Abstract
• Background and Aims The floral display influences the composition of pollinators interacting with a plant species. Geographic and temporal variation in pollinator composition complicates the understanding of the evolutionary consequences of floral display variation. This paper analyses the relationships between Silene acutifolia, a hermaphroditic perennial herb, and its pollinators, based on field studies in the north-west of Spain.
• Methods Studies were conducted over three years (1997–1999). Firstly, the main pollinators of this species were determined for two years in one population. Secondly, pollen limitation in fruit and seed production was analysed by supplementary hand pollinations, and counting the pollen grains and tubes growing in styles for two different-sized populations. Finally, the effect of flower size and number on the rate of visitation and total seed number was examined for 15 marked plants.
• Results and Conclusions The primary pollinators were long-tongued insects, including Hymenoptera, Lepidoptera and Diptera, but the composition and visitation frequencies differed between years. Pollen limitation occurred in one of the years of study. There was between-population variation in the number of pollen grains and pollen tubes found in styles, suggesting pollen limitation in one population. Overall, pollinators visited plants with more open flowers more frequently, and pollinated more flowers within these plants. Conversely, petal and calyx sizes had no effect on insect visitation. Plants with higher rates of visits produced higher number of seeds, suggesting that pollinator-mediated limitation of seed and fruit production may be important in some years.
Keywords: Anthophora, Bombus, Caryophyllaceae, female reproductive success, floral display, mutualism, pollen limitations, pollination ecology, Silene acutifolia
INTRODUCTION
Plant mutualisms and the fidelity of the relationships among plant and animal species has been the object of interest since Sprengel (1995), and dates back more than 200 years. The role of specialized versus generalized pollination has been controversial (e.g. Waser et al., 1996; Fenster et al., 2004), and is one of the current debates in pollination biology (Johnson and Steiner, 2000). In spite of the proposal that the general evolutionary trend is toward specialization (Stebbins, 1970), there is an increasing recognition that most flowering plants are pollinated by a diverse number of species (Schemske and Horvitz, 1984; Herrera, 1996; Waser et al., 1996; Gómez and Zamora, 1999; Herrera, 2005), forming a continuum from highly specialized, to moderate and extremely generalized systems. In general, plants with specialized flower morphologies, e.g. bilateral symmetry, narrow corolla tubes or spurs, have been shown as examples of adaptation to specific pollinators. Among the best examples of tight specialization, are the diverse species of Ficus and Yucca, Lophocereus schottii (Fleming and Holland, 1998) and Disa draconis (Johnson and Steiner, 1997).
Plants that depend on animals for pollen dispersal are under selection to increase their attractiveness. Nevertheless, pollinator-mediated phenotypic selection on flower attractive characters can only occur when fitness is limited by pollinator visitation and when there is a relationship between the floral trait and pollinator attraction (Totland, 2001). An increase in pollinator visitation, pollen export and pollen deposition are the most common response with larger inflorescences, more open flowers or an enlargement in the size of the attractive parts of flowers (Pellmyr, 2002; Delph et al., 2004). However, a trade-off between floral number and size sometimes exists (Delph et al., 2004). Female fitness may be limited by pollen supply and/or resources available for maturing seeds. In the first case, selection for increasing fecundity would be through floral traits that influence pollen deposition. Selection should favour those individuals assigning fewer resources to pollen attraction in the case of resource limitation (Bierzychudek, 1981; Stephenson, 1981; Haig and Westoby, 1988; Zimmerman and Pike, 1988; Ågren and Willson, 1992; Burd, 1994).
The occurrence or extent of pollen limitation varies among populations and among flowering seasons, and different studies have examined the effects of supplemental hand-pollination at both spatial and temporal scales (Dieringer, 1992; Dudash and Fenster, 1997; Baker et al., 2000; Ramsey and Vaughton, 2000). There have been remarkably few attempts to demonstrate that contrasting ecological conditions are associated with different degrees of pollen limitation between species or populations within species (Larson and Barrett, 2000). In small populations, pollen limitation could be due to reduction in the number of potential mates (Allee effect), reduction in floral display and in parallel number of effective pollinators or reduction in pollen quality in species with self-incompatibility mechanisms. Plants in smaller populations or less dense patches may receive fewer visits from pollinators and the availability of pollen may become limiting for seed set (Lamont et al., 1993).
Silene is an interesting genus in which to investigate the evolution of pollination systems because of the diverse mechanisms found within it. Different congeners show diverse flower colours (from red to pink, and white); flower orientation to pollinators, petal sizes and shapes, nectar and scent. In addition to this high inter-specific variation, Silene presents high intra-specific variation in floral display (Delph et al., 2002). Phylogenetically closed species (see Desfeux et al., 1996) have different pollination systems. This is particularly striking in the sister species S. latifolia, S. diclinis and S. dioica, clustered in the same clade, and with different pollination systems: Silene latifolia, with moth pollination syndrome, has nocturnal pollinators, although it can be pollinated by other diurnal pollinators (Jürgens et al., 1996; Young, 2002); S. diclinis is a generalist pollinated by Apis, Bombus, hoverflies and Lepidoptera (Bañares et al., 2003); and S. dioica is mainly pollinated by bumblebees in some populations (Carlsson-Granér et al., 1998), but pollinated by muscid flies and syrphid flies in other studies (Westerbergh and Saura, 1994). Species with nocturnal pollination have been widely studied because of the pollination–predation relationships of the nocturnal pollinator Hadena bicruris, which has the potential to show either mutualistic or parasitic relationships with Silene and related genera.
This paper focuses on the mutualistic relationships of Silene acutifolia, a plant that grows on rocks in the mountains of the north-west of Spain, and north and central Portugal. This species is endemic to this area, and its mutualistic relationships are interesting with respect to the possible selective pressures exerted by pollinators. Silene acutifolia flowers are tubular, with well-marked three-dimensional form, characters sometimes associated with bee-pollinated flowers, although in general bees have no single pollination syndrome (Proctor et al., 1996). On the other hand, S. acutifolia has some traits commonly associated with pollination by butterflies, such as diurnal flowering time and purplish-pink flowers, and also calyx length.
