Abstract
• Background and Aims Information on the influence of wounding on lignin synthesis and distribution in differentiating xylem tissue is still scarce. The present paper provides information on cell modifications with regard to wall ultrastructure and lignin distribution on cellular and subcellular levels in poplar after wounding.
• Methods Xylem of Populus spp. close to a wound was collected and processed for light microscopy, transmission electron microscopy and cellular UV microspectrophotometry. Cell wall modification with respect to lignin distribution was examined at different stages of wound tissue development. Scanning UV microspectrophotometry and point measurements were used to determine the lignin distribution.
• Key Results Xylem fibres within a transition zone between differentiated xylem laid down prior to wounding and the tissues formed after wounding developed distinctively thickened secondary cell walls. Those modified walls and cell corners showed, on average, a higher lignin content and an inhomogeneous lignin distribution within the individual wall layers.
• Conclusions The work presented shows that wounding of the xylem may induce a modified wall architecture and lignin distribution in tissues differentiating at the time of wounding. An increasing lignin content and distinctively thickened walls can contribute to improved resistance as part of the compartmentalization process.
Keywords: Wound reaction, fibre cell wall, UV microspectrophotometry, lignin distribution, Populus spp
INTRODUCTION
In trees, wounding induces specific reactions which have been the subject of numerous investigations at macroscopic and microscopic levels (e.g. Sharon, 1973; Mullick, 1977; Shortle, 1979; Shigo, 1984; Schmitt and Liese, 1990, 1992a, b; Liese and Dujesiefken, 1996; Pearce, 2000). Depending on the depth of wounding, the reactions can be restricted to the bark or they can also extend to the cambium and xylem. The wound reactions can either occur close to the wound or affect larger portions of tissue adjacent to the wound. This response depends on the extent of wounding, the season and tree vitality (Schmitt and Liese, 1992a). Lesions affecting the cambial region often lead to the formation of a wound callus and subsequently modified xylem and phloem (e.g. Liese and Dujesiefken, 1989; Fink, 1999; Grünwald et al., 2002). Within the xylem a combination of different reactions taking place compartmentalizes the inflicted area (Sharon, 1973; Shigo and Marx, 1977; Bauch et al., 1980; Rademacher et al., 1984; Shigo, 1984; Lowerts et al., 1986; Liese and Dujesiefken, 1996). The macro- and microscopically most prominent mechanism involved in compartmentalization is the formation of a so called boundary layer. This layer is mainly characterized by the synthesis of phenolic compounds in parenchyma cells with their ensuing extrusion into neighbouring cells and vessel occlusions (e.g. Schmitt and Liese, 1990; Pearce, 2000).
Research on wound reactions in poplar has been mostly carried out by conventional light microscopy (e.g. Buntrock, 1989). Earlier studies mainly focused on wound reactions of the bark, such as the formation of the ligno-suberized zone, of new periderms, and new bark (Kaufert, 1937; Soe, 1959; Trockenbrodt and Liese, 1991). The influence of pressure on tissue differentiation on longitudinal bark strips, separated from the bole, has been examined in detail (Brown and Sax, 1962). Bucciarelli et al. (1999) compared, over a 96-h period, the wound responses of the bark of resistant and susceptible Populus tremuloides genotypes, inoculated with Entoleuca mammata, using histochemical and microspectrophotometric analyses.
The objective of the present study was to provide detailed information on the modification of xylem elements within a transition zone between differentiated xylem laid down prior to wounding and the tissues laid down after wounding. The xylem elements in this transition zone often develop distinct wall thickenings. A particular emphasis is given to the lignin distribution on a cellular and subcellular level. For this purpose, the unaffected xylem and modified xylem were investigated by transmission electron microscopy (TEM) and cellular UV microspectrophotometry.
MATERIALS AND METHODS
Investigations were carried out with four 25-year-old Populus tremula L. × Populus tremuloides Michx. trees and one 35-year-old Populus tremula L. tree, growing in the east of Hamburg. Rectangular wounds of 10 × 10 cm2 were set on 7 Jul. 2002 and 23 Jun. 2003 by removing the bark from the stems using a saw and a chisel. The edges of the debarked areas were smoothed and the wound surfaces scraped with a razor blade to remove remnants of the cambium and the differentiating xylem. The trees were wounded at stem heights between 50 and 100 cm and the wounds were facing different directions.
