Abstract
Water is an effective promoter of the endo-selective opening of trisubstituted epoxides, enabling related cascades leading to a variety of substituted ladder polyether structures. When used in conjunction with a tetrahydropyran-templated nucleophile, water can overcome the powerful electronic directing effect of a methyl substituent at either site of the epoxide, making water a uniquely versatile medium and promoter for epoxide opening.
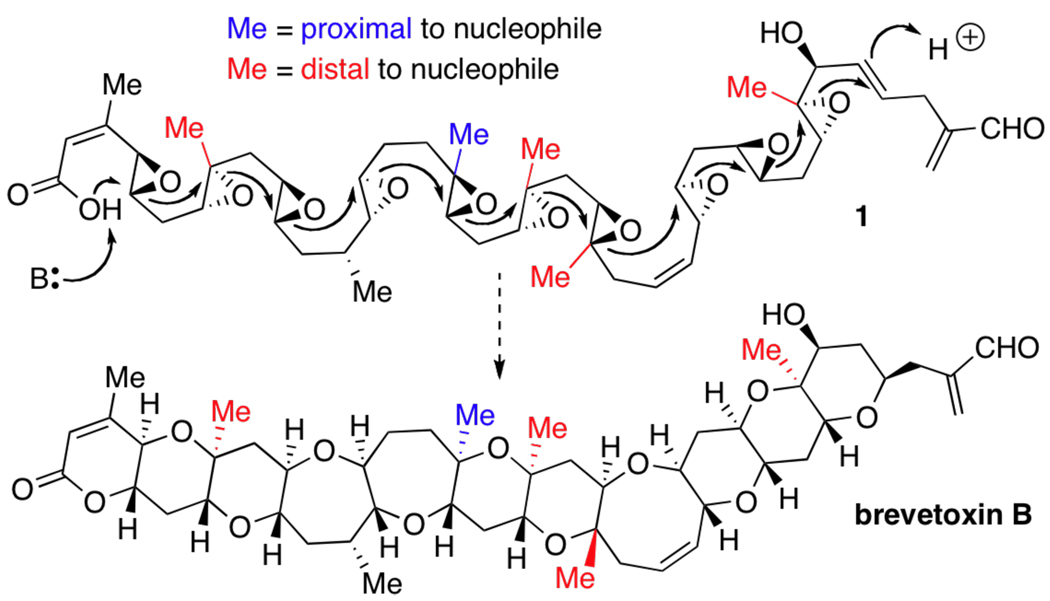
In 1985 Nakanishi advanced a concise and appealing proposal for the biosynthesis of the ladder polyether family of natural products, a synthesis that culminates in a cascade of regio- and stereoselective epoxide openings (Scheme 1).1 Our group recently reported an emulation of the Nakanishi hypothesis, wherein water serves as the superior promoter of endo-selective epoxide-opening cascades.2 This earlier account was limited to cascades of trans-disubstituted epoxides, and we herein report that water (as solvent) is also a simple and general solution for cascades involving trisubstituted epoxides. Overcome by this method is the well documented, strong directing effect that methyl (Me) groups have on epoxide ring-opening reactions,3 and thus enabled is the rapid assembly of multiple patterns of substituted ladder polyether subunits.
Scheme 1.
Proposed Biosynthetic Cascade to Brevetoxin B
An angular Me group is the only substituent other than hydrogen (H) observed at ladder polyether ring junctions, and every structure in this large family of natural products possesses at least one Me group. Nature has conceived two variations of this substitution, requiring two chemically quite different kinds of (E)-trisubstituted epoxides in the corresponding polyepoxide precursors. For example, in the hypothesized precursor (1) to brevetoxin B (Scheme 1), Me groups are observed both  and
and  4 to the internal nucleophile; the putative cascade must tolerate both possibilities. In fact, nearly all ladders bearing more than one Me group, including the brevetoxins, maitotoxin, gambierol, and gymnocin B, are proposed to arise from similar polyepoxides bearing an “out-of-register” mixture of both distally and proximally substituted epoxides.
4 to the internal nucleophile; the putative cascade must tolerate both possibilities. In fact, nearly all ladders bearing more than one Me group, including the brevetoxins, maitotoxin, gambierol, and gymnocin B, are proposed to arise from similar polyepoxides bearing an “out-of-register” mixture of both distally and proximally substituted epoxides.