The main aims of this study are: (a) to examine the pollinator assemblage of S. acutifolia, and evaluate whether it constitutes a specialist or generalist pollination system; (b) to analyse if there is pollen limitation per flower among populations and years; (c) to ascertain if there are between population differences in the number of pollen grains on stigmas or pollen tubes on the styles, as a measure of pollen limitation; and (d) to evaluate the relationship between number of open flowers and floral size with pollinator visitation, and the latter with total seeds produced per plant.
MATERIALS AND METHODS
Plant and study area
Silene acutifolia Link ex Rohrb. is a polycarpic herb, endemic to the north-west of Spain, and north and central Portugal. The purplish-pink flowers are hermaphroditic, and self-compatible, although they do not reach high fruit or seed sets without the co-operation of pollinators (Buide and Guitián, 2002). The inflorescences are dichasium-type. Typical inflorescences have a primary flower with one flower on each side of the central axis (secondary flowers) and another one on each side of the lateral axis (tertiary flowers). Within-inflorescence variation in flower size, fruit and seed set is very common (Buide, 2004). The flowers have five free petals forming a functional tube enclosed by the tubular calyx. Thus, calyx length is a measure of the distance to nectar, which in Caryophyllaceae is located in a disc at the base of stamens. The petals have a claw, enclosed by the calyx, and a limb. The claw has a small fringe at the top. Since the claws are inside the calyx tube, the attractive part of the corolla is the limb. The number of flower stems is very variable among plants, and as a result, the number of open flowers is also very different.
This study was carried out in the two areas marked in Fig. 1. The smallest population (Queixa, 42°14′N 07°26′W; Ourense, north-west Spain) consists of only 50 reproductive individuals. The biggest area corresponds to the river Sil canyon (‘Cañones del Sil’ hereafter, 42°26′N 07°42′W; Peares, Ourense, north-west Spain), and detailed numbers of reproductive plants are shown in parenthesis. Black dots indicate sites were experiments were carried out. Silene acutifolia grows in sparse groups through the granite rock faces, and gene flow among the different sites may result when seeds drop the top of rock faces, and also by pollen carried by pollinators.
Fig. 1.
Distribution of the two study areas in the north-west of Spain, and location of sites where S. acutifolia grows along a transect alongside the road, in Cañones del Sil. In parenthesis are shown the number of reproductive individuals. The black dots denote the sites where experiments were conducted.
Pollinators assemblage
To determine the pollination system of S. acutifolia, visits of pollinators were recorded during the flowering peak, considering only those visitors that effectively contact anthers and/or stigmas during flower visits. Therefore, all insect species recorded can be considered as pollinators. Measurements of the pollinator activity took place in 1997 and 1998 in Cañones del Sil, within a 28 m2 area of high density of reproductive individuals of S. acutifolia (site B in Fig. 1). The observations were carried out every day at different times (0700–0800 h, 1000–1100 h, 1300–1400 h and 1600–1700 h). The total number of observation hours was 27 in 1997 and 28 in 1998. The number of flowers visited by every potential pollinator was recorded during each census. The observations were performed on clear or partly cloudy days, and in similar temperature conditions. At the beginning and at the end of each census, the temperature and relative humidity were measured by using a thermohygrometer.
Three species of Anthophora Latreille, 1803; subgenus Amegilla Friese, 1897 occurred on S. acutifolia. Most of the collected individuals were A. (Amegilla) crassipes Lepeletier, 1841, while the others represent two unidentified species of this genus.
Pollen limitation
Pollen limitation experiments were carried out in Cañones del Sil during 1997, 1998 and 1999). In 1997, 12 plants were marked and flowers from different positions within inflorescences were hand-pollinated to test for pollen limitation (seven from primary flowers, 17 from secondary flowers and 14 from tertiary flowers). Flowers from the same positions within inflorescences but on other plants on the same site were marked as controls. In 1998 and 1999 hand-pollinated flowers were compared with controls marked in the same plant and position, but within different inflorescences (n = 10 in 1998, n = 17 in 1999).
The pollen was collected from flowers in a separate group of plants (but in the same site), and applied to exerted and receptive styles of flowers in the female phase. To ensure that the pollination had indeed taken place, every flower was hand-pollinated for two successive days. The pollen limitation index [L = 1 – (Po/Ps)] was calculated following Larson and Barrett (2000), Po is the percentage of fruit set or seed set taken from open-pollinated controls while Ps is the same quantity for the fruit set or seed set from plants that had received supplemental cross pollen. L = 0 indicates no pollen limitation in the population under study.
Fruit set differences between hand-pollinated flowers and controls were analysed by Fisher's exact test. In 1997, variation in seed set with pollen supplementation and position in the inflorescence was analysed by two-way ANOVA (treatment and position were treated as fixed factors). In 1998 and 1999, the effect of pollen supplementation on seed set was analysed using a paired t-test since the control flowers were chosen on the same plant.
Population differences in pollen deposition on style–stigma
The differences in pollen deposition between the populations of Cañones del Sil and Queixa were analysed in 1998. In Cañones del Sil, 15 plants were marked, five in each of the three sites (A, B and D in Fig. 1). Site B was separated by approx. 1·5 km from A, and D approx. 2 km from B. In total, 70 flowers with developed style–stigmas were gathered from 14 different plants (five flowers per plant). The five flowers on each plant were chosen from different positions within the inflorescence (primary, secondary and tertiary). In Queixa, 30 flowers were gathered from ten different plants (three flowers per plant: one from each primary, secondary and tertiary position). The flowers were fixed in FAA (a mixture of 9 % ethanol:distilled water:37–40 % formaldehyde:glacial acetic acid; 10:7:2:1, v/v/v) and the adhered pollen grains together with the number of well-developed pollen tubes (i.e. pollen tubes extending at least halfway down the style) were counted by means of an epifluorescence photomicroscope (see methods in Buide and Guitián, 2002). Petal-limb and calyx lengths were quantified in the same 70 flowers. For each flower, the petal-limb and calyx length were taken as the mean of the five petals.
