Abstract
• Background and Aims Dwarf mistletoes (Arceuthobium; Viscaceae) are highly specialized dioecious angiosperms parasitic on many gymnosperm hosts in the northern hemisphere. Several dwarf mistletoe species are capable of inducing an unusual form of isophasic infection in which the internal (endophytic) system proliferates even into the apical buds of its hosts. Studies of the internal endophytic system have, for the most part, focused on the parasite within secondary host tissues. The present anatomical and ultrastructural study characterizes the growth pattern of the isophasic endophytic system of Arceuthobium douglasii within the dormant apical buds of Pseudotsuga menziesii.
• Methods Semi-thin serial sections from dwarf mistletoe-infected host apical buds were mounted, stained and micrographed. Graphic files were created from the serial micrographs and these files were stacked. These stacked files were utilized to describe the pattern of growth of the endophyte within the host tissue. The interface between cells of the mistletoe and host was also examined at the ultrastructural level by transmission electron microscopy.
• Key Results By utilizing a novel technique of superimposed graphics, the current study reveals an organized pattern of mistletoe distribution that penetrates further into host tissues than previously known. A consistent pattern of growth occurring even into the preformed leaves of the host is documented.
• Conclusions The apparently non-intrusive growth of the parasite appears to be developmentally synchronized with that of the host. No symplastic connections were observed in the ultrastructural examination of the parasite/host interface within the apical buds of Pseudotsuga menziesii parasitized by A. douglasii or of Pinus contorta parasitized by A. americanum.
Keywords: Anatomy, Arceuthobium, British Columbia, dwarf mistletoe, Douglas fir, endophytic, isophasic, Viscaceae
INTRODUCTION
The mistletoe genus Arceuthobium (Viscaceae) includes several species that exhibit a highly specialized parasitic strategy known as isophasic parasitism. This strategy involves a growth synchrony between host and parasite, among other things, resulting in a predictable shoot-emergence pattern of the mistletoe with respect to host morphology. It also involves the permanent presence of the ultimate portions of the endophyte in the buds of the host (Kuijt, 1960). Only some species exhibit this remarkable form of parasitism, and only with respect to certain host species. For example, isophasic parasitism normally develops on Pinus contorta when A. americanum attacks it; the same species parasitizing Picea glauca results in a fundamentally different interaction and endophytic distribution. Each case of isophasic parasitism results in the development of a broom-like formation on the host, where all buds contain the youngest endophytic elements.
Three species capable of isophasic growth are native to temperate North America: A. americanum, A. pusillum and A. douglasii. A fourth species, A. minutissimum, endemic to the Himalayas, also produces isophasic brooming. Additional species have been reported to induce isophasic brooming (Hawksworth and Wiens, 1972, 1996; Kiu, 1984), but as morphological descriptions of their symptomatology have not been made, uncertainty persists.
The endophyte of Arceuthobium initially consists of uniseriate filaments distributed through various host tissues. Under isophasic conditions, many of these filaments are aligned parallel to the host branch. Filaments in older portions thicken to become cortical strands positioned in the phloem or inner cortical region of the host and produce radial sinkers which become encased by additional host xylem. It is from cortical strands that emergent mistletoe shoots are formed.
Extensive literature exists on the Arceuthobium endophyte as it develops in the mature branches of the host (e.g. Cohen, 1954; Alosi and Calvin, 1984, 1985; Sadik et al., 1986a, b; Calvin and Wilson, 1996). Most studies deal with conditions that are not isophasic, but a few researchers have studied isophasic endophytes (Thoday and Johnson, 1930; Kuijt, 1960; Srivastava and Esau, 1961; Tainter, 1971). Only Thoday and Johnson (1930) and Kuijt (1960) report the occurrence of the endophyte within apical buds of isophasic brooms, though the former authors are somewhat equivocal. The purpose of this study was to confirm the presence of endophytic isophasic filaments of A. douglasii in the apical buds of Pseudotsuga menziesii as well as in the A. americanum–Pinus contorta association, and to chart the precise position of the A. douglasii endophyte within these buds. A detailed anatomical analysis will contribute to a more thorough understanding of this unique isophasic growth pattern.
MATERIALS AND METHODS
Collection
Shoots from isophasic brooms of Pseudotsuga menziesii (Mirb.) Franco (Douglas fir) infected with Arceuthobium douglasii Engelm. were collected six times in southern British Columbia. Collections were made in June and October 1999 just north of Olalla on Hwy 3a; the remaining four were made between July 2002 and November 2003 on Green Mountain Road (49°20'15.8'N, 119°48'57.3W, 700 m a.s.l.). Shoots from isophasic brooms of Pinus contorta Loudon (lodgepole pine) infected with A. americanum Nutt. Ex Engelm. were collected seven times between July 1999 and November 2003. The latter were collected near the road to Lightning Lakes 1.5 km south-east of the junction with Hwy 3 at Manning Lodge, 64 km east of Hope, British Columbia (49°03'46.7'N, 120°47'55.7W, 1200 m a.s.l.). The lodgepole pine in this area has since been decimated by a bark beetle infestation and several of the larger trees utilized for collections had succumbed along with the mistletoe by November 2003.
Specimens were kept on ice and transported to laboratory facilities at the Canadian Food Inspection Agency, Centre for Plant Health, Sidney, BC, or the University of Victoria, Department of Biology, Victoria, BC, until dissected and fixed approx. 18–24 h after collection. Examinations of the shoot emergence patterns of the mistletoes were made within 48–72 h of collection. Digital micrographs of most collections were made under a dissecting microscope with a Nikon Coolpix 885 camera (Nikon Imaging, Tokyo, Japan), or a Sony Power HAD RGB camera (Sony Electronics, Tokyo, Japan) coupled to an Olympus SZX9 dissecting microscope (Olympus Corporation, Tokyo, Japan).
Sample preparation
Apical buds of mistletoe-infected Douglas fir and lodgepole pine were dissected, aspirated and fixed in glutaraldehyde [2 % glutaraldehyde in 0.05 m sodium cacodylate (pH 7·3) with 1 mm CaCl2 and 4 % sucrose], post-fixed in 1 % osmium tetroxide for 1 h at room temperature, dehydrated with acetone or ethanol and propylene oxide then infiltrated and embedded in Spurr's resin (Spurr, 1969). Mature needles from 1- and 2-year-old flushes were also fixed and embedded in the same manner.
Serial, longitudinal, semi-thin sections (0·5 μm) through portions of embedded buds were made utilizing knives of ultramicrotome glass cut with a Leica EMKMR2 knife maker (Leica Microsystems AG, Wetzlar, Germany). Semi-thin sections were produced with Leica Ultracut microtomes (Leica Microsystems AG). At approx. 10-μm intervals, three or four sections were mounted on a glass slide and stained with Richardson's stain (Methylene Blue and Azure II) (Richardson et al., 1960; Ruzin, 1999) or Toluidine Blue O (Ruzin, 1999).
