Abstract
• Background and Aims Moss roses are old garden roses covered with a mossy growth on flower pedicel and calyx. This moss releases a pine-scented oleoresin that is very sticky and odoriferous. Rosa × centifolia ‘muscosa’ was the first moss rose to be obtained by bud-mutation but, interestingly, R. × damascena ‘Quatre Saisons Blanc Mousseux’ was the first repeat-blooming cultivar, thus interesting breeders. In the present study, the anatomy of these sports (i.e. bud-mutations) is characterized and the volatile organic compounds (VOCs) produced by the moss versus the petals are identified. They are compared between the two lines and their respective parents.
• Methods Anatomy of the moss is studied by environmental scanning electron microscopy and histochemical light microscopy. Sudan Red IV and Fluorol Yellow 088 are used to detect lipids, and 1-naphthol reaction with N,N-dimethyl-p-phenylenediamine to detect terpenes (Nadi reaction). Head-space or solid/liquid extraction followed by gas chromatography and mass spectrometry are used to identify VOCs in moss, trichomes and petals.
• Key Results Moss of the two cultivars has the same structure with trichomes on other trichomes but not exactly the same VOCs. These VOCs are specific to the moss, with lots of terpenes. An identical VOC composition is found in leaves but not in petals. They are nearly the same in the moss mutants and in the respective wild types.
• Conclusions Sepals of moss roses and their parents have a specific VOC pattern, different from that of the petals. The moss corresponds to a heterochronic mutation with trichomes developing on other trichomes. Such a mutation has probably appeared twice and independently in the two lines.
Keywords: Rosa × damascena ‘bifera’, Rosa × damascena ‘Quatre Saisons Blanc Mousseux’, Rosa × centifolia, Rosa × centifolia ‘muscosa’, moss roses, glandular trichomes, histochemistry, volatile organic compounds, gas chromatography, sport, terpenoids, benzenoids
INTRODUCTION
Moss roses are old garden roses belonging to the subgenus Eurosa sect. Gallicanae also named subgenus Rosa sect. Rosa (e.g. Millan et al., 1996; Raymond et al., 2000; Wissemann, 2003, and references therein). Their flower pedicel and calyx are covered with a green to brown mossy growth. This mossy structure releases a pine-scented oleoresin that is very sticky and odoriferous. Among these moss roses, Rosa × damascena ‘Quatre Saisons Blanc Mousseux’ (syn. ‘Perpetual White Moss’ or ‘Rosier de Thionville’) is a repeat-blooming shrub up to 1·5 m tall, with delicate brown thorns on the stem. The double-petal flower is white with, sometimes, a pink tint. It was created by an anonymous breeder in Thionville (France) in 1829 and probably not by Laffay in 1835 (François Joyaux 53470 Commer France, pers. comm.). It is a sport or bud-mutation of R. × damascena ‘bifera’ (syn. ‘Quatre Saisons’, ‘Autumn damask’ or ‘semperflorens’). Rosa × damascena ‘bifera’ is a repeat-blooming hybrid of R. × damascena, the Damask rose.
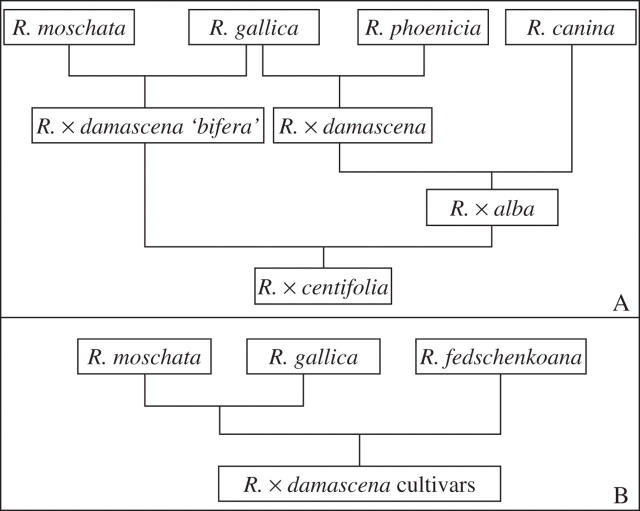
Historically, the first moss roses to be obtained were not sported from R. × damascena ‘bifera’ but from R. × centifolia (cabbage rose). Even though some French and English authors state that moss roses were known before the 18th century, the first clear and indisputable reference to a moss rose is that of Boerhaave in 1720 (cited by Hurst and Breeze, 1922) who described a ‘Rosa rubra plena, spinosissima, pedunculo muscoso’, now known as Rosa × centifolia ‘muscosa’. At this time, these full-petal flowers were sterile due to the development of stamens into petals. Fertile moss roses, with simple- or double-petal flowers, were obtained at the beginning of the 19th century and were then used to create most of the hybrids now known. Furthermore, the creation of the first repeat-blooming cultivar of moss roses, R. × damascena ‘Quatre Saisons Blanc Mousseux’, in the beginning of the 19th century, encouraged breeders to use this valuable horticultural trait. Unfortunately, nobody knows the exact genetic relationship between these two founder cultivars of moss roses: R. × damascena ‘bifera’ and R. × centifolia. The only published phylogeny is based on caryological and morphological data (Fig. 1A; Hurst, 1941) and has never been confirmed by other analyses. However, other information concerning the origin of R. × damascena cultivars largely contradicts this phylogeny. For example, R. × damascena ‘bifera’, which, according to Hurst (1941), has a biparental origin (R. moschata and R. gallica) was also proposed to have a triparental origin. According to Iwata et al. (2000), the parents could be R. moschata × gallica and R. fedschenkoana (Fig. 1B), even though not confirmed by other authors. By the same token, the extent to which the two founder cultivars of moss roses, R. × damascena ‘Quatre Saisons Blanc Mousseux’ and R. × centifolia ‘muscosa’, share the same genetic background is not known, even though Centifolia and Damask roses have recently been suggested to have close phylogenetic relationship (Martin et al., 2001).
