Abstract
• Background and Aims The effect of heating and cooling on cambial activity and cell differentiation in part of the stem of Norway spruce (Picea abies) was investigated.
• Methods A heating experiment (23–25 °C) was carried out in spring, before normal reactivation of the cambium, and cooling (9–11 °C) at the height of cambial activity in summer. The cambium, xylem and phloem were investigated by means of light- and transmission electron microscopy and UV-microspectrophotometry in tissues sampled from living trees.
• Key Results Localized heating for 10 d initiated cambial divisions on the phloem side and after 20 d also on the xylem side. In a control tree, regular cambial activity started after 30 d. In the heat-treated sample, up to 15 earlywood cells undergoing differentiation were found to be present. The response of the cambium to stem cooling was less pronounced, and no anatomical differences were detected between the control and cool-treated samples after 10 or 20 d. After 30 d, latewood started to form in the sample exposed to cooling. In addition, almost no radially expanding tracheids were observed and the cambium consisted of only five layers of cells. Low temperatures reduced cambial activity, as indicated by the decreased proportion of latewood. On the phloem side, no alterations were observed among cool-treated and non-treated samples.
• Conclusions Heating and cooling can influence cambial activity and cell differentiation in Norway spruce. However, at the ultrastructural and topochemical levels, no changes were observed in the pattern of secondary cell-wall formation and lignification or in lignin structure, respectively.
Keywords: Norway spruce, Picea abies, cambium, xylem, phloem, cell differentiation, heating, cooling, light microscopy, transmission electron microscopy, UV-microspectrophotometry
INTRODUCTION
The growth and development of trees is controlled by internal and external factors. External factors (temperature, precipitation, radiation, daylength, etc.) regulate the initiation, termination, rate and extent of growth, as well as developmental processes in plants. In temperate climatic zones, vascular cambium in conifers exhibits seasonal periods of activity and dormancy. Cambial activity usually ceases in late summer and resumes in spring with cell division followed by differentiation into xylem and phloem (Denne and Dodd, 1981; Savidge, 1996, 2000; Kozlowsky and Pallardy, 1997; Lachaud et al., 1999; Wodzicki, 2001; Larcher, 2003).
In order to characterize the factors enabling cambial growth in conifers, shoots, stem cuttings or intact stem portions have been exposed to controlled conditions during cambial dormancy and/or activity (Little and Bonga, 1974; Little, 1981; Savidge and Wareing, 1981; Riding and Little, 1984, 1986; Mellerowicz et al., 1992; Savidge and Barnett, 1993; Barnett and Miller, 1994; Oribe and Kubo, 1997; Oribe et al., 2001, 2003, 2004; Rensing and Samuels, 2004). Experiments revealed differences in cambial response to heat treatment among different conifers (Barnett and Miller, 1994; Oribe et al., 2001, 2003, 2004). In the deciduous Larix leptolepis, no cell division was observed in the cambium of warmed stems until the natural resumption of cambial activity occurring after bud break. In dormant evergreen Picea sitchensis saplings, cambial reactivation occurred only in the heated region of the stem if needles and buds were left intact (Barnett and Miller, 1994). In the evergreen Cryptomeria japonica, cambial reactivation often occurred in the heated portion of the stem. This response gradually increased as the dormant season passed from winter to spring. Heating also induced localized reactivation of the cambium in treated portions of the stem in the evergreen conifer Abies sachalinensis. However, the cells in the reactivated cambium stopped dividing soon after only a few cells had been generated and no differentiating xylem cells were observed. It has been suggested that heating directly triggers the breaking of cambial dormancy in evergreen conifers (Oribe and Kubo, 1997). By contrast, the influence of cooling of tree stems on cambium activity during the growing season has received relatively little attention. Moreover, increased knowledge on the influence of temperature on cell-wall formation, especially lignification, would contribute to a better understanding of the environmental factors regulating wood formation in trees.
The purpose of this preliminary research was to evaluate the response of dormant cambium of Norway spruce (Picea abies) to applied heat before its regular reactivation in early spring 2004, as well as to observe the response of the active cambium to experimental cooling at the presumed height of cambial activity. The response of the cambium to both treatments was investigated every 10 d by means of light microscopy (LM), transmission electron microscopy (TEM) and UV-microspectrophotometry (UMSP). In order to reveal the effect of treatments on the general anatomy of xylem and phloem growth rings, samples were taken at the end of the vegetation period in 2004.
MATERIAL AND METHODS
Three 70-year-old Norway spruce test trees (dbh 35 cm) were selected in an urban Norway spruce/beech forest at Rožnik in Ljubljana (46 °03′N, 14 °28′E, 323 m a.s.l.).
