Abstract
• Background and Aims It is stated in many recent publications that nitrate ( ) acts as a signal to regulate dry matter partitioning between the shoot and root of higher plants. Here we challenge this hypothesis and present evidence for the viewpoint that
) acts as a signal to regulate dry matter partitioning between the shoot and root of higher plants. Here we challenge this hypothesis and present evidence for the viewpoint that  and other environmental effects on the shoot : root dry weight ratio (S:R) of higher plants are often related mechanistically to changes in shoot protein concentration.
and other environmental effects on the shoot : root dry weight ratio (S:R) of higher plants are often related mechanistically to changes in shoot protein concentration.
• Methods The literature on environmental effects on S:R is reviewed, focusing on relationships between S:R, growth and leaf  and protein concentrations. A series of experiments carried out to test the proposal that S:R is dependent on shoot protein concentration is highlighted and new data are presented for tobacco (Nicotiana tabacum).
and protein concentrations. A series of experiments carried out to test the proposal that S:R is dependent on shoot protein concentration is highlighted and new data are presented for tobacco (Nicotiana tabacum).
• Key Results/Evidence Results from the literature and new data for tobacco show that S:R and leaf  concentration are not significantly correlated over a range of environmental conditions. A mechanism involving the relative availability of C and N substrates for growth in shoots can explain how shoot protein concentration can influence shoot growth and hence root growth and S:R. Generally, results in the literature are compatible with the hypothesis that macronutrients, water, irradiance and CO2 affect S:R through changes in shoot protein concentration. In detailed studies on several species, including tobacco, a linear regression model incorporating leaf soluble protein concentration and plant dry weight could explain the greater proportion of the variation in S:R within and between treatments over a wide range of conditions.
concentration are not significantly correlated over a range of environmental conditions. A mechanism involving the relative availability of C and N substrates for growth in shoots can explain how shoot protein concentration can influence shoot growth and hence root growth and S:R. Generally, results in the literature are compatible with the hypothesis that macronutrients, water, irradiance and CO2 affect S:R through changes in shoot protein concentration. In detailed studies on several species, including tobacco, a linear regression model incorporating leaf soluble protein concentration and plant dry weight could explain the greater proportion of the variation in S:R within and between treatments over a wide range of conditions.
• Conclusions It is concluded that if  can influence the S:R of higher plants, it does so only over a narrow range of conditions. Evidence is strong that environmental effects on S:R are often related mechanistically to their effects on shoot protein concentration.
can influence the S:R of higher plants, it does so only over a narrow range of conditions. Evidence is strong that environmental effects on S:R are often related mechanistically to their effects on shoot protein concentration.
Keywords: Dry matter partitioning, nitrate signalling, nitrogen, protein, Nicotiana tabacum, tobacco, shoot : root ratio
INTRODUCTION
Changes in irradiance level, photoperiod and supply of CO2, water and inorganic nutrients can affect the partitioning of dry matter between the shoot and root of higher plants (Andrews et al., 2001; Raven et al., 2005). Consistently, the shoot : root dry weight ratio (S:R) increases with increased 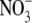 supply over the range likely to occur in natural and agricultural soils and it is stated in many recent publications that
supply over the range likely to occur in natural and agricultural soils and it is stated in many recent publications that 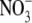 acts as a signal to regulate dry matter partitioning between the shoot and root of higher plants (e.g. Forde, 2002; Kruse et al., 2002; Foyer et al., 2003; Santi et al., 2003; Scheible et al., 2004). This response of S:R to
acts as a signal to regulate dry matter partitioning between the shoot and root of higher plants (e.g. Forde, 2002; Kruse et al., 2002; Foyer et al., 2003; Santi et al., 2003; Scheible et al., 2004). This response of S:R to 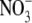 is viewed by some as one of several
is viewed by some as one of several 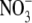 -specific effects which contribute to the regulation of plant metabolism and plant architecture (Stitt and Scheible, 1999; Forde, 2002; Scheible et al., 2004).
-specific effects which contribute to the regulation of plant metabolism and plant architecture (Stitt and Scheible, 1999; Forde, 2002; Scheible et al., 2004).
Here, firstly, we challenge the hypothesis that 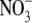 acts as a signal to regulate S:R. Secondly, we present evidence for the viewpoint that
acts as a signal to regulate S:R. Secondly, we present evidence for the viewpoint that 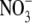 and other environmental effects on S:R of higher plants, are often related mechanistically to changes in shoot protein concentration. Results from the literature and new data for tobacco (Nicotiana tabacum) are utilized in our discussion.
and other environmental effects on S:R of higher plants, are often related mechanistically to changes in shoot protein concentration. Results from the literature and new data for tobacco (Nicotiana tabacum) are utilized in our discussion.
MATERIALS AND METHODS
The initial and repeat experiments were carried out between 1–2 Sep. and 23–24 Oct. and 8–9 Sep. and 30–31 Oct. 2004 in a glasshouse under natural daylight at the University of Sunderland. The temperature was maintained above 15 °C, day and night. Seeds of Nicotiana tabacum L. ‘Petit Havana SR1’ were germinated in sieved John Innes seed compost (John Innes Manufacturers Association, Harrogate, UK) in the glasshouse. After 2–3 weeks, seedlings of approximately equal size were transferred to liquid culture and the different nutrient and irradiance treatments imposed. The treatments were complete nutrient solution (control) and low N, P, S, K and Mg as described in Andrews et al. (1999); low irradiance (6 % open ground PAR, complete nutrient medium) as described in Andrews et al. (2005); and different N forms, where 4 mol m−3 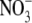 in the complete nutrient solution was replaced with 0·5 mol m−3 urea, 0·5 mol m−3 glutamine or 0·5 mol m−3 NH4NO3. In all treatments, concentrations of all macronutrients except the deficient nutrient were made equal to those in the control by the addition of the appropriate Na or Cl salt as required (Andrews et al., 1999). Plants were harvested at the onset of flowering and leaf soluble protein and NO3-concentrations and shoot and root dry weight were determined as described in Andrews et al. (1999, 2005). Both experiments were of completely randomized design with three replicates for all treatments. Data from the two experiments were pooled for statistical analysis and presentation using one-way analysis of variance with nutrient/irradiance treatment as the variable. Linear and quadratic regression analysis was carried out on S:R v plant dry weight, leaf soluble protein concentration and leaf
in the complete nutrient solution was replaced with 0·5 mol m−3 urea, 0·5 mol m−3 glutamine or 0·5 mol m−3 NH4NO3. In all treatments, concentrations of all macronutrients except the deficient nutrient were made equal to those in the control by the addition of the appropriate Na or Cl salt as required (Andrews et al., 1999). Plants were harvested at the onset of flowering and leaf soluble protein and NO3-concentrations and shoot and root dry weight were determined as described in Andrews et al. (1999, 2005). Both experiments were of completely randomized design with three replicates for all treatments. Data from the two experiments were pooled for statistical analysis and presentation using one-way analysis of variance with nutrient/irradiance treatment as the variable. Linear and quadratic regression analysis was carried out on S:R v plant dry weight, leaf soluble protein concentration and leaf 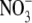 concentration. Variability quoted in the text is the standard error. Before giving these experimental data, evidence from the literature will be reviewed and discussed.
concentration. Variability quoted in the text is the standard error. Before giving these experimental data, evidence from the literature will be reviewed and discussed.
