Abstract
• Background and Aims The assessment of the genetic variability and the identification of isolated populations within a given species represent important information to plan conservation strategies on a genetic basis. In this work, the genetic variability in five natural populations of Juniperus phoenicea, three from Sardinia, one from Cyprus and the last one in the Maritime Alps was analysed by means of ISSRs, on the hypothesis that the latter could have been a refugial one during the last glaciation.
• Methods ISSRs were chosen because of their ability to detect variation without any prior sequence information. The use of three primers yielded 45 reproducible, polymorphic bands, which were utilized to estimate the basic parameters of genetic variability and diversity.
• Key Results All of the populations analysed harboured an adequate amount of genetic variability, with HS = 0·1299. The proportion of genetic diversity between populations has been estimated by GST = 0·12. The three Sardinian populations are separated, as tested by AMOVA, from the Cyprus and the continental ones.
• Conclusions The results indicate that geographical isolation has represented a major barrier to gene flow in Juniperus phoenicea. This work represents a first step towards a full genetic characterization of a conifer from the Mediterranean, a world biodiversity hotspot confronted with climate change, and thus contributes towards the planning of genetics-informed conservation strategies.
Keywords: Juniperus phoenicea L, genetic variation, ISSR, conservation, Cupressaceae
INTRODUCTION
The combination of a wide geographical range and of the presence of dispersed populations make it possible for a plant species to harbour a vast amount of genetic variability. This genetic richness can be present either in the form of allelic variability or allelic ‘uniqueness’ of some populations (Petit et al., 1998). The occurrence of peculiar allelic combinations, which could have been selected in order to adapt to particular environmental conditions, not to be found in other areas of the distribution range, can be exploited in planning conservation strategies, such as reforestation. Thus, the identification of relict populations, or of refugia, can be a valuable tool for conservation purposes in plants.
Juniperus phoenicea (family Cupressaceae) is a small monoecious or dioecious Mediterranean tree native to coastal sites, as the species name implies. Its distribution, ranging from Portugal to Saudi Arabia, covers the whole Mediterranean basin, where the species is present mainly with scattered populations in littoral sites. This is reflected also in the many uncertainties about the presence of intraspecific taxa, based upon morphological (Gaussen, 1968), biochemical (LeBreton and Thivend, 1981) and molecular (Adams et al., 2002) evidence. Even without entering taxonomic details, this is diagnostic of a vast amount of genetic variability present in the species. Attention was focussed on a small population of Juniperus phoenicea present in the area of Valdieri on the Italian side of the Maritime Alps, where a natural preserve has been created. The main attribute of this population is the geographical position, in a secondary valley of the Alps, at an altitude of 1200–1500 m a.s.l. The area of the Maritime Alps, characterized also by the presence of the Argentera-Mercantour National Park, is of interest because of the presence of the highest number (32) of exclusive endemic species of the whole Alpine range (Pawlowski, 1970). In particular, the very site of Valdieri is relevant because of the presence of a small population of Norway spruce that has been targeted as a possible refugial population by genetic analysis (Scotti et al., 2000). The purpose of this study was to ascertain whether the juniper ‘mountain’ population of Valdieri showed characteristics of a refugial population in the comparison with other populations of the Mediterranean basin, which is one of the world's most important areas for biodiversity now experiencing the first effects of climate change. By means of assessing the amount and distribution of genetic variability in this species, this preliminary study represents a first step towards developing conservation guidelines and strategies which can be of more general interest. ISSRs (inter-simple sequence repeats) were used to detect variation at the DNA level in samples collected from Valdieri, from three Sardinian populations and from one population in Cyprus.
Given the preliminary character of this study, ISSRs were the choice among the many classes of available molecular markers because they do not require preliminary sequence information, they are less prone to laboratory conditions than RAPDs, have been successfully used in similar studies in plant species, among them Juniperus (Adams et al., 2003) and, last but not least, are amenable to be upgraded to co-dominant markers in the form of microsatellites (van der Nest et al., 2000).
MATERIALS AND METHODS
Plant material and DNA extraction
The plants used in this study were sampled from five natural populations of Juniperus phoenicea L. located in the Maritime Alps [Valdieri (JV), 22 trees], Sardinia [Capo Caccia (JS), 15 trees; Capo d'Orso (JO), 21 trees; Asinara island (JA), 31 trees] and Cyprus [Akra Gkreco (JC), 20 trees)]. The locations of sampled populations are shown in Fig. 1 and their characteristics are reported in Table 1. All populations grew on rocky soil and at the sea level, with the exception of the JV population. Bud material was collected and stored at 4 °C until DNA extraction, and stored thence at −80 °C.
