Abstract
• Aims Arbuscular mycorrhizae are formed between >80 % of land plants and arbuscular mycorrhizal (AM) fungi. This Botanical Briefing highlights the chemical identification of strigolactones as a host-recognition signal for AM fungi, and their role in the establishment of arbuscular mycorrhizae as well as in the seed germination of parasitic weeds.
• Scope Hyphal branching has long been described as the first morphological event in host recognition by AM fungi during the pre-infection stages. Host roots release signalling molecules called ‘branching factors’ that induce extensive hyphal branching in AM fungi. Strigolactones exuded from host roots have recently been identified as an inducer of hyphal branching in AM fungi. Strigolactones are a group of sesquiterpenes, previously isolated as seed germination stimulants for the parasitic weeds Striga and Orobanche. Parasitic weeds might find their potential hosts by detecting strigolactones, which are released from plant roots upon phosphate deficiency in communication with AM fungi. In addition to acting as a signalling molecule, strigolactones might stimulate the production of fungal symbiotic signals called ‘Myc factors’ in AM fungi.
• Conclusions Isolation and identification of plant symbiotic signals open up new ways for studying the molecular basis of plant–AM-fungus interactions. This discovery provides a clear answer to a long-standing question in parasitic plant biology: what is the natural role for germination stimulants? It could also provide a new strategy for the management and control of beneficial fungal symbionts and of devastating parasitic weeds in agriculture and natural ecosystems.
Keywords: Sesquiterpene lactone, Lotus japonicus, Gigaspora margarita, root exudate, Striga, Orobanche, phosphate nutrition
INTRODUCTION
Mycorrhizae are symbiotic associations between soil fungi and plant roots (Smith and Read, 1997). Arbuscular mycorrhizae, formed between >80 % of land plants and arbuscular mycorrhizal (AM) fungi belonging to the Glomeromycota (Schüssler et al., 2001), are the most common and widespread symbiosis on our planet (Fig. 1A) (Gianinazzi-Pearson, 1996; Harrison, 2005). AM fungi are obligate symbionts incapable of completing their life cycle in the absence of a host root. The fungi penetrate and colonize plant roots, where they differentiate into highly branched structures known as arbuscules, which are thought to be the principal sites of nutrient exchange between the two organisms. Concomitant development of extra-radical hyphae outside the plant roots allows the fungi to supply the host with essential nutrients such as phosphate, nitrate and other minerals from the soil. In return, AM fungi receive carbohydrates derived from photosynthesis in the host. AM symbiosis also confers resistance to the plant against pathogens and environmental stresses. Species composition and richness of AM fungi have been shown to contribute greatly to plant biodiversity as well as to the variability and productivity of natural ecosystems (van der Heijden et al., 1998). The fossil records from the Ordovician and Devonian eras indicate the existence of AM symbioses over 460 million years ago, suggesting that the fungi played a crucial role in facilitating the colonization of land by plants (Remy et al., 1994; Redecker et al., 2000). Despite the central importance of AM symbiosis in both agriculture and natural ecosystems, the mechanisms for the formation of a functional symbiosis between plants and AM fungi are largely unknown. A major factor hampering studies on AM fungi is their obligately biotrophic nature; the fungi have not been cultured in the absence of a plant host.
Fig. 1.
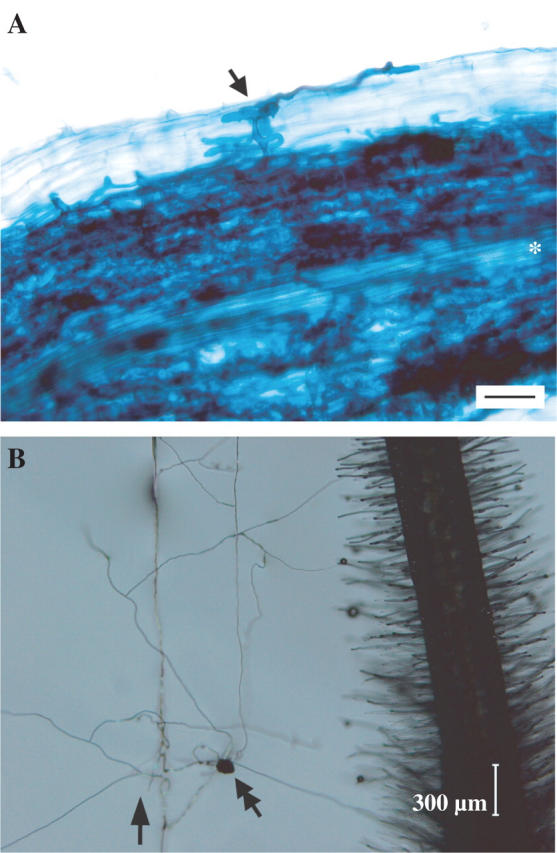
Symbiotic interactions between the model legume Lotus japonicus and the AM fungus Gigaspora margarita. (A) Arbuscular mycorrhizae formed between L. japonicus and G. margarita. Fungal hyphae are stained with trypan blue after the root has been cleared by heating in KOH. Arrow, appressorium formed on root surface; asterisk, root's stele. Scale bar = 60 μm. (B) Hyphal branching of G. margarita in the vicinity of roots of L. japonicus. Secondary, tertiary, fourth and fifth hyphae emerge from primary hypha. Arrow, direction of growth of primary hypha (germ tube); double arrow, auxiliary cells of fungus. Scale bar = 300 μm.