A single representative Douglas fir apical bud was longitudinally sectioned in 0·5-μm increments and 129 successive digital micrographs were made. Each micrograph was examined, the image cropped and the background eliminated. The host bud (without bud scales) was outlined, the individual cells of the parasite were identified and highlighted, and the host tissue was masked leaving only the outlines of the host and of the mistletoe cells (Fig. 1). The individual graphic representations generated from the micrographs were superimposed or stacked in series emphasizing and clarifying the pattern of endophyte growth (Figs 1 and 2).
Fig. 1.
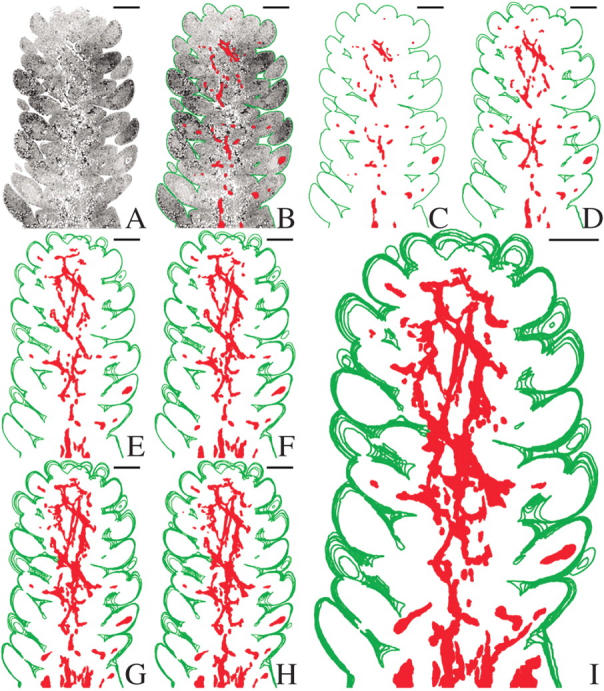
Stacked tracings of section profiles showing the distribution of the mistletoe endophyte in the cortical region near the periphery of a dormant Douglas fir apical bud. (A) Light micrograph of a longitudinal section of a dormant A. douglasii-infected Douglas fir apical bud. (B) Same as (A) but with host outlined in green and mistletoe cells highlighted in red. (C) Same as (A) and (B), but with all host cells masked to create a tracing representation of the mistletoe present in a single section. (D–I) Serial tracing representations, at 10-μm intervals, are stacked to illustrate the pattern of distribution of the endophyte in the cortex of the host. In (I) note the presence of the mistletoe in primordial leaves. Scale bars = 250 μm.
Fig. 2.
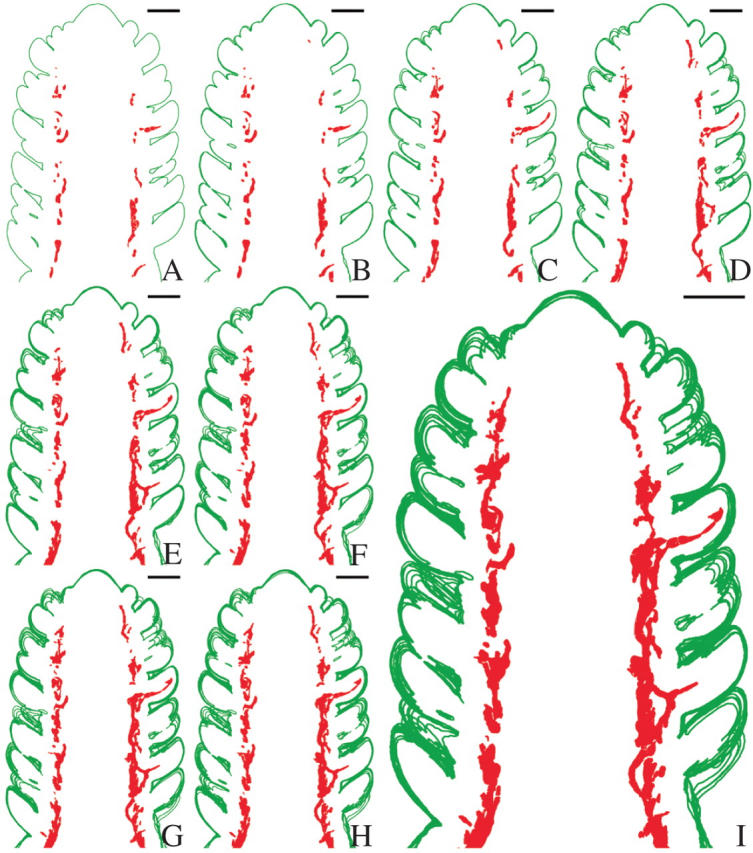
Stacked tracings of section profiles showing the distribution of the mistletoe endophyte in the median region of a dormant Douglas fir apical bud with host outlined in green and mistletoe cells highlighted in red. (A) Tracing representation of a single median longitudinal section. (B–I) Serial tracing representations, at 10-μm intervals, are stacked to illustrate the pattern of distribution of the endophyte in the host. In (I) note the presence of the mistletoe in primordial leaves and its limitation to the cortex of the host apical bud elsewhere. Scale bars = 250 μm.
Prior to trimming specimen blocks for transmission electron microscopy (TEM) study, 1-μm semi-thin sections were prepared as above, mounted on glass coverslips, stained with 5 % uranyl acetate in 50 % ethanol for 1 h and 5 % aqueous lead citrate for 1 h, mounted on aluminium stubs and carbon coated. These samples were examined at low magnification with a Hitachi S-3500N scanning electron microscope (SEM) (Hitachi Science Systems Ltd, Ibaraki, Japan) and TEM-like digital images were created (Fig. 3A and B). The specimen blocks were then trimmed in preparation for ultra-thin sectioning.
Fig. 3.