LEAF 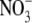 REGULATES S:R: LIMITATIONS
REGULATES S:R: LIMITATIONS
The assertion that 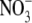 regulates S:R is primarily based on results from studies on mutants and transformants of tobacco with decreased expression of nitrate reductase (NR). In particular, a highly significant positive correlation between leaf
regulates S:R is primarily based on results from studies on mutants and transformants of tobacco with decreased expression of nitrate reductase (NR). In particular, a highly significant positive correlation between leaf 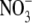 content and S:R was found for eight different genotypes growing at a wide range of
content and S:R was found for eight different genotypes growing at a wide range of 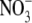 supply (Scheible et al., 1997). However, plants can take up and utilize a range of forms of N, and S:R increases with N supply regardless of N form (Andrews et al., 1985, 1999, 2001, 2004a, b). There is evidence that nitrification of reduced N can occur within shoot tissue of some species (Watt and Cresswell, 1987; Hipkin et al., 2004) and, although levels of
supply (Scheible et al., 1997). However, plants can take up and utilize a range of forms of N, and S:R increases with N supply regardless of N form (Andrews et al., 1985, 1999, 2001, 2004a, b). There is evidence that nitrification of reduced N can occur within shoot tissue of some species (Watt and Cresswell, 1987; Hipkin et al., 2004) and, although levels of 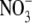 produced are likely to be low, it cannot be discounted that they are high enough to act as a ‘signal’ in some processes such as stomatal closure (Raven, 2003). However, it is stressed that S:R changes with
produced are likely to be low, it cannot be discounted that they are high enough to act as a ‘signal’ in some processes such as stomatal closure (Raven, 2003). However, it is stressed that S:R changes with 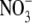 supply over the range which affects growth (dry matter production). Indeed, there are several reports that S:R increases with increased
supply over the range which affects growth (dry matter production). Indeed, there are several reports that S:R increases with increased 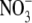 supply above that which gives maximum growth: this effect is associated with increased tissue reduced N (total N –
supply above that which gives maximum growth: this effect is associated with increased tissue reduced N (total N – 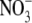 /NO2 – N) concentration (Andrews et al., 1999). For example, for common bean (Phaseolus vulgaris), plant dry weight increased with increased
/NO2 – N) concentration (Andrews et al., 1999). For example, for common bean (Phaseolus vulgaris), plant dry weight increased with increased 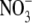 supply from 0·5 to 4–6 mol m−3, then decreased with a further increase in
supply from 0·5 to 4–6 mol m−3, then decreased with a further increase in 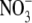 supply to 10 mol m−3, but S:R and plant reduced-N content increased with increased
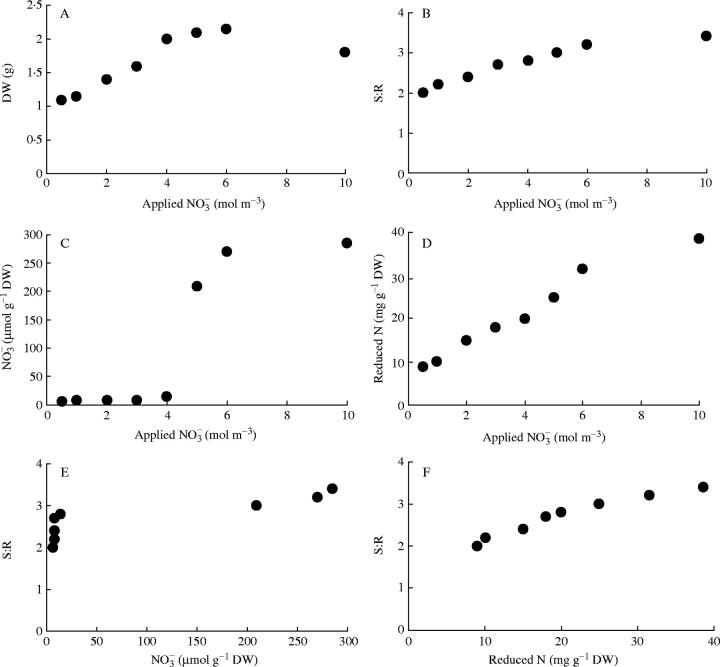
supply to 10 mol m−3, but S:R and plant reduced-N content increased with increased 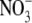 supply throughout (Fig. 1). There was a strong positive correlation between S:R and tissue reduced N concentration (r=0·97, P < 0·0001). Leaf
supply throughout (Fig. 1). There was a strong positive correlation between S:R and tissue reduced N concentration (r=0·97, P < 0·0001). Leaf 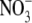 concentration differed to S:R in its response to
concentration differed to S:R in its response to 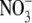 supply and ranged from 6 to 14 µmol g−1 d. wt at 0·5–4 mol m−3
supply and ranged from 6 to 14 µmol g−1 d. wt at 0·5–4 mol m−3 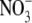 then increased 20-fold with increased
then increased 20-fold with increased 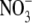 supply from 4·0 to 6·0 mol m−3, the range of
supply from 4·0 to 6·0 mol m−3, the range of 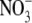 supply where growth reached a maximum (Fig. 1). There are reports in the literature, for several other species, that leaf
supply where growth reached a maximum (Fig. 1). There are reports in the literature, for several other species, that leaf 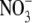 concentrations are low and change little with increased leaf
concentrations are low and change little with increased leaf  supply until maximum growth is reached (Khamis and Lamaze, 1990; Zhen and Leigh, 1990; Dastgheib et al., 1995), although this is not always the case (Andrews et al., 1992).
supply until maximum growth is reached (Khamis and Lamaze, 1990; Zhen and Leigh, 1990; Dastgheib et al., 1995), although this is not always the case (Andrews et al., 1992).
Fig. 1.
The effect of different applied 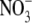 concentrations on total plant dry weight (DW), shoot to root dry weight ratio (S:R), leaf
concentrations on total plant dry weight (DW), shoot to root dry weight ratio (S:R), leaf 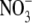 concentration and total plant reduced N concentration of common bean. Taken from Andrews et al. (1995).
concentration and total plant reduced N concentration of common bean. Taken from Andrews et al. (1995).
In the study of Scheible et al. (1997), the major proportion of the change in S:R of tobacco was associated with exceptionally high leaf 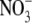 concentrations, which would rarely occur under natural or agricultural conditions. Specifically, values for S:R ranged from around 2 to 10, with those above 3·5 associated with leaf
concentrations, which would rarely occur under natural or agricultural conditions. Specifically, values for S:R ranged from around 2 to 10, with those above 3·5 associated with leaf 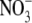 concentrations of 500–3000 µmol g−1 d. wt. Also, there were deviations from the strong relationship between leaf
concentrations of 500–3000 µmol g−1 d. wt. Also, there were deviations from the strong relationship between leaf 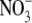 content and S:R at low leaf
content and S:R at low leaf 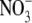 concentrations. Plants that were grown on low
concentrations. Plants that were grown on low 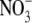 had S:R values that lay below the regression line, while plants that were grown on
had S:R values that lay below the regression line, while plants that were grown on 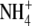 alone or NH4NO3 had S:R values above the regression line. In addition, the relationship between leaf
alone or NH4NO3 had S:R values above the regression line. In addition, the relationship between leaf 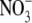 content and S:R did not hold under P deficiency. In relation to
content and S:R did not hold under P deficiency. In relation to 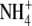 nutrition, it was suggested that discrepancies might be due to a restriction of root growth as a result of acidification; competition between root growth and
nutrition, it was suggested that discrepancies might be due to a restriction of root growth as a result of acidification; competition between root growth and 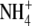 assimilation in the roots or a separate signal from
assimilation in the roots or a separate signal from 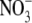 deficiency that is generated in N metabolism. It was argued that P deficiency acts via a separate signal from
deficiency that is generated in N metabolism. It was argued that P deficiency acts via a separate signal from 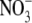 deficiency. Subsequent work on NR transformants of tobacco has shown that the relationship between S:R and leaf
deficiency. Subsequent work on NR transformants of tobacco has shown that the relationship between S:R and leaf 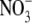 concentration does not hold at a twice ambient CO2 concentration (Kruse et al., 2002).
concentration does not hold at a twice ambient CO2 concentration (Kruse et al., 2002).
