Abstract
Our aim was to investigate the patterns of functional inputs and outputs from individual barrels in the mouse somatosensory cortex, and to test the hypothesis that individual barrels in layer IV are functionally independent of direct inputs from neighboring barrels. In a mouse in vitro slice preparation of the barrel cortex, we recorded voltage-sensitive dye signals evoked in response to microstimulation of a single barrel. Activity propagated from the stimulated barrel to the supragranular layers, where it spread to activate several barrel columns. However, in no instance did activity propagate directly from the stimulated barrel to neighboring barrels. Neither suppression of GABAergic inhibition, nor activation of N-methyl-D-aspartate receptors, revealed direct interbarrel interactions. By contrast, microstimulation in the supra- or infragranular layers resulted in direct propagation of activity to neighboring barrel columns. We conclude that the neurons within individual barrels are functionally independent of direct inputs from neighboring barrels. This suggests that the response properties of layer IV barrel neurons are shaped primarily by their presynaptic thalamic afferents and by intrabarrel interactions, and that these responses are independent of direct inputs from neighboring barrels.
INTRODUCTION
Processing of sensory information in the cerebral cortex is described as a hierarchical process, in which inputs from “specific” thalamic nuclei are integrated first by neurons in layer IV, which, in turn, provide inputs to neurons in other layers of the same functional column, and subsequently to adjacent columns (Gilbert 1992; Keller 1995; Rauschecker et al. 1997). Since layer IV is considered the first stage in this information processing scheme, it has been the focus of numerous studies attempting to describe the mechanisms that shape the responses of neurons in this layer. A key issue of debate is the relative contribution of thalamic versus intracortical inputs in shaping the response properties of layer IV neurons (see Miller et al. 2001). For example, in the visual cortex, a controversy exists regarding the roles of thalamic versus intracortical interactions in shaping orientation and direction tuning (see Roerig and Kao 1999; Worgotter and Koch 1991). A similar controversy exists in the “barrel” cortex regarding the relative contribution of thalamic versus intracortical inputs in shaping the responses of layer IV neurons.
The barrel region of the somatosensory cortex contains representations of the whiskers, which map in a topographic manner onto corresponding arrays of cellular aggregates termed barrels (Woolsey and Van der Loos 1970). Neurons in a layer IV barrel that corresponds to a particular whisker respond preferentially to stimulation of that “principal” whisker, and these responses are determined primarily by inputs from a single thalamic barreloid (reviewed by Armstrong-James 1995; Simons 1995). Most layer IV (barrel) neurons respond also to activation of whiskers neighboring the principal whisker (the “surround receptive fields;” SRF), but these responses are less vigorous, more temporally dispersed, and of longer onset latency than the responses to the principal whisker (see Armstrong-James 1995; Simons 1995). Two alternative hypotheses have been proposed to explain the mechanisms generating the SRFs of barrel neurons. One hypothesis argues that SRFs are shaped by direct synaptic interactions between neurons in adjacent barrels (Armstrong-James 1995). This hypothesis is supported by data from tract-tracing studies (Bernardo et al. 1990; Hoeflinger et al. 1995) and from experiments demonstrating that lesions of a barrel eliminate responses to the homologous whisker in the remaining barrels (Armstrong-James et al. 1991). A conflicting hypothesis states that SRFs are shaped by thalamic and local synaptic interactions within a single barrel, and that barrel neurons receive no direct inputs from neighboring barrels (Simons 1995). This hypothesis has support from morphological analyses of individual neurons (Lübke et al. 2000; Simons and Woolsey 1984), from recordings of pairs of barrel neurons (Feldmeyer et al. 1999; Petersen and Sakmann 2000), and, surprisingly, from experiments demonstrating that lesions of a barrel have no effect on SRFs in neighboring barrels (Goldreich et al. 1999).
Although some of the discrepant findings described above are likely due to differences in experimental approaches (see DISCUSSION in Goldreich et al. 1999), the crux of this controversy is whether or not neighboring barrels communicate directly with each other. In an attempt to address this question, we took advantage of functional imaging techniques to systematically assess the sources of synaptic inputs to layer IV barrel neurons. Our results demonstrate that individual barrel neurons receive little or no direct inputs from neighboring barrels, and that most inputs to barrel neurons originate from neurons located in their parent barrel column. Some of these results appeared previously in abstract form (Laaris et al. 2000b).
METHODS
Slice preparation
Animal protocols used in this study complied with all pertinent institutional and Federal regulations. Young-adult CD-1 male mice (n = 26), 21–30 days old, were anesthetized with Ketamine hydrochloride (30 mg/kg). The brains were removed, and 400-µM-thick slices were prepared as previously described (Gottlieb and Keller 1997). Because intracortical connections occur preferentially among barrel rows (Bernardo et al. 1990; Keller and Carlson 1999), and to preserve as many of these intracortical connections as possible, we prepared slices that were parallel to the orientation of barrel rows. For this purpose, slices were cut in a frontal oblique plane, perpendicular to the dorsal aspect of the cortex and oriented posteromedially at 55° to the medial surface of the hemisphere. This plane is approximately parallel to the orientation of barrel rows (Gottlieb and Keller 1997). Slices were kept in a holding chamber that contained artificial cerebrospinal fluid (ACSF) at 30°C, aerated with 95% O2-5% CO2. Following a 1-h recovery period, the slices were maintained in the same chamber at room temperature. ACSF was composed of (in mM) 124 NaCl, 25 NaHCO3, 5 N,N-bis[2-hydroxyethyl]-2-aminoethane-sulfonic acid (BES), 3 KCl, 1.3 MgSO4, 2.0 CaCl2, and 15 glucose.
