Abstract
• Background and Aims The xylem plays an important role in strengthening plant bodies. Past studies on xylem formation in tension woods in poplar and also in clinorotated Prunus tree stems lead to the suggestion that changes in the gravitational conditions affect morphology and mechanical properties of xylem vessels. The aim of this study was to examine effects of hypergravity stimulus on morphology and development of primary xylem vessels and on mechanical properties of isolated secondary wall preparations in inflorescence stems of arabidopsis.
• Methods Morphology of primary xylem was examined under a light microscope on cross-sections of inflorescence stems of arabidopsis plants, which had been grown for 3–5 d after exposure to hypergravity at 300 g for 24 h. Extensibility of secondary cell wall preparation, isolated from inflorescence stems by enzyme digestion of primary cell wall components (mainly composed of metaxylem elements), was examined. Plants were treated with gadolinium chloride, a blocker of mechanoreceptors, to test the involvement of mechanoreceptors in the responses to hypergravity.
• Key Results Number of metaxylem elements per xylem, apparent thickness of the secondary thickenings, and cross-section area of metaxylem elements in inflorescence stems increased in response to hypergravity. Gadolinium chloride suppressed the effect of hypergravity on the increase both in the thickness of secondary thickenings and in the cross-section area of metaxylem elements, while it did not suppress the effect of hypergravity on the increase in the number of metaxylem elements. Extensibility of secondary cell wall preparation decreased in response to hypergravity. Gadolinium chloride suppressed the effect of hypergravity on cell wall extensibility.
• Conclusions Hypergravity stimulus promotes metaxylem development and decreases extensibility of secondary cell walls, and mechanoreceptors were suggested to be involved in these processes.
Keywords: Arabidopsis thaliana, metaxylem, protoxylem, hypergravity, mechanoreceptor, secondary cell wall, secondary thickening, mechanical property, cell wall extensibility
INTRODUCTION
Primary and secondary cell walls play a major role in sustaining the aerial parts of land plants under normal gravity conditions (1 g) on earth. Chemical and mechanical properties of primary cell walls have been much investigated under altered gravity conditions. The content of matrix and cellulosic polysaccharides increases in terms of unit length in shoots under hypergravity conditions (Hoson et al., 1996; Soga et al., 1999a, b). On the other hand, the content of some cell wall components such as cellulose (Cowles et al., 1984; Nedukha, 1996; Hoson et al., 2002) and matrix polysaccharides (Hoson et al., 2002; Soga et al., 2002) decreases under microgravity conditions in space.
In addition to these polysaccharides in cell walls, lignin in the secondary cell wall gives rigidity to plant bodies. Development of vascular tissues, having intensively lignified secondary cell walls, has been postulated to be essential for the evolution of land plants for both physical and physiological reasons, i.e. for resistance to 1 g gravity and for adaptation to the dry environment on land. Previous studies indicate that lignin formation is suppressed under microgravity conditions in space (Cowles et al., 1984; Nedukha, 1996; Levine et al., 2001). Recently, it was found that prolonged hypergravity conditions promote formation of secondary cell walls and deposition of acetylbromide-extractable lignins in arabidopsis inflorescence stems (Tamaoki et al., 2006).
Gravitational force has been suggested to promote xylem formation in poplar (Populus tremula), when tension woods are formed (Andersson-Gunneras et al., 2003). Three-dimensional clinorotation has been demonstrated to reduce both the number of fibre cells in secondary xylem and the angle of cellulose microfibrils in the secondary cell wall in Prunus spachiana (Nakamura et al., 1999), and to change the morphology of fibre cells and vessel elements as well (Yoneyama et al., 2004). These observations lead to the suggestion that changes in gravitational conditions affect morphology and mechanical properties of xylem vessels.
Xylem plays an important role in strengthening plant bodies as well as in transporting water and minerals. It is a complex tissue composed of vessels, tracheids, fibres and parenchyma. In arabidopsis, secondary xylem does not develop in immature fluorescence stems shorter than 10 cm, although primary xylem does exist in them (Ko et al., 2004).
The weight of the inflorescence stem itself has been shown to be responsible for the induction of secondary xylem development in mature arabidopsis inflorescence stems, because a weight (2·5 g) placed on the top of the immature inflorescence stem facilitates secondary xylem development (Ko et al., 2004). These facts together with the finding that formation of secondary cell walls and lignin deposition are stimulated under hypergravity conditions (Tamaoki et al., 2006) suggest that gravitational stimulus promotes development of primary xylem as well as secondary xylem.
Both lanthanum and gadolinium ions, which are inhibitors of stretch-activated mechanosensitive ion channels, eliminated the inhibitory effect of hypergravity on shoot elongation in azuki bean (Vigna angularis) epicotyls and arabidopsis hypocotyls (Soga et al., 2004). It was demonstrated that gadolinium chloride suppressed the promoting effect of prolonged hypergravity on lignification in the immature arabidopsis inflorescence stem (Tamaoki et al., 2006). These findings indicate a possible involvement of mechanoreceptors in the perception of hypergravity stimulus in plants.
