Abstract
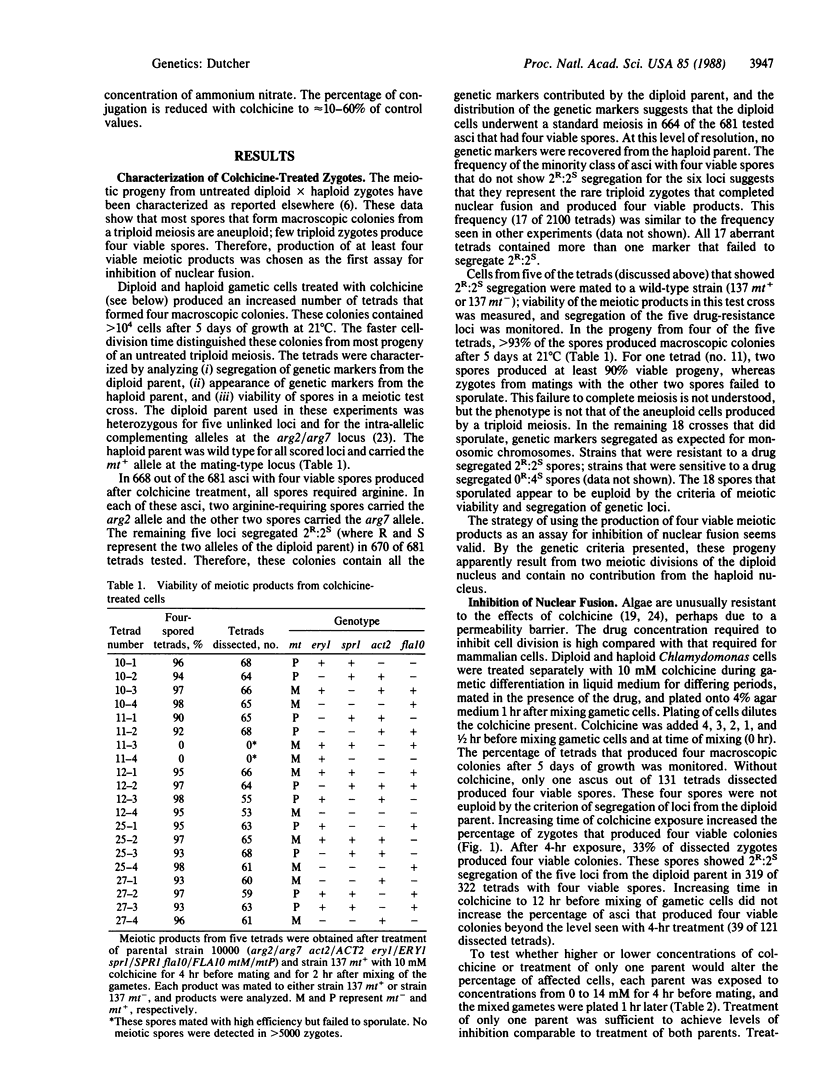
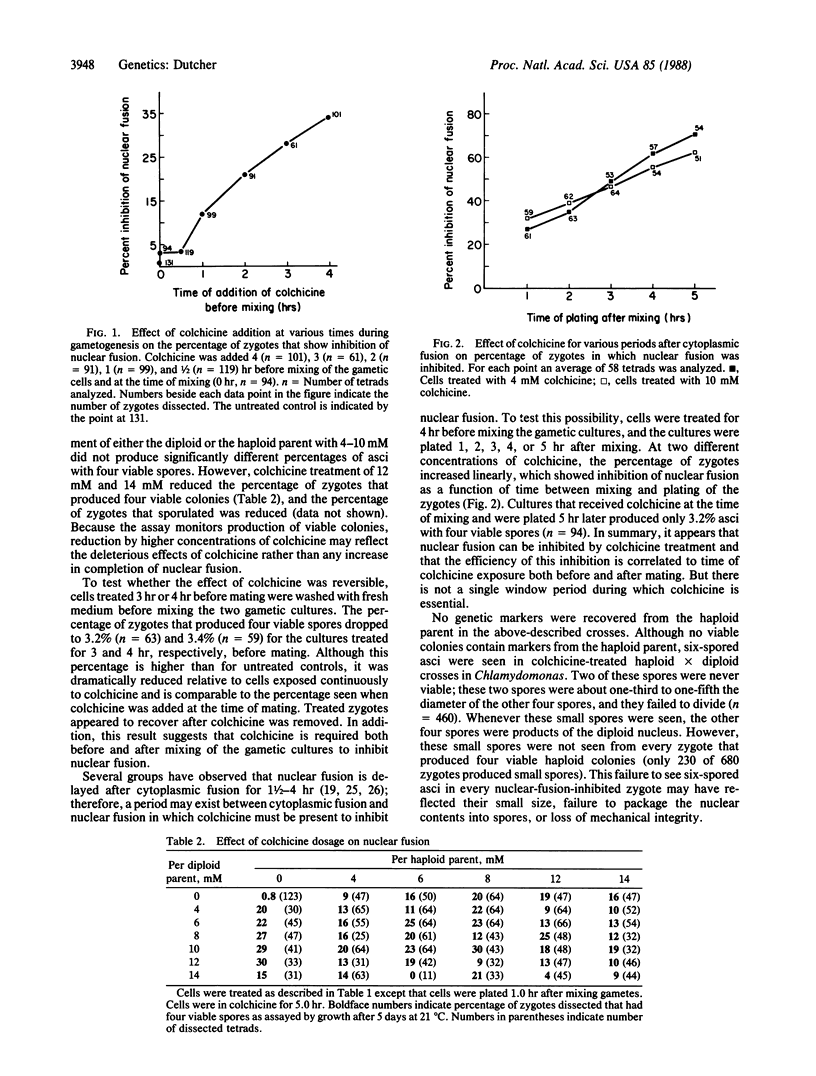
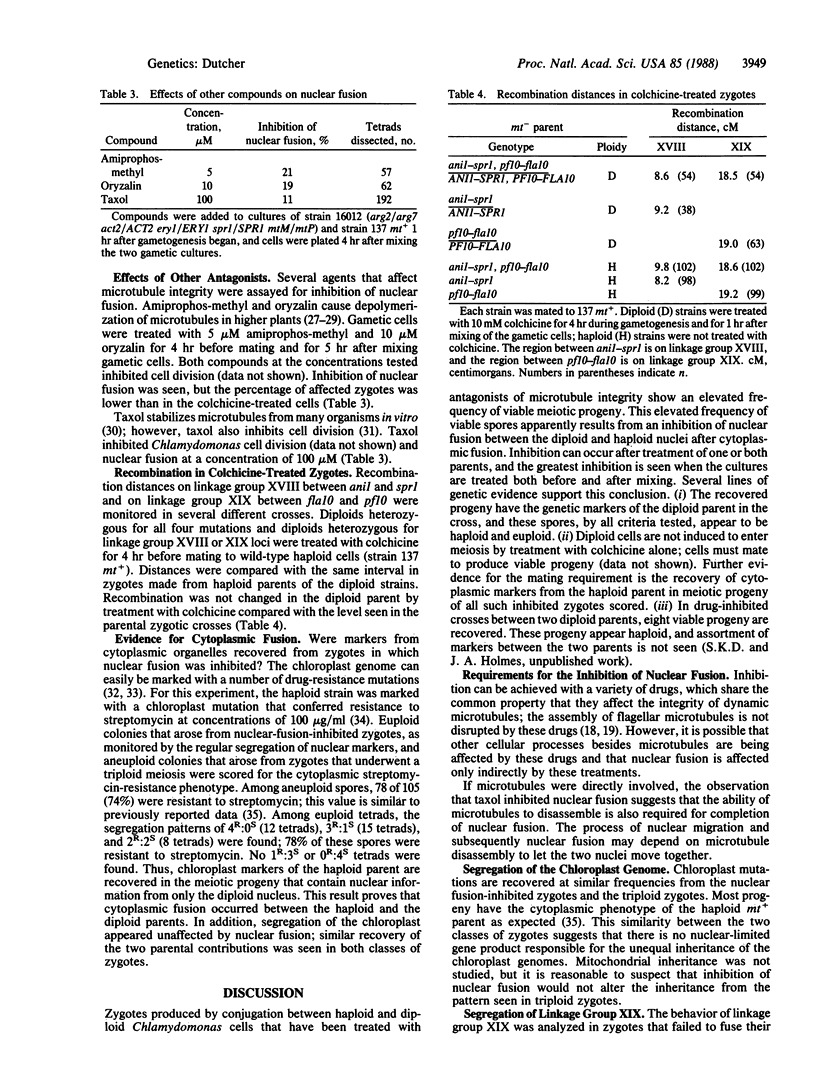
Nuclear fusion in newly formed Chlamydomonas reinhardtii zygotes can be inhibited by drugs that affect microtubule stability, which include colchicine, amiprophosmethyl, oryzalin, and taxol. This inhibition can be monitored genetically by the production of haploid meiotic products from conjugations between haploid and diploid parents. Such zygotes would normally produce aneuploid progeny. Inhibition of nuclear fusion by colchicine requires treatment of gametic cells both before conjugation and after formation of the zygotes. These results suggest that nuclear fusion requires dynamic microtubules. Treated zygotes formed from a haploid-diploid mating can produce six spores, but only four spores germinate to form viable haploid colonies. No contribution from the nuclear genome of the haploid parent is recovered, whereas all loci from the diploid parent are recovered. The four viable products from the diploid parent of inhibited zygotes show normal segregation of loci located on linkage groups segregating according to Mendelian laws. Levels of meiotic recombination were tested for pairs of loci on linkage groups XVIII and XIX and found to be unchanged by inhibition of nuclear fusion. Thus, similar to Saccharomyces cerevisiae, C. reinhardtii mating-type functions necessary for nuclear fusion are not nuclear limited and can act through the cytoplasm. Inhibition of nuclear fusion can be used to analyze diploid Chlamydomonas that cannot enter meiosis. This technique permits direct analysis of dominant mutations, dominant suppressors and enhancers, and new alleles of identified loci that have been isolated in diploid strains.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams G. M., Wright R. L., Jarvik J. W. Defective temporal and spatial control of flagellar assembly in a mutant of Chlamydomonas reinhardtii with variable flagellar number. J Cell Biol. 1985 Mar;100(3):955–964. doi: 10.1083/jcb.100.3.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajer A. S., Molè-Bajer J. Drugs with colchicine-like effects that specifically disassemble plant but not animal microtubules. Ann N Y Acad Sci. 1986;466:767–784. doi: 10.1111/j.1749-6632.1986.tb38458.x. [DOI] [PubMed] [Google Scholar]