Fig. 1.
Geographical map showing the location of the five populations of Juniperus phoenicea studied. JV, Valdieri; JA, Asinara island; JS, Capo Caccia; JO, Capo d'Orso; JC, Akra Gkreco.
Table 1.
Natural populations of Juniperus phoenicea used in this study
| Population name | Locality | Country | Latitude (N) | Longitude (E) | No. of plants |
|---|---|---|---|---|---|
| JV | Valdieri | Italy | 44°17′ | 07°24′ | 20 |
| JC | Cape Gkreco | Cyprus | 34°57′ | 34°05′ | 15 |
| JS | Capo Caccia | Italy | 40°34′ | 08°09′ | 14 |
| JO | Capo d'Orso | Italy | 41°10′ | 09°25′ | 21 |
| JA | Asinara | Italy | 41°03′ | 08°15′ | 31 |
Total genomic DNA was extracted from 100 mg of tissue of each plant using QIAGEN DNeasy Plant Mini kit according to the manufacturer's instructions, after grinding in a mortar in liquid nitrogen. DNA was suspended in 100 µL of sterile, distilled water and stored at 4 °C.
ISSR amplification and analysis
An initial set of 16 primers (see Table 2) was screened to select those generating good amplification patterns. Primers were 18-mer and were randomly designed using the most frequent dinucleotide microsatellites in plants flanked by a dinucleotide sequence 3′ anchored.
Table 2.
Sequences of the 16 ISSR primers used in the preliminary screening on the five populations of Juniperus phoenicea
| ISSR | Primer sequences (5′→3′) |
|---|---|
| ISSR(AG)8–1 | agagagagagagagagTC |
| ISSR(AG)8–2 | agagagagagagagagGT |
| ISSR(AG)8–3 | agagagagagagagagGC |
| ISSR(AG)8–4 | agagagagagagagagCA |
| ISSR(AC)8–1 | acacacacacacacacAG |
| ISSR(AC)8–2 | acacacacacacacacCG |
| ISSR(AC)8–3 | acacacacacacacacGA |
| ISSR(AC)8–4 | acacacacacacacacTG |
| ISSR(AT)8–1 | atatatatatatatatTT |
| ISSR(AT)8–2 | atatatatatatatatGC |
| ISSR(AT)8–3 | atatatatatatatatCT |
| ISSR(AT)8–4 | atatatatatatatatAG |
| ISSR(CT)8–1 | ctctctctctctctctAC |
| ISSR(CT)8–2 | ctctctctctctctctGC |
| ISSR(CT)8–3 | ctctctctctctctctAG |
| ISSR(CT)8–4 | ctctctctctctctctGT |
The three primers used for genotyping are indicated in bold.
PCR amplifications were performed in a final volume of 20 µL containing 30 ng of genomic DNA, 50 mM of KCl, 10 mM of Tris–HCl pH 8·3, 0·01 % gelatin, 400 µM of each dNTP, 1–2·5 mM of MgCl2, 1 µM of the primer and 1 U of RED Taq polymerase (Sigma).
Reactions were carried out in a MJ Research PTC-100 thermal cycler using the following profile: 94 °C for 4 min followed by 45 cycles of 94 °C for 30 s, 52 °C for 45 s, 72 °C for 2 min and ended with 72 °C for 7 min.
All amplification reactions were repeated at least three times and only three primers exhibiting specific PCR products unequivocally scorable and reproducible in successive amplifications were used for the final studies (see Table 3 for the annealing temperature and the concentration of MgCl2 that yielded the best results).
Table 3.
Amplification parameters and number of reproducible bands for each of the three ISSR primers used in this study
| ISSR | Annealing temperature (°C) | [MgCl2] (mM) | No. of loci detected |
|---|---|---|---|
| ISSR(AG)8–4 | 52 | 2 | 17 |
| ISSR(AC)8–4 | 52 | 2 | 21 |
| ISSR(AC)8–1 | 52 | 1·5 | 7 |
Amplification products were resolved electrophoretically on 2 % agarose gels run at 3 V cm−1 in TBE 1X buffer. After staining with ethidium bromide, gels were visualized on a UV lamp and photographed. Molecular weights were estimated by comparison with a 100-bp DNA ladder, a 1-kb DNA ladder and pBlueScript digested by HpaII. PCR products were scored for band presence or absence.