One adult tree was used for the heating experiment and one for the cool treatment. The third tree was not treated and served as a control. The number of test trees was defined in accordance with experimental limitations when working with large forest trees. Average, maximum and minimum daily air temperatures were recorded by a weather station located 50 m from the test trees during both experiments. Localized heat treatment was applied to the tree in the period between 29 March and 3 May, 2004. The stem portion of the first Norway spruce was heated with a 15-m-long electric heating cable [FSM-17 (Freeze Stop Warming Band; Jowitherm, The Netherlands), 17 W/+5 °C, 11 W/25 °C, 230 V] wrapped around 1 m of the length of the stem. The lower part of the heating system was 70 cm above the ground. Insulation material was wrapped around the electric heating cable to prevent energy loss. The temperature between the bark and the insulation was adjusted to 23–25 °C and monitored daily with a thermometer sensor. Localized cooling of the stem of the second experimental spruce was performed between 14 June and 20 July, 2004. A pump circulated cooled water through copper tubes, which were wrapped around 1 m of the length of the stem. The system was carefully insulated. The temperature between the stem and the insulation was set to 9–11 °C, which was monitored and regulated daily with a capillary thermostat.
Blocks of tissue (10 × 10 × 30 mm3) containing inner phloem, cambium and outer xylem were taken at the beginning of each of the treatments and thereafter at 10-d intervals. Samples were taken at breast height (1·3 m above the ground) from treated trees and the control tree. The insulation was carefully placed in the original position after each sampling. Additionally, samples were taken approximately 10 cm above the treated and insulated portions of the stems to investigate the effect of temperature on cambial activity along the stem from the site of its application. Samples were taken again on 12 December, 2004 (winter) to investigate the effect of cooling and heating on the anatomy and incremental width of xylem and phloem. After sampling, blocks of tissue were immediately fixed in FAA (formaldehyde–ethanol–acetic acid solution) and, after 1 week, were dehydrated through a graded series of ethanol. For LM, permanent cross-sections of 25 μm thickness were prepared on a microtome Leica SM 2000R, stained with safranin and astra blue and mounted in Euparal. A Nikon Eclipse 800E light microscope was used for anatomical observations. Radially flattened cells of dormant cambium were easily distinguished from terminal latewood tracheids and differentiated secondary phloem. An increase in the number of cells in the cambium indicated divisional activity of the cambium. Expanding cells with thin primary walls between the cambium and a zone of xylem and phloem cells with secondary walls were considered to be xylem and sieve cells in the post-cambial growth stage. Determining the initiation of secondary wall thickening was based on birefringence in the cell walls under polarized light. Blue-stained cell walls and the protoplasmic content in the cell lumina indicated incompletely developed tracheids. The fully matured tracheids had red-stained cell walls and empty lumina.
In addition, semi-thin sections of about 1 μm thickness were prepared with an Ultracut S ultramicrotome and stained with 1 % toluidine blue solution. For this purpose, samples for TEM and UMSP were dehydrated through a graded series of ethanol and water-free acetone and then embedded in Spurr’s epoxy resin (Spurr, 1969). For TEM, ultra-thin transverse sections (80–100 nm thick) were stained with potassium permanganate according to the method of Donaldson (1992). Examination was carried out with a Philips CM 12 transmission electron microscope at accelerating voltages of 40 or 60 kV to enhance contrast. For UV spectroscopic measurements, unstained transverse sections (1 μm thick) were prepared from the epoxy resin-embedded samples, transferred to quartz slides, immersed in a drop of non-UV-absorbing glycerine and covered with quartz coverslips. Cell-wall analyses were performed using a UV-microspectrophotometer (UMSP 80, Zeiss) equipped with a scanning stage enabling the determination of image profiles at defined wavelength. The specimens were first conventionally investigated by point measurements with a spot size of 1 μm2 using the program LAMWIN® (Zeiss). Spectra were taken at wavelengths of 240–400 nm in 2-nm steps. The measurements were automatically repeated 50 times for each point analysis. Additionally, specimens were scanned at a defined wavelength of 280 nm for conifer lignin, using the scan program APAMOS® (Zeiss). The scan program digitizes rectangular fields with a local geometrical resolution of 0.25 μm2 and a photometrical resolution of 4096 greyscale levels, which are converted in 14 basic colours to visualize absorbance intensities (Koch and Kleist, 2001; Koch and Grünwald, 2004).
RESULTS
Climatic conditions during experiment
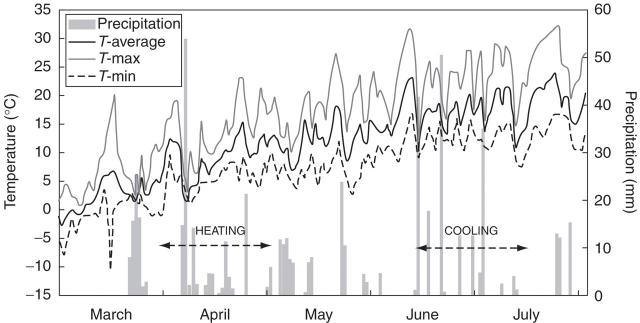
Daily average, maximum and minimum air temperatures and amount of precipitation recorded in the area from 1 March to 31 July, 2004 are given in Fig. 1. Conditions during March and April were relatively mild, with minimum air temperatures falling below freezing only at the beginning of the heating experiment. Air temperatures gradually increased but never exceeded 25 °C during the heating experiment. June and July were much warmer, with maximum temperatures rarely above 30 °C and minimum temperatures never below 5 °C. Thus, as expected, air temperatures during the cooling experiment were much higher than those during the heating experiment.
