Abstract
• Background and Aims Sunflower cultivars exhibit a wide range of oil content in the mature achene, but the relationship between this and the dynamics of oil deposition in the achene during grain filling is not known. Information on the progress, during the whole achene growth period, of the formation of oil bodies in the components of the achene and its relationship with variations in final oil content is also lacking.
• Methods The biomass dynamics of achene components (pericarp, embryo, oil) in three cultivars of very different final oil concentration (30–56 % oil) were studied. In parallel, anatomical sections were used to follow the formation of oil and protein bodies in the embryo, and to observe pericarp anatomy.
• Key Results In all cultivars, oil bodies were first observed in the embryo 6–7 daa after anthesis (daa). The per-cell number of oil bodies increased rapidly from 10–12 daa until 25–30 daa. Oil bodies were absent from the outer cell layers of young fruit and from mature pericarps. In mature embryos, the proportion of cell cross-sectional area occupied by protein bodies increased with decreasing embryo oil concentration. The sclerenchymatic layer of the mature pericarp decreased in thickness and number of cell layers from the low-oil cultivar to the high-oil cultivar. Different patterns of oil accumulation in the embryo across cultivars were also found, leading to variations in ripe embryo oil concentration. In the high-oil cultivar, the end of oil deposition coincided with cessation of embryo growth, while in the other two cultivars oil ceased to accumulate before the embryo achieved maximum weight.
• Conclusions Cultivar differences in mature achene oil concentration reflect variations in pericarp proportion and thickness and mature embryo oil concentration. Cultivar differences in protein body proportion and embryo and oil mass dynamics during achene growth underlie variations in embryo oil concentration.
Keywords: Sunflower, oil body, lipid accumulation, protein body, pericarp
INTRODUCTION
Oil concentration of the sunflower fruit (achene) at maturity is known to vary among cultivars, and much effort by plant breeders has been aimed at increasing the oil concentration in mature achenes of modern oilseed cultivars (e.g. López Pereira et al., 2000; León et al., 2003). Attempts have also been made to identify the effects of factors such as radiation (Dosio et al., 2000; Aguirrezábal et al., 2003), temperature (Harris and Bojinger, 1980; Rondanini et al., 2003) and time of sowing (de la Vega and Hall, 2002) on this trait. A limited amount of attention has been paid to the dynamics of oil deposition in the achene in individual cultivars throughout the whole of the achene growth phase in single cultivars (e.g. Villalobos et al., 1996). However, studies of particular issues affecting final oil concentration (e.g. the formation of oil and protein bodies, embryo/pericarp proportions, etc.) have usually covered only part of the achene growth phase in single cultivars or related only to the situation found in the mature achene. Some work has been done on the changes in achene histology during oil and protein body formation (Tzen et al., 1993; Bolyakina and Raikhman, 1999), but again coverage of these processes during the achene growth phase is only partial and cultivar differences have usually not been an object of this research. Consequently, although much is known about oil and protein deposition in sunflower achenes at levels which range from the biochemical to gross achene morphology, and about some of the factors which control achene final oil concentration, there is only partial understanding of the origin of cultivar differences. The work reported here aimed at documenting the growth of achene components (pericarp, embryo, oil) throughout the whole of the achene growth phase in three cultivars with contrasting final achene oil concentration and at providing a histological basis for the observed changes in component mass.
Gross anatomy of the sunflower fruit, particularly the pericarp, is relatively well known (Hanausek, 1902; Roth, 1977; Sáenz, 1981; Percie Du Sert and Durrieu, 1988; Denis et al., 1994; Lindström et al., 2000). Genetic factors are important in determining the constitution of the pericarp, although environmental and management practices also affect it (Baldini and Vannozzi, 1996). In a study of 27 different populations of sunflower, McWilliam and English (1978) found that the embryo : achene ratio is a good indicator of achene oil concentration, and changes in embryo : pericarp ratios have been a feature of breeding for modern oilseed sunflower cultivars (Sadras and Villalobos, 1994; López Pereira et al., 2000). However, it is not clear how these variations in mature achene pericarp : embryo ratios between cultivars arise during the growth of the achene, as dynamics of these components of the achene have usually been studied in individual cultivars (e.g. Villalobos et al., 1996; Ploschuk and Hall, 1997).
In sunflower, lipid accumulation rate in the embryo is low in the days following anthesis, and later increases to a fairly stable rate which is maintained until close to physiological maturity (Villalobos et al., 1996; Connor and Hall, 1997; Rondanini et al., 2003). Available biochemical evidence is consistent with these observations. Thus, Garcés and Mancha (1989) observed that the incorporation of radioactive precursors into polar lipids (components of the cellular membranes) predominated until 12 d after anthesis (daa), preferential incorporation in triacylglycerol synthesis taking place only from 12 to 25 daa. Reserve lipids in sunflower and other oilseeds are laid down in subcellular structures known as oil bodies, formed by a hydrophobic matrix of triacylglycerols surrounded by a layer of phospholipids, on which proteins called (oleosins and caleosins) are deposited (Huang, 1992; Murphy, 2001). Oleosins and the phospholipid layer confer stability to the oil bodies and mantain them as separate entities. There has been considerable interest in the ontogeny of oil bodies (Tzen et al., 1993; Murphy, 2001). A recent study in sunflower (Beaudoin et al., 1999) revealed that oleosins are synthesized on endoplasmic reticulum-bound ribosomes and later transferred to areas of the endoplasmic reticulum involved in oil-body biosynthesis, and Mazhar et al. (1998) also found that oleosin synthesis began in early stages of embryo growth. Tzen et al. (1993) found average diameters of between 0·65 and 2·0 μm for rape, mustard, cotton, linseed, corn, peanuts and sesame seed oil bodies, and reported that oil-body size in 11 more species (including sunflower) were within this range. What is still lacking is a comparative study of the dynamics of oil deposition in achene structures across cultivars and the provision of histological information on the connections between oil-body formation in the various parts of the achene and overall progress of oil deposition in the achene.
Besides oil, the embryo cells of sunflower contain protein bodies as a reserve component (Vintéjoux, 1992; Shoar-Ghafari and Vintéjoux, 1997; Bolyakina and Raikhman, 1999). Steer et al. (1984), Goffner et al. (1988) and Ploschuk and Hall (1997) found that protein accumulation in sunflower occurs at a greater rate during the first part of the achene filling period. Achene oil and protein concentrations in the mature achene are often found to exhibit negative associations (Blanchet and Merrien, 1982; Connor and Sadras, 1992; López Pereira et al., 2000). Across cultivars, however, higher mature achene oil concentrations are only partially associated with reduced protein production; the increase in oil concentration at achene maturity was somewhat more in proportion than the decrease in protein concentration (López Pereira et al., 2000). The connections between oil body and protein body density in embryo tissues of cultivars of differing final oil concentration has not been explored.
Work reported in this paper aimed at studying the dynamics of the mass of achene components (pericarp, embryo, oil) in three sunflower cultivars differing in final oil concentration. Highest growth rates for each achene component are staggered in time and the pericarp ceases to grow well before the other components achieve maximum mass (e.g. Villalobos et al., 1996). Consequently, particular attention was paid to the timing of initiation and when the growth of each component ended, and to the rate of growth of each component during its major phase of growth. In parallel, the dynamics of oil-body formation in the embryo were followed, the area of protein bodies and oil bodies was compared in sections of embryo tissues, and cultivar differences in pericarp histology were documented.