The effect of floral traits on pollen deposition on stigmas and pollen tube development were analysed separately by population, and each response variable (pollen grains or pollen tubes) by means of generalized linear modelling (GLM) techniques (McCullagh and Nelder, 1989). Based on deviance residual plots, models assuming a negative binomial error structure with a log link function were found to be the most appropriate for pollen grain data sets, and models assuming a quasipoisson error structure for pollen tube data sets. Calculations were made using the R software (Ihaka and Gentleman, 1996).
Floral traits and pollinator visitation
The visitation rate of pollinators per plant and the number of flowers visited were measured during 1998 at the Cañones del Sil area. The 15 marked plants were the same as those for pollen deposition on the style–stigma. Ten censuses of 10 min were carried out haphazardly for each plant, at different times of day, during the flowering peak. For each plant, the mean number of visits each plant receives (visitation rate per plant, Np), the number of floral visits each plant receives (mean number of flowers visited per plant, Nf), and the number of visits received by an individual flower (visitation rate per plant, tv), was calculated following Ohashi and Yahara (1998). The measures of petal-limb and calyx lengths were the same as for population differences in pollen deposition on the style–stigma.
The relationships among floral display and the different variables measuring visits of pollinators (Nf, Np and tv) were analysed by means of simple linear regressions with SPSS. The variable Np showed normal distribution (Kolmogorov–Smirnov test, P = 0·137; Shapiro–Wilk test, P = 0·359), Nf and tv were transformed [x′ = log (x + 1)]. Linear regressions were also applied to analyse the effects of petal-limb and calyx lengths on pollinator visitation.
If the pollinators are exerting a selective pressure, the most visited flowers should show an increase in their fitness.
Pollinator visitation and female reproductive success
The effect of number of pollinator visits on female fitness was measured through total number of seeds (TS) produced by each plant at the end of the flowering period. The total number of seeds was estimated by multiplying fruit set by mean of seeds per fruit, by total number of flowers. The total number of flowers was estimated from both the number of senescent flowers (that remain on the plant), and the fruits produced. Statistical significance was determined by means of a series of linear regressions of Nf, Np and tv on total seed production of each marked plant (TS). To meet the normal distribution requirement for linear regression, TS was logarithmically transformed.
RESULTS
Pollinators assemblage
Mean temperatures were lower in 1997 than in 1998, especially before midday (Table 1). The mean number of visits per hour was higher in 1998 (127) than in 1997 (89), but differences were not statistically significant (ANOVA, P = 0·082). The pollinators increased their visits throughout the day, with the highest mean number of visits between 1600 h and 1700 h in both years. In 1997, the main pollinators were bees from the genus Anthophora (mainly A. crassipes), which carried out 66 % of the floral visits. The second more frequent pollinators were beeflies (Bombylius spp.) with 16 % of the visits, followed by butterflies, especially Hemaris fuciformis (7 %). In 1998, bees were also the main pollinators, but the most frequent species was Bombus hortorum, which realized 60 % of the floral visits. That year, B. pascuorum was responsible for 26 % of the visits (Table 2).
Table 1.
Mean temperatures at the census area in the population of Cañones del Sil, and number of flowers of Silene acutifolia visited per hour during the two years of study
| Mean temperature (°C) | Mean visited flowers/hour (mean ± s.e.) | |||
|---|---|---|---|---|
| Time | 1997 | 1998 | 1997 | 1998 |
| 0700–0800 | 16·4 | 23·4 | 31·57 ± 11·77 | 45·29 ± 11·62 |
| 1000–1100 | 23·3 | 31·6 | 86·0 ± 13·55 | 166·14 ± 38·72 |
| 1300–1400 | 23·9 | 24·7 | 108·0 ± 19·09 | 122·0 ± 28·74 |
| 1600–1700 | 23·8 | 22·1 | 138·00 ± 22·16 | 175·86 ± 42·40 |
Table 2.
Pollinators of Silene acutifolia in the two years of study (Cañones del Sil population)
| % visited flowers (n) | ||
|---|---|---|
| Pollinators | 1997 | 1998 |
| Hymenoptera | ||
| Apis mellifera | 0·2 (4) | 0·1 (5) |
| Bombus pascuorum | 0·7 (16) | 25·6 (970) |
| Bombus hortorum | 5·9 (143) | 59·6 (2257) |
| Bombus terrestris | 0 | 0·1 (2) |
| Bombus unidentified | _ | 1·2 (44) |
| Anthophora crassipes, A. spp. | 66·4 (1597) | 1·5 (56) |
| Chelostoma spp. | 0 | 0·03 (1) |
| Hymenoptera S.I. | 1·4 (34) | 1·1 (42) |
| Lepidoptera | ||
| Macroglossum stellatarum | 0·6 (14) | 0·7 (27) |
| Hemaris fuciformis | 6·9 (165) | 1·1 (42) |
| Gonepterix rhamni | 0·7 (18) | 0·3 (13) |
| Unidentified | _ | 0·1 (2) |
| Diptera | ||
| Bombylius spp. | 15·9 (382) | 2·5 (96) |
| Syrphidae | 0·3 (8) | 0·1 (3) |
| Unidentified | 1·0 (23) | 0·1 (5) |
| Nectar robbers | ||
| Anthophora sp. | 0 | 5·9 (222) |
The values represent percentage of visited flowers, in parenthesis number of flowers visited per insect taxa (27 h in 1997 and 28 h in 1998).
Pollen limitations
In 1997, all hand-pollinated primary-position flowers set fruit, compared with only 86 % in controls, but that difference was not statistically significant (Fisher test, P = 1·000, n = 7). Hand-pollinated flowers from the secondary position set significantly more fruits than controls (Fisher test, P = 0·0184, n = 17), as did tertiary flowers (Fisher test, P = 0·0063, n = 14). On the other hand, seed set in that year was significantly higher in hand-pollinated flowers than in controls, in every position of the inflorescence. At the same time, seed set was significantly reduced depending on the inflorescence position (primary-position fruits yielded higher seed sets than secondary, and those higher than tertiary) (Table 3). The interaction was not statistically significant, showing that reduction in seed set with position was produced in control and hand-pollinated flowers in the same way.