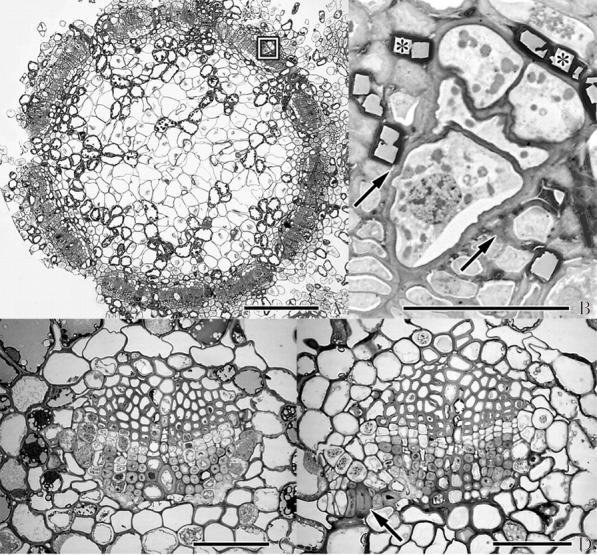
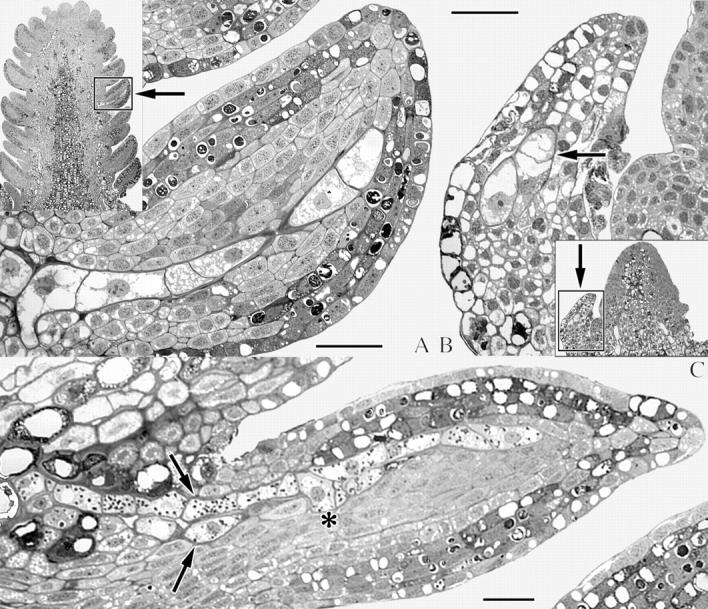
(A, B) Cross-section of branch and (C, D) needles of A. douglasii-infected Douglas fir. (A) SEM micrograph of cross-section through an infected branch proximal to the crown region of a dormant bud. (B) Close-up of box outlined in (A) in the region of a vascular bundle showing presumed sinker initiation by a multiseriate strand of mistletoe. Arrows indicate cambial zone. Primary xylem is at bottom of micrograph. Oxalic acid crystals are identified by asterisks. (C) Light micrograph of a cross-section of a vascular bundle of a healthy needle. (D) Same as (C) but with possible endophytic strand indicated by arrow near phloem. Scale bars: A = 250 μm; B–D = 50 μm.
Ultra-thin (70–80 nm) sections were cut with a 2·1-mm Diatome diamond knife (Diatome US, Hatfield, PA, USA) installed in a Leica Ultracut ultramichrotome, mounted on carbon-coated Formvar (1 % Formvar in 1,2-dichloroethane) copper mesh grids or slotted brass grids and stained with 5 % uranyl acetate in 50 % ethanol for 30 min and 5 % aqueous lead citrate for 10 min.
Sample imaging
Semi-thin serial sections were examined and photographed utilizing several different microscope and digital camera combinations. These included a Zeiss Universal epi-fluorescence microscope (Carl Zeiss AG, Göttingen, Germany) coupled with a Nikon Coolpix 990 (Nikon Imaging) or a Spot® model 7.0 camera with Spot® version 4.0 imaging software (Diagnostics Instruments Inc., Sterling Heights, MI, USA) and a Zeiss Axioskop microscope (Carl Zeiss AG, Göttingen, Germany) coupled to a DVC still digital camera (DVC Company, Austin, TX, USA) with Northern Eclipse version 5.0 imaging software (Empix Imaging Inc., Mississauga, Ontario, Canada). All digital micrographs were subsequently viewed and edited utilizing Adobe Photoshop CS (Adobe Systems Inc., San Jose, CA, USA).
Grids were viewed with a Hitachi 7000 TEM (Hitachi Science Systems Ltd) at 75-kV acceleration voltage. Images were captured on Kodak electron microscope film 4489 (Kodak, Rochester, NY, USA) and scanned with a Polaroid SprintScan 45 scanner with Polacolor Insight software (Polaroid, Waltham, MA, USA). All subsequent digital editing was performed utilizing Adobe Photoshop CS software (Adobe Systems Inc.) as above.
RESULTS AND DISCUSSION
Endophyte identification
An essential early step in establishing the pattern of endophyte distribution within host apical buds was the identification of cellular features in which parasite and host differed. Five distinctive characteristics were created from the literature and from direct observation. All were clearly visible in the 0·5- to 1·0-μm sections (Fig. 4).
Fig. 4.
Light micrograph of cross-section of a multiseriate strand of the endophyte of A. douglasii infecting Pseudotsuga menziesii illustrating the five characteristics utilized to differentiate the parasite from its host: (1) thickened external endophyte cell walls; (2) cell size and shape; (3) chromocentric nucleus; (4) presence of lipids (asterisks); and (5) plasmolysis (arrows) and vacuolization. Scale bar = 50 μm.
(1) Thickened external endophyte cell walls
Wherever the non-lignified cell walls of the endophyte contact host cells they are characterized by a pronounced and uniform thickening. These thick outer walls are clearly observed in the early illustrations of cross-sections of small Arceuthobium strands in Solms-Laubach (1867–1868, figs 6–10) and Cohen (1954, figs 7–9) and are usually two to three times as thick as those of the host parenchyma (Kuijt and Toth, 1976; Calvin and Wilson, 1996). This thickening contrasts sharply with the much thinner internal transverse walls observed in uniseriate filaments and in transverse and longitudinal walls of the older tiered, multiseriate filaments (Kuijt, 1960).
(2) Cell size and shape
Mistletoe cells in host branches are generally larger than the surrounding host cells, and in young uniseriate filaments are two to three times as long as they are wide (Thoday and Johnson, 1930). This is consistent with observations made in this study except that parasite cells are often four or more times as long as they are wide.
(3) Nucleus
Plant nuclei are of two basic types: chromocentric, characterized by the presence of dense chromatin masses; and reticulate, with chromatin evenly distributed throughout (Lafontaine, 1974). During interphase, the chromocentric A. douglasii nuclei contain a fine meshwork of chromatin and conspicuous chromatin masses: those of Pseudotsuga menziesii have a more reticulate chromatin distribution and lack the masses (Alosi and Calvin, 1984, 1985). Bhandari and Nanda (1970) observe that the relative chromaticity of the nuclei of the host (Pinus excelsa) and parasite (A. minutissimum) is distinct. Kuijt (1960), in differentiating parasite from host, relied on the conspicuous nucleus and the dark staining nucleoli of the cells of the endophyte. All of the studies mentioned above were made on Pinaceae; Sadik et al. (1986a), however, in their study of A. oxycedri infecting Juniperus (Cupressaceae), note that nuclear contrasts were not sufficiently pronounced to differentiate host from parasite.