The plant–AM-fungus interaction is initiated by mutual signal exchange between the two partners during pre-infection stages (Harrison, 2005). Host roots release signal molecules called ‘branching factors’ (BFs) that induce extensive hyphal branching in AM fungi. AM fungi have long been postulated to produce signal molecules called ‘myc factors’ (MFs) that induce the molecular and cellular responses leading to successful root colonization by AM fungi. Neither of these signals had been isolated and chemically identified until recently when a BF was isolated from the root exudates of the model legume Lotus japonicus, and was identified as a strigolactone, 5-deoxy-strigol (Akiyama et al., 2005). Strigolactones are a group of sesquiterpene lactones, previously isolated as seed-germination stimulants for the root-parasitic weeds Striga and Orobanche (Bouwmeester et al., 2003). It now turns out that the same compounds are detected by beneficial fungal symbionts and by devastating parasitic weeds as host-derived signals.
HYPHAL BRANCHING: HOST RECOGNITION RESPONSE OF AM FUNGI
The critical developmental step in the life cycle of AM fungi is hyphal branching. Although AM fungi cannot complete their life cycle in the absence of a host root, their spores can germinate independently of host plants as long as some physical and physiological conditions are fulfilled. However, the hyphal growth is very limited, and ceases long before consumption of spore reserves if a host root is not present in the environment (Bécard and Piché, 1989). In the presence of host roots, the hyphae of AM fungi differentiate into specific morphological structures characterized by extensive hyphal branching, which help the fungi to ensure contact with the root and the establishment of symbiosis (Fig. 1B). These hyphal branching phenomena were first described by Mosse and Hepper (1975) in the first report of the in vitro co-culture of a clover root and an AM fungus. They called these structures ‘arbuscule-like branches’. Powell (1976) found that hyphae, recently germinated from spores, branch repeatedly to form septate ‘fan-like structures’ in the close vicinity of onion roots before appressorium formation, and suggested that the fan-like pre-infection hyphae are the site of cytological changes necessary before hyphae from spores become physiologically infective. Giovannetti et al. (1993, 1994) used a membrane sandwich system to show that this differential hyphal morphogenesis is a host recognition response of AM fungi. AM fungal hyphae, grown on membranes overlying AM host roots, exhibited extensive hyphal branching, while no morphogenetic event was elicited by the roots of non-hosts (including hosts of ecto, arbutoid and ericoid mycorrhizae as well as non-mycorrhizal plants). These findings strongly indicated the existence of chemical signals, emitted exclusively by host roots, acting as early cues for differential hyphal branching.
BRANCHING FACTORS: LIPOPHILIC LOW-MOLECULAR-WEIGHT COMPOUNDS EXUDED FROM HOST ROOTS
The identity of BFs, exuded from host roots, has been the object of considerable research. Dialysis membranes have been used in the sandwich system described above to determine that the BF exuded from growing roots of common basil (Ocimum basilicum), which elicits hyphal branching in Glomus mosseae, is a low-molecular-weight compound <500 Da (Giovannetti et al., 1996). The development of an in-vitro bioassay for hyphal branching in germinating spores of the genus Gigaspora (Nagahashi and Douds, 1999) has facilitated the analysis of the chemical characteristics and distribution of BF in the plant kingdom (Buee et al., 2000; Nagahashi and Douds, 2000). BF was present in root exudates of all host plants of AM fungi, but absent in those of non-hosts. The discovery that BF is partitioned into ethyl acetate from an aqueous root exudate (Buee et al., 2000), and is retained on a C18 reverse-phase resin (Nagahashi and Douds, 2000), indicates that BF is a lipophilic compound. The presence of several BFs in host plants was suggested by C18 reverse-phase column and preparative thin-layer chromatography (Nagahashi and Douds, 2000). Root exudates from plants grown under phosphate-limited conditions are more active than those from plants with sufficient phosphate nutrition, suggesting that the production of BF in roots and its exudation are regulated by phosphate availability (Nagahashi and Douds, 2000).
Phenolics including flavonoids have been strong candidates for BF because these compounds play an active role in the regulation of symbiotic and pathogenic interactions with microbes (Peters and Varma, 1990). In fact, some flavonoids such as quercetin have been reported to promote spore germination, hyphal elongation and hyphal branching (Gianinazzi-Pearson et al., 1989; Tsai and Phillips, 1991; Bécard et al., 1992). However, flavonoids have been ruled out as BF candidates because root exudates of maize mutants deficient in chalcone synthase, necessary for the biosynthesis of flavonoids, showed comparable activity to those of the wild type in the hyphal branching assay (Buee et al., 2000). This is further supported by the evidence that the same maize mutants are equally colonized by AM fungi as the wild-type maize (Bécard et al., 1995) and that quercetin shows no activity in the branching bioassay (Buee et al., 2000; Nagahashi and Douds, 2000). In addition, significant amount of quercetin, myricetin and kaempferol, flavonoids with strong stimulatory activity on AM fungi, have been detected in non-mycorrhizal plants such as Arabidopsis thaliana (Burbulis et al., 1996).