In the present study, using immature inflorescence stems of arabidopsis plants, it was investigated whether a change in the magnitude of gravitational force, particularly hypergravity stimulus, promotes primary xylem development and whether gadolinium chloride influences the effect of hypergravity on primary xylem formation.
MATERIALS AND METHODS
Plant material and hypergravity treatment
Arabidopsis thaliana (L.) Heynh ecotype ‘Columbia’ was used for the experiments. After surface sterilization with 95 % (v/v) ethanol for 10 s, each seed was planted on 1·0 % (w/v) agar containing Murashige and Skoog medium (Wako, Tokyo, Japan) in a test tube (15 mm in diameter, 105 mm in length), and kept at 4 °C for 3 d, and then allowed to grow at 22 °C for 20–26 d under continuous white light provided with a bank of fluorescent tubes (Mellow white 20 W daylight type; Toshiba, Tokyo, Japan), intensity being 130 µmol m−2 s−1 at plant level. Arabidopsis plants having inflorescence stems of 10 mm in length (arabidopsis growth stage number 5; Boyes et al., 2001) were selected, and then exposed to hypergravity at 300 g in the direction from shoot to root for 24 h at 25 °C in the dark using a centrifuge (SL-05A, Sakuma Seisakusho, Tokyo, Japan). After centrifugation, plants were grown at 22 °C for another 3 d for morphological analysis and 5 d for analysis of mechanical properties of the secondary cell wall preparation. For 1 g control (i.e. normal gravity), test tubes having plants with a 10-mm inflorescence stem were placed in the dark without centrifugation.
Treatment with gadolinium chloride was performed as follows: arabidopsis plants grown for 20–26 d having inflorescence stems 10 mm in length were selected to examine the effect of gadolinium chloride on stem thickening and vessel formation. An aliquot of 10 µL of an aqueous solution containing 40 mm gadolinium chloride or deionized distilled water was added to the agar medium to give a final concentration of 0·1 mm. At 3 h after application of gadolinium chloride, plants were exposed to hypergravity at 300 g for 24 h at 25 °C in the presence or absence of gadolinium chloride in the dark using the centrifuge. Subsequently, they were grown at 22 °C with or without gadolinium chloride for another 3 or 5 d at 22 °C under continuous white light as described above.
Clearing of inflorescence stems and fluorescence microscopy
The basal 10-mm part of the inflorescence stems of hypergravity-treated arabidopsis plants was cut and fixed in FAA solution containing 5 % (v/v) formalin, 5 % (v/v) acetic acid and 50 % (v/v) ethanol, for 12 h at room temperature, and cleared in 10 % (w/v) solution of KOH at 105 °C for 1 min. The cleared stems were rinsed in dH2O for 10 min and mounted on a glass slide, and then observed under a fluorescence microscope (BX-50 FLA; Olympus, Tokyo, Japan) equipped with a filter assembly for excitation by ultraviolet light (U-MWU: excitation filter, BP330-385; absorption filter, BA420; dichroic mirror, DM-400; Olympus). For fluorescence micrographs, frozen sections (50 µm thick) were prepared as described previously (Karahara and Shibaoka, 1992). Light photomicrographs were taken with a digital camera (Cool Snap cf, Nippon Roper, Tokyo, Japan) fitted to the microscope.
Embedding inflorescence stems in resin and quantitative morphological analysis of xylem vessels
Basal and apical 10-mm parts of the inflorescence stems of hypergravity-treated plants were cut and fixed in FAA solution as described above. The fixed segments were washed, dehydrated by passage through a graded ethanol and propylene oxide series, and embedded in Spurr's resin (Spurr, 1969) or Quetol resin (Nisshin EM, Tokyo, Japan). Transverse semi-thin sections (1 µm thick) were cut and observed under a bright field or a fluorescence microscope. Photomicrographs were taken as described above.
Quantitative morphological analysis of primary xylem vessels was carried out on digital fluorescence photomicrographs of cross-sections using software Openlab Darkroom (Improvision, Coventry, UK). Numbers of metaxylem elements were counted in each xylem in inflorescence stems. Leaf trace xylems were excluded from counting. The cross-section area of a xylem element, a measure of radial growth, was determined as an area surrounded by the outer margin of the secondary wall thickening. Apparent thickness of the secondary wall thickening of a xylem element was calculated as follows: besides the cross-section area of a xylem element, the cross-section area of the lumen of the xylem element was measured as the area surrounded by the inner margin of the secondary wall thickening. Apparent radius of a xylem element and of its lumen was calculated from the corresponding cross-section area, assuming that these areas were true circles. For the protoxylem vessels, apparent thickness of the secondary wall thickenings was not examined, since the protoxylem elements had helical thickenings and, thus, the cross-section area of the thickening depends on an angle formed between the thickening and the observed section, which is variable among protoxylem elements.
Because of the limited capacity of the centrifuge, the number of samples that could be handled in one experiment was too small to produce meaningful results for a quantitative morphological analysis. Thus, the experiment was repeated two or three times and the data were combined. For that reason, the biological repetition is once. The sample size, or the number of plants, presented in each table and figure is a sum of the number of plants examined in two or three separate experiments. The Mann–Whitney U-test (two tailed) was employed to determine whether the effects of the treatments were statistically significant.
