Abstract
•Background and Aims In the dry tropics, vegetative phenology varies widely with tree characteristics and soil conditions. The present work aims to document the phenological diversity of flowering and fruiting with reference to leafing events in Indian dry-tropical tree species.
•Methods Nine tree species, including one leaf-exchanging and eight deciduous showing varying leafless periods, were studied. Monthly counts of leaves, flowers and fruits were made on 160 tagged twigs on ten individuals of each species for initiation, completion and duration of different phenological events through two annual cycles.
•Key Results Variation in flowering relative to leaf flushing (which occurred just prior to or during a hot, dry summer) revealed five flowering types: summer flowering (on foliated shoots), rainy-season flowering (on foliated shoots following significant rains), autumn flowering (on shoots with mature leaves), winter flowering (on shoots undergoing leaf fall) and dry-season flowering (on leafless shoots). Duration of the fruiting phenophase was shortest (3–4 months) in dry-season and winter-flowering species, 6–9 months in rainy-and autumn-flowering species, and maximum (11 months) in summer-flowering species. A wide range of time lag (<1 to >8 months) between the start of vegetative (first-leaf flush) and reproductive (first-visible flower) phases was recorded in deciduous species; this time lag was correlated with the extent of the leafless period. A synthesis of available phenological information on 119 Indian tropical trees showed that summer-flowering species were most abundant (56 % of total species) amongst the five types recognized.
•Conclusions The wide diversity of seasonal flowering and fruiting with linkages to leaf flush time and leafless period reflect the fact that variable reproductive and survival strategies evolved in tree species under a monsoonic bioclimate. Flowering periodicity has evolved as an adaptation to an annual leafless period and the time required for the fruit to develop. The direct relationship between leafless period (inverse of growing period) and time lag between onset of vegetative and reproductive phases reflects the partitioning of resource use for supporting these phases. Predominance of summer flowering coupled with summer leaf flushing seems to be a unique adaptation in trees to survive under a strongly seasonal tropical climate.
Keywords: Tropical tree phenology, flowering types, fruiting, asynchrony, leafless period, semi-evergreen species, summer flowering, summer leaf flushing
INTRODUCTION
Seasonal duration of leafing, flowering and fruiting mainly determine phenological behaviour in tropical trees. These phenological events are not mutually independent in woody species, and flowering may be partly or wholly dependent on leafing activity (van Schaik et al., 1993). Nevertheless, tree species with similar leaf phenology often differ in the timing of their flowering and fruiting (Seghieri et al., 1995). Many deciduous tree species show flowering and fruiting during the leafless period, exhibiting wide separation between leafing and flowering phenophases. In many evergreen and in some deciduous species leaf flush and flowering occur close in time on the same new shoot. An analysis of the proximate controls of flowering in tropical deciduous forest species indicates that the timing of vegetative phenology strongly determines the flowering periods, and thus flowering at least depends indirectly on environmental periodicity (Rivera et al., 2002). Variation in flowering time relative to vegetative phenology, induced by a variety of factors (significant rain in winter/summer, decreasing or increasing photoperiod, or drought-induced leaf fall), results in a number of flowering patterns in tropical trees (Borchert et al., 2004).
The seasonality of tropical tree phenology is mainly determined by the duration and intensity of seasonal drought (Mooney et al., 1995) and even conspecific trees often experience differing degrees of drought stress (Singh and Kushwaha, 2005a). The severe drought experienced by different tree species may be reliably indicated by the leafless period (deciduousness), which reflects an integrated effect of seasonal drought, tree characteristics and soil moisture conditions (Singh and Kushwaha, 2005b). During seasonal drought the leafless condition in trees helps in the rehydration of the stem/twig, a prerequisite for the subsequent flowering or leaf flushing (Borchert, 1996; Borchert et al., 2002). Reproductive events generally occur during the period of low photosynthetic activity or after the period of high rates of reserve accumulation (Fenner, 1998). In view of the prolonged drought in the dry tropics, the predominant tree species are expected to be deciduous, showing early dry-season leaf-fall, leaf-flushing after the first rains, and the onset of the reproductive phase (flowering) in the early dry period following cessation of rains. The tropical dry forests harbour several phenological functional types showing widely varying leafless periods (reciprocal to growth period) and rates of resource use during vegetative growth (which increases with greater deciduousness) (Kushwaha and Singh, 2005). Although most tropical trees show a fairly well-defined short flowering period during a particular time of the year which coincides with specific phases of leafing phenology, yet flowering and fruiting phenologies of tropical trees have mainly been reported without reference to leafing (Schongart et al., 2002). Several questions are raised with respect to tropical deciduous forest trees differing in degree of deciduousness and seasonal flowering time. What are the basic flowering and fruiting patterns in different species? Are there any relationships between flowering time, fruiting duration and extent of leafless period in deciduous tree species? Are survival adaptations related to vegetative growth (leafless period) and reproductive growth (time lag between onset of leafing and flowering) related to each other?
Global climate change may force variation in timing, duration and synchronization of phenological events in tropical forests (Reich, 1995). Tropical trees are expected to respond variously to changes in rainfall and temperature because they differ widely with respect to adaptations to seasonal drought and cues for bud break of vegetative and flower buds (Singh and Kushwaha, 2005b). Several studies have shown significant variation (advanced or delayed) in onset dates of flowering (Fitter and Fitter, 2002) and fruiting responses (Chapman et al., 2005) in tree species as a result of climatic change. Probably the climate change impact can be better assessed at the level of functional types based on the duration of deciduousness and timing of onset of the reproductive phase (first-visible-flower). The need for functional types has been emphasized to evaluate and predict the nature of vegetation responses to future global change (Box, 1996).
