Abstract
• Background and Aims Unlike the dispersal mechanisms of many desert plants, the whole dead skeleton of Anastatica hierochuntica is involved in seed dispersal and preservation. This process depends on the hygrochastic nature of the lignified conducting tissue that bends when dry and straightens under wet conditions. An anatomical interpretation of this mechanical movement was investigated.
• Methods An anatomical study of the stem was conducted on the juvenile plants raised under different water treatments and on the branch-orders of adult A. hierochuntica size-classes.
• Key Results In the juvenile stem of A. hierochuntica, the area of cortex, conducting tissue and pith increased with water availability. However, the hydraulic conductance decreased, resulting in a better withdrawal of water in water-stressed plants. The anatomical investigation of the hygrochastic mechanism revealed an asymmetric distribution of the cortical tissues, with the conducting tissues of the stem of juvenile and adult plants being larger in the lower side. The hydraulic conductance was better in the basal and middle branch-orders than the terminal ones, permitting better conductance of water to the subsequent branch-orders.
• Conclusions The lignified conducting tissue of the whole stem, having a hygrochastic nature, controls the movement of the branches. The greater amount of conducting tissue associated with a higher density of wide xylem vessels was observed in the lower side of the stem as compared with the upper side. Consequently, the conducting tissue in the lower side of the stem was suggested to be more effective in the opening process of the curled dry branches through better and more rapid conductance of water. Alternatively, due to the few narrow xylem vessels in the upper side of the stem, it was likely that the conducting tissue in the upper side is more effective in the closing process by providing more rapid drying. The mechanical rise of water and the related hygrochastic efficiency were maximized in the basal and middle branch-orders that are mostly involved in the mechanical movement.
Keywords: Anatomy, hygrochasy, seed dispersal, hydraulic conductance, Anastatica hierochuntica
INTRODUCTION
The hygrochastic and seed dispersal ecology of desert plants reveals part of the mystery associated with their persistence in arid desert environments. This subject has been investigated by several authors, e.g. Murbeck (1919, 1943), Zohary (1937), Fahn (1947), Gutterman et al. (1967), Witztum et al. (1969), Fahn and Werker (1972), Gutterman (1990, 1993b, 1994), Van Rooyen et al. (1990) and Gutterman and Shem-Tov (1997). As a desert environment becomes more extreme, many plant species relate seed dispersal to rain events (ombrohydrochory), so as to ensure successful germination and establishment in the proper space and time (Gutterman, 1993a). Hygrochasy is one of the most efficient dispersal ombrohydrochoric mechanisms restricting seed dispersal to rain events. Van Oudtshoorn and Van Rooyen (1999) summarized the advantages of hygrochasy for desert species, including protection from predators, spreading the risk of dispersal and germination over several years, regulating the timing of germination and deposition of seeds in a suitable place.
The plant organ responsible for the hygrochastic movement is usually lignified and varies among annual and perennial desert plant species that disperse seeds hygrochastically. The hygrochastic organ may be a capsule enclosing seeds, e.g. Mesembryanthemum species and Aizoon hispanicum (Aizoaceae; Gutterman, 1980/81, 1990) and Aptosimum spinescens (Scrophulariaceae; Günster, 1992); the woody hygrochastic involucre of the capitula in Asteriscus pygmaeus (Asteraceae; Gutterman and Ginott, 1994); the hygrochastic peduncles of the inflorescence of Plantago coronopus (Plantaginaceae; Fahn and Werker, 1972); the pedicels and sepals in Salvia harmonium and Salvia viridis (Lamiaceae; Fahn and Werker, 1972); and the hygrochastic umbel rays in Ammi visnaga (Abiaceae; Gutterman, 1990). For Anastatica hierochuntica, the seed remains on the dry skeletons protected by the curled branches of dead plants around the infructescences (fruiting branches). Seed dispersal is performed by the hygrochastic uncurling of the dead branches, then by the force of rain causing the opening of the fruit valves and the release of seeds (Friedman et al., 1978). Obviously, the hygrochastic stem branches are involved in the dispersal process.
The aim of the present work was to: (a) investigate the anatomical mechanism of the hygrochastic movement of the stem by studying the anatomical peculiarities of the stem and their relationship to the hygrochastic efficiency and the mechanical rise of water; (b) test whether the distribution of the different types of tissues (thin walled and mechanical tissues) is an inherent character, i.e. manifested from the juvenile stage before the stem branches or whether a redistribution of these tissues occurs at an older stage; and (c) study the anatomical features of the response of A. hierochuntica to variable rainfall treatments.
MATERIALS AND METHODS
Study species
Anastatica hierochuntica is a desert annual (Fig. 1A) characterized by a highly efficient mechanism of seed dispersal (Friedman et al., 1978; Danin, 1983; Van Oudtshoorn and Van Rooyen, 1999). This mechanism depends on the hygrochastic nature of the dead skeletons. After senescence, the dry lignified stem branches are curled around the enclosed fruits (Fig. 1B). These branches uncurl hygrochastically when wetted by dew interception and rainfall (Fig. 1C). Seeds are released by the force of rain drops on the fruit valves and by repeated curling and uncurling of the stem branches.
Fig. 1.
Anastatica hierochuntica green plant (A), dry curled skeleton (B), and hydrated uncurled skeleton (C).