Isolation of secondary cell wall preparation
The basal 10-mm part of the inflorescence stems of hypergravity-treated arabidopsis plants was cut and their apical ends were glued with a chemical instant adhesive (aron alpha; Toagosei, Tokyo, Japan) so that isolated xylems would not separate from each other after treatment with an enzyme solution. For isolation of secondary walls, the segments were treated with the enzyme solution containing 1 % (w/v) Sumizyme C (Shin Nihon Chemical, Anjo, Japan), 0·05 % (w/v) Pectlyase Y-23 (Kikkoman, Tokyo, Japan), 5 mm MES-KOH (pH 5·5) and 5 mg L−1 (w/v) chloramphenicol (Nacalai Tesque, Kyoto, Japan) for 5 d at 23 °C.
Measurement of the mechanical properties of secondary cell walls
Cell wall extensibility was measured with a tensile tester (Tensilon RTM-25, Toyo Baldwin, Tokyo, Japan) (Parvez et al., 1996). The isolated secondary cell wall preparations from the inflorescence stems of hypergravity-treated plants were washed with deionized water, immersed in methanol for at least 1 h and stored in methanol until use. The sample was rehydrated before the measurement by being immersed in deionized water. After rehydration, the secondary cell wall preparation was fixed between two clamps (the distance between the clamps was 3 mm) and stretched by lowering the bottom clamp at a speed of 20 mm min−1, until a load of 20 g was produced. The cell wall extensibility (strain stress−1, µm g−1) was measured with the load-extension curve immediately before the load of 20 g was produced.
The experiment to measure the mechanical properties of secondary cell walls was repeated twice. The Mann–Whitney U-test (two tailed) was employed to determine whether the effects of hypergravity were statistically significant.
RESULTS
Morphology of primary xylem vessels
Quantitative morphological analysis was performed for proto- and metaxylem elements in arabidopsis inflorescence stems (Fig. 1). In a part closer to the base of the stem (Table 1), the number of metaxylem elements per xylem in inflorescence stems significantly increased in response to the hypergravity stimulus of 300 g for 24 h. In addition, radial growth of metaxylem elements represented by an increase in the cross-section area of metaxylem elements, and the apparent thickness of the secondary wall thickenings of metaxylem elements substantially increased under hypergravity conditions. In a part closer to the apex of inflorescence stems (Table 2), hypergravity increased the number, the apparent thickness of the thickenings and the cross-section area of metaxylem elements, as observed in the part closer to the base of the inflorescence stem (Table 1). On the other hand, neither the number nor the cross-section area of protoxylem elements increased in the part closer to the base of the inflorescence stem in response to the hypergravity stimulus (Table 3).
Fig. 1.
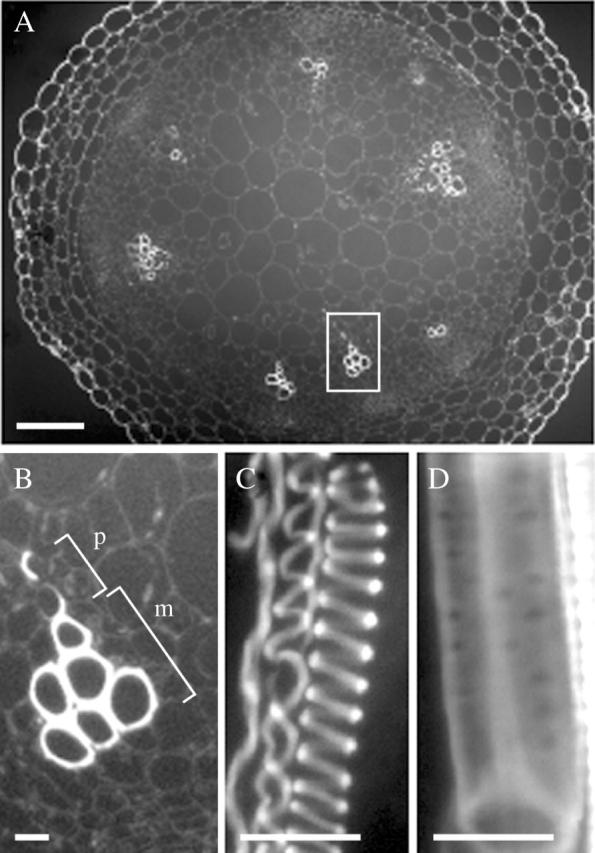
Xylem vessels in the basal region of inflorescence stems of arabidopsis observed under a fluorescence microscope. (A) Overview of a cross section of an inflorescence stem cut in the basal region. Scale bar = 100 µm. (B) Detail of xylem vessels in the area outlined in (A). m, Metaxylem; p, protoxylem. (C and D) Longitudinal view of the protoxylem (C) and the metaxylem (D) in a cleared stem. Scale bars = 10 µm.
Table 1.