Generally, 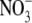 levels in plant tissues are positively related to
levels in plant tissues are positively related to  uptake but this need not necessarily be the case. It has been suggested that it is the influx of
uptake but this need not necessarily be the case. It has been suggested that it is the influx of 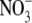 into the shoot, or xylem loading of
into the shoot, or xylem loading of 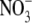 which determines S:R (Stitt and Krapp, 1999; Kruse et al., 2002). However, neither of these hypotheses can explain the effects of different N forms on S:R. Also, in the study of Kruse et al. (2002), S:R and xylem sap
which determines S:R (Stitt and Krapp, 1999; Kruse et al., 2002). However, neither of these hypotheses can explain the effects of different N forms on S:R. Also, in the study of Kruse et al. (2002), S:R and xylem sap 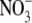 concentrations were not significantly correlated across genotype and CO2 concentrations. New data for tobacco, presented and discussed below, further emphasize the limitations of the proposal that leaf
concentrations were not significantly correlated across genotype and CO2 concentrations. New data for tobacco, presented and discussed below, further emphasize the limitations of the proposal that leaf 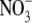 concentration regulates S:R (Table 1).
concentration regulates S:R (Table 1).
Table 1.
Shoot : root dry weight ratio (S:R), total plant dry weight and leaf soluble protein and 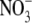 concentrations for tobacco supplied basal nutrient solution (control,
concentrations for tobacco supplied basal nutrient solution (control, 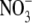 as N source); basal nutrient solution deficient in N, P, S, K or Mg; basal nutrient solution with
as N source); basal nutrient solution deficient in N, P, S, K or Mg; basal nutrient solution with 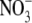 replaced with urea, glutamine (Gln) or NH4NO3 as N source and basal nutrient solution under low irradiance (Ir)
replaced with urea, glutamine (Gln) or NH4NO3 as N source and basal nutrient solution under low irradiance (Ir)
| S:R | Dry weight | Protein (mg g−1 d. wt) |
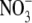 (µmol g−1 d. wt) (µmol g−1 d. wt) |
|
|---|---|---|---|---|
| Low Mg | 7·02 | 3·60 | 211 | 540 ± 36 |
| Control | 6·79 | 5·36 | 170 | 112 ± 3 |
| Low K | 6·26 | 1·38 | 140 | 409 ± 18 |
| Low Ir | 5·92 | 0·82 | 129 | 1801 ± 87 |
| NH4NO3 | 4·51 | 2·23 | 79·8 | 59 ± 5 |
| Gln | 4·37 | 1·08 | 97·7 | 4 ± 1 |
| Low S | 4·24 | 3·47 | 74·8 | 159 ± 18 |
| Low P | 3·47 | 2·61 | 108 | 411 ± 14 |
| Low N | 2·58 | 1·58 | 34·3 | 21 ± 4 |
| Urea | 2·48 | 0·97 | 35·5 | 4 ± 1 |
| LSD | 0·275 | 0·412 | 13·48 |
Variability quoted for 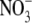 concentration is the standard error.
concentration is the standard error.
VIEWPOINT: N AND OTHER RESOURCES AFFECT S:R THROUGH EFFECTS ON SHOOT PROTEIN
The mechanism
Various mechanisms other than 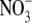 signalling have been proposed to explain the N effect on S:R (Bastow-Wilson, 1988; Andrews et al., 1999, 2001). Bastow-Wilson (1988) reviewed models for the control of S:R and concluded that changes in S:R in response to deficits of macronutrients, water, irradiance and CO2 usually conform to the Thornley (1972) model. In this model, the factors that determine S:R are the supply of C and N substrates by the shoot and root, respectively, transport of these substrates between shoot and root and their incorporation into plant structure. It was argued that structural growth of shoot and root is co-limited by the local C and N substrate concentrations and that this growth acts as a sink for substrates to which further substrates diffuse from the points of supply. It was further argued that the rate of transport of C and N substrate from shoot to root and root to shoot, respectively, is proportional to the concentration gradient divided by a resistance. Hence, a decrease in C substrate acquisition would result in an increase in S:R while a decrease in N substrate acquisition would cause S:R to decrease. A weakness of this model is that, although there is strong evidence that transport of C from shoot to root is driven by a concentration gradient of C substrate, N transport from root to shoot occurs primarily via mass flow through the xylem, driven by transpiration (Pate, 1980; Dewar, 1993).
signalling have been proposed to explain the N effect on S:R (Bastow-Wilson, 1988; Andrews et al., 1999, 2001). Bastow-Wilson (1988) reviewed models for the control of S:R and concluded that changes in S:R in response to deficits of macronutrients, water, irradiance and CO2 usually conform to the Thornley (1972) model. In this model, the factors that determine S:R are the supply of C and N substrates by the shoot and root, respectively, transport of these substrates between shoot and root and their incorporation into plant structure. It was argued that structural growth of shoot and root is co-limited by the local C and N substrate concentrations and that this growth acts as a sink for substrates to which further substrates diffuse from the points of supply. It was further argued that the rate of transport of C and N substrate from shoot to root and root to shoot, respectively, is proportional to the concentration gradient divided by a resistance. Hence, a decrease in C substrate acquisition would result in an increase in S:R while a decrease in N substrate acquisition would cause S:R to decrease. A weakness of this model is that, although there is strong evidence that transport of C from shoot to root is driven by a concentration gradient of C substrate, N transport from root to shoot occurs primarily via mass flow through the xylem, driven by transpiration (Pate, 1980; Dewar, 1993).
Dewar (1993) developed the Thornley (1972) model such that a fraction of the N taken up by the root is translocated in the xylem transpiration stream from the root to the shoot where it is transferred laterally to the shoot phloem. The remaining fraction of the N taken up is transferred directly to the root phloem. Also, a fraction of the N translocated to the shoot is subsequently translocated back to the root in the phloem at a rate determined by the shoot to root gradient of labile C in accordance with the Münch pressure flow mechanism. Shoot and root growth rates are considered to be functions of local water potentials and labile C and N concentrations. It is assumed that the plant water balance is in instantaneous equilibrium for given values of shoot and root structure, so that the rate of shoot transpiration is equal to the rate of uptake of water by the root. Shoot and root water potential are calculated directly in terms of shoot and root dry matter and the rate of transpiration. Similarly, the proportion of N taken up that is allocated to the shoot is in direct proportion to the fraction of plant biomass contained in the shoot. Shoot N substrate is carried to the root as phloem translocate at a rate determined by the gradient of C substrate concentration. It was argued that as long as the fraction of N taken up that is transported in the xylem to the shoot is less than, or equal to, the shoot fraction then the Münch pressure flow mechanism of phloem translocation would always ensure that there is a higher labile N concentration in the root than in the shoot, opposite to the concentration gradient of labile C. The Dewar model (Dewar, 1993), as with that of Thornley (1972), relies on the existence of a gradient of N substrate between the root and shoot with the N substrate concentration greater in root than shoot. This will not always be the case. For example, although under 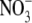 nutrition, the root is the main site of
nutrition, the root is the main site of 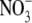 uptake, the site of
uptake, the site of 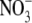 assimilation (production of amino acids) is the source of N that will be used for growth. Considerable data indicate that for many higher plants, the shoot is the main site of
assimilation (production of amino acids) is the source of N that will be used for growth. Considerable data indicate that for many higher plants, the shoot is the main site of 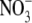 assimilation at low and high external
assimilation at low and high external 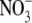 concentrations (Andrews, 1986). Also, typically, there is little
concentrations (Andrews, 1986). Also, typically, there is little 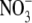 transported in the phloem (Pate, 1980; Andrews et al., 2004a). The Thornley and Dewar models (Thornley, 1972; Dewar, 1993) cannot explain a decrease in S:R, with decreased
transported in the phloem (Pate, 1980; Andrews et al., 2004a). The Thornley and Dewar models (Thornley, 1972; Dewar, 1993) cannot explain a decrease in S:R, with decreased 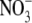 supply, for species which have the shoot as their main site of
supply, for species which have the shoot as their main site of 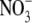 assimilation at low and high
assimilation at low and high 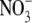 supply. Nevertheless, generally, predictions made from the Thornley/Dewar models, relating S:R to the relative availability of C and N substrate for growth and empirical/functional equilibrium/optimization models relating S:R to tissue N concentration, are in good agreement with experimental data (e.g. Ågren and Ingestad, 1987; Levin et al., 1989; Hilbert and Reynolds, 1991; Gleeson, 1993; Ågren and Franklin, 2003). However, as discussed below, across N form or different macronutrient treatments, S:R is more closely correlated with leaf soluble protein concentration than with leaf, shoot or plant N concentration.