Electrical stimulation
To focally microstimulate restricted regions in the slice, we used double-barrel micropipettes (5- to 10-µM tip diameter) filled with ACSF (1.0–2.8 MΩ). Constant-current pulses (3–10 µA, 150 µs) were delivered through an optically isolated stimulus isolator (Grass PSIU6, Quincy, MA) driven by a pulse generator (Grass S48). The pulse generator was also used to trigger image acquisition by the A/D converter (see following text). Glass pipettes (filled with 2 M NaCl, 2–5MΩ) were placed in layer IV to record thalamic-evoked field potentials. These recordings were digitized on-line and stored on an Apple Macintosh computer.
Imaging voltage-sensitive dye signals
One hour after they were prepared, individual slices were stained with the voltage-sensitive dye RH-414 (100 µM; Molecular Probes, Eugene, OR). The dye was dissolved in ACSF, and a single slice was placed in a static bath containing this solution, continuously aerated with 95% O2-5% CO2 for 30–45 min. The stained slice was then transferred to an immersion-type recording chamber and continuously perfused at 2 ml/min with ACSF at room temperature.
Methods used for recording voltage-sensitive optical signals are similar to those described in detail elsewhere (Keller et al. 1998; Laaris et al. 2000a; Wu and Cohen 1993). To washout unbound dye, stained slices were perfused with ACSF for at least 15 min before initiating the optical recording. The recording chamber was mounted on a fixed stage upright microscope (Olympus BX50WI), rigidly mounted on a vibration-isolation table. A stabilized DC power source was used to power a 100-W tungsten-halogen lamp, and the light from this lamp was band-limited with interference and heat filters (≥700 nm); unless otherwise indicated, a 540 ± 30 nm band-pass interference filter was used (Omega Optical, Brattleboro, VT). Light reflected from the preparation was collected through a × 10 (0.3 NA, Olympus) water-immersion objective and projected onto a hexagonal 464-element array of photodiodes (NeuroPlex, OptImaging, Fairfield, CT). Each photodiode sampled optical signals from a region of approximately 60 × 60 µm2. The current output from each photodiode was separately converted to voltages and amplified in two separate stages (× 1,000), multiplexed, and digitized at 12-bit resolution with an A/D converter. Optical signals were filtered at 500 Hz before digitizing. All electronic components are part of the commercial NeuroPlex system. Data were collected and stored on a personal computer controlled by NeuroPlex software (OptImaging).
To precisely identify the regions in the slice from which optical recordings were collected, a custom-designed beam splitting device (Microscope Services, Rockville, MD) was used to simultaneously project the images of the slice and light from light-emitting diodes embedded in the photodiode array onto the image plane of a charge-coupled device (CCD) camera (Dage CCD72, Michigan City, IN). This allowed us to demarcate the locations of individual barrels, and of laminar boundaries. These anatomical features, observed in unstained slices, correlated well with their appearance in Nissl-stained sections.
Unless otherwise indicated, all recordings were obtained at a sample rate of 1.63 kHz. Optical responses depicted represent the average of five consecutive traces, collected at 20-s intervals. To correct for spatial differences in illumination intensity and light path length, the signal recorded from each detector was divided by the resting light intensity calculated for the corresponding detector. The resting light intensity for each detector was calculated by subtracting the intensity values recorded while the shutter was closed from those recorded while the shutter was open, when no stimulation was applied. The resulting signal amplitudes are expressed as a fractional change in fluorescence, relative to baseline fluorescence levels (ΔF/F 0). To quantify relative changes in light absorption, we calculated the mean and SD of the ΔF/F 0 during the 50 ms preceding the stimulus; poststimulus signal amplitudes are expressed as the number of SDs above these mean baseline values. Optical responses that are at least 2 SDs above this mean were considered significant.
Analyses of data were performed on an Apple Macintosh computer, using routines developed in Igor (WaveMetrics, Lake Oswego, OR). The unpaired Student's t-test was used for statistical analyses. Numerical data are presented as means ± SD of the samples.
Pharmacological and ionic manipulations
Pharmacological agents were prepared immediately before use from stock solutions and dissolved in ACSF. The following agents were obtained from RBI-Sigma (Natick, MA): d(−)-2-amino-5-phosphonopentanoic acid (AP5), tetrodotoxin (TTX), and gabazine. Nominally Ca2+-free solutions were prepared by replacing Ca2+ with equimolar concentrations of Mg2+; nominally Mg2+-free solutions were prepared by replacing Mg2+ with equimolar concentrations of Ca2+.
RESULTS
To determine the spatiotemporal propagation of activity within the barrel cortex, we took advantage of voltage-sensitive dye signals to assay the responses of a population of cortical neurons to stimulation of different cortical sites. As in our previous studies (Keller et al. 1998; Laaris et al. 2000a), we characterized the origin of the recorded signals by pharmacologically isolating different components of the responses to microstimulation of layer IV. To suppress synaptic transmission, we bathed the slices in nominally Ca2+-free solutions (“0 Ca2+”). This resulted in complete suppression of the optical responses, except for responses recorded at the stimulation site (n = 11). Optical responses were completely abolished following application of the Na+ channel antagonist TTX (0.5 µM; n = 4). Application of the N-methyl-D-aspartate (NMDA) receptor antagonist AP5 (50–100 µM; n = 6) resulted in a 53 ± 7% (mean ± SD) reduction in the amplitude of responses recorded at the stimulation site, and a corresponding 33 ± 9% reduction in responses recorded in the supragranular layers. Application of both AP5 and the non-NMDA receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; 25–50 µM; n = 7) resulted in complete suppression of the responses in the supragranular layers. These results indicate that the optical responses (except at the stimulation site) represent synaptic activation of neurons postsynaptic to cells at the stimulation site, mediated by activation of glutamate receptors.