Precise phenological information with respect to flowering and fruiting, evaluated against leafing and leafless periods, is scarce in tropical deciduous forests in India, which account for approx. 46 % of the forested land in the country (Singh and Singh, 1988). So is the information on onset dates of different phenological events, duration of events and asynchrony in tropical forest trees. In these forests common tree species show a wide range of leafless periods due to differing timings of leaf fall within the annual cycle (Kushwaha and Singh, 2005). The hypothesis was tested that in tropical deciduous forest trees flowering periodicity has evolved as an adaptation to the annual leafless duration (affecting rate and period of vegetative growth) and the time required for fruit development. The specific objectives of the present study were to: (a) document the seasonal patterns of flowering and fruiting in relation to leafing events in common tree species in a tropical dry deciduous forest; (b) quantify the levels of asynchrony for flowering and fruiting events in these tree species; and (c) evaluate the interrelationship between flowering and leaf phenophases. The emerging flowering types in this study were compared with an analysis of the descriptive phenological notes on Indian tropical trees given by Troup (1921).
MATERIALS AND METHODS
Study site
The present study was carried out in Hathinala Forest (24°18′N, 83°6′E; 315–485 m a.s.l.), which is spread over the Vindhyan plateau, in the Sonbhadra district of Uttar Pradesh, India. At Hathinala, several widespread low hills, with an undulating ridge top and a plateau, rise 20–40 m above the general terrain.
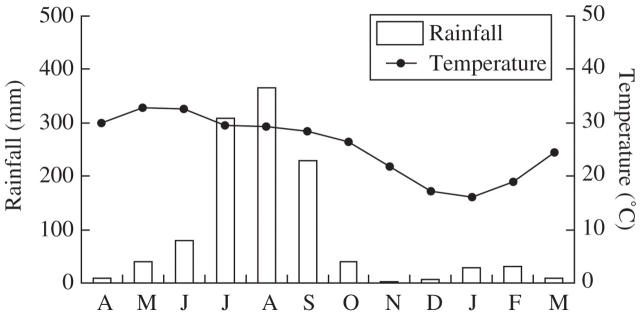
Climate is characterized by three seasons: warm-wet rainy (mid-June–September); cool-dry winter (November–February); and hot-dry summer (April–June). March and October represent the transitional months between seasons, the major part of both being closer to the season following them. The maximum day temperature varies from 20 °C in January to 42 °C in June, and the minimum night temperature from 10 °C in January to 30 °C in May. About 85 % of the annual rainfall (approx. 1035 mm) occurs during the rainy season, and 7–8 dry months occur during the annual cycle (Fig. 1).
Fig. 1.
Long-term climatic conditions at Hathinala, India.
The vegetation of Hathinala forest belongs to Northern Tropical Dry Deciduous Forest type (subgroup 5-B of Champion and Seth, 1968). The top-storey trees form a nearly continuous 15–20 m high canopy, underlain by a discontinuous lower storey of trees, a thin shrub layer and a seasonal herb layer which includes several grasses. The combined stem density of the common tree species, listed in Table 1, was 75 % of the total tree density at the study site. In the rainy season the forest becomes lush green due to the presence of fully expanded leaves of trees and shrubs and a dense herbaceous layer. The summer aspect is pale and parched because of the leafless trees and shrubs and a dried-up herb layer. A detailed description of vegetation, climate and soil at the study site is given in Kushwaha and Singh (2005).
Table 1.
Asynchrony indices for flowering, fruiting and fruit-fall phenological events in common tree species in tropical dry deciduous forest at Hathinala, India
| Asynchrony index | |||
|---|---|---|---|
| Functional type/species | Flowering | Fruiting | Fruit fall |
| Semi-evergreen | |||
| Shorea robusta (Dipterocarpaceae) | 0.20 | 0.19 | 0.36 |
| <2-months-deciduous | |||
| Hardwickia binata (Caesalpiniaceae) | 0.34 | 0.34 | 0.24 |
| Diospyros melanoxylon (Ebenaceae) | 0.12 | 0.21 | 0.30 |
| Anogeissus latifolia (Combretaceae) | 0.30 | 0.24 | 0.20 |
| 2–4-months-deciduous | |||
| Terminalia tomentosa (Combretaceae) | 0.25 | 0.33 | 0.33 |
| Acacia catechu (Mimosaceae) | 0.35 | 0.30 | 0.39 |
| Lagerstroemia parviflora (Lythraceae) | 0.34 | 0.18 | 0.38 |
| >4-months-deciduous | |||
| Boswellia serrata (Burseraceae) | 0.32 | 0.21 | 0.16 |
| Lannea coromandelica (Anacardiaceae) | 0.43 | 0.37 | 0.33 |
Values are mean of two annual cycles.
Experimental design
To document the phenological diversity and asynchrony in common tree species a 2-ha permanent plot was marked at the Hathinala site. Ten adult individuals (>30 cm girth) each of the species listed in Table 1 were marked. On each marked individual, four major branches (one in each direction) were selected, and on each branch four twigs (currently growing shoots of last-order branches) were marked with metal tags. Thus, for each species 160 twigs were selected to represent the whole plant canopy. On these twigs monthly counts of leaves, flowers and fruits were made for two consecutive annual cycles (May 2001 to June 2003). In addition, ten other adult individuals of each species were marked within the permanent plot for visual observations of phenological events.
The following phenological events were derived in all conspecific trees from the monthly counts of leaves, flowers and fruits: leaf flush initiation; leaf flush completion; leaf fall initiation; leaf fall completion; leafless period; initiation of flowering; completion of flowering; time lag between start of vegetative (first-leaf flush) and reproductive (first-visible flower) phases; initiation of fruiting; completion of fruiting; fruit-fall initiation; and completion of fruit fall. Detailed information on leaf phenology has been published elsewhere (Kushwaha and Singh, 2005). Depending on the leaf phenology four plant functional types (semi-evergreen, <2-months-deciduous, 2–4-months-deciduous and >4-months-deciduous) were recognized (Table 1).