The crux of the problem is that the Me group is generally a strong director of epoxide-opening regioselectivity, particularly under acid catalysis. Valuable methods for endo-selective opening, epitomized by those developed by the McDonald group,3a–c take advantage of this directing effect,3 but these necessarily accommodate only  Me substitution. Moreover, distal Me substitution at every epoxide is generally vital for high regioselectivity and yield.5 The endo-selective opening of epoxides with a Me or other simple alkyl group6
Me substitution. Moreover, distal Me substitution at every epoxide is generally vital for high regioselectivity and yield.5 The endo-selective opening of epoxides with a Me or other simple alkyl group6  to the pendent nucleophile has not been documented except under enzyme catalysis7 or when a stronger directing group at the distal site of the epoxide was used.8 We conjectured that tetrahydropyran (THP)-templated, water-promoted cascades might prove relatively insensitive to the electronic effects and afford a general solution to the problem of Me substitution, particularly for the challenging case of proximal Me substitution noted above.
to the pendent nucleophile has not been documented except under enzyme catalysis7 or when a stronger directing group at the distal site of the epoxide was used.8 We conjectured that tetrahydropyran (THP)-templated, water-promoted cascades might prove relatively insensitive to the electronic effects and afford a general solution to the problem of Me substitution, particularly for the challenging case of proximal Me substitution noted above.
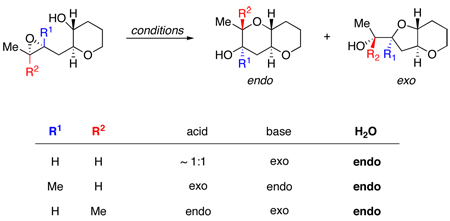
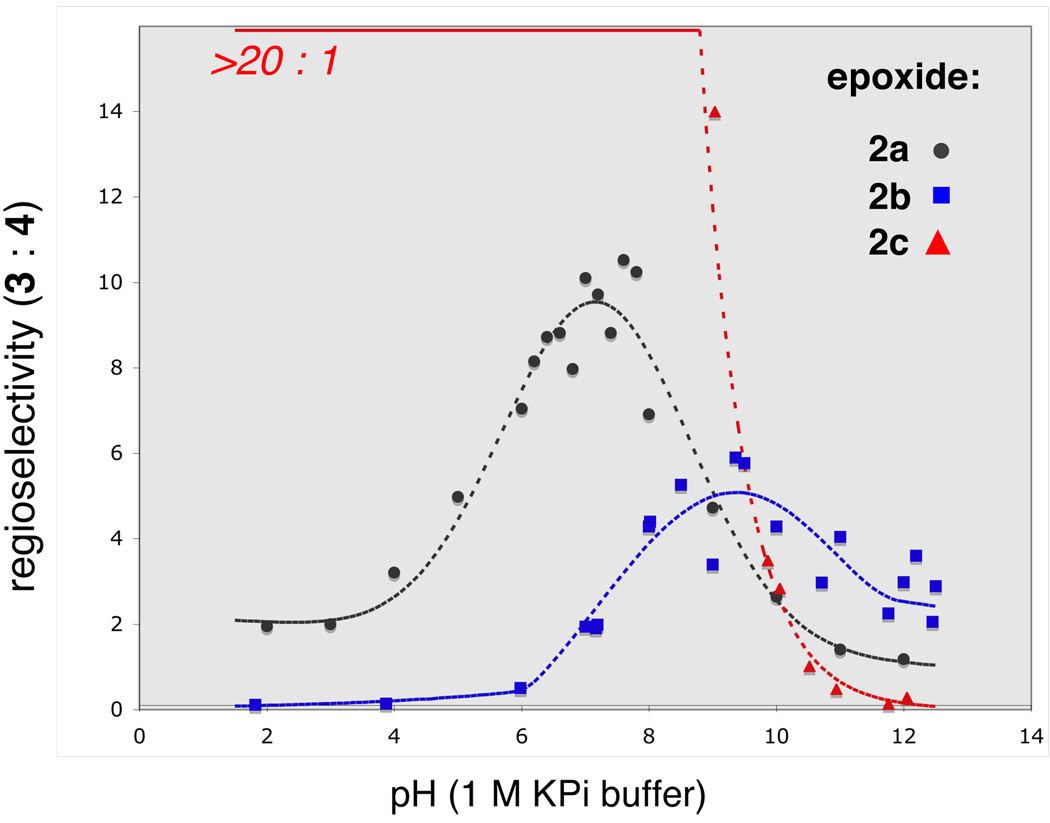
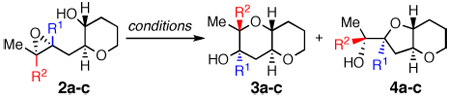
We accordingly began our investigation with proximally Me-substituted monoepoxide 2b (Table 1). Unsurprisingly, both Brønsted (CSA) and Lewis (BF3) acids were highly exo selective, affording the undesired 4b. Conversely, Brønsted base activation by Cs2CO3 provided desired bis-THP product 3b, with moderate endo selectivity apparently arising from the alkoxide’s preference for the less sterically hindered site of attack. Most striking was water, which effected cyclization with nearly 5:1 selectivity for 3b over 4b. Furthermore, the selectivity of cyclizations of 2b improved to almost 6:1 endo:exo in potassium phosphate buffer within a pH range of 8 to 10 (Chart 1); intriguingly, this selectivity drops again as pH increases past 10.
