexandre C, Vincent JP. Producing cells retain and recycle Wingless in Drosophila embryos. Curr Biol. 2002;12:957–962. doi: 10.1016/s0960-9822(02)00867-9. [DOI] [PubMed] [Google Scholar]
- Romanowska M, Evans A, Kellock D, Bray SE, McLean K, Donandt S, et al. Wnt5a exhibits layer-specific expression in adult skin, is upregulated in psoriasis, and synergizes with type 1 interferon. PLoS One. 2009;4:e5354. doi: 10.1371/journal.pone.0005354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safholm A, Leandersson K, Dejmek J, Nielsen CK, Villoutreix BO, Andersson T. A formylated hexapeptide ligand mimics the ability of Wnt-5a to impair migration of human breast epithelial cells. J Biol Chem. 2006;281:2740–2749. doi: 10.1074/jbc.M508386200. [DOI] [PubMed] [Google Scholar]
- Safholm A, Tuomela J, Rosenkvist J, Dejmek J, Harkonen P, Andersson T. The Wnt-5a-derived hexapeptide Foxy-5 inhibits breast cancer metastasis in vivo by targeting cell motility. Clin Cancer Res. 2008;14:6556–6563. doi: 10.1158/1078-0432.CCR-08-0711. [DOI] [PubMed] [Google Scholar]
- Saldanha J, Singh J, Mahadevan D. Identification of a Frizzled-like cysteine rich domain in the extracellular region of developmental receptor tyrosine kinases. Protein Sci. 1998;7:1632–1635. doi: 10.1002/pro.5560070718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen M, Chamorro M, Reifert J, Corr M, Carson DA. Blockade of Wnt-5A/frizzled 5 signaling inhibits rheumatoid synoviocyte activation. Arthritis Rheum. 2001;44:772–781. doi: 10.1002/1529-0131(200104)44:4<772::AID-ANR133>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Song E, Lee SK, Wang J, Ince N, Ouyang N, Min J, et al. RNA interference targeting Fas protects mice from fulminant hepatitis. Nat Med. 2003;9:347–351. doi: 10.1038/nm828. [DOI] [PubMed] [Google Scholar]
- van Bokhoven H, Celli J, Kayserili H, van Beusekom E, Balci S, Brussel W, et al. Mutation of the gene encoding the ROR2 tyrosine kinase causes autosomal recessive Robinow syndrome. Nat Genet. 2000;25:423–426. doi: 10.1038/78113. [DOI] [PubMed] [Google Scholar]
- Weeraratna AT, Arnold JT, George DJ, DeMarzo A, Isaacs JT. Rational basis for Trk inhibition therapy for prostate cancer. Prostate. 2000;45:140–148. doi: 10.1002/1097-0045(20001001)45:2<140::aid-pros8>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Weeraratna AT, Dalrymple SL, Lamb JC, Denmeade SR, Miknyoczki S, Dionne CA, et al. Pan-trk inhibition decreases metastasis and enhances host survival in experimental models as a result of its selective induction of apoptosis of prostate cancer cells. Clin Cancer Res. 2001;7:2237–2245. [PubMed] [Google Scholar]
- Weeraratna AT, Jiang Y, Hostetter G, Rosenblatt K, Duray P, Bittner M, et al. Wnt5a signaling directly affects cell motility and invasion of metastatic melanoma. Cancer Cell. 2002;1:279–288. doi: 10.1016/s1535-6108(02)00045-4. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Yoo SK, Nishita M, Kikuchi A, Minami Y. Wnt5a modulates glycogen synthase kinase 3 to induce phosphorylation of receptor tyrosine kinase Ror2. Genes Cells. 2007;12:1215–1223. doi: 10.1111/j.1365-2443.2007.01128.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.