(4) Presence of lipids
The presence of lipids in the endophyte cells has been discussed by Alosi and Calvin (1984, 1985), Calvin and Wilson (1996), Kuijt and Toth (1976), Sadik et al. (1986a, b), Tainter (1971) and Thoday and Johnson (1930). Arceuthobium douglasii parenchyma often contains large, regular, more or less spherical, dark-staining lipids; in Douglas fir, lipid droplets, when present, were generally smaller and less abundant.
(5) Plasmolysis and vacuolization
Kuijt (1960) observed that the endophyte cytoplasm shows more severe plasmolysis than its host, owing to fixation. In the material of this study a similar tendency was observed, but the distribution of cytoplasm and presence of vacuole membranes indicated vacuolization in addition to plasmolysis.
A great deal of seasonal variation in all of the above characteristics was observed with the possible exception of the thickened endophyte cell walls. In late spring, as dormancy is replaced by active growth, host cells undergo a very rapid increase in size; the expanding bud breaks through the protective layer of bud scales and produces the current year's flush of growth. During the bud break stage, the host cells appeared much closer in size to the mistletoe cells. Patterns of chromatin distribution also varied seasonally, in both host and parasite, but remained sufficiently distinct to provide an effective means of differentiation. During summer, when the host apical meristem is initiating the next season's growth, endophyte cells within the very small buds are heavily vacuolated and lipids are largely absent. It was often difficult to apply all five characteristics to any single cell (Fig. 4); as such, three or four characteristics commonly sufficed for identification. There were instances in which the differentiation of the parasite from the host was difficult as in Fig. 5C. When this occurred, questionable cells were treated as belonging to the host.
Fig. 5.
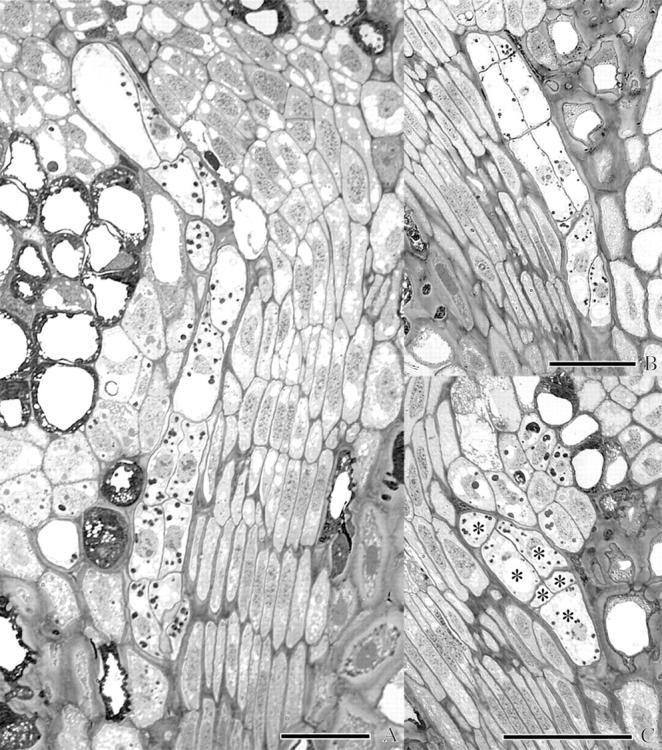
Light micrographs of endophytic filaments of A. douglasii near the crown region in the dormant apical buds of Douglas fir illustrating the tiered arrangement of cells characteristic of multiseriate strands. In each case, the endophytic filament is contiguous with and adjacent to the procambium. (A) Procambium is to the right of the endophyte in the lower two-thirds of the micrograph. This filament ultimately terminates in a leaf primordium. (B) Procambium is to the left of this clearly defined example of a multiseriate filament. (C) This micrograph demonstrates the ambiguity encountered in differentiating mistletoe from host. Only cells labelled with an asterisk were positively identified as endophyte. Scale bars = 50 μm.
The uniseriate strands of the endophyte were immediately recognizable in the first 0.5-μm sections through the cortical region of dormant apical buds of Douglas fir infected with A. douglasii. However, the profusion of mistletoe tissue present and its complexity made it necessary to develop a method of visually separating parasite from host. Initially, a single micrograph was examined, the mistletoe cells were identified and the host tissue masked to highlight the endophyte. If the host tissue was eliminated entirely, the course of the ramifying strands of the mistletoe were clarified (Fig. 6).
Fig. 6.
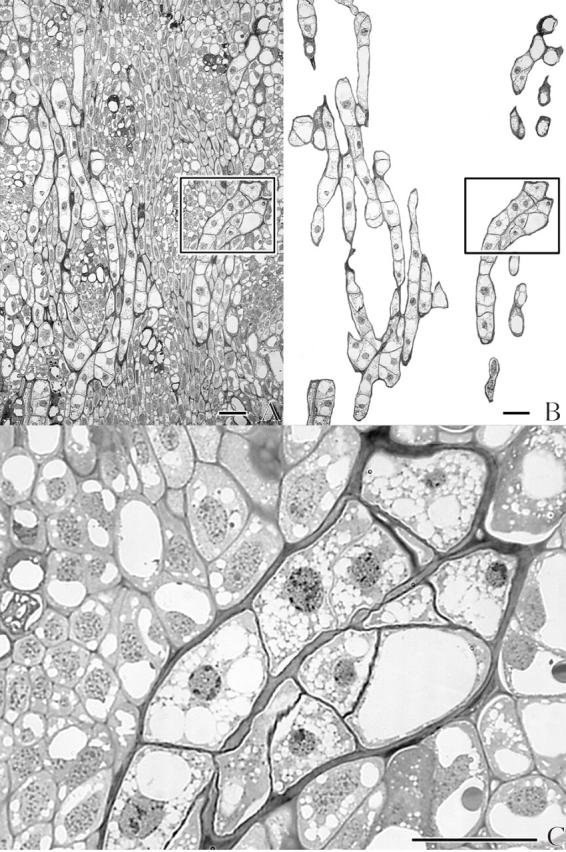
Light micrographs of a longitudinal section through the cortex of an infected dormant Douglas fir apical bud. (A) Unedited micrograph. (B) Same as (A), but with the host tissue hidden by an opaque mask. (C) Close-up of area outlined in (A) and (B) with the host partially masked to emphasize the two converged uniseriate strands of the endophyte. Scale bars = 50 μm.