Effects of hypergravity on the morphology of metaxylem elements in the basal region of arabidopsis inflorescence stems
| 1 g |
300 g |
||||
|---|---|---|---|---|---|
| Mean ± s.e. | n | Mean ± s.e. | n | Probability P | |
| Number of xylem elements (per xylem) | 5·17 ± 0·46 | 52 | 8·02 ± 0·70 | 54 | < 0·01 |
| Cross-section area of the elements (µm2) | 90·07 ± 2·39 | 277 | 113·1 ± 3·97 | 416 | < 0·0001 |
| Apparent thickness of the secondary thickenings (µm) | 1·26 ± 0·02 | 277 | 1·56 ± 0·03 | 416 | < 0·001 |
The number of plants analysed was 9–11, which is the sum of the number of plants examined in two separate experiments.
Table 2.
Effects of hypergravity on the morphology of metaxylem elements in the apical region of arabidopsis inflorescence stems
| 1 g |
300 g |
||||
|---|---|---|---|---|---|
| Mean ± s.e. | n | Mean ± s.e. | n | Probability P | |
| Number of xylem elements (per xylem) | 2·20 ± 0·25 | 40 | 3·30 ± 0·43 | 50 | = 0·048 |
| Cross-section area of the elements (µm2) | 25·38 ± 1·24 | 86 | 54·93 ± 3·94 | 166 | < 0·0001 |
| Apparent thickness of the secondary thickenings (µm) | 0·70 ± 0·01 | 86 | 0·87 ± 0·03 | 166 | < 0·001 |
The number of plants analysed was 9–10, which is the sum of the number of plants examined in two separate experiments.
Table 3.
Effects of hypergravity on the morphology of protoxylem elements in the basal region of arabidopsis inflorescence stems
| 1 g |
300 g |
||||
|---|---|---|---|---|---|
| Mean ± s.e. | n | Mean ± s.e. | n | Probability P | |
| Number of xylem elements (per xylem) | 2·71 ± 0·37 | 21 | 2·85 ± 0·44 | 27 | = 0·8136 |
| Cross-section area of the elements (µm2) | 45·3 ± 2·52 | 57 | 45·1 ± 2·63 | 77 | = 0·7138 |
The number of plants analysed was five, which is the sum of the number of plants examined in two separate experiments.
Extensibility of secondary cell wall preparation
Increases in the number of metaxylem elements per xylem, the apparent thickness of secondary thickenings and the cross-section area of metaxylem elements by the hypergravity stimulus may increase the physical strength of xylem vessels. To test this possibility, extensibility of secondary cell walls was measured using enzymatically isolated secondary cell wall preparations, which mainly consist of isolated xylem and, in some cases, interfascicular strands as well.
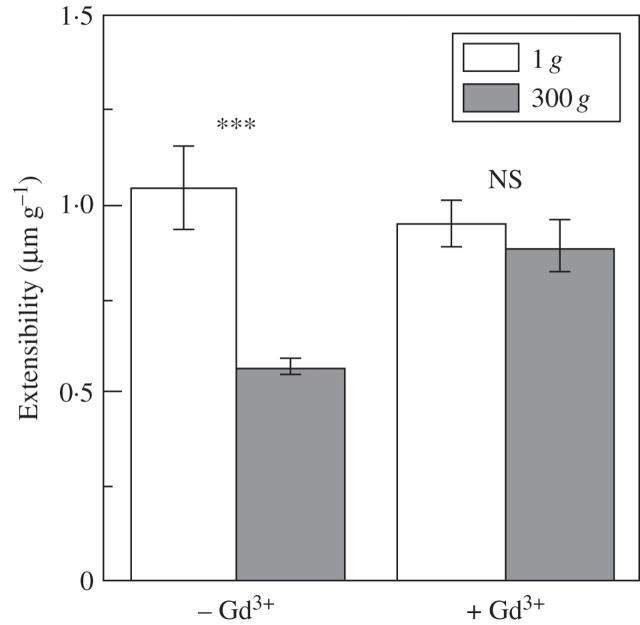
Physical strength of secondary cell wall preparations prepared from inflorescence stems of arabidopsis plants grown for 3 d after centrifugation at 300 g was found to be too weak to determine the mechanical properties of cell walls by applying 20 g strain using a tensile tester. So, the measurement of cell wall extensibility was done using secondary cell wall preparations obtained from plants grown for another 5 d after centrifugation. The extensibility of secondary cell wall preparations significantly decreased in response to hypergravity (see Fig. 3).
