Abstract
• Background and Aims Previous studies on grass leaf tensile properties (behaviour during mechanical stress) have focused on agricultural applications such as resistance to trampling and palatability; no investigations have directly addressed mechanical properties during water stress, and hence these are the subject of this study.
• Methods Critical (lethal) relative water contents were determined for three species of grass in the genus Eragrostis varying in their tolerance to drought. Measurements were taken for leaf tensile strength, elastic modulus, toughness and failure load under different conditions of hydration, and light microscopy and histochemical analyses were undertaken.
• Key Results Leaf tensile strength of fully hydrated leaves for the drought-intolerant E. capensis, the moderately drought-tolerant E. tef and the drought-tolerant E. curvula correlated well with drought tolerance (critical relative water content). Eragrostis curvula had higher tensile strength values than E. tef, which in turn had higher values than E. capensis. Measurements on the drought-tolerant grass E. curvula when fully hydrated and when dried to below its turgor loss point showed that tensile strength, toughness and the elastic modulus all increased under conditions of turgor loss, while the failure load remained unchanged. Additional tests of 100 mm segments along the lamina of E. curvula showed that tensile strength, toughness and the elastic modulus all decreased with distance from the base of the lamina, while again the failure load was unaffected. This decrease in mechanical parameters correlated with a reduction in the size of the vascular bundles and the amount of lignification, as viewed in lamina cross-sections.
• Conclusions The results confirm that leaf mechanical properties are affected by both water status and position along the lamina, and suggest a positive correlation between leaf internal architecture, tensile strength, cell wall chemistry and tolerance to dehydration for grasses.
Keywords: Biomechanics, drought, Eragrostis, lignification, tensile strength, water stress
INTRODUCTION
Mechanical studies on grasses have largely focused on agricultural applications such as the relationship of tensile and shear strength to palatability (De Sousa et al., 1982; Easton, 1989; O'Reagain, 1993) and resistance to trampling (Sun and Liddle, 1993). Studies on the tensile properties (primarily failure load and tensile strength) of common forage grasses of semi-arid regions have suggested that the primary variability between leaf blades within and between species was due to age and growth stage (Kneebone, 1960; Sun and Liddle, 1993). However, there is some controversy in the literature regarding the impact that leaf morphology, leaf relative water content (RWC) and the relative position along the lamina have on the tensile properties of grass leaves. For example, early studies on the relationship of lamina hydration levels to mechanical properties concluded that ‘dry or wilted leaves tend to be a little tougher than more turgid, succulent ones (Kneebone, 1960)’. Studies by Vincent (1983) on the common forage grass Lolium perenne at various water contents suggest that at <20 % RWC, the stiffness (resistance in bending) of mesophyll cells increases >7-fold. Further, he reported that the quality of fracture (perpendicular to the lamina vs. parallel along the bundle sheath veins) differed between laminas of >50 % RWC (clean breaks) and those of <50 % RWC (ragged breaks along the vascular bundles). A subsequent study on this species failed to find any significant differences in tensile strength, elastic modulus or toughness (resistance to fracture) when comparing field, greenhouse and fully ‘soaked’ leaves (Greenberg et al., 1989).
Most studies have reported that leaf tensile properties decrease from the base to the tip (Kneebone, 1960; Martens and Booysen, 1968). However, Greenberg et al. (1989) show in L. perenne that this is not always the case. In addition, while eight out of nine species studied by Sun and Liddle (1993) agreed with the earlier studies, one species, Cynodon dactylon, exhibited an increase in stiffness (resistance to bending) from the base to the tip of the lamina. The number of fibres and vascular bundles has been suggested as possible reasons for the latter discrepancy, but no anatomical data were included (Sun and Liddle, 1993).