Plant material
Seeds obtained from dry A. hierochuntica skeletons collected from Wadi Hagoul (Cairo–Suez desert road) were raised to the juvenile stage (expansion of the first two foliage leaves) under four different water treatments. Each treatment was represented by five pots filled with soil collected near field-grown A. hierochuntica populations. The water treatments were equivalent to 100, 200, 500 and 1000 mm annual rainfall, which amounts to 5, 10, 15 and 20 mm rainfall at the juvenile stage. Plant materials were collected and fixed in formalin–acetic acid–alcohol (9 : 5 : 5) mixture. The materials were transferred later to 50 % ethanol and then processed for the anatomical investigation.
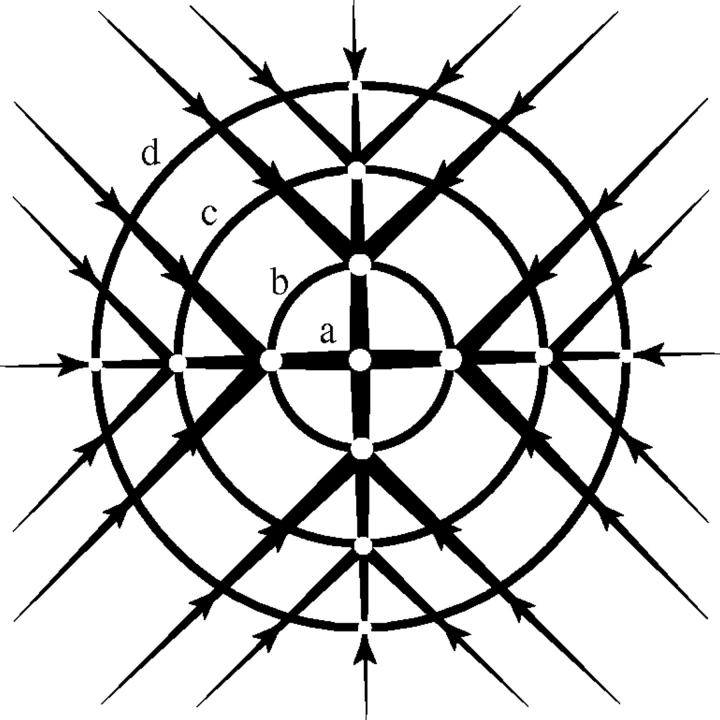
For adult plants, the anatomical study was conducted on the different branch-orders of living individual A. hierochuntica plants growing naturally in the field. The number of a size-class indicates the number of branch-orders present, i.e. an individual belonging to size-class IV consists of four branch-orders, the first branch-order corresponding to the basal segment and the fourth branch-order to the terminal segment of the stem (Fig. 2).
Fig. 2.
Ramification of A. hierochuntica plant belonging to size-class IV, showing branching orders (a, first; b, second; c, third; d, fourth). Light dots on the main branching position represent the bases of infructescences. Short branches at the ramification positions are degenerated branches that turned to the ground (after Friedman et al., 1978).
The anatomical features of the stem, in the case of juvenile plants and size-classes, were chosen for study because the stem is the organ responsible for the hygrochastic movement resulting in seed dispersal. The lower side of the stem in the text indicates the outer or abaxial side, while the upper side of the stem indicates the inner or adaxial side.
Measurements and data analysis
The anatomical features of the stem were performed using a light microscope equipped with an ocular micrometer. Anatomical parameters describing the thin-walled cells (epidermis and cortex) and lignified cells (conducting tissue) were measured for juvenile plants raised under different water treatments and for the different branch-orders of the adult plants.
The parameters investigated were the dimensions of epidermal cells on the lower and upper sides of the stem, thickness of the cortical layer and density (number of cells mm−2) of the cortical cells in the lower and upper sides of the stem, the thickness of the conducting tissue layer, and the density and diameter of xylem vessels in the lower and upper sides of the stem. The ratios of the lower to upper values of these parameters were calculated. Ratios between parameters were used as an estimate of the degree of difference between the lower and upper sides of the stem. Analysis of variance was used to test the significance of differences between means of stem parameters and ratios for water-treated juvenile plants.
Because water flux through the conducting tissue is an important factor in hygrochastic movement, the ratio of the conducting tissue area to the total area occupied by the cortex and pith tissues and the total area of the conducting tissue in stem transverse sections were considered an indirect measure of the hygrochastic efficiency of the stem. Moreover, the theoretical hydraulic conductance was considered to be a direct estimate of the efficiency of the mechanical rise of water through the xylem conduits, assuming they are not closed by air bubbles. The theoretical hydraulic conductance (Kh) was estimated from anatomical measurements using the Hagen–Poiseuille law (Gibson, 1984; Fahmy, 1997):
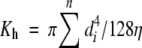 |
where d is the diameter (m) of the ith vessel in cross-section and η is the viscosity of water (MPa).