IDENTIFICATION OF STRIGOLACTONES AS INDUCERS OF HYPHAL BRANCHING IN AM FUNGI
Purification of the BFs has been severely hampered by the extremely low concentrations produced and exuded by roots as well as their chemical instability. For the first time, a BF has been successfully isolated from the root exudates of L. japonicus and identified (Akiyama et al., 2005). BF from L. japonicus grown hydroponically under low-phosphate conditions is a lipophilic, neutral compound as revealed by solvent partition between ethyl acetate and acidic or basic aqueous solutions. To obtain sufficient amounts of BF for spectroscopic analysis, a BF-enrichment procedure was developed, in which the hydroponic solution containing BF was continuously pumped through an activated charcoal cartridge. The BF adsorbed to the surface of activated charcoal was eluted with acetone, and was partitioned to yield an ethyl acetate-soluble neutral fraction. Hyphal branching assay-guided purification of the neutral fraction by column chromatography over silica gel and semi-preparative C18 reverse-phase HPLC resulted in the isolation of a BF. The BF was identified as a strigolactone, 5-deoxy-strigol, by spectroscopic analysis and chemical synthesis (Fig. 2A). Natural 5-deoxy-strigol induced extensive hyphal branching in germinating spores of Gigaspora margarita even if only 30 pg were applied per disc (Fig. 3A, B). The hyphal branching activity of racemic 5-deoxy-strigol (prepared by chemical synthesis) was comparable to that of the natural compound, confirming that the activity of the natural 5-deoxy-strigol is not due to contaminants in the purified sample.
Fig. 2.
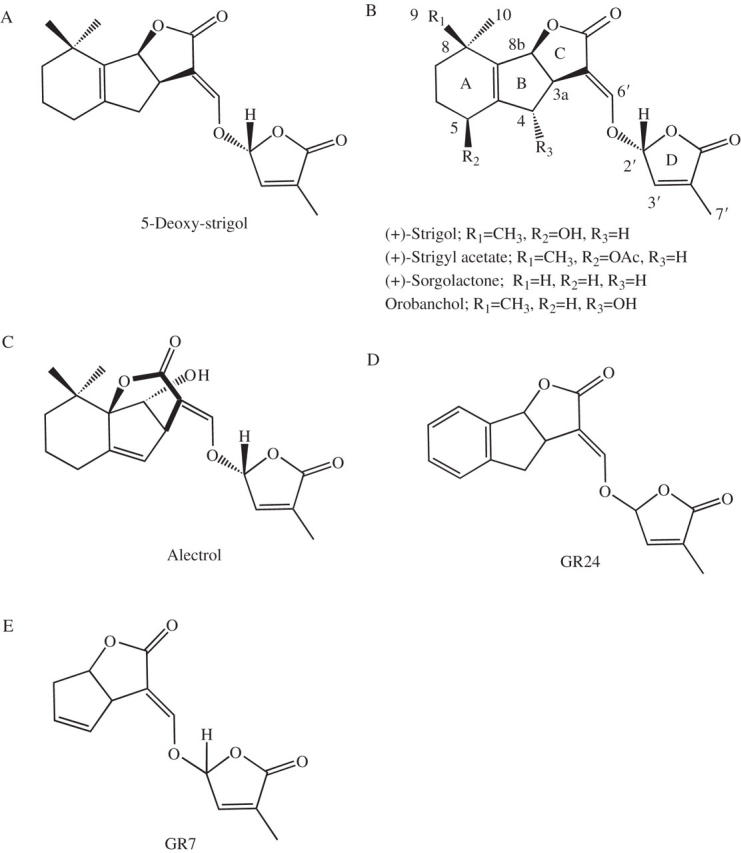
Chemical structures of natural strigolactones and synthetic analogues: (A) 5-deoxy-strigol; (B) four natural strigolactones; (C) alectrol (tentative structure); (D) synthetic analogue GR24; (E) synthetic analogue GR7. The absolute configuration of the natural 5-deoxy-strigol (A), (+)-strigol and (+)-sorgolactone (B) is 3a(R), 8b(S), 2'(R).
Fig. 3.