Leaf tensile properties may prove to be a useful tool to study plant response to drought stress (Balsamo et al., 2003a). Species within the genus Eragrostis differ greatly in their ability to tolerate water stress, and as such provide a good model to study water relationships and drought (Vander Willigen et al., 2001; Balsamo et al., 2003b). In this study, we test the hypothesis that dehydration tolerance is positively correlated with the tensile strength of leaves. First, the critical RWCs (cRWCs) are determined for three species of Eragrostis. These values are compared with the tensile strengths for each species under fully hydrated conditions. A qualitative assessment of leaf internal architecture and the degree of lignification of leaf tissues from these species was made using light microscope sections and staining with the metachromatic stain toluidine blue O, which stains lignified tissues blue/green and non-lignified tissues purple (Lawson and Poethig, 1995; Orkwiszewski and Poethig, 2000). Subsequently, we focus on the most drought-tolerant species, E. curvula, to characterize leaf tensile properties (tensile strength, elastic modulus, toughness and failure strain) in more detail. Tensile properties of leaves held above and below the turgor loss point (TLP) (point of lamina wilting) are measured to determine the effects of hydration at physiologically relevant water contents. Finally, tensile properties along the length of the lamina (fully hydrated) are measured to ascertain any differences in tensile properties due to position along the lamina. An assessment of leaf architecture and degree of lignification is made along the lamina for qualitative comparison with mechanical properties. We conclude that leaf tensile properties in these experiments were strongly influenced by hydration levels and position along the lamina. The results also suggest a positive correlation between leaf tensile properties and drought tolerance, and that increased tensile strength in drought-tolerant species also correlates with aspects of tissue architecture and cell wall chemistry.
MATERIALS AND METHODS
Plant materials
Eragrostis curvula (Schrad.) Nees Ermelo, E. tef (Zucc.) Trotter and E. capensis (Thunb.) Trin. were sown in seedling flats (1 : 1 potting soil : river sand mix) and maintained in a glasshouse at the University of Cape Town, South Africa for 3 months. During the course of the studies, plants were kept in a controlled environmental chamber [14 h light (500 µmol m–1 s–2) at 25 °C; 10 h dark at 18 °C, 50–65 % relative humidity]. For the subsequent studies on E. curvula, seeds were planted and seedlings grown for 3–4 months in a 1 : 1 mixture of potting soil and sand. Plants were maintained in a glasshouse at Villanova University (Villanova, PA, USA). Glasshouse conditions were 25 °C/20 °C day/night and held to approx. 70 % relative humidity. Three-month-old plants were watered and covered with a polyethylene bag 12 h prior to mechanical measurements for the fully hydrated treatment. Dry plants (plants where the leaf RWC was below the TLP of the tissue) were sampled 1 week after withholding water. Water potentials of all leaves examined were determined using a PMS plant pressure chamber (Corvallis, OR, USA).
Measurements of leaf morphology and mechanical properties
For the initial experiments using E. curvula, E. tef and E. capensis, tensometers were constructed using 10, 25 and 50 Newton (N) Pesola scales (Baar, Switzerland), a mounting bracket, clamps, weather stripping, a metal beaker and duct tape (see Balsamo et al., 2004 for details). Only the basal 100 mm of lamina of dry plants were tested. For hydrated plants, the first four segments (each 100 mm in length) of laminas (between 400 and 500 mm in total length) were tested. For all experiments, n = 20 laminas per treatment data point were tested from 10 different plants.
Whole laminas were excised from plants and cut into 100 mm segments. The width and thickness of each segment were measured halfway down the length to ±0·001 mm using a ProMax digital caliper (Fowler instruments, Boston, MA, USA). Laminas were then secured in the clamps and 1 cent coins (Republic of South Africa) of approx. 1·33 g each added to the beaker until the lamina fractured between the clamps. The tensile strength was calculated by dividing the failure load by the cross-sectional area of the lamina (N mm–2 = MPa). While this technique precluded the collection of the toughness and modulus of elasticity values (due to the lack of continuous output monitoring), it is very portable and reproducible, and comparisons with more sophisticated laboratory equipment (outlined below) did not yield significant differences in failure load or tensile strength values for similarly treated materials (data not shown).