RESULTS
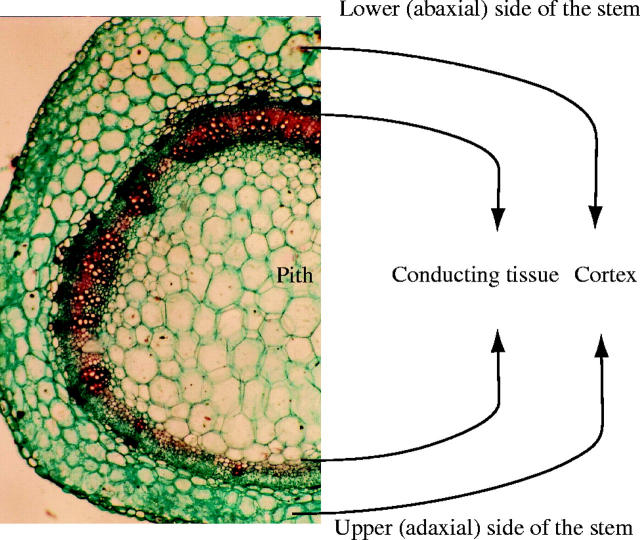
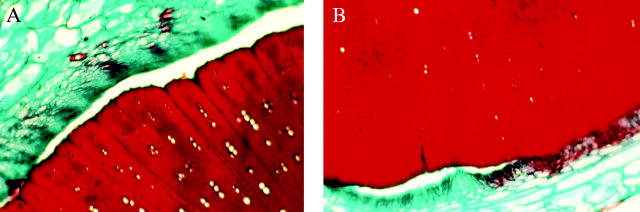
The crown of Anastatica hierochuntica consists of repeated ramifications of four main branches at the base of the crown. In transverse sections (Fig. 3), the stem at any order of branching is characterized by asymmetric distribution of cortex and conducting tissues. The cortical layer in the lower (abaxial) side of the stem is generally wider than that in the upper (adaxial) side. Similarly, the conducting tissue layer is thicker in the lower side of the stem and frequently narrower in the upper side or towards the right and left sides of the stem (Fig. 4). After senescence, complete decortication of the stem occurs, where the outer tissues (phloem, cortex and epidermis) are shed leaving the stem consisting of the conducting tissue surrounding the pith.
Fig. 3.
Transverse section of A. hierochuntica juvenile stem showing distribution of cortex and conducting tissue layers in the lower and upper sides of the stem.
Fig. 4.
Transverse section of A. hierochuntica adult stem showing the conducting tissue layer in the lower (A) and upper (B) sides of the stem.
Thin-walled cell tissues
The epidermis and cortex are formed of thin-walled cells. The stem of A. hierochuntica juvenile plants increased in diameter with the increased amounts of water supplied. Significant differences were observed between the 1000-mm rainfall treatment and the treatments with equivalently lower amounts of rain, with an approx. 0·5-mm increase in stem diameter of the juvenile plants (Table 1).
Table 1.
Measured and calculated conducting tissue anatomical parameters of Anastatica hierochuntica juvenile plants raised under different water treatments
| Treatment (mm rainfall) |
||||
|---|---|---|---|---|
| Parameter | 100 | 200 | 500 | 1000 |
| Diameter of stem (mm) | 1·28 ± 0·16a | 1·36 ± 0·20a | 1·45 ± 0·04a | 1·80 ± 0·08b |
| Total conducting tissue area (mm2) | 0·42 ± 0·01a | 0·44 ± 0·02b | 0·55 ± 0·02c | 0·62 ± 0·02d |
| Ratio of conducting tissue area to total cortex and pith areas | 0·53 ± 0·01a | 0·51 ± 0·02a | 0·44 ± 0·04b | 0·39 ± 0·01b |
| Total number of xylem vessels per stem | 386·3 ± 15·18a | 456·3 ± 24·70b | 556·6 ± 57·74c | 600·0 ± 70·00c |
| Hydraulic conductance (m4 MPa−1 s−1 × 10−10) | 127·7 ± 27·37a | 98·24 ± 19·94b | 76·37 ± 18·96c | 40·53 ± 10·53d |
Values followed by different superscript letters are significantly different within the same row at P < 0·05.
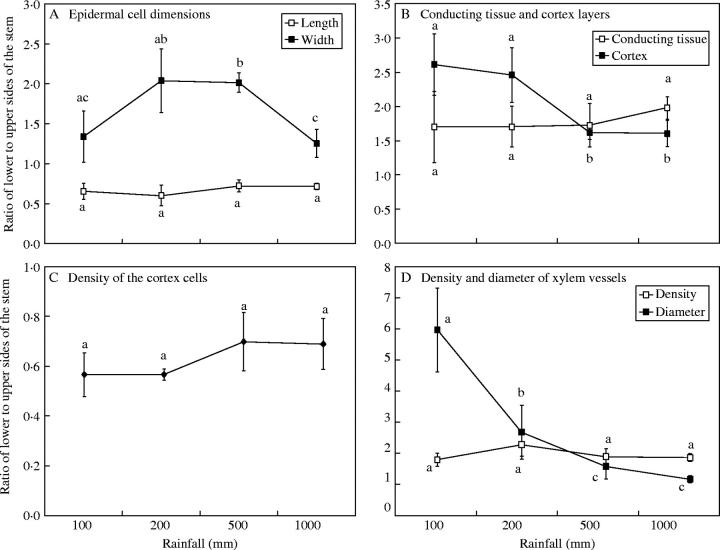
The ratios of the lower to upper length of epidermal cells were lower than unity, while the cell width ratios were greater than unity (Fig. 5A). The ratio of lower to upper thickness of cortex (Fig. 5B), is greater than 1·5 for all water treatments. This ratio attained a maximum of 2·6 under the 100-mm rainfall treatment and then decreased as the amounts of water increased, the lower water treatments attaining significantly higher ratios than the 500- and 1000-mm rainfall treatments. The ratio of lower to upper density of cortical cells (Fig. 5C) was more or less the same for all water treatments and ranged between 0·57 and 0·70. This low ratio (less than unity) denotes a smaller size of cells in the upper side than in the lower side of the stem under all treatments.