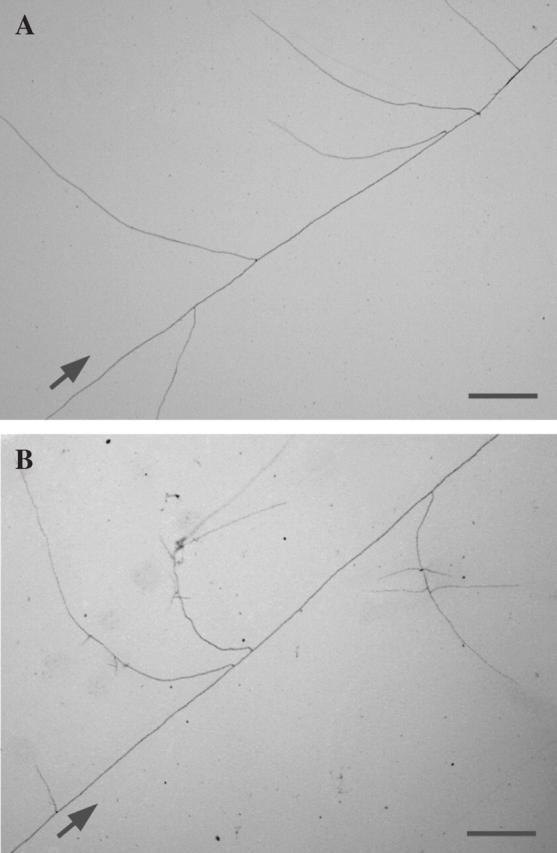
Hyphal branching of Gigaspora margarita induced by 5-deoxy-strigol using the paper disc diffusion method: (A) control (70 % ethanol in water); (B) natural 5-deoxy-strigol (30 pg per disc). Extensive formation of hyphal branches from secondary hyphae is induced by treatment with 5-deoxy-strigol. Control hyphae, which treated with 70 % ethanol in water, form no hyphal branches from secondary hyphae. Arrows indicate direction of growth of primary hyphae. Scale bars = 1 mm.
Strigolactones are a group of sesquiterpene lactones, previously isolated as seed-germination stimulants for the parasitic weeds Striga and Orobanche spp. The known natural strigolactones (sorgolactone and strigol; Fig. 2B) and a synthetic analogue (GR24; Fig. 2D) induced hyphal branching at picogram to nanogram levels in the assay. Orobanchol (Fig. 2B) is also highly active on G. margarita (K. Akiyama and H. Hayashi, unpublished results). Thus, surprisingly, strigolactones are revealed to be BFs. Recently, Bécard et al. (2005) also reported the stimulatory effects of the synthetic strigolactone analogues GR24 and GR7 (Fig. 2E) on hyphal branching in Gigaspora rosea. They also found that these two analogues activate cellular respiration in G. rosea and Glomus intraradices.
STRIGOLACTONES: GERMINATION STIMULANTS FOR PARASITIC WEEDS
The parasitic weeds Striga and Orobanche are among the most damaging agricultural pests in large parts of the world (Bouwmeester et al., 2003). The roots of parasitic weeds can attack the roots of their plant hosts and rob them of water and nutrients. The lives of millions of people in Africa, India and the Middle East are affected by severe harvest reductions due to heavy infestations of susceptible crops with these parasites. Most species are obligate parasites incapable of completing their life cycle in the absence of a host. The first important step in their life cycle is seed germination. Seeds of the parasites remain dormant until germination is stimulated by a chemical produced and exuded by host roots. For development of a control programme, considerable efforts have been made in the identification of the germination stimulants. Thus, to date, five natural strigolactones have been isolated, and a number of structural analogues have been synthesized (Bouwmeester et al., 2003). 5-Deoxy-strigol is the sixth natural strigolactone; this compound has been prepared by organic synthesis as a derivative of strigol, but has not been isolated from any natural source (Frischmuth et al., 1991). This strigolactone was reported to be approximately one-third as active as (+)-strigol on Orobanche crenata seed germination (Bergmann et al., 1993).
Strigolactones are highly active on parasitic weeds, inducing 50 % seed germination at picomolar concentrations. The connection of the C and D rings to each other via an enol ether bond (see chemical structure shown in Fig. 2B) was shown to be necessary for germination stimulation (Mangnus and Zwanenburg, 1992). The inherent instability of strigolactones is principally due to easy cleavage of the enol ether bond by nucleophilic agents including water. Taken with the observations that all strigolactones tested were highly active on AM fungi, and that their activity drastically decreased after concentration or storage of a solution of strigolactones in nucleophilic solvents such as pure or aqueous methanol, it appears that the C–D part is also essential for the effect of strigolactones on AM fungi (K. Akiyama and H. Hayashi, unpubl. res.). The chemical lifetime of strigolactones under natural soil conditions can be very short, enabling these chemicals to convey positional information about the roots of living host plants to AM fungi (and also parasitic weeds). Given the facts that production of strigolactones by red clover roots is stimulated under low phosphate conditions, and that parasitic weeds prevail in areas with limited phosphate availability in the soil (Yoneyama et al., 2001), it is tempting to speculate that parasitic weeds might find their potential hosts by detecting strigolactones, which are released from plant roots upon phosphate deficiency in communication with AM fungi.