- Cavalier-Smith T. Basal body and flagellar development during the vegetative cell cycle and the sexual cycle of Chlamydomonas reinhardii. J Cell Sci. 1974 Dec;16(3):529–556. doi: 10.1242/jcs.16.3.529. [DOI] [PubMed] [Google Scholar]
- Conde J., Fink G. R. A mutant of Saccharomyces cerevisiae defective for nuclear fusion. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3651–3655. doi: 10.1073/pnas.73.10.3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado M. A., Conde J. Benomyl prevents nuclear fusion in Saccharomyces cerevisiae. Mol Gen Genet. 1984;193(1):188–189. doi: 10.1007/BF00327435. [DOI] [PubMed] [Google Scholar]
- Dutcher S. K. Internuclear transfer of genetic information in kar1-1/KAR1 heterokaryons in Saccharomyces cerevisiae. Mol Cell Biol. 1981 Mar;1(3):245–253. doi: 10.1128/mcb.1.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebersold W. T. Chlamydomonas reinhardi: heterozygous diploid strains. Science. 1967 Jul 28;157(3787):447–449. doi: 10.1126/science.157.3787.447. [DOI] [PubMed] [Google Scholar]
- Galloway R. E., Goodenough U. W. Genetic analysis of mating locus linked mutations in Chlamydomonas reinhardii. Genetics. 1985 Nov;111(3):447–461. doi: 10.1093/genetics/111.3.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant D. M., Gillham N. W., Boynton J. E. Inheritance of chloroplast DNA in Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6067–6071. doi: 10.1073/pnas.77.10.6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton E. P., Suhr-Jessen P. B. Autoradiographic evidence for self-fertilization in Tetrahymena thermophila. Exp Cell Res. 1980 Apr;126(2):391–396. doi: 10.1016/0014-4827(80)90278-5. [DOI] [PubMed] [Google Scholar]
- Klar A. J. Mating-Type Functions for Meiosis and Sporulation in Yeast Act through Cytoplasm. Genetics. 1980 Mar;94(3):597–605. doi: 10.1093/genetics/94.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINE R. P., EBERSOLD W. T. The genetics and cytology of Chlamydomonas. Annu Rev Microbiol. 1960;14:197–216. doi: 10.1146/annurev.mi.14.100160.001213. [DOI] [PubMed] [Google Scholar]