Fig. 5.
Comparison of ratios of lower to upper sides of the juvenile stem of Anastatica hierochuntica raised under different water treatments.
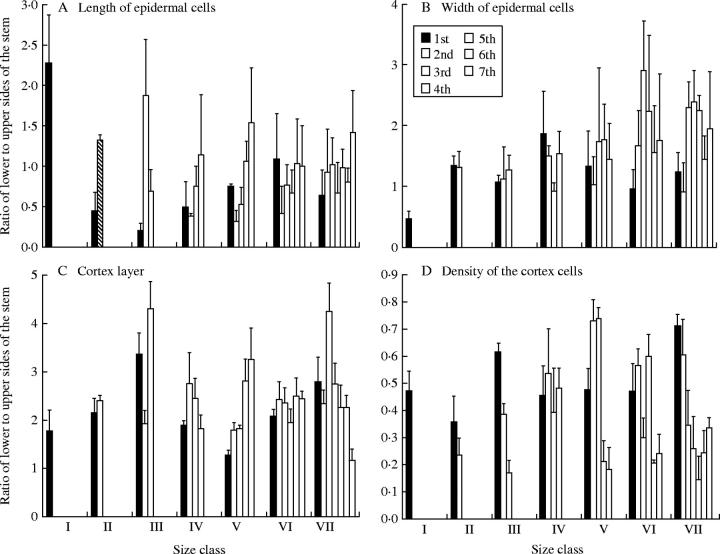
Examination of the outer to inner anatomical ratios of different orders in the size-classes of the adult A. hierochuntica individuals studied revealed that dimensions of the epidermal cells in the lower side of the stem are generally shorter and wider than those in the upper side (Fig. 6A and B). In most of the branch-orders, the ratio of lower to upper length of epidermal cells is lower than unity, while the ratio of outer to inner width is greater than unity. The dimensions of the epidermal cells in the first size-class attained the greatest length and shortest width among all classes.
Fig. 6.
The ratios of lower to upper epidermal and cortical anatomical parameters of adult stems for different orders (columns) of Anastatica hierochuntica size-classes.
Similarly, the cortical layer in the lower side of the stem was thicker and had larger cells than that in the upper side of the stem (Fig. 6C and D). This is drawn from the ratio of lower to upper thickness of the cortical layer (greater than unity) and that of the density of cortical cells (lower than unity).
Lignified cell tissues
The total area of the lignified conducting tissues seen in a cross-section of a stem significantly increased with increasing water treatment, reaching 0·62 mm2 under the 1000-mm rainfall treatment (Table 1). The increase in the total area of conducting tissues is associated with a progressive decrease in the ratio of conducting tissue area to the total cortex and pith areas, attaining a minimum of 0·39 mm2 under the 1000-mm rainfall treatment (Table 1). Furthermore, the ratio of the lower to upper layers of conducting tissues was greater than 1·5 (Fig. 5B) for all rainfall treatments, reflecting a greater amount of conducting tissue in the lower side of the stem.
The ratio of lower to upper density of xylem vessels (Fig. 5D) seems not to be affected by water treatments. The total number of xylem vessels increased significantly with increasing amounts of water, ranging between 386·3 vessels under the 100-mm rainfall treatment and 600 vessels under the 1000-mm rainfall treatment (Table 1). The diameter of xylem vessels was larger in the lower side than in the upper side of the stem (ratios of outer- to upper-side values greater than unity) for all water treatments (Fig. 5D). This ratio decreased with the increase in amounts of water where a significant difference occurred between the 100-mm (5·96) and 1000-mm (1·17) water treatments.
The mechanical rise of water as manifested by the theoretical calculation of hydraulic conductance, attained a significant decrease with the increase in amount of water supplied (Table 1). A value of 127·7 m4 MPa−1 s−1 × 10−10 was estimated for the 100-mm rainfall treatment that decreased to 40·53 m4 MPa−1 s−1 × 10−10 in the 1000-mm rainfall treatment.
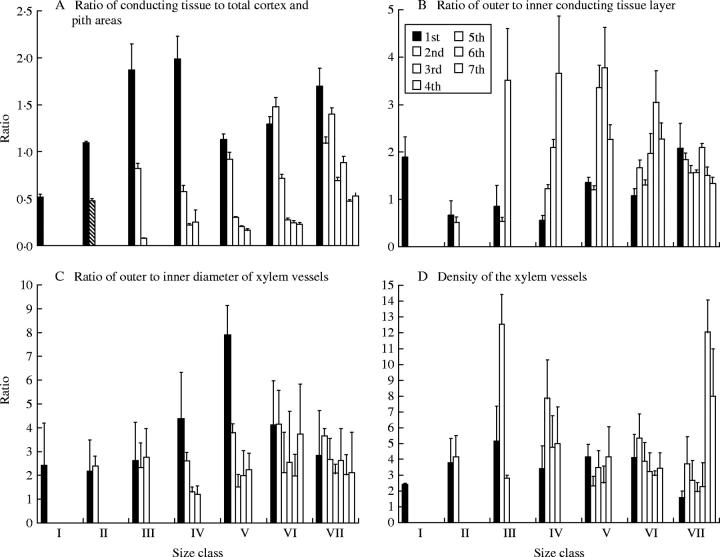
As the stem diameter decreased from the basal to the terminal branch-orders in the size-classes of the adult A. hierochuntica individuals studied, the total conducting tissue area and the total number of xylem vessels decreased (Table 2). The ratio of the conducting tissue area to the total areas of the cortex and pith (Fig. 7A) is generally greater than unity in the basal branch-orders as compared with other orders of the same class. Alternatively, the ratio of lower to upper thickness of conducting tissue is generally greater at the terminal branches (Fig. 7B). It is noteworthy that this ratio is greater than unity (i.e. thicker in the lower side of the stem) for most branch-orders, and attained 3·78 in the fourth branch-order of the fifth size-class.