Fig. 1.
Average, maximum and minimum daily air temperatures and amount of precipitation in Ljubljana (46°03′N, 14°28′E, 323 m a.s.l.) during the two experimental periods. The duration of the heating and cooling experiments is indicated by arrows.
Heating experiment
Examination of the material removed on 29 March, 2004 revealed that the heating experiment was set before regular initiation of cell divisions in the cambium. Radial series of dormant cambium consisted of 4–5 radially flattened cells (Fig. 2D). After 10 d of heat treatment, 7–8 layers of dividing cambial cells and three layers of early phloem sieve cells were observed in samples from the heated portion of the stem (Fig. 2A). The cambium was still dormant above the treated portion of the stem and in the control tree. Heating the stem portion for 20 d caused xylem formation, but no reactivation of the cambium 10 cm above the treated region was observed. Similarly, the cambium of the control tree remained dormant, with up to five layers of cells. In the heat-treated sample, the cambium comprised 7–8 layers of cells, with an increase of 3–4 layers of early phloem sieve cells, and an increase in xylem of 3–5 layers of earlywood tracheids in the post-cambial growth (Fig. 2B). After 30 d of heat application, on 3 May, 2004, regular cambial activity had started in the non-heated part of the stem and in the control tree (Fig. 2E). The cambial zone was 7–8 layers of cells wide, the phloem consisted of four layers of early phloem sieve cells and the xylem of four layers of radially expanding earlywood tracheids. In the heat-treated stem portion, up to 15 layers of undifferentiated earlywood tracheids were present on the xylem side (Fig. 2C). Five to six layers of cells were in the stage of post-cambial growth and 7–9 layers of tracheids were undergoing secondary wall formation and lignification. Tracheids adjacent to the terminal latewood tracheids of the previous growth ring (2003) were in the final stages of cell differentiation. No completely matured earlywood tracheids had formed in the 30 d of heat treatment. Current phloem growth consisted of a more or less continuous tangential band of axial parenchyma, followed by 5–6 layers of early phloem sieve cells. Heat-treated and non-treated samples taken at the end of the vegetation period (12 December, 2004) revealed comparable widths of xylem growth rings and a similar proportion of latewood (approx. 40 %) (see Fig. 4 below). No differences were detected in terms of the anatomy and width of the phloem growth increments during 2004 between treated and non-treated samples. Phloem growth increments comprised of 3–5 late phloem sieve cells, followed by a tangential band of axial parenchyma cells and a layer of 3–4 early phloem sieve cells.
Fig. 2.
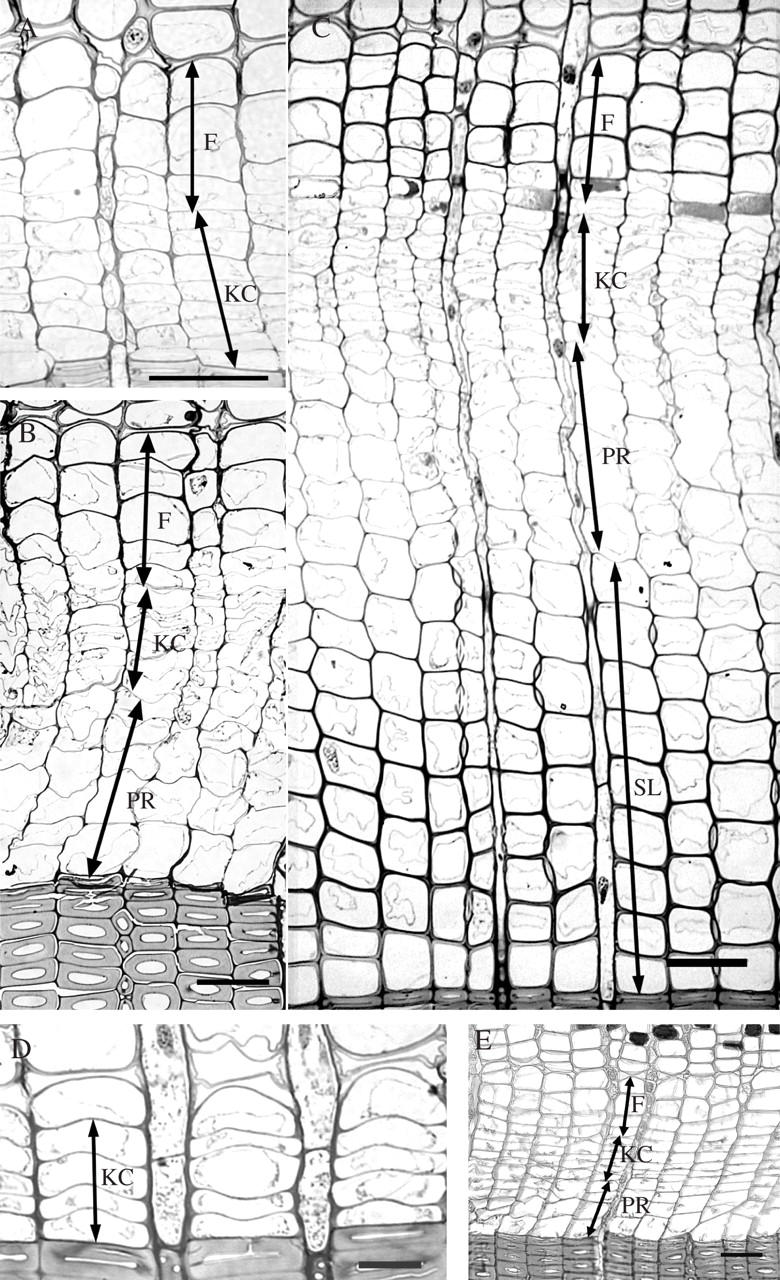
The response of cambium in Norway spruce to experimental heating, as viewed on cross-sections by light microscopy. (A) Divisionally active cambium (KC) and phloem cells (F) formed after 10 d of heating. (B) Phloem increment (F) and xylem cells in post-cambial growth (PR) formed as a response to 20 d of heating. (C) Phloem (F) and xylem cells in post-cambial growth (PR) and phase of secondary cell wall formation and lignification (SL) formed after 30 d of heating. (D) Dormant cambium (KC) in a control sample at the beginning of the experiment. (E) Regular cambial reactivation in a control sample after 30 d of the experiment. Scale bars = 50 μm.