Fig. 1.
Lineages of old garden roses proposed by (A) Hurst (1941) and (B) Iwata et al. (2000).
Chemical analyses of volatile organic compounds (VOCs) of moss roses have not been published, though they are for the parent cultivars (Tucker and Maciarello, 1988; Picone et al., 2004). Indeed, cultivars of R. × damascena and R. × centifolia are used to produce essential oil by hydrodistillation or solvent extraction of petals. The oil obtained by hydrodistillation contains high levels of monoterpene alcohols, citronellol, geraniol and their derivatives as acetates, for example (Kovatz, 1987; Lawrence, 1997; Jirovetz et al., 2002). It also contains a noticeable proportion of paraffin derivatives. VOCs of these roses have also been studied by supercritical CO2 extraction or solid/liquid phase extraction in pentane/dichloromethane mixtures. In these cases, the volatile composition is a little different: 2-phenylethanol is generally the major constituent, followed by monoterpene alcohols (Antonelli et al., 1997; Boelens, 1997). It is probably because 2-phenylethanol is lost in rose water during hydrodistillation. In addition to the analysis of the chemical composition of various rose oils, more recent studies have focused on VOCs emitted by flowers. For example, Picone et al. (2004) made an in-depth analysis of the rhythmic emission of floral volatiles from Rosa × damascena ‘bifera’. In the study, 2-phenylethanol was the most abundant emitted compound. It was found in mixture with monoterpene alcohols, oxidized monoterpenes and aromatic compounds. Although the floral volatiles of these roses are well known, the chemical composition of the mossy organs has never been described.
In all moss roses, the sport character is reversible. Specimens of R. × damascena ‘Quatre Saisons Blanc Mousseux’ with only one pink flower devoid of moss in the shrubs have been observed. As far as is known, the sport character has never been described from a histological or a chemical point of view.
In this paper, the anatomy of R. × damascena ‘Quatre Saisons Blanc Mousseux’ and R. × centifolia ‘muscosa’ are studied. VOCs produced by the mossy trichomes are compared with VOCs emitted by petals. The anatomy of the moss sport is compared with the trichomes of the cultivars from which they originated, R. × damascena ‘bifera’ and R. × centifolia.
MATERIALS AND METHODS
Rose cultivation
Rose cultivars were cultivated outside in four locations: Université Jean Monnet de Saint-Etienne, Ecole Normale Supérieure de Lyon, Jardin Botanique de la Ville de Lyon and Roseraie de Saint-Galmier.
Environmental scanning electron microscopy (ESEM)
Pieces of leaves were directly pasted onto a stage in the low-pressure chamber of an S-3000N Hitachi microscope (Tokyo, Japan). Samples were then cooled from +4 °C to a minimum of –20 °C by the Pelletier effect. Pressure was set to 110 Pa and electron voltage to 15 kV for observation and micrographs.
Light microscopy and histochemistry
Observations of sepals were made with a Leitz DMRB microscope. To reveal lipids, pieces of sepals were rinsed in 50 % ethanol, stained for 20 min in Sudan Red IV in 70 % ethanol, rinsed again in 50 % ethanol and observed (Jensen, 1962). Fluorol Yellow 088 was also used to visualize lipids (Brundrett et al., 1991). A 5·10−3 % (w/v) solution in 50 % (v/v) PEG 400 and 45 % (v/v) glycerol was prepared for stock. Pieces of sepals were then stained for 1–10 min by immersion in this solution diluted 1000 times or more and then directly observed by fluorescence (excitation filter 340–380 nm and barrier filter 420 nm). For the Nadi reaction (David and Carde, 1964), fresh sections were placed for 0·5–1 h in a freshly made mixture of 0·001 % 1-naphthol, 0·001 % N,N-dimethyl-p-phenylenediamine dihydrochloride and 0·4 % ethanol in 100 mm sodium cacodylate-HCl buffer (pH 7·2) and then observed directly. Lipophilic droplets are then blue, or purple when they contain terpenes.