Table 2.
Measured and calculated conducting tissue anatomical parameters of stem branches of adult individuals of Anastatica hierochuntica
| Size-class | Branch-order | Diameter of stem (mm) | Total conducting tissue area (mm2) | Total number of xylem vessels | Hydraulic conductance (m4 MPa−1 s−1 × 10−10) |
|---|---|---|---|---|---|
| I | 1st | 1·43 ± 0·07 | 0·62 ± 0·02 | 288·0 ± 13·75 | 151·1 ± 35·3 |
| II | 1st | 2·01 ± 0·07 | 0·98 ± 0·03 | 303·3 ± 15·28 | 403·1 ± 158·2 |
| 2nd | 1·70 ± 0·04 | 0·78 ± 0·03 | 270·0 ± 20·00 | 21·37 ± 13·64 | |
| III | 1st | 2·18 ± 0·16 | 2·35 ± 0·15 | 268·3 ± 7·64 | 215·3 ± 46·0 |
| 2nd | 1·86 ± 0·07 | 0·73 ± 0·02 | 181·6 ± 10·15 | 0·43 ± 0·15 | |
| 3rd | 0·97 ± 0·05 | 0·09 ± 0·01 | 141·0 ± 7·64 | 0·35 ± 0·07 | |
| IV | 1st | 2·36 ± 0·13 | 4·02 ± 0·23 | 240·0 ± 55·68 | 30·62 ± 9·89 |
| 2nd | 1·93 ± 0·17 | 1·18 ± 0·12 | 229·0 ± 4·58 | 6·64 ± 2·89 | |
| 3rd | 1·42 ± 0·11 | 0·35 ± 0·05 | 180·3 ± 5·51 | 0·92 ± 0·29 | |
| 4th | 0·92 ± 0·15 | 0·09 ± 0·01 | 101·0 ± 6·56 | 0·04 ± 0·01 | |
| V | 1st | 3·74 ± 0·34 | 6·92 ± 0·38 | 856·6 ± 40·41 | 69877 ± 8436·8 |
| 2nd | 3·28 ± 0·19 | 3·88 ± 0·13 | 603·3 ± 15·28 | 14580 ± 2265·6 | |
| 3rd | 3·00 ± 0·07 | 1·45 ± 0·05 | 440·0 ± 45·83 | 966·18 ± 245·7 | |
| 4th | 1·96 ± 0·15 | 0·68 ± 0·03 | 226·6 ± 25·17 | 23·35 ± 7·26 | |
| 5th | 1·39 ± 0·08 | 0·28 ± 0·03 | 189·6 ± 9·50 | 17·06 ± 5·34 | |
| VI | 1st | 4·14 ± 0·12 | 7·67 ± 0·21 | 1000 ± 50·00 | 243766 ± 33281·9 |
| 2nd | 3·26 ± 0·18 | 6·10 ± 0·36 | 690·0 ± 36·06 | 31151 ± 3928·8 | |
| 3rd | 3·02 ± 0·07 | 2·83 ± 0·15 | 573·3 ± 15·28 | 843·2 ± 132·9 | |
| 4th | 1·83 ± 0·12 | 0·55 ± 0·05 | 306·6 ± 40·41 | 17·56 ± 5·87 | |
| 5th | 1·63 ± 0·04 | 0·41 ± 0·04 | 229·0 ± 8·54 | 7·73 ± 10·40 | |
| 6th | 1·08 ± 0·05 | 0·17 ± 0·03 | 157·3 ± 7·51 | 0·75 ± 0·34 | |
| VII | 1st | 5·57 ± 0·083 | 9·75 ± 0·13 | 1030 ± 60·83 | 57360 ± 14340 |
| 2nd | 4·73 ± 0·51 | 6·80 ± 0·26 | 1016 ± 50·00 | 23700 ± 1374·7 | |
| 3rd | 3·85 ± 0·41 | 5·10 ± 0·10 | 906·6 ± 40·41 | 28242 ± 3259 | |
| 4th | 2·63 ± 0·44 | 2·87 ± 0·15 | 653·3 ± 55·08 | 8196 ± 1715 | |
| 5th | 2·51 ± 0·07 | 2·30 ± 0·20 | 386·6 ± 70·95 | 708·6 ± 172·1 | |
| 6th | 1·80 ± 0·23 | 0·91 ± 0·04 | 381·6 ± 10·41 | 61·44 ± 21·25 | |
| 7th | 1·43 ± 0·08 | 0·68 ± 0·03 | 226·6 ± 20·82 | 9·78 ± 3·13 |
Fig. 7.