Table 1.
Dependence of Regioselectivity under Various Epoxide-Opening Conditions
 | ||||||
|---|---|---|---|---|---|---|
| conditions and regioselectivity (3:4)a |
||||||
| epoxide | Cs2CO3, MeOHb |
CSA, CH2Cl2c |
BF3•OEt2, CH2Cl2d |
H2Oe | ||
| 2a | H | H | 1 : 2.7 | 1 : 1.2 | 1.4 : 1 | 10 : 1 |
| 2b | Me | H | 3.0 : 1 | 1 : 5.2 | 1 : 11 | 4.9 : 1 |
| 2c | H | Me | 1 : 17 | 5.8 : 1f | >20 : 1f | >20 : 1 |
ratios determined by 1H NMR spectroscopy.
30 equiv Cs2CO3, rt, 0.02 M.
1 equiv (+/−)-CSA, rt, 0.02 M.
0.25 equiv BF3•OEt2, −78° to rt, 0.02 M.
deionized water, rt, 0.02 M.
Isopropyl ketone side product also isolated, see Supporting Information.
Chart 1.
Dependence of Regioselectivity on pH
Epoxides with distal Me substituents have been shown to open with high endo regioselectivity with a variety of acidic promoters.3a–e Indeed, exposure of epoxy alcohol 2c to both BF3•OEt2 and CSA induced selective cyclization to bis-THP 3c, but considerable amounts (up to 20%) of isomerization of 2c to an isopropyl ketone side product were also observed under these conditions. A cleaner reaction was achieved in deionized water, which smoothly transformed 2c to 3c with >20:1 endo:exo selectivity and no trace of the ketone byproduct. A pH screen (Chart 1) revealed that very high selectivity holds under acidic, neutral, and mildly basic conditions; only at pH >9 does selectivity drop below 10:1.
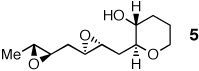
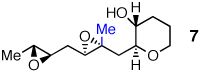
Optimistic that cascades of substituted epoxides should be possible in aqueous media, we prepared diepoxy alcohol 7 (Table 2) bearing a proximal Me group. Stirring 7 in warm water produced the desired tris-THP triad 8 in 32% yield.9 To the best of our knowledge, this transformation represents the first endo-selective epoxide-opening cascade to accommodate a proximal Me substituent. Cascades with base or acid in organic solvent supplied no trace of 8.
Table 2.
Cascades of Methyl-Substituted Diepoxytetrahydropyranols under Various Epoxide-Opening Conditions
| conditions and isolated yielda of desired product: |
|||||
|---|---|---|---|---|---|
| substrate | desired product | Cs2CO3 in MeOHb |
CSA in CH2Cl2c |
BF3•OEt2 CH2Cl2d |
H2Oe |
 |
 |
0% | tracef | tracef | 74% |
 |
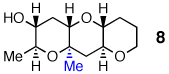 |
0% | 0% | 0% | 32% |
 |
 |
0% | 46% | 63% | 67% |
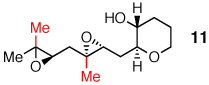 |
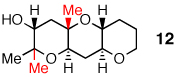 |
0% | 43% | 61% | 54% |
Corrected for diastereomeric purity of starting material (between 7.5:1 and 20:1 for all cases, see Supporting Information); average of at least two experiments.