For all microscopic work, samples were collected from the lateral wound edge 2, 4, 10, 17, 62 and 95 weeks after wounding. Callus tissue and the adjacent modified xylem were removed with chisel and razor blades. Unaffected xylem of the same poplar trees served as reference material.
Light microscopy
For light microscopy, samples were cut into 10 × 10 × 8 mm3 pieces, fixed for 3 d in 4 % buffered formol (Lillie, 1954) washed in distilled water, and embedded in polyethylene-glycol. Transverse sections, 10 μm thick, were cut with a sliding microtome, stained with a standard safranine/astra blue solution and mounted onto glass slides.
Transmission electron microscopy
For TEM, the samples from the same wounds as used for light microscopy were cut to a final size of 3 × 3 × 8 mm3 (Fig. 1), fixed for 1 d in a buffered mixture of glutaraldehyde and paraformaldehyde according to Karnovsky (1965), partly postfixed with a 1% osmium tetroxide solution, washed in 0·1 m cacodylate buffer (pH 7·3), serially dehydrated in a graded series of acetone and embedded in Spurr's epoxy resin (Spurr, 1969).
Fig. 1.
Diagram of a transverse section through a lateral wound callus showing the area containing thick-walled xylem fibres (hatching) within the transition zone (arrows) and the position of tissue sampling for TEM and UV microspectrophotometry.
Ultrathin (80–100 nm) transverse sections were cut with diamond knives, placed on copper grids and either double-stained with uranyl acetate and lead citrate or with potassium permanganate. The samples were examined with a Philips CM 12 TEM at an accelerating voltage of 60 or 80 kV.
UV microspectrophotometry
Samples for UV microspectrophotometry were prepared in the same way as for TEM. Semi-thin (1-μm) transverse sections were cut on an ultramicrotome using a diamond knife. The semi-thin sections were transferred to quartz slides, immersed in a drop of non-UV absorbing glycerine and covered with a quartz cover slip. For the microscopic investigations the ultrafluar objectives 32 : 1 and 100 : 1 were used.
Scanning UV microspectrophotometry was carried out using a ZEISS UMSP 80 microspectrophotometer equipped with an Osram high-pressure xenon lamp, an ultrafluar quartz condenser and a scanning stage enabling the determination of image profiles at a constant wavelength of 280 nm using the scan program APAMOS (Automatic-Photometric-Analysis of Microscopic Objects by Scanning, Zeiss). This wavelength represents the typical absorbance maximum of lignified cell walls. The scan program digitizes rectangular tissue portions with a local geometrical resolution of 0.25 μm2 and a photometrical resolution of 4096 grey scale levels, converted into 14 basic colours representing the measured absorbance intensities (Koch and Kleist, 2001).
The specimens were also analysed by UV microspectrophotometry point measurements with a spot size of 1 μm2. UV-spectra were taken at wavelengths from 240 to 400 nm in 2-nm steps using the program LAMWIN (Zeiss) (Takabe, 2002). These point measurements, which were used for a semi-quantitative determination of lignin content, were automatically repeated 50 times at each spot for individual wall layers: (a) compound middle lamella, (b) S2 layers of the secondary wall and (c) cell corners.
RESULTS
Light microscopy revealed that wounding induced the formation of xylem cells with modified characteristics in the transition zone between the xylem laid down prior to wounding and the tissues laid down after wounding (Figs 1 and 2). Within this zone the xylem cells were still differentiating at the time of wounding. The formation of distinctively thick-walled fibres was the most prominent structural pattern within this zone. Electron microscopy showed that these fibres deposited additional secondary wall (S2) material leading to extremely thick walls compared with regular xylem cells (Figs 3–5). This wound-induced reaction resulted in three modes of wall-thickening. One group of xylem fibres showed the presence of a supplementary S2 layer (Fig. 3); the second group of fibres developed a continuously thickened secondary cell wall (Fig. 4) and in the third, smaller group, fibre walls developed a sclereid-like sublayering (Fig. 5). Cell corners within the transition zone predominantly consisted of material with a high electron density, which is unusual for poplar (Fig. 4).
Fig. 2.

Transverse section (light microscopy) through a wound callus and the modified xylem that is located within a transition zone between xylem laid down prior to and after wounding containing fibres with wound-induced wall thickenings (arrows). Scale bar = 10 mm.
Fig. 3.