CHEMICAL DIVERSITY AND DISTRIBUTION OF STRIGOLACTONES AMONG PLANTS
Strigolactones have been isolated from root exudates of a variety of plants, including the monocots sorghum, maize and proso millet, and the dicots cotton, cowpea, red clover, Menispermum dauricum and Lotus japonicus (Fig. 2A–C) (Cook et al., 1966, 1972; Hauck et al., 1992; Müller et al., 1992; Siame et al., 1993; Yokota et al., 1998; Yasuda et al., 2003; Akiyama et al., 2005). The isolation of (+)-strigol from an aseptic root organ culture of M. dauricum has unambiguously demonstrated that this strigolactone is of plant origin (Yasuda et al., 2003). Although strigolactones are suggested to be more widely distributed in the plant kingdom, the isolation and characterization of strigolactones in root exudates, as in the case of this BF, have been hampered by the extremely low concentrations produced and exuded by host roots as well as their relative instability. Theoretical considerations based on the ubiquitous occurrence of AM symbiosis suggest that strigolactones are produced by almost all plants, including angiosperms, gymnosperms, pteridophytes including psilotophytes and lycopods and some mosses, although all the strigolactones identified so far have been isolated exclusively from herbaceous higher plants. It is noteworthy that Arabidopsis thaliana, belonging to the non-mycotrophic family Brassicaceae, has been found to produce seed germination stimulants, but at much lower concentrations than in the crop plants carrot and tobacco, which are hosts of AM fungi (Westwood, 2000). This broad distribution of strigolactones in the plant kingdom and their levels in plant root exudates are consistent with the host specificity of AM fungi.
Given that the C–D part of strigolactones is essential for hyphal branching activity, and that modifications in the A and B rings do not appear to affect their ability to induce hyphal branching in AM fungi, >100 strigolactone derivatives can be predicted to exist in the plant kingdom. This is not surprising if one considers the several hundred million years of co-evolution of plants and AM fungi. Recent development of an analytical method using HPLC connected to tandem mass spectrometry enables identification of known strigolactones, as well as the search for novel ones in root exudates from a relatively small number of plants (Sato et al., 2003, 2005). The enrichment procedure with activated charcoal, which was used for the isolation of 5-deoxy-strigol from L. japonicus, can provide sufficient amounts of strigolactone for spectroscopic analysis. Novel unidentified strigolactones can be isolated and identified by combining these two methods.
Very little is known about the biogenetic origin of strigolactones in plants, although they have been regarded to be sesquiterpenoids (isoprenoids consisting of three isoprene units). Isoprenoids are biosynthesized via two independent pathways: the cytosolic mevalonic acid pathway and the plastidic non-mevalonate, methylerythritol phosphate (MEP) pathway. The tricyclic ABC ring of strigolactones has recently been revealed to be formed by cleavage of C40-carotenoids originating from the MEP pathway, as shown for the plant hormone abscisic acid (Matusova et al., 2005). Coupling of the D (methylbutenolide) ring to the ABC ring via the enol ether will lead to 5-deoxy-strigol, which itself is the first product in the strigolactone biosynthesis capable of acting both as a BF on AM fungi and as a germination stimulant on parasitic weeds. Isolated as a natural product for the first time, 5-deoxy-strigol could be further converted to strigol and orobanchol by hydroxylation at C-5 and C-4, respectively. Sorgolactone could also be biosynthesized from 5-deoxy-strigol via oxidative demethylation at C-9. Taken together, 5-deoxy-strigol is likely to be a branching point in strigolactone biosynthesis.
AM FUNGAL RESPONSES TO STRIGOLACTONES
It is clear that strigolactones exuded from host roots can trigger a cascade of molecular and cellular events leading to the formation of pre-infection hyphal branching structures in AM fungi. In the last few years, a growing number of studies has been conducted on the molecular changes occurring in AM fungi during pre-symbiotic stages (Jun et al., 2002; Breuninger and Requena, 2004). A semi-purified exudate from carrot root organ cultures was shown to induce the expression of mitochondrial-related genes and, in turn, fungal respiratory activity before intense hyphal branching (Tamasloukht et al., 2003). This cellular respiration is also activated by the chemically pure synthetic analogues GR24 and GR7 in Gigaspora rosea and Glomus intraradices (Bécard et al., 2005). These findings indicate that respiration is a primary target of AM fungal metabolism induced by the root factor. A slight induction of GmarCuZnSOD (Gigaspora margarita CuZn superoxide dismutase) gene expression was observed in germinated spores of G. margarita exposed to a semi-purified root exudate fraction from L. japonicus (Lanfranco et al., 2005). Strigolactones also appear to act as chemo-attractants: fungal hyphae of Glomus mosseae exhibited chemotropic growth towards roots at a distance of at least 910 μm in response to host-derived signals, possibly strigolactones (Sbrana and Giovannetti, 2005).