Because no observations were made during the interval between two sampling dates (usually 30 d), it was assumed that in a tree a particular phenophase began before, or continued beyond, the date of the first/last record by a one-half-sampling interval. For example, in this study the flowering period for each species was calculated from 15 d before the date on which the event was recorded for the first time in any individual to 15 d after the date on which the event was recorded the last time amongst individuals. Fruiting period of a species was the duration (days) from the first fruit formation to the last amongst its individuals. In the same way, the fruit-fall period of a species represented the time duration from the first fruit fall amongst individuals to the last. For each individual of a species flowering, fruiting and fruit-fall durations were calculated from 15 d before the date on which the event was recorded for the first time to 15 d after the date on which the event was recorded for the last time.
The synchrony index for flowering, fruiting and fruit-fall phenophases of each species was calculated as the ratio between the individual's mean duration of a phenological phase and the overall duration of the phase (Devineau, 1999). The higher the ratio, the greater the coincidence between different individuals of a species (i.e. the ratio 1·0 denotes perfect synchrony amongst individuals and as the ratio decreases from 1·0 asynchrony increases). The value of the synchrony index was subtracted from 1·0 to get the asynchrony index. Synchrony indices based on the duration of phenological phases represent the whole population and vary minimally with the number of conspecific individuals under observation; thus, it can be compared in terms of overall intra-site and inter-site synchrony.
RESULTS
Flowering and the leaf phenological state
Spring flushing, semi-evergreen Shorea robusta began flowering with the onset of leaf fall in winter. Its flowering (January–April) coincided with the leaf transitional state (leaf fall, leaf initiation), and fruit formation and leaf flushing both were supported at the same time (Fig. 2). Greater flowering and fruiting occurred in this species during the first annual cycle. In the eight summer-flushing deciduous species the initiation of flowering was seasonally separated. The <2-months-deciduous, Diospyros melanoxylon flowered in the hot-dry period on new shoots (summer flowering) showing virtual synchronization of leaf flushing with flowering. Hardwickia binata did not flower in the first annual cycle, but during the second annual cycle 70 % individuals flowered. Rainy season flowering in <2-months-deciduous (Hardwickia binata) and 2–4-months-deciduous species (Lagerstroemia parviflora and Acacia catechu) coincided with their leaf-flushing phenophase. Autumn flowering in <2-months-deciduous (Anogeissus latifolia) and 2–4-months-deciduous (Terminalia tomentosa) species occurred at the end of leaf flushing (on growing shoots). Cool dry-season flowering (December–March) in >4-months-deciduous Boswellia serrata and Lannea coromandelica occurred on leafless twigs after a long interval following completion of leaf flush (approx. 3–5 month) and leaf fall (approx. 1–3 month).
Fig. 2.
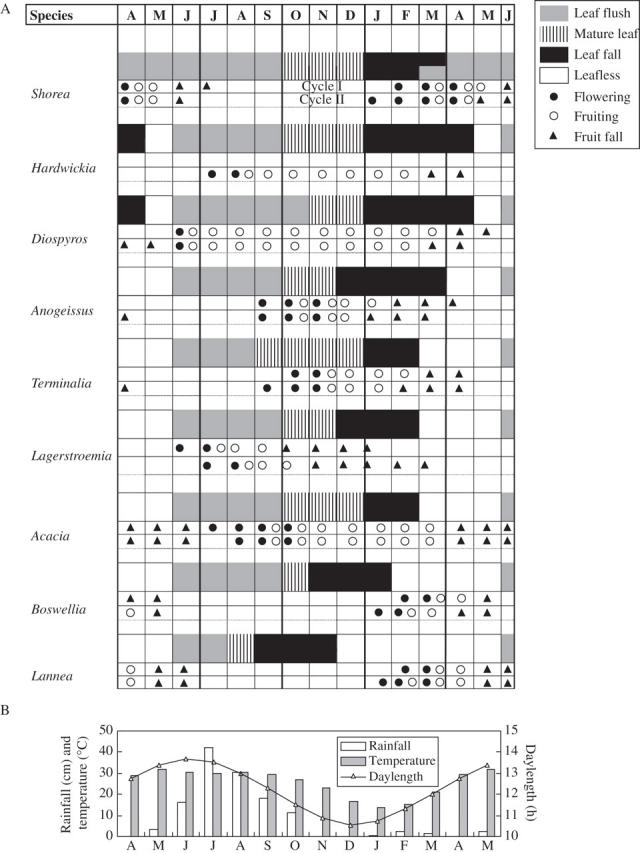
Flowering, fruiting and leafing phenology of nine tree species (A), and climatic conditions (B) as observed during two consecutive annual cycles in tropical dry deciduous forest at Hathinala, India. Values of April, May and half of June are shown twice to clearly depict the leafless period.
Flowering time and fruiting duration
Species flowering in different seasons exhibited varying fruiting durations (Fig. 3). Formation of fruits continued through 2–3 months following the peak flowering time in different species. However, the time required for fruit maturation varied considerably with species. Winter-flowering Shorea showed a 3- to 4-month-long fruiting phenophase. Amongst the deciduous species, Diospyros, which flowers in summer before the onset of rains, showed the longest fruiting phenophase (11 months). In other deciduous species flowering in succeeding seasons, fruiting duration was invariably shortened. Rainy season flowering species (Hardwickia, Lagerstroemia and Acacia) showed a 7- to 9-month-long fruiting phenophase. Autumn-flowering Anogeissus and Terminalia showed 5–8 months of fruiting. In the dry-season-flowering Boswellia and Lannea, fruiting extended through 3–4 months. Fruit fall occurred in most species during the last 2–3 months of the fruiting phenophase (excepting Lagerstroemia, 6 months). Generally, fruit fall was completed in the April–June period, <2 months before the rains began.
Fig. 3.