Transverse section (TEM) through a modified xylem fibre with very thick, lamellate secondary wall (arrows), ray parenchyma cell (RP). Scale bar = 5 μm. Duration of wound response 10 weeks.
Fig. 4.

Transverse section (TEM) through modified xylem of the transition zone. Fibres with continuous secondary cell wall thickenings (without lamellation); cell corners contain material with extremely high electron density (arrow). Scale bar = 2.5 μm. Duration of wound response 4 weeks.
Fig. 5.

Transverse section (TEM) through wound-induced fibres with a distinct polylamellate structure of the secondary wall (arrows). Scale bar = 2.5 μm. Duration of wound response 4 weeks.
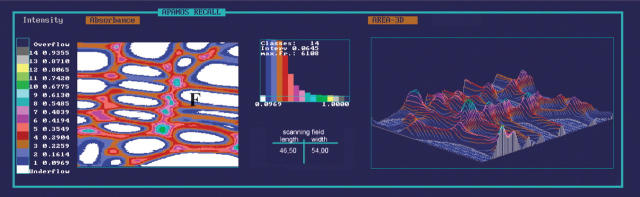
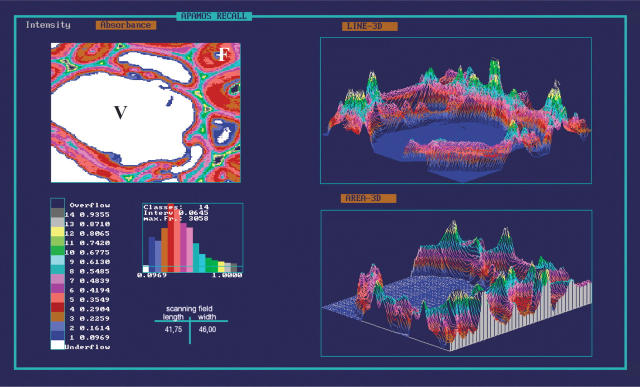
To determine the lignin distribution within different wall layers of unmodified and modified tissues, these were first analysed by scanning UV microspectrophotometry at a constant wavelength (λ) of 280 nm. Figures 6 and 7 show representative two- and three-dimensional (2D and 3D, respectively) UV image profiles of unaffected mature secondary poplar xylem. Figure 6 shows the distribution pattern of lignin in earlywood fibres and vessel elements characterized by thin S2 wall layers of relatively low uniform absorbance values (Abs280nm 0·09–0·16) and increasing values in the compound middle lamella regions (Abs280nm 0·16–0·23). The highest absorbencies of Abs280nm 0·35–0·67 were found in some cell corners of vessel elements. Terminal latewood fibres (Fig. 7) showed slightly higher absorbance values especially in the compound middle lamella (Abs280nm 0.23–0.35), as compared with earlywood. The broader S2 layer revealed absorbance values of Abs280nm 0·09–0·23, the cell corners of Abs280nm 0·35–0·67.
Fig. 6.
UV micrograph and a 3D UV microscopic scanning profile of unaffected xylem tissue. The coloured pixels represent different UV-absorbance values within the cell wall layers of vessel elements (V) and fibres (F) at 280 nm wavelength.
Fig. 7.
UV micrograph and a 3D image profile of unaffected latewood fibres (F) at the annual ring border.
The influence of lesions on the lignin distribution within walls of modified xylem cells of the transition zone is presented in Figs 8–11. As early as 4 weeks after wounding, many fibres and vessel elements displayed different stages of secondary wall thickening (Fig. 8). The secondary wall of the scanned modified fibres showed microscopically invisible concentric sublayers with increasing absorbance values from Abs280nm 0·09 to 0·48. The lowest values always occurred in the wall regions adjacent to lumen. High absorbance values were commonly recorded in the compound middle lamella (Abs280nm 0·55–0·68) between fibres and also in the cell corners (Abs280nm 0·81–0·94). The absorbance values of vessel walls also indicated a heterogeneous lignin distribution with unusually high values of Abs280nm 0·67 up to 0·87 in the compound middle lamella between vessels and neighbouring fibres (Fig. 8).
Fig. 8.
UV micrograph and a 3D image profile of a small vessel element (V) and fibres (F) in the transition zone 4 weeks after wounding.
Fig. 9.
UV micrograph and a 3D image profile of fibres showing strongly developed secondary wall thickenings within a band of fibres close to the former wound surface, 62 weeks after wounding. Some fibre lumina are filled with phenolic compounds (arrow).