For the subsequent experiments using only E. curvula, leaves were tested using an MTS Bionix 100 mechanical testing system (New Prarie, MN, USA); for details see Balsamo et al. (2003a). Briefly, 100 mm sections of leaves were placed into grips 30 mm apart (to avoid edge effects and distortion of the modulus of elasticity values; Niklas, 1992) and stretched at a rate of 20 mm min–1. At the point of catastrophic failure (the break point or failure load), tensile strength (σm) and failure strain (ɛf) were determined. Force and displacement were recorded continuously using an accompanying software package (MTS Testworks 4) and subsequently normalized to stress (force per cross-sectional area at t = 0 reported as MPa) and strain (increment in length/initial length). The modulus of elasticity (E = a measure of material stiffness) was calculated from the slope of the linear portion of the resultant stress–strain curve. The toughness (W = total area under the stress–strain curve required to break a unit volume of material) was also determined.
Relative water content (RWC)
The RWC was calculated as the water content divided by the water content estimated at full turgor, determined as described in Vander Willigen et al. (2001). Means of the water content of leaves at full turgor were recorded for each species using >20 representative leaf samples from plants which had been hydrated fully overnight in plastic bags. Water contents were determined gravimetrically by oven drying at 70 °C for 48 h. The cRWC (point at which recovery from water deficit stress was no longer possible) was determined as the RWC at which there was a sudden considerable increase in electrolyte leakage (CM100 conductivity meter, Reid and Associates, Durban, South Africa) suggesting membrane damage (described by Berjak et al., 1993). This lethal tissue damage was confirmed with the tetrazolium test for cell viability (International Rules of Seed Testing, 1999).
Microscopy and histochemistry
Leaf sections of E. capensis, E. tef and E. curvula were sliced with a clean razor blade and fixed in 2·5 % (v/v) glutaraldehyde in 50 mm PO4 buffer (pH 7·0) for 12 h at 4 °C. Sections were then washed twice in 50 mm PO4 buffer (pH 7·0) followed by 50 mm Na-cacodylate buffer (pH 7·0) and post-fixation in 1 % (w/v) OsO4 in 50 mm Na-cacodylate buffer (pH 7·0). Sections were then dehydrated in acetone and embedded in Spurr's resin. Sections (0·25 µm) were cut on a Sorvall MT 6000 ultramicrotome, stained with 1 % (w/v) aqueous toluidine blue O stain, and viewed and photographed on an Olympus BX60 light microscope using an Olympus SC35 SLR 35 mm camera. As E. tef and E. curvula appeared quite similar in cross-section, only E. capensis and E. curvula appear in Fig. 2.
Statistics
Results were analysed using analysis of variance (ANOVA) and Student's t-test using SAS student's version statistical package or Excel 6.0.
RESULTS
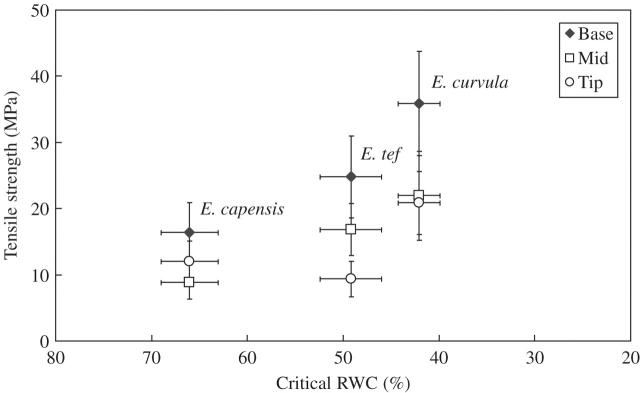
Analysis of the cRWC, beyond which recovery from drought is not possible, revealed that E. curvula was the most drought-tolerant species in this study, with a cRWC of approx. 41 %. This was followed by E. tef (cRWC = 49·5 %), with E.capensis (cRWC = 66 %) being the most drought sensitive (Fig. 1). The same trend was found in the mean leaf tensile strengths of the grasses at full turgor, with E. curvula > E. tef > E. capensis. Consequently, there was a positive correlation between drought tolerance and leaf tensile strength (Fig.1).
Fig. 1.
Leaf tensile strength measurements of hydrated leaves from three Eragrostis spp. plotted against their critical (lethal) RWCs. Leaf base (diamonds), mid-sections (squares) and tips (circles) were significantly different (P < 0·05) among species. Values with bars = means ± s.d. (for both x- and y-axes), n = 20.