In addition to dye-related signals, optical responses may also arise from sources intrinsic to the slice (Grinvald et al. 1988; Yuste et al. 1997). To determine whether such intrinsic signals contributed to the waveforms recorded in our study, we performed experiments in slices that were not stained with the voltage-sensitive dyes (n = 3); no optical signals were detected in these control experiments. We also tested the dependence of these signals on the illumination wavelength. When the illumination was changed to a wavelength outside the absorption spectrum of the dye (≥800 nm), no optical signal was detected in the barrel cortex. This suggests that the optical signals represent dye-related responses and are not the result of intrinsic optical signals.
Dye-related optical signals may also originate from activation of glial cells. In this case, the optical responses recorded from glial cells are expected to exhibit a slow time course (> 1 s), compared with that of neuronal responses (Konnerth et al. 1987). However, optical signals recorded in the barrel cortex had only a single depolarizing component, whose duration was 65.32 ± 24.40 ms (n = 14). These findings suggest that the dye-related optical signals analyzed in the present study reflect neuronal responses and are not related to signals originating from glial cells.
Responses to stimulation of individual barrels
To selectively activate neurons within a single layer IV barrel, we used low-current (3–10 µA) microstimulation delivered via a double-barrel glass pipette (see METHODS). We oriented the tips of these stimulating pipettes to target the hollow of a layer IV barrel. To estimate the effective current spread, we analyzed responses recorded when synaptic transmission was suppressed (in 0 Ca2+). Under these conditions, optical signals were observed only at the stimulation site and did not propagate. We accepted only cases in which responses in 0 Ca2+ were restricted to a single barrel, to restrict our analyses to responses to stimulation of a single barrel. To analyze the spatial propagation of the optical responses, we generated color-coded activity maps representing the instantaneous amplitude of the responses recorded by each photodiode (Fig. 1). In these maps, response amplitude is represented as the number of SDs above mean baseline values, calculated separately for each photodiode (see METHODS). To identify the locus of these responses, we overlaid on the activity maps drawings of the laminar and barrel boundaries, obtained from videographic images and from Nissl-stained sections.
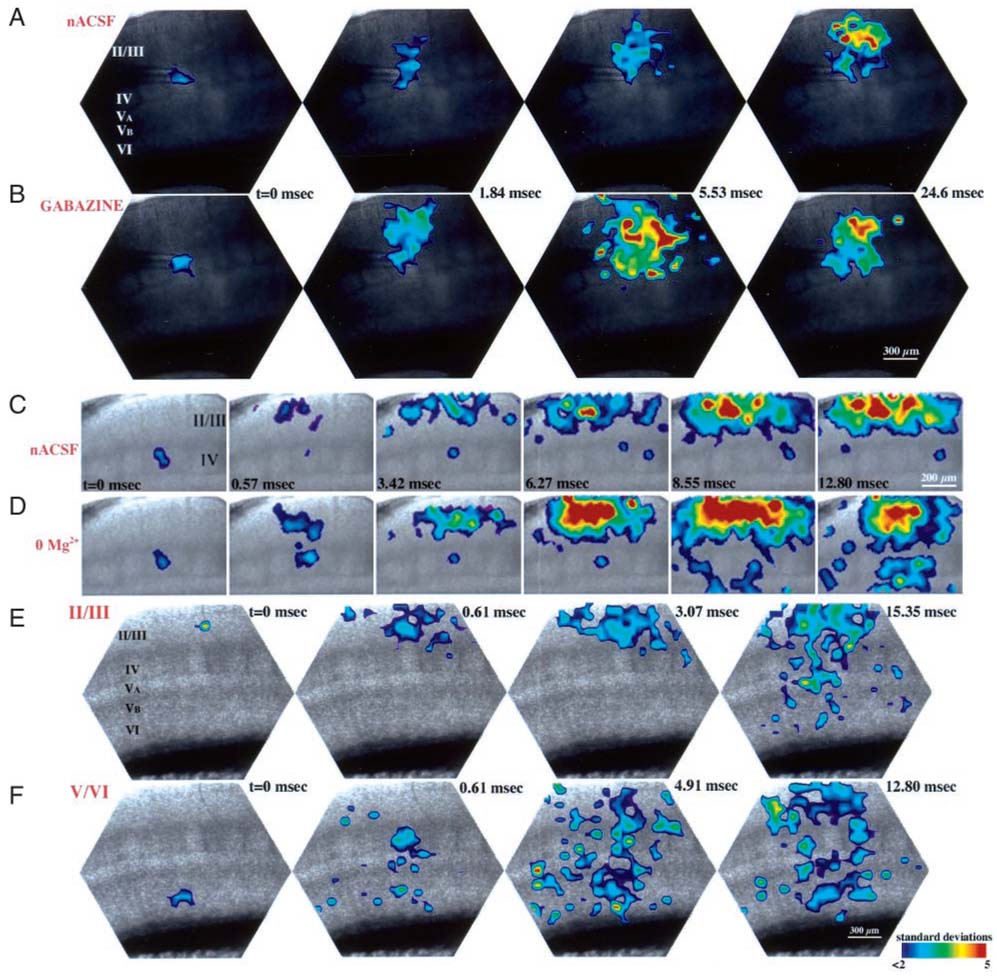
FIG. 1.