MATERIALS AND METHODS
Crop management and treatments
Experiments were carried out using three commercial sunflower cultivars of contrasting mature achene oil concentration (‘Dakar’, approx. 30 % oil; ‘11051’, approx. 44 % oil; and ‘Prosol 35’, approx. 56 % oil) (Table 1). Seeds of the first two cultivars were provided by Advanta Argentina, and the last by Produsem SA, Pergamino Argentina. Crops were hand sown on 17 November 1997 on a vertic argiudol at the Facultad de Agronomía, Universidad de Buenos Aires, using a fully randomized complete block design with three replicates (3·5 m × 3·5 m plots). The plots were totally surrounded by two border rows. Crop density was 4·8 plants m−2, with 0·3 m between plants in the rows and 0·7 m between rows. Nitrogen was applied as urea, at 50 kg ha−1, 20 d after emergence. Insects were controlled with carbofuran (5 L ha−1 at 48 %) applied before sowing, and plots were weeded manually until the canopy closed. When rainfall was insufficient, irrigation was applied. During the achene filling period the mean daily temperature was 22·5 °C and mean daily global radiation 19·8 MJ m−2d−1.
Table 1.
Peripheral achene final mature dry weight in experiments conducted during the summers of 1994–95, 1996–97 and 1997–98 for three cultivars of sunflower differing in oil content at maturity
| Mature achene dry weight (mg) |
Mature achene oil concentration (%, d. wt basis) |
|||||
|---|---|---|---|---|---|---|
| Cultivar | 1994–95 | 1996–97 | 1997–98 | 1994–95 | 1996–97 | 1997–98 |
| ‘Dakar’ | 164·5 ± 0·92a | 134·3 ± 1·35b | 158·5 ± 0·53c | [33·1 ± 0·19a] | [33·3 ± 1·06a] | 30·4 ± 0·13b |
| ‘11051’ | 84·3 ± 2·03a | 93·5 ± 1·56b | 112 ± 0·23c | [44·9 ± 0·71a] | [42·7 ± 0·93a] | 44 ± 0·38a |
| ‘Prosol 35’ | 70·5 ± 0·77a | 89·5 ± 0·75b | 93·4 ± 0·46c | [44·7 ± 0·44a] | [50·5 ± 1·43b] | 55·8 ± 0·22c |
Values of the mature achene oil concentration shown in the last three columns were determined for achenes from all positions on the capitulum in 1994–95 and 1996–97 (values in square brackets) and from achenes from the peripheral position only in 1997–98. Values are means (n = 3) ± s.e. and are derived from bilinear functions fitted to experimental data.
Within each column, values followed by different letters are significantly (P < 0·05) different.
Sampling protocol
Approximately 2 months after sowing (16 January 1998 for the low and high concentration cultivars, and 19 January 1998 for the medium oil cultivar), 40 plants per plot were tagged at the start of anthesis of each plant. Twenty of these plants were assigned to samplings for achene component mass dynamics and achene histology, the remaining 20 to samplings for achene oil concentration. To study growth in mass of the pericarp and embryo, plants were sampled three times a week over a period of 5 weeks, commencing 0 days after anthesis (daa) for a total of 16 samplings. At each sampling five fruits per plant from each of three plants were taken from the outer region of the capitulum (first four rings). Fruit were removed using fine forceps, taking care not to damage the head. The same head was not sampled more than five times and fruit was never taken from areas close to a previous sample on the same head. Embryos were manually separated from the surrounding ovary tissue and the dry weight of embryos and pericarps determined. To determine achene oil content dynamics, all fruit from the outermost four rings of the head were collected from one plant per replicate on the same dates as the samples used for embryo and pericarp mass dynamics. Each plant was sampled only once. Achenes were dried for at least 48 h at 60 °C, and achene oil percentage was determined on 10-g subsamples of entire dried seeds using nuclear magnetic resonance (NMR) analysis. These data were used to calculate embryo oil content and concentration (on a dry-weight basis) using the equations:
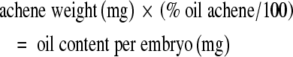 |
(1) |
 |
(2) |
All values for oil concentration in the whole achene or in the embryo shown in this paper are on a dry-weight basis.
At 1, 4–5, 6–7, 8–9, 10–12, 14–15, 25–30 and 40–45 daa, additional samples were taken for achene histology from plants assigned to sampling for embryo and pericarp mass dynamics. Particular care was taken to ensure that these fruits were representative (in size and appearance) of the achenes that formed part of the larger sample taken for achene component mass dynamics. Groups of three peripheral flowers/developing fruits were sampled from three different plants at each sampling date. Embryo sections were prepared for all samples starting with the 4–5 daa harvest, and pericarp sections were prepared from samples harvested 1, 8–9 and 40–45 daa.
During the summers of 1994–95 and 1996–97 experiments similar to the one described above were conducted at the same site using the same protocol with a single exception : achene oil contents were determined on samples made up of all achenes on the capitulum instead of being restricted to peripheral achenes only.
Estimation of duration and rates of growth of embryo, pericarp and oil
Duration of, and rates of, mass increase for embryo, pericarp and oil were estimated using the non-linear routine of SYSTAT (Wilkinson, 1986) to fit bilinear regressions to the data. This routine uses an optimization technique to determine the breakpoint between linear sections, the slope and intercept of an initial straight line defined by: p = a1 + bx for x < x0; and of a second straight line defined by: p = a2 for x > x0; where p is the weight of pericarp, embryo or oil, a1 and a2 are the intercepts and b the slope. x is expressed in units of daa and x0 is the point of intersection between the two straight lines indicating the end of the growth of the achene component. The duration of achene component growth periods was defined as the interval between anthesis of the flowers on the peripheral portion of the head and x0 (pericarp); or the interval between the intercept of the initial line on the time axis and x0 (embryo). The intercepts on the time axis of the bilinear functions were taken as the effective initiation of embryo or oil deposition and the slopes of the initial time as the effective rates of growth of these achene components. Embryo dry weight increase and oil deposition begin with some delay in respect to anthesis, so values for the first three samples (5, 8 and 10 daa) were not used when fitting bilinear functions. Because durations and rates of achene component growth can be affected by temperature, data were also expressed on a thermal time basis with a base temperature of −1 °C (Chimenti et al., 2001) and bi-linear functions fitted to mass/thermal time relationships. Data for air temperatures during the experiment were obtained from a standard meteorological station situated 500 m from the plots.
Dynamics of oil-body formation
Freshly collected fruits were dissected and either whole embryos or small cubical portions (sides 0·5–1 mm long) were cut from the different embryo parts. Theses were placed in 3 % glutaraldehyde in phosphate buffer (pH 7·2), dehydrated in an ascending series (70–95 %) of ethanol concentrations and included in JB4 resin (Polysciences Inc., Warrington, USA) for optical microscopy, or in Spurr resin (Spurr, 1969) for electron microscopy, following the standard methodology of O'Brien and McCully (1981). Semi-thin (1 μm) and ultra-thin (70–80 Å) sections were obtained with a Ultracut III Reichert ultramicrotome (Reichert-Jung, Austria). Semi-thin sections of early (low lipid content in cells) samplings stained best with 0·1 % Sudan black, and later (higher lipid content) sections stained better with 0·5 % toluidine blue. Consequently, staining procedures were adjusted according to achene age. Sections were photographed with a Zeiss Axioplan (Oberkochen, Germany) optical microscope. Ultra-thin sections were stained in uranile acetate and lead citrate (Reynolds, 1963) and photographed with a Jeol JEM/1200 EXII (Jeol, Japan) transmission electron microscope.