supply. Nevertheless, generally, predictions made from the Thornley/Dewar models, relating S:R to the relative availability of C and N substrate for growth and empirical/functional equilibrium/optimization models relating S:R to tissue N concentration, are in good agreement with experimental data (e.g. Ågren and Ingestad, 1987; Levin et al., 1989; Hilbert and Reynolds, 1991; Gleeson, 1993; Ågren and Franklin, 2003). However, as discussed below, across N form or different macronutrient treatments, S:R is more closely correlated with leaf soluble protein concentration than with leaf, shoot or plant N concentration.
In a solid substrate (e.g. soil, sand, vermiculite/perlite), the growth rate for a range of plant species increased with increased 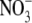 supply, from a very low value at 0·1 mol m−3 applied
supply, from a very low value at 0·1 mol m−3 applied 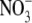 to a maximum in the range 1–5 mol m−3
to a maximum in the range 1–5 mol m−3 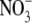 , then changed little or decreased with increased
, then changed little or decreased with increased 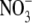 supply to 20 mol m−3, whilst tissue N concentration and S:R increased with increased
supply to 20 mol m−3, whilst tissue N concentration and S:R increased with increased 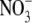 throughout (Andrews et al., 2001; Fig. 1). For several species grown on
throughout (Andrews et al., 2001; Fig. 1). For several species grown on 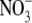 , a significant, a positive linear relationship was found between S:R and whole plant or shoot N concentration per unit dry weight. There are reports that for plants of similar dry weight, S:R is greater with
, a significant, a positive linear relationship was found between S:R and whole plant or shoot N concentration per unit dry weight. There are reports that for plants of similar dry weight, S:R is greater with 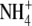 than with
than with 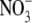 as N source (Scheible et al., 1997; Andrews et al., 2001, and references therein). However, where tested, the tissue N concentration for plants of similar dry weight was also greater with
as N source (Scheible et al., 1997; Andrews et al., 2001, and references therein). However, where tested, the tissue N concentration for plants of similar dry weight was also greater with 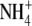 than with
than with 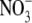 . For barley (Hordeum vulgare) and non-N2-fixing common bean (Phaseolus vulgaris), the relationship between S:R and plant N concentration was similar with
. For barley (Hordeum vulgare) and non-N2-fixing common bean (Phaseolus vulgaris), the relationship between S:R and plant N concentration was similar with 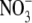 or
or 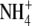 as N source (Andrews et al., 1999). However, for non-N2-fixing pea (Pisum sativum) there was a strong positive correlation between S:R and plant and shoot N concentration with
as N source (Andrews et al., 1999). However, for non-N2-fixing pea (Pisum sativum) there was a strong positive correlation between S:R and plant and shoot N concentration with 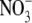 or
or 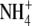 as N source, but the relationships between S:R and plant and shoot N concentration were substantially different with the two N forms (Andrews et al., 1999). For pea, the relationship between S:R and leaf soluble protein concentration was similar with the two N forms.
as N source, but the relationships between S:R and plant and shoot N concentration were substantially different with the two N forms (Andrews et al., 1999). For pea, the relationship between S:R and leaf soluble protein concentration was similar with the two N forms.
There are several reports for grain legumes that S:R is greater for N2-fixing plants than for 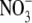 -fed plants of similar dry weight; this difference was related to an increased sink for photosynthate imposed by the nodules (Marschner, 1995). Andrews et al. (2004b) examined the relationships between S:R, growth and leaf soluble protein concentrations for pea inoculated with Rhizobium leguminosarum and supplied with low (0·5 mol m−3)
-fed plants of similar dry weight; this difference was related to an increased sink for photosynthate imposed by the nodules (Marschner, 1995). Andrews et al. (2004b) examined the relationships between S:R, growth and leaf soluble protein concentrations for pea inoculated with Rhizobium leguminosarum and supplied with low (0·5 mol m−3)  and uninoculated plants supplied with a range of
and uninoculated plants supplied with a range of 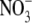 concentrations from 0·5 to 10 mol m−3. Inoculation and increased
concentrations from 0·5 to 10 mol m−3. Inoculation and increased 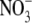 supply to 4 mol m−3, resulted in increases in S:R, growth and leaf soluble protein concentration. S:R and leaf protein concentration were as great for inoculated plants as for plants on 4 mol m−3
supply to 4 mol m−3, resulted in increases in S:R, growth and leaf soluble protein concentration. S:R and leaf protein concentration were as great for inoculated plants as for plants on 4 mol m−3 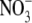 supply, although plant dry weight was 55 % greater with the
supply, although plant dry weight was 55 % greater with the 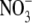 treatment. A linear regression model incorporating leaf soluble protein concentration and plant dry weight could explain 78 % of the variation in S:R of plants within and between the inoculated and uninoculated plant treatments. Omission of data for the inoculated plants from this analysis reduced this value by 2 % to 76 %. Thus, if there is a nodulation-specific effect on S:R, it appears to be insignificant outside the effects of nodulation on leaf protein concentration and growth.
treatment. A linear regression model incorporating leaf soluble protein concentration and plant dry weight could explain 78 % of the variation in S:R of plants within and between the inoculated and uninoculated plant treatments. Omission of data for the inoculated plants from this analysis reduced this value by 2 % to 76 %. Thus, if there is a nodulation-specific effect on S:R, it appears to be insignificant outside the effects of nodulation on leaf protein concentration and growth.
Our view is that the increase in S:R with increased N supply, regardless of its effect on growth (but excluding 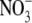 or
or 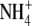 toxicity effects), is due to an increase in N relative to C substrate for shoot growth in conjunction with the proximity of the shoot to the C supply. Specifically, increased N supply results in increases in N uptake, N assimilation and tissue organic N concentration. The increase in organic N concentration is likely to be due to increases in a range of N-containing molecules, but mainly amino acids, soluble protein and insoluble membrane-bound proteins with the relative proportions of each dependent on environmental conditions (Millard, 1988; Evans and Seemann, 1989; Andrews et al., 1999). Nitrogen uptake, N assimilation and protein synthesis are energy-requiring processes, hence the increase in organic N concentration reflects an increased proportion of energy/C derived from photosynthesis being utilized in processing N. However, N is a component of chlorophyll and photosynthetic enzymes and hence can influence photosynthesis greatly (Lawlor, 2002). If increased processing of N results in increased photosynthate available for growth, shoot dry weight will increase relative to root dry weight due to proximity of the shoot to the C source and increased organic N available for growth. Also, if growth increases, part of the N effect on S:R may be a growth/ontogenetic effect, although for several species under steady-state nutrition (constant internal N and constant relative growth rate), S:R was found to remain constant at a value dependent on tissue N concentration (Ågren and Ingestad, 1987; Ingestad and Ågren, 1991; Ågren and Franklin, 2003). Thus growth-related changes in S:R may be due to how nutrients are applied over time. Possible effects of growth on S:R need further testing. Nitrogen productivity (C gain per unit N per unit time) decreases with increased organic N concentration. If organic N concentration increases but the photosynthate available for growth changes little or decreases, S:R will still increase as again the shoot will realize a greater proportion of its growth potential due to its proximity to the source of C and the availability of reduced N for growth. It is proposed that shoot protein concentration is of particular importance as this reflects the availability of N substrate and N catalyst for shoot growth (Andrews et al., 1999, 2001). This hypothesis is independent of the form of N nutrition and the site of N assimilation and is similar to the Thornley model (Thornley, 1972), in that structural growth is co-limited by local C and N substrate concentrations and C transport from shoot to root is driven by a concentration gradient of C substrate, but it does not rely on a gradient of N between root and shoot. It is our view that, as for N, other environmental effects on S:R are often primarily mediated through effects on leaf protein concentration and hence shoot and then plant growth. The evidence for this hypothesis is now discussed.