Table 3.
Two-factor analysis of variance for the effect of treatment (pollen addition), and inflorescence position (primary, secondary and tertiary flowers) on seed set of Silene acutifolia
| Source of variation | d.f. | MS | F | P |
|---|---|---|---|---|
| Inflorescence position | 2 | 1079·5 | 4·448 | 0·015 |
| Treatment | 1 | 1767·0 | 7·281 | 0·009 |
| Inflorescence position × treatment | 2 | 27·2 | 0·112 | 0·894 |
| Error | 66 | 242·7 |
In 1998, all hand-pollinated flowers set fruit, and the same happened with controls, which reached 100 % of fruit set. No significant differences in the seed set between hand-pollinated flowers and controls were detected (Fisher test = 1·417).
In 1999, there was no statistically significant differences between hand-pollinated flowers and controls (Fisher test = 1·000). Also no significant differences were found in the seed set (Fisher test = 0·229).
Values of the pollen limitation index were low (Table 4), especially in the fruit and seed set in 1998 and 1999. The highest values were for fruit set (0·37) and seed set (0·13) in 1997.
Table 4.
Pollen limitation index (L) for Silene acutifolia
| Pollen limitation index | ||
|---|---|---|
| Year | Fruit set | Seed set |
| 1997 | 0·37 | 0·13 |
| 1998 | 0 | 0·042 |
| 1999 | 0·056 | 0·005 |
L = 1 – (Po/Ps), Po is the percentage fruit set or seed set of open-pollinated controls and Ps is the percentage fruit set or seed set by plants that received supplemental cross pollen. L = 0 indicates no pollen limitation in the population under study.
Population differences in pollen deposition on the style–stigma
The mean number of pollen grains found in the stigmas of flowers collected in Cañones del Sil was markedly higher than in Queixa (Table 5). Pollen deposition was highly variable, e.g. ranging from zero to the highest number of 1003 pollen grains in Cañones del Sil, against 99 in Queixa. The mean number of pollen tubes developed in the styles was considerably lower than pollen grains adhered, in both populations. In Queixa it was especially low (mean of only 3.2), relative to ovule number. The flowers collected had a mean of 45 ovules in Cañones del Sil, and 51 in Queixa. Therefore, the number of pollen grains in the flowers collected in Queixa was not enough to fertilize the ovules. Since mean style lengths of flowers from Queixa were higher than mean style length in Cañones del Sil (enough to consider the flowers to be in the female phase; see Buide and Guitián, 2002), the difference in pollen germination is not due to non-receptive flowers in Queixa.
Table 5.
Mean number of pollen grains adhering to the stigma, pollen tubes developed in the style, style length, and ovule number per flower in flowers in female phase gathered from the populations of Cañones del Sil and Queixa during 1998
| Population | ||
|---|---|---|
| Cañones del Sil | Queixa | |
| Pollen grains adhering to the stigma | 104 ± 180·24 (1003–0); n = 67 | 14·5 ± 25·0 (99–0); n = 30 |
| Pollen tubes developed in the style | 16·19 ± 23·91 (124–0); n = 67 | 3·17 ± 10·06 (54–0); n = 30 |
| Style length (mm) | 8·12 ± 1·25 (9·93–3·10); n = 75 | 8·94 ± 0·78 (10·40–7·20); n = 30 |
| Ovule number per flower | 45·1 ± 5·40 (57–34); n = 75 | 51·0 ± 5·64 (61–39); n = 30 |
Values represent mean ± standard deviation, interval and number of flowers measured.
The floral traits analysed had a variable association with number of pollen grains and with the pollen tubes (Table 6). In Queixa, the petal-limb differences in length significantly explained variation in the number of pollen grains on the stigma (P = 0·025); and both petal-limb and calyx length significantly explain variation in pollen tubes in the style (P = 0·02). On the other hand, in Cañones del Sil both traits had a significant effect on the number of pollen grains (petal-limb length P = 0·001, and calyx length P = 0·022), but only petal-limb length had a significant effect on pollen tubes (P = 0·04).
Table 6.
Effects of petal-limb length and calyx length on number of pollen grains deposited in stigmas, and pollen tubes developing in styles shown by analysis of deviance tables* for the populations of Queixa and Cañones del Sil, and the two response variables
| Population | Response variable | d.f. | Deviance difference | Res. d.f. | Residual deviance | P | |
|---|---|---|---|---|---|---|---|
| Queixa | Pollen grains | Null | 29 | 38·396 | |||
| Petal-limb length | 1 | 5·022 | 28 | 33·374 | 0·025 | ||
| Calyx length | 1 | 0·317 | 27 | 33·058 | 0·574 | ||
| Limb : calyx | 1 | 0·001 | 26 | 33·056 | 0·970 | ||
| Pollen tubes | Null | 29 | 371·63 | ||||
| Petal-limb length | 1 | 77·89 | 28 | 293·73 | 0·02 | ||
| Calyx length | 1 | 78·06 | 27 | 215·67 | 0·02 | ||
| Limb : calyx | 1 | 19·06 | 26 | 196·61 | 0·24 | ||
| Cañones del Sil | Pollen grains | Null | 66 | 96·589 | |||
| Petal-limb length | 1 | 10·099 | 65 | 86·490 | 0·001 | ||
| Calyx length | 1 | 5·209 | 64 | 81·280 | 0·022 | ||
| Limb : calyx | 1 | 0·298 | 63 | 80·983 | 0·585 | ||
| Pollen tubes | Null | 66 | 2054·60 | ||||
| Petal-limb length | 1 | 141·68 | 65 | 1912·91 | 0·04 | ||
| Calyx length | 1 | 28·88 | 64 | 1884·04 | 0·34 | ||
| Limb : calyx | 1 | 11·99 | 63 | 1872·05 | 0·54 |
* The models applied were negative binomial for number of pollen, and quasipoisson for pollen tubes.