Intra-embryo distribution and mean size of oil bodies at 40–45 daa
Cotyledons, shoot axis and radicle of mature embryos were sectioned freehand approx. 25–30 μm thick. The sections were stained in 0·1 % aqueous nile blue and viewed with a Zeiss fluorescence microscope (Axioplan, Oberkochen, Germany) to record the distribution pattern of oil bodies among the various parts of the embryo. To determine oil body final size, the isolation procedure of Tzen et al. (1993) was followed with slight modifications. The tissue (2·5 g of cotyledon of mature embryos per 10 mL grinding medium) was ground with a mortar at 20 °C in a grinding medium containing 0·6 m sucrose, 1 mm EDTA, 10 mM KCl, 1 mm MgCl2, 2 mm DTT and 0·5 m Tricine buffer adjusted to pH 7·5 with KOH. The homogenate was filtered and a 7·5-mL portion of the filtrate was placed in a15-mL centrifuge tube, and 7·5 mL of a flotation medium (grinding medium containing 0·4 m instead of 0·6 m sucrose) was layered on top. The tube was centrifuged at 11 000 g for 30 min in a Sorvall RC5C ultracentrifuge. The supernatant was collected and resuspended in 7·5 mL of grinding medium containing an additional 7·5 mL 2 m NaCl. The resuspended material was placed in a 15-mL centrifuge tube, 7·5 mL of flotation medium (grinding medium containing 7·5 mL 2 m NaCl and 0·25 m instead of 0·6 m sucrose) was layered on top, and the tube was centrifuged at 11 000 g for 30 min. The supernatant was collected, and the oil bodies were stained with 0·1 % aqueous nile blue and examined by fluorescence microscopy.
Distribution, size and relative importance of protein bodies in the mature achene
Whole mature embryos harvested at 40–45 daa were embedded in paraffin and serially cut at 10–15 μm with a Minot-type rotary microtome. Sections were stained with safranin-fast green (Johansen, 1940) to observe the protein bodies. Photographs of selected embryo tissues (mesophyll of cotyledon, and parenchyma of the shoot–radicle axis) were taken with a Zeiss fluorescence microscope. The photographs were digitized with a Hewlett Packard Scan Jet 4c scanner, and images of at least ten cells per tissue per cultivar were analysed with Image Tool 1.28 for Windows (Wilcox et al., 1996) to determine per-cell average sectional area of protein bodies, and whole cell sectional area.
Pericarp structure
Thin slices (approx. 2 mm thick) were cut from the central region of young developing fruits (1 daa and 8–9 daa) and mature fruits (40–45 daa) of all three cultivars. Slices were placed in 3 % glutaraldehyde in phosphate buffer (pH 7·2), dehydrated in an ascending series (70–95 %) of ethanol concentrations, and included in Historesin (Leica) (young fruits) or JB4 resin (Polysciences, Inc.) (mature fruits). Semi-thin (1–3 μm) sections were obtained with a Ultracut III Reichert ultramicrotome and stained with 0·1 % cresyl violet. In addition, portions of mature fruits of all three cultivars were embedded in paraffin and serially cut at 10–15 μm with a Minot-type rotary microtome, and sections were stained with safranin-fast green (Johansen, 1940) to observe pericarp thickness. Two measurements of mature pericarp thickness were made on each of five different samples per cultivar.
Statistical analyses
Standard analyses of variance were performed on the data, and the Tukey test was used to determine significance of differences between means (Steel and Torrie, 1980). In the text, means are given ± s.e.
RESULTS
Embryo structure and oil-body dynamics
At 5–6 daa embryo length was 0·5–0·8 mm in ‘11051’ and ‘Prosol 35’, and 0·8–1 mm in ‘Dakar’, and the cotyledons and the hypocotyl–radicle axis were already discernible. Maximum embryo length was reached at 16–17 daa, ranging from 8·5–10 mm in ‘11051’ and ‘Prosol 35’, and 13–16 mm in ‘Dakar’. Mature achene weights decreased in the order ‘Dakar’ → ‘11051’ → ‘Prosol 35’, while mature achene oil concentration increased in the same order (Table 1). At a very early growth stage (4–5 daa) cells in all tissues were generally isodiametric and scarcely differentiated, with a dense central nucleus and optically clear cytoplasm (Fig. 1A). During the following 4 weeks cells expanded and tissues attained moderate histological differentiation. Cell walls remained thin and intercellular spaces scarce, while large quantities of oil and reserve protein accumulated in oil bodies and protein bodies.
Fig. 1.
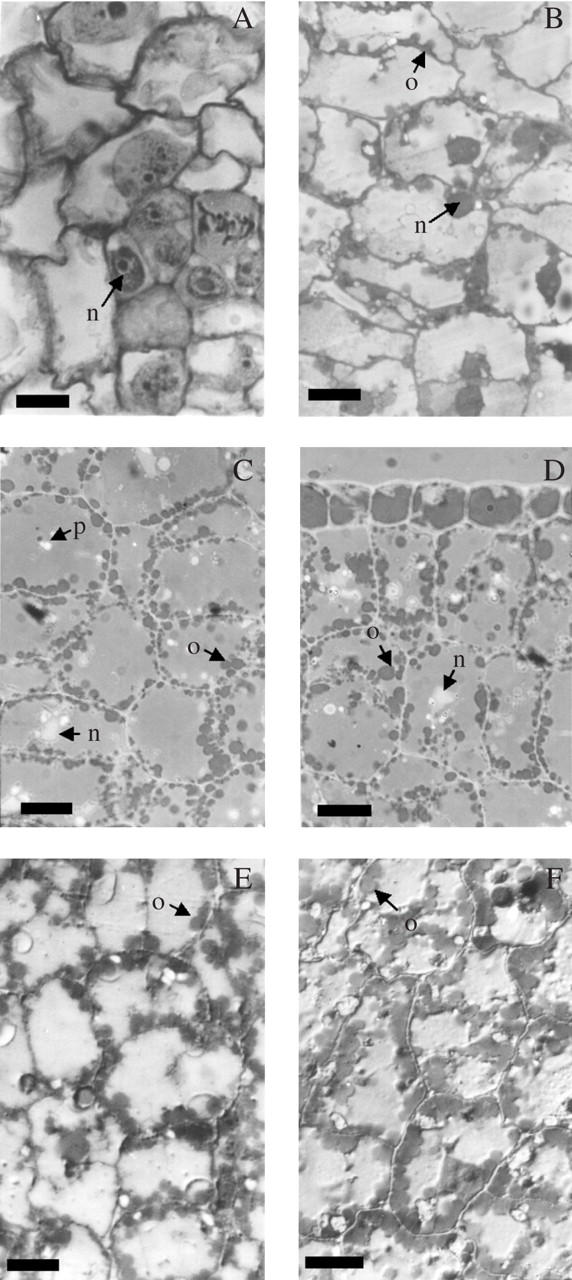
Transections of sunflower cotyledons at early developmental stages: (A) ‘Prosol 35’ at 4–5 daa—cells show meristematic features throughout the tissue; (B) ‘Prosol 35’ at 6–7 daa—oil bodies become visible; (C) and (D) ‘Dakar’ and ‘11051’, respectively—at 8–9 daa oil bodies occupy only the cell periphery; (E) and (F) ‘Prosol 35’ at 10–12 daa and 14–15 daa, respectively. Fixation with 3 % glutaraldehyde, included in JB4 resin and stained with Sudan black 0·1 %. n, Nucleus; o, oil body; p, protein body. Scale bars = 10 μm.
In all three cultivars the dynamics of oil-body formation and distribution among embryo tissues were similar. The first oil bodies (up to ten per cell transection) appeared in the cotyledons at 6–7 daa as relatively uniform globular bodies approx. 2·3 μm in diameter, adjacent to the cell wall but distinctly separated from each other (Fig. 1B). At 8–9 daa oil-body number per cell had increased (Fig. 1C–D). Oil bodies appeared simultaneously in the epidermis and mesophyll of the cotyledons (Fig. 1D). At 10–12 daa (Fig.1E) and 14–15 daa (Fig. 1F) oil bodies (15–20 per cell transection) occupied much of the periphery of the cell and started appearing in the centre (Fig. 1F) of the cell. At 25–30 daa there were 30–35 oil bodies per transection, and oil bodies occupied most of the cell transection (Fig. 2A). There was no detectable difference in oil body number per cell transection between samples taken at 25–30 daa and at 40–45 daa (Fig. 2B–D).
Fig. 2.