Comparison of ratio of conducting tissue area to total cortex and pith areas (A) and the ratios of lower to upper conducting tissue parameters of adult stems (B–D) for different orders (columns) of Anastatica hierochuntica size-classes.
The diameter and density of xylem vessels (Fig. 7C and D) are generally twice as high in the lower as in the upper sides of the stem in the same branching order, reflecting a greater water supply to the lower side of the stem. Within the same size-class, the hydraulic conductance of the xylem vessels (Table 2) decreased from the basal branch-orders towards the terminal orders, indicating better conductance in the basal ones. The highest value of hydraulic conductance (243 766 m4 MPa−1 s−1 × 10−10) was recorded for the basal branch-order of the sixth size-class. It was noticed that the values of the hydraulic conductance in the terminal branch-orders are minute as compared with those in the basal ones. This can be explained by the big differences found in the total area of the xylem conduits in these branch orders.
DISCUSSION
The study species, Anastatica hierochuntica, differs from resurrection plants in that the curling and uncurling processes of its skeletons are purely mechanical and do not involve desiccation tolerance of cellular constituents in the dry state then recovery of vital processes upon dehydration. After senescence, the dry skeletons of A. hierochuntica undergo shedding of leaves and decortication. Consequently, these dry skeletons cannot resume life again in the same way as resurrection plants. The dry skeletons may be considered as seed reservoirs with a seed-dispersal function (Friedman et al., 1978; Danin, 1983; Van Oudtshoorn and Van Rooyen, 1999; Kabiel, 2005).
The hygrochastic movement of fruit parts and/or associated plant organs has been investigated (Zohary and Fahn, 1941; Fahn, 1947; Fahn and Werker, 1972). Two mechanisms were proposed, which may either work separately or in combination. The first mechanism, ‘cohesion mechanism’, depends on the ability of specialized thin-walled cells to absorb and accumulate water in their lumen, which results in these cells swelling (shrinkage of these cells occurs upon losing water). The act of opening and closing of the dispersal apparatus or plant organ is caused by alternating swelling and shrinkage of these cells. In the case of A. hierochuntica, thin-walled cells are represented by the epidermal and cortical cells. The asymmetric distribution of the epidermis (thinner and more stretched cells in the upper side of the stem) and cortex (thinner layer with smaller cells in the upper side of the stem) in the lower and upper sides of the stem seems to have no role in the process of repeated curling and uncurling of the hygrochastic branches, because of the complete decortication of the branches after senescence. This asymmetric distribution may facilitate the first closure of the branches where the resistance of the cortex to curvature is minimized by the presence of a thin cortex layer in the upper side. It is noteworthy that all of the infructescences (fruiting branches) are borne on the upper side of the branches and this feature may be a reason for a less well-developed cortical layer on this side of the stem.
The second mechanism, ‘imbibition mechanism’, is based on the water content of the thick lignified cell walls causing swelling and shrinkage of cells in a direction perpendicular to that of the cellulosic microfibrils (Zohary and Fahn, 1941; Fahn, 1947; Fahn and Werker, 1972). Another type of tissue, ‘resistant lignified cells’, which makes little contribution to the hygrochastic movement, was reported by Fahn (1947). These cells have cellulosic microfibrils in a direction parallel to the direction of movement and are present in combination in the active tissue. The active and resistant tissues involved in the hygrochastic movement were reported to be present in different hygrochastic organs, e.g. bracts of inflorescences (Anvillea gracini), pedicels (Ziziphora capitata) and the lignified cells of the central cylinder in the case of Teucrium lamiifolium and Salvia harmonium (Fahn, 1947).
Hygrochastic mechanism
The imbibition of water by the lignified conducting tissue of A. hierochuntica explains the hygrochastic movement of the dry branches. Effectively, the thickness of the conducting tissue in the lower side of the stem is generally greater than that in the upper side in all branch-orders of the skeletons. Moreover, the conducting tissue in the lower side of the stem has wider xylem vessels with a higher density than that in the upper side of the stem, where values in the lower side of the stem are generally more than double the corresponding values in the upper side. Remarkably, in the upper side of the stem, xylem vessels are generally concentrated towards the pith leaving a wide area for the conducting tissue, which consists of lignified cells, sometimes with widely separated narrow vessels.
Considering all these anatomical facts, it seems that two mechanisms work in combination for the hygrochastic movement of A. hierochuntica branches. The conducting tissue in the lower side of the stem seems to be more effective in the opening process than the conducting tissue in the upper side. This view is supported by the fact that numerous xylem vessels with a larger diameter conduct larger amounts of water in the upward direction to the lower side of the stem, and then water diffuses through lateral pits to hydrate the lignified cells in the upper side of the stem. This suggestion is supported by the observations of Friedman et al. (1978), in which the maximum opening of A. hierochuntica skeletons resulting from root emersion in water was 11 % of that obtained from shoot emersion and required a period of 3 h compared with 2 h in the case of shoot emersion. In the case of shoot emersion the lignified cells in the outer and upper sides of the stem contribute equally and simultaneously to the opening process, resulting in a faster opening of the skeletons. On the other hand, the conducting tissue in the upper side of the stem, having fewer vessels with smaller diameters that are generally restricted to the parts adjacent to the pith, leaving a mass of lignified cells towards the upper side of the stem, is more apt to dehydrate faster than that in the lower side and, hence, may be considered to be more effective in the closing movement. This asymmetric system is apparently responsible for the hygrochastic movement.