Floral traits and pollinator visitation
The marked plants produced a mean of 126 flowers, ranging from 35 to 317. Pollinators visited plants with larger floral displays more frequently. In spite of high variability, plants with more flowers open during censuses received a higher number of pollinator visits per census (Fig. 2A, P = 0·011). The number of visits was also influenced by location of the plant. Plants in site D showed fewer visits than plants located in site B (P < 0·05, post hoc Tukey test). The same significant tendency is evident when the response variable is the number of flowers visited per census (Fig. 2B; P < 0·001). The number of visits also had a significant effect on the visitation rate per flower (Fig. 2C; P = 0·013).
Fig. 2.
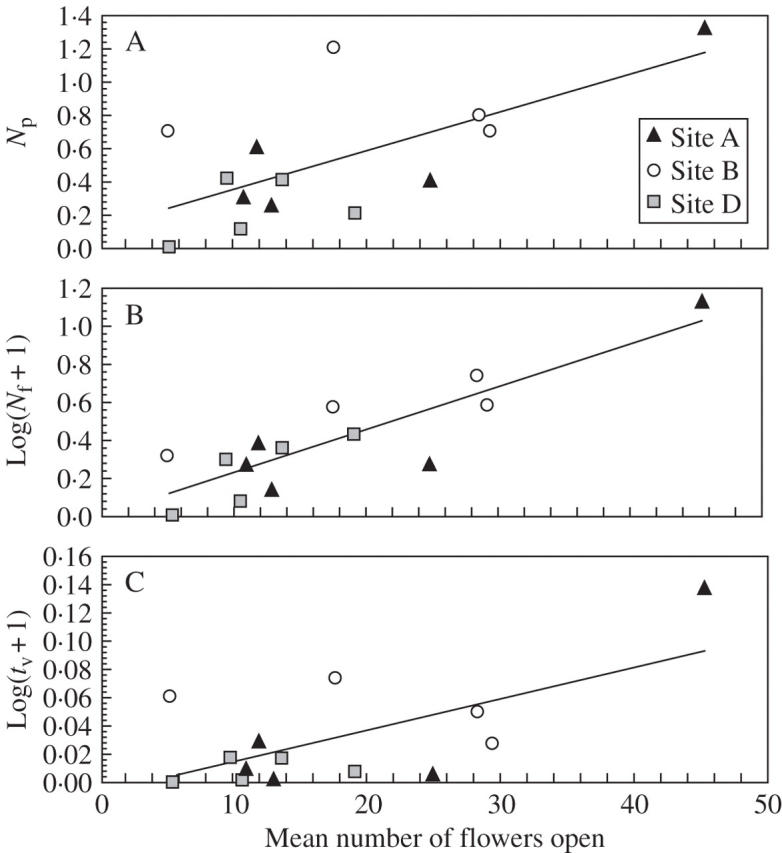
(A) Regression of mean number of flowers open per plant on the visitation rate of pollinators per plant (Np), (B) logarithmic transformed mean number of flowers visited per plant [log(Nf + 1)], and (C) logarithmic transformed visitation rate per plant [log(tv + 1)]. Regression equations: (A) y = 0·126 + 0·023x, R2 = 0·43, P = 0·011; (B) y = 0·0048 + 0·023x; R2 = 0·76, P = 0·000; (C) y = –0·00699 + 0·0022x, R2 = 0·42, P = 0·013. Different sites in Cañones del Sil are represented by solid triangles (site A), open circles (site B) and shaded squares (site D).
The linear regressions demonstrate that the petal and calyx sizes had no effect on pollinator attraction. In particular, petal-limb length had no significant effect on Np (P = 0·273), or on log(Nf + 1) (P = 0·364) or on log(tv + 1) (P = 0·319). Similarly, calyx length had no significant effect on Np (P = 0·513), or on log(Nf + 1) (P = 0·553) or on log(tv + 1) (P = 0·439).
Pollinator visitation and female reproductive success
A scatterplot of the regression of Np, Nf and tv on the female reproductive success (total number of seeds produced per marked plant, TS) shows that plants located in site D had lower values of visits per census (Fig. 3A). For example, the highest value in the site D plants was 0·4 visits per 10-min census. The plants growing in site B showed in general a higher number of visits per census. The exception was one plant in site A that had the highest Np. The tendency is to significantly increase total number of seeds with increasing Np values (P < 0·016). The plant with the highest Np had the highest estimates for the total number of seeds produced (1135 seeds). The only plant with no visits in censuses had the lowest estimate (988 seeds). Secondly, the tendency of plants with more flowers visited per census is to produce more seeds (Fig. 3B, P < 0·001). Finally, visitation rate per flower, tv (the number of visits an individual flower received in a 10-min census), was only a marginally significant predictor of TS (P = 0·051) (Fig. 3C).
Fig. 3.

(A) Regression of the visitation rate of pollinators per plant (Np), (B) logarithmic transformed mean number of flowers visited per plant [log(Nf + 1)], and (C) logarithmic transformed visitation rate per plant [log(tv + 1)] on the logarithmic transformed seeds produced per plant (TS). Regression equations: (A) y = 3·218 + 0·389x, R2 = 0·39, P = 0·016; (B) y = 3·154 + 0·674x, R2 = 0·65, P = 0·001; (C) y = 3·316 + 3·362x, R2 = 0·28, P = 0·051. Different sites in Cañones del Sil are represented by solid triangles (site A), open circles (site B) and shaded squares (site D).