Propagation of optically recorded responses in the barrel cortex, in response to focal microstimulation in the hollow of a layer IV barrel (pipette tips are obscured). Each panel depicts the amplitude of optical responses, recorded by each of the 464 photodiodes. Signal amplitudes, expressed as SDs above the mean baseline signals, are color-coded and interpolated; color scale at bottom right applies to all panels. Time intervals are relative to the onset of stimulation. Optical signals were acquired at a temporal resolution of 1.63 kHz, except data in C and D in which a region of interest was acquired at 3.51 kHz. Laminae are indicated with Roman numerals. In response to microstimulation of a single barrel, activity propagates to the supragranular layers, where it propagates horizontally before descending to activate neighboring barrels (A and C). Note that activity does not propagate directly from the stimulated barrel to neighboring barrels. Application of the GABAA receptor antagonist gabazine results in enhanced responses in the supragranular layers, but fails to reveal direct interbarrel interactions (B). Similarly, enhancement of N-methyl-d-aspartate (NMDA) receptors by removal of Mg2+ from the extracellular solution results in enhanced activity in the supra- and infragranular layers, without activating direct interbarrel interactions (D). Microstimulation in the supragranular layers (E) results in rapid propagation of activity across these layers, and subsequent activation of individual barrels and the infragranular layers. Microstimulation in the infragranular layers (F), presumably activating both intracortical and thalamocortical pathways, activates long-range horizontal connections in the infragranular layers as well as intracolumnar pathways.
Figure 1, A and C , depicts representative activity maps in response to low-intensity (3–5 µA) microstimulation of a single layer IV barrel. Within 0.6 ± 0.03 ms following the stimulation, activity propagated to the supragranular layers, where it spread horizontally across two to four barrel columns. Significantly, in no case did activity propagate directly from the stimulated barrel to neighboring barrels (Fig. 1, A and C ). Low-amplitude optical signals were recorded in adjacent barrels, but their onset latency was 5.79 ± 2.2 ms following the stimulus, significantly later than the responses recorded in the supragranular layers. Furthermore, in every case, activation in an adjacent barrel was preceded by activation of layers II/III immediately above that barrel. These findings suggest that responses in neighboring barrels were evoked by feed-forward inputs from layers II/III.
Intracortical synaptic interactions in the barrel cortex are dependent, in large part, on activation of glutamatergic NMDA receptors (Gil and Amitai 1996; Huang et al. 1998; Thomson and Deuchars 1997). Indeed, in a previous study we demonstrated that “unmasking” of NMDA receptors—by bathing slices in a nominally Mg2+-free solution (“0 Mg2+”) can dramatically enhance the propagation of optical signals recorded in the barrel cortex in response to thalamic stimulation (Laaris et al. 2000a). To test whether a similar NMDA receptor–mediated mechanism regulates interbarrel interactions, we recorded optical responses to stimulation of a single barrel in 0 Mg2+ (n = 11), a manipulation that enhances the activation of NMDA receptors (Collingridge and Bliss 1995). As depicted in Fig. 1, C and D , this manipulation did not reveal the existence of direct inputs between neighboring barrels, nor did it alter the onset latency of responses recorded in different regions. Thus in 0 Mg2+, activity propagated from the stimulated barrel to layers II/III, and finally to neighboring barrels, with the same temporal sequence as in control experiments. Application of 0 Mg2+ did, however, affect the duration of optical responses recorded in the supragranular layers; in 8 of 11 slices the duration of these responses increased by 61 ± 13%, whereas in 3 additional slices no significant change was observed.
We also tested the possibility that direct interbarrel interactions are suppressed by GABAergic inhibition, by repeating these experiments in the presence of the GABAA receptor antagonist gabazine (1.25–3.0 µM, n = 4). At these concentrations, gabazine is reported to result in complete and specific suppression of GABAA receptor–mediated responses (Hamann et al. 1988; Soltesz and Mody 1994). As depicted in Fig. 1B , the spatiotemporal patterns of optical signals recorded in gabazine are indistinguishable from those recorded in control conditions. In both cases activation of neighboring barrels occurs only after activation of the supragranular layers, and no direct interbarrel interactions are observed. This is in agreement with our previous findings, demonstrating that suppression of inhibition does not affect the spatiotemporal propagation of thalamic-evoked activity in the barrel cortex (Laaris et al. 2000a). Suppression of GABAA receptors significantly enhanced the amplitude of responses recorded in the supragranular layers (by 94 ± 23%) and, consequently, also enhanced the later responses recorded in neighboring barrels (by 33 ± 16%).
Responses to stimulation of other layers
When focal microstimulation (3–5 µA) was applied in layers II or III, activity propagated rapidly across the supragranular layers, activating four to six barrel columns (Fig. 1E ). We estimated the propagation velocity by measuring the delay between the onset of optical responses at two points in the slice, and dividing this value by the distance between these points. The propagation of activity across layers II/III was 0.13 ± 0.03 m/s (n = 9). Because our calculation omits the contribution of synaptic delays, and because we cannot determine the number of synaptic delays between the two points measured, this value may be lower than the actual conduction velocity of intracortical axons. Nevertheless, this value is similar to that calculated from measurements of the propagation of intracortical field potentials in the neocortex in vitro (Aroniadou and Keller 1993), and from estimations of intracortical conduction velocities in the barrel cortex in vivo (Armstrong-James 1995). Within 0.72 ± 0.04 ms (n = 6) following micro-stimulation in layers II/III, optical signals appeared in layer IV barrels, followed by activation of layer Va, and distributed activity in layers Vb and VI (Fig. 1E ). Similar spatiotemporal activity patterns were recorded in six slices, in response to stimulation of either the superficial or deep portions of layers II/III.