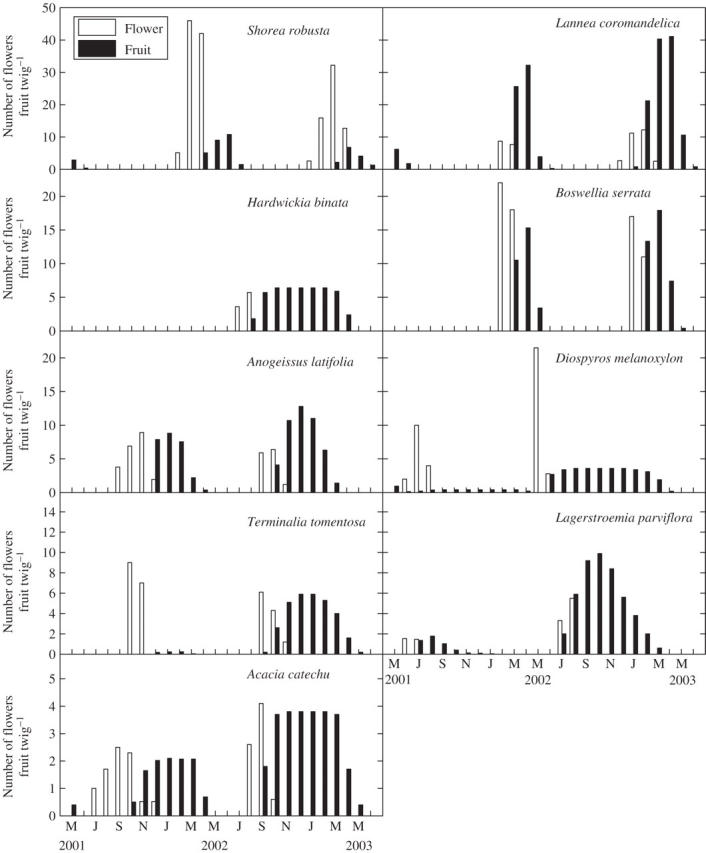
Seasonal variation in the number of flowers and fruits on twigs in nine tree species of tropical dry deciduous forest at Hathinala, India. Each bar represents the mean of 160 twigs.
Intra-species asynchrony of phenophases
Wide variations were noticed among tree species with respect to intra-species asynchrony for flowering, fruiting and fruit-fall phenophases (Table 1). Even the asynchrony indices for the three phenophases of the same species differed considerably from each other. The least asynchrony index (i.e. high synchrony) for the flowering phenophase was recorded in Diospyros, and the highest asynchrony occurred in Lannea. Individuals of Diospyros and Lagerstroemia showed least asynchrony in fruit initiation. The highest asynchrony occurred amongst the individuals of Lannea. The least asynchronized fruit fall occurred in Boswellia and the highest asynchrony was recorded in Acacia.
Time lag between reproductive and vegetative phases
All deciduous tree species showed a distinct time lag between the estimated dates of initiation of the vegetative phase (first-leaf flush) and the succeeding reproductive phase (first-visible flower) (Table 2). In <2-months-deciduous (Hardwickia, Diospyros and Anogeissus) and 2–4-months-deciduous (Terminalia, Lagerstroemia and Acacia) species the time lag ranged from 0·8 to 4·5 months. The >4-months-deciduous, Boswellia and Lannea showed approx. 8 month time lag. The time lag between the start of vegetative and reproductive growth phases was significantly positively correlated with the duration of leafless period in deciduous species (r = 0·86, P < 0·01). Besides, with an increase in the above time lag the fruiting duration decreased in these species.
Table 2.
Characteristics of different flowering types in common tree species in tropical dry deciduous forest at Hathinala, India
| Flowering type/time/species | Possible flowering cue | Vegetative phenology | Time lag* (months) | Leafless period (days) | Fruiting duration (months) |
|---|---|---|---|---|---|
| Deciduous species | |||||
| Summer (March–June) (Diospyros) | Increasing day length and/or temperature | Leaf flushing | 0.8 | 33 | 11 |
| Rainy (June–August) (Acacia, Hardwickia, Lagerstroemia) | First significant rain | Leaf flushing | 1.3–2.7 | 18–78 | 7–9 |
| Autumn (September–December) (Anogeissus, Terminalia) | Decreasing day length | End of leaf flushing, shoots with mature leaves | 4.2–4.5 | 62–69 | 6–7 |
| Dry season (December–March) (Lannea, Boswellia) | Leaf fall or winter rains | Leafless shoots | 7.7–8.2 | 138–201 | 3–4 |
| Semi-evergreen (leaf-exchanging) species | |||||
| Winter (January–March) (Shorea) | Drought induced leaf shedding | During and after leaf exchange | 0 | 0 | 3–4 |
Estimated time lag between first leaf flush to first visible flower.
Flowering types in Indian tropical trees
The majority of species initiate flowering at the beginning of any one of the three seasons, with varying leaf phenological state, and the flowering period is virtually superimposed over that season. Some species flower towards the end of the rainy season, continuing until early winter (apparently coinciding with declining photoperiod). According to flowering time, their possible flowering cue(s), and state of leaf phenology during flowering, the following five flowering types are distinguishable amongst the presently studied species (Table 2): (1) summer flowering, on foliated shoots during the hot-dry period (March–June), probable flowering cue increasing day length/temperature; (2) rainy flowering, on foliated shoots during the warm-wet period (June–August), first significant rains may act as flowering cue; (3) autumn flowering, on shoots with mature leaves (September–December) during the period of decreasing day length; (4) winter flowering, on foliated shoots with concurrent leaf fall and leaf flush during the cool dry season (January–March), leaf-fall may act as a cue to flowering; and (5) dry-season flowering, occurring on leafless twigs during cool dry season (December–March) soon after leaf shedding or after sporadic winter rains. While winter flowering occurs in leaf-exchanging semi-evergreen species, the deciduous species show four flowering types. These flowering types show differing leafless periods, fruiting durations and vegetative-to-reproductive time lag. Their probable flowering cues also vary.