Fig. 10.
UV micrograph and a 3D image profile of fibres (F) and vessel elements (V), close to the wound surface, 95 weeks after wounding.
Fig. 11.
UV micrograph and a 3D image profile of radially flattened fibres with secondary wall thickenings and ray parenchyma cells (Rp), some of them filled with phenolic compounds (arrow), 95 weeks after wounding.
Figure 8 displays the lignin distribution in modified fibres with extremely thickened secondary walls close to a wound surface after 62 weeks of response. The lumina in most of these fibres were extremely reduced by the thickened wall and in some cases the remaining lumina were filled with strongly UV-absorbing deposits (Fig. 9). The inhomogeneously distributed absorbance values within the thickened S2 varied between Abs280nm 0·09 and increased maxima of Abs280nm 0·42, both within the S2 of an individual fibre and between fibres. Compound middle lamella absorbance values ranged from Abs280nm 0·61 to 0·81 and in cell corners from Abs280nm 0·81 up to Abs280nm over 0·94.
Figures 10 and 11 show the lignin distribution patterns for vessel elements, fibres and ray parenchyma cells within the transition zone after nearly 2 years of wound response. The image profiles display distinctly high absorbance values across the entire thickened walls of fibres and vessels (Fig. 10). The S2 layer of vessels showed absorbance values between Abs280nm 0·23 and Abs280nm 0·42. For compound middle lamella regions UV absorbance ranged from Abs280nm 0·48 to 0·68 and for the cell corners from Abs280nm 0·55 to 0·94. The absorbance values of the S2 in fibre walls varied between Abs280nm 0·16 and Abs280nm 0·35. The distribution of the values was inhomogeneous. The absorbance values for the compound middle lamella and the cell corners were Abs280nm 0·23–0·55 and Abs280nm 0·42–0·94, respectively (Figs 10 and 11).
To characterize the UV-absorbance behaviour of previously scanned tissue portions, point measurements with a spot size of 1 μm and a wavelength between 240 and 400 nm were carried out. In Fig. 12, representative UV absorbance spectra of unaffected and modified fibre wall layers are shown. Those point measurements of wall layers from unaffected early- and latewood fibres were characterized by mean absorption maxima at a wavelength of 270/272 nm for the S2 and the compound middle lamella, and of 278 nm for the cell corners (Fig. 12A). In distinctively thickened secondary wall regions of modified fibres, absorption maxima at wavelengths between 272 and 274 nm were regularly detected. The spectra taken in middle lamella regions of modified fibres also shifted towards higher wavelengths of 274–276 nm when compared with unaffected fibres. The absorbance maxima of cell corner regions of transition zone fibres with wavelengths of 276–280 nm were not notably different from cell corners of fibres from the unaffected references (Fig. 12B–F).
Fig. 12.

Representative UV absorbance spectra of fibre cell wall layers of unaffected controls (A) and after different periods of wound response (B–F); (B) 2, (C) 4, (D) 17, (E) 62 and (F) 95 weeks after wounding. Vertical line indicates the 272 nm wavelength.
The ratio of UV absorbance at 280 nm to that at 260 nm (A280 : A260) for the S2 layer, compound middle lamella and cell corner areas of the modified tissue and the reference material are listed in Table 1. This ratio combined with the characteristic wavelength of the lignin absorption maxima (Fig. 12) can be used as an indicator for the proportion of the different lignin moieties and p-hydroxy benzoic acid residues associated with them.
Table 1.
Ultraviolet absorbance ratio A280 : A260 of different cell wall layers
| A280 : A260 |
|||
|---|---|---|---|
| Sample | S2 layer | Compound middle lamella | Cell corner region |
| Unaffected fibres | 1·02 | 1·09 | 1·29 |
| Affected fibres after 2 weeks of wound response | 1·20 | 1·25 | 1·29 |
| Affected fibres after 4 weeks of wound response | 1·18 | 1·20 | 1·25 |
| Affected fibres after 17 weeks of wound response | 1·20 | 1·29 | 1·26 |
| Affected fibres after 62 weeks of wound response | 1·16 | 1·19 | 1·25 |
| Affected fibres after 95 weeks of wound response | 1·34 | 1·33 | 1·33 |
The spectra of the detected accessory phenolic extractives in fibres and ray parenchyma cells had much higher absorbance values (Abs280nm 1·38) than cell wall lignins. Furthermore, their absorbance maxima displayed a bathochromic shift to a wavelength of 284–286 nm and a slower decrease of the absorbance, when compared with lignin.