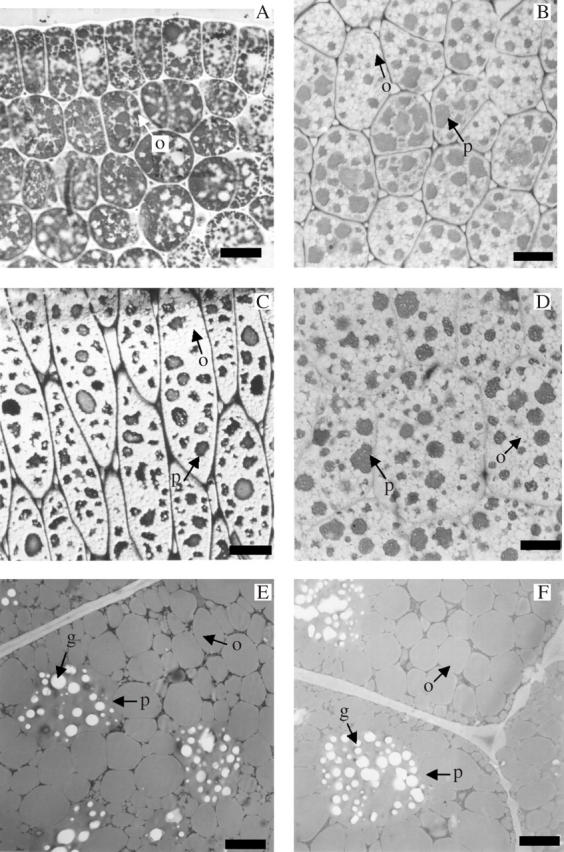
Transections of sunflower cotyledons at late developmental stages: (A) ‘Prosol 25’ at 30 daa—the number of oil bodies increases considerably and the light intercellular areas correspond to the cytoplasm and protein bodies (fixation with 3 % glutaraldehyde, inclusion in JB4 resin and coloration with Sudan black 0·1 %); (B), (C) and (D) 40–45 daa (‘Dakar’, ‘11051’ and ‘Prosol 35’, respectively)—the protein bodies are stained dark, oil bodies are lighter due to the staining used (0·5 % toluidine blue) (fixation with glutaraldehyde 3 %, inclusion in JB4 resin. (E) and (F) Ultra-thin sections showing ultrastructural details of the cotyledon at 40–45 daa (‘Dakar’ and ‘Prosol 35’, respectively). Note the protein bodies, the compression of the oil bodies and the reduced area occupied by other cytoplasm fractions. Fixed with glutaraldehyde 3 %, inclusion in Spurr resin and stained with uranile acetate and lead citrate. g, Globoids; o, oil body; p, protein body. Scale bars: A–D = 10 μm; E and F = 2 μm.
In the mature embryos (40–45 daa) the nucleus, the oil bodies and the protein bodies were the most conspicuous cell components (Fig. 2B–D). The oil bodies in most cells were tightly packed and became somewhat polygonal because of compression, but their individuality was still preserved (Fig. 2E, F). Oil bodies were present in the epidermis and mesophyll of the cotyledons, and in the epidermis, cortex and central cylinder of the hypocotyl–radicle axis of mature embryos of all three cultivars (data not shown), with apparently similar abundance in all tissues. Oil bodies isolated from embryos of the three cultivars had similar shapes and diameters ranging from 1 to 4 μm. Mean oil-body diameter tended to increase in the sequence low-oil to medium-oil to high-oil cultivar (1·96 ± 0·16 μm, 2·13 ± 0·18 μm and 2·5 ± 0·3 μm, respectively) but these differences were not statistically significant. The diameters of isolated oil bodies were consistent with those observed in cotyledon sections of the three cultivars.
Protein bodies
Protein bodies became visible at 8–9 daa as numerous, small unstained bodies which contrasted with the moderately stained cytoplasm and the Sudan-black dark-stained oil bodies (Fig. 1C, D). At embryo maturity, toluidine blue-stained protein bodies were easily distinguishable as large, dark, approximately globular structures with irregular contours due to pressure from oil bodies (Fig. 2B–2F). In ultra-thin sections protein bodies showed many electron-clear globoids (Fig. 2B–2F). Protein bodies appeared to be less abundant in mature embryos of the high-oil than in the low-oil cultivar (cf. Fig. 2B with Fig. 2C). The average areas occupied by protein bodies in the whole cell sections of the mature embryo were 3·72 ± 1·16 μm2 cell–1, 2·81 ± 1·06 μm2 cell–1 and 2·86 ± 1·45 μm2 cell–1, equivalent to 35·8 % ± 1·94, 23·6 % ± 1·38 and 24·9 % ± 1·23 of the whole cell area, in the low-, medium- and high-oil cultivars, respectively. These differences in percentage of cell area occupied by protein bodies were statistically significant (P < 0·05).
Pericarp anatomy
Transections of very young developing fruits (1 daa, Fig. 3A; 8–9 daa, Fig. 3B) showed no traces of oil bodies at any pericarp layer. Sections of mature achenes (Fig. 3C–E) showed several layers (epidermis, hypodermis, black layer or pigmented cells, sclerenchymatic tissue and parenchymatic tissue, the latter adjacent to the seed), but oil bodies were not present in any of these cell types. The sclerenchymatic layer was noticeable due to the massive amount of thick-walled cells, distributed in discrete areas separated by thin parenchymatic radii. The thickness of secondary walls decreased towards the pericarp interior (best seen in Fig. 3D and E). The average thickness of the sclerenchymatic layer decreased from the low-oil cultivar to the medium- and high- oil cultivars (62·3 ± 0·35 μm, 34·1 ± 0·3 μm and 23·9 ± 0·5 μm, respectively). These values reflected differences in the number of cell layers, which was higher in the low-oil cultivar (18·6 ± 0·2, Fig. 3C) than in the medium-oil (11·6 ± 0·3, Fig. 4D) and in the high-oil cultivars (6·8 ± 0·1, Fig. 4E). The differences among cultivars for pericarp thickness and number of cell layers were highly significant (P < 0·01).
Fig. 3.
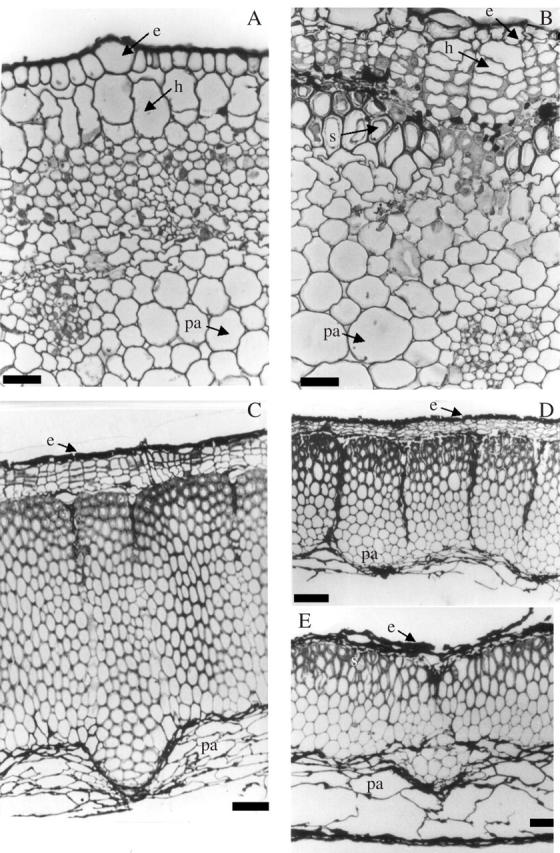
Transections of sunflower pericarp: (A and B) early developmental stages of ‘Prosol 35’. (C), (D) and (E) mature pericarp (‘Dakar’, ‘11051’ and ‘Prosol 35’, respectively). Note differences in thickness of sclerenchymatic tissue between cultivars. Fixation with 3 % glutaraldehyde, inclusion in JB4 resin and stained with 0·1 % cresyl violet. e, Epidermis; h, hypodermis; pa, parenchyma; s, sclerenchyma. Scale bars: A and B = 10 μm; C = 100 μm; D and E = 45 μm.