toxicity effects), is due to an increase in N relative to C substrate for shoot growth in conjunction with the proximity of the shoot to the C supply. Specifically, increased N supply results in increases in N uptake, N assimilation and tissue organic N concentration. The increase in organic N concentration is likely to be due to increases in a range of N-containing molecules, but mainly amino acids, soluble protein and insoluble membrane-bound proteins with the relative proportions of each dependent on environmental conditions (Millard, 1988; Evans and Seemann, 1989; Andrews et al., 1999). Nitrogen uptake, N assimilation and protein synthesis are energy-requiring processes, hence the increase in organic N concentration reflects an increased proportion of energy/C derived from photosynthesis being utilized in processing N. However, N is a component of chlorophyll and photosynthetic enzymes and hence can influence photosynthesis greatly (Lawlor, 2002). If increased processing of N results in increased photosynthate available for growth, shoot dry weight will increase relative to root dry weight due to proximity of the shoot to the C source and increased organic N available for growth. Also, if growth increases, part of the N effect on S:R may be a growth/ontogenetic effect, although for several species under steady-state nutrition (constant internal N and constant relative growth rate), S:R was found to remain constant at a value dependent on tissue N concentration (Ågren and Ingestad, 1987; Ingestad and Ågren, 1991; Ågren and Franklin, 2003). Thus growth-related changes in S:R may be due to how nutrients are applied over time. Possible effects of growth on S:R need further testing. Nitrogen productivity (C gain per unit N per unit time) decreases with increased organic N concentration. If organic N concentration increases but the photosynthate available for growth changes little or decreases, S:R will still increase as again the shoot will realize a greater proportion of its growth potential due to its proximity to the source of C and the availability of reduced N for growth. It is proposed that shoot protein concentration is of particular importance as this reflects the availability of N substrate and N catalyst for shoot growth (Andrews et al., 1999, 2001). This hypothesis is independent of the form of N nutrition and the site of N assimilation and is similar to the Thornley model (Thornley, 1972), in that structural growth is co-limited by local C and N substrate concentrations and C transport from shoot to root is driven by a concentration gradient of C substrate, but it does not rely on a gradient of N between root and shoot. It is our view that, as for N, other environmental effects on S:R are often primarily mediated through effects on leaf protein concentration and hence shoot and then plant growth. The evidence for this hypothesis is now discussed.
The evidence: literature on root-acquired resources
A series of studies has been carried out to test the proposal that root-acquired resources affect S:R through effects on shoot protein concentration; leaf soluble protein concentration was used as a measure of shoot protein status. If this proposal is correct, then across different environmental variables, there should be a positive correlation between S:R and shoot protein concentration. Andrews et al. (1999) examined relationships between S:R, total plant dry weight, shoot and plant N concentration and leaf soluble protein concentration for pea, common bean and wheat (Triticum aestivum) under different nutrient deficiencies. The effect of nutrient deficiency on S:R was dependent on plant species, specific nutrient and experiment. For example, for all species, in all experiments, S:R decreased with decreased N or P supply while, for Mg deficiency, S:R consistently increased substantially with pea or bean but did not change or decreased with wheat, depending on the experiment. However, despite these differences, a linear regression model incorporating leaf soluble protein concentration and plant dry weight could explain >80 % of the variation in S:R within and between treatments for pea supplied with different concentrations of 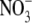 or
or 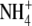 , pea and common bean supplied with different concentrations of N, P, K and Mg, and wheat supplied with different concentrations of N, P, K, Mg, Ca and S. Similarly for annual ryegrass (Lolium multiflorum) in a separate study, in which S:R decreased under N, P or S deficiency but increased under Mg, K or Ca deficiency, or when
, pea and common bean supplied with different concentrations of N, P, K and Mg, and wheat supplied with different concentrations of N, P, K, Mg, Ca and S. Similarly for annual ryegrass (Lolium multiflorum) in a separate study, in which S:R decreased under N, P or S deficiency but increased under Mg, K or Ca deficiency, or when 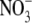 was replaced by
was replaced by 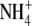 in the complete nutrient medium, a linear regression model incorporating leaf soluble protein concentration could explain 84 % of the variation in S:R within and across treatments (Andrews et al., 2001). In the study of Andrews et al. (1999), the relationship between S:R and leaf soluble protein concentration was, generally, much stronger than that between S:R and leaf N, shoot N or plant N concentration. This indicates that leaf soluble protein concentration is more important than overall plant N status in determining S:R.
in the complete nutrient medium, a linear regression model incorporating leaf soluble protein concentration could explain 84 % of the variation in S:R within and across treatments (Andrews et al., 2001). In the study of Andrews et al. (1999), the relationship between S:R and leaf soluble protein concentration was, generally, much stronger than that between S:R and leaf N, shoot N or plant N concentration. This indicates that leaf soluble protein concentration is more important than overall plant N status in determining S:R.
Generally, S:R increases with increased water supply over the range which causes increased growth (McDonald and Davies, 1996; Andrews et al., 2001). Often this response is likely to have been at least in part a growth/development effect but there are reports for many species that protein synthesis decreases under limiting water supply (Lawlor and Cornic, 2002), thus water could act on S:R via its effect on protein synthesis as well as growth. When tested, results obtained were consistent with this proposal (Andrews et al., 2001). For example, for Himalayan balsam (Impatiens glandulifera) supplied with 0·05–0·4 ml water g−1 substrate, plant dry weight increased with water supply to 0·25 ml g−1 substrate and then decreased with increased water supply thereafter (Andrews et al., 2001). Here, S:R and leaf protein concentration changed little with increased water supply to 0·15 ml g−1 substrate, then decreased steadily with increased water supply thereafter. A linear regression model using leaf soluble protein concentration could explain 84 % of the variation in S:R across water treatments. Thus, results are consistent with the proposal that N form, macronutrient, and water effects on S:R are often primarily mediated through their effects on protein synthesis and growth. Leaf 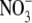 concentration was not measured in these studies but for pea and annual ryegrass supplied with different concentrations of
concentration was not measured in these studies but for pea and annual ryegrass supplied with different concentrations of 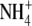 or
or 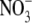 (Andrews et al., 1999, 2001) and inoculated and uninoculated pea (Andrews et al., 2004b), it seems unlikely that S:R and leaf
(Andrews et al., 1999, 2001) and inoculated and uninoculated pea (Andrews et al., 2004b), it seems unlikely that S:R and leaf 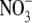 concentration would have been strongly correlated.
concentration would have been strongly correlated.