Microstimulation (3–5 µA; n = 5) in either layer V or VI also resulted in horizontal propagation of activity (Fig. 1 F ). However, unlike the relatively homogenous activation of supragranular sites following stimulation in layers II/III (Fig. 1E ), stimulation of the deep layers resulted in activation of several noncontiguous sites in the infragranular layers. Optical signals were recorded also in the layer IV barrels and in the supragranular layers. However, because stimulation in the infragranular layers is likely to activate both neurons residing in that layer as well as cortical afferents, it is not possible to determine whether activity in these layers represents postsynaptic responses to stimulation of infragranular neurons or, for example, responses to stimulation of thalamocortical afferents (Laaris et al. 2000a).
DISCUSSION
Technical considerations
Because the main goal of this study was to test the hypothesis that individual barrels are functionally independent, it is essential to consider potential technical limitations that may have confounded our ability to identify interbarrel interactions.
The functional imaging paradigm we employed is advantageous in that it permits analyses, at a high resolution, of the spatiotemporal propagation of activity within the barrel cortex. These optical signals are particularly sensitive to suprathreshold (i.e., spiking) activity (Laaris et al. 2000a), and subthreshold activity in a synchronously activated population of neurons. This is because dye-related optical responses have a relatively low signal-to-noise ratio (Wu and Cohen 1993). It is therefore possible that under our recording conditions, subthreshold responses of individual neurons were not detected. A second limitation is that electrical microstimulation may inadvertently activate axons-of-passage, including those belonging to cortical afferents and to antidromically activated neurons.1 This concern is unlikely to affect the main conclusion of this study; whether or not axons-of-passage were stimulated, the optical recordings demonstrate that neurons in neighboring barrels do not interact directly with each other.
Finally, we recognize that a reduced slice preparation may not retain all the anatomical and functional characteristics of an in vivo preparation.
Interbarrel interactions
The results described in this study support the conclusion that neurons in neighboring barrels do not communicate directly with each other. Furthermore, our data suggest that GABAergic or NMDA receptor–mediated mechanisms do not obscure functional interactions between neighboring barrels.
These results are in agreement with previous anatomical studies of Golgi-impregnated barrel neurons, demonstrating that both the axonal and dendritic arbors of layer IV neurons are largely confined to their parent barrel (Harris and Woolsey 1983; Simons and Woolsey 1984; Woolsey et al. 1975). However, due to the capriciousness of the Golgi-impregnation method (Millhouse 1981), the possibility remained that a selection bias prevented the identification of interbarrel interactions. It can be argued that a similar selection bias also affected analyses of the morphology of intracellularly labeled neurons (Lübke et al. 2000), and of dual recordings from pairs of neurons (Feldmeyer et al. 1999; Petersen and Sakmann 2000), the results of which also argue against the existence of direct interbarrel interactions. However, in conjunction with the present results, the preponderance of evidence suggests that barrel neurons do not receive direct inputs from neighboring barrels. This conclusion is also supported by findings that microstimulation of thalamocortical axons in an in vitro slice preparation results in spatiotemporal propagation of activity from layer IV barrels, but that direct propagation between neighboring barrels does not occur, even when GABAergic inhibition or NMDA receptors are suppressed (Laaris et al. 2000a).
Inputs to layer IV barrels
Data reported here demonstrate that barrel neurons receive inputs from presynaptic neurons in layers II/III of the barrel cortex. Significantly, these inputs originate preferentially from neurons in their parent column. These findings are in agreement with previous anatomical analyses, demonstrating that axons belonging to supragranular neurons have sparse projections to layer IV, and that these axons preferentially target barrels associated with the parent column (Gottlieb and Keller 1997; Yabuta et al. 2000). Similarly, infragranular neurons provide relatively sparse direct inputs to barrel neurons, and these inputs also originate primarily from the parent column, although some infragranular neurons may target neurons in neighboring barrels (Gottlieb and Keller 1997). Thus, although most inputs to a barrel originate from their parent column, intercolumnar interactions do exist, and these may be involved in shaping the long-latency components of the SRF of barrel neurons.
Outputs from layer IV barrel neurons
Like the patterns of inputs to individual barrels, the outputs from a barrel are also directed preferentially to neurons residing within the parent column (Laaris et al. 2000a). Our present data suggest that the main output from a barrel is to the supragranular layers immediately above it. In addition, we recently obtained preliminary evidence, with the use of photostimulation and whole cell recording techniques, that layer IV neurons project also to the infragranular layers below their parent barrel, albeit these projections are more sparse than the layer IV inputs to the supragranular layers (Laaris et al. 2000b). These results are in agreement with descriptions of the morphology of layer IV spiny stellate cells, whose axons ramify within a narrow vertical domain in layers II–VI (Lübke et al. 2000; Petersen and Sakmann 2000).
Activity originating from a single barrel rapidly propagates, via feed-forward mechanisms, for long distances in the supra- and infragranular layers, to activate neurons located in a number of barrel columns (see also Laaris et al. 2000a). The likely anatomical substrate for this horizontal propagation are the long axon collaterals of supra- and infragranular pyramidal cells (Bernardo et al. 1990; Gottlieb and Keller 1997).