Fig. 4.
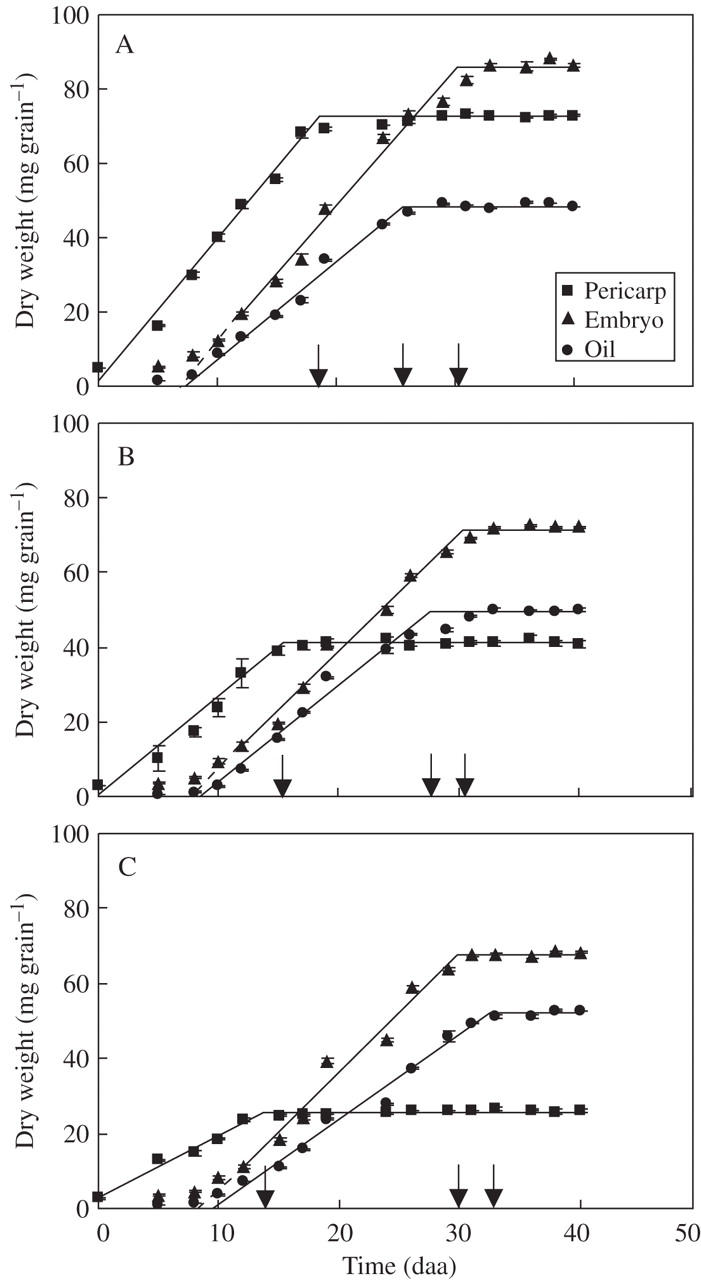
Dynamics of pericarp, embryo and oil weights per achene for three sunflower cultivars ‘Dakar’ (A), ‘11051’ (B) and ‘Prosol 35’ (C). Each point represents the mean of three replicates, vertical bars are ± 1 s.e. The continuous lines show the adjusted bi-linear functions (r2 range 0·89–0·99) and the dashed lines show the extrapolations used to estimate the start of the effective filling. The arrows indicate the end of the oil growth periods for the three achene components. Mean values for timing of initiation and rates and durations of growth for each achene component are given in Table 2–Table 4.
Pericarp and embryo growth and oil deposition
The growth patterns of achene components (pericarp, embryo, oil) were broadly similar across the three cultivars, with the pericarp growing first and rapid increases of embryo and oil mass taking place later, and the pericarp ceasing to grow before the embryo and oil components had reached their maximum values (Fig. 4). The detail of these patterns, however, varied greatly between cultivars (Tables 2–4). Differences in initial mass and effective rates and durations of growth of the pericarp were the basis for very different final pericarp weights across cultivars (Table 2 and Fig. 4). The effective durations of embryo growth did not differ among cultivars, but embryos of ‘Dakar’ grew faster than those of the remaining two cultivars (Table 3). Embryo weights at the first harvest at which this component could be distinguished (5 daa) were 5·3 ± 0·31, 3·4 ± 0·24 and 3·5 ± 0·33 mg for low-, mid- and high-oil cultivars, respectively. The outcome of these differences in initial mass and rate of growth was that mature embryo weights decreased from the low oil cultivar to the high oil one, although the range between the largest and smallest embryos was much more restricted than for the corresponding pericarps. There was some indication that initiation of rapid embryo growth lagged further behind anthesis in the mid- and high-oil cultivars with respect to the low-oil one (Table 3). However, the overall duration of achene growth (i.e. time until effective initiation of embryo growth + effective duration of embryo growth) was very similar between cultivars, unlike what was found for the pericarps (Fig. 4 and Table 2). Consequently, the final proportion of embryo in the achene differed widely across cultivars (Fig. 4 and Table 3).
Table 2.
Dynamics of the growth of pericarp in the three cultivars of sunflower differing in oil content at maturity
| Pericarp weight (mg) |
Duration of growth |
Growth rate |
||||
|---|---|---|---|---|---|---|
| Cultivar | Initial (0 daa) | Final | daa | °Cd | mg d−1 | mg (°Cd)−1 |
| ‘Dakar’ | 4·9 ± 0·90a | 72·7 ± 1·12a | 18·6 ± 0·21a | [429·2 ± 8·7a] | 3·7 ± 0·07a | [0·16 ± 0·002a] |
| ‘11051’ | 3·0 ± 0·62b | 40·7 ± 0·71b | 15·4 ± 0·35b | [352·4 ± 7·9b] | 2·7 ± 0·11b | [0·10 ± 0·002b] |
| ‘Prosol 35’ | 2·7 ± 0·66b | 26·0 ± 0·08c | 13·7 ± 0·29c | [323·4 ± 8·4c] | 1·8 ± 0·06c | [0·07 ± 0·003c] |
Data in square brackets are values of duration and rates on a thermal time basis (base temperature = −1 °C).
Values are means (n = 3) ± s.e. and are derived from bilinear functions fitted to experimental data.
Within each column, values followed by different letters are significantly (P < 0·05) different.
Table 3.
Dynamics of the growth of the embryo in the three cultivars of sunflower differing in oil content at maturity
| Initiation of effective period of growth |
Effective duration of growth |
Effective growth rate |
Total duration of growth |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Final embryo weight (mg) | daa | °Cd | d | °Cd | mg d−1 | mg (°Cd)−1 | daa | °Cd | % embryo in achene (d. wt basis) | |
| ‘Dakar’ | 85·8 ± 0·74a | 6·8 ± 0·96a | [154·9 ± 23·1a] | 23·4 ± 0·50a | [560·8 ± 11·5a] | 3·6 ± 0·14a | [0·17 ± 0·004a] | 30·2 ± 0·41a | [715·7 ± 9·4a] | 54·3 ± 0·20a |
| ‘11051’ | 71·3 ± 0·27b | 7·7 ± 1·05a | [171·0 ± 25·3a] | 22·7 ± 0·49a | [541·4 ± 11·2a] | 3·1 ± 0·16b | [0·15 ± 0·003b] | 30·4 ± 0·37a | [712·4 ± 8·5a] | 63·8 ± 0·48b |
| ‘Prosol 35’ | 67·4 ± 0·43c | 9·5 ± 0·64a | [194·0 ± 15·4a] | 21·3 ± 0·46a | [517·7 ± 10·5a] | 3·1 ± 0·13b | [0·15 ± 0·003b] | 29·8 ± 0·42a | [711·7 ± 9·7a] | 72·4 ± 0·25c |
Data in square brackets are values of duration and rates on a thermal time basis (base temperature = −1 °C).