The ratios (A280 : A260) of S2 and compound middle lamella regions from transition zone fibres with thickened walls were higher when compared with unmodified fibre walls. For cell corner regions of affected tissue portions no such trend for the ratio (A280 : A260) could be obtained, because the values were slightly higher and also lower as in the references.
In summary, the microscopic analyses showed that xylem fibres close to a wound and within a transition zone between differentiated xylem laid down prior to and tissue laid down after wounding developed a distinctively thicker secondary wall. These modified fibre walls regularly showed a higher lignin content and an inhomogeneous lignin distribution in the middle lamella and the secondary wall.
DISCUSSION
Microscopy
Light and electron microscopy showed that cells that were differentiating at the time of wounding were affected in the deposition of wall material. These cells were localized in a transition zone consisting of differentiating xylem cells at the time of wounding. Depending on their stage of differentiation at the time of wounding the different cell types respond in a distinctive manner. Within phloem tissue the wound-induced formation of modified fibres and sclereids as well as the lignification of inflicted areas is commonly reported (e.g. Soe, 1959; Brown and Sax, 1962; Mullick, 1977; Trockenbrodt and Liese, 1991; Bucciarelli et al., 1999; Fink, 1999). The formation of a tangential band of thick-walled, flattened xylem fibres within a zone between the modified and regular xylem after wounding through ‘pinning’, was observed in beech wood by Schmitt et al. (2000). A distinctive feature of wound-associated wood of yellow poplar (Liriodendron tulipifera) is the presence of sclereids (Lowerts et al., 1986). It is assumed for poplar that besides modified fibres with a lamellar S2 some cells with a less axial extension and a sublayering within the S2 are sclereids. These structural alterations within the existing tissue, as observed in poplar and also in beech and yellow poplar xylem (Lowerts et al., 1986, Schmitt et al., 1999), supplement in some cases the well-known compartmentalization processes in these species (e.g. Shigo and Marx, 1977; Shortle, 1979; Shigo, 1984; Buntrock, 1989; Liese and Dujesiefken, 1996).
Topochemical characterization
UV microspectrophotometry has proven to be a reliable technique to study lignin distribution and its semiquantitative determination under various conditions and in different tree species (e.g. Bland and Hillis, 1969; Scott et al., 1969; Fergus and Goring, 1970a, b; Musha and Goring, 1975; Bauch et al., 1976; Fukazawa, 1992; Okuyama et al., 1998; Bucciarelli et al., 1999; Grünwald et al., 2001, 2002a, b; Koch and Kleist, 2001; Takabe, 2002; Koch and Grünwald, 2004). The following discussion is based on the observed UV absorbance behaviour of tissues in response to wounding.
Both guaiacyl and syringyl model compounds show a minimum absorbance at wavelengths from 250 to 260 nm and a maximum from 270 to 280 nm which is characteristic for hardwood lignin. This is in contrast to a strong maximum absorbance of p-hydroxy benzoic acid residues at 260 nm. With an increasing guaiacyl lignin content, the absorbance maximum shifts closer to 280 nm (Fergus and Goring, 1970a, b; Musha and Goring, 1975; Terashima et al., 1986b; Fukazawa, 1992; Koch and Kleist, 2001; Takabe, 2002).