Strigolactones show potent activity at very low concentrations, suggesting a highly sensitive perception system for strigolactones present in AM fungi. Such a system should be a prerequisite for this obligate biotrophic organism to survive for over 460 million years under natural conditions. The induction of seed germination in parasitic weeds is thought to proceed via a receptor-mediated mechanism. A tentative molecular mechanism proposed for the stimulation of seed germination involves the addition of a nucleophilic species, present at a putative receptor site, to the enol ether carbon double bond in a Michael fashion, followed by elimination of the D ring (Mangnus and Zwanenburg, 1992). Labelled strigolactone analogues were synthesized for isolation and purification of the strigolactone receptor by affinity chromatography (Reizelman et al., 2003), though the receptor has not yet been isolated. Further study will provide insights into the origin and evolution of the putative receptors in AM fungi and parasitic weeds.
Some solid evidence has been presented for AM fungal production of a long-hypothesized symbiotic signal, the MF, in response to the plant symbiotic signal strigolactones. Fungal hyphae of the genus Gigaspora growing in the vicinity of host roots, but separated from the roots by a membrane, release a diffusible substance that induces the expression of a symbiosis-specific gene, MtENOD11 (Medicago truncatula early nodulin 11), in Medicago truncatula roots (Kosuta et al., 2003). This expression was correlated both spatially and temporally with the appearance of hyphal branching, and was not observed when hyphal branching was absent. These findings strongly suggest that strigolactones may be required for synthesis of the diffusible AM factor. Fungal exudates from Gigaspora rosea, Gigaspora margarita and Glomus intraradices were also found to stimulate lateral root formation (Oláh et al., 2005). Activation of the promoter of a symbiosis-specific gene, LjCbp1 (Lotus japonicus calcium-binding protein 1), was observed not only in arbuscule-containing cells but also in cells which are not in contact with fugal hyphae, suggesting that this gene promoter can be used as a molecular marker to detect the diffusible AM factor (Kistner et al., 2005). With the aid of strigolactones, which might stimulate the production of the signal molecule in AM fungi in the absence of a host root, MF will be purified and characterized by bioassay based on these molecular and morphological responses in the near future.
CONCLUSIONS AND FUTURE PERSPECTIVES
Isolation and identification of plant symbiotic signals open up new ways for studying the molecular basis of plant–AM-fungus interactions. The model legume L. japonicus, which was used for identification of the chemical signals, enables a smooth transition of our chemical research results to molecular analysis of the signal molecule-mediated events in the AM symbiosis as well as the strigolactone biosynthetic pathway and its regulation in plants (Parniske, 2004). Availability of strigolactone and its analogues will facilitate basic research on AM fungal responses to this signal molecule, and also applied research on the development of novel techniques for in vitro propagation of AM fungi and increased AM colonization in plants. The discovery described in this paper also provides a clear answer to a long-standing question in parasitic plant biology: ‘what is the natural role for germination stimulants?’ Another implication is that crop protection strategies based on eliminating host production of strigolactones should be reconsidered so as to balance the disruption of parasite germination signalling with preservation of vital AM symbiosis.
Acknowledgments
K.A. was supported by Core Research for Evolutional Science and Technology (CREST), Japan Science and Technology Agency.
LITERATURE CITED
- Akiyama K, Matsuzaki K. Hayashi H. 2005. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 435: 824–827. [DOI] [PubMed] [Google Scholar]
- Bécard G, Piché Y. 1989. New aspects on the acquisition of biotrophic status by a vesicular-arbuscular mycorrhizal fungus, Gigaspora margarita. New Phytologist 112: 77–83. [Google Scholar]
- Bécard G, Douds DD, Pfeffer PE. 1992. Extensive in vitro hyphal growth of vesicular-arbuscular mycorrhizal fungi in the presence of CO2 and flavonols. Applied and Environmental Microbiology 58: 821–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bécard G, Taylor LP, Douds DD, Pfeffer PE, Doner LW. 1995. Flavonoids are not necessary plant signal compounds in arbuscular mycorrhizal symbioses. Molecular Plant-Microbe Interactions 8: 252–258. [Google Scholar]
- Bécard G, Roux C, Sejalon-Delmas N, Puech V, Sébastien R. 2005. Modulators of the development of mycorrhizal fungi with arbuscules, and uses thereof. WO Patent 2005/077177 A2.