Values are means (n = 3) ± standard error and are derived from bilinear functions fitted to experimental data.
Within each column, values followed by different letters are significantly (P < 0·05) different.
Table 4.
Dynamics of oil mass in the embryo in the tree cultivars of sunflower differing in oil content at maturity
| Initiation of effective period of oil formation |
Effective duration of oil content growth |
Effective rate of oil content growth |
Total duration of oil content growth |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cultivar | Final oil weight (mg per embryo) | daa | °Cd | d | °Cd | mg d−1 | mg (°Cd)−1 | daa | °Cd | % oil in embryo (d. wt basis) |
| ‘Dakar’ | 48·4 ± 0·78a | 7·3 ± 0·57a | [168·4 ± 13·7a] | 18·3 ± 0·30a | [455·3 ± 6·9a] | 2·6 ± 0·04a | [0·11 ± 0·003a] | 25·6 ± 0·37a | [623·0 ± 8·5a] | 55·9 ± 0·26a |
| ‘11051’ | 49·4 ± 0·20a | 8·5 ± 0·83a | [195·4 ± 20·0a] | 19·1 ± 0·66a | [481·6 ± 15·1a] | 2·5 ± 0·06a | [0·11 ± 0·003a] | 27·6 ± 0·47b | [677·0 ± 10·8b] | 69·3 ± 0·61b |
| ‘Prosol 35’ | 52·0 ± 0·37b | 9·5 ± 0·44a | [216·0 ± 10·6a] | 23·0 ± 0·68b | [512·0 ± 15·6a] | 2·2 ± 0·05b | [0·10 ± 0·004a] | 32·4 ± 0·44c | [728·0 ± 10·1c] | 72·4 ± 0·25c |
Data in square brackets are values of duration and rates on a thermal time basis (base temperature = −1 °C). Values are means (n = 3) ± standard error and are derived from bilinear functions fitted to experimental data.
Within each column, values followed by different letters are significantly (P < 0·05) different.
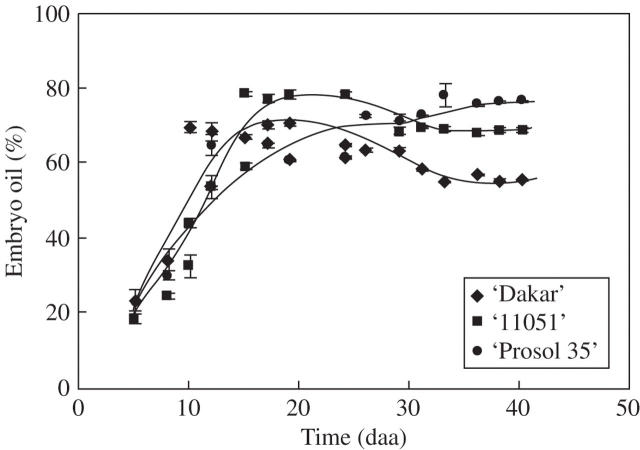
Final oil mass per embryo was rather similar across cultivars, although in the high-oil cultivar it was significantly higher than the other two (Table 4). Cultivar ranking for rates of oil deposition during the effective growth period for this variable was similar to that found for embryo growth rates. However, the effective duration of achene oil mass increase was significantly (P < 0·05) greater in ‘Prosol 35’ (Table 4), in contrast to the effective duration of embryo growth which was similar across cultivars (Table 3). An important consequence of this was that embryo oil concentration (%) actually fell during the last phase of achene filling in the low- and medium-oil cultivars (Fig. 5). In the high-oil cultivar, embryo oil concentration (%) remained stable in the middle third of the oil deposition phase, but rose again in the final phase of oil deposition (Fig. 5). These interactions between the patterns of growth of embryo and oil masses outweighed the effects of later initiation of the effective period of oil deposition in the mid- and high-oil cultivars and determined quite large differences in embryo final oil concentration (%) (Table 4). Results for the experiments conducted in 1995–96 and 1996–97 were broadly consistent, for peripheral achenes of all three cultivars, with those presented here (Fig. 4) for patterns of growth of the pericarp, the embryo and the whole achene, and for cultivar rankings for mature achene weight, mature achene oil concentration and mature embryo oil concentration (data not shown). Achene oil content in these early experiments was determined on samples drawn from all achene positions on the head, rather than on peripheral achenes only as in present results, hence patterns of oil mass per achene dynamics are not comparable to present data. Anthesis (and, consequently, the start of oil deposition in the achene) progresses from the periphery to the centre of the head over a period of 5–7 d. Consequently, mean oil content per achene in samples drawn from all positions of the head continues to increase over a longer period than that found in peripheral achenes only. In spite of this effect, ‘apparent’ relative peripheral embryo oil concentration (i.e., that estimated using oil content values measured on achenes from all positions) fell during the final stages of achene filling in ‘Dakar’ in both early experiments, and in one out of two years in ‘11051’ (Mantese, 1998), exhibiting an effect similar to that seen in Fig. 5 for these two cultivars. This result indicates that the fall in embryo oil concentration shown in Fig. 5 for ‘Dakar’ and ‘11051’ is a consistent feature of these cultivars that is stable across years.
Fig. 5.
Dynamics of embryo oil concentration for three sunflower cultivars: ‘Dakar’ (diamonds), ‘11051’ (squares) and ‘Prosol 35’ (circles). Each point represents the mean of three replicates, vertical bars are ± 1 s.e. The lines are fitted by eye.
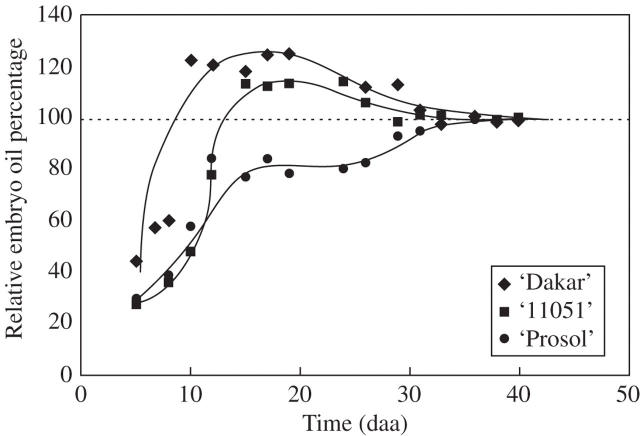
Figure 6 illustrates the contrasting patterns of relative embryo oil concentration dynamics in the three cultivars using data normalized to remove the effect of cultivar differences in embryo oil concentration. The dynamics of relative embryo oil content may be of agronomic interest, particularly when crops are exposed to stresses which may curtail achene filling.
Fig. 6.
Relative (to final values) embryo oil percentage for three sunflower cultivars: ‘Dakar’ (diamonds), ‘11051’ (squares) and ‘Prosol 35’ (circles). Each point represents the mean of three replicates. The lines are fitted by eye.
DISCUSSION
The present temporally and histologically referenced study of oil accumulation in the sunflower achene across cultivars of very different final oil concentration has produced some results that are consistent with studies using mature achenes or those based on a single cultivar. It has also served to highlight the pitfalls inherent in extrapolating results obtained in a single cultivar to others.