DISCUSSION
The first goal of this study was to evaluate the pollination system of Silene acutifolia. This study presents a moderately generalized pollination system similar to that proposed as commonplace in nature (Waser et al., 1996). The pollinators observed belong to three different insect orders (Hymenoptera, Diptera and Lepidoptera). Therefore, it is not possible to conclude that it is a specialist pollination system in the narrowest sense of the term. Pollinator specialization by long-tongued bees and bee-like insects may be considered in a broader sense, because this group includes the most frequent pollinators in both years (Anthophora and Bombylius in 1997, Bombus hortorum and B. pascuorum in 1998). However, it cannot be forgotten that butterflies make a contribution to the visiting fauna as well (Macroglossum stellatarum, Hemaris fuciformis and Gonepterix rhamni), and the proportion of Lepidoptera could change in other populations. In the theoretical moderately generalized pollination model, specialization becomes less likely in the presence of temporal variation in pollinator services. This apparent contradiction between the prevalence of generalized pollination systems in the current angiosperms and the evolution of floral diversity is not completed resolved, and several hypotheses have been proposed (Ollerton, 1996). Moreover, when the availability of any given pollinator fluctuates through time and space, generalization may be adaptive (Fishbein and Venable, 1995; C. M. Herrera, 1988; J.Herrera, 1988; Fenster and Dudash, 2001). Current evidence suggests that in nature there is a continuum from very specialized systems to those with multiple pollinators.
Some members of Silene have been considered to be pollinated by moths and butterflies, yet others have shorter and wider tubes more typical of flowers associated with bees and long-tongued flies (Proctor et al., 1996). Fenster and Dudash (2001) documented in Silene virginica a concordance between floral traits, associated with the hummingbird pollination syndrome, and the main pollinator in that study. These authors found that hummingbirds are the most important pollinator of that species, but also found among-year and between-site variation. Silene acutifolia has some traits commonly associated with pollination by butterflies, such as diurnal flowering time and purplish-pink flowers. The mean calyx length (16·1 mm, n = 78) permits the proboscis of some common European butterflies an easy access to the nectar located at the base of stamens, inside the tube formed by the calyx. For example, Macroglossum stellatarum, one of the butterflies pollinating S. acutifolia in this study, has a proboscis length of 25–28 mm (Proctor et al., 1996). Long-tongued bees in the genus Anthophora have shorter probosces (8–13 mm depending on the species, Proctor et al., 1996), but Anthophora uses the lower petals as a landing platform and introduces the head inside the calyx tube, simultaneously accessing the nectar and collecting the pollen from elongated stamens with dehiscent anthers. Thus, tubular flowers with nectar at the base of stamens in S. acutifolia have the nectar accessible not only to butterflies, but also to long-tongued bees and flies with shorter probosces, diversifying the spectrum of pollinators found in this study. In conclusion, S. acutifolia has traits that facilitate long-tongued bee pollination and also butterfly pollination.
Pollen limitation is an important factor influencing seed set in plants (Ashman et al., 2004). Silene acutifolia produces low seed sets based on autonomous selfing (<40 % in contrast to >80 % in controls; Buide and Guitián, 2002), thus fitness is limited by pollinator visitation. The importance of pollen limitation in populations of S. acutifolia varied between years, with populations exhibiting an effect of pollen supplementation of fruit set in 1997 that was not detected in later years of this study. This variation in the pollen limitation between years may reflect variation in climatic conditions. The mean temperatures in the months before flowering were high, and flowering time started earlier in 1997 than in the following years. On the other hand, rainfall during the flowering period was higher than average. These special conditions of temperature and rainfall appear to affect the flowering of S. acutifolia (Buide et al., 2002), as well as pollinators behaviour. Navarro (2000) made similar observations in the north of the Iberian Peninsula, concluding that these weather conditions, and their associated resource shortage early in the season, could lead to local extinction of bumblebees. Differences in pollinator composition between 1997 and 1998 may also be a consequence of different weather conditions. On the other hand, although no pollen limitation was found in 1998 and 1999 at the floral level, other flowers of the same plant may exhibit a deficit in pollen. For that reason, it could be interesting to hand-pollinate all the flowers of the plant, and measure the effect on total production of seeds (Zimmerman and Pyke, 1988).
With respect to population variations in the potential for pollen limitation of seed set, this study shows a marked difference between the Cañones del Sil and Queixa sites, both in the number of pollen grains deposited on the stigmas, and in the number of pollen tubes growing in the styles. The average number of pollen grains in Cañones del Sil was 104, against 15 in Queixa. The low number of pollen grains in Queixa is below that necessary to fertilize the mean number of ovules in this species. A low number of reproductive individuals suggests that insufficient pollen is available, at least in the year of the study, although it could also be a deficit of pollinators. Heterospecific pollen belonging to the families Ericaceae, Cruciferae, and to the genus Geranium was discovered in the styles of Queixa flowers of S. acutifolia, supporting the hypothesis of a deficit in potential mates. In future studies, knowledge of the pollinator composition at these sites will be important, since spatio-temporal variation in pollen limitation occurs in other species (Dieringer, 1992; Baker et al., 2000). In Silene virginica, Dudash and Fenster (1997) found pollen limitation mediated by hummingbirds in only one of two sites. The differences detected by Dudash (1993) also depend on plant size. Overall, pollen limitation can vary among years and populations, and with plant traits and phenology, making long-term studies that consider ecological differences important. For example, Navarro (1996) failed to detect pollen limitation in Petrocoptis grandiflora and P. viscosa, two Caryophyllaceae considered closely related to Silene (Mayol and Roselló, 1999).
Pollinators visited the plants of S. acutifolia with more open flowers more frequently. The number of flowers visited in each plant also increased with higher floral display. Conversely, flowers with longer petal-limbs or calyx did not attract more pollinators. In contrast, petal-limb length positively affected the number of pollen grains in styles of both populations. Larger corollas increased pollen deposition, but no relationship was found between petal-limb length and seed set. A possible explanation for this apparent contradiction is simultaneous constraints in reproductive output from pollen availability and other environmental factors (Haig and Westoby, 1988). As a consequence, selection on floral traits involved in pollinator attraction may be relaxed because a positive relationship between pollen deposition and the subsequent seed number does not exist (Totland, 2001).
