Abstract
• Background and Aims Plant cyanogenesis is the release of toxic cyanide from endogenous cyanide-containing compounds, typically cyanogenic glycosides. Despite a large body of phytochemical, taxonomic and ecological work on cyanogenic species, little is known of their frequency in natural plant communities. This study aimed to investigate the frequency of cyanogenesis in Australian tropical rainforests. Secondary aims were to quantify the cyanogenic glycoside content of tissues, to investigate intra-plant and intra-population variation in cyanogenic glycoside concentration and to appraise the potential chemotaxonomic significance of any findings in relation to the distribution of cyanogenesis in related taxa.
• Methods All species in six 200 m2 plots at each of five sites across lowland, upland and highland tropical rainforest were screened for cyanogenesis using Feigl–Anger indicator papers. The concentrations of cyanogenic glycosides were accurately determined for all cyanogenic individuals.
• Key Results Over 400 species from 87 plant families were screened. Overall, 18 species (4·5 %) were cyanogenic, accounting for 7·3 % of total stem basal area. Cyanogenesis has not previously been reported for 17 of the 18 species, 13 of which are endemic to Australia. Several species belong to plant families or orders in which cyanogenesis has been little reported, if at all (e.g. Elaeocarpaceae, Myrsinaceae, Araliaceae and Lamiaceae). A number of species contained concentrations of cyanogenic glycosides among the highest ever reported for mature leaves—up to 5·2 mg CN g−1 d. wt, for example, in leaves of Elaeocarpus sericopetalus. There was significant variation in cyanogenic glycoside concentration within individuals; young leaves and reproductive tissues typically had higher cyanogen content. In addition, there was substantial variation in cyanogenic glycoside content within populations of single species.
• Conclusions This study expands the limited knowledge of the frequency of cyanogenesis in natural plant communities, includes novel reports of cyanogenesis among a range of taxa and characterizes patterns in intra-plant and intra-population variation of cyanogensis.
Keywords: Australia, β-glycosidase, chemotaxonomy, cyanogenic glycoside, cyanogenesis, defence, hydrogen cyanide, polymorphism, Queensland, screening, secondary metabolite, tropical rainforest
INTRODUCTION
Cyanogenesis is the ability to release toxic hydrogen cyanide (HCN) from endogenous cyanide-containing compounds. It has long been recognized in plants (Conn, 1991; Seigler, 1991), and has been recorded in ferns, fern-allies, gymnosperms, as well as monocotyledonous and dicotyledonous angiosperms from >550 genera and 130 plant families (Conn, 1981; Poulton, 1990; Jones, 1998). Cyanogenesis in plants requires the presence of either an unstable cyanohydrin, or of a stable cyanogen and its degradative enzymes (Seigler, 1991). While cyanolipids have been identified from a few taxa (Mikolajczak et al., 1970), cyanogenesis in plants most commonly results from the hydrolysis of cyanogenic glycosides (Conn, 1981). Autotoxicity in intact plants is prevented by the spatial separation—either at the subcellular or at the tissue level—of the cyanogenic glycoside and catabolic enzymes (Kojima et al., 1979; Selmar, 1993a; Poulton and Li, 1994; Zheng and Poulton, 1995; Hickel et al., 1996). The catabolism of cyanogenic glycosides is therefore initiated upon tissue disruption, due to mechanical damage or ingestion by herbivores, for example, which enables mixing of enzymes and cyanogenic substrate (Wajant et al., 1994; Patton et al., 1997).
Little is known about the frequency of cyanogenesis in natural plant communities. This is despite a large body of literature documenting cyanogenesis in >2650 species of angiosperms worldwide (Lechtenberg and Nahrstedt, 1999). Indeed, as many as 11 % of all plant species are predicted to be cyanogenic (Jones, 1998). Historically, much of the interest in cyanogenesis centred around recording toxic plants with the potential for stock and human poisoning, the high frequency of cyanogenesis among food plants (Jones, 1998), and the potential utility of cyanogenesis and the structure of specific cyanogens in elucidating phylogenetic relationships between taxa (e.g. Gibbs, 1974). As a consequence, much of what is known about the frequency of cyanogenesis comes from surveys of regional floras, or of specific taxonomic groups. There are a number of substantial chemotaxonomic works incorporating information on cyanogenesis (see Hegnauer, 1966, 1973, 1986, 1989, 1990; Gibbs, 1974), several smaller and more specific chemotaxonomic works (e.g. Tjon Sie Fat, 1978, 1979b; Spencer and Seigler, 1985; van Wyk and Whitehead, 1990; Seigler, 1994), including work on Australian Acacia spp. (Conn et al., 1985; Maslin et al., 1988), and numerous inventories of cyanogenic species (e.g. Rosenthaler, 1919; Seigler, 1976a, 1976b; Tjon Sie Fat, 1979a; Francisco and Pinotti, 2000). Several Australian researchers were active in the field early last century, reporting a number of cyanogenic species and the specific cyanogenic constituents involved (e.g. Petrie, 1912, 1913, 1914, 1920; Finnemore and Cox, 1928, 1929; Finnemore and Cooper, 1936, 1938). More than 700 species in the Queensland flora were screened by Smith and White (1918). Further phytochemical screening of the Queensland flora was conducted by Webb (1948, 1949); however, there was negligible testing among tropical taxa. Importantly, many of these records are based on qualitative tests performed using herbarium specimens, and tests of this kind using dried material are by no means conclusive. One further limitation of these data in terms of extrapolating to natural plant communities is that negative results were often not reported.
Tropical rainforests are of particular interest in the study of plant chemical defences. Elevated herbivore pressure in tropical environments is hypothesized to have favoured both a diverse array of defences and high levels of investment in chemical defence (Levin, 1976; Levin and York, 1978; Coley and Barone, 1996; Kursar and Coley, 2003). Indeed, in the field of plant secondary chemistry, the tropical rainforest has been the focus of intense interest in co-evolutionary relationships between plants and herbivores, and the extraordinary inter-specific and intra-plant variation in chemical defence strategies (Feeny, 1976; Levin, 1976; Coley et al., 1985; Coley and Kursar, 1996).
On the whole, community-level studies of the distribution of chemical defences have focused on a small set of Asian and African rainforests (McKey et al., 1978; Gartlan et al., 1980; Davies et al., 1988; Waterman et al., 1988). In addition, these and other studies have tended to focus on C-based ‘quantitative’ defences (e.g. Coley, 1983, 1988). Among N-based defences, the greater awareness of the frequency of alkaloid-bearing plants in tropical and other ecosystems is a consequence of extensive phytochemical screening for bioactive compounds (e.g. Swanholm et al., 1960; Hartley et al., 1973; Smolenski et al., 1974; Collins et al., 1990; Hadi and Bremmer, 2001) or ecological research into specific plant–herbivore interactions (e.g. Janzen and Waterman, 1984; Hartmann et al., 1997), rather than systematic studies within natural communities (but see Mali and Borges, 2003).
There are some hypothesis-driven surveys for cyanogenesis. Two studies have investigated the frequency of cyanogenesis in floras, investigating evolutionary and ecological hypotheses about exposure to herbivory (see Kaplan et al., 1983; Adsersen et al., 1988; Adsersen and Adsersen, 1993). However, only the survey of Thomsen and Brimer (1997) in Costa Rican rainforest has been conducted in a systematic fashion, well defined with respect to forest area and plant size. As with other N-based defences, work on cyanogenic tropical rainforest species worldwide is limited, and there has been no work on cyanogenesis in Australian tropical rainforests, or any other form of chemical defence. The lack of work on cyanogenesis in diverse tropical rainforests is further surprising, as it is a readily detectable and constitutive chemical defence (Gleadow and Woodrow, 2000b).
Here we report some of the findings of a large-scale quantitative survey for cyanogenesis in Australian tropical rainforests. First, we investigated the frequency of cyanogenesis and the contribution of cyanogenic species to biomass (basal area) in lowland, upland and highland tropical rainforest in north Queensland, Australia. Conducting the survey in a standardized fashion will enable comparison with other communities (e.g. Thomsen and Brimer, 1997). Secondly, intra-plant and intra-population variation in concentrations of cyanogenic glycosides was quantified. The high level of endemism among the Australian tropical rainforest flora (Webb and Tracey, 1981) and the number of rainforest taxa previously untested for cyanogenesis underscore the potential for novel reports of cyanogenesis within different taxonomic groups. Finally, therefore, this study aimed to investigate the potential taxonomic significance of cyanogenesis reported here. Overall, this study aimed to expand the limited knowledge of the frequency of cyanogenesis in the Australian flora and in natural plant communities.
MATERIALS AND METHODS
Field sites
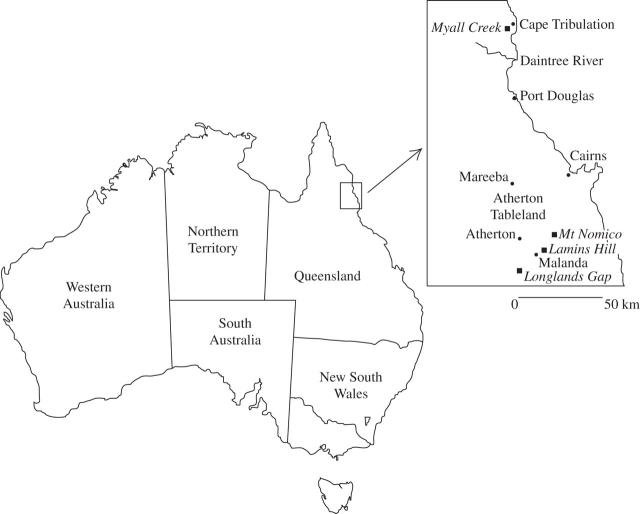
Field work was conducted between July 1999 and September 2002. Six 200 m2 plots (20 × 10 m) were established at each of five sites (total area 1200 m2 per site) in lowland and upland rainforest in the tropics of north east Queensland, Australia (Fig. 1). Six plots were selected for two reasons: first, the number of new species captured with each additional plot had reached a plateau at around five species and, secondly, it was a realistic sample size given the time and resources available. Sites were selected to capture maximum species diversity as on the Atherton Tablelands, forest type and species composition vary both with substrate and with altitude (Tracey, 1982). Further, to enable comparison of forests occurring on different soil types, two pairs of sites with similar altitude and rainfall were selected. The first pair comprised a site on soil derived from basalt at Lamins Hill and one on soil derived from granite at Mt Nomico (Fig. 1). The second pair comprised sites on soils derived from basalt and rhyolite at Longlands Gap (Fig. 1). A fifth site in lowland rainforest near Cape Tribulation and Myall Creek, on soil derived from metamorphic substrate, was also surveyed (Fig. 1). The distribution of cyanogenic species in relation to resource availability (soil nutrients) will be addressed elsewhere (see Miller, 2004).
