Abstract
• Background and Aims New approaches are needed to evaluate the various hypotheses of phyllotaxis, and an examination of anodic leaf asymmetry may be one such approach.
• Methods Data were collected on the direction of midrib curvature and leaf coil in Syngonium podophyllum, the location of floral buds in Acalypha virginica, the position of secondary leaves of Croton variegatus ‘Banana’ and the relative size of half-lamina in Aglaonema crispum and Calathea ornata.
• Key Results All five features were exclusively anodic with respect to the direction of the genetic spiral regardless of whether the spiral was clockwise or counterclockwise.
• Conclusions Any phyllotactic mechanism must include some asymmetric component which cannot be explained by the prevalent hypotheses of contact parastichies, inhibitory fields, available space, pressure waves and auxin transport. The most favourable hypothesis is the primary vasculature explanation as it includes an asymmetric feature.
Keywords: Acalypha virginica, Aglaonema crispum, anodic, asymmetry, Calathea ornata, Croton variegatus, phyllotaxy, secondary leaves, Syngonium podophyllum
INTRODUCTION
The arrangement of leaves, scales and inflorescences along an axis, or phyllotaxis, has been examined intensively in many directions. There have been studies on mathematical description (Schimper, 1830; Church, 1904; van Iterson, 1907; Richards, 1948), by theoretical explanation (Schwender, 1878; Schoute, 1913; Esau, 1943; Plantefol, 1948; Green et al., 1996), by experimental investigations (Snow and Snow, 1931; Wardlaw, 1949; Meicenheimer, 1981; Reinhardt et al., 2001), using physical models (Airy, 1873; Douady and Couder, 1996) and from an evolutionary approach (Niklas, 1988). Schwabe (1984) lists 32 theories of phyllotaxis, and many of them, although quite interesting, are often supported by equivocal data. For example, Snow and Snow separated leaf primordia by surgical cutting and the result supported their theory of minimal available space, but it could also support Schoute's hypothesis of inhibitory fields. Also, waves of influence of Turing's morphogen can also be generated by physical stress (Green et al., 1996).
Despite all these studies, the mechanism behind phyllotaxis remains unknown (Jean, 1984, p. 175; Veit, 1998; Reinhardt, 2000). Jean recommends that theories need to be more elaborately explained, especially mathematically, so that they can be tested with more critical data, whereas Veit and Reinhardt look to molecular biology for answers. Another approach to eliminate hypotheses is to examine cases of patterns paralleling that of phyllotaxis, namely features that are anodic, oriented in the direction up the genetic spiral towards the younger end, or cathodic, running down the genetic spiral. Skutch (1927) in banana (Musaceae) noted that convolute vernation is cathodic to the genetic spiral; Cutter (1959–60) found inflorescences on the anodic side of the leaf axil in Victoria (Nymphaceae); and Carr (1984) noted anodic axillary buds in several eucalypt species (Eucalyptaceae). Recently, Obara et al. (2004) found two variant leaf patterns in the leaf lateral asymmetry 1 mutant in rice, one of which is characterized by a reduced half lamina on the side facing the next younger primordium. The study here reports on 10 more cases of traits paralleling the direction of the genetic spiral, namely they are anodic, including the direction of midrib curvature, location of secondary blade, direction of leaf coiling, position of axillary floral buds and size of a half-lamina. The significance of these patterns to the mechanisms of leaf arrangement will be explored.
MATERIALS AND METHODS
Plants of Syngonium podophyllum (= Nephthytis Afzelii Schott., family Araceae) and Croton variegatus ‘Banana’ (family Euphorbiaceae) were purchased from Nanz and Kraft Florists in Louisville, KY, USA, and those of Acalypha virginica, three-sided mercury (family Euphorbiaceae), were growing as weeds in the University's greenhouse and in the author's back garden. Several plants of Aglaonema crispum (family Araceae) and Calathea ornata (family Marantaceae) were purchased at a local Lowe's Home Improvement store. Terms used are anodic, meaning that side of an organ facing the ascending direction, or the younger region, of a spiral, and cathodic which is facing the descending direction, or older region, of a spiral.