Selection on flower size in insect-pollinated species can only take place if the number or quality of pollinator visits limit the reproductive success of plants, and if pollinators must discriminate between flowers of different sizes or at least visit certain phenotypes more often than others (Totland, 2001). Broad within-inflorescence variation occurs in S. acutifolia (M. L. Buide, unpubl. res.) such that plants display at the same time a diverse array of floral sizes, potentially obscuring a relationship between petal and calyx lengths and insect visitation. Alternatively, differences in the proboscis length of known pollinators are considerable, and may help explain the diverse floral sizes.
Furthermore, visitation rate of pollinators per plant, and mean number of flowers visited per plant, but not visitation rate per plant, are related to total seed production. These outcomes suggest that in S. acutifolia the selective pressures exerted by pollinators through female fitness may tend to increase the number of flowers per plant, and not the size of flowers. In S. latifolia, Wright and Meagher (2004) proposed that larger flowers do not necessarily attract more pollinators in all years and sites, and found no evidence for selection on female floral characters for one site/year combination, despite selection on one male floral character.
In conclusion, this paper presents a moderately generalized pollination system in the hermaphroditic perennial herb S. acutifolia. The relative importance of the main pollinators varies with years, as does the extent of pollen limitation on seed set. A clear deficit of pollen was found in the population with less reproductive individuals, and reflects the lower number of pollen grains and pollen tubes found in female-phase flowers collected from this site. Clearly the floral display has an effect on pollinator attraction, but this appears to occur only in relation to the number of open flowers rather than to flower size. Finally, it must be remarked that selection on floral traits was only partially documented, and the effects of floral trait variation could make a difference to male reproductive success.
Acknowledgments
The author thanks J. Guitián for useful discussions in the previous versions of this manuscript, and J. A. Díaz-Peromingo, for field assistance. I acknowledge J. A. Andrés for advice on statistics, and J. C. Ornosa-Gallego for identification of Anthophora species. I especially thank Susan Kephart, and anonymous reviewers for very helpful comments on this paper. M.L.B. was supported during the field work by a doctoral fellowship from the Galician Government.
LITERATURE CITED
- Ågren J, Willson MF. 1992. Determinants of seed production in Geranium maculatum. Oecologia 92: 177–182. [DOI] [PubMed] [Google Scholar]
- Ashman TL, Knight TM, Steets JA, Amarasekare P, Burd M, Campbell D, et al. 2004. Pollen limitation of plant reproduction: ecological and evolutionary causes and consequences. Ecology 85: 2408–2421. [Google Scholar]
- Baker AM, Barrett SCH, Thompson JD. 2000. Variation of pollen limitation in the early flowering Mediterranean geophyte Narcissus assoanus (Amaryllidaceae). Oecologia 124: 529–535. [DOI] [PubMed] [Google Scholar]
- Bañares Á, Blanca G, Güemes J, Moreno JC, Ortiz S. 2003. Atlas y Libro Rojo de la Flora Vascular Amenazada de Espana. Madrid: Dirección General de Conservación de la Naturaleza.
- Bierzychudek P. 1981. Pollinator limitation of plant reproductive effort. The American Naturalist 117: 838–840. [Google Scholar]
- Buide ML. 2004. Intra-inflorescence variation in floral traits and reproductive success of the hermaphrodite Silene acutifolia. Annals of Botany 94: 441–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buide ML, Guitián J. 2002. Breeding system in the dichogamous hermaphrodite Silene acutifolia (Caryophyllaceae). Annals of Botany 90: 691–699. [DOI] [PMC free article] [PubMed]
- Buide ML, Díaz-Peromingo JA, Guitián, J. 2002. Flowering phenology and female reproductive success in Silene acutifolia Link ex Rohrb. Plant Ecology 163: 93–103. [Google Scholar]
- Burd M. 1994. Bateman's principle and plant reproduction: the role of pollen limitation in fruit and seed set. The Botanical Review 60: 83–139.
- Carlsson-Granér U, Elmqvist T, Ågren J, Gardfjell H. 1998. Floral sex ratios, disease and seed set in dioecious Silene dioica. Journal of Ecology 86: 79–91. [Google Scholar]
- Delph LF, Frey FM, Steven JC, Gehring JL. 2004. Investigating the independent evolution of the size of floral organs via G-matrix estimation and artificial selection. Evolution and Development 6:438–448. [DOI] [PubMed] [Google Scholar]
- Delph LF, Knapczyk FN, Taylor DR. 2002. Among-population variation and correlations in sexually dimorphic traits of Silene latifolia. Journal of Evolutionary Biology 15: 1011–1020. [Google Scholar]
- Desfeux C, Maurice S, Henry JP, Lejeune B, Gouyon PH. 1996. Evolution of reproductive systems in the genus Silene. Proceedings of the Royal Society of London B. Biological Sciences 263: 409–414. [DOI] [PubMed]
- Dieringer G. 1992. Pollinator limitation in populations of Agalinis strictifolia (Scrophulariaceae). Bulletin of the Torrey Botanical Club 119: 131–136. [Google Scholar]
- Dudash MR. 1993. Variation in pollen limitation in populations of Agalinis strictifolia (Scrophulariaceae). Bulletin of the Torrey Botanical Club 119: 131–136. [Google Scholar]
- Dudash MR, Fenster CB. 1997. Multiyear study of pollen limitation and cost of reproduction in the iteroparous Silene virginica. Ecology 78: 484–493. [Google Scholar]
- Fenster CB, Dudash MR. 2001. Spatiotemporal variation in the role of hummingbirds as pollinators of Silene virginica. Ecology 82: 844–851. [Google Scholar]