Cooling experiment
Light microscopy of tissues removed from the control tree and from the tree prepared for cooling showed that on 14 June, 2004, when the experiment began, the cambium consisted of 8–10 layers of dividing cells. The phloic increment comprised 1–2 layers of late phloem sieve cells adjacent to the cambium, a tangential band of axial parenchyma cells and 3–4 layers of early phloem sieve cells. On the xylem side, 6–8 layers of earlywood cells were in post-cambial growth stage, 9–10 layers of the tracheids in the process of secondary wall formation and lignification, and 4–6 layers of earlywood tracheids were fully developed (Fig. 3A). After 10 and 20 d of the cooling experiment, no differences at the anatomical level could be detected among the samples taken from the cool-treated portion of the stem, a sample taken above the treated part of the stem or a sample removed from the control tree. Anatomical differences among tissues exposed to cooling and control samples were conspicuous after 30 d of cooling, on 20 July, 2004. Latewood started to form, as concluded from the narrow radial dimensions of the recently formed tracheids near the cambium of the cool-treated stem (Fig. 3C). Only up to two layers of cells were in the stage of post-cambial growth. The cambium was up to five layers of cells wide in the cooled sample (Fig. 3D). Above the cool-treated stem portion and in the sample from the control tree, the cambium was considerably wider, consisting of 7–8 cells (Fig. 3B). The non-treated samples showed the formation of earlywood or transition wood, while 6–7 layers of tracheids were in the stage of post-cambial growth. Observation of phloem anatomy revealed no differences between the cool-treated and control samples. At the end of the vegetation period, the cool-treated sample exhibited a reduced proportion of latewood (approx. 20 %) as compared with the control sample (approx. 40 %), although the widths of the xylem growth rings were comparable (Fig. 4). The structure and width of the phloem growth increments were similar in control and treated trees.
Fig. 3.
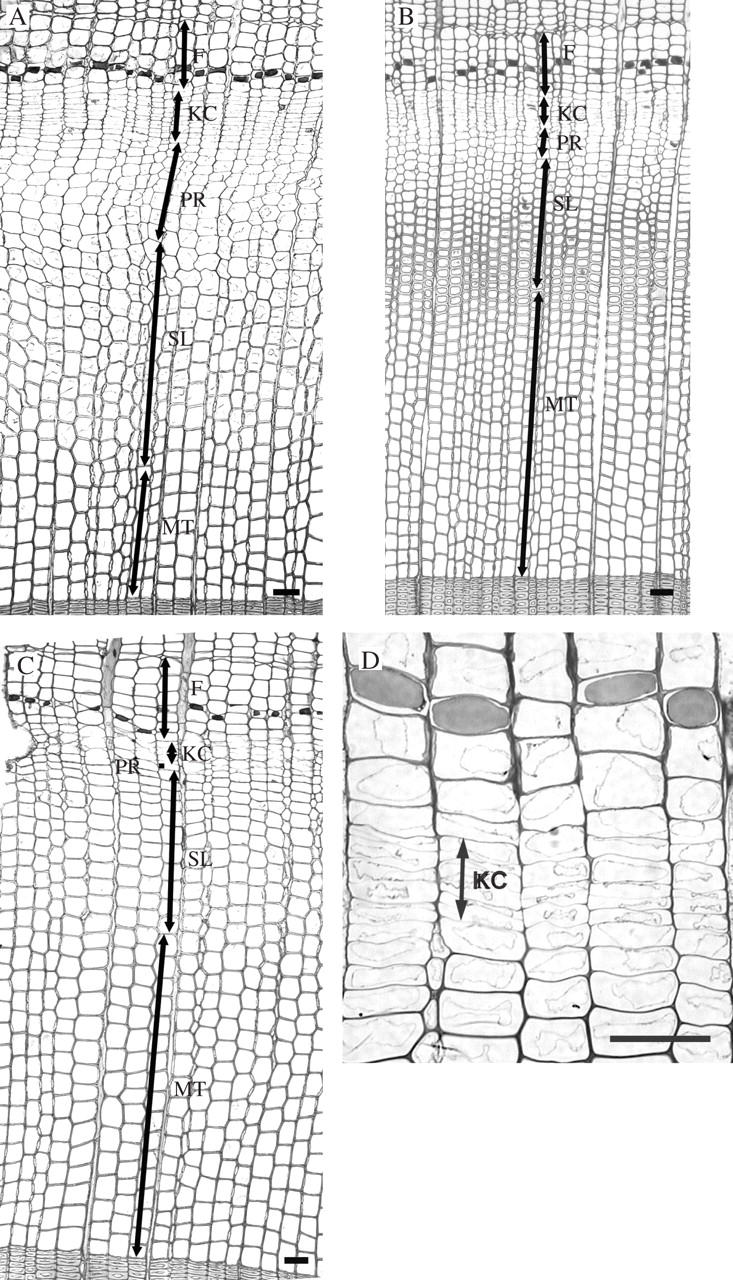