RESULTS
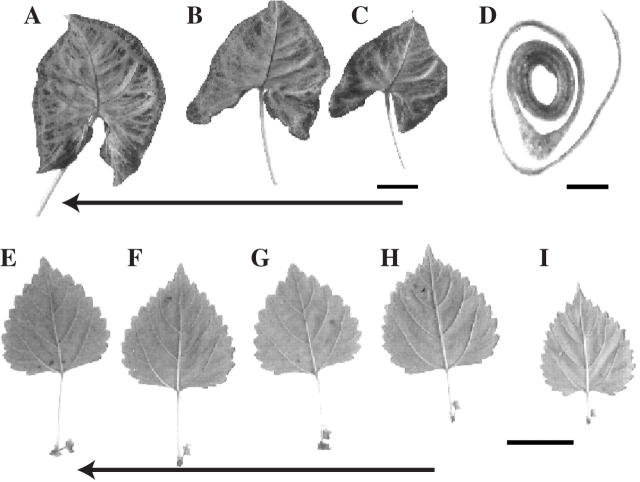
Syngonium podophyllum, or arrowhead vine, exhibits two anodic traits: first, the midrib curves gently towards the anodic side of the leaf blade in all of 160 leaves examined and, secondly, the young leaf also coils in the anodic direction in the great majority of cases (Table 1; Fig. 1A–D). Shoots of A. virginica, or three-seeded mercury, bear two axillary floral clusters with one much larger than the other. Again, the larger cluster was always on the cathodic side and the smaller buds are on the anodic side of the subtending leaf (Table 1; Fig. 1E–I). The anodic trait in C. varietagus ‘Banana’ is the appearance of a secondary blade with its own midrib arising from a primary leaf, or more particularly from a primary blade, (Table 1; Fig. 2B, D, E), a feature that seems to be new to plant morphology. About a quarter of all leaves examined have this secondary blade and all 243 secondary blades examined were on the anodic side of the primary leaf. Branch shoots change the direction of spiralling, and the location of these secondary blades also changed in 14 of 14 mother–daughter shoot pairs to remain anodic.
Table 1.
Features of anodic leaves and inflorescences
| No. of shoots |
||||||||
|---|---|---|---|---|---|---|---|---|
| Species | No. of plants | Clockwise | Counterclockwise | Total | Feature | Anodic | Neutral | Cathodic |
| Syngonium | 10 | 10 | 16 | 26 | Direction of midrib | 160 | 0 | 0 |
| Leaf coiling | 32 | 0 | 3 | |||||
| Acalypha | 23 | 13 | 10 | 23 | Smaller axillary bud | 119 | 0 | 0 |
| Croton | 46 | 39 | 42 | 81 | Secondary blade | 243 | 0 | 0 |
| Aglaonema | 6 | 12 | 9 | 21 | Smaller half of leaf | 103 | 2 | 0 |
| Calathea | 9 | 0 | 26 | 26 | Smaller half of leaf | 132 | 0 | 0 |
Fig. 1.
(A–D) Syngonium podophyllum (arrowleaf vine). (A–C) Three consecutive leaves from right to left having midribs curved to the left. The arrow shows the direction of the genetic spiral. Scale bar = 30 mm. (D) Leaf coiling to the left, i.e. anodic. Scale bar = 25 mm. (E–I) Acalypha virginica (three-sided mercury). Consecutive leaves from right to left in the direction of the genetic spiral (arrow) with one of two floral bud clusters visible on the cathodic side of petioles and the smaller bud on the anodic side of petioles. Scale bar = 10 mm.
Fig. 2.

(A–F) Croton variegates ‘Banana’ consecutive primary leaves in the direction of the genetic spiral (arrow), and secondary leaf blades (B, D and E) are anodic to the genetic spiral. Scale bar = 30 mm. (G–L) Series of asymmetric half blade sizes in Aglaonema crispum, Chinese evergreen. Scale bar = 30 mm.
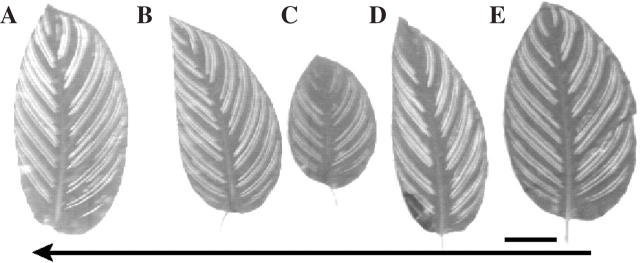
Aglaonema crispum, Chinese evergreen, is similar to Syngonium in that one side of the leaf is larger than the other but without a curved midrib. Of six plants with 21 shoots, 103 leaves had the noticeably smaller side in the anodic direction as seen by macroscopic examination, with only two leaves seemingly having identical halves (Table 1, Fig. 2G–L). Calathea ornata is another plant with asymmetric leaves. Of nine plants with 26 shoots examined, all had a counterclockwise genetic spiral and all 132 leaves scored had the smaller half in the anodic direction (Table 1; Fig. 3A–E).