- Fenster C, Armbruster W, Willson P, Dudash M, Thomson J. 2004. Pollination syndromes and floral specialization. Annual Review of Ecology and Systematics 35: 375–403. [Google Scholar]
- Fishbein M, Venable DL. 1995. Diversity and temporal change in the effective pollinators of Asclepias tuberosa. Ecology 77: 1061–1073. [Google Scholar]
- Fleming TH, Holland JN. 1998. The evolution of obligate pollination mutualism: senita cactus and senita moth. Oecologia 114: 368–375. [DOI] [PubMed] [Google Scholar]
- Gómez JM, Zamora R. 1999. Generalization vs. specialization in the pollination system of Hormathophyla spinosa (Cruciferae). Ecology 80: 796–805. [Google Scholar]
- Haig D, Westoby M. 1988. On limits to seed production. The American Naturalist 131: 757–759. [Google Scholar]
- Herrera CM. 1988. Variation in mutualism: the spatiotemporal mosaic of a pollinator assemblage. Biological Journal of the Linnean Society 35: 95–125. [Google Scholar]
- Herrera CM. 1996. Floral traits and plant adaptation to insect pollinators: a devil's advocate approach. In: Lloyd DG, Barrett SCH, eds. Floral biology. New York: Chapman and Hall, 65–87
- Herrera CM. 2005. Plant generalization on pollinators: species property or local phenomenon? American Journal of Botany 92: 13–20. [DOI] [PubMed] [Google Scholar]
- Herrera J. 1988. Pollination relationships in southern Spanish Mediterranean shrublands. Journal of Ecology 76: 274–287. [Google Scholar]
- Ihaka R, Gentleman R. 1996. R: a language for data analysis and graphics. Journal of Computational and Graphical Statistics 5: 299–314. [Google Scholar]
- Johnson SD, Steiner KE. 1997. Long-tongued fly pollination and evolution of floral spur length in the Disa draconis complex (Orchidaceae). Evolution 51: 45–53. [DOI] [PubMed] [Google Scholar]
- Johnson SD, Steiner KE. 2000. Generalization versus specialization in plant pollination systems. Trends in Ecology and Evolution 15: 140–143. [DOI] [PubMed] [Google Scholar]
- Jürgens A, Witt T, Gottsberger G. 1996. Reproduction and pollination in Central European populations of Silene and Saponaria species. Botanica Acta 109: 316–324. [Google Scholar]
- Lamont BB, Klinkhamer PGL, Witkowski ETF. 1993. Population fragmentation may reduce fertility to zero in Banksia goodii – a demonstration of the Allee effect. Oecologia 94: 446–450. [DOI] [PubMed] [Google Scholar]
- Larson BMH, Barrett SCH. 2000. A comparative analysis of pollen limitation in flowering plants. Biological Journal of the Linnean Society 69: 503–520. [Google Scholar]
- McCullagh P, Nelder JA. 1989. Generalized linear models. London: Chapman and Hall.
- Mayol M, Rosselló JA. 1999. A synopsis of Silene subgenus Petrocoptis (Caryophyllaceae). Taxon 48: 471–482. [Google Scholar]
- Navarro L. 1996. Biología reproductiva y conservación de dos endemismos del noroccidente ibérico: Petrocoptis grandiflora Rothm. y Petrocoptis viscosa Rothm. (Caryophyllaceae). PhD Thesis, University of Santiago de Compostela, Spain.
- Navarro L. 2000. Pollination ecology of Anthyllis vulneraria subsp. vulgaris (Fabaceae): nectar robbers as pollinators. American Journal of Botany 87: 980–985. [PubMed] [Google Scholar]
- Ohashi K, Yahara T. 1998. Effects of variation in flower number on pollinator visits in Cirsium purpuratum (Asteraceae). American Journal of Botany 85: 219–224. [PubMed] [Google Scholar]
- Ollerton J. 1996. Reconciling ecological processes with phylogenetic patterns: the apparent paradox of plant–pollinator systems. Journal of Ecology 84: 767–769. [Google Scholar]
- Pellmyr O. 2002. Pollination by animals. In: Herrera CM, Pellmyr O, eds. Plant–animal interactions. An evolutionary approach. Oxford: Blackwell Science.
- Proctor M, Yeo P, Lack A. 1996. The natural history of pollination. Portland, OH: Timber Press.
- Ramsey M, Vaughton G. 2000. Pollen quality limits seed set in Burchardia umbellata (Colchicaceae). American Journal of Botany 87: 845–852. [PubMed] [Google Scholar]
- Schemske DW, Horvitz CC. 1984. Variation among floral visitors in pollination ability: a precondition for mutualism specialization. Science 225: 519–521. [DOI] [PubMed] [Google Scholar]
- Sprengel CK. 1995. Discovery of the secret of nature in the structure and fertilization of flowers. In: Lloyd DG, Barrett SCH, eds. Floral biology. Chapman and Hall: New York, 3–43 [translation by P. Haase of the introduction by C. K. Sprengel (1793) to Das entdeckte Geheimniss der Natur im Bau und in der Befruchtung der Blumen. I. Berlin: Vieweg].
- Stebbins GL. 1970. Adaptive radiation of reproductive characteristics in angiosperms. I. Pollination mechanisms. Annual Review of Ecology and Systematics 1: 307–326. [Google Scholar]
- Stephenson AG. 1981. Flower and fruit abortion: proximate causes and ultimate functions. Annual Review of Ecology and Systematics 12: 253–279. [Google Scholar]
- Totland Ø. 2001. Environment-dependent pollen limitation and selection on floral traits in an alpine species. Ecology 82: 2233–2244. [Google Scholar]
- Waser NM, Chitka L, Price MV, Williams NM, Ollerson J. 1996. Generalization in pollination systems, and why it matters. Ecology 77: 1043–1060. [Google Scholar]
- Westerbergh A, Saura A. 1994. Gene flow and pollinator behaviour in Silene dioica populations. Oikos 71: 215–224. [Google Scholar]
- Wright JW, Meagher TR. 2004. Selection on floral characters in natural Spanish populations of Silene latifolia. Journal of Evolutionary Biology 17: 382–395. [DOI] [PubMed] [Google Scholar]
- Young HJ. 2002. Diurnal and nocturnal pollination of Silene alba (Caryophyllaceae). American Journal of Botany 89: 433–440. [DOI] [PubMed] [Google Scholar]
- Zimmerman M, Pyke GH. 1988. Reproduction in Polemonium: assessing the factors limiting seed set. The American Naturalist 131: 723–738. [Google Scholar]