Fig. 1.
Location of study sites in tropical rainforest in far north east Queensland, Australia. There were two pairs of sites in upland rainforest on the Atherton Tableland: Lamins Hill (17°22·4′S, 145°42·5′E) and Mt Nomico (17°13·3′S, 145°40·4′E), and two sites at Longlands Gap (17°27′S, 145°28′E). A fifth site was in lowland rainforest near Myall Creek and Cape Tribulation (16°06·2′S, 145°26·9′E).
It was not possible to control strictly for logging history; all sites on the Atherton Tablelands had been selectively logged prior to the declaration of the Wet Tropics World Heritage Area in 1988. Detailed site descriptions are provided in Miller (2004).
All individuals (palms, trees and vines) with a diameter at breast height (dbh) of ≥5 cm were tagged and identified. All additional species (dbh <5 cm) present in lower strata, with the exception of herbaceous ground species, were also tagged and identified.
Samples of all cyanogenic species, as well as species dominant at each site, and some rare species were pressed and lodged at the University of Melbourne (MELU) and Brisbane (BRI) Herbaria. Accession numbers, where assigned, are listed (Appendix).
Climate
The climate in far north Queensland is characterized by a marked wet season from December to April. Climate recording stations in the study area are scattered, therefore data specific to each site were not available. While located in the tropical latitudes, because of its higher altitude, the climate of the Atherton Tableland is semi-tropical. Mean annual temperature within the study area on the Tableland is 22 °C (Nix, 1991), with a minimum of 10 °C (Hall et al., 1981; see Graham et al., 1995). All the upland and highland rainforests surveyed on the Atherton Tableland have high average annual rainfall, generally in the range 2000–3000 mm plus cloud interception (Tracey, 1982). For example, the average annual rainfall at Lamins Hill is 3584 mm, based on 30 years of records from the Queensland Bureau of Meteorology (see Osunkoya et al., 1993). In contrast, the climate in the coastal lowland tropical rainforest near Cape Tribulation is characterized by higher temperatures. Mean daily temperatures range from 28 °C in January to 22 °C in July, and temperatures may reach the mid to high 30 s during the summer months. Average annual rainfall is also high, at 3928 mm recorded at Cape Tribulation (based on 65 years of records from the Queensland Bureau of Meteorology).
Sites
Upland and highland rainforest
The upland rainforest at Lamins Hill [17°22·4′S, 145°42·5′E; altitude 850 m above sea level (a.s.l.); Fig. 1] is classified as complex mesophyll vine forest on basalt (type 1b; Tracey, 1982; Tracey and Webb, 1975). This forest type typically occurs on upland sites (400–800 m), on high fertility kraznozem soils derived from basalt, with high rainfall (Tracey, 1982). It is characterized by a closed canopy with multiple tree layers, and an uneven canopy with height ranging from 35 to 45 m (Tracey 1982).
The upland rainforest at Mt Nomico (17°13·3′S, 145°40·4′E, 900 m a.s.l.; Fig. 1) is within the Gillies Range State Forest, and is on low nutrient soil derived from granite. The forest is classified as complex notophyll vine forest, typical of upland granitic soils (type 6; Tracey, 1982; Tracey and Webb, 1975).
In the highland rainforest of Longlands Gap State Forest (altitude 1100–1200 m a.s.l.), there is a sharp boundary in forest type defined by basalt (17°27·7′S; 145°28·5′E) and rhyolite (17°27·3′S, 14528·6′E) parent substrates. On basalt, the forest is complex notophyll vine forest (type 5a; Tracey, 1982; Tracey and Webb, 1975), a forest type characterized by an uneven canopy (20–40 m high) and numerous tree layers, typical of basaltic cool wet uplands and highlands (Tracey, 1982). On rhyolite, the forest is simple microphyll vine forest (type 9; Tracey, 1982), which is common on upland granitic soils (800–1300 m), and is characterized by an uneven canopy 20–25 m high, with emergent Agathis atropurpurea (up to 35 m).
Lowland rainforest
The fifth site was in lowland rainforest in the Daintree World Heritage Area, near Cape Tribulation (Fig. 1). The site was near to Thompson and Myall Creeks (16°06·2′S, 145°26·9′E; altitude 40 m a.s.l.). The forest is complex mesophyll vine forest (type 1a; Tracey, 1982), the canopy is irregular, from 25 to 33 m in height, and supports a great diversity of species and life forms, including many palms and lianas. The floristic composition is patchy, with considerable variation in canopy and understorey dominants over small distances (Webb et al., 1972; Tracey, 1982). The soil is relatively nutrient-poor red clay loam podsol derived from metamorphic substrate.
Sampling for detection of cyanogenesis
Whole leaf samples (1–2 g f. wt) were taken from individuals with a dbh ≥5 cm, and from all additional species in the lower strata, until at least three individuals of each species had been sampled. Where possible, the youngest fully expanded leaves without epiphyllous communities were selected; however, in the case of samples acquired by pruning shears attached to extension poles or by slingshot and rope from the canopy, it was not always possible to be selective. In instances where it was not possible to obtain samples from large canopy trees, samples were taken from nearby individuals of the same species. Fresh whole leaf samples used for cyanide testing were stored in air-tight bags on ice until tested for cyanide 2–6 h later using Feigl–Anger (FA) papers (Feigl and Anger, 1966). Individuals of rare species, and those with little foliage, were sampled only once for analysis in the laboratory where a quantitative assay was used to test for cyanogenesis using freeze-dried ground tissue. Because cyanogenesis is known to be a polymorphic trait (e.g. Hughes, 1991; Aikman et al., 1996), a minimum of three individuals of all species was tested, more where species were common. Owing to the diverse and heterogeneous nature of the forests, multiple individuals of each species were not always represented in the plots. Therefore, for these rare species, testing individuals located outside the plots was required, and still there were several species for which only single samples were obtained.
A range of other factors was taken into consideration when sampling. For example, given that young leaves of tropical species, in particular, are typically more highly defended than older leaves (Coley, 1983; Coley and Barone, 1996), both young (soft expanding leaves) and old (recent fully expanded) leaves were tested for cyanogenesis, where possible. In addition, depending on availability, fruit and flowers from several species were tested. Owing to the large number of species and samples in the survey, it was not possible to examine seasonal trends in defence; however, individuals of the majority of species were tested in wet and dry seasons for qualitative changes in cyanogenic status, as there is some evidence for seasonal variation in cyanogenesis (Seigler, 1976b; Janzen et al., 1980; Kaplan et al., 1983). Finally, because edaphic factors have been reported to affect the expression of cyanogenesis (e.g. Urbanska, 1982), species found on more than one substrate type were tested at each site.
Sample collection, handling and storage for quantitative analysis
In addition to samples for fresh cyanide tests, whole leaf samples (again recent fully expanded leaves) were taken for quantification of cyanogenic glycosides. All individuals of species that produced a positive result and individuals of rare species were sampled. Depending on sampling conditions, samples were either placed immediately into liquid nitrogen, or placed in a sealed air-tight bag and kept on ice for 2–6 h until snap frozen in liquid nitrogen. Frozen samples were transported to the laboratory on dry ice, freeze-dried and stored on desiccant at −20 °C for analysis. Freeze-dried samples were ground using either a cooled IKA Labortechnic A10 Analytical Mill (Janke and Kunkel, Stanfen, Germany) or, for smaller samples, an Ultramat 2 Dental Grinder (Southern Dental Industries Ltd, Bayswater, Victoria, Australia).
Chemical analyses
Detection of cyanogenesis: Feigl–Anger papers
The presence of cyanogenic compounds in fresh field samples was determined using FA indicator papers (Feigl and Anger, 1966). FA papers were selected in preference to picrate papers because FA papers are more sensitive (Nahrstedt, 1980) and less prone to giving false-positive results (Brinker and Seigler, 1989). FA test papers were prepared according to Brinker and Seigler (1989). Because FA papers can be sensitive to moisture and light (Brinker and Seigler, 1989), they were stored in the dark and on desiccant until use.
Fresh leaves (approx. 1–2 g f. wt) were crushed in duplicate screw-top vials. Old and young foliage samples were tested separately. To facilitate cyanogenesis, 0·5 mL of water was added to one of the vials, and pectinase from Rhizopus spp. (Macerase® Pectinase, 441201 Calbiochem®, Calbiochem-Novabiochem Corp., La Jolla CA, USA) (0·4 g L−1) in 0·1 m Tris–HCl (pH 6·8) was added to the other. Pectinase has been found to have non-specific β-glycosidase activity (Brimer et al., 1995) and, therefore, in the absence of sufficient endogenous β-glycosidase, enables tests for the presence of cyanogenic glycosides to be made. The indicator papers were suspended above the tissue by means of the screw-top lid, and vials were left at room temperature and checked after 12 and 24 h. This 24 h time period, used in other surveys (e.g. Dickenmann, 1982; Thomsen and Brimer, 1997; Buhrmester et al., 2000; Lewis and Zona, 2000), was selected to avoid spurious test results due to substantial bacterial contamination which may occur beyond 24 h (Saupe et al., 1982). Tissue of known cyanogenic species, Prunus turneriana or Ryparosa javanica, was used as a positive control. Moderate to strongly cyanogenic samples gave a positive result within a few minutes or up to a few hours, while more weakly cyanogenic samples took several more hours. In accordance with the recommendations of Brinker and Seigler (1989), a new test was conducted for any samples producing a slow positive response (24 h) in case of interference by microbial cyanogenesis. In addition, any samples with inconclusive colour change were re-tested. An individual was considered cyanogenic if a positive, repeatable result was obtained, and a species was considered cyanogenic if at least one individual produced a consistent and repeatable positive result. A negative test result indicates the absence of a cyanogenic glycoside, or of the specific cyanogenic β-glycosidase, or both.