Light micrographs showing the response of the cambium to the cooling experiment. (A) Current phloem and xylem increment at the beginning of the experiment in mid-June. (B) Earlywood or transition wood (PR, SL) formed in the control tree after 30 d: PR, post-cambial growth; SL, secondary wall formation and lignification; F, phloem cells. (C) Formation of latewood (PR, SL) in a cool-treated sample after 30 d. (D) Detail of the cambial region (KC) in the cooled sample after 30 d. The cambium is up to five layers of cells (KC) wide. Scale bars = 50 μm.
Fig. 4.

Light micrographs of current xylem growth rings at the end of the vegetation period. (A) Heated sample; (B) cooled sample with a reduced portion of latewood; (C) control sample, Scale bars = 100 μm.
UV spectrophotometry and TEM
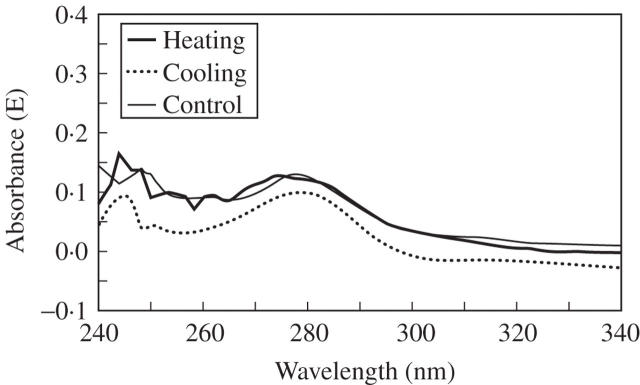
UV-spectrophotometry was used for examination of possible changes in the structure of lignin resulting from the heating or cooling treatments (Fergus and Goring, 1970; Fukazawa and Imagawa, 1981; Fengel and Wegener, 1989; Takabe et al., 1992; Koch and Kleist, 2001; Koch and Grünwald, 2004). Treated and non-treated samples showed UV-spectra typical of softwood lignin, with a distinct maximum at wavelengths of 280 nm and a local minimum at approx. 260 nm (Fig. 5). Furthermore, spectral scanning of the heat-treated xylem tissue revealed no alterations in the differentiation process of the cell walls. Absorbance at 280 nm increased with increasing distance from the cambium, indicating a lower lignin content in the cell walls of younger developing cells. Lignification extended from the cell corners toward the inner parts of the secondary cell walls (Fig. 6). On the basis of the UMSP observations, no changes in the pattern of lignin incorporation and its structure between treated and non-treated samples were found after 30 d of the experiment.
Fig. 5.
UV spectra measured in secondary cell-wall regions of tracheids in heat-treated, cool-treated and control samples showed a typical absorption maximum at 280 nm. Lower values than typically measured indicated incomplete development of the xylem cells.
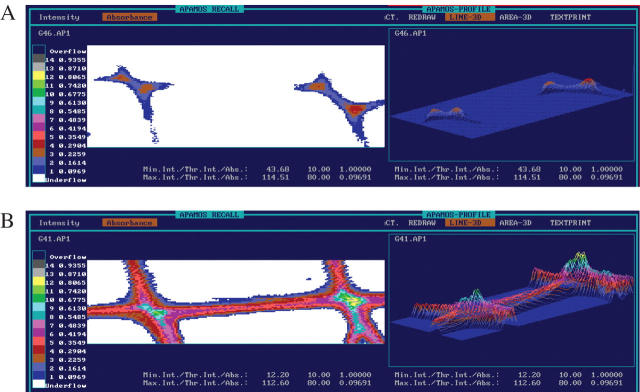
Fig. 6.
UV micrographs and three-dimensional profiles of cell walls of (A) fourth and fifth and (B) eighth and ninth earlywood tracheids in heat-treated Norway spruce scanned with a geometrical resolution of 0.25 μm2. The scales indicate different UV-absorbance values at a wavelength of 280 nm.