The evidence: literature on shoot-acquired resources
Often, but not invariably, the S:R and leaf protein concentration per unit dry weight of higher plants decrease with the increased growth associated with increased irradiance level or photoperiod (Andrews et al., 2001, and references therein). Thus irradiance could affect S:R via its effect on shoot protein concentration in accordance with our hypothesis. Detailed studies on irradiance effects on growth, S:R and tissue N and protein concentrations provide evidence that this is the case. For example, Ingestad and McDonald (1989) found that for birch (Betula pendula), dry weight increased but S:R and tissue N concentration decreased with increased irradiance over a wide range of 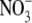 supply, and concluded that irradiance affected S:R to an extent corresponding to its effect on the N status of the plant. Also, for Tradescantia fluminensis supplied with 5 mol m−3
supply, and concluded that irradiance affected S:R to an extent corresponding to its effect on the N status of the plant. Also, for Tradescantia fluminensis supplied with 5 mol m−3 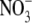 , plant dry weight increased with increased irradiance from 1 % to around 50 % relative irradiance (Ir; open ground irradiance = 100 % Ir), then changed little with increased irradiance thereafter (Maule et al., 1995). Here, the S:R and leaf soluble protein concentration increased sharply with increased irradiance to around 10 % Ir, then decreased steadily with increased irradiance to 50 % Ir. A linear regression model utilizing leaf soluble protein concentration and plant dry weight could explain 87 % of the variation in S:R across irradiance levels. Similarly, for Himalayan balsam supplied with 1 or 5 mol m−3
, plant dry weight increased with increased irradiance from 1 % to around 50 % relative irradiance (Ir; open ground irradiance = 100 % Ir), then changed little with increased irradiance thereafter (Maule et al., 1995). Here, the S:R and leaf soluble protein concentration increased sharply with increased irradiance to around 10 % Ir, then decreased steadily with increased irradiance to 50 % Ir. A linear regression model utilizing leaf soluble protein concentration and plant dry weight could explain 87 % of the variation in S:R across irradiance levels. Similarly, for Himalayan balsam supplied with 1 or 5 mol m−3 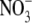 , at a range of relative irradiance levels (1–55 % Ir), plant dry weight increased with irradiance from 1 % to 8 % Ir and from 1 % to 28 % Ir at the lower and higher
, at a range of relative irradiance levels (1–55 % Ir), plant dry weight increased with irradiance from 1 % to 8 % Ir and from 1 % to 28 % Ir at the lower and higher 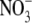 concentrations, respectively (Maule, 2000). In general, S:R decreased with increased irradiance throughout and at similar irradiance levels was greater at 5 than 1 mol m−3
concentrations, respectively (Maule, 2000). In general, S:R decreased with increased irradiance throughout and at similar irradiance levels was greater at 5 than 1 mol m−3 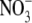 . The S:R was not significantly related to plant dry weight but was significantly related to leaf soluble protein concentration. A linear regression model incorporating leaf soluble protein concentration and plant dry weight could explain 60 % of the variation in S:R within and across treatments and 92 % of the variation across treatment means.
. The S:R was not significantly related to plant dry weight but was significantly related to leaf soluble protein concentration. A linear regression model incorporating leaf soluble protein concentration and plant dry weight could explain 60 % of the variation in S:R within and across treatments and 92 % of the variation across treatment means.
Generally, S:R changes little or decreases with increased growth associated with increased CO2 concentration (Stulen et al., 1998; Poorter and Nagel, 2000). We have not tested the relationships between CO2 supply, growth, S:R and leaf protein concentration but the available data are consistent with our proposal that CO2 affects S:R through effects on shoot protein concentration. Specifically, where tested, decreased S:R with increased CO2 was usually associated with decreased leaf N and/or protein concentration. It has been argued by several workers that CO2 affects S:R via its effect on N status and if nutrient supply is maintained at optimal level then S:R is little affected by CO2 supply (Stulen et al., 1998; Poorter and Nagel, 2000).
Increased S:R associated with decreased irradiance is likely to be associated with increased leaf 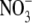 concentration but the magnitude of the increase in leaf
concentration but the magnitude of the increase in leaf 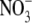 concentration in shade is often much greater than that associated with high
concentration in shade is often much greater than that associated with high 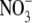 supply (Maule, 2000; Andrews et al., 2005; Table 1). The relationship between leaf
supply (Maule, 2000; Andrews et al., 2005; Table 1). The relationship between leaf 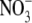 concentration and S:R does not hold for NR transformants of tobacco at twice ambient CO2 concentration (Kruse et al., 2002).
concentration and S:R does not hold for NR transformants of tobacco at twice ambient CO2 concentration (Kruse et al., 2002).
The evidence: new data for tobacco
Table 1 shows S:R, total plant dry weight and leaf soluble protein and 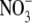 concentrations for tobacco under different nutrient deficiencies, low irradiance and when
concentrations for tobacco under different nutrient deficiencies, low irradiance and when 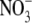 in the complete nutrient solution was replaced with other N forms; all measurements varied greatly depending on treatment (P < 0·001). In relation to the different N form treatments, the nutrient solutions used were not sterilized and there is likely to have been a degree of N transformation within the pots. Nevertheless, the data shown in Table 1 indicate major differences in relationships between total plant dry weight and leaf soluble protein and
in the complete nutrient solution was replaced with other N forms; all measurements varied greatly depending on treatment (P < 0·001). In relation to the different N form treatments, the nutrient solutions used were not sterilized and there is likely to have been a degree of N transformation within the pots. Nevertheless, the data shown in Table 1 indicate major differences in relationships between total plant dry weight and leaf soluble protein and 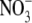 concentrations across N-form treatment which provides evidence that there were differences in the major form of N taken up and assimilated. For example, leaf soluble protein concentration was almost three times greater with glutamine than with low
concentrations across N-form treatment which provides evidence that there were differences in the major form of N taken up and assimilated. For example, leaf soluble protein concentration was almost three times greater with glutamine than with low 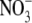 , but total plant dry weight was around 50 % greater with low
, but total plant dry weight was around 50 % greater with low 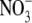 . Across all treatments, S:R was not significantly correlated with plant dry weight or leaf
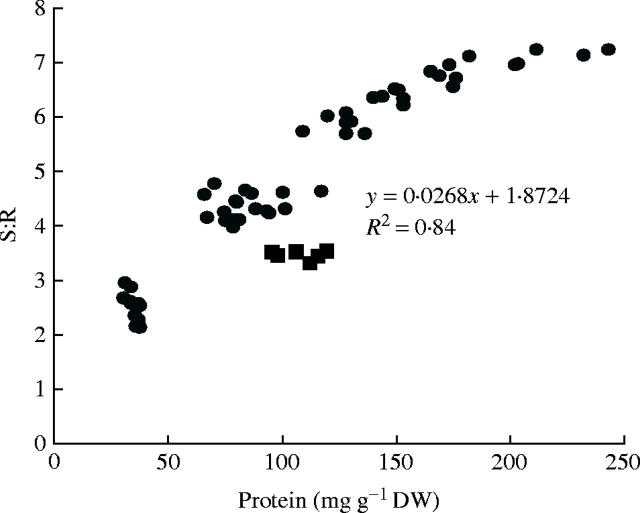
. Across all treatments, S:R was not significantly correlated with plant dry weight or leaf 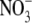 concentration but there was a strong positive relationship between S:R and leaf soluble protein concentration (Fig. 2). The linear component could explain 82 % of the variation in S:R within and across the treatments. Only the values for the low P treatment obviously fell outside this line. When the low P treatment values were omitted from the analysis, the linear component could explain 91 % of the variation in S:R within and across treatments, although there is an indication that the curve is ‘flattening off’ and a quadratic model gave an R2 value of 96 %. Such a strong relationship between S:R and leaf protein concentration over such a wide range of conditions is further evidence that leaf protein concentration often plays an important role in the regulation of S:R.
concentration but there was a strong positive relationship between S:R and leaf soluble protein concentration (Fig. 2). The linear component could explain 82 % of the variation in S:R within and across the treatments. Only the values for the low P treatment obviously fell outside this line. When the low P treatment values were omitted from the analysis, the linear component could explain 91 % of the variation in S:R within and across treatments, although there is an indication that the curve is ‘flattening off’ and a quadratic model gave an R2 value of 96 %. Such a strong relationship between S:R and leaf protein concentration over such a wide range of conditions is further evidence that leaf protein concentration often plays an important role in the regulation of S:R.
Fig. 2.
The relationship between S:R and leaf soluble protein concentration for tobacco under different nutrient and low irradiance conditions (see Table 1): replicate values are presented. Filled squares indicate values for P-deficient plants.