- Longo F. J., Anderson E. The fine structure of pronuclear development and fusion in the sea urchin, Arbacia punctulata. J Cell Biol. 1968 Nov;39(2):339–368. doi: 10.1083/jcb.39.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck D., Piperno G., Ramanis Z., Huang B. Flagellar mutants of Chlamydomonas: studies of radial spoke-defective strains by dikaryon and revertant analysis. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3456–3460. doi: 10.1073/pnas.74.8.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matagne R. F. Fine structure of the arg-7 ciston in chlamydomonas reinhardi. Complementation between arg-7 mutants defective in argininosuccinate lyase. Mol Gen Genet. 1978 Mar 20;160(1):95–99. [PubMed] [Google Scholar]
- Matagne R. F., Mathieu D. Transmission of chloroplast genes in triploid and tetraploid zygospores of Chlamydomonas reinhardtii: Roles of mating-type gene dosage and gametic chloroplast DNA content. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4780–4783. doi: 10.1073/pnas.80.15.4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mets L. J., Geist L. J. Linkage of a Known Chloroplast Gene Mutation to the Uniparental Genome of CHLAMYDOMONAS REINHARDII. Genetics. 1983 Nov;105(3):559–579. doi: 10.1093/genetics/105.3.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami S. A., Goodenough U. W. Novel glycopolypeptide synthesis induced by gametic cell fusion in Chlamydomonas reinhardtii. J Cell Biol. 1978 Apr;77(1):165–181. doi: 10.1083/jcb.77.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morejohn L. C., Fosket D. E. Inhibition of Plant Microtubule Polymerization in vitro by the Phosphoric Amide Herbicide Amiprophos-Methyl. Science. 1984 May 25;224(4651):874–876. doi: 10.1126/science.224.4651.874. [DOI] [PubMed] [Google Scholar]
- Morejohn L. C., Fosket D. E. Taxol-induced rose microtubule polymerization in vitro and its inhibition by colchicine. J Cell Biol. 1984 Jul;99(1 Pt 1):141–147. doi: 10.1083/jcb.99.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orias J. D., Hamilton E. P., Orias E. A microtubule meshwork associated with gametic pronucleus transfer across a cell-cell junction. Science. 1983 Oct 14;222(4620):181–184. doi: 10.1126/science.6623070. [DOI] [PubMed] [Google Scholar]