TEM of heated samples revealed that at the end of April, i.e. prior to the regular onset of wood formation, several layers of differentiating xylem cell rows had developed. Young cells close to the cambium appeared with thin walls and were still undergoing enlargement. A few rows away from the cambium, cells showed the beginning of deposition of secondary wall material. With increasing distance to the cambium, the width of the secondary cell wall increased (Fig. 7A, B). Potassium permanganate staining clearly demonstrated that lignification was delayed as compared with cellulose deposition, and started in the cell corner and middle lamella regions. Thereafter, lignification extended towards the outer portions of the secondary wall and finally to the inner portions.
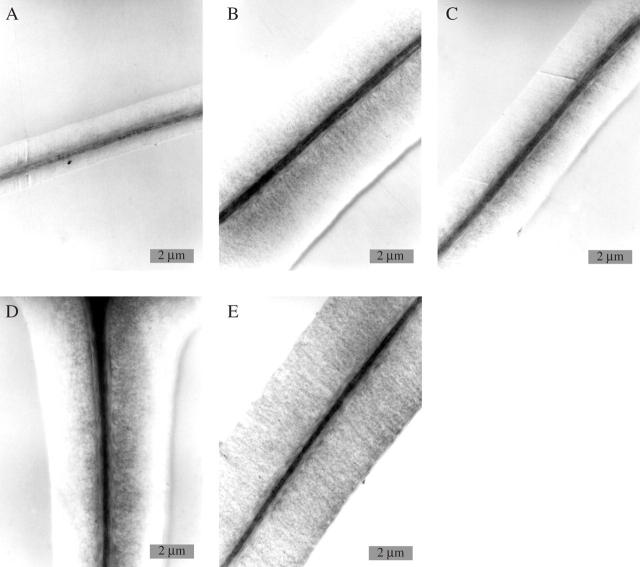
Fig. 7.
Electron micrographs of tangential tracheid cell walls of treated and non-treated samples. (A) Heated sample after 30 d, showing earlywood cells with thin walls close to the cambium: middle lamella, S1, and outer S2, lignified, inner S2 without lignin. (B) Heated sample after 30 d, of the eighth and ninth tracheid rows from the cambium; the width of the secondary wall has increased. (C) Cooled sample after 30 d, showing the ninth and tenth tracheid rows incompletely lignified. (D) Control tree from the heating experiment after 30 d, showing the ninth and tenth tracheid rows incompletely lignified. (E) Cooled sample after 30 d, showing the 13th and 14th tracheid rows with distinct lignification of the whole cell wall.
Neither control nor cooled stem portions sampled during the vegetation period showed fine structural variations with regard to xylem cell development. As described above for the heated samples, all cell development stages were seen, starting close to the cambium with primary wall stages during cell enlargement, and further away from the cambium with secondary wall deposition and delayed onset of lignification (Fig. 7C, D). Lignification of the entire secondary wall became finalized in cell rows 13–14 from the cambium (Fig. 7E).
DISCUSSION
The experiments described herein have demonstrated that heating or cooling can cause alterations in regular cambial activity. This is in agreement with previous observations of cambial reactivation in locally heated stems of several conifers in winter (Oribe and Kubo, 1997; Oribe et al., 2001, 2004). Applying heat stimulated divisions in the cambium and led to xylem and phloem formation. Cambial reactivation in the stem of Norway spruce occurred on the phloem side first, which supports the observations of Oribe et al. (2001, 2003, 2004) in other evergreen conifer species. In locally heated regions of stems of Abies sachalinensis during cambial dormancy, a few fusiform cambial cells on the phloem side of the cambium differentiated into phloem cells prior to the re-initiation of cambial cell divisions, and only minimal differentiation of xylem occurred after cambial reactivation. According to Larson (1994), there are no significant differences among fusiform cambial cells. Furthermore, storage starch is not accumulated in fusiform cambial cells during dormancy (Larson, 1994; Oribe et al., 2001). One of the reasons for earlier cambial reactivation on the phloem side in the heated part of the stem might be a perception of a gradient of external triggers in the cambium (Oribe et al., 2001). During heat treatment, there might be two main pathways for translocation of nutrients: radial, from phloem parenchyma cells; and tangential, from ray cambial cells. In this respect, the supply of nutrients to the cambial cells adjacent to the phloem is greater than on the xylem side, resulting in earlier reactivation of the cambial cell on the phloem side (Oribe et al., 2001). That the cambium remained dormant elsewhere in the stem and that its reactivation was restricted to the heated region confirmed the observations of Barnett and Miller (1994) regarding the non-transference of temperature along the stem from the site of its application.
During winter cambial dormancy, cambial growth potential varies with species, evergreen and deciduous habit, and habitat of conifers (Oribe and Kubo, 1997; Oribe et al., 2001, 2003). Cambial cells of evergreen conifers at the quiescent stage of cambial dormancy, a stage that is imposed by external factors, can re-initiate cell division independently of the growth of new shoots and the development of buds in spring (Oribe and Kubo, 1997; Oribe et al., 2001, 2003). Localized heating of stems of Abies sachalinensis during late winter induced localized reactivation of the cambium. However, the effect of localized heating on the extent of cell division in heat-reactivated cambium was not as distinct as that in naturally reactivated cambium. In addition, heated reactivated cambium ceased cell division soon after a few cells had been produced. Oribe et al. (2003) suggested that in Abies sachalinensis, continuous cell divisions in heated-reactivated cambium require additional conditions, which appear to be satisfied in naturally reactivated cambium. In the evergreen Cryptomeria japonica, cambial reactivation often occurred in the heated portion of the stem (Oribe and Kube, 1997). This might reflect a dormancy stage of the cambium, showing that reactivation is more likely during environmentally imposed dormancy. No cambial response to heat treatment in the deciduous Larix leptolepis indicated that cambial reactivation in this species is limited by several factors associated with bud break (Oribe and Kubo, 1997).