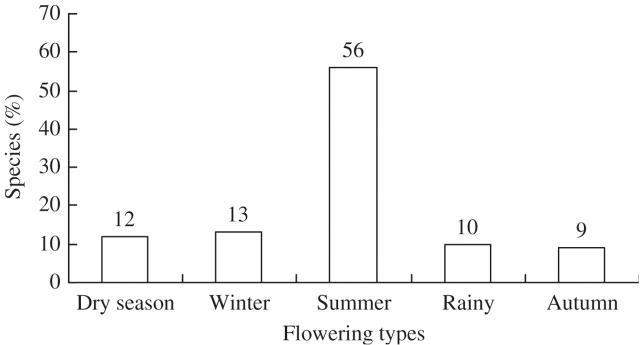
To verify the ubiquitousness of these categories in a large species data set, the phenological notes included in silvicultural descriptions of 119 Indian tropical trees (Troup, 1921) were brought together as shown in Appendix 1. Although these species are distributed in dry to moist tropical regions, taking into consideration the timing and duration of leafing and flowering, deduced from these descriptions, it is evident that these 119 species are easily categorized within the five flowering types suggested from the present study. While the majority of tree species (56 %) are summer flowering, the remaining four categories account for 9–13 % species each (Fig. 4). Summer-flowering species mostly produce flowers along with leaf flushing, but about 18 % of these species may flower on leafless shoots.
Fig. 4.
Proportion of the five flowering types in 119 Indian tropical trees.
The available description of the flowering time and fruit maturation in 119 tropical tree species (Appendix 1) also indicated that fruiting duration in most of the reported Indian trees is related to the particular flowering type. The fruiting duration in these species generally decreased in the order: summer-flowering, rainy-flowering, autumn-flowering, winter-flowering and dry-season-flowering species. It is interesting that in species flowering during the dry period of the annual cycle (dry-season flowering, winter flowering, summer flowering), the fruit type was distributed among pods (13–22 %), drupe (19–26 %), capsule (6–21 %), berry (9–14 %) and follicles (4–14 %). However, in rainy- and autumn-flowering species about one-half of the species showed pods as their fruit type.
DISCUSSION
Diversity of seasonal flowering types
Flowering and fruiting in tropical deciduous forest trees typically occur throughout the year on one species or another. Synchronization of flowering with a particular season of the annual cycle by many species appears to be under the control of prevailing climatic conditions of that season. The detection of several flowering types in Indian tropical trees revealed that a variety of strategies have evolved to ensure survival and reproduction under a monsoonic bioclimate. The predominant role of leaf shedding in triggering flowering as a result of stem rehydration is indicated in winter-flowering, leaf-exchanging Shorea and in >4-months-deciduous dry-season-flowering Boswellia and Lannea. In many species, flower buds formed during the growing season remain dormant until bud expansion is triggered during the dry season by rehydration of leafless twigs caused by leaf shedding (Borchert, 2000); such bud endo-dormancy may represent a strategy to separate resource use for vegetative and reproductive phenophases. Potential adaptive significance of winter flowering in leaf-exchanging species (confined to moist micro-sites), having a slower resource-use rate during its long vegetative phase, may be to maximize the accumulation of photosynthate during a longer growing period and start the reproductive phase at a time when vegetative growth is at its minimum. The >4-months-deciduous species, generally showing shallow root systems and distribution in very dry sites, use the favourable rainy season for leafing to rapidly accumulate sufficient photosynthate, and initiate reproduction prior to the steep fall in soil water reserve during the progressively drier period of the annual cycle. Dry-season flowering on leafless shoots during the early part of the dry period may have evolved in response to rapid resource-use rate during their short growing period (mainly rainy season). Water storage in their trunk may enable maintenance of a high stem water potential and flowering during the dry season (Schongart et al., 2002).
Species with intermediate duration of deciduousness and resource-use rate show summer, rainy or autumn flowering possibly depending on resource requirement during their reproductive phase. Many species have been reported to regularly flower synchronously after the spring equinox during March–June (Van Devender et al., 2000; Felger et al., 2001). In such species, perhaps in Diospyros as well, increasing day length/temperature may induce flowering during hot dry summer. In species flowering during the rainy season (Hardwickia, Lagerstroemia, Acacia), the first heavy rain may act as a flowering cue. Autumn flowering occurs during early dry season at a time when carbohydrate reserves are in plenty and climate conditions are favourable for reproduction before depletion of soil water reserves. Declining day length may induce synchronous development of flowers in autumn-flowering (September–November) species (Anogeissus and Terminalia). Flower development on foliated shoots after the autumn equinox indicates flower induction by declining day length (Rivera and Borchert, 2001). Intra-species asynchrony recorded in the presently studied tree species illustrates the plasticity of the individuals that should contribute, to a large extent, to population maintenance and expansion in the forest.
The proportion of species flowering during the dry period of the year varies widely among tropical deciduous forests bearing differing intensity of drought, e.g. Guanacaste (Costa Rica) 53 %, Yucatan (Mexico) 54 %, Jalisco (Mexico) 49 % and Sonora (Mexico) 76 % of the total species number (Borchert et al., 2004). In relatively moist tropical deciduous forests (e.g. Yucatan and Guanacaste; average rainfall 1223 and 1315 mm, thee and five dry months, respectively) about one-half of the tree species flower during or soon after the rainy season. However, in relatively drier tropical deciduous forests (Sonora and Hathinala; rainfall 660 and 1035 mm, seven and eight dry months), less than one-third of the species flower during the wet period of the annual cycle. The large fraction of species flowering in Indian tropical forests during the dry season (83 %, December–June) reflects the availability of water required for the growing organs (e.g. through sporadic winter rains, absorption from soil water reserves by leaf-exchanging species, or using stored stem water in stem succulents). Predominant summer flowering in the majority of Indian deciduous forest tree species (56 %), which are leafless for <1 to 4 months, in association with summer leaf flushing seems to be an unique adaptation to survive under a strongly seasonal climate with a short wet period (growth promoting) and a long dry (growth suppressive) period (Singh and Kushwaha, 2005b). Flowering during the dry part of annual cycle may also help in pollination and seed dispersion (Janzen, 1967; Wright, 1996). It has been reported that dry forests are characterized by a higher proportion of wind-dispersed species compared with moist forests (Bullock, 1995).