- Bergmann, C. Wegmann K, Frischmuth K, Samson E, Kranz A, Weigelt D, et al. 1993. Stimulation of Orobanche crenata seed germination by (+)-strigol and structural analogues dependence on constitution and configuration of the germination stimulants. Journal of Plant Physiology 142: 338–342. [Google Scholar]
- Bouwmeester HJ, Matusova R, Zhongkui S, Beale MH. 2003. Secondary metabolite signalling in host-parasitic plant interactions. Current Opinion in Plant Biology 6: 358–364. [DOI] [PubMed] [Google Scholar]
- Breuninger M, Requena N. 2004. Recognition events in AM symbiosis: analysis of fungal gene expression at the early appressorium stage. Fungal Genetics and Biology 41: 794–804. [DOI] [PubMed] [Google Scholar]
- Buee M, Rossignol M, Jauneau A, Ranjeva R, Bécard G. 2000. The pre-symbiotic growth of arbuscular mycorrhizal fungi is induced by a branching factor partially purified from plant root exudates. Molecular Plant-Microbe Interactions 13: 693–698. [DOI] [PubMed] [Google Scholar]
- Burbulis IE, Iacobucci M, Shirley BW. 1996. A null mutation in the first enzyme of flavonoid biosynthesis does not affect male fertility in Arabidopsis. The Plant Cell 8: 1013–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook CE, Whichard LP, Turner B, Wall ME, Egley GH. 1966. Germination of witchweed (Striga lutea Lour.): isolation and properties of a potent stimulant. Science 154: 1189–1190. [DOI] [PubMed] [Google Scholar]
- Cook CE, Whichard LP, Wall ME, Egley GH, Coggon P, Luhan PA, et al. 1972. Germination stimulants. II. The structure of strigol-A potent seed germination stimulant for witchweed (Striga lutea Lour.). Journal of the American Chemical Society 94: 6198–6199. [Google Scholar]
- Frischmuth K, Samson E, Kranz A, Welzel P, Meuer H, Sheldrick WS. 1991. Routes to derivatives of strigol (the witchweed germination factor) modified in the 5-position. Tetrahedron 47: 9793–9806. [Google Scholar]
- Gianinazzi-Pearson V. 1996. Plant cell responses to arbuscular mycorrhizal fungi: getting to the roots of the symbiosis. The Plant Cell 8: 1871–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianinazzi-Pearson V, Branzanti B, Gianinazzi S. 1989. In vitro enhancement of spore germination and early hyphal growth of a vesicular-arbuscular mycorrhizal fungus by host root exudates and plant flavonoids. Symbiosis 7: 243–255. [Google Scholar]
- Giovannetti M, Sbrana C, Avio L, Citernesi AS, Logi C. 1993. Differential hyphal morphogenesis in arbuscular mycorrhizal fungi during preinfection stages. New Phytologist. 125: 587–593. [DOI] [PubMed] [Google Scholar]
- Giovannetti M, Sbrana C, Logi C. 1994. Early process involved in host recognition by arbuscular mycorrhizal fungi. New Phytologist 127: 703–709. [DOI] [PubMed] [Google Scholar]
- Giovannetti M, Sbrana C, Silvia A, Avio L. 1996. Analysis of factors involved in fungal recognition response to host-derived signals by arbuscular mycorrhizal fungi. New Phytologist 133: 65–71. [Google Scholar]
- Harrison MJ. 2005. Signaling in the arbuscular mycorrhizal symbiosis. Annual Review of Microbiology 59: 19–42. [DOI] [PubMed] [Google Scholar]
- Hauck C, Müller S, Schildknecht H. 1992. A germination stimulant for parasitic flowering plants from Sorghum bicolor, a genuine host plant. Journal of Plant Physiology 139: 474–478. [Google Scholar]
- van der Heijden MGA, Klironomos JN, Ursic M, Moutoglis P, Streitwolf-Engel R, Boller T, et al. 1998. Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature 396: 69–72. [Google Scholar]
- Jun J, Abubaker J, Rehrer C, Pfeffer PE, Shachar-Hill Y, Lammers PJ. 2002. Expression in an arbuscular mycorrhizal fungus of genes putatively involved in metabolism, transport, the cytoskeleton and the cell cycle. Plant and Soil 244: 141–148. [Google Scholar]
- Kistner C, Winzer T, Pitzschke A, Mulder L, Sato S, Kaneko T, et al. 2005. Seven Lotus japonicus genes required for transcriptional reprogramming of the root during fungal and bacterial symbiosis. The Plant Cell 17: 2217–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosuta S, Chabaud M, Lougnon G, Gough C, Dénarié J, Barker DG, et al. 2003. A diffusible factor from arbuscular mycorrhizal fungi induces symbiosis-specific MtENOD11 expression in roots of Medicago truncatula. Plant Physiology 131: 952–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanfranco L, Novero M, Bonfante P. 2005. The mycorrhizal fungus Gigaspora margarita possesses a CuZn superoxide dismutase that is up-regulated during symbiosis with legume hosts. Plant Physiology 137: 1319–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangnus EM, Zwanenburg B. 1992. Tentative molecular mechanisms for germination stimulation of Striga and Orobanche seeds by strigol and its synthetic analogues. Journal of Agricultural and Food Chemistry 40: 1066–1070. [Google Scholar]