Effects of gadolinium chloride
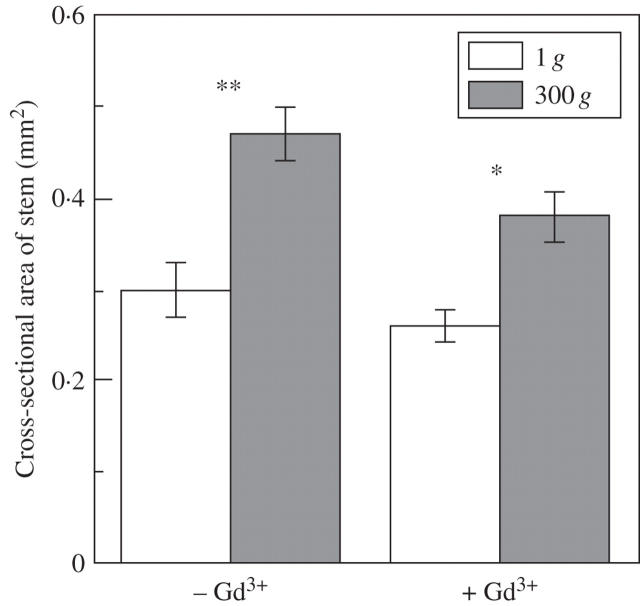
The cross-section area of inflorescence stems significantly increased in response to the stimulus. This effect of hypergravity was slightly suppressed by gadolinium chloride at 0·1 mm (Fig. 2). As shown in Table 4, on the other hand, gadolinium chloride suppressed the effect of hypergravity on an increase both in the apparent thickness of secondary thickenings and in the cross-section area of metaxylem elements in the basal region of inflorescence stems. The increase in the amount of xylem caused by hypergravity was not statistically significant but it tended to increase in the H2O control (Table 4). However, gadolinium chloride did not eliminate the effect of hypergravity on an increase in the number of metaxylem elements. Gadolinium chloride at this concentration suppressed the effect of hypergravity on a decrease in the extensibility of secondary cell wall preparation (Fig. 3).
Fig. 2.
Effects of hypergravity on cross-section area of arabidopsis inflorescence stems in the presence or absence of gadolinium chloride. White columns, 1 g; shaded columns, 300 g. Values are mean ± s.e. (n = 6–9, –Gd3+; n = 7, +Gd3+). *, P < 0·05; **, P < 0·01 (Mann–Whitney U-test, two tailed). The sample size is a sum of the number of plants examined in two separate experiments.
Table 4.
Effects of 0·1 mm gadolinium chloride on the morphology of metaxylem elements in the basal region of arabidopsis inflorescence stems
| 1 g |
300 g |
||||
|---|---|---|---|---|---|
| Mean ± s.e. | n | Mean ± s.e. | n | Probability P | |
| H2O | |||||
| The number of xylem elements (per xylem) | 6·07 ± 1·03 | 15 | 9·35 ± 1·39 | 17 | = 0·0684 |
| Cross-section area of the elements (µm2) | 89·34 ± 3·35 | 91 | 112·63 ± 4·47 | 159 | = 0·0078 |
| Apparent thickness of the secondary thickenings (µm) | 0·94 ± 0·02 | 91 | 1·11 ± 0·19 | 159 | < 0·0001 |
| Gadolinium chloride (0·1 mm) | |||||
| The number of xylem elements (per xylem) | 6·38 ± 0·63 | 37 | 8·63 ± 0·66 | 41 | = 0·017 |
| Cross-section area of the elements (µm2) | 79·58 ± 2·58 | 235 | 86·00 ± 3·39 | 354 | = 0·0628 |
| Apparent thickness of the secondary thickenings (µm) | 1·08 ± 0·02 | 235 | 1·09 ± 0·01 | 354 | = 0·7864 |
The number of plants analysed was three to seven, which is the sum of the number of plants examined in two separate experiments.
Fig. 3.
Effects of gadolinium chloride on hypergravity-induced decrease in extensibility of secondary wall preparation from arabidopsis inflorescence stems. White columns, 1 g; shaded columns, 300 g. Values are mean ± s.e. (n = 11–14, –Gd3+; n = 10–14, +Gd3+). ***, P < 0·005; NS, not significant (Mann–Whitney U-test, two tailed).). The samples were collected from three repetitions. The whole experiment was repeated twice to test reproducibility.
DISCUSSION
Plant growth and development under hypergravity
In general, intact mammals can survive about 8 g (Waldron and Brett, 1990). Higher plants, however, can withstand much greater gravity: pea (Pisum sativum) root tips can survive after centrifugation of up to 200 000 g for 5 min (Bouck, 1963). Pea can germinate and grow successibly at up to approx. 10 000 g (Waldron and Brett, 1990). In addition, the growth rate of root elongation in azuki bean is correlated with the logarithm of the magnitude of gravity, ranging from 1 to 300 g (Soga et al., 2005). In arabidopsis, hypergravity at 300 g substantially suppresses hypocotyl elongation and decreases cell wall extensibility, while such effects of hypergravity are not observed in the presence of gadolinium or lanthanum, an inhibitor of stretch-activated mechanosensitive ion channels (Soga et al., 2004). These facts indicate that hypergravity at 300 g used in the present study is not an extraordinary stimulus for arabidopsis plants.
Effects of hypergravity on primary xylem development
Xylem has two different origins; primary xylem differentiates from procambium derived from the apical meristem, and secondary xylem differentiates from vascular cambiums. In arabidopsis, the primary xylem is present in the inflorescence stem shorter than 10 cm in height, but secondary xylem does not occur in such immature stems (Ko et al., 2004). The present study focused on the analysis of the effect of hypergravity on the formation of primary xylem in arabidopsis using immature 10-mm-long inflorescence stems.