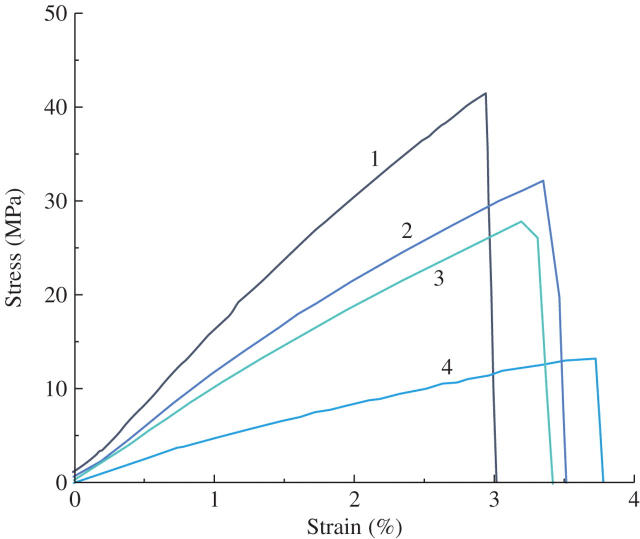
For the second set of experiments, the water potential of E. curvula was 0·76 ± 0·4 MPa for the fully hydrated treatment and 1·46 ± 0·5 MPa for plants dried to below the TLP (point of wilting). Leaf tensile parameters of hydrated vs. partially dehydrated leaf bases are summarized in Table 1. No shearing was observed. All values were greater for partial dehydration, with the exception of the failure strain. The water potential of whole laminas of the plants where leaves were mechanically tested along the length of the lamina averaged 0·85 ± 0·6 MPa. Leaf tensile strength, toughness and the elastic modulus all decreased by approx. 60 % from the segment closest to the base to the segment closest to the tip, while the failure strain remained unchanged (Table 2). An example of a typical set of stress–strain curves is illustrated in Fig. 2. Mechanical parameters determined from these tests are summarized in Table 2. All values decreased from the base to the tip of the lamina, except for failure strain, which remained unchanged. In addition, qualitatively, the lamina sections from the base tended to shatter, with the vascular bundles providing resistance to the perpetrations of the fracture, while sections from the tip generally broke perpendicular to the plane of the lamina (not shown).
Table 1.
A comparison of E. curvula leaves (first 100 mm from base) either fully hydrated (FH) or dried to below the turgor loss point of the leaves (<TLP)
| σm (MPa) | W (J cm–3) | E (MPa) | ɛf (%) | |
|---|---|---|---|---|
| FH | 23·73 ± 9·4 | 0·399 ± 0·21 | 1051·47 ± 335·7 | 2·78 ± 0·5 |
| <TLP | 42·68 ± 17·2 | 0·739 ± 0·35 | 1787·09 ± 683 | 2·91 ± 0·4 |
Tensile strength (σm), toughness (W), elastic modulus (E) and failure strain (ɛf), n = 20 laminas per treatment. Values = means ± s.d. All comparisons are significantly different (P < 0·001), except for failure strain.
Table 2.
Leaf mechanical properties along the lamina of E. curvula
| σm (MPa) | W (J m–3) | E (MPa) | ɛf (%) | |
|---|---|---|---|---|
| 1 | 53·14 ± 20·0a | 0·97 ± 0·5a | 2162·2 ± 709a | 2·95 ± 0·5a |
| 2 | 37·57 ± 15·4b | 0·68 ± 0·3b | 1498·8 ± 662b | 3·17 ± 0·6a |
| 3 | 29·16 ±11·1b | 0·51 ± 0·2bc | 1176·6 ± 481bc | 3·06 ± 0·4a |
| 4 | 21·13 ± 7·5c | 0·38 ± 0·1c | 854·4 ± 346c | 3·22 ± 0·6a |
Numbers (1–4) correspond to 100 mm segments of lamina from base (1) to tip (4). Tensile strength (σm), toughness (W), elastic modulus (E) and failure strain (ɛf), n = 20 replicates per segment. Values = means ± s.d. Lower case letters signify if samples are statistically different (P < 0·05) in a column.
Fig. 2.
Stress–strain curves from leaf tensile tests along the lamina of E. curvula. Numbers (1–4) correspond to 100 mm segments from the base to the tip where 1 = basal 100 mm segment, 2 = second 100 mm segment from the base, 3 = third 100 mm segment from the base, 4 = tip 100 mm segment.