Scheible et al. (1997) reported that on high 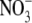 supply, tobacco transformants with very low NR activity (note this is with high S:R and leaf
supply, tobacco transformants with very low NR activity (note this is with high S:R and leaf 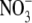 concentrations), had leaf protein concentrations similar to the
concentrations), had leaf protein concentrations similar to the 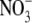 -limited wild type. This at first appears to be inconsistent with our proposal that
-limited wild type. This at first appears to be inconsistent with our proposal that 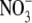 affects S:R through changes in shoot protein concentration. However, Scheible et al. (1997) presented protein concentrations on a fresh weight basis and it is likely that water content and hence protein per unit dry weight were substantially greater in the high
affects S:R through changes in shoot protein concentration. However, Scheible et al. (1997) presented protein concentrations on a fresh weight basis and it is likely that water content and hence protein per unit dry weight were substantially greater in the high 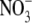 NR transformants than in the low
NR transformants than in the low 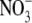 wild type due to the osmotic effect of
wild type due to the osmotic effect of 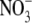 accumulation (Andrews et al., 2005). The finding of Scheible et al. (1997), that roots of tobacco transformants on high
accumulation (Andrews et al., 2005). The finding of Scheible et al. (1997), that roots of tobacco transformants on high 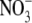 supply with lower
supply with lower 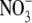 accumulation than in shoots contained high levels of protein, support this proposal. Also, although the tobacco transformants resembled the N-deficient wild type with respect to starch content, starch turnover and sugar levels when grown on low
accumulation than in shoots contained high levels of protein, support this proposal. Also, although the tobacco transformants resembled the N-deficient wild type with respect to starch content, starch turnover and sugar levels when grown on low 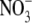 supply, they behaved differently on high
supply, they behaved differently on high 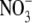 supply. Here, when
supply. Here, when 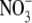 accumulated to high levels, the leaves contained much less starch and greater sugar concentrations than expected in an N-deficient plant. The potential magnitude of the effect of
accumulated to high levels, the leaves contained much less starch and greater sugar concentrations than expected in an N-deficient plant. The potential magnitude of the effect of 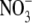 accumulation on the difference between protein levels per unit fresh weight, or dry weight, is highlighted using the data obtained for tobacco here. At low N and low irradiance, respectively, protein concentrations were 4·13±0·15 and 5·25 ± 0·19 mg g−1 f. wt leaf but, on a dry weight basis, values were almost four times greater with the low irradiance treatment (Table 1). Recent work that examined starch mobilization induced by
accumulation on the difference between protein levels per unit fresh weight, or dry weight, is highlighted using the data obtained for tobacco here. At low N and low irradiance, respectively, protein concentrations were 4·13±0·15 and 5·25 ± 0·19 mg g−1 f. wt leaf but, on a dry weight basis, values were almost four times greater with the low irradiance treatment (Table 1). Recent work that examined starch mobilization induced by 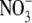 resupply to N-starvedArabidopsis plants found that this process was blocked in an NR-null mutant (Wang et al., 2004). As
resupply to N-starvedArabidopsis plants found that this process was blocked in an NR-null mutant (Wang et al., 2004). As 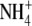 and glutamine induced starch mobilization in the wild type and mutant, it was concluded that
and glutamine induced starch mobilization in the wild type and mutant, it was concluded that 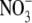 reduction was necessary for this process to occur. Wang et al. (2004) highlighted that their findings were not consistent with respect to the results reported by Scheible et al. (1997) for NR deficient tobacco, where starch mobilization was similar in the wild-type and mutant lines. It was proposed that residual
reduction was necessary for this process to occur. Wang et al. (2004) highlighted that their findings were not consistent with respect to the results reported by Scheible et al. (1997) for NR deficient tobacco, where starch mobilization was similar in the wild-type and mutant lines. It was proposed that residual 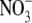 reduction in the NR-deficient tobacco accounted for the mobilization of starch in these experiments.
reduction in the NR-deficient tobacco accounted for the mobilization of starch in these experiments.
CONCLUSIONS
Results from the literature and new data for tobacco show that S:R and leaf 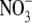 concentrations are not significantly correlated over a wide range of conditions. A mechanism involving the relative availability of C and N substrates for growth in shoots can explain how shoot protein concentration affects shoot growth and, hence, root growth and S:R. Generally, results in the literature are compatible with the hypothesis that macro-nutrients, water, irradiance and CO2 affect S:R through effects on shoot protein concentration. In detailed studies on several species, including tobacco, a linear regression model incorporating leaf soluble protein concentration and plant dry weight could explain the greater proportion of the variation in S:R within and between treatments over a wide range of conditions. It is concluded that evidence is strong that environmental effects on S:R are often related mechanistically to their effects on leaf protein concentration and not leaf
concentrations are not significantly correlated over a wide range of conditions. A mechanism involving the relative availability of C and N substrates for growth in shoots can explain how shoot protein concentration affects shoot growth and, hence, root growth and S:R. Generally, results in the literature are compatible with the hypothesis that macro-nutrients, water, irradiance and CO2 affect S:R through effects on shoot protein concentration. In detailed studies on several species, including tobacco, a linear regression model incorporating leaf soluble protein concentration and plant dry weight could explain the greater proportion of the variation in S:R within and between treatments over a wide range of conditions. It is concluded that evidence is strong that environmental effects on S:R are often related mechanistically to their effects on leaf protein concentration and not leaf 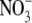 concentration. It is recommended that leaf protein concentration is measured in studies where environmental effects on dry matter partitioning are investigated.
concentration. It is recommended that leaf protein concentration is measured in studies where environmental effects on dry matter partitioning are investigated.
LITERATURE CITED
- Ågren GI, Franklin O. 2003. Root:shoot ratios, optimization and nitrogen productivity. Annals of Botany 92: 795–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ågren GI, Ingestad T. 1987. Root:shoot ratio as a balance between nitrogen productivity and photosynthesis. Plant, Cell and Environment 10: 579–586. [Google Scholar]
- Andrews M. 1986. The partitioning of nitrate assimilation between root and shoot of higher plants. Plant, Cell and Environment 9: 511–519. [Google Scholar]
- Andrews M, MacFarlane JJ, Sprent JI. 1985. Carbon and nitrogen assimilation by Vicia faba L. at low temperature: the importance of concentration and form of applied-N. Annals of Botany 56: 651–658. [Google Scholar]
- Andrews M, Morton JD, Lieffering M, Bisset L. 1992. The partitioning of nitrate assimilation between root and shoot of a range of temperate cereals and pasture grasses. Annals of Botany 70: 271–276. [Google Scholar]
- Andrews M, Zerihun A, Watson C. 1995. Nitrogen form effects on the partitioning of dry matter between root and shoot of Phaseolus vulgaris L. In: Proceedings of the 2nd European Conference on Grain Legumes. Paris, France: AEP (l'Association Européene de Recherche sur les Protéagineuse), 60–61.
- Andrews M, Sprent JI, Raven JA, Eady PE. 1999. Relationships between shoot to root ratio, growth and leaf soluble protein concentration of Pisum sativum, Phaseolus vulgaris and Triticum aestivum under different nutrient deficiencies. Plant, Cell and Environment 22: 949–958. [Google Scholar]
- Andrews M, Raven JA, Sprent JI. 2001. Environmental effects on dry matter partitioning between shoot and root of crop plants: relations with growth and shoot protein concentration. Annals of Applied Biology 138: 57–68. [Google Scholar]
- Andrews M, Lea PJ, Raven JA, Lindsey K. 2004a. Can genetic manipulation of plant nitrogen assimilation enzymes result in increased crop yield and greater N-use efficiency? An assessment. Annals of Applied Biology 145: 25–40. [Google Scholar]
- Andrews M, Raven JA, James EK, Sprent JI. 2004b. Environmental, developmental and rhizobial effects on dry matter partitioning between shoot and root of grain legumes. In: Proceedings of the 5th European Conference on Grain Legumes/2nd International Conference of Legume Genomics and Genetics. Paris, France: AEP (l'Association Européene de Recherche sur les Protéagineuse), 121–124.