Primary xylem is subdivided into protoxylem and metaxylem (Esau, 1977). Protoxylem, which differentiates and matures earlier than metaxylem, is made up of small cells with an extensible wall thickening pattern, which is annular or helical, supporting continued cell elongation. On the other hand, the metaxylem, which differentiates after cell elongation has ceased, is a large cell with reticulate, scalariform or pitted wall pattern. Thus, the metaxylem walls rather than the protoxylem ones are considered to be responsible for strengthening plant bodies under gravitational conditions. As shown in Table 1, the stimulus of hypergravity at 300 g for 24 h substantially promoted the development of metaxylem elements in the immature inflorescence stem, but not of protoxylem elements (Table 3).
As demonstrated in Prunus tree stems, the number of fibre cells decreases in response to clinorotation (Nakamura et al., 1999). On the other hand, the number of metaxylem elements per xylem in both basal and apical regions of arabidopsis inflorescence stem increased in response to hypergravity stimulus (Tables 1 and 2). These findings indicate that an altered gravity environment affects the differentiation of metaxylem elements. A transcription factor gene ATHB-8, which is positively regulated by auxin, has been shown previously to modulate positively the activity of procambium and the differentiation of cambial cells (Baima et al., 2001). It will be interesting to see if ATHB-8 gene and auxin are also involved in the hypergravity-induced increase in metaxylem elements.
Although the number of metaxylem elements per xylem was larger in a region closer to the base of inflorescence stem (Table 1) than in a region closer to the apex (Table 2), there was no significant difference in the effect of hypergravity on the percentage increase in the number of metaxylems per xylem (Table 5). These results suggest that the effect of hypergravity on procambium activity is similar between apical and basal regions of arabidopsis inflorescence stems.
Table 5.
Percentage increase in the morphological parameters of metaxylem elements by hypergravity in the apical and basal regions of arabidopsis inflorescence stems
| Apical | Basal | |
|---|---|---|
| Number of xylem elements | 152 | 155 |
| Cross-section area of the elements | 213 | 126 |
| Apparent thickness of the secondary thickenings | 124 | 124 |
Radial growth of metaxylem elements, represented by an increase in the cross-section area of metaxylem elements, of the 1 g control was significantly smaller in the apical region than in the basal region in arabidopsis inflorescence stems (Tables 1 and 2), indicating that xylem vessels are less-developed in the apical region than the basal region. An increase in cross-section area of the metaxylem elements was promoted by hypergravity stimulus both in a region closer to the base (Table 1) and in a region closer to the apex (Table 2). Unlike an increase in the proportion of metaxylem in the xylem, the promoting effect of hypergravity on radial growth of metaxylem elements was much larger in a region closer to the apex than in a region closer to the base (Table 5), indicating that radial growth is more sensitive to the promoting effect of hypergravity in the apical region than in the basal region of the inflorescence stem.
The thickness of cell walls in metaxylem elements increased under hypergravity conditions both in the apical and the basal region of arabidopsis inflorescence stem (Tables 1 and 2). Unlike radial growth of metaxylem elements, there was no significant difference in the effect of hypergravity on the percentage stimulation of cell wall thickening in metaxylem elements in either the apical or the basal region. These results indicate that the thickening of cell walls in metaxylem elements is stimulated under hypergravity conditions, irrespective of whether they are in the apical or the basal regions of the inflorescence stems.
The mechanism by which hypergravity induces the thickening of cell walls in metaxylem elements is unknown. A possible mechanism might be involvement of the RDH3 gene, mutation of which causes a drastic reduction in wall thickness in fibres and vessels (Hu et al., 2003). In addition, RDH3 is also responsible for organization of the actin cytoskeleton (Hu et al., 2003). Disruption of the actin cytoskeleton by latrunculin B promotes a gravitropic response in maize roots (Hou et al., 2003). It may be possible that a hypergravity stimulus promotes the thickening of cell walls in metaxylem elements in arabidopsis inflorescence stems through influencing the expression of the RDH3 gene.
Effects of hypergravity on an increase in a diameter of the stem
It has been reported that a shorter period of hypergravity stimulus applied in the direction from shoot to root suppresses shoot elongation and increases the amount of cell wall polysaccharides per unit length in cress (Lepidium sativum) hypocotyls (Hoson et al., 1996) and in azuki bean epicotyls (Soga et al., 2003). In the inflorescence stem of arabidopsis, it had been found previously that prolonged hypergravity inhibits stem elongation, and increases the content of primary and secondary cell walls per unit length (Tamaoki et al., 2006). The present study demonstrated that the cross-section area of inflorescence stems increased 1·6-fold by hypergravity stimulus compared with the 1 g control (Fig. 2). These results, together with the fact that the dry mass of the major components in the shoot is cell wall material, suggest that a hypergravity-induced increase in the cross-section area of arabidopsis inflorescence stems is partly caused by enhanced formation of thickened cell walls.