The spectra obtained in the present study for thin S2 layers in unaffected fibres of poplar with a relative low absorbance maximum at 270–272 nm and a less distinct peak correspond to spectra obtained in earlier studies (Fergus and Goring, 1970a, b; Musha and Goring, 1975; Fukazawa, 1992), although the ratio A280 : A260 is slightly higher than detected in other poplar species. Thus, the measurements indicate a low content of predominant syringyl-type lignin associated with p-hydroxy benzoic acid residues. The relatively low lignin content in the S2 layer, as compared with compound middle lamella and cell corner regions, as well as the uniform distribution within this layer, are in good agreement with general concepts for cell wall compositions of hardwoods (e.g. Fergus and Goring, 1970a, b; Musha and Goring, 1975; Terashima et al., 1986a, b, 1993; Donaldson, 2001; Donaldson et al., 2001; Koch and Kleist, 2001; Grünwald et al., 2002a; Takabe, 2002). The peak absorbance at 272 nm in fibre secondary walls and the only slightly higher absorbance of the compound middle lamella indicate increased lignin content with a nearly unmodified lignin composition. Grünwald et al. (2002a) reported alterations in the lignin composition in middle lamella regions of xylem fibres with increasing distance from the cambium, as indicated by a shift of the peak absorbance towards 272 nm at the end of cell differentiation due to an increasing amount of syringyl and p-hydroxyphenyl subunits. More frequently, however, a higher relative abundance of guaiacyl lignin moieties in hardwood middle lamellae has been reported (e.g. Fergus and Goring, 1970b; Musha and Goring, 1975; Terashima, 1986a, b, 1993). The present scannings of middle lamella regions showed a more speckled heterogeneous lignin distribution, whereas in Hevea brasiliensis the intercorner and cell corner middle lamella showed a mostly mottled lignin distribution, as observed by Singh and Schmitt (2000). The spectra obtained for cell corner regions showed the highest lignin concentrations of predominantly guaiacyl lignin, which is in good agreement with general lignification concepts for soft- and hardwoods (e.g. Fergus and Goring, 1970b; Terashima et al., 1986b, 1993; Fukazawa, 1992; Donaldson, 2001; Donaldson et al., 2001).
The spectra obtained from cells located in the transition zone showed that wounding may induce modification in lignin composition and distribution. These alterations were mainly restricted to secondary wall and compound middle lamella regions, whereas cell corner lignin remained nearly unaffected. Within thickened secondary walls the average peak absorbance shifted slightly towards higher wavelength and the ratio A280 : A260 simultaneously increased, verifying a higher amount of guaiacyl moieties and a reduced amount of associated p-hydroxy benzoic acid residues. The same effect was evidenced by UV spectra from middle lamella regions. More detailed information on the microdistribution of lignin was provided by UV scannings which also displayed elevated lignin content with highly variable maximum values in fibre and vessel walls. It was found that at an early stage of wound response the lignin content increases in concentric sublayers within the S2-layer. It is more likely that this distribution resembles phloem sclereids in hardwoods (authors' observations) as well as in bamboo fibres as shown by Koch and Kleist (2001). Normal hardwood fibres have a uniform lignin distribution across the entire S2 layer (Saka and Goring, 1988; Koch and Kleist, 2001). During later stages of wound response the lignin distribution within the secondary wall appeared more inhomogeneous. The lignin content in compound middle lamella and cell corner regions, however, was also significantly higher in late stages of wound response.
As mentioned before, the syringyl : guaiacyl ratio and the condensation of methoxyl groups determines the position of the absorbance maximum and also the absorbance intensity. With decreasing MeO/C9 values, the peak position shifts towards higher wavelength and the intensity of absorbance increases, whereas the overall lignin concentration may still be constant (Musha and Goring, 1975). This phenomenon therefore restricts semiquantitative interpretations of maxima differences between inflicted and unaffected cells.
From these results obtained for poplar, it can be stated that wounding induces an increased wall thickness and a modified lignin topochemistry in xylem fibres differentiating at the time of wounding. It is assumed that this wound response is part of the compartmentalization and adds a further mechanism contributing to an increased resistance.
Acknowledgments
We thank Prof. Dr D. Eckstein and Dr A. Singh for critically reviewing the manuscript and C. Waitkus for helping with the photographic work. The work was partly funded by the ‘Deutsche Forschungsgemeinschaft/DFG’ (GR 1788/2-1).
LITERATURE CITED
- Bauch J, Seehann G, Fitzner H. 1976. Microspectrophotometrical investigations on lignin of decayed wood. Material und Organismen, Beiheft 3: 141–152. [Google Scholar]
- Bauch J, Shigo AL, Starck M. 1980. Wound effects in the xylem of acer and betula species. Holzforschung 34: 153–160. [Google Scholar]
- Bland DE, Hillis WE. 1969. Microspectrophotometric investigations of lignin and polyphenol distribution in wood sections. Appita 23: 204–210. [Google Scholar]
- Brown CL, Sax K. 1962. The influence of pressure on the differentiation of secondary tissues. American Journal of Botany 49: 683–691. [Google Scholar]
- Bucciarelli B, Ostry ME, Fulcher RG, Anderson NA, Vance CP. 1999. Histochemical and microspectrophotometric analyses of early wound responses of resistant and susceptible Populus tremuloides inoculated with Entoleuca mammata (≡ Hypoxylon mammatum). Canadian Journal of Botany 77: 548–555. [Google Scholar]
- Buntrock M. 1989. Anatomische Untersuchungen über die Wundreaktion der Pappel. Diploma Thesis, University of Hamburg, Germany.