As more buds were examined two important facts emerged. First, it became apparent that the endophytic strands were distributed in a more or less predictable pattern within the host. Second, and of perhaps greater interest, the endophyte was present even in some of the preformed leaves within the dormant buds (Fig. 7A, B).
Fig. 7.
Light micrographs of endophytic filaments of A. douglasii in preformed leaves and bud scales of longitudinal sections of Pseudotsuga menziesii. (A) Uniseriate filament in a preformed leaf of a dormant bud. (B) Oblique section of a uniseriate filament in a bud scale of an actively growing early summer bud. Endophyte cells are indicated by the arrow. (C) Preformed leaf of a dormant bud illustrating the presence of two uniseriate filaments (arrows) and initiation of branching (asterisk). Scale bars = 50 μm.
The entire bud identified as E-22-2 was serially sectioned and photographed. The micrographs were manipulated to create graphic representations of the endophyte's position within the host, and a crude animation created. Although this method could not be used to generate a three-dimensional representation, this simple animation proved to be a useful tool for visualizing and describing the pattern of endophyte distribution.
Lipid distribution and plasmolysis
Lipids were generally more abundant immediately above the ‘crown region’ (see Allen and Owen, 1972) and in the basal region of the cortex of the host bud. Endophyte cells near the bud apex often contained no lipids at all, consistent with the assertion of Alosi and Calvin (1985) that meristematic cells have few lipid bodies. Sadik et al. (1986a) describe sinkers in host secondary tissue, noting that lipids were abundant in sinkers but that more lipid inclusions were present at the tips of sinkers. Both Thoday and Johnson (1930; A. pusillum) and Parke (1951; A. douglasii) theorized that sinker cells are generated from an intercalary meristem located at the host cambium. If this is true, then the cells at the tips of the sinkers are developmentally older than those at the base; thus lipids are concentrated in the older endophyte cells, consistent with findings reported in this study. Plasmolysis and vacuolization increased as the abundance of lipids decreased. Mistletoe cells near the apical meristems of host buds often had little cytoplasm present and vacuoles were the most prominent cellular feature.
Endophyte distribution
The endophyte in host apical buds is, for the most part, composed of a series of parenchymatous uniseriate strands that appear to grow in fairly regular helical patterns within the host cortex. Like the helix observed in the arrangement of the leaf primordia, the endophytic strands spiral in both clockwise and anticlockwise directions from the crown region to apex. In his discussion of A. americanum parasitizing P. contorta, Kuijt (1960) suggests that the endophyte forms a loose wreath of filaments just below the host crown region. In this study no evidence of a wreath of filaments was found. Contrary to the indications in Kuijt (1960), the occurrence of mistletoe endophyte was not observed in the pith of either species; it occurred exclusively in the cortex, except as noted below.
A series of uniseriate and multiseriate strands thus seems to be located between the procambial strands and the epidermis centrifugal to the host crown region. Above the crown region a complex network of largely uniseriate strands ramifies throughout the cortex centrifugal to the pith, extending into some of the embryonic bud scales and preformed leaves of the host. The transverse cell walls separating endophyte cells within these strands are generally uniformly thin. By contrast the outer cell walls of the mistletoe are much thicker where they interface with host cells (Figs 3B, 4, 5, 6C, 7 and 8A, B). What appear to be multiseriate strands are quite common when viewed in longitudinal sections. These strands are of two very different forms.
Fig. 8.
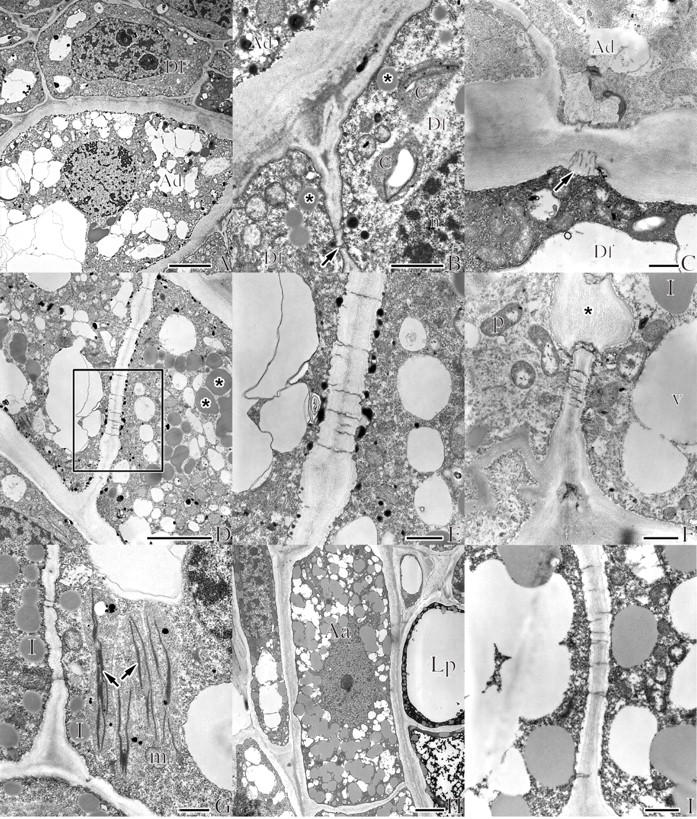
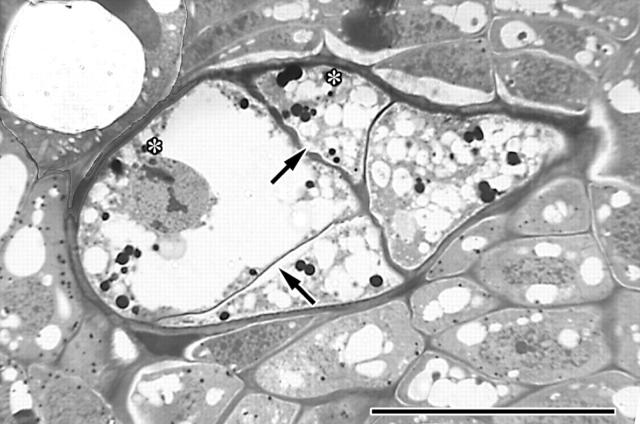
Transmission electron micrographs of A. douglasii (Ad) in dormant apical buds of Douglas fir (Df) (A–G) and of A. americanum (Aa) in the apical buds of lodgepole pine (Lp) (H and I). (A) Micrograph illustrating some of the most common ultrastructural features used to differentiate parasite from host, including the distinctive thick external cell wall, relatively larger cell size and increased vacuolization of the mistletoe cell (Ad) contrasted to the host cell (Df). Scale bar = 5 μm. (B) Close-up of the lower right corner of (A) illustrating some of the ultrastructural details of the host (Df), including chloroplasts with characteristic thylakoid membranes (c), lipids (asterisks) and plasmodesma (arrow). Scale bar = 1 μm. (C) Half plasmodesmata on the host side of the thickened external endophyte cell. Scale bar = 1 μm. (D) A portion of a uniseriate endophyte filament with characteristic thickened external cell wall, numerous lipids (asterisks) and plasmodesmata of the internal cell wall. Scale bar = 5 μm. (E) Area outlined in (D) illustrating plasmodesmata and small dark-stained starch granules. Scale bar = 1 μm. (F) Endophyte illustrating proplastids (p), lipids (l), vacuole (v) and an internal wall with numerous plasmodesmata and large cellulosic cell wall thickening (asterisk). Scale bar = 1 μm. (G) Ultrastructural details within a multiseriate strand of mistletoe with lipids (l), mitochondria (m) and endoplasmic reticulum (arrows). Scale bar = 1 μm. (H) A single cell of a uniseriate strand of the mistletoe endophyte; the cytoplasm is filled with numerous lipid bodies characteristic of late dormancy. Scale bar = 5 μm. (I) Close-up of an internal cell wall of the endophyte with the numerous plasmodesmata common to both of the Arceuthobium species studied. Scale bar = 1 μm.