- Perkins D D. The Detection of Linkage in Tetrad Analysis. Genetics. 1953 Mar;38(2):187–197. doi: 10.1093/genetics/38.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett-Heaps J. D., Spurck T. P. Studies on kinetochore function in mitosis. I. The effects of colchicine and cytochalasin on mitosis in the diatom Hantzschia amphioxys. Eur J Cell Biol. 1982 Aug;28(1):77–82. [PubMed] [Google Scholar]
- Ramanis Z., Luck D. J. Loci affecting flagellar assembly and function map to an unusual linkage group in Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A. 1986 Jan;83(2):423–426. doi: 10.1073/pnas.83.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum J. L., Moulder J. E., Ringo D. L. Flagellar elongation and shortening in Chlamydomonas. The use of cycloheximide and colchicine to study the synthesis and assembly of flagellar proteins. J Cell Biol. 1969 May;41(2):600–619. doi: 10.1083/jcb.41.2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAGER R., GRANICK S. Nutritional studies with Chlamydomonas reinhardi. Ann N Y Acad Sci. 1953 Oct 14;56(5):831–838. doi: 10.1111/j.1749-6632.1953.tb30261.x. [DOI] [PubMed] [Google Scholar]
- Sager R. MENDELIAN AND NON-MENDELIAN INHERITANCE OF STREPTOMYCIN RESISTANCE IN CHLAMYDOMONAS REINHARDI. Proc Natl Acad Sci U S A. 1954 May;40(5):356–363. doi: 10.1073/pnas.40.5.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager R. Inheritance in the Green Alga Chlamydomonas Reinhardi. Genetics. 1955 Jul;40(4):476–489. doi: 10.1093/genetics/40.4.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff P. B., Fant J., Horwitz S. B. Promotion of microtubule assembly in vitro by taxol. Nature. 1979 Feb 22;277(5698):665–667. doi: 10.1038/277665a0. [DOI] [PubMed] [Google Scholar]
- Schiff P. B., Horwitz S. B. Taxol stabilizes microtubules in mouse fibroblast cells. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1561–1565. doi: 10.1073/pnas.77.3.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. M., Regnery D. C. Inheritance of Sexuality in Chlamydomonas Reinhardi. Proc Natl Acad Sci U S A. 1950 Apr;36(4):246–248. doi: 10.1073/pnas.36.4.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman A. M., Zimmerman S. Action of colcemid in sea urchin eggs. J Cell Biol. 1967 Aug;34(2):483–488. doi: 10.1083/jcb.34.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]