Fig. 3.
(A–E) Calathea ornata leaf series with unequal half-blades. Scale bar = 30 mm.
Other plants with asymmetric sized lamina halves like Aglaonema with the smaller half on the anodic side but only casually observed include Dieffenbachia maculate and D. amoena (Araceae), Ficus benjamina (Moraceae) and Epipremnum aureum (Araceae).
These four additional plants briefly noticed, five others analysed in detail and three more cited in the literature total 12 examples from 11 cultivars in four families. This anodic feature from about 125 species with spiral phyllotaxy inspected in this study is sufficiently common to consider it as reflecting some fundamental character related to the phyllotactic mechanism.
DISCUSSION
The five directional features examined here, leaf midrib, leaf coiling, axillary buds, secondary leaves and half-lamina size, are easily recognized, and their direction with respect to the genetic spiral is also easily determined. Dormer (1972, p. 123) noted that any grossly displayed asymmetry of leaves is associated with the phyllotaxic direction, a generalization in total agreement with the data found here. The curvature of all midribs of Syngonium leaves is anodic, as is the coiling direction of most leaves (Table 1, 32 out of 35). Also, the smaller of two axillary buds in Acalypha and the location of secondary blades in Croton are always anodic. The siting of leaves is the expression of the fundamental mechanism of phyllotaxis and that these five asymmetric traits are associated with this phyllotaxy mechanism indicates an asymmetric aspect to this phyllotaxy mechanism.
These asymmetries have been recorded in other plants. Banana always has counterclockwise phyllotaxis and its leaves are also always coiled anodically (Skutch, 1927), identical to the pattern of the smaller half-lamina size found here in Aglaonema. Most plants have equal occurrence of stems with clockwise and counterclockwise spirals, hence banana and Calathea are curious exceptions to the phyllotactic rule and still the anodic feature occurs. A more typical case of both types of spirals is that in Victoria described by Cutter (1959–60) where axillary buds are anodic; she was interested in whether these buds are axillary or extra-axillary not in what this relationship means to leaf siting. Carr (1988) also described axillary buds on shoots with both types of shoot spirals in several Eucalyptus species which he used as taxonomic criteria, while the relationship of this phenomenon to the phyllotaxic mechanism was not considered. A subtle feature of Spiraea leaves was noted by Brett and Dormer (1960). They found a larger or smaller than average number of marginal serrations follow certain parastichy sequences rather than along the genetic spiral, and they concluded that some physical relationship exists between leaves.
The five traits examined here can be associated with some defect on the anodic side of leaves. The curvature of the midrib and the coiling of the young leaf of Syngonium as well as the smaller size of the anodic half-lamina are likely to be due to delayed growth of the anodic side of the leaf blade. Normal timing of growth on the cathodic side causes the midrib to be pushed to the other side, particularly at the tip where there is more freedom of displacement. Also, the direction of leaf coiling possibly occurs by the cathodic side expanding first and around the midrib, and later the anodic side coils but is forced to roll within the cathodic leaf side and its edge extends progressively more within itself (Fig. 1D). The three exceptional leaves of Syngonium that coiled in the cathodic direction (Table 1) can be explained by these rare leaves (three out of 35) being only slightly asymmetric, resulting in the coiling direction occurring in either direction by chance alone. Similarly, the two exceptional leaves of 105 examined in Aglaonema that are symmetric instead of anodic (Table 1) may be only slightly asymmetric but are swamped out by the normal variability of growth. In both exceptional cases, small quantitative differences may result in qualitative switching of direction. The cathodic location of larger axillary buds in Acalypha suggests delayed growth of the anodic buds. Finally, the appearance of secondary blades in Croton only on the anodic side of the leaf could be from some breakdown of suppression of leaf initiation resulting in one leaf arising atypically on a parental leaf. This extension appears to be a blade because it clearly has a midrib, unlike smaller localized lateral blade growths common in other cultivars of Croton.