Cross-sectional evaluation of the three species revealed structural differences between E. capensis, and E. tef and E. curvula. The midrib of E. capensis has vascular bundle extensions on both the adaxial and abaxial side that extend to the epidermis (Fig. 3A). Both the bundle sheath extension cells and the xylem stain positive for lignin as evidenced by the light blue colour (non-lignified tissues will stain purple). The lamina has large, multilayered bulliform cells on the adaxial surface that penetrate deeply into the mesophyll almost to the abaxial epidermal layer (Fig. 3B). In contrast, the midribs of E. curvula and E. tef (E. tef not shown) exhibit a robust, lignified region of collenchyma cells (sclerenchyma) above the central vascular bundle and phloem fibres below (Fig. 3C). In the lamina, every third to fifth vascular bundle exhibits vascular bundle extensions of collenchyma and fibres (Fig. 3D).
Fig. 3.
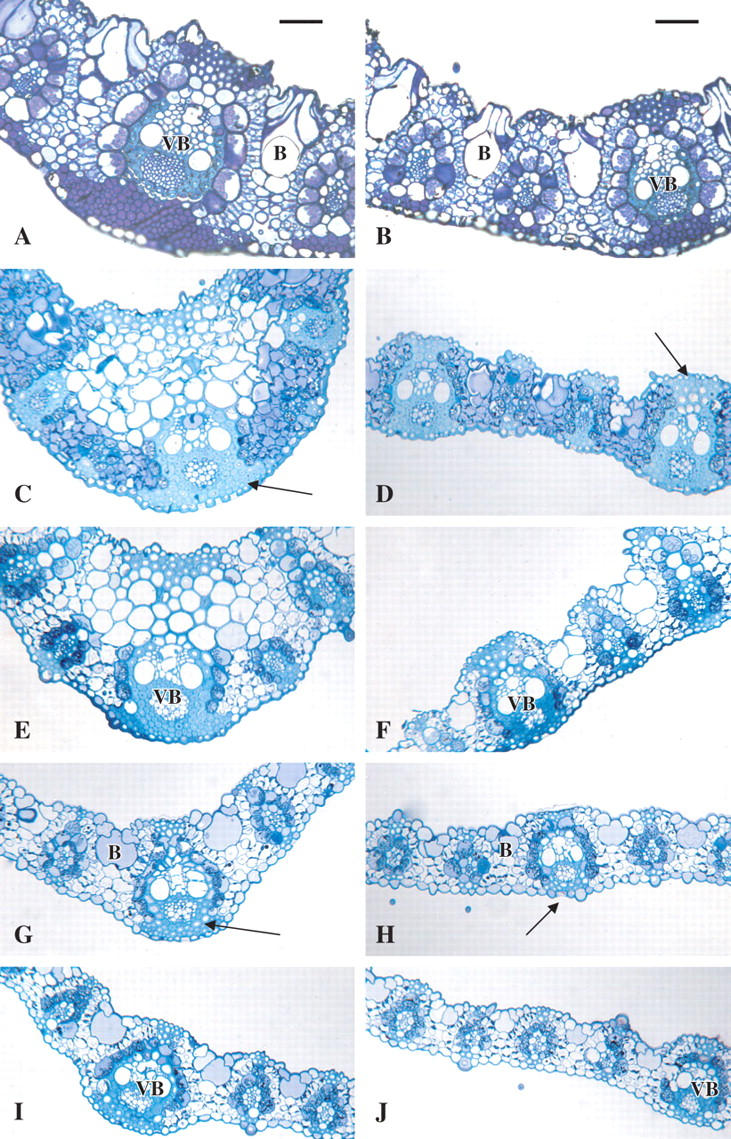
Leaf cross-sections of E. capensis near the base and tips of the lamina, and E. curvula along the length of the lamina. Sections of lamina are shown, with the adaxial side towards the top of the micrographs. All sections were stained with toluidine blue O. (A) Two cm from the base of E. capensis. (B) Two cm from the tip of E. capensis. (C, E, G and I) Midrib cross-sections along the leaf blade of E. curvula C = 50 mm from the base, E = 150 mm, G = 250 mm and I = 350 mm. (D, F, H and J) Lamina cross-sections along the leaf blade of E. curvula D = 50 mm from the base, F = 150 mm, H = 250 mm and J = 350 mm. For all micrographs, B = bulliform cells, VB = vascular bundle; lignified areas of bundle sheath extensions (with cells a light blue colour) are indicated by arrows. Non-lignified areas are stained dark purple. Bars in (A) and (B) indicate 100 µm. All sections were photographed at 50× and printed at 100×.