The response of the cambium to the drop in temperature in our experiment was less pronounced and was not visible on the xylem side until 30 d of cooling. Cooling part of the Norway spruce stem caused earlier formation of latewood. Adjacent to the cambium, only 1–2 radially expanding xylem cells were observed. In addition, the number of cells in the cambium decreased to four or five, which was typical of dormant cambium. The drop in air temperatures presumably shortened cambial activity, resulting in a lower portion of latewood in the current xylem increment. It appears that the heating and cooling treatments did not influence the widths or the structure of the phloem growth increments. Moreover, at the ultrastructural and topochemical levels, no alterations were observed in the pattern of secondary cell wall formation and lignification or in lignin structure among cooled, heated and control samples.
The results presented herein and the results obtained by others confirm the importance of external factors on cambial activity and corresponding cell differentiation. However, internal factors, such as phytohormones and sugars, must also be taken into consideration (Little and Bonga, 1974; Riding and Little, 1984, 1986; Mellerowicz et al., 1992; Savidge and Barnett, 1993; Savidge, 1996, 2000; Kozlowsky and Pallardy, 1997; Lachaud et al., 1999; Aloni et al., 2000; Krabel, 2000; Uggla et al., 2001; Wodzicki, 2001; Oribe et al., 2003). The extent of cell divisions and cell differentiation in the cambium might depend on the supply of sucrose from the storage tissue to the cambium (Oribe et al., 2003). The cessation of cell divisions in the heat-reactivated cambium could be a result of the absence of sucrose. Oribe et al. (2003) hypothesized that the continuation of cell division in the cambium after cambial reactivation requires a continuous supply of sucrose. Moreover, an inability of cambial derivatives to proceed with differentiation might cause a deficiency of indole acetic acid (IAA) in the cambial region of locally heated stems during cambial dormancy. During regular cambial activity, a continuous supply of IAA from elongating shoots and/or expanding buds can maintain cambial divisions and the subsequent cell development (Oribe et al., 2003). Local cooling of the stem could reduce supply and the level of carbohydrates and IAA to differentiating xylem cells, and hence trigger earlier formation of latewood tracheids and thus earlier cessation of cambial activity than under natural conditions. Long-term experiments are required to provide more detail of the effects of low or high temperatures on the rate of cell division, the histochemical response and possible disturbances in cellular differentiation.
Acknowledgments
The authors gratefully acknowledge the help of Professor Jasna Štrus and her team at the Department of Biology, University of Ljubljana, and Tanja Potsch from the Institute for Wood Biology and Wood Protection, Federal Research Centre for Forestry and Forest Products, Hamburg. We are grateful to Peter Cunder and Maks Merela for helpful field assistance. We are indebted to the Slovenian Forestry Institute for enabling experimental work in the field. We thank Dr Tom Levanič from the Slovenian Forestry Institute for providing the meteorological data. This work was funded by the COST Action E28 (Genosilva: European Forest Genomics Network), the Ministry of Education, Science and Sport of the Republic of Slovenia and EU PINE project (V. FP EVK2-CT-2002-00136).
LITERATURE CITED
- Aloni R, Feigenbaum P, Kalev N, Rozovsky S. 2000. Hormonal control of vascular differentiation in plants: the physiological basis of cambium ontogeny and xylem evolution. In: Savidge RA, Barnett JR, Napier R, eds. Cell and molecular biology of wood formation. Oxford: BIOS Scientific Publishers Ltd, 223–236.
- Barnett JR, Miller H. 1994. The effect of applied heat on graft union formation in dormant Picea sitchensis (Bong.) Carr. Journal of Experimental Botany 45: 135–143. [Google Scholar]
- Denne MP, Dodd RS. 1981. The environmental control of xylem differentiation. In: Barnett JR, ed. Xylem cell development. Tunbridge Wells, UK: Castle House Publications Ltd, 236–255.
- Donaldson LA. 1992. Lignin distribution during latewood formation in Pinus radiata. IAWA Bulletin n.s. 12: 381–387. [Google Scholar]
- Fengel D, Wegener G. 1989. Wood: chemistry, ultrastructure, reactions. Berlin: Walter de Gruyter.