In the few instances where insufficient tissue was available for both FA paper tests using fresh leaves and subsequent laboratory analysis, cyanogenesis was determined based on the quantitative assay of freeze-dried ground leaf tissue (as described below) by comparison with a negative tissue control (e.g. Alstonia scholaris or Aglaia meridionalis).
In this study, tests for cyanogenesis used approx. 1–2 g f. wt of leaves, which is larger than tissue samples tested in previous surveys (e.g. 50 mg f. wt by Lewis and Zona, 2000; and 200 mg f. wt by Thomsen and Brimer, 1997; Buhrmester et al., 2000). According to Dickenmann (1982) who used 500 mg f. wt tissue, a weak positive reaction with FA papers, where part of the paper turns blue, indicated approx. 2–20 mg HCN kg−1 f. wt, while a strong reaction indicated >50 mg HCN kg−1 f. wt. These lower values equate to just over 6–60 µg HCN g−1 d. wt using a conversion based on the mean foliar water content of several species in this study, which was 70 %. In this study, based on fresh leaf tests and quantitative analysis of freeze-dried tissue from the same sample, the threshold sensitivity of FA papers was similar, within the range 5–8 µg HCN g−1 d. wt. This threshold sensitivity of FA papers corresponds well to the criteria of Adsersen et al. (1988) for classifying individuals as cyanogenic, where individuals with <93 nmol HCN g−1 f. wt (approximately equivalent to 8 µg HCN g−1 d. wt) were considered acyanogenic.
Polymorphism and confirmation of acyanogenesis
For the purposes of the survey, a species was considered cyanogenic if at least one individual produced a repeatable and positive test result. For a few species, not all individuals produced positive results using FA papers; therefore, some further investigation of the polymorphism for cyanogenesis in these species was conducted. First, to confirm the acyanogenic character of the individuals producing negative results with FA papers, a greater mass of freeze-dried tissue (100 mg) was assayed quantitatively for the evolution of cyanide (see below) and compared with a non-cyanogenic species, A. scholaris, as a control for baseline noise in the assay. Based on the criteria of Adsersen et al. (1988), if <8 µg HCN g−1 d. wt was detected, then that individual was considered acyanogenic.
Quantification of cyanogenic glycosides
The concentration of cyanogenic glycoside in plant tissues was measured by trapping the cyanide (CN), liberated following hydrolysis of the glycoside, in a well containing 1 m NaOH (Brinker and Seigler, 1989; Gleadow et al., 1998). Freeze-dried, ground plant tissue (30–50 mg) was incubated for 20–24 h at 37 °C with 1 mL of 0·1 m citrate–HCl (pH 5·5). Cyanide in the NaOH well was determined using the method of Gleadow and Woodrow (2002) adapted from Brinker and Seigler (1989) for use with a photometric microplate reader (Labsystems Multiskan® Ascent, with incubator, Labsystems, Helsinki, Finland). The method is highly sensitive; the determination of CN in NaOH is relatively specific for cyanide, and can detect as little as 5 µg L−1 (Brinker and Seigler, 1989). The absorbance was measured at 590 nm with NaCN as the standard.
RESULTS
Survey for cyanogenesis
In total, fresh leaf material from 401 woody plant species from 87 families was tested for cyanogenesis (Appendix). Cyanogenesis was found in 18 species from 13 families, representing 4·5 % of species occurring within 1200 m2 of rainforest at each of five sites. In each case, the addition of pectinase during incubation did not result in any additional positive results. The test with added enzyme therefore effectively served as a replicate test for each sample. A further two species were found to be cyanogenic but were not included in the analysis—the non-woody Alocasia brisbanensis, and Helicia nortoniana which occurred outside the plots (Table 1). The number of cyanogenic species at each site ranged from four to 10. The cyanogenic species ranged in life form from climbing monocotyledons such as the supplejack, Flagellaria indica, to 30 m canopy trees such as the northern silky oak, Cardwellia sublimis. The proportion of cyanogenic species ranged from 4·7 % in upland rainforest at Mt Nomico, to 6·5 % in lowland rainforest near Cape Tribulation. Overall, cyanogenic species accounted for 7·3 % of total basal area (range 1·2–13·4 %). The highest proportion was in highland rainforest on basalt soil at Longlands Gap, while forest on rhyolite in adjacent forest had the lowest proportion. In lowland rainforest, the contribution of cyanogenic species to biomass was also high, at 11·6 %, compared with 3·3 and 7·1 % at Mt Nomico and Lamins Hill sites, respectively.
Table 1.
Summary of the concentration of cyanogenic glycosides in leaves and other plant tissues from cyanogenic species found in six plots in upland/highland (U) and lowland (L) tropical rainforest at five sites: high nutrient basalt sites at Lamins Hill (B1) and Longlands Gap (B2), and low nutrient sites on granite at Mt Nomico (G), on rhyolite at Longlands Gap (R) and on metamorphic substrate near Cape Tribulation (M)
| Concentration of cyanogenic glycosides in different plant parts (mg CN g−1 d. wt) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Form | Species | Family | Forest | Site | Leaf | Stem | Floral parts | Fruit/seed |
| H | Alocasia brisbanensis (F.M.Bailey) Domin* | Araceae | U | B1 | – | – | – | – |
| T | Beilschmiedia collina B.Hyland | Lauraceae | U | B1, B2, G, R | Old: 28·4–1263 (n = 36) | – | – | – |
| Yg: 1039–1391 | ||||||||
| T | Brombya platynema F.Muell. | Rutaceae | L | M | 156·4–1285 (n = 20) | – | – | – |
| 0–10·5 (n = 27) | ||||||||
| T | Cardwellia sublimis F.Muell. | Proteaceae | U, L | B1, B1, G, R, M | Old:11·5–69·8 (n = 21) | – | Bud: 779–851 | Seed: approx.8 |
| Yg: 733·1, tips: 219·1 | Flwr: 130–334 | |||||||
| T | Cleistanthus myrianthus (Hassk.) Kurz | Euphorbiaceae | L | M | Old: 1·1–17·3 (n = 41) | – | Flwr: 30·7 | Mature: 160–377 |
| Yg: 78·4–80·4 (n = 3) | Immature: 821 | |||||||
| T | Clerodendrum grayi Munir | Verbenaceae | U | B1, B2, G | Old: 1325–4800 (n = 6) | – | Bud: 733 | – |
| Flwr 440–942 | ||||||||
| T | Elaeocarpus sericopetalus F.Muell. | Elaeocarpaceae | U | G, R | 1100–5056 (n = 10) | Sdlg: 1739–2679 | – | 1028 |
| Tree: 135 | ||||||||
| V | Embelia grayi S.T.Reynolds | Myrsinaceae | U | B1, B2 | 10–81 (n = 3) | – | – | – |
| V | Flagellaria indica L. | Flagellariaceae | U, L | B1, G, M | 11–177·3 (n = 6) | – | – | – |
| T | Helicia australasica F.Muell. | Proteaceae | L | M | 42·2–155·2 (n = 8)† | – | – | – |
| T | Helicia blakei Foreman | Proteaceae | U | B1 | Mean: 17·9 (n = 2) | – | – | – |
| T | Helicia nortoniana (F.M.Bailey) F.M.Bailey* | Proteaceae | L | M | – | – | – | – |
| V | Passiflora sp. (Kuranda BH12896) | Passifloraceae | L | M | 313–922 (n = 4) | – | – | – |
| T | Mischocarpus exangulatus (F.Muell.) Radlk. | Sapindaceae | U | B1 | Old: 133; yg: 222 (n = 1) | – | – | – |
| T | Mischocarpus grandissimus (F.Muell.) Radlk. | Sapindaceae | U, L | G, M | 49–680 (n = 2) 761–2006 (n = 4)‡ | – | – | – |
| T | Opisthiolepis heterophylla L.S.Sm. | Proteaceae | U | B1, B2 | Old: 10–208 (n = 7) | – | – | – |
| Yg: 2000 | ||||||||
| V | Parsonsia latifolia (Benth.) S.T.Blake | Apocynaceae | U | B1, G, R | 765–4835 (n = 3) | – | – | – |
| T | Polyscias australiana (F.Muell.) Philipson | Araliaceae | U, L | B1, B2, G, R, M | Old: <6·8 (n = 30); 10–28·5 (n = 16) | – | – | – |
| Yg: 10·8–29·6; tips: 259 | ||||||||
| T | Prunus turneriana (F.M.Bailey) Kalkman | Rosaceae | U, L | B1, M | old: 2472–4888 (n = 4) | 560–1100 (n = 2) | – | Mature seed: 400 |
| Yg: 3128–6464; Tip: 6000–8498 | Root: approx. 2000 | Yg fruit/seed: 8950 | ||||||
| T | Ryparosa javanica (Blume) Kurz ex Koord. & Valeton** | Achariaceae | L | M | 1749 (n = 2) | – | – | Mature seed: 5430 |
| Yg lf: 2560 (n = 5) | Yg seed: 7760 (n = 5) | |||||||
Means and ranges of cyanogenic glycosides concentration are given for fully expanded (old) leaves where not specified, and for some young (yg) leaves, for n individuals. Life form: herb (H), tree (T), vine (V). Cyanogenic glycoside concentrations were measured as evolved cyanide, following hydrolysis of freeze-dried tissue, without the addition of non-specific β-glycosidase (e.g. emulsin/pectinase).