Functional implications
A functional column in the neocortex is defined as the vertical aggregate of neurons that respond, with similar latencies, preferentially to inputs from a cohort of thalamic afferents that relay information related to the same sensory modality and receptive field structure (Mountcastle 1997). In the barrel cortex, thalamic afferents related to the same whisker terminate almost exclusively in a single barrel (Jensen and Killackey 1987; Senft and Woolsey 1991). The absence of direct inter-barrel interactions, and the fact that most intracortical inputs and outputs from a barrel involve cells located immediately above and below it, support the conclusion that a functional column in the barrel cortex is centered on an individual layer IV barrel. This conclusion is in agreement with findings that neurons throughout a barrel column respond preferentially to activation of the same whisker (reviewed by Armstrong-James 1995; Simons 1995).
Application of GABAA receptor antagonists in vivo results in an expansion of the surround receptive field size of layer IV barrel neurons, such that the responses of these cells to non-principal whiskers is dramatically enhanced (Brumberg et al. 1996; Kyriazi et al. 1996). Furthermore, Kelly et al. (1999) demonstrated that removal of a whisker results in immediate disinhibition of layer IV receptive fields. Our finding that suppression of GABAergic inhibition does not reveal direct interbarrel interaction suggests that the receptive field expansions observed in vivo are related to modulation of synaptic interactions within individual barrels, and are not dependent on changes in intercolumnar interactions.
The absence of interbarrel interactions and the paucity of inputs to a barrel from neighboring columns suggest that the response properties of layer IV neurons are shaped primarily by the properties of their presynaptic thalamic afferents, and by synaptic interactions within a barrel. Indeed, within a barrel, interactions among excitatory neurons (Feldmeyer et al. 1999), inhibitory cells (Gibson et al. 1999), and between excitatory and inhibitory neurons (Keller and White 1989) are thought to be highly prevalent, reliable, and efficacious. Thus as first posited by Simons and collaborators (Kyriazi and Simons 1993; Simons and Carvell 1989), the network embedded in a single barrel can extract information related to activation of both the principal and adjacent whiskers from the activity patterns of their presynaptic thalamic afferents, without relying on direct inputs from adjacent barrel columns. This conclusion is supported also by findings that adjacent-whisker responses of neurons in a barrel are unaffected by lesions of neighboring barrels (Goldreich et al. 1999), and that movements of both the principal and adjacent whiskers activate barrel neurons at short latencies (Moore and Nelson 1998; Petersen and Diamond 2000). At longer latencies, barrel neurons may also respond to inputs originating from adjacent barrel columns, mediated through neurons in layer V, and, to a lesser extent, by cells in layers II/III (see above). However, since the barrel microcircuit is critically sensitive to the initial firing synchrony of afferent thalamic inputs (Pinto et al. 2000), and since these inputs rapidly engage potent inhibitory interactions that suppress later responses (Simons and Carvell 1989; Swadlow et al. 1998), the role of these long-latency, intercolumnar interactions remains unknown.
In conclusion, the present findings, together with the preponderance of previous anatomical and functional data, are consistent with the hypothesis that individual barrels function as independent parallel networks, each extracting information on the deflection parameters (e.g., direction and velocity) of multiple whiskers. Individual barrels then relay this information to other cortical layers, where intercolumnar integration occurs via long-range horizontal interactions.
Acknowledgments
We are grateful to A. Thompson for outstanding technical expertise.
This work was supported by National Institute of Neurological Disorders and Stroke Grant NS-31078.
Footnotes
We attempted to address this potential pitfall by recording optical responses to either photostimulation of caged glutamate or iontophoretic application of glutamate. However, the optical signals evoked using these techniques were below the detection level of our imaging system. Presumably, these stimulation paradigms activated only a small number of presynaptic neurons, which were insufficient to evoke detectable optical postsynaptic responses.
REFERENCES
- Armstrong-James M. The nature and plasticity of sensory processing within adult rat barrel cortex. In: Jones EG, Diamond IT, editors. Cerebral Cortex. The Barrel Cortex of Rodents. vol. 11. New York: Plenum; 1995. pp. 333–373. [Google Scholar]
- Armstrong-James M, Callahan CA, Friedman MA. Thalamo-cortical processing of vibrissal information in the rat. I. Intracortical origins of surround but not centre-receptive fields of layer IV neurones in the rat S1 barrel field cortex. J Comp Neurol. 1991;303:193–210. doi: 10.1002/cne.903030203. [DOI] [PubMed] [Google Scholar]
- Aroniadou VA, Keller A. The patterns and synaptic properties of horizontal intracortical connections in the rat motor cortex. J Neurophysiol. 1993;70:1493–1553. doi: 10.1152/jn.1993.70.4.1553. [DOI] [PubMed] [Google Scholar]