Microscopic and histochemical examination of leaf cross-sections along the length of the lamina (Fig. 3C–J) revealed several trends. First, the size of the vascular bundles of the midrib (Fig. 3C, E, G and I) and lamina (Fig. 3D, F, H and J) decreased from the base to the tip of the lamina. Secondly, the degree of secondary wall thickenings decreased from the base to the tip. Thirdly, the degree of lignification as evidenced by differential staining with toluidine blue O decreased from the base to the tip. Finally, the proportion of vascular bundle tissue to bulliform cells in leaf cross-section decreased from the base to the tip.
DISCUSSION
Increased tensile strength correlated to increased tolerance to drought (survival with loss of RWC) among the three Eragrostis species in this study (Fig. 1). While these results agree with previous studies investigating the relationship of leaf sclerophylly to drought tolerance in dicotyledonous chaparral shrubs (Balsamo et al., 2003a), this is the first such study using monocots as a model to investigate the relationship between leaf tensile properties, internal architecture and drought tolerance. While there were no obvious structural differences between the moderately drought-tolerant E. tef and the drought-tolerant E. curvula, a more detailed analysis of the structural components and a quantification of the the amount of lignified tissues might be useful. However, there were obvious structural differences between the leaves of the two drought-tolerant species (E. curvula and E. tef) and the relatively mesic E. capensis (Fig. 3). Both the midrib and lamina vascular bundles in E. capensis had fewer robust bundle sheath extensions and fibrous regions, and the bulliform cells were several layers thick. Overall it appears as if the bulliform cells are a much larger component of the lamina than is the case with the drought-tolerant species (Fig. 3).
Tensile strength measurements on E. curvula (Table 1) agree with previous studies of this species (Kneebone, 1960; O'Reagain, 1993). There was a significant difference in tensile properties between leaves above and below their TLP which fills a gap of previous studies on grasses concentrating on either the difference between field and fully hydrated leaves (Greenberg et al., 1989) or very dry (and dead) leaves (Vincent, 1983). Tensile strength increased after wilting and decreased dramatically from the base to the tip of the lamina. In addition, the elastic modulus and leaf toughness also increased or decreased in proportion to the tensile strength values reported. However, the failure strain was not significantly different between fully hydrated and partially dried laminas. Additionally, similar to other reports on grass leaves (Kneebone, 1960; Martens and Booysen, 1968), the tensile properties of the three species of Eragrostis in the present study decreased from the base to the tip (Table 2). However, unlike most of the species reported by Martens and Booysen (1968), the changes in tensile strength appear linear (Table 2). In addition, the toughness and elastic modulus also decrease in a linear fashion. Interestingly, there is not a significant difference in the failure strain, suggesting that parallel vein architecture (vs. reticulate as is the case for most dicotyledonous species) has a major impact on this property (Table 2).
Compared with dicotyledonous species (Balsamo et al., 2003a), the measured failure strain of E. curvula was quite low, indicating that the lamina of this species does not stretch very much before breaking. This agrees with previous qualitative studies on another forage grass, L. perenne L. (Vincent, 1982; Greenberg et al., 1989). Stress–strain curves generated by the Testworks 4 software package were all of the elastic/plastic type (Niklas, 1992). However, there were no clear plastic break points evident in any of the curves (Fig. 2). Additionally, the descending curve following catastrophic failure of the material remained linear for all sections of the lamina, indicating that the majority of the vascular bundles responsible for the tensile strength were breaking at about the same time. If mesophyll or small vascular bundles were breaking first, the descending curve would be ragged (Balsamo et al., 2003a). These results may be due to the type of venation typical of monocotyledons (parallel) vs. dicotyledons (reticulate). This may also explain the comparatively high values for the elastic modulus (a measure of stiffness) when compared with dicotyledonous species.