Data analysis
All the amplified bands were treated as dominant genetic markers. For each sample ISSR bands were scored as 1 (present) or 0 (absent) and these binary data were used to assemble a rectangular matrix. Under the assumption that each band corresponds to a genetic locus, allelic frequencies were estimated as q = square root of the frequency of ‘0’ phenotypes, using the correction of Lynch and Milligan (1994) as implemented by the software AFLPsurv (Vekemans, 2002). For principal co-ordinate analysis (PCoA), the method for dominant data by Huff and colleagues (Huff et al., 1993) as implemented by GenAlEx (Peakall and Smouse, 2001) was followed, where the distance equals D = [1 − 2nxy/2n] with 2nxy the number of shared bands between two individuals and 2n the total number of banding positions. By the same software an analysis of molecular variance (AMOVA) was performed to partition the total genetic variation among regions and among populations within regions (Excoffier et al., 1992; Huff et al., 1993). The test of significance for the AMOVA was carried out on 999 permutations of the data.
Basic population parameters such as polymorphism (P) and heterozygosity (H) were calculated for each population based on the banding pattern observed. The amount and distribution of genetic variation were analysed in several ways. First, standard Nei's measures were estimated starting with single locus heterozygosity He = 1 − (p2 + q2), where p represents the frequency of the ‘presence of the band’ allele and q the frequency of the ‘null’ allele, mean heterozygosity within populations HS, total heterozygosity between populations HT, the diversity among populations DST = HT − HS and the coefficient of genetic differentiation GST = DST/HT. Nei's genetic distances D were also computed between the populations studied (Nei, 1978) and their significance assessed by bootstrap. UPGMA clustering was made by NEIGHBOR of the Phylip package (Felsenstein, 2004) and a consensus tree obtained by CONSENSE of the same package. Finally, the estimate Nm of gene flow, as the number of migrants entering a population in each generation, was made according to Wright (1931), Nm = (1 − FST)/4FST, where the conceptually equivalent GST is used as an approximation for FST.
RESULTS
Genetic variability
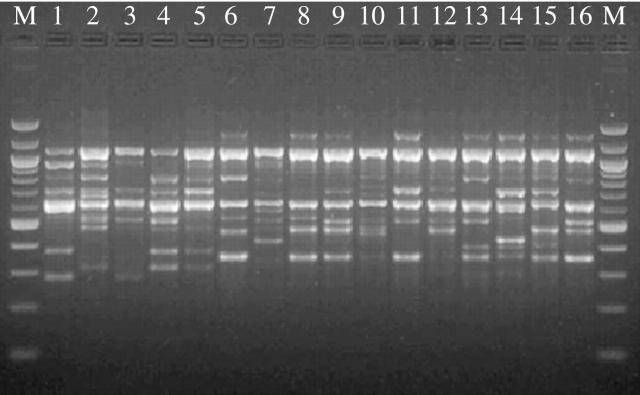
The information obtained by the analysis of the banding pattern is summarized in Table 4. Three selected ISSR primers yielded 45 reproducible amplification products in the range 260–1350 bp after amplification of five populations of Juniperus phoenicea. Some ISSR bands were population-specific: four out of 17 bands yielded by the primer (AG)8–4 (Fig. 2) and three out of seven of those produced by (AC)8–1 were found only in the three ‘Sardinian’ populations, while six out of 21 bands of the (AC)8–4 primer were specific to JV and JC. Under the assumption that each amplification product corresponds to a genetic biallelic locus, allelic frequencies were estimated after the Lynch and Milligan correction from the frequency of trees showing the ‘0’ phenotype. The degree of polymorphism ranged from 24·4 % (JC) to 62·2 % (JO).
Table 4.
Genetic variability and diversity for Juniperus phoenicea in the populations studied
| JV | JC | JS | JO | JA | All | |
|---|---|---|---|---|---|---|
| P | 48·9 | 24·4 | 35·6 | 62·2 | 53·3 | |
| He | 0·133 | 0·101 | 0·142 | 0·146 | 0·128 | |
| HT | 0·148 | |||||
| HS | 0·130 | |||||
| DST | 0·018 | |||||
| GST | 0·120 | |||||
| Nm | 1·8 |
P, Percentage polymorphism; He, Nei's heterozygosity; HT, total heterozygosity; HS, heterozygosity within population; DST, heterozygosity between populations; GST, degree of population differentiation; Nm, number of migrants per generation.