In the first type, the cells occur in longitudinal pairs, separated by the thin wall described above (Fig. 5). This tiered arrangement of cells, indicative of their initially uniseriate structure, is consistent with descriptions of the cortical strands of the endophytic system as it occurs in the secondary host tissues (Heinricher, 1921; Thoday and Johnson, 1930; Parke, 1951). These multiseriate strands were more common near the base of the bud, especially in the crown region. Within the uniseriate and tiered multiseriate endophytic strands plasmodesmata were commonly observed (Fig. 8D-G, I), however, none were ever observed between parasite and host.
In the second type, the tiered arrangement of cells is absent; the walls between mistletoe cells often appear for the most part more like the thick mistletoe–host interface. This non-tiered multiseriate appearance is, in part, due to the meandering course described by the uniseriate strands; as they make their convolute way through the host cortex they are often found to be immediately adjacent to one another. In many cases, two or three individual strands will meet at a single point in the cortex and form an intertwining mass of tissues. Although difficult to interpret without study of consecutive serial sections, what appear to be relatively irregular multiseriate strands are presumed to be a series of fused, intertwined uniseriate strands. In some of the places where these endophytic strands grow within close contact of one another, instead of the thick cell wall normally seen on the strand periphery, alternating thin and thick areas of cell wall occur (Fig. 6C). This may represent reabsorption of the cell wall and a re-establishment of symplasty where two or more of the uniseriate strands contact one another within the cortex. In this study, no plasmodesmata were observed in these areas of possible reabsorption. Extensive ultrastructural study of these regions would be required to determine whether secondarily formed plasmodesmata establish a symplastic connection between individual strands of the endophyte.
Branching of the uniseriate strands of the endophyte was common, consistent with the descriptions of A. pusillum by Thoday and Johnson (1930), A. douglasii by Parke (1951) and A. minutissimum by Bhandari and Nanda (1970). Often a bulge arises from the side of a sub-terminal cell. Eventually an oblique or longitudinal division wall forms and the meristematic cell separated from the mother strand gives rise to a new strand, through a series of regular transverse divisions, indistinguishable from that from which it has arisen. This branching was observed throughout the cortex of dormant host buds and at times even occurred in preformed leaves (Fig. 7C).
Mistletoe was observed in preformed leaves (Fig. 7A and C) and embryonic bud scales (Fig. 7B) of dormant buds. Usually, but not exclusively, a single uniseriate strand of the endophyte, with its origin in the cortex, had penetrated the preformed leaf of the host. Although at this stage of development the method employed did not allow a distinctive staining of the provascular tissue within the primordial leaves, in each case the endophyte was situated toward the centre of the needle in the region that will ultimately give rise to the vascular tissue of the leaf. In many cases, the parasitic strand reached to within a few cells of the primordial leaf apex (Fig. 7A). In several instances, sub-apical branching of the endophyte was observed even within the newly expanding leaf (Fig. 7C) and, occasionally, two individual uniseriate strands were observed within a single preformed leaf.
Kuijt (1960), in the expanding material of an actively growing apical bud of Douglas fir, reported several instances where the apical cell of a single filament of A. douglasii endophyte actually broke through the epidermis of the host. His micrograph (fig. 39) clearly shows this eruption of a filament in the axil of a bud scale subtending a primordial leaf. The exact time of collection was not recorded, but the developmental state of the bud scale and leaf primordium in his micrograph are indicative of material collected in mid-summer. During the present study, no such emergences were observed in comparable material, even though the endophyte was observed within embryonic bud scales (Fig. 7B), often in close proximity to the epidermis immediately adaxial to the insertion point of the bud scale. In order to prepare apical buds for fixation it was necessary to excise the bud scales; it is probable that the emerging mistletoe filaments that Kuijt observed were present in the study material but were removed with the bud scales prior to fixation.
The endophyte was not present in every preformed leaf; however, infected leaves did occur with consistency in every bud examined. In an attempt to quantify the number of infected leaves, the single serially sectioned apical bud, described above, was examined at 10-μm intervals. Of the 120 total leaf primordia present, 37 contained mistletoe tissue. Portions of four additional Douglas fir buds were serially sectioned and the number of infected primordia recorded. Only fully sectioned leaves were included in calculations. This recognizes the fact that partially sectioned needles may contain the endophyte in the unsectioned and therefore unexamined portion. No recognizable pattern of primordial leaf infection was apparent in any apical buds examined; infected needles appeared to be randomly distributed over all regions of the bud. The results indicate that the mistletoe appears to infect approximately one-quarter to one-half of all the preformed leaves within a dormant bud.
The occurrence of the endophyte in primordial leaves raised the question of the possible presence of the parasite in mature host leaves. Although mature needles from all sample collections made in 2003 were examined, no evidence was found of the mistletoe, with one exception. In a single needle petiole cross-section, an unusual group of cells was noted immediately adjacent to the primary phloem of the host (Fig. 3D). The thick cell walls and unusual position suggests that the group of cells may have been associated with the tip of the endophytic strand. However, it is also possible that this unusual cell group was the early stage of astrosclereid formation, not unusual in the mesophyll of mature needles of Douglas fir (Al-Talib and Torrey, 1961; Apple et al., 2002). Nevertheless, this explanation seems unlikely as Douglas fir astrosclereids normally occur singly in more mature needles.