Collection of volatiles, gas chromatography and gas chromatography mass spectrometry (GC-FID and GC-MS)
All samplings were made at the same hour of the day (10 a.m.) to minimize effects of rhythmic emissions. Fragrance volatiles were extracted overnight at 4 °C by soaking 1 g of tissue in 2 mL of hexane containing 40 mg L−1 of camphor as an internal standard. This solid/liquid extraction was made on sepals and petals of fully opened flowers and on leaves. Alternatively, a head-space system was used to draw off volatile organic compounds (Heath and Manukian, 1994; Grison-Pigé et al., 2001). Briefly, fully opened flowers were enclosed in a polyethylene terephthalate (Nalophan) bag equipped with inlet and outlet. Vacuum pumps were used to draw purified air (charcoal cartridges Orbo32, Supelco) through the enclosed bag. Purified air was blown at 400 mL min−1 and pulled out at 300 mL min−1. At the outlet, the head-space volatiles were collected for 1 h on a glass cartridge (75 mm × 4 mm) containing 30 mg Tenax (ARS Inc., Gainesville, FL, USA). Volatile compounds were eluted from Tenax with hexane in which camphor had been added as an internal standard. GC-FID analyses were performed on an Agilent 6850 gas chromatograph equipped with a flame ionization detector (FID). Nitrogen was used as the carrier gas at a flow rate of 1 mL min−1. A glass HP-Innowax capillary column (30 m × 0·25 mm) was employed under the following conditions: 3 min at 40 °C then 2 °C min−1 up to 160 °C and 12 °C min−1 to 240 °C with 2 min hold time. Injection was in split mode with a 10:1 ratio. Volatile components were identified on the basis of retention time with authentic compounds, when available. Parallel analyses for identification of compounds were carried out by chromatography and mass spectrometry on an Agilent 6890 gas chromatograph/mass spectrometer (CNRS, Wiley 275 and Wist 98 mass spectrum databases) with helium as the carrier gas. Analysis parameters were as follows: injected volume 1 μl; split ratio 10:1; temperature of injector and detector, 250 °C; film thickness 0·25 μm; temperature of ion-source and the interface and ionizing voltage, 230 °C, ei-mode, 70 eV; mass scan rate 2·94 scans s−1 for 50–550 m z−1. All experiments were performed at least three times.
RESULTS
VOC analysis and origin in R. × damascena ‘Quatre Saisons Blanc Mousseux’
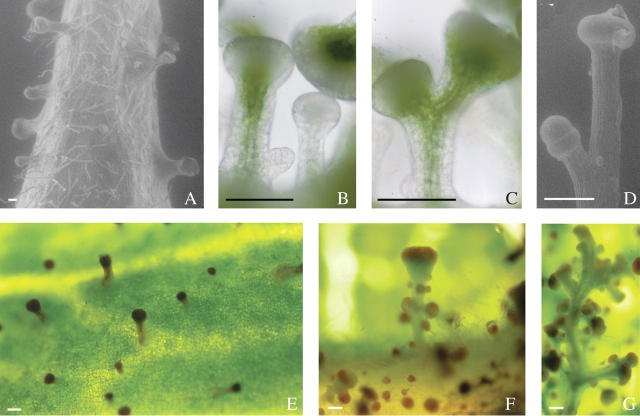
During the floral transition, the moss of R. × damascena ‘Quatre Saisons Blanc Mousseux’ becomes more and more visible and is most abundant on flower buds. On its parent, R. × damascena ‘bifera’, the mossy structure never appears (Fig. 2A). A comparison of flower buds of R. × damascena ‘bifera’ and its mossy sport clearly shows the difference (Fig. 2B). This moss is composed of a multitude of long sticky trichomes along the pedicel and the sepals (Fig. 2C). The resin-like compounds are produced by glands densely scattered on the mossy structure (Fig. 2D) and dead insects are often glued on them. These secreting trichomes have a long and branched stalk and more or less red heads topped by a sticky droplet (Fig. 2E–F). The red pigment (Fig. 2F) is vacuolar, probably an anthocyanin. These glandular trichomes are pluricellular with chlorophyll present in the centre cells of the stalk. Sticky droplets are secreted by the head cells (Fig. 2). Sudan Red IV histochemical staining shows that they contain lipids (Fig. 2G), which is confirmed by the fluorescence of Fluorol Yellow 088 (Fig. 2H). Furthermore, the purple colour obtained after the Nadi reaction, clearly indicates that they also contain terpenes (Fig. 2I). Such droplets vary in size and sometimes drip along the stalk.
Fig. 2.
(A–D and F) photographs, (E) ESEM photomicrograph and (G–I) histochemical photomicrographs showing the morphology and chemical secretion of trichomes: (A) R. × damascena `bifera', (C–I) R. × damascena ‘Quatre Saisons Blanc Mousseux', and (B) both cultivars for comparison. Trichome exudates are stained with (G) Sudan Red IV, (H) Fluorol Yellow 088 in epifluorescence and (I) Nadi reagent. Scale bars: E and F = 100 μm; G–I = 50 μm.
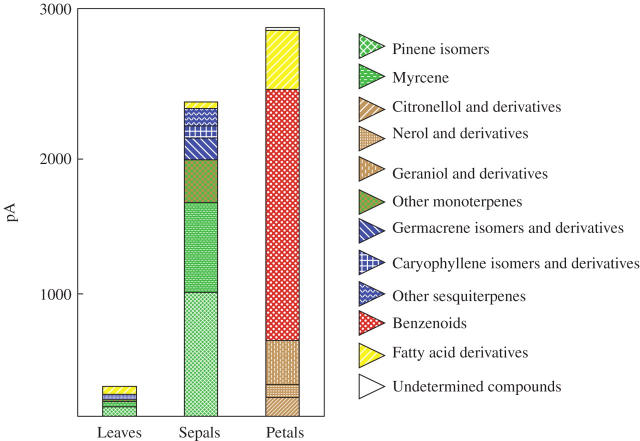
During opening, the whole flower smells like R. × damascena (rosy odour) with a resin note as confirmed by head-space analysis (Fig. 3) which reveals chemicals characteristic of R. × damascena: 2-phenylethanol, citronellol, geraniol, nerol and derivatives. 2-Phenylethanol is a benzenoid, and citronellol, geraniol and nerol are monoterpene alcohols. Other monoterpenoids (myrcene and sabinene) were also detected. To analyse the contribution of the different organs of the flower to the scent and to know more about the composition of the lipidic glue, GC-FID and GC-MS analyses on solid/liquid extracts were conducted (Fig. 4). Sepals were compared with leaves and petals. Collectively, results indicate a very low level of VOCs in leaves (10 times less than sepals) and the highest level in petals (around 1·2 times more than sepals). In sepals, the majority of compounds were monoterpenoids such as pinene isomers and myrcene. Sesquiterpenoids and fatty acid derivatives were also detected.