Fig. 2.
Amplification profiles of 16 Juniperus phoenicea trees from the JC population with the primer ISSR(AG)8–4. Lanes M, 100-bp marker.
Genetic diversity
Total heterozygosity (HT) for Juniperus phoenicea, as estimated by three ISSR primers for a total of 45 loci, was 0·148, compared with an average heterozygosity within populations (average HS) of 0·l30 and to a diversity between populations (DST) of 0·018. The proportion of genetic variation contributed by the differences between populations (GST) is 0·12, thus leaving 88 % of the total genetic variation harboured within the populations analysed.
The amount of gene flow, calculated as Nm = (1 − FST)/4FST, is 1·8. The genetic distances between the five populations, as estimated by Nei's D, were used to build a UPGMA dendrogram (Fig. 3), after bootstrapping the data 1000 times. The clustering of the three Sardinian populations is the most evident feature of the tree.
Fig. 3.

UPGMA tree of Nei's genetic distances between the five populations studied. The percentage of resampling runs supporting each node is shown. JV, Valdieri; JA, Asinara island; JS, Capo Caccia; JO, Capo d'Orso; JC, Akra Gkreco.
AMOVA
The total amount of genetic variation detected has been partitioned into its components due to the subdivision between regions and between populations within regions. The populations were divided into three geographical regions: ‘Sardinia’, comprising JA, JS and JO, ‘Alps’ with JV and ‘Cyprus’ with JC. The results of AMOVA (Table 5) show that a small but significant amount of genetic variation (7 % of the total) is due to differences among regions and that another, larger, significant amount (16 % of the total) is due to differences among populations within regions.
Table 5.
Analysis of molecular variance (AMOVA) based on 45 ISSR markers for the five populations of Juniperus phoenicea
| Source of variation | d.f. | Variance component | % variation explained | P |
|---|---|---|---|---|
| Among regions | 2 | 0·364 | 0·071 | 0·001 |
| Among populations/within regions | 2 | 0·846 | 0·164 | 0·001 |
| Within populations | 93 | 3·943 | 0·765 | 0·001 |
Regions analysed are ‘Sardinia’ with three populations and ‘Valdieri’ and ‘Cyprus’ with one population each.
P-values were estimated by a permutation procedure based on 999 replicates.
Ordination analysis
A multivariate ordering (PCoA), based on pairwise genetic distances between the individuals of all the five populations studied, was used to assess levels of intra-population genetic structuring and gave the results shown in Fig. 4, where each tree is plotted according to the first two principal co-ordinates. In general, the clusters formed by trees of the same population are easily recognizable, if not so well defined. The first two co-ordinates explain 21·44 % of the total variability (11·09 % + 10·35 %).
Fig. 4.
Principal co-ordinate analysis based on the multilocus genotype for the 101 trees of this study. The percentage of the total variability explained by the two first components is 11·09 % for PC1, 10·35 % for PC2. Each symbol represents a single tree as indicated (JV, Valdieri; JA, Asinara island; JS, Capo Caccia; JO, Capo d'Orso; JC, Akra Gkreco).
DISCUSSION
Genetic variability and diversity
Five populations of Juniperus phoenicea were analysed by three ISSR primers yielding 45 scorable bands. This number is lower than in similar studies based on ISSRs in other plant species, but given the somewhat preliminary flavour of this study it proves large enough to give some useful insights. Under the assumption that each band represents a genetic locus, it was possible to estimate allelic frequencies and therefore to proceed further into estimating classical population parameters.
The five populations show a percentage of polymorphism ranging from 24·4 % (JC) to 62·2 % (JO), while the overall species polymorphism was 45 %. In a ISSR-based study on the rare endemic species Lactoris fernandeziana, whose geographical range is limited to a lonely island of the Pacific Ocean, the polymorphism within populations varied from 0 % to 37 % and was 53 % at the species level (Crawford et al., 2001). In other plant species with a large distribution, ISSRs yielded values of polymorphism between 72 % and 95 % in Penstemon (Wolfe et al., 1998) and 81 % in Primula obconica (Nan et al., 2003), but these studies were based on a greater number of individuals and ISSR bands.
Estimates of total genetic diversity (Nei's He) are very similar across populations (range 0·101–0·146; extremes are for JC and JO populations, respectively). The populations seem therefore to bear the mark of some colonization or isolation event; the Valdieri population, in particular, shows He = 0·133, similar to the Sardinian ones, indicating that its characteristics are compatible with those of other isolated populations, thus probably sharing an analogue ecological history.