The asymmetric distribution of cortex and conducting tissue in the lower and upper sides of the stem (greater in the lower side) seems to be an inherent character, which is manifested from the juvenile stage even before the branching of the main stem. As the plant grows, new tissues are added following the same trend. Obviously, the amount of water supplied did not affect this general trend at the juvenile stage.
Mechanical rise of water and hygrochastic efficiency
The mechanical rise of water through xylem vessels is of great importance to the upward conductance of water to the hygrochastic branches of A. hierochuntica. Capillary forces, together with hydraulic conductance, provide the potential to refill the empty xylem conduits, until the whole skeleton is hydrated. It is noteworthy that the Hagen–Poiseuille law of hydraulic conductance is not restricted to use in the soil–plant–atmosphere system, but is also valid for the dead skeletons of A. hierochuntica and even in the study of the flow of water or fluids through rock pores (Bernabe et al., 1982). The efficiency of the mechanical rise of water is represented here by the hydraulic conductance of the stem that relates xylem structure (diameter and number of vessels) to function (conductance), which is positively correlated (Zimmermann, 1971; Gibson et al., 1984). The hydraulic conductance of the stem decreases in the direction from the basal to the terminal branch-orders in each size-class. This indicates a maximum capacity in the mechanical rise of water in the basal branches, which is needed for an efficient rise of water to the subsequent branch-orders.
The hydraulic conductance of the stem of A. hierochuntica juvenile plants increases with water stress. Despite the significant decrease of conducting tissue area with the decreased amounts of water, the increase in the hydraulic conductance is mainly caused by a significant increase in the diameter of xylem vessels in the lower side of the stem. This may permit a more efficient rise of water for water-stressed plants at the juvenile stage. The presence of wider xylem vessels was sometimes reported for water-stressed plants and species, from environments where water is available only episodically, to maximize water uptake when water is available, by reducing the resistance associated with the smaller xylem vessels (Castro-Diez et al., 1998; Nicotra et al., 2002).
The hygrochastic nature of the lignified cells of the conducting tissue is responsible for the movement of A. hierochuntica branches. Therefore, in addition to the role of the hydraulic conductance, better hygrochastic efficiency is indicated by the greater total area of the conducting tissue in cross-section and the higher ratio of conducting tissue area to the total areas of cortex and pith. In essence, the hygrochastic efficiency decreased from the basal branch-orders to the terminal ones for all size-classes. The maximum hygrochastic efficiency in basal branch-orders permits appreciable and rapid opening and closing of the whole structure, helped by the high hygrochastic efficiency of the middle branch-orders.
An increase in hygrochastic efficiency, as represented by the increase in the area of conducting tissue, was observed with the increase in water supply. This is coupled with an increase in cortex and pith areas as a direct response to the increased growth associated with increasing amounts of water, resulting in a smaller ratio of conducting tissue area to the total areas of cortex and pith. Nicotra et al. (2002) reported a smaller area of root conducting tissues in seedling of species of low-rainfall environments relative to species of high-rainfall environments.
CONCLUSIONS
The hygrochastic anatomy of A. hierochuntica stems showed an asymmetric distribution of the cortex and conducting tissue. The lower side of the stem is endowed with a greater proportion of the cortex and conducting tissue layers than the upper side. The thinner cortical layer in the upper side of the stem plays a limited role in decreasing the resistance of the first skeleton curling after senescence but for repeated curling and uncurling over years, such a role is lost due to decortication. The thick layer of conducting tissue in the lower side of the stem, associated with the high density of wide xylem vessels, is more effective in the opening process (uncurling) of skeletons by permitting rapid movement of water, which diffuses thereafter through lateral pits to the conducting tissues in the upper side of the stem. The thin layer of conducting tissue in the upper side of the stem is more effective in the closing process (curling) of skeletons, where xylem vessels are few, narrow and restricted to the periphery of the conducting tissue towards the pith.
The refilling of the xylem vessels relies on capillary forces and hydraulic conductance, although the hydration effect of the dry skeletons, resulting from a possible direct exposure of the hygrochastic branches to water, contributes most to the hygrochastic movement. For a definite size-class, the hydraulic conductance attains maximum values in the basal branch-orders and decreases towards the terminal ones, ensuring an efficient upward mechanical rise of water. The anatomy of A. hierochuntica juvenile plants showed an increased hydraulic conductance with decreased amounts of water, an adaptation to maximize the uptake of water in dry environments.
The hygrochastic efficiency, as estimated by the total area of the stem cross-section occupied by conducting tissue, and the ratio of the conducting tissue area to the total cortex and pith areas, followed the same trend as the hydraulic conductance, being highest at the basal branch-orders, permitting effective movement of the branches. Juvenile plants showed a greater area of conducting tissue with increased water supply. The ratio of the conducting tissue area to the cortex and pith areas, in the juvenile plants, showed no consistent difference with the amount of water supplied.
LITERATURE CITED
- Bernabe Y, Brace WF, Evans B. 1982. Permeability, porosity and pore geometry of hot-pressed calcite. Mechanics of Materials 1: 173–183. [Google Scholar]
- Castro-Diez P, Puyravaud JP, Cornelissen JHC, Villar-Salvador P. 1998. Stem anatomy and relative growth rate of woody plant species and types. Oecologia 116: 57–66. [DOI] [PubMed] [Google Scholar]
- Danin A. 1983. Desert vegetation of Israel and Sinai. Jerusalem: Cana Publishing House.