Fig. 3.
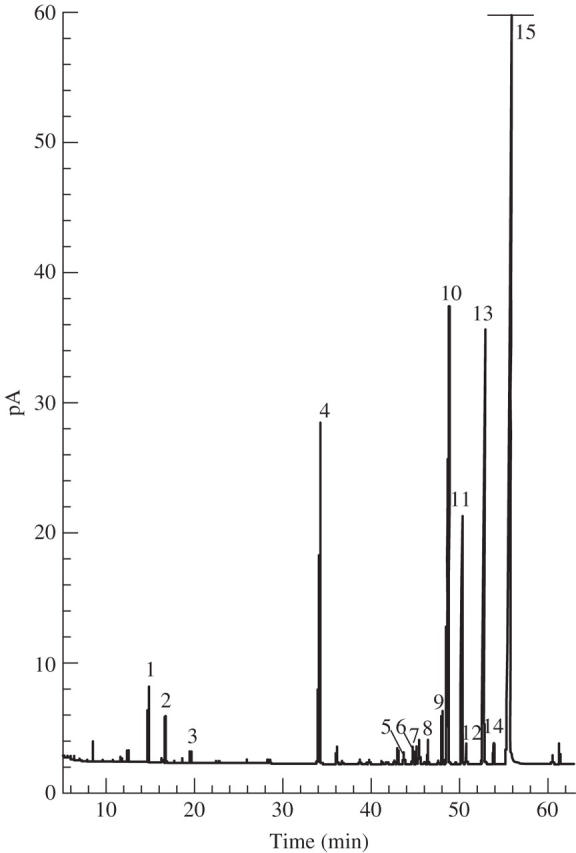
Head-space, GC-FID and GC-MS analysis of R × damascena ‘Quatre Saisons Blanc Mousseux’. 1, Myrcene; 2, β-phellandrene; 3, ocimene isomers; 4, internal standard (camphor); 5, neral; 6, germacrene D; 7, heptadecane; 8, geranial; 9, geranylacetate; 10, citronellol; 11, nerol; 12, β-phenylethylacetate; 13, geraniol; 14, benzylalcohol; 15, 2-phenylethanol (184·1 pA).
Fig. 4.
GC-FID and GC-MS analysis on solid/liquid extracts of leaves, sepals and petals of R. × damascena ‘Quatre Saisons Blanc Mousseux’.
In these analyses, monoterpenoids were the most diversified (Fig. 4 and Table 1). In sepals and leaves, α-pinene and myrcene were the most abundant but β-pinene, sabinene and β-ocimene isomers were also important. Lots of monoterpenoids were also detected in sepals but not in other organs (linalool, 1,8-cineole, β-phellandrene sabinene hydrate and terpinolene). Some specific monoterpenoids were found in petals: geraniol was the most abundant but citronellol and nerol were also very important. The three organs contained sesquiterpenoids that were particularly abundant in leaves and sepals. Germacrene D and β-caryophyllene were present in petals, sepals and leaves. Some other sesquiterpenes, such as α-humulene and α-farnesene, were specific to sepals and leaves.
Table 1.
Percentages of each monoterpene in monoterpenoid GC-FID analyses of solid/liquid extracts of leaves, sepals and petals of R. × damascena ‘Quatre Saisons Blanc Mousseux’
| Percentages |
||||
|---|---|---|---|---|
| Monoterpenoids | Retention time (min) | Leaves | Sepals | Petals |
| α-Pinene | 8·15 | 47·2 | 42·3 | – |
| β-Pinene | 11·48 | 6·9 | 6·1 | – |
| Myrcene | 14·60 | 30·2 | 35·2 | 0·1 |
| Sabinene | 12·20 | 6·5 | 7·0 | – |
| Sabinene hydrate* | 31·62 | 1·4 | 0·8 | – |
| Cis-β-ocimene | 18·47 | 1·4 | 1·5 | – |
| Trans-β-ocimene | 19·37 | 4·8 | 4·5 | – |
| Limonene | 16·13 | 1·5 | 1·6 | – |
| β-Phellandrene | 16·57 | – | 0·4 | – |
| Terpinolene | 20·90 | – | 0·2 | – |
| Linalool | 36·75 | – | 0·2 | – |
| 1,8-Cineole | 16·42 | – | 0·1 | – |
| Camphene | 9·69 | – | 0·1 | – |
| Geraniol | 52·48 | – | – | 55·3 |
| Geranial | 46·22 | – | – | 1·9 |
| Geranyl acetate | 47·80 | – | – | 0·5 |
| Citronellol | 48·39 | – | – | 24·8 |
| Citronellyl acetate | 42·62 | – | – | 0·3 |
| Nerol | 50·03 | – | – | 16·6 |
| Neral | 43·49 | – | – | 0·5 |
–, Not detected.