- Bernardo KL, Mccasland JS, Woolsey TA, Strominger RN. Local intra- and interlaminar connections in mouse barrel cortex. J Comp Neurol. 1990;291:231–255. doi: 10.1002/cne.902910207. [DOI] [PubMed] [Google Scholar]
- Brumberg JC, Pinto DJ, Simons DJ. Spatial gradients and inhibitory summation in the rat whisker barrel system. J Neurophysiol. 1996;76:130–140. doi: 10.1152/jn.1996.76.1.130. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Bliss TVP. Memories of NMDA receptors and LTP. Trends Neurosci. 1995;18:54–56. [PubMed] [Google Scholar]
- Feldmeyer D, Egger V, Lubke J, Sakmann B. Reliable synaptic connections between pairs of excitatory layer 4 neurones within a single ‘barrel’ of developing rat somatosensory cortex. J Physiol (Lond) 1999;521:169–190. doi: 10.1111/j.1469-7793.1999.00169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature. 1999;402:75–79. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- Gil Z, Amitai Y. Properties of convergent thalamocortical and intracortical synaptic potentials in single neurons of neocortex. J Neurosci. 1996;16:6567–6578. doi: 10.1523/JNEUROSCI.16-20-06567.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert CD. Horizontal integration and cortical dynamics. Neuron. 1992;9:1–13. doi: 10.1016/0896-6273(92)90215-y. [DOI] [PubMed] [Google Scholar]
- Goldreich D, Kyriazi HT, Simons D. Functional independance of layer IV barrels in rodent somatosensory cortex. J Neurophysiol. 1999;82:1311–1316. doi: 10.1152/jn.1999.82.3.1311. [DOI] [PubMed] [Google Scholar]
- Gottlieb JP, Keller A. Intrinsic circuitry and physiological properties of pyramidal neurons in rat barrel cortex. Exp Brain Res. 1997;115:47–60. doi: 10.1007/pl00005684. [DOI] [PubMed] [Google Scholar]
- Grinvald A, Frostig RD, Lieke E, Hildesheim R. Optical imaging of neuronal activity. Physiol Rev. 1988;68:1285–1366. doi: 10.1152/physrev.1988.68.4.1285. [DOI] [PubMed] [Google Scholar]
- Hamann M, Desarmenien M, Desaulles E, Bader MF, Feltz P. Quantitative evaluation of the properties of a pyridazinyl GABA derivative (SR 95531) as a GABAA competitive antagonist. An electrophysiological approach. Brain Res. 1988;442:287–296. doi: 10.1016/0006-8993(88)91514-4. [DOI] [PubMed] [Google Scholar]
- Harris RM, Woolsey TA. Computer-assisted analyses of barrel neuron axons and their putative synaptic contacts. J Comp Neurol. 1983;220:63–79. doi: 10.1002/cne.902200107. [DOI] [PubMed] [Google Scholar]
- Hoeflinger BF, Bennett-Clarke CA, Chiaia NL, Killackey HP, Rhoades RW. Patterning of local intracortical projections within the vibrissae representation of rat primary somatosensory cortex. J Comp Neurol. 1995;354:551–563. doi: 10.1002/cne.903540406. [DOI] [PubMed] [Google Scholar]
- Huang W, Armstrong-James M, Rema V, Diamond ME, Ebner FF. Contribution of supragranular layers to sensory processing and plasticity in adult barrel cortex. J Neurophysiol. 1998;80:3261–3271. doi: 10.1152/jn.1998.80.6.3261. [DOI] [PubMed] [Google Scholar]
- Jensen KF, Killackey HP. Terminal arbors of axons projecting to the somatosensory cortex of the adult rat. I. The normal morphology of specific thalamocortical afferents. J Neurosci. 1987;7:3529–3543. doi: 10.1523/JNEUROSCI.07-11-03529.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller A. Synaptic organization of the barrel cortex. In: Jones EG, Diamond IT, editors. Cerebral Cortex. The Barrel Cortex of Rodents. vol. 11. New York: Plenum; 1995. pp. 221–262. [Google Scholar]
- Keller A, Carlson GC. Neonatal whisker clipping alters intracortical, but not thalamocortical projections in rat barrel cortex. J Comp Neurol. 1999;412:83–94. doi: 10.1002/(sici)1096-9861(19990913)412:1<83::aid-cne6>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Keller A, White EL. Triads: a synaptic network component in the cerebral cortex. Brain Res. 1989;496:105–112. doi: 10.1016/0006-8993(89)91056-1. [DOI] [PubMed] [Google Scholar]
- Keller A, Yagodin S, Aroniadou-Anderjaska V, Zimmer LA, Ennis M, Sheppard NF, Shipley MT. Synaptic physiology of rat olfactory bulb glomeruli revealed by optical imaging. J Neurosci. 1998;18:2602–2612. doi: 10.1523/JNEUROSCI.18-07-02602.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MK, Carvell GE, Kodger JM, Simons DJ. Sensory loss by selected whisker removal produces immediate disinhibition in the somatosensory cortex of behaving rats. J Neurosci. 1999;19:9117–9125. doi: 10.1523/JNEUROSCI.19-20-09117.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konnerth A, Obaid AL, Salzberg DM. Optical recording of electrical activity from parallel fibers and other cell types in skate cerebellar slices in vitro. J Physiol (Lond) 1987;393:681–702. doi: 10.1113/jphysiol.1987.sp016848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriazi HT, Carvell GE, Brumberg JC, Simons DJ. Quantitative effects of GABA and bicuculline methiodide on receptive field properties of neurons in real and simulated barrels. J Neurophysiol. 1996;75:547–560. doi: 10.1152/jn.1996.75.2.547. [DOI] [PubMed] [Google Scholar]