Effects of hypergravity on cell wall extensibility of isolated xylems
Hypergravity has been shown to decrease the extensibility of cell walls in cress hypocotyls (Hoson et al., 1996) and in azuki bean epicotyls (Soga et al., 2001). Prolonged hypergravity significantly decreased extensibility of secondary cell walls prepared from arabidopsis inflorescence stems by enzyme digestion of the primary cell wall components (Fig. 3). The mechanism by which hypergravity modifies the mechanical properties of cell walls may be different between primary and secondary cell walls. In the primary cell wall of azuki bean epicotyls, hypergravity-induced decrease in cell wall extensibility is related to the metabolism of xyloglucan (Soga et al., 2004). On the other hand, a decrease in cell wall extensibility of the secondary cell wall preparations isolated from arabidopsis inflorescence (Fig. 3) may be caused by promoted formation of lignin under hypergravity conditions (Tamaoki et al., 2006). In addition to lignification, an increase in the number of metaxylem elements per xylem and cell wall thickening in metaxylem elements may also be responsible for a decrease in extensibility of secondary cell wall preparations under hypergravity conditions (Fig. 3).
Effects of gadolinium chloride
The inhibitory effect of a short period of hypergravity on epicotyl elongation in azuki bean seedlings is suppressed by application of gadolinium chloride (Soga et al., 2004), which blocks stretch-activated mechanosensitive ion channels (mechanosensors) in various organisms including plants (Ding and Pickard, 1993). Gadolinium chloride also eliminated the effect of hypergravity on the extensibility of the primary cell walls in azuki bean epicotyls (Soga et al., 2004). The present results revealed that 0·1 mm gadolinium chloride eliminated the inhibitory effect of prolonged hypergravity on cell wall extensibility in isolated secondary cell wall preparations mainly consisting of xylem (Fig. 3), suggesting the involvement of mechanoreceptors in this process.
Gadolinium chloride eliminated the promoting effect of prolonged hypergravity on an increase in the cross-section area of metaxylem elements and in the apparent thickness of secondary thickenings (Table 4). These findings strongly suggest that mechanosensors perceive gravitational stimulus and metaxylem elements may utilize it to influence radial growth and cell wall thickening of metaxylems in arabidopsis inflorescence stems. Gadolinium chloride, however, did not eliminate the promoting effect of the hypergravity stimulus on an increase in the number of metaxylems. These results indicate that the procambium activity causing an increase in the number of metaxylems may not be under the control of gadolinium-sensitive mechanoreceptors.
Radial growth of arabidopsis inflorescence stems represented by an increase in cross-section area was stimulated by prolonged hypergravity stimulus (Fig. 2). This stimulation of radial growth of the stem by the hypergravity condition was partially suppressed by gadolinium chloride (Fig. 2). In the present study, it has been demonstrated that the increase in the cross-section area of metaxylem elements and in the apparent thickness of the secondary thickenings was suppressed by gadolinium chloride. It has previously been shown that the amount of cell wall polysaccharide in a unit length increases in response to a short period of hypergravity, and this effect of hypergravity is eliminated by gadolinium chloride (Soga et al., 2004). Taken together, the increase in the content of cell wall polysaccharides, the increase in the cross-section area of metaxylem elements and in the apparent thickness of secondary thickenings in response to hypergravity conditions are suggested to contribute to the promotion of stem radial growth.
Acknowledgments
This study was supported, in part, by ‘Ground-Based Research Announcement for Space Utilization’ funded by the Japan Space Forum. We thank Shin Nihon Chemical Co. for kindly providing Sumizyme C.
LITERATURE CITED
- Andersson-Gunneras S, Hellgren JM, Bjorklund S, Regan S, Moritz T, Sundberg B. 2003. Asymmetric expression of a poplar ACC oxidase controls ethylene production during gravitational induction of tension wood. The Plant Journal 34: 339–349. [DOI] [PubMed] [Google Scholar]
- Baima S, Possenti M, Matteucci A, Wisman E, Altamura M, Ruberti I, Morelli G. 2001. The Arabidopsis ATHB-8 HD-Zip protein acts as a differentiation-promoting transcription factor of the vascular meristems. Plant Physiology 126: 643–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouck GB. 1963. An examination of the effects of ultracentrifugation on the organelles in living root tip cells. American Journal of Botany 59: 636–640. [Google Scholar]
- Boyes DC, Zayed AM, Ascenzi R, McCaskill AJ, Hoffman NE, Davis K, et al. 2001. Growth stage–based phenotypic analysis of Arabidopsis: a model for high throughput functional genomics in plants. The Plant Cell 13: 1499–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowles JR, Scheld HW, Lemay R, Peterson C. 1984. Growth and lignification of seedlings exposed to eight days of microgravity. Annals of Botany 54: 33–48. [DOI] [PubMed] [Google Scholar]
- Ding JP, Pickard BG. 1993. Mechanosensory calcium-selective cation channels in epidermal cells. The Plant Journal 3: 83–110. [DOI] [PubMed] [Google Scholar]
- Esau K. 1977. Anatomy of seed plants, 2nd edn. New York: Wiley.