Correct isomer not identified.
Benzenoids, mostly 2-phenylethanol, were barely detected in leaves and sepals but they accounted for almost 70 % of the VOCs in petals (Fig. 4). Smaller quantities of benzyl alcohol were also detected.
Fatty acid derivatives were very abundant in leaves (Fig. 4); they represented 26 % of the VOCs in leaves but they did not exceed 15 % in sepals and petals. Furthermore, they were not exactly the same in the different organs. In petals, the most abundant compounds were aliphatic hydrocarbons such as nonadecane and nonadecene-1 while other fatty acid derivatives were in traces. In sepals and leaves, the ‘green leaf volatiles’ were very prominent. Cis-3-hexenol and trans-2-hexenal were the major compounds (respectively 16 % and 16 % in sepals, and 34 % and 8 % in leaves) but trans-2-hexenol and hexanal were also abundant (respectively 3 % and 3 % in sepals, and 5 % and 1 % in leaves).
Comparison of R. × damascena ‘Quatre Saisons Blanc Mousseux’ with R. × centifolia ‘muscosa’ and their parents
In order to know whether the same moss sport has appeared twice, in two different rose lineages, histochemical and chemical analysis were performed on R. × damascena ‘Quatre Saisons Blanc Mousseux’ and its parent, R. × damascena ‘bifera’, and on R. × centifolia ‘muscosa’ and its parent, R. × centifolia.
Trichomes of R. × damascena ‘bifera’ have non-branched and short stalks (Fig. 5A). At the contrary, glandular trichomes of R. × damascena ‘Quatre Saisons Blanc Mousseux’ are generally very long and highly branched (Fig. 2). In fact, new glandular trichomes develop on older ones (Fig. 5B) suggesting a repetition of the developmental programme. The head can grow rapidly (Fig. 5C) before the stalk (Fig. 5D). R. × centifolia trichomes resemble those of R. × damascena ‘bifera’. One difference is that they seem to have redder head cells (Fig. 5E): trichomes of R. × centifolia ‘mucosa’ are highly branched (Fig. 5F) and may be very long (Fig. 5G).
Fig. 5.
(A and D) ESEM photomicrographs and (B, C and E–G) light photomicrographs of (A) R. × damascena ‘bifera’, (B–D) R. × damascena ‘Quatre Saisons Blanc Mousseux’, (E) R. × centifolia and (F and G) R. × centifolia ‘muscosa’. Scale bars = 100 μm.
Chemical analysis of the scent of R. × centifolia ‘muscosa’ and R. × damascena ‘Quatre Saisons Blanc Mousseux’ revealed similar composition of VOCs in the petals and some slight differences in the sepals (Fig. 6). In petals of R. × centifolia ‘muscosa’, benzenoids (mostly 2-phenylethanol) made up nearly 60 % of the volatile compounds, as in R. × damascena ‘Quatre Saisons Blanc Mousseux’. Other chemicals were geraniol, nerol, citronellol and their derivatives. Fatty acid derivatives (mostly nonadecane) were also present in both cultivars. In sepals, a noticeable difference was the presence of some specific sesquiterpenes in R. × centifolia ‘muscosa’ (β-farnesene, for example).
Fig. 6.
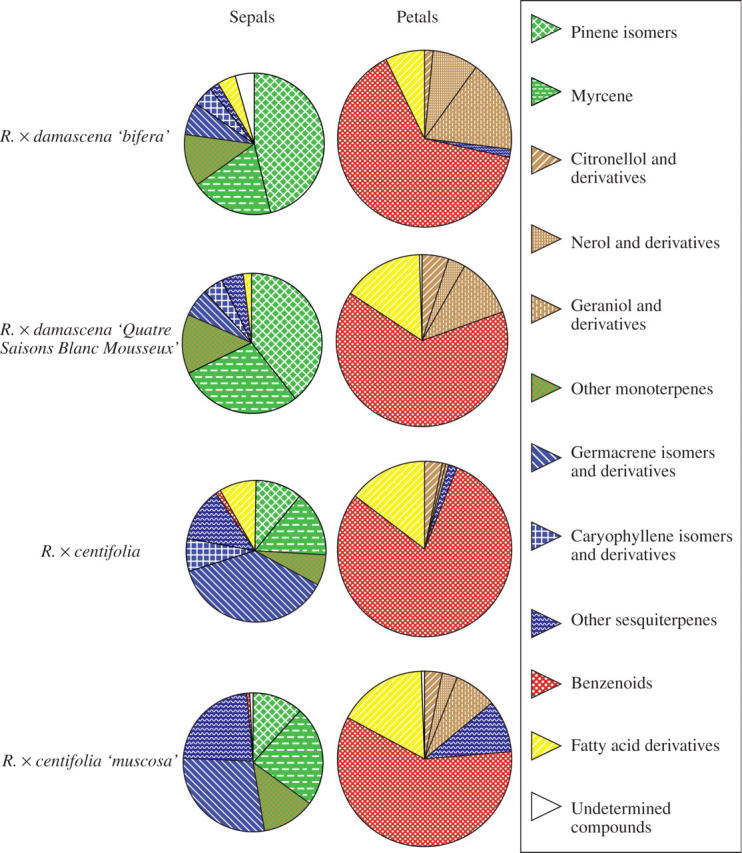
GC-FID and GC-MS analysis on solid/liquid extracts of sepals and petals of two moss roses and their parents.