The lag between anthesis and the appearance of the first oil bodies in the embryo cells at 6–7 daa is consistent with both the present observations (e.g. Fig. 4) and those of others (e.g. Villalobos et al., 1996) on the dynamics of oil deposition in sunflower achenes. They are also consistent with biochemical evidence (Garcés and Mancha, 1989) that lipid synthesis early in achene filling (up to 12 daa) is associated more with cell membranes than with the deposition of reserve triacylgycerols. In sunflower, all cell types in the embryo formed oil bodies, including the epidermal cells. Oil-body diameters, measured in both isolated oil bodies and in cell sections, were slightly larger (range 1·96–2·5 μm) than the upper limit (2 μm) of the range found by Tzen et al. (1993) in their survey of 18 species (including sunflower) and the 0·5–2·0 μm range suggested by Murphy (2001) for orthodox seeds. No evidence was found for changes in oil-body size in different tissues of the embryo, unlike maize in which oil bodies from the scutellum are larger than those from the embryonic axis (Trelease, 1969). The appearance of the first oil bodies in sunflower embryos takes place fairly early during achene filling, earlier than the 2 weeks after anthesis reported for rapeseed (Murphy, 1990). However, this species difference may be more apparent than real in ontogenic terms, as achene-filling takes place in rapeseed over a longer period of time. There has been some uncertainty about the presence, or otherwise, of reserve lipids in the pericarp of the sunflower achene. It was not possible to find any indication of oil bodies in sections taken from pericarp samples harvested up to 8–9 daa or in those from mature achenes. Harris et al. (1978) assumed 3 % lipids in pericarps, presumably associated with cell membranes. However, even if this were a reasonable assumption when applied to the early stages of achene growth, it is unlikely to remain so throughout achene filling. While sections of pericarp from very young achenes showed nucleated cells and cytoplasm (Fig. 3A, B), both of these cell components were absent in sections taken from mature pericarp. An additional issue here is that the proportion of sclerenchyma (with presumably low membrane : wall ratios) in the pericarp increases rapidly after 8–9 daa, and in some cultivars more than others. Although this whole issue could do with more attention, the available information suggests that any lipids extracted from the pericarp are associated with membranes, and the contribution of these lipids to oil extracted using solvents is likely to lose importance as achene growth progresses. Results presented here (Figs 4–6) are based on the assumption that all lipids are located in the embryo, but it should be noted that other assumptions (e.g. a constant 2 % of pericarp mass assigned to lipids, or 2 % until 12 daa followed by a linear decrease to 0 % at physiological maturity) does not alter the temporal patterns or the differences between cultivars shown in these figures.
Protein bodies also appeared in embryo cells early (8–9 daa) during embryo growth, which is consistent with observations by Goffner et al. (1988) and Mazhar et al. (1998) about deposition of reserve proteins in sunflower embryos. The present data also show that the proportion of mature embryo cell sectional area occupied by protein bodies was significantly greater in the low-oil cultivar than in the other two, at least partially consistent with the often noted trade-off between achene oil and protein concentrations (Blanchet and Merrien, 1982; Connor and Sadras, 1992; López Pereira et al., 2000). Interestingly, however, the proportions of embryo cell cross-sectional areas taken up by protein bodies was similar between the mid- and high oil embryos, in spite of the much greater embryo oil concentration of the latter cultivar.
The order in which rapid growth of the components (pericarp, embryo and oil) of the achene initiated and ceased was broadly similar, across all three cultivars, to those observed in previous studies using single cultivars (e.g. Villalobos et al., 1996; Rondanini et al., 2003). However, it is also clear that the origins of cultivar differences in mature achene oil concentration are complex and strongly linked to the patterns of growth of each component and its interactions with the remaining ones. Importantly, this study has demonstrated that variations in initial size and rate and duration of growth of the pericarp can impact strongly on the proportion of the embryo in the achene and, following on from that, mature achene oil concentration. Equally, the data also show that although the proportion of embryo in the achene is an important source of cultivar differences in final achene oil concentration, cultivar differences in embryo oil concentration also contribute to this effect. Finally the degree of synchrony between cessation of growth of the whole embryo and of oil has been shown to be a factor in altering final oil concentration of the embryo and achenes (Figs 5 and 6), in addition to differences in rates and durations of oil deposition and embryo growth (Tables 3 and 4). When only the final oil concentration of the achene is considered, interactions of the type shown in this work are masked. Taken as a whole, the present information also fills the previously existing void about the anatomical bases of observations derived exclusively from mature achenes (Percie Du Sert and Durrieu, 1988; Shoar-Ghafari and Vintéjoux, 1997; Bolyakina and Raikhman, 1999). Some interest has been shown in the patterns of gene expression at the start of achene growth (Mazhar et al., 1998); present results suggest that attention also needs to be paid to the switching off of genes close to the end of achene filling and the factors which may control the latter.
Data in Tables 3 and 4 show that oil weight per embryo was fairly stable across the three cultivars, and that increases in the percentages of oil concentration in the embryo were associated with decreases in embryo weight. Two of the avenues for increased achene oil concentration through breeding therefore appear to be reduction in pericarp weight (Table 2) and reduction in embryo weight (Table 4), both of which lead to a smaller achene. In an analysis of changes in the attributes of sunflower oilseed cultivars released by breeders to farmers in Argentina over the period 1930–95 López Pereira et al. (1999) showed that this trend did, in fact, occur. Further exploitation of these two avenues could be limited if yield component compensations between achene weight and achene number (López Pereira et al., 1999) were insufficient to maintain (or increase) yield potential. Not much is known of the genetic bases of biomass (e.g. Jofuku et al., 2005) or oil yield in sunflower or other species. León et al. (2003) isolated eight quantitative trait loci (QTLs) for grain oil concentration (one with a large effect), and Laurie et al. (2004) report a discouragingly large number (>50) of QTLs for oil concentration in maize kernels none of which had major effects. Notwithstanding this last result [the oil content of the maize kernel is likely to be a more complex system than a sunflower achene in which, unlike maize, there is essentially no (non-oil containing) endosperm in the mature grain], further work aimed in this direction is required. Some of the features of oil accumulation in the sunflower achene revealed in the present work may provide part of the framework needed for this purpose.
The differences, among cultivars, of the dynamics of embryo oil concentrations (Figs 5 and 6) close to the end of achene filling may help to explain some genotype × environment interactions for achene oil concentration that can arise in breeders' multiple environment field trials when some, but not all, of a set of trials are exposed to terminal stresses. Terminal stress can shorten grain-filling duration, and this could alter genotype rankings for oil content if continuing growth of the embryo biomass after reaching its maximum oil content were also curtailed.
Acknowledgments
This work was supported by a grant from of the University of Buenos Aires to A.I.M. We thank Dr G. Laguna-Hernández (Laboratory of Cytology, UNAM, México City, México) for help with electron microscopy.
LITERATURE CITED
- Aguirrezábal LAN, Lavaud Y, Dosio GAA, Izquierdo NG, Andrade FH, González LM. 2003. Intercepted solar radiation during seed filling determines sunflower weight per seed and oil concentration. Crop Science 43: 152–161. [Google Scholar]
- Baldini M, Vannozzi G. 1996. Crop management practice and environmental effects on hullability in sunflower hybrids. Helia 19: 47–62. [Google Scholar]
- Beaudoin F, Lacey D, Napier A. 1999. The biogenesis of the plant seed oil body : oleosin protein is synthesised by ER-bound ribosomes. Plant Physiology and Biochemistry 37: 481–490. [Google Scholar]
- Blanchet R, Merrien A. 1982. Influence of water supply on assimilation, yield components and oil-protein production of sunflower. Proceedings of the Sunflower Workshop, EEC Plant Protein Programme. Athens, Greece, 23–24 November. Plant Breeding Institute, University of Bari, Italy, 185–201.
- Bolyakina Yu, Raikhman L. 1999. Structural aspects of protein accumulation in developing sunflower seeds. Russian Journal of Plant Physiology 46: 340–348. [Google Scholar]
- Chimenti C, Hall A, López M. 2001. Embryo growth rate and duration in sunflower as affected by temperature. Field Crops Research 69: 81–88. [Google Scholar]
- Connor D, Hall A. 1997. Sunflower physiology. In: Schneiter AA, ed. Sunflower technology and production. Madison, WI: American Society of Agronomy, Crop Science Society of America, Soil Science Society of America, 113–182.