Deciduousness and leafing-flowering time lag
Very few studies have examined the possible functional significance of an interrelationship between leafing and flowering/fruiting phenophases in tropical trees (van Schaik et al., 1993). Occurrence of leaf flushing (vegetative phase) and flowering (reproductive phase) requires the availability of substantial amounts of resources within the trees. For instance, flower production and maintenance requires considerable expense of energy to form non-photosynthetic tissues and nectar (Ashman and Schoen, 1997). Besides, the time lag between these two events reflects partitioning of resource use for vegetative and reproductive functions. Various physiologically active sites or sinks (e.g. leaf buds and leaves, flower buds and flowers, and fruit) may compete for water, nutrients and metabolites (Lieberman, 1982), and such internal competition may lead to the partitioning in time of plant functions like leafing and flowering. While the two survival adaptations (leafless period and flowering time) are linked with capacity adaptation (e.g. resource-use rate), the duration of deciduousness and time lag between onset of leafing and flowering help trees in making maximum use of available resources for growth and reproduction. Seasonally dry forests are characterized by sudden increase (after rain onset) and slow reduction (after rain cessation) in resource availability and, under such conditions, optimization of vegetative growth is crucial for tree survival and reproduction. It is suggested that flowering time and time lag between the onset of leafing and flowering affect the degree of separation of resource use for vegetative and reproductive events within trees. Variation in flowering time in different species may be related to resource-use rate during vegetative growth (which depends on the duration of deciduousness) and the time required for fruit development. In the deciduous tree species in the present study, the longer the leafless duration, the more delayed (greater time lag) is the initiation of flowering relative to leaf flushing. The leafless period is an adaptation to avoid water stress, and water stress affects flowering time in tropical forest trees (Bullock, 1995). Increase in leafless period in deciduous species results in reduction in the vegetative growth period, and drought stress is not only reflected in terms of leafless period, but is also evident from the greater seasonal separation between the two phases. In <2-months-deciduous and 2–4-months-deciduous species both phases begin relatively close in time, possibly due to slower resource-use rate (reflected by longer leaf flush period) and greater tolerance to water stress (suggested by short leafless period) than the >4-months-deciduous tree species (having a short leaf flush period and longer leafless period). The temporal separation of leafing and flowering in tropical deciduous tree species serves as an important adaptation to a strongly seasonal, dry climate, where optimization of vegetative growth during the short growing season may be crucial for tree survival.
Fruiting duration and flowering time
Different flowering types are related to varying durations of fruiting phenophase (summer-flowering species, approx. 11 months; rainy species, 7–9 months; autumn species, 6–7 months; winter and dry-season species, 3–4 months). Thus, all flowering types complete the fruiting phenophase during late dry season before the onset of the succeeding rainy season, ensuring that some, if not all, seeds are available for germination when the soil is sufficiently moist. Fruit maturation and suitable conditions for dispersal are closely synchronized in tropical dry forests because of the pronounced differences of biotic and abiotic conditions between dry and rainy seasons (Griz and Machado, 2001). It is suggested that in dry tropical trees the duration of fruiting phenophase depends, at least to some extent, on the time of flowering and the leafless period during the annual cycle. Species with longer fruiting duration, flower in the hot-dry summer and take full advantage of rainy season for fruit development. For instance, Diospyros, flowering in the summer, has a fruit maturation period of approx. 11 months, ending in a fleshy fruit. It is recognized that fleshy-fruited species bear fruit when the moisture levels are sufficient (mainly during the wet season) to allow fruit growth and maturation (Lieberman, 1982). Primack (1985) reported that species with larger fruits (requiring a longer time for fruit development) flowered earlier than species with small fruits. Phenological events in tropical trees may depend on preceding and successive stages (Fenner, 1998), hence the fruiting duration may impose a constraint on flowering time. Although fruiting duration is related to flowering time, fruit type is independent of flowering time, especially in species flowering during the dry part of the year. The predominance of pods as fruit type in rainy- and autumn-flowering species is mainly due to abundance of species belonging to the families Mimosaceae, Fabaceae and Caesalpiniaceae.
Possible climate change impact
The importance of understanding the determinants of phenological patterns has been emphasized to predict responses of specific communities to global climate change (vanVliet and Schwartz, 2002). Trends of erratic precipitation and increasing temperature are likely to alter the length of the growing season (hence the extent of leafless period) by affecting the timing of leaf-flush and leaf-fall, though to varying extents in different functional types. The competition among species (or functional types) is likely to become modified, if their phenological behaviour differs in sensitivity to the environmental conditions (Rotzor et al., 2004). Climate change will force deviations in the length of the growing period, and competition among species may alter the resource use patterns in different species. In dry tropics water stress has frequently been cited as a primary trigger for leaf shedding, but very little is known about its effect on reproductive phenology or flowering (Diaz and Granadillo, 2005). In winter-flowering leaf-exchanging species, deviations in summer and/or winter rains may affect the timings of leaf exchange and flowering through drought-induced leaf fall and depletion of sub-soil water reserves. In summer-flushing and summer-flowering trees, which are most abundant in India, the bud break will be independent of rainfall patterns, but survival of young leaves and flowering and fruit setting may be affected adversely if the rainfall period is shifted. The timing of rainy flowering will vary greatly according to the onset of the rainy season (earlier or delayed); this will affect their reproductive success. In the semi-arid region of north-western Venezuela, the varying timing of episodic rains triggers variable flowering in several tree species (Diaz and Granadillo, 2005). The direct effect of climate change will be less serious than the effect of changed phenology on pollinators and seed-dispersal agents (Corlett and Lafrankie, 1998). A rainy spell during the dry season or drought during the rainy season may cause a shift in leaf flushing and/or flowering. In seasonally dry forests of Gunacaste, Costa Rica, a 10-week-long severe drought (caused by El-Nino) during the mid-rainy season forced phenological deviations such as the replacement of deciduous species leaves by the new leaves during the rainy season and shortening of leaf life-span, abscission of flower buds in some species, precocious leaf exchange in evergreen species during drought and recurrent flushing after drought; however, no notable change occurred in phenology of spring-flushing brevideciduous and stem-succulent trees (Borchert et al., 2002). Studies designed to alter the timing of early season rainfall have shown that some species differentially respond in terms of growth and/or phenology to change in the timing of soil wetting (Tissue and Wright, 1995; Priya and Bhat, 1999).