Compared with their respective parents, each cultivar had the same qualitative composition but not exactly the same quantities of VOCs. Both had differences in fatty acid derivatives and R. × centifolia ‘muscosa’ had a lower proportion of benzenoids than R. × centifolia.
As shown before, the VOCs of the sepals were quite different from those of the petals. Rosa × damascena ‘Quatre Saisons Blanc Mousseux’ had nearly the same sepal composition as its parent except for a higher quantity of myrcene. Rosa × centifolia ‘muscosa’ also had the VOC composition of its parent except that fatty acid derivatives (cis-3-hexenol, trans-2-hexenal, trans-2-hexenol and hexanal) were replaced by an increased quantity of myrcene and other monoterpenes. An interesting observation is the large amount of α-pinene detected in R. × damascena ‘bifera’ and R. × damascena ‘Quatre Saisons Blanc Mousseux’ (30–40 %) compared with the two other cultivars (around 10 %).
Collectively, these results do not reveal any difference between the moss sports of these two different cultivars. The same repeat-programme of trichome development seems to occur in R. × damascena ‘Quatre Saisons Blanc Mousseux’ and R. × centifolia ‘muscosa’ in full bloom.
DISCUSSION
Rosa × damascena ‘Quatre Saisons Blanc Mousseux’ is a sport of R. × damascena ‘bifera’. It has been shown that the mossy structure has the characteristics of a heterochronic mutation. Indeed, trichomes of this moss rose are exactly the same as those of its parent's trichomes, except that there is a repetitive development of trichomes on pre-existing trichomes. VOC composition and quantities are also similar. Histochemical staining shows that the sticky droplets secreted by the head cells may drip along the stalk and contain lipids and terpenes. Such composition is often found in secreting cells (Proctor et al., 1996; Fahn, 2000; Caissard et al., 2004). Furthermore, the VOCs emitted by sepals, thus by trichomes, are nearly the same in both cultivars but much more abundant in the moss sport. They contain a high amount of pinene isomers, nearly 25 % of myrcene and nearly 25 % of caryophyllene isomers and other sesquiterpenes such as germacrene D.
Compared with the scent composition of other rose cultivars, all these VOCs have already been detected in the flower head-space or in the essential oil (Knudsen et al., 1993; Weiss, 1997; Oka et al., 1998; Hayashi et al., 2004; Shalit et al., 2004). Nevertheless, in one study (Mihailova et al., 1977), the composition of the ‘chalice leaves’ of R. × damascena ‘Kazanlik’ has been described as nearly identical to the composition of the petals, but it is not clear whether ‘chalice leaves’ means calyx, bracts or last leaves before the full bloom. Nevertheless, this hybrid being genetically related to R. × damascena ‘bifera’ (Widrlechner, 1981; Weiss, 1997; Iwata et al., 2000), these results are not in agreement with the present analysis and with the odour that can be smelt. In all the present analyses, petals have a very different composition from sepals, with geraniol, nerol, citronellol, their derivatives and a very high amount of 2-phenylethanol. Furthermore, in another species with glandular trichomes, R. rugosa, it has also been shown that the chemical composition of combined sepals and gynoecium (i.e. non-dissected receptacle) is different from the one of petals (Dobson et al., 1990). Indeed, in petals, high levels of 2-phenylethanol, geraniol, geranial, citronellol and nerol are detected but in sepals/gynoecium only low levels of these VOCs are present. They are replaced by α-farnesene and miscellaneous sesquiterpenes. The characteristic scent composition of each floral organ has also been shown in another species, Boronia megastigma (MacTavish and Menary, 1997). Authors interpret these differences of VOCs between floral whorls as a protection of the flower bud against insects before and during flowering, and as a guide inside the flower after anthesis. This hypothesis is in agreement with the toxic or repellent function attributed to glandular trichomes and to the attractive function of petals (Levin, 1973; Wagner, 1990; Proctor et al., 1996; Dudareva et al., 2000; Pichersky and Gershenzon, 2002). It could also explain why these plants use very different pathways of secondary metabolite biosynthesis in different flower whorls, each whorl undergoing a different selection pressure. For example, fatty acid derivatives detected in leaves and sepals (cis-3-hexenol, trans-2-hexenol, trans-2-hexenal, hexanal) are known to be involved in indirect defence (Paré and Tumlinson, 1999; Baldwin et al., 2001).