The preformed leaves within the dormant bud are generally <500 μm in length and in some cases the endophyte grows to within a few cells of the leaf primordium apex (Fig. 7A). The failure to detect the mistletoe in the emerged needle suggests that the growth of the endophyte may not keep pace with the rapidly elongating needle. Alternatively, the characteristics of the endophyte may change to such an extent that they cannot be recognized by the techniques employed in this study.
Even though the main focus of this study was the endophyte within the primary tissues of host apical buds, observations of the crown region were also made. Kuijt (1960, fig. 6) illustrates a loose wreath of endophytic filaments girdling the base of the bud and repeated just subtending the apex of the bud of Pinus contorta infected with A. americanum. He further theorizes a similar pattern of endophyte distribution for Pseudotsuga menziesii infected with A. douglasii. In this study, no such wreaths of Douglas fir mistletoe cells were observed; instead, numerous individual filaments were observed, always in the cortical region centrifugal to primary xylem of the vascular bundles. Many of the strands were uniseriate, but many multiseriate strands were also observed that displayed the tiered arrangement described above.
In the discussion of endophyte identification, reference was made to the presence of an intercalary meristem in the Arceuthobium endophyte located at the vascular cambium region of the host which generates sinker cells (Thoday and Johnson, 1930; Parke, 1951). Gill (1935), Kuijt (1960) and Srivastava and Esau (1961) agree with this interpretation; however, all of these studies have been in the secondary tissues of the host. When a cross-section through the crown region of an infected Douglas fir bud is examined, the uniseriate and multiseriate strands of the endophyte are clearly visible. These strands are always located in or near the primary phloem area of the vascular traces or in the newly developing secondary phloem centrifugal to the vascular cambium. In several cases, a configuration of cells was observed which strongly suggested the initiation of a sinker penetrating, or being encased in, the primary xylem of the host (Fig. 3A, B). The wedge-shaped endophyte does not penetrate into host xylem cells; rather it appears to be pushing the files of primary xylem cells apart. This early ontogeny of sinker initiation supports Kuijt's (1960) argument that the radial development of sinkers is not dependent on the endophytic contact with phloem rays as suggested by others.
Ultrastructural observations
Although the main emphasis of this study was anatomical, limited ultrastructural observations of the parasite and of the host/parasite interface were made. Previous ultrastructural studies of the Arceuthobium endophyte have been within the context of the host secondary tissues. Electron micrographs were made of the endophyte of A. douglasii in Pseudotsuga menziesii and of A. americanum in Pinus contorta, in primary tissue of the host apical buds, bud scales and leaf primordia.
Writing on the ultrastructure of A. pusillum, Tainter (1971) established the presence of chloroplasts in parenchyma cells of aerial shoots and endophyte. Chloroplasts within the endophyte were also observed in A. oxycedri by Heinricher (1921) and Sadik et al. (1986a); Solms-Laubach (1867–1868) also states that he observed chloroplasts. In their ultrastructural studies, Alosi and Calvin (1984, 1985) and Calvin and Wilson (1996) make no mention of the presence of chloroplasts within endophytic tissue of Arceuthobium although they document the presence of plastids.
This study found plastids to be abundant in the endophyte of A. douglasii and A. americanum (Fig. 8). Chloroplasts were frequently observed in host cells (Fig. 8B) but none were observed in the cells of the endophyte. Lipids were common in both mistletoe species and were especially abundant in A. americanum collected in late spring (Fig. 8H). In addition to numerous lipids, small dark-staining starch granules were also observed, most commonly in association with the cell wall of A. douglasii (Fig. 8D, E).
All of the researchers mentioned, except Tainter (1971), agree that no true plasmodesmata connect mistletoe cells with those of their hosts, although Alosi and Calvin (1985) do note the presence of half plasmodesmata extending from the protoplast of host cells to the middle lamella region separating host from parasite. Tainter (1971), in his examination of a spruce broom, states that he observed plasmodesmata in restricted areas between cells of the endophytic system of A. pusillum and host needle trace phloem parenchyma of Picea mariana, but his micrograph, figure 6b, fails to show complete plasmodesmata (Alosi and Calvin, 1985) and it seems possible that an error may have been made in cell identification (Kuijt and Toth, 1976). In the present study no plasmodesmata were observed connecting host to parasite. Half plasmodesmata were documented (Fig. 8C) and plasmodesmata connecting endophyte cells within uniseriate strands were very common in both of the host/parasite associations studied (Fig. 8D–G, I). Transverse primary walls between mistletoe cells occasionally contained massively thickened areas (Fig. 8F) referred to as peculiar beadlike thickenings by Kuijt (1960). These thickenings, with their clearly evident microfibril structure, resemble the cellulosic primary cell wall of the endophyte, suggesting that they are not composed of callose.
Within the apical buds studied very little physical evidence of crushing or tearing of host tissue was observed suggesting that little or no intrusive growth was occurring. Indeed conjoint cell walls of parasite and host are fused so completely that it is usually impossible to discern the precise location of any middle lamella. This implies that the uniseriate strands of the endophyte must be capable of a very rapid longitudinal growth in early spring when host shoot elongation occurs. The formation of transverse division walls was commonly observed in the cells composing uniseriate strands; these division walls were not confined to apical cells but were observed along the entire strand. This frequent division may explain, in part, the ability of the mistletoe to keep pace with host growth. Alternatively, the very thick cellulosic, primary wall of the mistletoe cell, where it interfaces with the host, might accommodate the stretching of the filament during the rapid elongation of the host branch.
Suggestions for further study
As mentioned in the Introduction, isophasic infection is not limited to the two parasite/host combinations discussed above. It would be useful to expand the methodology employed in this study into the two other Arceuthobium species known to induce an isophasic host response. The technique could also be utilized to confirm the isophasic status of A. guatemalense, A. chinense, A. sichuanense, A. pini and A. abietis-religiosae. By employing the polymerase chain reaction test to detect Arceuthobium douglasii, as published by Marler et al. (1999), molecular confirmation could be added to the anatomical information presented in this study. The test could provide an effective method for rapidly identifying the presence or absence of the endophyte in species thought to be isophasic and for determining if the parasite is present in host needles.
Conclusions
The endophyte of isophasic A. douglasii extends much further into host tissues than previously known. Throughout the undifferentiated areas where the host prepares for the next year's longitudinal growth, the filaments of the parasite are already present, ready to extend with host tissues and lay the foundations for future emergent shoots. Mistletoe tissues make up a significant portion of the central core of the dormant host bud even extending into some of the preformed leaves. There is no evidence of symplastic continuity between host and parasite at least in the primary tissues. No other known parasitic angiosperm has accommodated their growth pattern more completely to the vegetative growth cycles of their hosts than isophasic dwarf mistletoes.