Conclusions
The wide diversity of seasonal flowering time and fruiting duration, with linkages to leafing and leafless durations, observed in tropical deciduous forest tree species suggest a variety of reproductive and survival strategies evolved under a monsoonic bioclimate in India. The drought stress is not only reflected in terms of the leafless period, but is also evident from greater seasonal separation between leafing and flowering. Flowering time and time lag between the onset of leafing and flowering affect the degree of separation of resource use for vegetative and reproductive events within trees. In tropical deciduous tree species flowering periodicity has evolved as an adaptation to annual leafless duration (affecting rate and period of vegetative growth) and time required for fruit development. Predominance of summer flowering in association with summer leaf flushing seems to be a unique adaptation to survive under a seasonal climate. Since environmental characteristics affect flowering and fruiting either directly (e.g. through conditions in the habitat) or indirectly (e.g. through the leafless period), probable global climatic change will have serious implications on future reproductive success of dry-tropical trees.
APPENDIX 1.
Synthesis of seasonal leafing, flowering and fruiting events in 119 Indian tropical forest tree species categorized under five flowering types (based on phenological notes included in silvicultural descriptions given by Troup, 1921). Grey shading shows the main flowering period of each type. Leafing data were not available in some species. Plant names are as originally listed, but the names of families have been updated where necessary. Key: ↓, leaf fall; +, leaf flush; *, flowering; §, fruit maturation
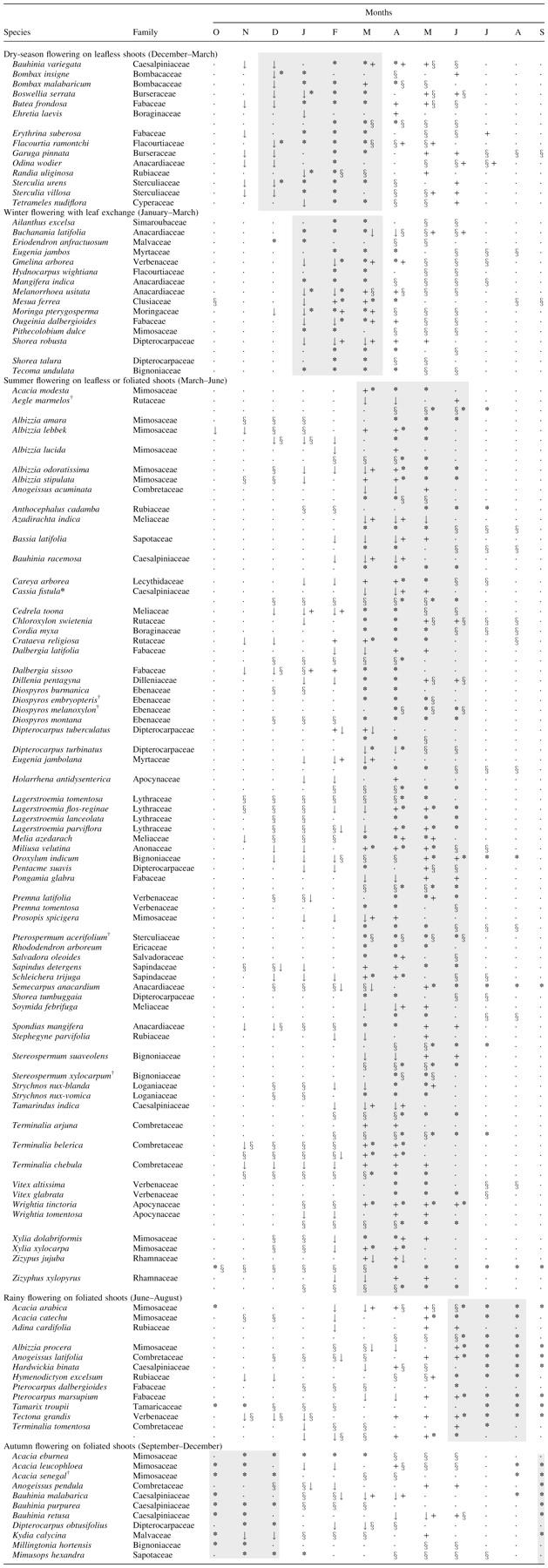
Fruit maturation in the next year.
Acknowledgments
The authors are grateful to the Head, Department of Botany and the Coordinator, Centre of Advanced Study in Botany, for laboratory and library facilities. Financial support was provided initially by the Ministry of Environment and Forests, New Delhi, and later by the Council for Scientific and Industrial Research, New Delhi, in the form of Research Associateship to C. P. Kushwaha.
LITERATURE CITED
- Ashman TL, Schoen DJ. 1997. The cost of floral longevity in Clarkia tembloriensis: an experimental investigation. Evolutionary Ecology 11: 289–300. [Google Scholar]
- Borchert R. 1996. Phenology and flowering periodicity of neotropical dry forest species: evidence from herbarium collections. Journal of Tropical Ecology 12: 65–80. [Google Scholar]
- Borchert R. 2000. Organismic and environmental controls of bud growth in tropical trees. In: Viemont JD, Crabbe J, eds. Dormancy in plants: from whole plant behavior to cellular control. Wallingford: CAB International, 87–107.
- Borchert R, Rivera G, Hagnauer W. 2002. Modification of vegetative phenology in a tropical semi-deciduous forest by abnormal drought and rain. Biotropica 34: 27–39. [Google Scholar]
- Borchert R, Meyer SA, Felger RS, Porter-Bolland L. 2004. Environmental control of flowering periodicity in Costa Rican and Mexican tropical dry forests. Global Ecology and Biogeography 13: 409–425. [Google Scholar]
- Box EO. 1996. Plant functional types and climate at global scale. Journal of Vegetation Science 7: 309–320. [Google Scholar]
- Bullock SH. 1995. Plant reproduction in neotropical dry forests. In: Bullock SH, Mooney HA, Medina E, eds. Seasonally dry tropical forests. Cambridge: Cambridge University Press, 277–303.