Rosa × centifolia ‘muscosa’ is a mossy sport derived from R. × centifolia. It has been shown that the mossy structure has the characteristics of a heterochronic mutation. Indeed, the moss corresponds to trichomes developed on other trichomes. These trichomes have similar head cells except that they are redder than those of R. × damascena cultivars. They also have the same VOC composition and quantities, except for a higher level of fatty acid derivatives in R. × centifolia sepals. Compared with R. × damascena cultivars, pinene isomers and myrcene are less abundant. Finally, it seems that the mutations of R. × centifolia ‘muscosa’ and R. × damascena ‘Quatre Saisons Blanc Mousseux’ are really identical but that they appeared twice in different rose lines. Nevertheless, in Hurst's phylogeny (Hurst, 1941), R. × damascena ‘bifera’ is a parent of R. × centifolia. Even if this phylogeny is contested, R. × centifolia and R. × damascena cultivars are both in the section Gallicanae and are genetically related (Weiss, 1997; Martin et al., 2001; Cairns, 2003; Wissemann, 2003). Thus, these cultivars could have preserved some traits of their common ancestor, R. gallica. Indeed, they have the same kind of glandular trichomes on leaves and sepals and nearly the same VOCs in sepals (data not shown). On the other hand, Iwata et al. (2000) hypothesized that the moss of R. × damascena ‘Quatre Saisons Blanc Mousseux’ could be explained by R. fedschenkoana being an ancestor. The question remains open.
In summary, it can be affirmed that sepals of moss roses and their parents have a specific VOC pattern, different from that of the petals. Furthermore, the moss trichomes of R. × damascena ‘Quatre Saisons Blanc Mousseux’ correspond to a heterochronic mutation of the trichomes of R. × damascena ‘bifera’. A similar mutation occurred in R. × centifolia ‘muscosa’, a sport of R. × centifolia. It probably happened in a close genetic background twice, i.e. independently in the two moss cultivars rather than ones followed by introgression during breeding. Their most direct ancestor generated glandular trichomes on sepals, a phenotypic trait of botanical species R. gallica and R. fedschenkoana of the section Gallicanae. Additional studies of the phylogenetic relationships within this section could clarify if both species are the direct ancestors of R. × centifolia and R. × damascena.
Acknowledgments
We are grateful to Laurent Legendre (Laboratoire BVpam) for critical reading of the manuscript. We thank Isabelle Anselme-Bertrand (Centre de Microscopie Electronique Stéphanois) for her help on the ESEM. We are indebted to Marie-Charlotte Anstett, Martine Hossaert-McKey (Centre d'Ecologie Fonctionnelle et Evolutive, CNRS, Montpellier) and Frédéric Jullien (Laboratoire BVpam) for the help with the head-space apparatus. We also thank Christian Dumas (Ecole Normale Supérieure de Lyon), Frédéric Pautz (Jardin Botanique de la Ville de Lyon) and Louise Bouchardon (Roseraie de Saint-Galmier) who allowed us to cut roses in their collection and François Joyaux (Association ‘Rosa gallica’, Roseraie de la Cour de Commer) who sent us roses and literature. We thank Leopold Jirovetz, Chris Hawes and Mike Venis for reviewing the manuscript.
LITERATURE CITED
- Antonelli A, Fabbri C, Giorgioni ME, Bazzocchi R. 1997. Characterization of 24 old garden roses from their volatile compositions. Journal of Agricultural and Food Chemistry 45: 4435–4439. [Google Scholar]
- Baldwin IT, Halitschke R, Kessler A, Schittko U. 2001. Merging molecular and ecological approaches in plant–insect interactions. Current Opinion in Plant Biology 4: 351–358. [DOI] [PubMed] [Google Scholar]
- Boelens MH. 1997. Differences in chemical and sensory properties of orange flower and rose oils obtained from hydrodistillation and from supercritical CO2 extraction. Perfumer and Flavorist 22: 31–35. [Google Scholar]
- Brundrett MC, Kendrick B, Peterson CA. 1991. Efficient lipid staining in plant material with Sudan Red 7B or Fluoral Yellow 088 in polyethylene glycol-glycerol. Biotechnic and Histochemistry 66: 111–116. [DOI] [PubMed] [Google Scholar]
- Cairns T. 2003. Horticultural classification schemes. In: Roberts AV, Debener T, Gudin S, eds. Encyclopedia of rose science. Amsterdam: Elsevier, 117–124.
- Caissard JC, Joly C, Bergougnoux V, Hugueney P, Mauriat M, Baudino S. 2004. Secretion mechanisms of volatile organic compounds in specialized cells of aromatic plants. Recent Research Development in Cell Biology 2: 1–15. [Google Scholar]
- David R, Carde JP. 1964. Coloration différentielle des inclusions lipidiques et terpéniques des pseudophylles du Pin maritime au moyen du réactif Nadi. Compte-Rendu de l'Académie des Sciences de Paris 258:1338–1340. [Google Scholar]
- Dobson HEM, Bergström G, Groth I. 1990. Differences in fragrance chemistry between flower parts of Rosa rugosa Thunb. (Rosaceae). Israel Journal of Botany 39: 143–156. [Google Scholar]
- Dudareva N, Piechulla B, Pichersky E. 2000. Biogenesis of floral scent. Horticultural Reviews 24: 31–54. [Google Scholar]
- Fahn A. 2000. Structure and function of secretory cells. Advances in Botanical Research 31: 37–75. [Google Scholar]
- Grison-Pigé L, Bessière JM, Turlings TCJ, Kjellberg F, Roy J, Hossaert-McKey M. 2001. Limited intersex mimicry of floral odour in Ficus carica. Functional Ecology 15: 551–558. [Google Scholar]
- Hayashi S, Yagi K, Ishikawa T, Kawasaki M, Asai T, Picone J, et al. 2004. Emission of 2-phenylethanol from its β-D-glucopyranoside and the biogenesis of these compounds from [2H8] L-phenylalanine in rose flowers. Tetrahedron 60: 7005–7013. [Google Scholar]
- Heath RR, Manukian A. 1994. An automated system for use in collecting volatile chemicals released from plants. Journal of Chemical Ecology 20: 593–607. [DOI] [PubMed] [Google Scholar]
- Hurst CC. 1941. Notes on the origin and evolution of our garden roses. Journal of the Royal Horticultural Society 66: 73–82, 242,–250, 282–289. [Google Scholar]
- Hurst CC, Breeze MSG. 1922. Notes on the origin of the moss-rose. Journal of the Royal Horticultural Society 47: 1–16. [Google Scholar]
- Iwata H, Kato T, Ohno S. 2000. Triparental origin of Damask roses. Gene 259: 53–59. [DOI] [PubMed] [Google Scholar]
- Jensen WA. 1962. Botanical histochemistry, principles and practice. San Francisco: W.H. Freeman and Company.