30 equiv Cs2CO3, rt, 0.02 M.
1 equiv (+/−)-CSA, rt, 0.02 M.
0.25 equiv BF3•OEt2, −78° to rt, 0.02 M.
60°, 0.02 M.
<5% (1H NMR).
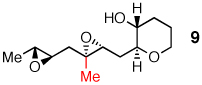
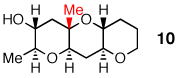
A distal Me group was incorporated into diepoxide 9, and water again proved amenable, affording triad 10 in 67% yield. A somewhat lower 54% yield of triad 12 was obtained in the aqueous reaction of diepoxide 11, in which both epoxides now bear distal Me substituents. In promoting cascades of 9 and 11, CSA and BF3 were competitive with water, with water slightly better than BF3 in reactions of 9 and the reverse observed in reactions of 11.
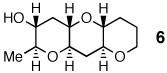
Reactions of 5,2 the parent system containing two trans-disubstituted epoxides, revealed that only water provides a significant quantity (74%) of the desired triad 6. Thus, water-promoted cyclizations provide a uniquely versatile strategy for the construction of all three epoxide substitution patterns found in the Nakanishi hypothesis and clearly proceed by a mechanism fundamentally different from those operating under simple acid or simple base catalysis.
Supplementary Material
Acknowledgment
C.J.M. thanks the George Büchi Graduate Fellowship for support. We thank Ivan Vilotijevic for cyclization data on 2a, Dr. Jeffery A. Byers for preparation of 2a, Dr. Peter Müller (MIT) for X-ray crystallography, and Li Li (MIT) for mass spectrometry.
Footnotes
Supporting Information Available: Experimental procedures and data for all new compounds (PDF). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Nakanishi K. Toxicon. 1985;23:473. doi: 10.1016/0041-0101(85)90031-5. [DOI] [PubMed] [Google Scholar]
- 2.Vilotijevic I, Jamison TF. Science. 2007;317:1189. doi: 10.1126/science.1146421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Bravo F, McDonald FE, Neiwert WA, Do B, Hardcastle KI. Org Lett. 2003;5:2123. doi: 10.1021/ol034539o. [DOI] [PubMed] [Google Scholar]; (b) Bravo F, McDonald FE, Neiwert WA, Hardcastle KI. Org Lett. 2004;6:4487. doi: 10.1021/ol048212e. [DOI] [PubMed] [Google Scholar]; (c) Valentine JC, McDonald FE, Neiwert WA, Hardcastle KI. J. Am. Chem. Soc. 2005;127:4586. doi: 10.1021/ja050013i. [DOI] [PubMed] [Google Scholar]; (d) Wan S, Gunaydin H, Houk KN, Floreancig PE. J. Am. Chem. Soc. 2007;129:7915. doi: 10.1021/ja0709674. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Morimoto Y, Nishikawa Y, Ueba C, Tanaka T. Angew. Chem. Int. Ed. 2006;45:810. doi: 10.1002/anie.200503143. [DOI] [PubMed] [Google Scholar]; (f) Xiong Z, Corey EJ. J. Am. Chem. Soc. 2000;122:9328. [Google Scholar]
-
4.
 and
and  Me groups are shown in
Me groups are shown in  and
and  , respectively.
, respectively.
- 5.In cascades to form polyoxepanes, trans-disubstituted epoxides can be accommodated to some extent, generally with lower yields; see references 4c and 4d.
- 6.Endo-selective cyclization with methoxymethyl substitution on the epoxide proximal to the pendent nucleophile was reported by Murai and coworkers; see: Fujiwara K, Tokiwano T, Murai A. Tetrahedron Lett. 1995;36:8063. Fujiwara K, Mishima H, Amano A, Tokiwano T, Murai A. Tetrahedron Lett. 1998;39:393.
- 7.Smith L, Hong H, Spencer JB, Leadlay PF. ChemBioChem. 2008;9:2967. doi: 10.1002/cbic.200800585. [DOI] [PubMed] [Google Scholar]
- 8.Matsukura H, Morimoto M, Koshino H, Nakata T. Tetrahedron Lett. 1997;38:5545. [Google Scholar]
- 9.A 6,5-fused side product arising from exo opening was also collected, in 39% yield; see Supporting Information.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.