Alocasia brisbanensis (a herb), and Helicia nortoniana which occurred outside the plots, were not included in the analysis, nor sampled for quantitative analysis.
Concentrations of cyanogenic glycosides in seed, flower and young leaves of Ryparosa javanica determined by B. L. Webber (unpubl data, 2004; Webber and Woodrow, 2004).
Only one individual of Helicia australasica occurred within the plots; seven additional saplings/trees were opportunistically tested external to the plots; all were cyanogenic.
Only one individual of Mischocarpus grandissimus occurred inside plots at Mt Nomico; additional individuals were sampled outside plots, including four in lowland rainforest, all were cyanogenic.
The cyanogenic species and concentrations of cyanogenic glycosides in leaves and other plant parts are summarized in Table 1. The foliar concentrations of cyanogenic glycosides among species ranged from around 8 µg CN g−1 d. wt—the concentration below which individuals were considered acyanogenic based on the criteria of Adsersen et al. (1988) and the limits of FA paper detection—to very high concentrations in excess of several mg CN g−1 d. wt (Table 1). For example, fully expanded leaves of numerous individuals of Polyscias australiana tested negative for cyanide, and contained <8 µg CN g−1 d. wt, while others tested positive and were in the range of 10–28 µg CN g−1 d. wt. Even in the weakly cyanogenic samples, only in very few cases did the coloration of the test paper change from 12 to 24 h in a qualitative sense. Individuals of numerous species had high concentrations of cyanogenic glycosides in excess of 1 mg CN g−1 d. wt (e.g. Mischocarpus grandissimus, Brombya platynema and Beilschmiedia collina), and several species contained extremely high concentrations of cyanogenic glycosides in mature tree leaves. Notably, fully expanded leaves from the tree species Elaeocarpus sericopetalus, Clerodendrum grayi and Prunus turneriana had concentrations of cyanogenic glycosides up to 5·2, 4·9 and 4·8 mg CN g−1 d. wt, respectively (Table 1).
Qualitative and quantitative variation in cyanogenesis
Intra-population variation in cyanogenic glycosides
For the majority of species initially identified as being cyanogenic, all individuals tested were cyanogenic; however, the concentrations of cyanogenic glycosides varied markedly between conspecific individuals (Table 1). For example, all saplings and trees of B. collina at each site tested positive for cyanogenesis, but the concentration of cyanogenic glycosides in fully expanded leaves from saplings (n = 19) ranged from 23·2 to 1263·4 µg CN g−1 d. wt at Mt Nomico alone, where B. collina was most abundant. In contrast to this quantitative phenotypic variation in cyanogenesis, conspecific individuals of a small number of species produced both positive and negative FA paper test results. This apparent qualitative polymorphism was most notable in B. platynema, a subcanopy tree species restricted to the lowland rainforest. Within the population of B. platynema, approx. 50 % of individuals tested produced negative FA test paper results for both young and old leaves, and had cyanogenic glycoside concentrations in the range 0·6–6·8 µg CN g−1 d. wt (Table 1.). The addition of buffered pectinase during testing did not alter the qualitative FA paper test result for any individual. Among cyanogenic B. platynema, the concentrations of cyanogenic glycosides varied over orders of magnitude from 10·5 to 1285·9 µg CN g−1 d. wt (Table 1).
Negative results with FA papers were obtained for two other species, Cleistanthus myrianthus and Polyscias australiana. Qualitative and quantitative analysis of old leaves from individuals of these species indicated a high frequency of acyanogenic individuals; however, unlike B. platynema, the phenotypic expression of cyanogenesis varied qualitatively with leaf age such that young leaves and leaf tips from these same individuals were consistently cyanogenic, as were reproductive tissues from C. myrianthus (Table 1).
Even among less common species with small sample sizes, the concentrations of cyanogenic glycosides varied markedly between individuals. For example, in Mischocarpus grandissimus, the concentration among six individuals from two sites ranged from 49 to 680 µg CN g−1 d. wt at Mt Nomico and from 637 to 2006 µg CN g−1 d. wt in lowland rainforest, and among three individuals of the woody vine Parsonsia latifolia, concentrations ranged from 765 to 4835 µg CN g−1 d. wt. There was no detectable affect of soil type on qualitative determination of cyanogenesis for individuals of the same species; where cyanogenic species were found on different substrates, all individuals tested were cyanogenic.
Intra-plant variation in cyanogenic glycoside concentration
In addition to cyanogenic polymorphism, both qualitative and quantitative, within populations of cyanogenic species, the concentration of cyanogenic glycosides varied with leaf age and plant part. In all cases where tested, young leaves or leaf tips had higher concentrations of cyanogenic glycosides than older leaves (Table 1). In some cases, the concentrations in young and old leaves differed by over an order of magnitude (e.g. C. sublimis and Opisthiolepis heterophylla; Table 1). In terms of determining the cyanogenic phenotype of an individual, this difference between leaves of different ages was most notable for C. myrianthus and P. australiana. Based on FA paper tests of mature fully expanded leaves of these species, there were frequent acyanogenic individuals; however, young leaves and leaf tips sampled from the same individuals of both species routinely tested positive for cyanogenesis. Furthermore, among mature trees of C. myrianthus, which had low levels of cyanogenic glycosides in old leaves (6–8 µg CN g−1 d. wt, i.e. considered acyanogenic), the fruit, in particular, had higher concentrations of cyanogenic glycosides (>800 µg CN g−1 d. wt; Table 1). Overall, few tests were made of seeds or other reproductive tissues as part of the survey; however, where reproductive tissues were tested from species with cyanogenic leaves, they were typically cyanogenic and contained higher concentrations of cyanogenic glycosides, although the concentration varied with the maturity of the fruit/seed. For example, the immature seed and fruit (combined) from Prunus turneriana had >8 mg CN g−1 d. wt compared with mature seed alone (<1 mg CN g−1 d. wt), while in R. javanica cyanogenic glycoside concentrations in flesh and seed of immature fruit decreased with maturity (Table 1; Webber and Woodrow, 2004). One exception was the papery wind-dispersed seeds of C. sublimis which yielded negligible cyanide (Table 1).
DISCUSSION
Cyanogenic species
Despite a substantial body of early work screening Australian flora, including some tropical flora, for cyanogenesis (e.g. Petrie, 1912; Smith and White, 1918; Finnemore and Cox, 1928; Hurst, 1942; Webb, 1948, 1949), few of the species tested in this study had previously been tested. Of the 18 species from 13 families found to be cyanogenic in this study, only one, F. indica, has previously been reported as cyanogenic (Petrie, 1912). Thirteen of the cyanogenic species are endemic to Queensland or Australia; several are restricted to small areas within north east Queensland. For example, Clerodendrum grayi is found only in a small area on the Atherton Tableland (Miller et al., 2006a), while R. javanica occurs in a limited area within lowland rainforest north of the Daintree River. Cyanogenesis is reported for the first time in the genera Beilschmiedia, Cardwellia, Cleistanthus, Elaeocarpus, Embelia, Mischocarpus, Opisthiolepis, Parsonsia and Polyscias. The number of new reports at the generic level may in part reflect the high level of endemism among the Australian tropical rainforest flora. In addition, several species are from families in which cyanogenesis has been rarely reported, if at all. For example, cyanogenesis is rare in Elaeocarpaceae, Lauraceae, Apocynaceae, Myrsinaceae and Araliaceae families. Following are descriptions of each cyanogenic species (listed alphabetically by family) discussed in relation to previous reports of cyanogenesis within the relevant taxonomic groups.
Ryparosa javanica (Blume) Kurz ex Koord. & Valeton (Achariaceae)
Ryparosa javanica is currently the subject of taxonomic revision (Webber, 2005). The Queensland Ryparosa sp. currently known as R. javanica is endemic to lowland rainforest north of the Daintree River to Cape Tribulation, north east Queensland. This species was found to be cyanogenic early in this survey, and has subsequently been the focus of extensive population-level studies of cyanogenesis (Webber, 2005). Reports of cyanogenesis in this genus are common; for example, R. javanica (sensu stricto) and R. caesia are cyanogenic (Rosenthaler, 1919). Cyanogenesis has been reported among other genera in Achariaceae, e.g. Hydnocarpus, Calancoba, Ceratiosicyos, Gynocardia, Erythrospermum, Pangium and Kiggelaria (Rosenthaler, 1919; Tjon Sie Fat, 1979a; Jensen and Nielsen, 1986). Many of these genera were previously in the Flacourtiaceae until recent revisions saw most cyanogenic genera assigned to the Achariaceae (Chase et al., 2002), including the tribe Pangieae of which Ryparosa is a member. Cyanogenesis was considered a useful taxonomic marker in Flacourtiaceae (Spencer and Seigler, 1985), this utility being apparent in the revision of the family (Chase et al., 2002). All individuals in sizeable populations of R. javanica were cyanogenic, and the cyanogenic glycoside in R. javanica has been identified as gynocardin (Webber, 2005), a cyclopentenoid cyanogenic glycoside typical of Achariaceae (Jaroszewski and Olafsdottir, 1987). Cyanogenic glycosides derived from valine/isoleucine have also been reported in species formerly in the Flacourtiaceae (Lechtenberg and Nahrstedt, 1999).