- Donaldson LA. 2001. Lignification and lignin topochemistry—an ultrastructural view. Phytochemistry 57: 859–873. [DOI] [PubMed] [Google Scholar]
- Donaldson LA, Hague J, Snell R. 2001. Lignin distribution in coppice poplar, linseed and wheat straw. Holzforschung 55: 379–385. [Google Scholar]
- Fergus BJ, Goring DAI. 1970a. The location of guaiacyl and syringyl lignins in birch xylem tissue. Holzforschung 24: 113–117. [Google Scholar]
- Fergus BJ, Goring DAI. 1970b. The distribution of lignin in birch wood as determined by ultraviolet microscopy. Holzforschung 24: 118–124. [Google Scholar]
- Fink S. 1999. Pathological and regenerative plant anatomy. Berlin/Stuttgart: Gebrüder Borntraeger.
- Fukazawa K. 1992. Ultraviolet microscopy. In: Lin SY, Dence CW, eds. Methods in lignin chemistry. Berlin: Springer Verlag, 110–121.
- Grünwald C, Ruel K, Joselau JP, Fladung M. 2001. Morphology, wood structure and cell wall composition of rolC transgenic and non-transformed aspen trees. Trees 15: 503–517. [Google Scholar]
- Grünwald C, Stobbe H, Schmitt U. 2002. Entwicklungsstufen der seitlichen Wundüberwallung von Laubgehölzen. Forstwissenschaftliches Zentralblatt 121: 50–58. [Google Scholar]
- Grünwald C, Ruel K, Kim YS, Schmitt U. 2002a. On the cytochemistry of cell wall formation in poplar trees. Plant Biology 4: 13–21. [Google Scholar]
- Grünwald C, Ruel K, Schmitt U. 2002b. Differentiation of xylem cells in rolC transgenic aspen trees—a study of secondary cell wall development. Annals of Forest Science 59: 679–685. [Google Scholar]
- Karnovsky MJ. 1965. A formaldehyde–glutaraldehyde fixative of high osmolality for use in electron microscopy. Journal of Cell Biology 27: 137–138. [Google Scholar]
- Kaufert F. 1937. Factors influencing the formation of periderm in Aspen. American Journal of Botany 24: 24–30. [Google Scholar]
- Koch G, Kleist G. 2001. Application of scanning UV microspectrophotometry to localise lignins and phenolic extractives in plant cell walls. Holzforschung 55: 563–567. [Google Scholar]
- Koch G, Grünwald C. 2004. Application of UV microspectrophotometry for topochemical detection of lignin and phenolic extractives in wood fibre cell walls. In: Schmitt U, Ander P, Barnett JR, Emons AMC, Jeronimidis G, Saranpää P, et al., eds. Wood fibre cell walls: methods to study their formation, structure and properties. Uppsala: Swedish University of Agricultural Science, 119–130.
- Liese W, Dujesiefken D. 1989. Wundreaktionen bei Bäumen. Tharandt: Tagungsbericht, 2. Symposium, Ausgewählte Probleme der Gehölzphysiologie—Gehölze, Mikroorganismen und Umwelt, 75–80.
- Liese W, Dujesiefken D. 1996. Wound reactions of trees. In: Raychaudhuri SP, Maramorosch, K, eds. Forest trees and palms: diseases and control. Oxford/New Delhi/Calcutta: IBH Publishing, 21–35.
- Lille RD. 1954. Histopathologic technique and practical histochemistry. 2nd edn. New York: McGraw-Hill.