- Connor D, Sadras V. 1992. Physiology of yield expression in sunflower. Field Crops Research 30: 333–389. [Google Scholar]
- Denis L, Coelho V, Vear F. 1994. Pericarp structure and hullability in sunflower inbred lines and hybrids. Agronomie 14: 453–461. [Google Scholar]
- Dosio GAA, Aguirrezábal LAN, Andrade FH, Pereyra VR. 2000. Solar radiation intercepted during seed filling and oil production in two sunflower hybrids. Crop Science 40: 1637–1644. [Google Scholar]
- Garcés R, Mancha M. 1989. Oleate desaturation in seeds of two genotypes of sunflower. Phytochemistry 28: 2593–2595. [Google Scholar]
- Goffner D, Cazalis R, Percie Du Sert C, Calmes J, Cavalie G. 1988. 14C photoassimilate partitioning in developing sunflower seeds. Journal of Experimental Botany 39: 1411–1420. [Google Scholar]
- Hanausek TF. 1902. Zur Entwicklungsgeschichte des Perikarps von Helianthus annuus. Berichte der Deutschen botanischen Gesellschaft 20: 449–454. [Google Scholar]
- Harris H, Bojinger V. 1980. Prediction of oil quality of sunflower from temperature probabilities in Eastern Australia. Australian Journal of Agricultural Research 31: 477–488. [Google Scholar]
- Harris H, McWilliam J, Mason W. 1978. Influence of temperature on oil content and composition of sunflower seed. Australian Journal of Agricultural Research 29: 1203–1212. [Google Scholar]
- Huang AH. 1992. Oil bodies and oleosins in seeds. Annual Review of Plant Physiology and Plant Molecular Biology 43: 177–200. [Google Scholar]
- Jofuku K, Omidyar P, Gee Z, Okamuro J. 2005. Control of seed mass and seed yield by the floral homeotic gene Apetala 2. Proceedings of the National Academy of Sciences of the USA 102: 3117–3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen DA. 1940. Plant microtechnique. New York, NY: McGraw-Hill.
- Laurie C, Chasalow S, LeDeaux J, McCarroll R, Bush D, Hauge B, et al. 2004. The genetic architecture of response to long-term artificial selection for oil concentration in the maize kernel. Genetics 168: 2141–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- León AJ, Andrade FH, Lee M. 2003. Genetic analysis of seed-oil concentrations across generations and environments in sunflower (Helianthus annuus L.). Crop Science 43: 135–140. [Google Scholar]
- Lindström L, Pellegrini C, Hernández L. 2000. Anatomía y desarrollo del pericarpo de distintos genotipos de girasol (Helianthus annuus L.). 15th International Sunflower Conference, Toulouse, France. 1: D13–18.
- López Pereira M, Sadras V, Trápani N. 1999. Genetic improvement of sunflower in Argentina between 1930 and 1050. I. Yield and its components. Field Crops Research 62: 157–166. [Google Scholar]
- López Pereira M, Trápani N, Sadras V. 2000. Genetic improvement of sunflower in Argentina between 1930 and 1995. Part III. Dry matter partitioning and achene composition. Field Crops Research 67: 215–221. [Google Scholar]
- Mantese A. 1998. Dinámica de la acumulación de lípidos en girasoles (Helianthus annuus L.) con diferentes rendimientos de aceite. Revista Facultad de Agronomía 18: 119–122. [Google Scholar]
- Mazhar H, Quayle R, Fido RJ, Stobart AK, Napier JA, Shewry PR. 1998. Synthesis of storage reserves in developing seeds of sunflower. Phytochemistry 48: 429–432. [Google Scholar]
- McWilliam JR, English SD. 1978. The effect of inflorescence size on seed characteristics and oil content of sunflower. Proceeding 8th International Sunflower Conference, Minneapolis. USA. Toowoomba, Australia: International Sunflower Association, 212–223.
- Murphy DJ. 1990. Storage lipid bodies in plants and other organisms. Progress in Lipid Research 29: 299–324. [PubMed] [Google Scholar]
- Murphy DJ. 2001. Biogenesis and functions of lipid bodies in animals, plants and microorganisms. Progress in lipid Research 40: 325–438. [DOI] [PubMed] [Google Scholar]
- O'Brien TP, McCully ME. 1981. The study of plant structure, principles and selected methods. Melbourne: Termarcarphi.
- Percie Du Sert C, Durrieu G. 1988. Édification de l' akène et de la achenee du tournesol (Helianthus annuus L.). Inf. Technique CETIOM 103: 12–20. [Google Scholar]
- Ploschuk E, Hall A. 1997. Maintenance respiration coefficient for sunflower achenes is less than that for the entire capitulum. Field Crops Research 49: 147–157. [Google Scholar]
- Reynolds ES. 1963. The use of lead citrate at high pH as an electron opaque stain in electron microscopy. Journal of Cell Biology 17: 208–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondanini D, Savin R, Hall AJ. 2003. Dynamics of fruit growth and oil quality of sunflower (Helianthus annuus L.) exposed to brief intervals of high temperature during achene filling. Field Crops Research 83: 79–90. [Google Scholar]
- Roth I. 1977. Fruits of angiosperms. Encyclopaedia of plant anatomy, Vol. 10. Berlin and Stuttgart: Gebrüder Borntraeger.
- Sadras V, Villalobos F. 1994. Physiological characteristics related to yield improvement in oil-seed sunflower (Helianthus annuus L.). In: Slafer G, ed. Genetic improvement of field crops: current status and development. New York, NY: Marcel Dekker, 287–320.
- Saenz AA. 1981. Anatomía y morfología de frutos de Heliantheae (Asteraceae). Darwiniana 23: 37–117. [Google Scholar]
- Shoar-Ghafari A, Vintéjoux C. 1997. Caractères et réactivité des achenes protéiques et de leurs globoïdes dans différents tissus des cotylédons de achenees d' Helianthus annuus L. (Asteraceae). Acta Botanica Gallica 144: 95–106. [Google Scholar]
- Spurr AR. 1969. A low viscosity epoxy resin embedding medium for electron microscopy. Journal of Ultrastructural Research 26: 31–43. [DOI] [PubMed] [Google Scholar]
- Steel RGD, Torrie JH. 1980. Principles and procedures of statistics, 2nd edn. New York, NY: McGraw-Hill.
- Steer T, Hocking P, Kortt A, Roxburgh C. 1984. Nitrogen nutrition of sunflower (Helianthus annuus L.): yield components, the timing of their establishment and seed characteristics in response to nitrogen supply. Field Crops Research 9: 219–236. [Google Scholar]
- Trelease RN. 1969. Changes and characteristics of lipid bodies during development. PhD Thesis, University of Texas, Austin.
- Tzen J, Cao Y, Laurent P, Ratnayake, Ch Huang AH. 1993. Lipids, proteins and structure of seed oil bodies from diverse species. Plant Physiology 101: 267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Vega AJ, Hall AJ. 2002. Effects of planting date, genotype and their interaction on sunflower yield. II. Components of oil yield. Crop Science 42: 1202–1210. [Google Scholar]
- Villalobos F, Hall A, Ritchie J, Orgaz F. 1996. OILCROP-SUN: a development, growth and yield model of sunflower crop. Agronomy Journal 88: 403–415. [Google Scholar]
- Vintéjoux C. 1992. Etude d' inclusions denses dans des cotylédons de achenees sèches (haricot, tournesol). Hétérogéneité structurale. Diversité de la composition élémentaire. Bulletin Société botanique de France, Actualities Botaniques. 139: 15–24. [Google Scholar]
- Wilcox C, Dove S, McDavid W, Greer D. 1996. Image Tool, Version 1.0. Department of Dental Diagnostics at the University of Texas Health Science Center, San Antonio, TX.
- Wilkinson L. 1986. SYSTAT for Windows. Version 5 Edition. Evanston, IL: SYSTAT.