- Fahmy GM. 1997. Leaf anatomy and its relation to the ecophysiology of some non-succulent desert plants from Egypt. Journal of Arid Environments 36: 499–525. [Google Scholar]
- Fahn A. 1947. Physico-anatomical investigation in the dispersal apparatus of some fruits. Palestine Journal of Botany 4: 36–45. [Google Scholar]
- Fahn A, Werker E. 1972. Anatomical mechanisms of seed dispersal. In: Koslowsky TT, ed. Seed biology. New York, NY: Academic Press, 152–221.
- Friedman J, Gunderman N, Ellis M. 1978. Water response of the hygrochastic skeletons of the true rose of Jericho (Anastatica hierochuntica L.). Oecologia 32: 289–301. [DOI] [PubMed] [Google Scholar]
- Gibson AC, Calkin HW, Nobel PS. 1984. Xylem anatomy, water flow, and hydraulic conductance in the fern Cyrtomium falcatum. American Journal of Botany 7: 564–574. [Google Scholar]
- Günster A. 1992. Aerial seed banks in the central Namid: distribution of serotinous plants in relation to climate and habitat. Journal of Biogeography 19: 563–572. [Google Scholar]
- Gutterman Y. 1980/81. Annual rhythm and position effect in the germinability of Mesembryanthemum nodiflorum. Israel Journal of Botany 29: 93–97. [Google Scholar]
- Gutterman Y. 1990. Seed dispersal by rain (ombrohydrochory) in some of the flowering desert plants in the deserts of Israel and the Sinai Peninsula. Mitteilung aus dem Institut fur Allgemeine Botanik Hamburg 22b: 841–852. [Google Scholar]
- Gutterman Y. 1993a. Seed Germination in desert plants (adaptation of desert organisms). Berlin, Germany: Springer-Verlag.
- Gutterman Y. 1993b. Dispersal and germination strategies affecting survival of desert plants. In: Come D, Corbineau F, eds. Basic and applied aspects of seed biology. Fourth International Workshop on Seeds, Vol. 1. Berlin, Germany: Springer-Verlag, 289–296.
- Gutterman Y. 1994. Strategies of seed dispersal and germination in plants inhabiting deserts. Botanical Review 60: 373–425. [Google Scholar]
- Gutterman Y, Ginott S. 1994. Long-term protected ‘seed bank’ in dry inflorescences of Asteriscus pygmaeus achene dispersal mechanism and germination. Journal of Arid Environments 26: 149–163. [Google Scholar]
- Gutterman Y, Shem-Tov S. 1997. Mucilaginous seed coat structure of Carrichtera annua and Anastatica hierochuntica from the Negev Desert highlands of Israel, and its adhesion to the soil crust. Journal of Arid Environments 35: 695–705. [Google Scholar]
- Gutterman Y, Witztum A, Evenari M. 1967. Seed dispersal and germination in Blepharis persica (Brum.) Kuntze. Israel Journal of Botany 16: 213–234. [Google Scholar]
- Kabiel HF. 2005. Adaptive significance of hygrochasy and growth for establishment and conservation ecology of Anastatica hierochuntica L. in Egypt. PhD Thesis, Cairo University.
- Murbeck S. 1919. Beiträge zur Biologie der Wütenpflanzen: Vorkommen und Bedeutung von Schleimabsonderung aus Smenhüllen. Lunds Universitet Arsskrift N. F. Adv 2 (15): 1–36. [Google Scholar]
- Murbeck S. 1943. Beiträge zur Biologie der Wüstenpflanzen: Vorkommen und Bedeutung von Schleimabson derung aus Samenhüllen. Lunds Universitet Arsskrift N. F. Adv. 2: 1–36. [Google Scholar]
- Nicotra AB, Babicka N, Westoby M. 2002. Seedling root anatomy and morphology: an examination of ecological differentiation with rainfall using phylogenetically independent contrasts. Oecologia 130: 136–145. [DOI] [PubMed] [Google Scholar]
- Van Oudtshoorn RK, Van Rooyen MW. 1999. Dispersal biology of desert plants. Berlin, Germany: Springer-Verlag.
- Van Rooyen MW, Theron GK, Grobbelaar N. 1990. Life form and dispersal spectra of the flora of Namaqualand, South Africa. Journal of Arid Environments 19: 133–145. [Google Scholar]
- Witztum A, Gutterman Y, Evenari M. 1969. Integumentary mucilage as an oxygen barrier during germination of Blepharis persica (Brum.) Kuntze. Botanical Gazette 130: 238–241. [Google Scholar]
- Zimmermann MH. 1971. Transport in the xylem. In: Zimmermann MH, Brawn CL, eds. Trees: structure and function. New York, NY: Springer-Verlag, 169–220.
- Zohary M. 1937. Die verbreitungsekologischen Verhältnisse der Flora Palästinas. I- Die Antitelechoristischen Erscheinungen. Beih. Bot. Cbl. Abt. A 56: 1–155. [Google Scholar]
- Zohary M, Fahn A. 1941. Anatomical-carpological observations in some hygrochastic plants of the oriental flora. Palestine Journal of Botany 2: 125–131. [Google Scholar]