Vincent (1982) states that the leaf vascular system accounts for >90 % of the tensile strength in grasses. A subsequent study (Vincent, 1983) came to a similar conclusion in leaves dried to below 20 % RWC. One possibility to explain the increase in tensile properties with water loss may be a loss of water in the vascular system, perhaps specifically in the fibres and xylem which tend to have significant secondary wall thickenings and also have the highest degree of lignification. However, Vincent also indicated that the tensile properties of mesophyll cells increased up to 7-fold in dry tissue when compared with tissue that was fully hydrated. Thus the observed increases in biomechanical properties at physiologically relevent RWCs as reported in the current study might also be explained by the partial dehydration of cellulose microfibrils of the mesophyll tissue. Assuming that total mesophyll cell wall accounts for up to 10 % of the measured leaf tensile strength for the leaves of E. curvula, the measured increases in tensile strength in this study under partially dehydrated conditions, as well as those in a previous study (Balsamo et al., 2005) that reported tensile strength values for this species from fully hydrated down to fully dried leaves, would support this latter hypothesis.
The decrease of mechanical properties from the base to the tip of the lamina suggests that leaf architecture and cell wall components of the cells of the vascular bundles play a major role in drought tolerance, perhaps by resisting implosion of the xylary conduits as hypothesized by Hacke et al. (2001) for woody tissues. Two of the species studied, E. tef and E. curvula, exhibited extensive lignification of the bundle sheath extensions (Fig. 3) which potentially may play a role in the stabilization of the lamina during periods of extended drought and loss of cellular water, thus preventing increased mechanical shear stress on the more delicate mesophyll cells. Another potential explanation for the differences in mechanical properties at the base and tip may be due to the manner in which monocot leaves develop with the meristem regions at the base. It is tempting to speculate that this may be an adaptation to herbivory, where the weakest, oldest parts of the leaves are the easiest to remove from the plant, leaving the tougher, basal region containing the meristem intact.
Qualitative assessment of stress–strain curves agreed with the observations of Vincent (1983) on L. perenne at different levels of hydration, namely that basal sections tended to shatter and break along the vascular bundles (like dry tissue) while those near the tip broke across the lamina (like more hydrated tissue), perhaps indicating a role for the midrib in mechanical stability. Indeed, in this species as well as many other grasses, often during drought the tip of the lamina is the first to dehydrate and die and the base the last (R. A. Balsamo, unpubl. res.). Further detailed studies on the relationship of mechanical properties such as tensile strength to drought tolerance on a wider variety of species (both monocotyledonous and dicotyledonous) appear warranted.
In conclusion, these studies have demonstrated a positive correlation between leaf tensile strength and drought tolerance for three monocotyledonous species within the genus Eragrostis. We show that leaf tensile strength among these species is strongly correlated to differences in leaf architecture and cell wall chemistry, as has been previously suggested for dicotyledonous plants. For the drought-tolerant E. curvula, we have confirmed previous studies that suggest that leaf tensile properties differ according to the measured position along the lamina. We provide data that suggest that these differences may be due to leaf internal architecture and cell wall chemistry (degree of lignification). Finally, we provide data suggesting that leaf tensile properties in mocotyledonous species are strongly influenced by the level of tissue hydration at physiologically relevant RWCs. Thus, in addition to altered physiological processes that are associated with drought tolerance, leaf architecture and cell wall chemistry may also play an important role, and we demonstrate that biomechanical studies are another useful way to investigate these phenomena.
Acknowledgments
We thank Keren Cooper for technical assistance with the microscopy, and the Claude Harris Leon Foundation for scholarship funding (for C.V.W.).
LITERATURE CITED
- Balsamo RA, Bauer AM, Davis SD, Rice BM. 2003a Leaf biomechanics, morphology, and anatomy of the deciduous mesophyte Prunus serrulata (Rosaceae) and the evergreen sclerophyllous shrub Heteromeles arbutifolia (Rosaceae). American Journal of Botany 90: 72–77. [DOI] [PubMed] [Google Scholar]
- Balsamo RA, Vander Willigen C, Farrant J. 2003b Relating leaf tensile properties to drought and desiccation tolerance for selected species of Eragrostis. 4th International Plant Biomechanics Conference July 20–25, 2003, Michigan State University, Lansing, MI, USA, 4: 17.