Parsonsia latifolia (Benth.) S.T.Blake (Apocynaceae)
This is the first report of cyanogenesis in the genus Parsonsia, a genus of woody or semi-woody climbers (130 species) distributed from Southeast Asia to Australia, New Caledonia and New Zealand. Members of the Apocynaceae family (220 genera, 2000 species) commonly have clear or milky latex, and frequently contain alkaloids (Mabberley, 1990; Collins et al., 1990). Parsonia latifolia (diameter up to 9 cm) has milky white latex, and is endemic to Australia (Forster and Williams, 1996). It is found in lowland and highland rainforest in noth east Queensland, parts of New South Wales and the Northern Territory (Hyland et al., 2003). Reports of cyanogenesis in Apocynaceae and the order Gentianales are few; Gibbs (1974), who reported negative results for Parsonsia eucalyptifolia and P. lanceolata, found only Alstonia scholaris (L.) R.Br. to be cyanogenic, and noted positive reports for four other species. Tests for cyanogenesis in the Apocynaceae are, however, apparently limited, with negative reports for only approx. 20 species (Gibbs, 1974; Adsersen et al., 1988; Thomsen and Brimer, 1997). In this study, leaf samples of all ages from multiple A. scholaris trees and seedlings gave negative FA paper results; quantitative assaying confirmed the absence of cyanogenesis. No cyanogenic constituents in the family have been characterized.
Polyscias australiana (F.Muell.) Philipson (Araliaceae)
This is the first report of cyanogenesis in the genus Polyscias, a genus of approx. 100 species distributed throughout Africa, Asia, Malesia, Australia and the Pacific Islands. In addition, cyanogenesis is very rare in the order Umbellales; Gibbs (1974) reported only negative results or some doubtful positive results in a few members of Araliaceae (Nothopanax sp. and Schefflera sp.) and the Umbelliferae. Subsequently, only Aralia spinosa L. (above-ground parts) has been reported as cyanogenic (Seigler, 1976b). Polyscias australiana is often considered a regrowth species in disturbed rainforest, and commonly occurs at rainforest margins. Concentrations of cyanogenic glycosides in this species were variable, and in mature leaves were commonly less than the threshold value used for classifying individuals as cyanogenic in this study (<8 µg CN g−1 d wt); however, the species was considered cyanogenic on the basis of repeatable positive results with FA papers for multiple individuals, in particular when analysing young foliage. Interestingly, in reporting cyanogenesis in A. spinosa, Seigler (1976b) noted substantial seasonal variation, in that the individual was cyanogenic at only one of three testing times throughout the year. Analysis of the distribution of cyanogenic glycosides in fruit, flowers and seasonal variation in foliar concentrations in P. australiana would better characterize cyanogenesis in this species.
Elaeocarpus sericopetalus F.Muell. (Elaeocarpaceae)
Elaeocarpus sericopetalus (‘Northern Quandong’) is a small to medium sized tree endemic to tropical rainforest within the Cook and Kennedy Districts of north eastern Queensland. This is the first report of cyanogenesis in the genus Elaeocarpus. Elaeocarpus sericopetalus was the only cyanogenic species identified among eight Elaeocarpus spp. and 11 species from the Elaeocarpaceae family tested in this study (Appendix). The extremely high foliar concentrations of cyanogenic glycosides (up to 5·2 mg CN g−1 d. wt in mature field-grown leaves) in E. sericopetalus rank it among the most cyanogenic tree species ever reported. Cyanogenesis is rare within the family Elaeocarpaceae and the order Malvales (Gibbs, 1974; Hegnauer, 1990; Lechtenberg and Nahrstedt, 1999), whereas alkaloids (e.g. indolizidine alkaloids) are considered common (Gibbs, 1974; Hegnauer, 1990; Mabberley, 1990). In the Elaeocarpaceae family, cyanogenesis has previously been reported in only two species: the leaves of Vallea stipularis ‘pyrifolia’ F.Ballard (Gibbs 1974), and the leaves [Greshoff (1898) cited in Hegnauer (1973)] and the bark (Pammel, 1911; Rosenthaler, 1919) of Sloanea sigun (Blume) K.Schum (syn. Echinocarpus sigun), were found to be cyanogenic. Sambunigrin was isolated from the leaves of S. sigun [R. Hegnauer and L. H. Fikenscher, unpubl. data (1983) cited in Hegnauer (1990)], the only previous report of a cyanogenic constituent in Elaeocarpaceae and Malvales. Characterization of the principal foliar cyanogenic glycoside—an apparently unusual phenylalanine-derived glycoside with an organic acid residue—is ongoing (Miller, 2004).
Cleistanthus myrianthus (Hassk.) Kurz (Euphorbiaceae)
This appears to be the first report of cyanogenesis in the genus Cleistanthus, which consists of 100–140 species. Cleistanthus myrianthus is a subcanopy rainforest tree (to 7 m), found in the lowland and foothills from the Daintree River to Rossville, north east Queensland (Cooper and Cooper, 1994). In Australia, it is classified as rare on the basis of this limited distribution, but it is also found in Southeast Asia and Malesia (Hyland et al., 2003). Cyanogenesis is especially common in Euphorbiaceae (300 genera, 7500 species), and is found in many genera including Bridelia, Euphorbia and Phyllanthus, as well as the economically important species Hevea brasiliensis (rubber tree) and Manihot esculenta (cassava) (e.g. Rosenthaler, 1919; Tjon Sie Fat, 1979a; Seigler et al., 1979; Adsersen et al., 1988).
The chemotaxonomic utility of cyanogenesis, as well as other secondary metabolites (e.g. alkaloids and terpenes), has been demonstrated in Euphorbiaceae (Seigler, 1994). Cyanogenic glycosides are useful at the infra-familial level in the Euphorbiaceae (van Valen, 1978; Seigler, 1994); the species in the subfamily Phyllanthoideae typically contain the tyrosine-derived cyanogenic glycosides dhurrin, taxiphyllin or triglochinin, while species in the subfamily Crotonoideae (sensu Pax and Hoffman, 1931; see van Valen, 1978), including Hevea and Manihot, produce cyanogenic glycosides derived from valine and isoleucine (e.g. linamarin and lotaustralin) (van Valen, 1978, Nahrstedt, 1987; Seigler, 1994). Given this pattern, one may predict Cleistanthus—assigned to the Phyllanthoideae—to contain cyanogens derived from tyrosine.
Flagellaria indica L. (Flagellariaceae)
Flagellaria indica (‘the supplejack’), a leaf tendril climber with cane-like stems, is known to be cyanogenic (Petrie, 1912; Webb, 1948; Gibbs, 1974; Morley and Toelken, 1983). Young shoots were suspected of poisoning stock in Australia (Everist, 1981), and it has also been reported to have medicinal properties (e.g. tumour inhibition; Collins et al., 1990). In other countries, it has been used as a hair wash, and as a contraceptive (see Hyland et al., 2003), but was not apparently used medicinally by aboriginal Australians (Morley and Toelken, 1983). Interestingly, monocotyledons are typically characterized by cyanogenic glycosides biosynthetically derived from tyrosine (Lechtenberg and Nahrstedt, 1999). Consistent with this, the tyrosine-derived cyanogenic glycoside triglochinin was isolated from the stem (and rhizome) of F. indica (L. H. Fikenscher unpubl. data, cited in Hegnauer, 1966), although meta-hydroxylated valine or isoleucine, and leucine-derived glycosides have also been found in monocotyledons (Nahrstedt, 1987; Lechtenberg and Nahrstedt, 1999).
Clerodendrum grayi Munir (Lamiaceae)
Clerodendrum grayi is a rare subcanopy tree endemic to the northern part of Queensland, Australia (Munir, 1989). Recent revisions of the division between Lamiaceae and Verbenaceae families (Cantino, 1992; Wagstaff et al., 1997, 1998) transferred the genus Clerodendrum, and others historically in the Verbenaceae family, to the Lamiaceae family. Cyanogenesis in Lamiaceae, and also Verbenaceae, has rarely been reported. Even within the order Lamiales, cyanogenesis is considered rare (Gibbs, 1974). In the Lamiaceae, known for its culinary and medicinal herbs [e.g. Lavandula (lavender); Mentha (mint)], typical constituents are monoterpenoids, diterpenes or triterpenes, as well as flavonoids and iridoid glycosides (Gibbs, 1974; Hegnauer, 1989; Taskova et al., 1997). In a survey of the flora of the Galapagos Islands, Clerodendrum molle var. molle was found to be cyanogenic (Adsersen et al., 1988; see also Gibbs, 1974, Tjon sie Fat, 1979a). In addition, several species of Clerodendrum are known to be toxic (Hurst, 1942; Webb, 1948; CFSAN, 2003); however, the poison is not detailed.
In this study, the extremely high foliar concentrations of cyanogenic compounds—up to 4·8 mg CN g−1 d. wt in mature field-grown tree leaves—are among the highest reported for tree leaves (Table 1). Two cyanogenic glycosides were purified from the leaf tissue of C. grayi (Miller et al., 2006a). Prunasin and its primerveroside, the rare diglycoside lucumin (Eyjólfsson, 1971), were found in the ratio 1 : 1·58 (mol:mol) (Miller et al., 2006a), the first reported co-occurrence of these glycosides, and the first confirmed report of lucumin in vegetative tissue (see Thomsen and Brimer, 1997). Given the relatively rarity of reports of cyanogenic glycosides from the Lamiaceae, and even within the order Lamiales, it is difficult to draw any conclusions about the biogenetic origins of glycosides within these taxonomic groups. Refer to Miller et al. (2006a) for a detailed discussion of cyanogenesis in C. grayi and associated taxa.