- Champion HG, Seth SK. 1968. A revised survey of the forest types of India. New Delhi: Manager of Publications, Government of India.
- Chapman CA, Chapman LJ, Struhsaker TT, Zanne AE, Clark CJ, Poulson JR. 2005. A long-term evaluation of fruiting phenology: importance of climate change. Journal of Tropical Ecology 21: 31–45. [Google Scholar]
- Corlett RT, Lafrankie JV. 1998. Potential impacts of climatic change on tropical Asian forests through an influence on phenology. Climatic Change 39: 439–453. [Google Scholar]
- Devineau J-L. 1999. Seasonal rhythms and phenological plasticity of savanna woody species in a fallow farming system (south-west Burkina Faso). Journal of Tropical Ecology 15: 497–513. [Google Scholar]
- Diaz M, Granadillo E. 2005. The significance of episodic rains for reproductive phenology and productivity in trees in semiarid regions of northwestern Venezuela. Trees 19: 336–348. [Google Scholar]
- Felger RS, Johnson MB, Wilson MF. 2001. The trees of Sonora, Mexico. Oxford: Oxford University Press.
- Fenner M. 1998. The phenology of growth and reproduction in plants. Perspectives in Plant Ecology, Evolution and Systematics 1: 78–91. [Google Scholar]
- Fitter AH, Fitter RSR. 2002. Rapid change in flowering time in British plants. Science 296: 1689–1692. [DOI] [PubMed] [Google Scholar]
- Griz LMS, Machado ICS. 2001. Fruiting phenology and seed dispersal syndromes in caatinga, a tropical dry forest in the northeast of Brazil. Journal of Tropical Ecology 17: 303–321. [Google Scholar]
- Janzen D. 1967. Synchronization of sexual reproduction of trees within the dry season in Central America. Evolution 21: 620–637. [DOI] [PubMed] [Google Scholar]
- Kushwaha CP, Singh KP. 2005. Diversity of leaf phenology in a tropical deciduous forest in India. Journal of Tropical Ecology 21: 47–56. [Google Scholar]
- Lieberman D. 1982. Seasonality and phenology in a dry tropical forest in Ghana. Ecology 70: 791–806. [Google Scholar]
- Mooney HA, Medina E, Bullock SH. 1995. Neotropical deciduous forests. New York: Academic Press.
- Primack RB. 1985. Patterns of flowering phenology in communities, populations, individuals and single flowers. In: White J, ed. The population structure of vegetation. Dordrecht: Junk, 571–593.
- Priya PB, Bhat KM. 1999. Influence of rainfall, irrigation, and age on the growth periodicity and wood structure of teak (Tectona grandis). IAWA Journal 20: 181–192. [Google Scholar]
- Reich PB. 1995. Phenology of tropical forests: patterns, causes, and consequences. Canadian Journal of Botany 73: 164–174. [Google Scholar]
- Rivera G, Borchert R. 2001. Induction of flowering in tropical trees by a 30-min reduction in photoperiod: evidence from field observations and herbarium specimens. Tree Physiology 21: 201–212. [DOI] [PubMed] [Google Scholar]
- Rivera G, Elliott H, Caldas LS, Nicolossi G, Coradin VTR, Borchert R. 2002. Increasing day-length induces spring flushing of tropical dry forest trees in the absence of rain. Trees 16: 445–456. [Google Scholar]
- Rotzer T, Grote R, Pretzsch H. 2004. The timing of bud burst and its effect on tree growth. International Journal of Biometeorology 48: 109–118. [DOI] [PubMed] [Google Scholar]
- van Schaik CP, Terborgh JW, Wright SJ. 1993. The phenology of tropical forests: adaptive significance and consequences for primary producers. Annual Review of Ecology and Systematics 24: 353–377. [Google Scholar]
- Schongart J, Piedade MTF, Ludwigshausen S, Horna V, Worbes M. 2002. Phenology and stem growth periodicity of tree species in Amazonian flood plain forests. Journal of Tropical Ecology 18: 581–597. [Google Scholar]
- Seghieri J, Floret Ch, Pontanier R. 1995. Plant phenology in relation to water availability: herbaceous and woody species in the savannas of northern Cameroon. Journal of Tropical Ecology 11: 237–254. [Google Scholar]
- Singh KP, Kushwaha CP. 2005a. Paradox of leaf phenology: Shorea robusta is a semi-evergreen species in tropical dry deciduous forests in India. Current Science 88: 1820–1824.
- Singh KP, Kushwaha CP. 2005b. Emerging paradigms of tree phenology in dry tropics. Current Science 89: 964–975.
- Singh KP, Singh JS. 1988. Certain structural and functional aspects of dry tropical forest and savanna. International Journal of Ecology and Environmental Sciences 14: 31–45. [Google Scholar]
- Tissue DT, Wright SJ. 1995. Effect of seasonal water availability on phenology and annual shoot carbohydrate cycle of tropical forest shrubs. Functional Ecology 9: 518–527. [Google Scholar]
- Troup RS. 1921. The silviculture of Indian trees, vols I and II. Oxford: Clarendon Press.
- Van Devender T, Sanders AC, Wilson RK, Meyer SA. 2000. Vegetation, flora and seasons of the Rio Cuchujaqui, a tropical deciduous forest near Alamos, Sonora. In: Robichaux RH, Yetman DA, eds. The tropical deciduous forest of Alamos. Tucson, AZ: The University of Arizona Press, 36–101.
- van Vliet AJH, Schwartz MD. 2002. Phenology and climate: the timing of life cycle events as indicators of climate variability and change. International Journal of Climatology 22: 1713–1714. [Google Scholar]
- Wright SJ. 1996. Phenological responses to seasonality in tropical forest plants. In: Mulkey SD, Chazdon RL, Smith AP, eds. Tropical forest ecophysiology. New York, NY: Chapman and Hall, 440–460.