The observations of the consistent pattern of A. douglasii distribution in the dormant apical buds of Pseudotsuga menziesii, particularly the position within preformed leaves and bud scales, and in close proximity to the leaf axils where axillary buds will develop, combined with the lack of ultrastructural or anatomical evidence of intrusive growth, suggest that the pattern of endophyte growth is truly isophasic, keeping pace with the cycles of seasonal growth experienced by the host. This distribution is closely correlated with the morphological pattern of shoot emergence observed in isophasic brooms.
Acknowledgments
Financial support, through a grant to J. Kuijt, was provided by the Natural Sciences and Engineering Research Council of Canada. The author thanks the Centre for Forest Biology, University of Victoria, The Canadian Food Inspection Agency, Centre for Plant Health, Sidney, BC, and Brent Gowen for EM assistance and Job Kuijt for his guidance and support.
LITERATURE CITED
- Allen GS, Owens JN. 1972. The life history of Douglas fir. Ottawa: Information Canada, Environment Canada Forestry Service. Cat. No. F042-4972.
- Alosi MC, Calvin CL. 1984. The anatomy and morphology of the endophytic system of Arceuthobium spp. In: Hawksworth FG, Scharpf RF, Technical coordinators, Biology of dwarf mistletoes: proceedings of the symposium. 8 August 1984; Fort Collins, CO. General Technical Report RM-111. Fort Collins, CO: US Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station, 40–52.
- Alosi MC, Calvin CL. 1985. The ultrastructure of dwarf mistletoe (Arceuthobium spp.) sinker cells in the region of the host secondary vasculature. Canadian Journal of Botany 63: 889–898. [Google Scholar]
- Al-Talib KH, Torrey JG. 1961. Sclereid distribution in the leaves of Pseudotsuga under natural and experimental conditions. American Journal of Botany 48: 71–79. [Google Scholar]
- Apple M, Tiekotter K, Snow M, Young J, Soeldner A, Phillips D, et al. 2002. Needle anatomy changes with decreasing tree age in Douglas-fir. Tree Physiology 22: 129–136. [DOI] [PubMed] [Google Scholar]
- Bhandari NN, Nanda K. 1970. The endophytic system of Arceuthobium minutissimum, the Indian dwarf mistletoe. Annals of Botany 34: 517–526. [Google Scholar]
- Calvin CL, Wilson CA. 1996. Endophytic system. In: Hawksworth FG, Wiens D. Dwarf mistletoes: biology, pathology, and systematics. Washington, DC: USDA, Forest Service, 113–122.
- Cohen LI. 1954. The anatomy and endophytic system of the dwarf mistletoe, Arceuthobium campylopodum. American Journal of Botany 41: 840–847. [Google Scholar]
- Gill LS. 1935. Arceuthobium in the United States. Transactions of the Connecticut Academy of Arts and Sciences 32: 111–245. [Google Scholar]
- Hawksworth FG, Wiens D. 1972. Biology and classification of dwarf mistletoe (Arceuthobium). Agriculture Handbook No. 401. Washington, DC: US Department of Agriculture, Forest Service.
- Hawksworth FG, Wiens D. 1996. Dwarf mistletoes: biology, pathology, and systematics. Agriculture Handbook No. 709. Washington, DC: US Department of Agriculture, Forest Service.
- Heinricher E. 1921. Das Absortionssystem von Arceuthobium oxycedri (DC.) M. Bieb. Berichte der Deutsche Botanische Gesellschaft 39: 20–25. [Google Scholar]
- Kiu HS. 1984. Arceuthobium and its hosts in southwestern China. In: Hawksworth FG, Scharpf RF, Technical coordinators. Biology of dwarf mistletoes: proceedings of the symposium. 8 August 1984, Fort Collins, CO. General Technical Report RM-111. Fort Collins, CO: US Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station, 18–19.
- Kuijt J. 1960. Morphological aspects of parasitism in the dwarf mistletoes (Arceuthobium). University of California Publications in Botany 30: 337–436. [Google Scholar]
- Kuijt J, Toth R. 1976. Ultrastructure of angiosperm haustoria – a review. Annals of Botany 40: 1121–1130. [Google Scholar]
- Lafontaine JG. 1974. The nucleus. In: Robards AW, ed. Dynamic aspects of plant ultrastructure. London: McGraw Hill.
- Marler M, Pedersen D, Mitchell-Olds T, Callaway RM. 1999. A polymerase chain reaction method for detecting dwarf mistletoe infection in Douglas-fir and western larch. Canadian Journal of Forest Research 29: 1317–1321. [Google Scholar]
- Parke RV. 1951. An investigation of the endophytic system of Arceuthobium douglasii (Engelm.). Masters Thesis, University of Washington, Seattle, WA, USA.
- Richardson KC, Jarett L, Finke EH. 1960. Embedding in epoxy resins for ultrathin sectioning in electron microscopy. Stain Technology 35: 313–323. [DOI] [PubMed] [Google Scholar]
- Ruzin SE. 1999. Plant microtechnique and microscopy. Oxford: Oxford University Press.
- Sadik A, Rey L, Renaudin S. 1986a. Le système endophytique d'Arceuthobium oxycedri. I. Organisation, étude cytologique et cytochemique. Canadian Journal of Botany 64: 1104–1111. [Google Scholar]
- Sadik A, Rey L, Renaudin S. 1986b. Le système endophytique d'Arceuthobium oxycedri. II. Aspects ultrastructuraux des zones de contact entre les tissues de l'hôte et du parasite. Canadian Journal of Botany 64: 2778–2784. [Google Scholar]
- Solms-Laubach H. 1867–1868. Ueber den Bau und die Entwicklung der Ernährungsorgane parasitischer Phanerogamen. Jahrbücher für wissenschaftliche Botanik 6: 615–621. [Google Scholar]
- Spurr AR. 1969. A low viscosity resin embedding medium for electron microscopy. Journal of Ultrastructural Research 26: 443–514. [DOI] [PubMed] [Google Scholar]
- Srivastava LM, Esau K. 1961. Relation of dwarf mistletoe (Arceuthobium) to the xylem tissue of conifers. I. Anatomy of parasite sinkers and their connection to host xylem. American Journal of Botany 44: 159–167. [Google Scholar]
- Tainter FH. 1971. The ultrastructure of Arceuthobium pusillum. Canadian Journal of Botany 49: 1615–1622. [Google Scholar]
- Thoday D, Johnson ET. 1930. On Arceuthobium pusillum Peck. I. The endophytic system. Annals of Botany 44: 393–413. [Google Scholar]