Beilschmiedia collina B.Hyland (Lauraceae)
Beilschmiedia collina (‘the mountain blush walnut’) is a tree species endemic to Queensland rainforest (Cooper and Cooper, 1994). Cyanogenesis is very rare in Lauraceae (Gibbs, 1974; Hegnauer, 1989), having only been reported from Cinnamomum camphora and Litsea glutinosa (Gibbs, 1974), with one other species (Nectranda megapotamica) reported to have cyanogenic glycosides but apparently lacks the catabolic enzymes, requiring further investigation (Francisco and Pinotti, 2000). The Lauraceae is better known for producing a range of alkaloids (e.g. Gibbs, 1974), and as a dominant family in the rainforest flora of Australia has been much studied for its toxic and potentially medicinal alkaloids (Webb, 1949, 1952; Collins et al., 1990). Of 39 species tested in the Lauraceae family in this survey, only B. collina was cyanogenic (Appendix). Given the apparent rarity of cyanogenesis in the family, and even the order Laurales, it is difficult to speculate as to the biosynthetic precursor of the cyanogenic constituent in B. collina. To the authors' knowledge, only the tyrosine-derived cyanogenic glycoside taxiphyllin has been reported in the Calycanthaceae family within the Laurales (Lechtenberg and Nahrstedt, 1999); tyrosine-derived glycosides are also found in Ranunculaceae in the order Ranunculales, which is in the same subclass Magnoliidae (sensu Cronquist, 1981) as Laurales.
Embelia grayi S.T. Reynolds (Myrsinaceae)
This is the first report of cyanogenesis in the genus Embelia—a genus of approx. 130 species—and among the first in the family Myrsinaceae. Furthermore, Gibbs (1974) considered cyanogenesis to be unknown in the order Primulales. Subsequent to Gibbs (1974), only two reports of cyanogenesis in Myrsinaceae could be found—for Rapanea parviflora (Kaplan et al., 1983), and for R. umbellata, which needs confirmation as cyanogenesis was only detected after 24 h of tissue incubation (Francisco and Pinotti, 2000). Overall, there are even a few negative test records for the family. Embelia grayi is a vine with diameter up to 9 cm, endemic to upland and highland rainforest in north east Queensland. Embelia caulialata, and three Rapanea spp., also tested here, were not cyanogenic. The order Primulales (sensu Cronquist, 1981) is in the subclass Dilleniidae which also includes the orders Malvales and Violales. Within the Violales, cyanogenesis is common in the Achariaceae (including Flacourtiaceae), Passifloraceae and Turneraceae families, which tend to contain cyanogenic glycosides of the cyclopentanoid series (Lechtenberg and Nahrstedt, 1999).
Passiflora sp. (Kuranda BH12896) (Passifloraceae)
Passiflora sp. (Kuranda BH12896) was the only species in the Passifloraceae tested in this study. It is a vine found in the lowland rainforests of north east Queensland; all Australian members of the Passifloraceae are climbers or sprawling shrubs (Morley and Toelken, 1983). Cyanogenesis is common within the family Passifloraceae (600 species, two genera worldwide), and the genus Passiflora (e.g. Rosenthaler, 1919; Tjon Sie Fat, 1979a; Adsersen et al., 1988; Olafsdottir et al., 1989). Several Australian Passiflora spp. were reported to be cyanogenic, including P. aurantia (Petrie, 1912; Smith and White, 1918; Webb, 1952; Gardner and Bennetts, 1956) and the endemic P. herbertiana Lindl. (Petrie, 1912), and implicated in poisoning stock (Smith and White, 1918). Within Passifloraceae, cyanogenesis has also been reported in Adenia spp. and Ophiocaulon spp. (Rosenthaler, 1919; Tjon Sie Fat, 1979a). The specific cyanogenic constituents may be taxonomically diagnostic at the infrafamilial level (e.g. occurrence of the rare glycoside passibiflorin; Adsersen et al., 1993). Cyanogenic Passifloraceae typically contain cyanogenic glycosides with cyclopentenoid ring structures (Seigler et al., 1982; Spencer and Seigler, 1985; Nahrstedt, 1987; Lechtenberg and Nahrstedt, 1999), although phenylalanine-derived glycosides (e.g. prunasin) have been isolated from Passiflora edulis (Spencer and Seigler, 1983; Chassagne et al., 1996; Seigler et al., 2002), and valine/isoleucine-derived glycosides (e.g. linamarin, lotaustralin) from several Passiflora spp. in the subgenus Plectostemma (Spencer et al., 1986).
Cyanogenesis in the Proteaceae family
Five of the 20 species tested in the Proteaceae family were found to be cyanogenic: C. sublimis F.Muell., O. heterophylla L.S.Sm., Helicia australasica F.Muell, H. blakei Foreman and H. nortoniana (F.M.Bailey) F.M.Bailey. The latter species was opportunistically tested as it was found only outside the plots. This is the first formal report of cyanogenesis in the monospecific genera Cardwellia and Opisthiolepis, while cyanogenesis has previously been reported in the genus Helicia (H. robusta; Gibbs, 1974).
Cardwellia sublimis (‘the northern silky oak’) is the only species in the tribe Cardwelliinae (Hoot and Douglas, 1998). This canopy species (to 30 m), an important timber tree, is endemic to north east Queensland, being widely distributed throughout well-developed lowland to highland rainforest (Hyland et al., 2003). Cardwellia sublimis was common to all sites in this study.
Opisthiolepis heterophylla (‘the blush silky oak’) is endemic and confined to north east Queensland, from the Kirrama Range to Cooktown. It grows to 30 m in lowland to highland rainforest, but is most common in upland and highland rainforest on the Atherton Tableland (Hyland et al., 2003). The flowers of O. heterophylla have been found to be cyanogenic (E. E. Conn, University of California, Davis, CA, USA, pers. comm.).
The genus Helicia (approx. 90 species) occurs throughout Asia and the Pacific, with nine species found naturally in Australia (Foreman, 1995). Helicia blakei (‘Blake's silky oak’) is endemic to north east Queensland, occurring as an understorey tree in well-developed upland rainforest (Hyland et al., 2003). Helicia australasica is a shrub or tree (3–20 m) widespread throughout northern Australian through to Papua New Guinea. It occurs as an understorey tree in well-developed rainforest, monsoon forest and dry rainforest (Foreman, 1995; Hyland et al., 2003). The fruit is known to be eaten by aborigines (Foreman, 1995). Helicia nortoniana is also an understorey tree (to 20 m), endemic to north east Queensland, and found in well-developed lowland and highland rainforest (Cooper and Cooper, 1994; Foreman, 1995; Hyland et al., 2003). These data support the preliminary results of tests on dried herbarium samples where only some samples of H. australasica and H. nortoniana tested positive for cyanide (E. E. Conn, University of California, Davis, CA, USA, pers. comm.). Tests of dried herbarium samples of H. blakei, however, gave only negative results (E. E. Conn, University of California, Davis, CA, USA, pers. comm.). The inconsistent findings by Conn are probably the result of using dried herbarium material.
Cyanogenesis is considered especially common in the Proteaceae (Swenson et al., 1989; Lechtenberg and Nahrstedt, 1999), known in a range of genera, but particularly in Grevillea and Hakea spp. In Australia, cyanogenic members of the Proteaceae have been implicated in stock poisoning (Gardner and Bennetts, 1956). Based on the reports of Gibbs (1974) and Tjon Sie Fat (1979a), and the study of Swenson et al. (1989) who found 44 of 155 proteaceous species tested to be cyanogenic, cyanogenesis is most widespread in the subfamily Grevilleoideae. For example, cyanogenesis has been reported in the genera Stenocarpus, Lomatia, Helicia, Xylomelum, Telopea, Macadamia, Hicksbeachia, Lambertia, Grevillea and Xylomelum (Petrie, 1912; Smith and White, 1918; Hurst, 1942; Gardner and Bennetts, 1956; Gibbs, 1974; Swenson et al., 1989; Lamont, 1993; E. E. Conn, University of California, Davis, CA, USA, pers. comm.), all of which are in the Grevilloiedeae (Hoot and Douglas, 1998). Consistent with this pattern, the cyanogenic genera reported here are all in the subfamily Grevilleoideae; Cardwellia in the subtribe Cardwelliinae (tribe Knightieae), Opisthiolepis in the subtribe Buckinghamiinae (tribe Embothrieae), and Helicia in the subtribe Heliciinae (tribe Heliceae). In contrast, there are only a few reports of cyanogenesis within the Proteoideae subfamily; cyanogenesis was only reported in single species of Conospermum, Petrophile and Protea (Gibbs, 1974; Swenson et al., 1989; E. E. Conn, University of California, Davis, CA, USA, pers. comm.).
The cyanogenic constituents, which have been identified in comparatively few species, appear to be biogenetically derived from tyrosine. Swenson et al. (1989) identified the cyanogenic glycosides in eight species; leaves and flowers of several Hakea, Leucadendron, Grevillea and Macadamia species were found to contain dhurrin and proteacin (see also Plouvier, 1964; Young and Hamilton, 1966). In this regard, the identity of the cyanogenic constituent in the monospecific genera in particular would be interesting.
Prunus turneriana (F.M.Bailey) Kalkman (Rosaceae)
Cyanogenesis is widespread within Rosaceae (Hegnauer, 1990), and has been much studied in the subfamily Prunoideae in particular, as it contains many cyanogenic cultivated species [e.g. Prunus domestica L. (plum); Armeniaca vulgaris Lam. (apricot)]. Cyanogenic Rosaceae (in particular Prunus spp.) are also a common source of poisoning in domestic animals (e.g. Poulton, 1983; Schuster and James, 1988). Prunus turneriana is one of only two Prunus species native to Australia and is a late successional canopy tree species in the lowland and upland rainforests of far north Queensland, Australia. The fruits of this canopy species are known to be toxic, yet are also eaten by cassowaries, fruit pigeons, Herbert river ringtail possums and musky-rat kangaroos (Cooper and Cooper, 1994). The flesh of the fruit was used raw by the Ngadjonji people—the original inhabitants of the rainforests on the Atherton Tablelands, north Queensland—for treating toothache, while the toxic kernels were processed for a starchy food (Huxley, 2003). Despite its common name ‘Almond bark’ and phytochemical surveys of Australian rainforest species (e.g. Webb, 1949), cyanogenesis in P. turneriana had not previously been reported. Cyanogenic glycosides were found to be distributed throughout all tissues and, consistent with other species in the Rosaceae, are biosynthetically derived from the amino acid phenylalanine (Møller and Seigler, 1999). Prunasin was identified as the major cyanogen in leaf, stem, root and seed tissues of P. turneriana, and amygdalin was restricted to the seed. What was unusual about P. turneriana was the presence of significant amounts of the (R)-prunasin epimer, (S)-sambunigrin, in leaf, stem and seed tissue, whereas root tissue contained only prunasin. Refer to Miller et al. (2004) for the detailed characterization of cyanogenesis in P. turneriana.