- Kyriazi HT, Simons DJ. Thalamocortical response transformations in simulated whisker barrels. J Neurosci. 1993;13:1601–1615. doi: 10.1523/JNEUROSCI.13-04-01601.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laaris N, Carlson GC, Keller A. Thalamic-evoked synaptic interactions in barrel cortex revealed by optical imaging. J Neurosci. 2000a;20:1529–1537. doi: 10.1523/JNEUROSCI.20-04-01529.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laaris N, Li Y, Kao JPY, Keller A. Functional independence of mouse cortical barrels. Soc Neurosci Abstr. 2000b;26:774.4. [Google Scholar]
- LüBke J, Egger V, Sakmann B, Feldmeyer D. Columnar organization of dendritic and axon arbors of synaptically coupled excitatory spiny neurons in layer 4 of the rat barrel cortex. J Neurosci. 2000;20:5300–5311. doi: 10.1523/JNEUROSCI.20-14-05300.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KD, Pinto DJ, Simons DJ. Processing in layer 4 of the neocortical circuit: new insights from visual and somatosensory cortex. Curr Opin Neurobiol. 2001;11:488–497. doi: 10.1016/s0959-4388(00)00239-7. [DOI] [PubMed] [Google Scholar]
- Millhouse OE. The Golgi methods. In: Heimer L, Robards MJ, editors. Neuroanatomical Tract-Tracing Methods. New York: Plenum; 1981. pp. 311–344. [Google Scholar]
- Moore CI, Nelson SB. Spatio-temporal subthreshold receptive fields in the vibrissa representation of rat primary somatosensory cortex. J Neurophysiol. 1998;80:2882–2892. doi: 10.1152/jn.1998.80.6.2882. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB. The columnar organization of the neocortex. Brain. 1997;120:701–722. doi: 10.1093/brain/120.4.701. [DOI] [PubMed] [Google Scholar]
- Petersen CCH, Sakmann B. The excitatory neuronal network of rat layer 4 barrel cortex. J Neurosci. 2000;20:7579–7586. doi: 10.1523/JNEUROSCI.20-20-07579.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RS, Diamond ME. Spatial-temporal distribution of whisker-evoked activity in rat somatosensory cortex and the coding of stimulus location. J Neurosci. 2000;20:6135–6143. doi: 10.1523/JNEUROSCI.20-16-06135.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto DJ, Brumberg JC, Simons DJ. Circuit dynamics and coding strategies in rodent somatosensory cortex. J Neurophysiol. 2000;83:1158–1166. doi: 10.1152/jn.2000.83.3.1158. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP, Tian B, Pons T, Mishkin M. Serial and parallel processing in rhesus monkey auditory cortex. J Comp Neurol. 1997;382:89–103. [PubMed] [Google Scholar]
- Roerig B, Kao JPY. Organization of intracortical circuits in relation to direction preference maps in ferret visual cortex. J Neurosci. 1999;19:RC1–RC5. doi: 10.1523/JNEUROSCI.19-24-j0002.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senft SL, Woolsey TA. Growth of thalamic afferents into the mouse barrel cortex. Cereb Cortex. 1991;1:308–335. doi: 10.1093/cercor/1.4.308. [DOI] [PubMed] [Google Scholar]
- Simons DJ. Neuronal integration in the somatosensory whisker/barrel cortex. In: Jones EG, Diamond IT, editors. Cerebral Cortex. The Barrel Cortex of Rodents. vol. 11. New York: Plenum; 1995. pp. 263–297. [Google Scholar]
- Simons DJ, Carvell GE. Thalamocortical response transformation in rat vibrissa/barrel system. J Neurophysiol. 1989;61:311–330. doi: 10.1152/jn.1989.61.2.311. [DOI] [PubMed] [Google Scholar]
- Simons DJ, Woolsey TA. Morphology of Golgi-Cox–impregnated barrel neurons in rat SmI cortex. J Comp Neurol. 1984;230:119–132. doi: 10.1002/cne.902300111. [DOI] [PubMed] [Google Scholar]
- Soltesz I, Mody I. Patch-clamp recordings reveal powerful GABAergic inhibition in dentate hilar neurons. J Neurosci. 1994;14:2365–2376. doi: 10.1523/JNEUROSCI.14-04-02365.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swadlow HA, Beloozerova IN, Sirota MG. Sharp, local synchrony among putative feed-forward inhibitory interneurons of rabbit somatosensory cortex. J Neurophysiol. 1998;79:567–582. doi: 10.1152/jn.1998.79.2.567. [DOI] [PubMed] [Google Scholar]
- Thomson AM, Deuchars J. Synaptic interactions in neocortical local circuits: dual intracellular recordings in vitro. Cereb Cortex. 1997;7:510–522. doi: 10.1093/cercor/7.6.510. [DOI] [PubMed] [Google Scholar]
- Woolsey TA, Dierker MK, Wann DF. Mouse SmI cortex. Qualitative and quantitative classification of Golgi-impregnated barrel neurons. Proc Natl Acad Sci USA. 1975;72:2165–2169. doi: 10.1073/pnas.72.6.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolsey TA, Van Der Loos H. The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. Brain Res. 1970;17:205–242. doi: 10.1016/0006-8993(70)90079-x. [DOI] [PubMed] [Google Scholar]
- Worgotter F, Koch C. A detailed model of the primary visual pathway in the cat. Comparison of afferent excitatory and intracortical inhibitory connection schemes for orientation selectivity. J Neurosci. 1991;11:1959–1979. doi: 10.1523/JNEUROSCI.11-07-01959.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J-Y, Cohen LB. Fast multisite optical measurement of membrane potential. In: Mason WT, editor. Fluorescent and Luminescent Probes for Biological Activity. London: Academic; 1993. pp. 389–404. [Google Scholar]
- Yabuta NH, Butler AK, Callaway EM. Laminar specificity of local circuits in barrel cortex of ephrin-A5 knockout mice. J Neurosci. 2000;20:7–10. doi: 10.1523/JNEUROSCI.20-15-j0005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuste R, Tank DW, Kleinfeld D. Functional study of the rat cortical microcircuitry with voltage-sensitive dye imaging of neocortical slices. Cereb Cortex. 1997;7:546–558. doi: 10.1093/cercor/7.6.546. [DOI] [PubMed] [Google Scholar]