- Matusova R, Rani K, Verstappen FW, Franssen MC, Beale MH, Bouwmeester HJ. 2005. The strigolactone germination stimulants of the plant-parasitic Striga and Orobanche spp. are derived from the carotenoid pathway. Plant Physiology 139: 920–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosse B, Hepper C. 1975. Vesicular-arbuscular mycorrhizal infections in root organ cultures. Physiological Plant Pathology 5: 215–223. [Google Scholar]
- Müller S, Hauck C, Schildknecht H. 1992. Germination stimulants produced by Vigna unguiculata Walp cv Saunders Upright. Journal of Plant Growth Regulation 11: 77–84. [Google Scholar]
- Nagahashi G, Douds DD. 1999. A rapid and sensitive bioassay with practical application for studies on interactions between root exudates and arbuscular mycorrhizal fungi. Biotechnology Techniques 13: 893–897. [Google Scholar]
- Nagahashi G, Douds DD. 2000. Partial separation of root exudate compounds and their effects upon the growth of germinated spores of AM fungi. Mycological Research 104: 1453–1464. [Google Scholar]
- Oláh B, Brière C, Bécard G, Dénarié J, Gough C. 2005. Nod factors and a diffusible factor from arbuscular mycorrhizal fungi stimulate lateral root formation in Medicago truncatula via the DMI1/DMI2 signalling pathway. The Plant Journal 44: 195–207. [DOI] [PubMed] [Google Scholar]
- Parniske M. 2004. Molecular genetics of the arbuscular mycorrhizal symbiosis. Current Opinion in Plant Biology 7: 414–421. [DOI] [PubMed] [Google Scholar]
- Peters NK, Varma DPS. 1990. Phenolic compounds as regulators of gene expression in plant-microbe interactions. Molecular Plant-Microbe Interactions 3: 4–8. [DOI] [PubMed] [Google Scholar]
- Powell CL. 1976. Development of mycorrhizal infections from Endogone spores and infected root segments. Transactions of the British Mycological Society 66: 439–445. [Google Scholar]
- Redecker D, Kodner R, Graham LE. 2000. Glomalean fungi from the Ordovician. Science 289: 1920–1921. [DOI] [PubMed] [Google Scholar]
- Reizelman A, Wigchert SCM, del-Bianco C, Zwanenburg B. 2003. Synthesis and bioactivity of labelled germination stimulants for the isolation and identification of the strigolactone receptor. Organic & Biomolecular Chemistry 1: 950–959. [DOI] [PubMed] [Google Scholar]
- Remy W, Taylor TN, Hass H, Kerp H. 1994. Four hundred-million-year-old vesicular arbuscular mycorrhizae. Proceedings of the National Academy of Sciences of the USA 91: 11841–11843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato D, Awad AA, Chae SH, Yokota T, Sugimoto Y, Takeuchi Y, et al. 2003. Analysis of strigolactones, germination stimulants for Striga and Orobanche, by high-performance liquid chromatography/tandem mass spectrometry. Journal of Agricultural and Food Chemistry 51: 1162–1168. [DOI] [PubMed] [Google Scholar]
- Sato D, Awad AA, Takeuchi Y, Yoneyama K. 2005. Confirmation and quantification of strigolactones, germination stimulants for root parasitic plants Striga and Orobanche, produced by cotton. Bioscience, Biotechnology, and Biochemistry 69: 98–102. [DOI] [PubMed] [Google Scholar]
- Sbrana C, Giovannetti M, 2005. Chemotropism in the arbuscular mycorrhizal fungus Glomus mosseae. Mycorrhiza 15: 539–545. [DOI] [PubMed] [Google Scholar]
- Schüssler A, Schwarzott D, Walker C. 2001. A new fungal phylum, the Glomeromycota: phylogeny and evolution. Mycological Research 105: 1413–1421. [Google Scholar]
- Siame BA, Weerasuriya Y, Wood K, Ejeta G, Butler L. 1993. Isolation of strigol, a germination stimulant for Striga asiatica, from host plants. Journal of Agricultural and Food Chemistry 41: 1486–1491. [Google Scholar]
- Smith SE, Read DJ. 1997. Mycorrhizal symbiosis. San Diego, CA: Academic Press.
- Tamasloukht M, Sejalon-Delmas N, Kluever A, Jauneau A, Roux C, Bécard G, et al. 2003. Root factors induce mitochondrial-related gene expression and fungal respiration during the developmental switch from asymbiosis to presymbiosis in the arbuscular mycorrhizal fungus Gigaspora rosea. Plant Physiology 131: 1468–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai SM, Phillips DA. 1991. Flavonoids released naturally from alfalfa promote development of symbiotic Glomus spores in vitro. Applied and Environmental Microbiology 57: 1485–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westwood JH. 2000. Characterization of the Orobanche-Arabidopsis system for studying parasite-host interactions. Weed Science 48: 742–748. [Google Scholar]
- Yasuda N, Sugimoto Y, Kato M, Inanaga S, Yoneyama K. 2003. (+)-Strigol, a witchweed seed germination stimulant, from Menispermum dauricum root culture. Phytochemistry 62: 1115–1119. [DOI] [PubMed] [Google Scholar]
- Yokota T, Sakai H, Okuno K, Yoneyama K, Takeuchi Y. 1998. Alectrol and orobanchol, germination stimulants for Orobanche minor, from its host red clover. Phytochemistry 49: 1967–1973. [Google Scholar]
- Yoneyama K, Takeuchi Y, Yokota T. 2001. Production of clover broomrape seed germination stimulants by red clover requires nitrate but is inhibited by phosphate and ammonium. Physiologia Plantarum 112: 25–30. [DOI] [PubMed] [Google Scholar]