Brombya platynema F.Muell. (Rutaceae)
This is the first report of cyanogenesis in the genus Brombya, which is a genus of 1–2 species endemic to Australia (Hyland et al., 2003). Brombya platynema is endemic to north east Queensland where it occurs as an understorey tree in well-developed forest (from sea level to 1100 m a.s.l.) (Hyland et al., 2003). The family (150 genera, 1500 species) includes many strongly scented shrubs and trees, and rutaceous species are known for their terpenoids and alkaloids (Price, 1963; Gibbs, 1974; Everist, 1981). Cyanogenesis is rare in Rutaceae, and has only been reported in Boronia bipinnate Lindl. (leaves; Rosenthaler, 1919), Zieria spp. (Hurst, 1942; Gibbs, 1974; Fikenscher and Hegnauer, 1977), Zanthoxylum fagara (Adsersen et al., 1988) and Loureira cochinchinensis Meissa (Gibbs, 1974). Even within the order Rutales, cyanogenesis is rare, with only a few additional definitive reports of cyanogenesis in the Tremandraceae family (Gibbs, 1974). The phenylalanine-derived cyanogenic glycosides prunasin/sambunigrin and zierin have been isolated from leaves of two Zieria spp. (Finnemore and Cooper, 1936; Fikenscher and Hegnauer, 1977). The rare meta-hydroxylated cyanogenic glycoside holocalin was recently identified as the principal cyanogen in leaves of B. platynema; traces of prunasin and amygdalin were also detected (Miller et al, 2006b). These data suggest the possibility that species in this family have cyanogenic glycosides biosynthetically derived from the amino acid phenylalanine.
Mischocarpus grandissimus (F.Muell.) Radlk. and Mischocarpus exangulatus (F.Muell.) Radlk. (Sapindaceae)
Two of the four species of Mischocarpus tested in this study were found to be cyanogenic—M. grandissimus and M. exangulatus—representing the first reports of cyanogenesis for the genus. Mischocarpus is a genus of 15 species found in Asia, Malesia and Australia; nine species occur naturally in Australia (Hyland et al., 2003). Both species are endemic to Queensland; M. grandissimus is restricted to north east Queensland, while M. exangulatus is also found on the Cape York Peninsula, Queensland. Mischocarpus grandissimus occurs as an understorey tree in well-developed lowland and upland rainforest (sea level to 750 m a.s.l.; Hyland et al., 2003). Similarly, M. exangulatus (‘the red bell mischocarp’) is an understorey tree to 15 m found in well-developed lowland and highland rainforest (sea level to 1100 m a.s.l.; Cooper and Cooper, 1994; Hyland et al., 2003). These were the only cyanogenic species identified among the 29 species in the Sapindaceae family tested in this study (Appendix). Cyanogenesis is known in the Sapindaceae family; however, the family is best known for cyanolipids in the seed oils of numerous species (e.g. Alectryon spp., Allophylus spp., Cardiospermum spp., Sapindus spp., Paullinia spp. and Ungnadia speciosa) (Mikolajczak et al., 1970; Seigler et al., 1971; Gowrikumar et al., 1976; Seigler and Kawahara, 1976). There are only a few reports of cyanogenesis in Australian indigenous members of Sapindaceae—for Dodonaea spp. (Hurst, 1942; Webb, 1949) and Alectryon spp. (Smith and White 1918; Finnemore and Cooper, 1938)—and, with the exception of Heterodendrum oleifolium Desf. [syn. Alectryon oleifolius (Desf.) S.Reyn], the cyanogenic constituents in these species have not been characterized. In addition to cyanolipids in the seeds, leucine-derived cyanogenic glycosides have been characterized from vegetative parts of sapindaceous species (Seigler et al., 1974; Hübel and Nahrstedt, 1975, 1979; Nahrstedt, and Hübel, 1978).
Further findings
Alocasia brisbanensis (Araceae) was also found to be cyanogenic, consistent with reports of cyanogenesis in numerous congeneric species (Rosenthaler, 1919; Tjon Sie Fat, 1979a). However, as a herb, it was not included in the analysis. The tyrosine-derived cyanogenic glycoside triglochinin has been isolated from the closely related A. macrorrhiza (Nahrstedt, 1975).
There were several findings which contradicted previous reports for species. In addition to negative results for A. scholaris noted above, Eupomatia laurina (Eupomatiaceae) was reported to be ‘doubtfully cyanogenic’ and Cananga odorata (Annonaceae) cyanogenic by Gibbs (1974), but neither species was found to be cyanogenic in this study (based on repeated tests of four and two individuals, respectively). Similarly, reports of cyanogenesis in fruit and leaves of the Australian endemic Davidson's plum (Davidsonia pruriens) (Petrie, 1912; Rosenthaler, 1929) were not corroborated, both mature leaves and fruit testing negative in this study. Gibbs (1974) also only found negative results for leaves of D. pruriens. In addition, negative test results for cyanogenesis in this study corroborate previous negative reports for Neolitsea dealbata (syn. Litsea dealbata), Cayratia acris, Morinda jasminoides and Sarcopetalum harveyanum (Gibbs, 1974; Appendix). A screening of Australian Proteaceae family, conducted primarily using dry herbarium material, was carried out by E. E. Conn (University of California, Davis, CA, USA, pers. comm.) and included several species tested in this study. As noted above, Conn had some inconsistent, and possibly therefore inconclusive, results for a number of species, which were confirmed by fresh sampling in this study. One of eight samples of Musgravea heterophylla gave a positive reaction after 12 h in Conn's survey, a species which was not found to be cyanogenic here. Further testing of M. heterophylla is warranted. As far as could be discerned, the majority of species tested in this study had not previously been tested, despite numerous screenings of Australian and Queensland plants, most notably by Webb (1948, 1949).
General findings: frequency of cyanogenesis
Of 401 species from 87 families tested in the survey, 18 (4·5 %) species from 13 families were cyanogenic. The proportion of cyanogenic species at the sites ranged from 4·7 to 6·5 %, values similar to the frequency of cyanogenic species found in a substantial survey of woody species in Costa Rican rainforest (Thomsen and Brimer, 1997)—the only previous study to report the frequency of cyanogenesis standardized with respect to plant size (dbh >10 cm) and forest area (7 × 1 ha plots). Overall, Thomsen and Brimer (1997) found that 4·0 % (range 2·1–5·7 % for plots) of 401 species from 68 families were cyanogenic, and that cyanogenic stems (dbh ≥ 5 cm) accounted for 3 % of total basal area (range 1·6–5·1 %). Here, the overall proportion of total basal area in cyanogenic stems was 7·3 % and ranged from 1·2 to 13·4 %. The highest proportions were in highland rainforest on basalt soil (13·4 %) and in lowland rainforest (11·6 %). Highland rainforest on rhyolite had the lowest proportion.
Overall, at a community level, there are few studies with which to compare frequencies of cyanogenesis reported here for tropical rainforest in north east Queensland. In the first instance, there have been few studies in tropical systems, but also, the screening methodology varies depending on the research question being addressed, be it taxonomic (e.g. examining chemical differences in relation to proposed phylogenetic relationships) or ecological (e.g. the role of secondary compounds in plant–animal interactions). For example, while the survey of Thomsen and Brimer (1997) was standardized with respect to plant size and forest area, the few surveys in other tropical systems have focused on plant–animal interactions, and have not screened in a standardized fashion. Only 2·3 % (one species) was found to be cyanogenic in the screening of >90 % of the flora (n = 43 species) in a species-poor seasonal cloud forest in India (Mali and Borges, 2003). In contrast, in a survey examining the frequency of cyanogenesis in relation to environmental factors and insect density along a transect from the shoreline to an inland lagoon in a neotropical woodland (‘restinga’), Kaplan et al. (1983) found 25 species (23 %) of 108 species screened to be cyanogenic. They also reported variable test results for a further 49 species (for n = 2–16 individuals), elevating the proportion of cyanogenic species to 68 %, a value which requires further examination before interpretation, as the sampling strategy (e.g. plant size, random sampling strategy, life form and transect area) was unclear, and some uncertainty with regard to picrate paper test results was expressed by the authors (Kaplan et al., 1983).
Adsersen et al. (1988) compared frequencies of cyanogenesis within the endemic and non-endemic flora of the Galapagos Islands, two floras subject to different suites of herbivores over a period of time. They screened fresh and herbarium specimens of a significant proportion (65 %) of the flora from the Archipelago, and reported 8·1 % of endemic species, and 5·3 % of native species—those which also occur on the South American mainland—to be cyanogenic. Interestingly, they also reported a further 22 % of native species and 30 % of endemic species to release cyanide in the presence of a crude mix of β-glycosidases (including 5 % β-glucuronidase from snails), suggesting that a large number of species contain cyanogenic glycosides but lack the catabolic β-glycoside enzyme. This contrasts with the findings of several other studies where the addition of non-specific β-glycosidases or pectinase during qualitative testing did not alter the frequency of positive results (e.g. Petrie, 1912; Thomsen and Brimer, 1997; Buhrmester et al., 2000; Lewis and Zona, 2000; but see Conn et al., 1985). Similarly, in this study, all samples were spontaneously cyanogenic without the addition of pectinase from Rhizopus spp., indicating that non-cyanogenic individuals probably lacked both the cyanogenic glycoside and β-glycosidase, or possibly that pectinase was not able to catalyse the cyanogenesis in these species. It is perhaps noteworthy that the greater frequency of cyanogenesis reported by Conn et al. (1985) in response to the addition of β-glycosidase (emulsin) was in a survey solely of the genus Acacia, indicating that such a response may vary among taxa.