- Andrews M, Maule HG, Raven JA, Mistry A. 2005. Extension growth of Impatiens glandulifera at low irradiance: importance of nitrate and potassium accumulation. Annals of Botany 95: 641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastow-Wilson J. 1988. A review of evidence on the control of shoot : root ratio, in relation to models. Annals of Botany 61: 433–449. [Google Scholar]
- Dastgheib F, Andrews M, Morton JD, Barnes MF. 1995. Mode of action of chlorsulfuron in a sensitive wheat (Triticum aestivum) cultivar: primary and secondary effects on nitrogen assimilation. Annals of Applied Biology 127: 125–135. [Google Scholar]
- Dewar RC. 1993. A root-shoot partitioning model based on carbon and nitrogen–water interactions and Münch phloem flow. Functional Ecology 7: 356–368. [Google Scholar]
- Evans JR, Seemann JR. 1989. The allocation of protein-nitrogen in the photosynthetic apparatus: costs, consequences and control. In: Briggs W, ed. Toward a broad understanding of photosynthesis. New York: AR Liss, 183–205.
- Forde BG. 2002. Local and long-range signaling pathways regulating plant responses to nitrate. Annual Review of Plant Biology 53: 203–204. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Parry M, Noctor G. 2003. Markers and signals associated with nitrogen assimilation in higher plants. Journal of Experimental Botany 54: 585–593. [DOI] [PubMed] [Google Scholar]
- Gleeson SK. 1993. Optimization of tissue nitrogen and root–shoot allocation. Annals of Botany 71: 23–31. [Google Scholar]
- Hilbert DW, Reynolds JF. 1991. A model allocating growth among leaf proteins, shoot structure and root biomass to produce balanced activity. Annals of Botany 68: 417–425. [Google Scholar]
- Hipkin CR, Simpson DJ, Wainwright SJ, Salem MA. 2004. Nitrification by plants that also fix nitrogen. Nature 430: 98–101. [DOI] [PubMed] [Google Scholar]
- Ingestad T, Ågren GI. 1991. The influence of plant nutrition on biomass allocation. Ecological Applications 1: 168–174. [DOI] [PubMed] [Google Scholar]
- Ingestad T, McDonald AJS. 1989. Interaction between nitrogen and photon flux density in birch seedlings at steady-state nutrition. Physiologia Plantarum 77: 1–11. [Google Scholar]
-
Khamis S, Lamaze T. 1990. Maximal biomass production can occur in corn (Zea mays) in the absence of
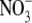 accumulation in either leaves or roots. Physiologia Plantarum 78: 388–394. [Google Scholar]
accumulation in either leaves or roots. Physiologia Plantarum 78: 388–394. [Google Scholar] - Kruse J, Hetzger I, Hansch R, Mendel R-R, Walch-Liu P, Engels C, et al. 2002. Elevated pCO2 favours nitrate reduction in the roots of wild-type tobacco (Nicotiana tabacum cv. Gat.) and significantly alters N-metabolism in transformants lacking functional nitrate reductase in the roots. Journal of Experimental Botany 53: 2351–2367. [DOI] [PubMed] [Google Scholar]
- Lawlor DW. 2002. Carbon and nitrogen assimilation in relation to yield: mechanisms are the key to understanding production systems. Journal of Experimental Botany 53: 773–787. [PubMed] [Google Scholar]
- Lawlor DW, Cornic G. 2002. Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant, Cell and Environment 25: 275–294. [DOI] [PubMed] [Google Scholar]
- Levin SA, Mooney HA, Field C. 1989. The dependence of plant root : shoot ratios on internal nitrogen concentration: Annals of Botany 64: 71–75. [Google Scholar]
- Marschner H. 1995. Mineral nutrition of higher plants. London: Academic Press.
- Maule HG. 2000. Ecological and physiological studies on Impatiens glandulifera. PhD Thesis, University of Sunderland, UK.
- Maule HG, Andrews M, Morton JD, Daly GT. 1995. Sun/shade acclimation and nitrogen nutrition of Tradescantia fluminensia, a problem weed in New Zealand native forest remnants. New Zealand Journal of Ecology 19: 35–46. [Google Scholar]
- McDonald AJS, Davies WJ. 1996. Keeping in touch: responses of the whole plant to deficits in water and nitrogen supply. Advances in Botanical Research 22: 229–300. [Google Scholar]
- Millard P. 1988. The accumulation and storage of nitrogen by herbaceous plants. Plant, Cell and Environment 11: 1–8. [Google Scholar]
- Pate JS. 1980. Transport and partitioning of nitrogenous solutes. Annual Review of Plant Physiology 31: 313–340. [Google Scholar]
- Poorter H, Nagel O. 2000. The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients and water: a quantitative review. Australian Journal of Plant Physiology 27: 595–607. [Google Scholar]
- Raven JA. 2003. Can plants rely on nitrate? Trends in Plant Science 8: 314–315. [DOI] [PubMed] [Google Scholar]
- Raven JA, Andrews M, Quigg A. 2005. The evolution of oligotrophy: implications for the breeding of crop plants for low input agricultural systems. Annals of Applied Biology 146: 261–280. [Google Scholar]
- Santi S, Locci G, Monte R, Pinton R, Varanini Z. 2003. Induction of nitrate uptake in maize roots: expression of a putative high-affinity nitrate transporter and plasma membrane H+-ATPase isoforms. Journal of Experimental Botany 54: 1851–1854. [DOI] [PubMed] [Google Scholar]
- Scheible W-R, Lauerer M, Schulze E-D, Caboche M, Stitt M. 1997. Accumulation of nitrate in the shoot acts as a signal to regulate shoot–root allocation in tobacco. The Plant Journal 11: 671–691. [Google Scholar]
- Scheible W-R, Morcuende R, Czechowski T, Fritz C, Osuna D, Palacios-Rojas N, et al. 2004. Genome-wide reprogramming of primary and secondary metabolism, protein synthesis, cellular growth processes, and the regulatory infrastructure of Arabidopsis in response to nitrogen. Plant Physiology 136: 2483–2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M, Krapp A. 1999. The interaction between elevated carbon dioxide and nitrogen nutrition: the physiological and molecular background. Plant, Cell and Environment 22: 583–621. [Google Scholar]
- Stitt M, Scheible W-R. 1999. Nitrate acts as a signal to control gene expression, metabolism and biomass allocation. In: Kruger N, Hill SA, Ratcliffe RG, eds. Regulation of metabolism. Dordrecht: Kluwer Academic Publishers, 275–306.
- Stulen I, den Hertog J, Fonseca F, Steg K, Posthumus F, van der Kooij TAW, et al. 1998. Impact of elevated atmospheric CO2 on plants. In: de Kok LJ, Stulen I, eds. Responses of plant metabolism to air pollution and global change. Leiden: Backhuys Publishers, 167–179.
- Thornley JHM. 1972. A balanced quantitative model for root : shoot ratios in vegetative plants. Annals of Botany 68: 211–216. [Google Scholar]
- Wang R, Tischner R, Gutiérrez RA, Hoffman M, Xing X, Chen M, et al. 2004. Genomic analysis of the nitrate response using a nitrate reductase-null mutant of Arabidopsis. Plant Physiology 136: 2512–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt MP, Cresswell CF. 1987. A comparison between the utilisation of storage protein and exogenous nitrate during seedling establishment in Zea mays L. Plant, Cell and Environment 10: 327–332. [Google Scholar]
- Zhen RG, Leigh RA. 1990. Nitrate accumulation by wheat (Triticum aestivum) in relation to growth and tissue concentrations. Plant and Soil 124: 157–160. [Google Scholar]