- Hoson T, Nishitani K, Miyamoto K, Ueda J, Kamisaka S, Yamamoto R, et al. 1996. Effect of hypergravity on growth and cell wall properties of cress hypocotyls. Journal of Experimental Botany 47: 513–517. [DOI] [PubMed] [Google Scholar]
- Hoson T, Soga K, Mori R, Saiki M, Nakamura Y, Wakabayashi K, et al. 2002. Stimulation of elongation growth and cell wall loosening in rice coleoptiles under microgravity conditions in space. Plant and Cell Physiology 43: 1067–1071. [DOI] [PubMed] [Google Scholar]
- Hou G, Mohamalawari DR, Blancaflor EB. 2003. Enhanced gravitoropism of roots with a disrupted cap actin cytoskeleton. Plant Physiology 131: 1360–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Zhong R, Marrison III WH, Ye ZH. 2003. The Arabidopsis RHD3 gene is required for cell wall biosynthesis and actin organization. Planta 217: 912–921. [DOI] [PubMed] [Google Scholar]
- Karahara I, Shibaoka H. 1992. Isolation of Casparian strips from pea roots. Plant and Cell Physiology 33: 555–561. [Google Scholar]
- Ko JH, Han KH, Park S, Yang J. 2004. Plant body weight-induced secondary growth in Arabidopsis and its transcription phenotype revealed by whole-transcriptome profiling. Plant Physiology 135: 1069–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine LH, Heyenga AG, Levine HG, Choi JW, Davin LB, Krikorian AD, et al. 2001. Cell-wall architecture and lignin composition of wheat developed in a microgravity environment. Phytochemistry 57: 835–846. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Sassa N, Kuroiwa E, Negishi Y, Hashimoto A, Yamashita M, et al. 1999. Growth of Prunus tree stems under simulated microgravity conditions. Advances in Space Research 23: 2017–2020. [DOI] [PubMed] [Google Scholar]
- Nedukha EM. 1996. Possible mechanisms of plant cell wall changes at microgravity. Advances in Space Research 17: 37–45. [DOI] [PubMed] [Google Scholar]
- Parvez MM, Wakabayashi K, Hoson T, Kamisaka S. 1996. Changes in cellular osmotic potential and mechanical properties of cell walls during light-induced inhibition of cell elongation in maize coleoptiles. Physiologia Plantarum 96: 179–185. [Google Scholar]
- Soga K, Wakabayashi K, Hoson T, Kamisaka S. 1999a. Hypergravity increases the molecular size of xyloglucans by decreasing xyloglucan-degrading activity in azuki bean epicotyls. Plant and Cell Physiology 40: 581–585. [DOI] [PubMed] [Google Scholar]
- Soga K, Harada K, Wakabayashi K, Hoson T, Kamisaka S. 1999b. Increased molecular mass of hemicellulosic polysaccharides is involved in growth inhibition of maize coleoptiles and mesocotyls under hypergravity conditions. Journal of Plant Research 112: 273–278. [DOI] [PubMed] [Google Scholar]
- Soga K, Wakabayashi K, Hoson T, Kamisaka S. 2001. Gravitational force regulates elongation growth of Arabidopsis hypocotyls by modifying xyloglucan metabolism. Advances in Space Research 27: 1011–1016. [DOI] [PubMed] [Google Scholar]
- Soga K, Wakabayashi K, Kamisaka S, Hoson T. 2002. Stimulation of elongation growth and xyloglucan breakdown in Arabidopsis hypocotyls under microgravity conditions in space. Planta 215: 1040–1046. [DOI] [PubMed] [Google Scholar]
- Soga K, Wakabayashi K, Kamisaka S, Hoson T. 2003. Growth restoration in azuki bean and maize seedlings by removal of hypergravity stimuli. Advances in Space Research 31: 2269–2274. [DOI] [PubMed] [Google Scholar]
- Soga K, Wakabayashi K, Kamisaka S, Hoson T. 2004. Graviperception in growth inhibition of plant shoots under hypergravity condition produced by centrifugation is independent of that in gravitropism and may involve mechanoreceptors. Planta 218: 1054–1061. [DOI] [PubMed] [Google Scholar]
- Soga K, Wakabayashi K, Kamisaka S, Hoson T. 2005. Mechanoreceptors rather than sedimentable amyloplasts perceive the gravity signal in hypergravity-induced inhibition of root growth in azuki bean. Functional Plant Biology 32: 175–179. [DOI] [PubMed] [Google Scholar]
- Spurr AR. 1969. A low-viscosity epoxy resin embedding medium for electron microscopy. Journal of Ultrastructural Research 26: 31–43. [DOI] [PubMed] [Google Scholar]
- Tamaoki D, Karahara I, Schreiber L, Wakasugi T, Yamada K, Kamisaka S. 2006. Effects of hypergravity conditions on elongation growth and lignin formation in the inflorescence stem of Arabidopsis thaliana L. Journal of Plant Research (in press). [DOI] [PubMed]
- Waldron KW, Brett CT. 1990. Effects of extreme acceleration on the germination, growth and cell wall composition of pea epicotyls. Journal of Experimental Botany 41: 71–77. [Google Scholar]
- Yoneyama E, Ishimoto-Negishi Y, Sano Y, Funada R, Yamada M, Nakamura T. 2004. Morphological changes in wood stem of Prunus jamasakura under simulated microgravity. Biological Sciences in Space 18: 3–6. [DOI] [PubMed] [Google Scholar]