Although these defective traits are anodic, the question is whether they are part of the genetic spiral or have their own course of development separate from but parallel to the genetic spiral. First, a one-type of spiral hypothesis assumes less detail than one with two types of spirals, leaf asymmetry and spiral direction, and, secondly, as the direction of spiral changes from mother to daughter shoots in Croton, the direction of the defect also shifts, as noted in all 14 cases examined. The asymmetry of these traits, i.e. secondary blades on the anodic side, the smaller size of the anodic half of the leaf blade, etc., suggests some asymmetric component of the phyllotaxy mechanism. Conservation of a laterally oriented asymmetry requires some spatial continuity between consecutive structures. Recall that Brett and Dormer (1960) found a correlation between serration number and certain parastichies which they concluded was based on physical connections between nodes and was not a feature of the nodes themselves.
How then do the prevailing major hypotheses satisfy the requirement of a laterally asymmetric continuity? The first available space hypothesis of Snow and Snow (1931) is insufficient to explain the data presented here because it includes no physical continuity between leaf sitings. Also, the pressure contact hypothesis of Schwendener (1878) and the mechanical pressure idea of Green et al. (1996) have leaf sites connected by transient pressure forces and, hence, are also inadequate. Schoute's (1913) inhibitory field notion is also insufficient because it involves a singular type of gradient originating from each primordium centre. The vascular hypothesis of Esau (1943) and to some extent that of Plantefol (1948) is based on physical continuity between leaves, with the procambium as the most promising connection. Reinhardt et al. (2000) have shown in tomato that auxin plays a critical role in leaf siting as experimental inhibition of polar transport of inole-3-acetic acid (IAA) prevents leaf initiation but the effect can be overcome by microapplication of auxin. Here is a new hypothesis of activator fields. Kang et al. (2003) found that new procambium at the apex is added acropetally, the opposite of basipetaly formed procambium when auxin is experimentally applied, lending credence to the vascular hypothesis and contradictory to the auxin transport hypothesis. The debate over leaf siting continues as before but with new data added to each view.
All the hypotheses of leaf arrangement other than the vascular hypothesis primarily attempt to explain Fibonacci numbers in either divergent angles or number of parastichies by theoretical considerations, whereas the vascular hypothesis is founded on empirical findings of a spatial relationship between leaf and primary veins. The most notable findings are certain sympodial systems such as the primary vasculature of Sequoia (Sterling, 1945). Here 13 open axial bundles in one case all branched in the anodic direction. Also, of the two leaf traces from different sympodia in the closed system of Kalenchoe uniflora (Step f) Raym-Hamet, one is more advanced than the other in ascending towards the apex (Jensen, 1968). Unfortunately, examination of six shoots shows this plant to have decussate phyllotaxy and symmetric leaves so it cannot be used to study the association between asymmetric leaves and vascular behaviour. This anodic direction of structural asymmetries may be only one more feature noted by the morphologist, if at all, but developmentally it reflects not only the presence of a complex mechanism but also one that is oriented to give lateral and not dorso-ventral symmetry.
Generally, the asymmetric feature associated with phyllotaxis can be explained in either of two ways. First, the vascular hypothesis has procambiam enlisting cells up into the apex thereby providing the physical continuity. In plants where two leaf traces from different sympodia fuse, the combined median trace determines, and enters, a new leaf primordium. Perhaps the trace on the cathodic side is more robust in feeding leaf development and the one on the anodic side is weaker and delayed in entering a primordium. Alternatively, the auxin transport hypothesis proposes that a high auxin concentration induces leaf initiation and it could also stimulate procambial enlistment activity lower in the apex, with one strand more responsive than the other. The tomato data of Reinhardt et al. (2000), while fostering the auxin interpretation, also support the vascular idea. Plants treated with a polar transport inhibitor to produce PIN stems having no leaves should still have normal vasculature according to the vascular hypothesis and no vasculature according to the polar transport idea. PIN stems have normal vasculature, thus supporting the vasculature hypothesis for leaf initiation.
It would be of interest to see if these anodic traits are associated with vasculature behaviour. Of the traits examined here, that of secondary blades in Croton, one new to plant morphology and which includes an additional midrib, is most promising in looking for some unusual anodic behaviour of leaf traces in the meristem as Jensen (1968) found in Kalanchoe.
LITERATURE CITED
- Airy H. 1873. On leaf-arrangement. Proceedings of the Royal Society 21: 176 [Google Scholar]
- Brett DW, Dormer KJ. 1960. Observations on a cyclic fluctuation in the leaf serrations of Spiraea salicifolia, and on the asymmetry of the leaf. New Phytologist 59: 104–108. [Google Scholar]
- Carr DJ. 1998. Systems of phyllotaxis in the genus Eucalyptus in relation to shoot architecture. In: Jean RV, Barabe D, eds. Symmetry in plants. Singaopore: World Scientific, 33–60.