The overall degree of genetic differentiation, as estimated by GST, is 0·12, slightly higher than the average for conifers (FST = 0·052 estimated for Picea abies across the whole of Europe by Lagercrantz and Ryman, 1990). The level of gene flow is estimated at one or two plants; gene flow of more than four migrants per generation is, in theory, sufficient (Slatkin, 1987) to prevent genetic differentiation between populations due to drift alone. In the present study the level of gene flow estimated is low enough not to rule out the possibility that some differentiation among the populations of Juniperus phoenicea can be due to isolation, as can also be suggested by the geographical position of the populations themselves.
In the present study, individuals from different populations display a lower genetic distance than trees within the same population, as indicated by the value of GST, meaning that almost 90 % of the species diversity resides within populations.
Another factor influencing the distribution of genetic diversity within a species is the distribution range; a species which has populations scattered over a wide range, as is the case of Juniperus phoenicea, is more prone to influence from the local environmental conditions and to display high values of population differentiation due to, at least in part, drift and inbreeding.
The population differentiation was estimated by means of Φ-statistics obtained from hierarchical analysis of the total genetic variation. Differentiation between populations was significant, accounting for 16 % of the total variation, and the differences between the ‘Sardinian’ and the other two populations were significant also, albeit accounting for a smaller amount (7 %) of genetic variation. These findings are consistent with the high levels of fragmentation of the species, probably enhanced by the sampling, which was made on five populations only and across a wide area of the Mediterranean. However, as far as is known, all but one of the natural populations of Juniperus phoenicea present in Italy was sampled; this means that the scattering of the populations is a biogeographical factor that must be taken into account when interpreting data or planning conservation strategies. Each population formed a genetically recognizable, if not so clear-cut, group according to ordination analysis. The relatively low amount (21 %) of genetic variation explained by the first two principal co-ordinates is in agreement with the limited, although significant, amount of genetic variation between populations partitioned by the AMOVA.
The AMOVA results are also corroborated by the phylogenetic tree built, based upon Nei's distances. Genetic distances separate, supported by high bootstrap values, the Sardinian populations from the Valdieri and the Cypriot ones. The distance between the latter ones is comparable with the distance estimates for the Sardinian populations (data not shown). The biological significance of this is far from clear and the use of more robust genetic markers (microsatellites or SNPs) and a true phylogeographical analysis based on cpDNA are required to unravel the situation.
Implications for conservation
This study, although limited to 45 ISSRs and a few geographical regions, has conservation implications for the species Juniperus phoenicea; in general, widespread species with geographically differentiated populations are prone to give rise to new sub-species or local ecotypes by means of isolation or local adaptation. In the present study, the analysis of the distribution of genetic variability, as estimated by ISSRs, suggests that 88 % of the total genetic variation is still harboured within populations. This leads to two conclusions: (1) the species is not endangered yet, and it probably will be sufficient, for conservation purposes, to maintain a few populations placed across the whole distribution range to ensure that the total genetic diversity is represented; (2) the Valdieri population, although peculiar for geographical position and environmental conditions, does not show evidence of a possible role as relict or refugial population. As Thompson (1999) pointed out, also in Mediterranean species, refugial populations should exhibit higher variability than those of the central distribution areas.
Given the climatic change that the Mediterranean region is experiencing, a continous monitoring of the species, including more populations, co-dominant genetic markers and the assessment of ‘adaptive’ genetic variation (e.g. by means of genes related to drought tolerance) will be useful to design effective conservation strategies.
Acknowledgments
This work was part of the PhD programme of M.M. and was partially funded by a grant of Parco Naturale del Monte Barro to D.P. We thank the Parco Nazionale delle Alpi Marittime, in the person of the Director, Dr Patrizia Rossi, for giving us access to the Juniperus phoenicea reserve, and Isabella Vanetti for her skillful technical assistance. We also thank Dr M. van Loo and another anonymous reviewer for suggestions that helped to improve the manuscript.