- Lowerts G, Wheeler EA, Kellison RC. 1986. Characteristics of wound-associated wood of Yellow-Poplar (Lirodendron tulipifera L.). Wood and Fiber Science 18: 537–552. [Google Scholar]
- Mullick DB. 1977. The non-specific nature of defence in bark and wood during wounding, insect and pathogen attack. Recent Advances in Phytochemistry 11: 395–441. [Google Scholar]
- Musha Y, Goring DAI. 1975. Distribution of syringyl and guaiacyl moieties in hardwoods as indicated by ultraviolet microscopy. Wood Science and Technology 9: 45–58. [Google Scholar]
- Okuyama T, Takeda H, Yamamoto H, Yoshida M. 1998. Relation between growth stress and lignin concentration in the cell wall: ultraviolet microscopic spectral analysis. Journal of Wood Science 44: 83–89. [Google Scholar]
- Pearce RB. 2000. Decay development and its restriction in trees. Journal of Arboriculture 26: 1–11. [Google Scholar]
- Rademacher P, Bauch J, Shigo AL. 1984. Characteristics of xylem formed after wounding in Acer, Betula and Fagus. IAWA Bulletin 5: 141–151. [Google Scholar]
- Saka S, Goring DAI. 1988. Localization of lignins in wood cell walls. In: Higuchi T, ed. Biosynthesis and biodegradation of wood components. New York, NY: Academic Press, 51–62.
- Schmitt U, Liese W. 1990. Wound reaction of the parenchyma in Betula. IAWA Bulletin 11: 413–420. [Google Scholar]
- Schmitt U, Liese W. 1992a. Seasonal influences on early wound reactions in Betula and Tilia. Wood Science and Technology 26: 405–412. [Google Scholar]
- Schmitt U, Liese W. 1992b. Response of xylem parenchyma by suberization in some hardwoods after mechanical injury. Trees 8: 23–30. [Google Scholar]
- Schmitt U, Möller R, Eckstein D. 2000. Seasonal wood formation dynamics of Beech (Fagus sylvatica L.) and Black Locust (Robinia pseudoacacia L.) as determined by the ‘Pinning’ technique. Journal of Applied Botany 74: 10–16. [Google Scholar]
- Scott JAN, Procter AR, Fergus BJ, Goring DAI. 1969. The application of ultraviolet microscopy to the distribution of lignin in wood: description and validity of the technique. Wood Science and Technology 3: 73–92. [Google Scholar]
- Sharon EM. 1973. Some histological features of Acer saccharum wood formed after wounding. Canadian Journal of Forest Research 3: 83–89. [Google Scholar]
- Shigo AL. 1984. Compartmentalization: a conceptual framework for understanding how trees grow and defend themselves. Annual Review of Phytopathology 22: 189–214. [Google Scholar]
- Shigo AL, Marx HG. 1977. Compartmentalization of decay in trees. USDA/Forest Service and Agriculture Information Bulletin 405: 1–73. [Google Scholar]
- Shortle WC. 1979. Compartmentalization of decay in red maple and hybrid poplar trees. Phytopathology 69: 410–413. [Google Scholar]
- Singh AP, Schmitt U. 2000. High variability in the distribution of lignin in the middle lamella of Rubber-wood (Hevea brasiliensis) cells. In: Kim YS, ed. New horizons in wood anatomy. Kwangju: Chonnam Nat′l University Press, 203–207.
- Soe K. 1959. Anatomical studies of bark regeneration following scoring. Journal of the Arnold Arboretum 40: 260–267. [Google Scholar]
- Spurr AR. 1969. A low viscosity epoxy resin embedding medium for electron microscopy. Journal of Ultrastructural Research 26: 31–43. [DOI] [PubMed] [Google Scholar]
- Takabe K. 2002. Cell walls of woody plants: autoradiography and ultraviolet microscopy. In: Chaffey N, ed. Wood formation in trees. London: Taylor & Francis, 159–177.
- Terashima N, Fukushima K, Takabe K. 1986a. Heterogeneity in formation of lignin. VIII. An autoradiographic study on the formation of guaiacyl and syringyl lignin in Magnolia kobus DC. Holzforschung 40: 101–105. [Google Scholar]
- Terashima N, Fukushima K, Tsuchiya S, Takabe K. 1986b. Heterogeneity in formation of lignin. VII. An autoradiographic study on the formation of guaiacyl and syringyl lignin in poplar. Journal of Wood Chemistry and Technology 6: 495–504. [Google Scholar]
- Terashima N, Fukushima K, Takabe K. 1993. Comprehensive model of the lignified plant cell wall. In: Jung HG, Buxton DR, Hatfield RD, Ralph J, eds. Forage cell wall structure and digestibility. Madison, WI: ASA-CSSA-SSSA, 247–270.
- Trockenbrodt M, Liese W. 1991. Untersuchungen zur Wundreaktion in der Rinde von Populus tremula L. und Platanus×acerifolia (Ait.) Willd.. Angewandte Botanik 65: 279–287. [Google Scholar]