- Church AH. 1904. On the relation of phyllotaxis to mechanical laws. London: Williams and Norgate.
- Cutter EG. 1959–60. The inception and distribution of flowers in the Nymphaceae. Proceedings of the Linean Society of London 172: 93–100. [Google Scholar]
- Dormer KJ. 1972. Shoot organization in vascular plants. Syracuse: Syracuse University Press.
- Douady S, Couder Y. 1996. Phyllotaxis as a dynamical self-organizing process. Part III. The simulation of the transient regimes of ontogeny. Journal of Theoretical Biology 178: 295– 312 [Google Scholar]
- Esau K. 1943. Vascular differentiation in the vegetative shoot of Linum. II. The first phloem and xylem. American Journal of Botany 30: 248–255. [Google Scholar]
- Green PB, Steele CS, Rennich SC. 1996. Phyllotactic patterns: a biophysical mechanism for their origin. Annals of Botany 77: 515–527. [Google Scholar]
- van Iterson J. 1907. Mathematische und Mikroskopisch-Anatomische studien über Battstellungen nebst Btrashtungen üer den Shalenbau der Mliolinen. Jena: Gustav Fisher.
- Jean RJ. 1984. Mathematical approach to pattern and form in plant growth. New York: John Wiley & Sons.
- Jensen LCW. 1968. Primary stem vascular patterns in three subfamilies of the Crassulaceae. American Journal of Botany 55: 553–563. [Google Scholar]
- Kang J, Tang, J, Donnelly P, Dengler N. 2003. Primary vascular pattern and expression of ATHB-8 in shoots of Arabidopsis. New Phytologist 158: 443–454. [DOI] [PubMed] [Google Scholar]
- Meicenheimer RD. 1981. Changes in Epilobium phyllotaxy induced by N-1-naphthylphtalmic acid and α-4-chlorophenoxyisobutyric acid. American Journal of Botany 68: 1139–1154. [Google Scholar]
- Niklas KJ. 1988. The role of phyllotactic pattern as a ‘development constraint’ on the inception of light by leaf surfaces Evolution 42: 1–16. [DOI] [PubMed] [Google Scholar]
- Obara M, Ikeda K, Itoh J-I, Nagato, Y. 2004. Characterization of leaf lateral symmetry 1 mutant in rice. Breeding Science 54: 157–163. [Google Scholar]
- Plantefol L. 1948. La théorie des hélices foliaires multiple. Paris: Masson.
- Reinhardt D, Mandel T, Kuhlemeier C. 2000. Auxin regulates the initiation and radial position of plant lateral organs. Plant Cell 12: 507–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards FJ. 1948. The geometry of phyllotaxis and its origin. Symposium of the Society of Experimental Biology 2: 217–245. [Google Scholar]
- Schoute JC. 1913.. Beitrage zur Blattstellunglehre. I. Die Theorie. Recueilde Travaux Botaniques Neerlandais 10: 153–339. [Google Scholar]
- Schawabe WW. 1984. Phyllotaxis. In: Barlow PW, Carr DJ. eds. Positional controls in plant development. Cambridge: Cambridge University Press, 403–440.
- Schimper CF. 1830. Beschreibung des Symphytgum Zeyheri und seiner zwei deutchen Verwandten der S. bulborum Schimper und S. tuberosum Jacqu. Geiger's Magazin für Pharmacie 29: 1–92.
- Schwendener BS. 1878. Mechanische Theorie der Blattstellungen. Leipzig: Englemann.
- Skutch AF. 1927. Anatomy of leaf of banana, Musa sapien L. var. Hort. Gros Michel. Botanical Gazette 84: 337–391. [Google Scholar]
- Snow M, Snow R. 1931. Experiments on phyllotaxis.1. The effects of isolating a primordium. Philosophical Transactions of the Royal Society 244: 483–513. [Google Scholar]
- Sterling C. 1945. Growth and vascular development in the shoot apex of Sequoia sempervirens (Lamb.) Endl. II. Vascular development in relation to phyllotaxis. American Journal of Botany 32: 380–386. [Google Scholar]
- Veit B. 1998. Leaf initiation: new developments in an expanding field. Plant Cell 10: 1407–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw CW. 1949. Experiments on organogenesis in ferns. Growth (Suppl.) 13: 93–131. [Google Scholar]