LITERATURE CITED
- Adams PA, Pandey N, Rezzi S, Casanova J. 2002. Geographic variation in the Random Amplified Polymorphic DNAs (RAPDs) of Juniperus phoenicea, J. p. var. canariensis, J. p. subsp. eumediterranea, and J. p. var. turbinata. Biochemical Systematic Ecology 30: 223–229. [Google Scholar]
- Adams PA, Schwarzbach AE, Naresh Pandey R. 2003. The concordance of terpenoid, ISSR and RAPD markers, and ITS sequence data sets among genotypes: an example from Juniperus. Biochemical Systematics and Ecology 31: 375–387. [Google Scholar]
- Crawford D, Tago-Nakazawa M, Stuessy TF, Anderson GJ, Bernardello G, Ruiz E, et al. 2001. Intersimple sequence repeat (ISSR) variation in Lactoris fernandeziana (Lactoridaceae), a rare endemic of the Juan Fernandez Archipelago, Chile. Plant Species Biology 16: 185–192. [Google Scholar]
- Excoffier L, Smouse PE, Quattro JM. 1992. Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics 131:479–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J. 2004. PHYLIP (Phylogeny Inference Package) version 3.6. Distributed by the author. Department of Genome Sciences, University of Washington, Seattle.
- Gaussen H. 1968. Les Cupressacees. In: Les Gymnospermes, Actuelles et Fossiles. Fasc. X. Laboratoire Forestienne, Universitè Toulouse, France,
- Huff DR, Peakall R, Smouse PE. 1993. RAPD variation within and among populations of outcrossing buffalograss (Buchloë dactyloides (Nutt.) Engelman). Theoretical and Applied Genetics 96: 827–834. [DOI] [PubMed] [Google Scholar]
- Lagercrantz U, Ryman N. 1990. Genetic structure of Norway spruce (Picea abies): concordance of morphological and allozymic variation. Evolution 44: 38–53. [DOI] [PubMed] [Google Scholar]
- LeBreton P, Thivend S. 1981. Sur une sous-espece du genevrier de Phenicie Juniperus phoenicea L., definie a partir de criteres biochimiques. Naturalia monspeleliensia series Botanique 47: 1–12. [Google Scholar]
- Lynch M, Milligan BG. 1994. Analysis of population genetic structure with RAPD markers. Molecular Ecology 3: 91–99. [DOI] [PubMed] [Google Scholar]
- Nan P, Shi S, Peng S, Tian C, Zhong Y. 2003. Genetic diversity in Primula obconica (Primulaceae) from Central and South-west China as revealed by ISSR markers. Annals of Botany 91: 329–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M. 1978. Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 89: 583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Nest MA, Steenkamp ET, Wingfield BD, Wingfield MJ. 2000. Development of simple sequence repeat (SSR) markers in Eucalyptus from amplified inter-simple sequence repeats (ISSR). Plant Breeding 119: 433–436. [Google Scholar]
- Pawlowski B. 1970. Remarques sur l'endemisme dans la flore des Alpes et des Carpates. Vegetatio 21: 181–243. [Google Scholar]
- Peakall R, Smouse PE. 2001. GenAlEx V5: Genetic Analysis in Excel. Population Genetic Software for teaching and research. Canberra: Australian National University. [DOI] [PMC free article] [PubMed]
- Petit RJ, El Mousadik A, Pons O. 1998. Identifying populations for conservation on the basis of genetic markers. Conservation Biology 12: 844–855. [Google Scholar]
- Scotti I, Vendramin GG, Matteotti L, Scarponi C, Sari-Gorla M, Binelli G. 2000. Post glacial recolonisation routes for Picea abies K. in Italy as suggested by the analysis of sequence-characterized amplified region (SCAR) markers. Molecular Ecology 9: 699–708. [DOI] [PubMed] [Google Scholar]
- Slatkin M. 1987. Gene flow and the geographic structure of natural populations. Science 236: 787–792. [DOI] [PubMed] [Google Scholar]
- Thompson JD. 1999. Population differentiation in Mediterranean plants: insights into colonization history and the evolution and conservation of endemic species. Heredity 82: 229–236. [DOI] [PubMed] [Google Scholar]
- Vekemans X. 2002. AFLP-SURV version 1·0. Distributed by the author. Laboratoire de Genetique et Ecologie Vegetale, Universitè Libre de Bruxelles, Belgium.
- Wolfe AD, Xiang Q-Y, Kephart SR. 1998. Assessing hybridization in natural populations of Penstemon (Scrophulariaceae) using hypervariable intersimple sequence repeat (ISSR) bands. Molecular Ecology 7: 1107–1125. [DOI] [PubMed] [Google Scholar]
- Wright S. 1931. Evolution in Mendelian populations. Genetics 16: 97–159. [DOI] [PMC free article] [PubMed] [Google Scholar]