- Balsamo RA, Hofmyer MD, Henen B, Bauer AM. 2004. Leaf biomechanics as a potential tool to predict feeding preferences of Psammobates geometricus (geometric tortoise). African Zoology 39: 175–181. [Google Scholar]
- Balsamo RA, Vander Willigen C, Boyko W, Farrant J. 2005. Anomalous leaf tensile properties during dehydration may help elucidate mechanisms of desiccation tolerance in Eragrostis nindensis. Physiologia Plantarum 124: 336–342. [Google Scholar]
- Berjak P, Vertucci CW, Pammenter NW. 1993. Desiccation-sensitive (recalcitrant) seeds: effects of developmental status and dehydration rate on characteristics of water and desiccation-sensitivity in Camelia sinensis. Seed Science Research 3: 155–166. [Google Scholar]
- De Sousa FB, Sleper DA, Belyea RL Matches AG. 1982. Leaf tensile strength, ‘in vitro’ digestibility, and fiber component relationships in tall fescue. Pesquisa Agropecuaria Brasiliera Brasilia 17: 1497–1504. [Google Scholar]
- Easton HS. 1989. Variability of leaf shear strength in perennial ryegrass. New Zealand Journal of Agricultural Research 32: 1–6. [Google Scholar]
- Farrant JM, Berjak P, Pammenter NW. 1985. The effect of drying rate on viability of retention of recalcitrant propagules of Avicennia marina. South African Journal of Botany 51: 432–438. [Google Scholar]
- Greenberg AR, Mehling A, Lee M, Bock JH. 1989. Tensile behaviour of grass. Journal of Materials Science 24: 2549–2554. [Google Scholar]
- Hacke UG, Sperry JS, Pockman WT, Davis SD, McCulloh KA. 2001. Trends in wood density and structure are linked to prevention of xylem implosion by negative pressure. Oecologia 126: 457–461. [DOI] [PubMed] [Google Scholar]
- International Rules for Seed Testing. 1999. Seed science and technology rule book. Proceedings of the International Seed Testing Association 27: 33–35. [Google Scholar]
- Kneebone WR. 1960. Tensile strength variations in leaves of weeping lovegrass (Eragrostis curvula (Schrad.) Nees.) and certain other grasses. Agronomy Journal 52: 539–542. [Google Scholar]
- Lawson EJR, Poethig RS. 1995. Shoot development in plants: time for a change. Trends in Genetics 11: 263–268. [DOI] [PubMed] [Google Scholar]
- Martens PO, Booysen P de V. 1968. A tensilmeter for the measurement of the tensile strength of grass leaf blades. Proceedings of the Grassland Society of South Africa 3: 51–56. [Google Scholar]
- Niklas K. 1992. Plant biomechanics: an engineering approach to plant form and function. Chicago: University of Chicago Press.
- O'Reagain PJ. 1993. Plant structure and the acceptability of different grasses to sheep. Journal of Range Management 46: 232–236. [Google Scholar]
- Orkwiszewski JAJ, Poethig RS. 2000. Phase identity of the maize leaf is determined after leaf initiation. Proceedings of the National Academy of Science of the USA 97: 10631–10636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Liddle MJ. 1993. Trampling resistance, stem flexibility and leaf strength in nine Australian grasses and herbs. Biological Conservation 65: 35–41. [Google Scholar]
- Vander Willigen C, Pammenter NW, Mundree SG, Farrant JM. 2001. Some physiological comparisons between the resurrection grass, Eragrostis nindensis, and the related desiccation-sensitive species, E. curvula. Plant Growth Regulation 35: 121–129. [Google Scholar]
- Vincent JFV. 1982. The mechanical design of grass. Journal of Materials Science 17: 856–860.
- Vincent JFV. 1983. The influence of water content on the stiffness and fracture properties of grass leaves. Grass and Forage Science 38: 107–114. [Google Scholar]