- Jirovetz L, Buchbauer G, Shahabi M. 2002. Comparative investigations of essential oils and their SPME headspace volatiles of Rosa damascena from Bulgaria and Rosa centifolia from Morocco using GC-FID, GC-MS and olfactometry. Journal of Essential Oil-Bearing Plants 5: 111–121. [Google Scholar]
- Knudsen JT, Tollsten L, Bergström G. 1993. Floral scents — a checklist of volatile compounds isolated by head-space techniques. Phytochemistry 33: 253–280. [Google Scholar]
- Kovatz E. 1987. Composition of essential oils. 7. Bulgarian oil of rose 5 (Rosa damascena Mill.). Journal of Chromatography 406: 185–222. [Google Scholar]
- Lawrence BM. 1997. Progress in essential oils. Perfumer and Flavourist 22: 57–74. [Google Scholar]
- Levin DA. 1973. The role of trichomes in plant defense. Quarterly Review of Biology 48: 3–15. [Google Scholar]
- MacTavish HS, Menary R. 1997. Volatiles in different floral organs, and effect of floral characteristics on yield of extract from Boronia megastigma (Nees). Annals of Botany 80: 305–311. [Google Scholar]
- Martin M, Piola F, Chessel D, Jay M, Heizmann P. 2001. The domestication process of the Modern Rose: genetic structure and allelic composition of the rose complex. Theoretical and Applied Genetics 102: 398–404. [Google Scholar]
- Mihailova J, Atanasova R, Balinova-Tsvetkova A. 1977. Direct gas chromatography of essential oil in the separate parts of the flower of the Kazanlik rose (Rosa damascena Mill. f. trigintipetala Dieck.). In: Proceedings of the 7th International Congress of Essential Oils, Kyoto, Japan: 219–221.
- Millan T, Osuna F, Cobos S, Torres AM, Cubero JI. 1996. Using RAPDs to study phylogenetic relationships in Rosa. Theoretical and Applied Genetics 92: 273–277. [DOI] [PubMed] [Google Scholar]
- Oka N, Ikegami A, Ohki M, Sakata K, Yagi A, Watanabe N. 1998. Citronellyl disaccharide glycoside as an aroma precursor from rose flowers. Phytochemistry 47: 1527–1529. [Google Scholar]
- Paré PW, Tumlinson JH. 1999. Plant volatiles as a defense against insect herbivores. Plant Physiology 121: 325–331. [PMC free article] [PubMed] [Google Scholar]
- Pichersky E, Gershenzon J. 2002. The formation of plant volatiles: perfumes for pollinator attraction and defense. Current Opinion in Plant Biology 5: 237–243. [DOI] [PubMed] [Google Scholar]
- Picone JM, Clery RA, Watanabe N, MacTavish HS, Turnbull CGN. 2004. Rhythmic emission of floral volatiles from Rosa damascena semperflorens cv. ‘Quatre Saisons’. Planta 219: 468–478. [DOI] [PubMed] [Google Scholar]
- Proctor M, Yeo P, Lack A. 1996. The natural history of pollination. Portland, OH: Timber Press.
- Raymond O, Fiasson JL, Jay M. 2000. Synthetic taxonomy of Rosa races using ACT-STATIS. Zeitschrift für Naturforschung 55c: 399–409. [DOI] [PubMed] [Google Scholar]
- Shalit M, Shafir S, Larkov O, Bar E, Kaslassi D, Adam Z, et al. 2004. Volatile compounds emitted by rose cultivars: fragrance perception by man and honeybees. Israel Journal of Plant Sciences 52: 245–255. [Google Scholar]
- Tucker AO, Maciarello MJ. 1988. Nomenclature and chemistry of the Kazanlik Damask rose and some potential alternatives from the horticultural trade of North America and Europe. In: Lawrence BM, Mookherjee BD, Willis BJ, eds. Proceedings of the 10th International Congress of Essential Oils, Fragrances and Flavors, Washington, DC, USA, 16–20 Nov. 1986. Amsterdam: Elsevier, 99–114.
- Wagner GJ. 1990. Secreting glandular trichomes: more than just hairs. Plant Physiology 96: 675–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss EA. 1997. Essential oil crops. Wallingford: CAB International.
- Widrlechner MP. 1981. History and utilization of R. damascena. Economic Botany 35: 42–58. [Google Scholar]
- Wissemann V. 2003. Conventional taxonomy (wild roses). In: Roberts AV, Debener T, Gudin S, eds. Encyclopedia of rose science. Amsterdam: Elsevier, 111–117.