- Fergus BJ, Goring DAI. 1970. The location of guaiacyl and syringyl lignins in birch xylem tissue. Holzforschung 24: 113–117. [Google Scholar]
- Fukazawa K, Imagawa H. 1981. Quantitative analysis of lignin using an UV microscopic image analyser. Variation within one growth increment. Wood Science and Technology 15: 45–55. [Google Scholar]
- Koch G, Kleist G. 2001. Application of scanning UV microspectrophotometry to localise lignins and phenolic extractives in plant cell walls. Holzforschung 55: 563–567. [Google Scholar]
- Koch G, Grünwald C. 2004. Application of UV microspectrophotometry for the topochemical detection of lignin and phenolic extractives in wood fibre cell walls. In: Schmitt U, Ander P, Barnett JR et al., eds. Wood fibre cell walls: methods to study their formation, structure and properties. Uppsala: Swedish University of Agricultural Sciences, 119–130.
- Kozlowsky TT, Pallardy SG. 1997. Growth control in woody plants. San Diego: Academic Press.
- Krabel D. 2000. Influence of sucrose on cambial activity. In: Savidge RA, Barnett JR, Napier R, eds. Cell and molecular biology of wood formation. Oxford, UK: BIOS Scientific Publishers Ltd, 113–125.
- Lachaud S, Catesson AM, Bonnemain JL. 1999. Structure and functions of the vascular cambium. Life Sciences 322: 633–650. [DOI] [PubMed] [Google Scholar]
- Larcher W. 2003. Physiological plant ecology. Ecophysiology and stress physiology of functional groups, 4th edn. Berlin: Springer-Verlag.
- Larson PR. 1994. The vascular cambium. Berlin: Springer-Verlag.
- Little CHA. 1981. Effect of cambial dormancy state on the transport of [1-14C] indol-3-ylacetic acid in Abies balsamea shoots. Canadian Journal of Botany 59: 342–348. [Google Scholar]
- Little CHA, Bonga JM. 1974. Rest in the cambium of Abies balsamea. Canadian Journal of Botany 52: 1723–1730. [Google Scholar]
- Mellerowicz EJ, Coleman WK, Riding RT, Little CHA. 1992. Periodicity of cambial activity in Abies balsamea. I. Effects of temperature and photoperiod on cambial dormancy and frost hardiness. Physiologia Plantarum 85: 515–525. [Google Scholar]
- Oribe Y, Kubo T. 1997. Effect of heat on cambial reactivation during winter dormancy in evergreen and deciduous conifers. Tree Physiology 17: 81–87. [DOI] [PubMed] [Google Scholar]
- Oribe Y, Funada R, Shibagaki M, Kubo T. 2001. Cambial reactivation in locally heated stems of the evergreen conifer Abies sachalinensis (Schmidt) Masters. Planta 212: 684–691. [DOI] [PubMed]
- Oribe Y, Funada R, Kubo T. 2003. Relationships between cambial activity, cell differentiation and the localization of starch in storage tissues around the cambium in locally heated stems of Abies sachalinensis (Schmidt) Masters. Trees 17: 185–192. [Google Scholar]
- Oribe Y, Funada R, Kubo T. 2004. Cambial activity in locally heated stems of evergreen and deciduous conifers during winter cambial dormancy. In: Baas P, Barnett JR, Carcaillet C et al., eds. Proceedings of the International Symposium on Wood Science organized by IAWA, IAWS and CIRAD, Montpelier, France: 47.
- Rensing KH, Samuels AL. 2004. Cellular changes associated with rest and quiscence in winter-dormant vascular cambium of Pinus contorta. Trees 18: 373–380. [Google Scholar]
- Riding RT, Little CHA. 1984. Anatomy and histochemistry of Abies balsamea cambial zone cells during the onset and breaking of dormancy. Canadian Journal of Botany 62: 2570–2579. [Google Scholar]
- Riding RT, Little CHA. 1986. Histochemistry of the dormant vascular cambium of Abies balsamea: changes associated with tree age and crown position. Canadian Journal of Botany 64: 2082–2087. [Google Scholar]
- Savidge RA. 1996. Xylogenesis, genetic and environmental regulation – a review. IAWA Journal 17: 269–310. [Google Scholar]
- Savidge RA. 2000. Intristic regulation of cambial growth. Journal of Plant Growth Regulation 20: 52–77. [Google Scholar]
- Savidge RA, Barnett JR. 1993. Protoplasmic changes in cambial cells induced by a tracheid-differentiation factor from pine needles. Journal of Experimental Botany 44: 395–407. [Google Scholar]
- Savidge RA, Wareing PF. 1981. A tracheid differentiation factor from pine needles. Planta 153: 395–404. [DOI] [PubMed] [Google Scholar]
- Spurr AR. 1969. A low viscosity embedding medium for electron microscopy. Journal of Ultrastructural Research 26: 31–43. [DOI] [PubMed] [Google Scholar]
- Takabe K, Miyauchi S, Tsunoda R, Fukazawa K. 1992. Distribution of guaiacyl and syringyl lignins in Japanese beech (Fagus crenata): variation within an annual ring. IAWA Bulletin n.s. 13: 105–112.
- Uggla C, Magel E, Moritz T, Sundberg B. 2001. Function and dynamics of auxin and carbohydrates during earlywood/latewood transition in Scots pine. Plant Physiology 125: 2029–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodzicki TJ. 2001. Natural factors affecting wood structure. Wood Science and Technology 35: 5–26. [Google Scholar]