It is important to note that the frequencies reported in all of these surveys were apparently based on the testing of single specimens of the vast majority of species (Adsersen et al., 1988; Thomsen and Brimer, 1997; Mali and Borges, 2003). Similarly, while the present study aimed to test at least three individuals of each species in duplicate (i.e. with and without added enzyme), only one individual of many species was encountered (Appendix). Furthermore most species here were also tested in both wet and dry seasons. A range of studies report variable positive and negative test results among sample sizes as small as n = 2 (e.g. Kaplan et al., 1983; Thomsen and Brimer, 1997). Given such polymorphism for cyanogenesis (see also Aikman et al., 1996), the reported frequencies in these surveys may underestimate overall the actual proportion of cyanogenic species in plant communities. The reported frequency of cyanogenesis may also vary with the plant part tested. In the Costa Rican study, Thomsen and Brimer (1997) reported a greater frequency of cyanogenesis among reproductive plant parts than leaves, as did Buhrmester et al. (2000) in populations of Sambucus canadensis (elderberry) in Illinois. Consistent with that trend, individuals of species with weakly cyanogenic leaves, including some which produced negative FA paper results, had higher concentrations of glycosides in flowers or fruits; however, overall, only a small number of reproductive tissues were tested, so limited comparison can be drawn (Table 1).
The frequency of cyanogenesis varies between taxonomic groups and with life form. Cyanogenesis is considered especially common in some plant families (e.g. Rosaceae, Euphorbiaceae, Passifloraceae and Proteaceae; Lechtenberg and Nahrstedt, 1999), and rare or absent in others (e.g. Lauraceae and Araliaceae; Gibbs, 1974; Hegnauer, 1989), a trend which may in part reflect differential intensity of testing among taxonomic groups. In this study, the frequency of cyanogenesis among the dominant plant families varied. In the Proteaceae family, five of 20 (25 %) species were cyanogenic, while two of 29 (6·9 %) species in the Sapindaceae, and one of 39 (2·5 %) species in the Lauraceae were cyanogenic (Table 1). In a screening of Australian Acacia, 6·9 % of 360 species were cyanogenic (Conn et al., 1985). Cyanogenesis appears to be rare among palms; only two species (1·2 %) of 155 species of palms (108 genera) were found to be cyanogenic (Lewis and Zona, 2000). No cyanogenic palm species were identified in this study.
Concentrations of cyanogenic glycosides
Several of the 18 cyanogenic species detected in this study contained concentrations of cyanogenic glycosides among the highest reported for leaves of woody species. Most notably, tree species E. sericopetalus, C. grayi and P. turneriana had foliar concentrations of cyanogenic glycosides up to 5·2, 4·9 and 4·8 mg CN g−1 d. wt, respectively (Table 1). Similarly, Webber (1999) recorded concentrations up to 5 mg CN g−1 d. wt in the tree species R. javanica; individuals of that species occurring within the survey area of this study had a lower mean concentration of 1·8 mg CN g−1 d. wt (Table 1). These high concentrations are substantially greater than the majority of values reported for foliage from a range of tropical and temperate taxa. For example, concentrations up to 1·1 mg HCN g−1 d. wt were reported in the tropical shrub Turnera ulmifolia (Shore and Obrist, 1992; Schappert and Shore, 1999), while the highest concentrations in naturally occurring populations of Australian Eucalyptus spp. were 2·59 mg CN g−1 d. wt and 3·16 mg CN g−1 d. wt for E. cladocalyx and E. yarraensis, respectively (Gleadow and Woodrow, 2000a; Goodger and Woodrow, 2002). Foliar concentrations of between 1·66 and 3·78 mg HCN g−1 d. wt have been recorded in cultivated Prunus spp. (Santamour, 1998). To our knowledge, possibly the highest foliar cyanogenic glycoside concentration among naturally occurring woody species was reported in the tropical proteaceous species Panopsis costaricensis by Thomsen and Brimer (1997), who measured 2150 mg HCN kg−1 f. wt (approximately equivalent to 7·2 mg HCN g−1 d. wt using a conversion based on the mean foliar water content of several species in this study, which was 70 %). The age of the leaves analysed was not specified, however, and it should also be noted that this value was determined using picrate papers and reflectrometry, a less dependable method more sensitive to the presence of interfering substances (Brinker and Seigler, 1989).
Assessing the ecological significance of the range of cyanogenic glycoside concentrations recorded here is difficult. While the high concentrations in several species (e.g. >2 mg CN g−1 d. wt; Table 1) would almost certainly constitute toxic levels important in defence—plants with >600 µg HCN g−1 d. wt are considered potentially dangerous to livestock, for example (Haskins et al., 1987)—the ecological significance of lower concentrations (e.g. approx. 8–50 µg CN g−1 d. wt) is harder to determine. This reflects the fact that despite the well-documented effectiveness of cyanogenesis in defence against generalist herbivores (e.g. Jones, 1998; Gleadow and Woodrow, 2002), overall there is no known particular concentration at which cyanogenic compounds are effective in herbivore deterrence. This is in part because unfortunately many herbivory studies only report the presence or absence of cyanogenesis, and not the actual concentrations of cyanogenic glycosides. Moreover, the efficacy of cyanogenesis as a defence depends not only on the concentration of cyanogenic glycosides, but also on the physiology, morphology and behaviour of the consumer (Gleadow and Woodrow, 2002).
Intra-plant variation in cyanogenic glycoside content
In tropical forests, it is estimated that up to 70 % of a leaf's lifetime damage occurs while expanding (Coley and Barone, 1996). This differential intensity of herbivory among old and young leaves, and the frequent observation that defence compounds tend to be concentrated in plant tissues of higher value to reproduction or growth (e.g. young leaves) is summarized in the Optimal Allocation Theory (OAT) of defence. This theory predicts that the most vulnerable and valuable plant parts—those susceptible to attack and most likely to contribute to growth and reproductive fitness such as reproductive structures and young leaves—will be more defended (McKey, 1974; Rhoades, 1979).
Results here add to the already substantial body of work on mostly temperate cyanogenic species consistent with the predictions of the OAT (e.g. Martin et al., 1938; Dement and Mooney, 1974; Cooper-Driver et al., 1977; Shore and Obrist, 1992; Dahler et al., 1995; Thomsen and Brimer, 1997; Gleadow et al., 1998; Gleadow and Woodrow, 2000b). In all species where young leaves were sampled, they contained significantly higher concentrations of cyanogenic glycosides than old leaves. This trend was most apparent in species that had low cyanogen content in old leaves (e.g. C. sublimis, C. myrianthus, O. heterophylla and P. australiana) (Table 1). Moreover, in some cases, individuals of these species appeared only to invest in cyanogenic glycoside defence in young leaves; acyanogenic old leaves were seemingly reliant more on physical toughness. Thus, leaf age is an important consideration when assigning the cyanogenic phenotype.
Again consistent with the OAT, reproductive tissues such as floral buds, flowers and fruits/seeds tended to have high total cyanogen content (Table 1). This pattern has been commonly reported among cyanogenic species (e.g. Spencer and Seigler, 1983; Selmar et al., 1991; Selmar, 1993b; Thomsen and Brimer, 1997; Webber, 1999). One notable exception to this was the low to negligible concentrations of cyanogenic glycosides in mature seeds of C. sublimis; unlike the fleshy seeds of many rainforest species, C. sublimis seeds are dry and papery. The absence of cyanogenesis in mature seeds of proteaceous Grevillea spp. was reported by Lamont (1993) in species with cyanogenic foliage and flowers. The higher concentrations in floral tissues of C. sublimis is consistent with previous reports for proteaceous species which tend to have high concentrations of cyanogenic glycosides in flowers, while leaves may have low total cyanogen content or be acyanogenic (e.g. Smith and White, 1918; Tjon Sie Fat, 1979a; Lamont, 1993).
Intra-population variation in cyanogenic glycoside content
All individuals of the majority of species were cyanogenic, albeit with low concentrations of cyanogenic glycosides in some instances. Negative results with FA papers for old leaves were only obtained for individuals of three of the 18 cyanogenic species. In the population of one of these species, B. platynema, 50 % of individuals were determined to be acyanogenic, with cyanogenic glycoside concentrations much less than the 8 µg CN g−1 d. wt threshold. The two other exceptions were C. myrianthus and P. australiana. Unlike B. platynema, cyanogenesis in individuals of these species varied qualitatively with leaf age and plant part. Thus, assigning the acyanogenic phenotype in these species was problematic.
This developmental trend towards differences in expression of cyanogenic potential has been reported previously; cyanogenesis is known be affected by plant age, growth phase, as well as the plant part used (Jones, 1972; Gibbs, 1974; Seigler, 1991). Consequently, as noted earlier, studies have reported a greater frequency of cyanogenesis when testing reproductive tissues, young foliage and shoots compared with old leaves (e.g. Gibbs, 1974; Aikman et al., 1996; Thomsen and Brimer, 1997; Buhrmester et al., 2000; Mali and Borges, 2003). These findings emphasize the importance of only comparing leaves of a similar age when classifying individuals according to the presence or absence of cyanogenesis.
Aside from the three species mentioned above, no acyanogenic individuals were identified in populations of other cyanogenic species. While the small sample sizes for most species reduced the likelihood of encountering an acyanogenic individual, even within populations of the more abundant species such as B. collina (n = 46 all sites), C. sublimis (n = 31 at all sites) and R. javanica (n = 249; Webber, 1999), no acyanogenic individuals were detected. In the latter example, the quantitative screening of >800 individuals of R. javanica failed to detect an acyanogenic individual in several distinct populations (Webber, 2005). This number (800) was substantially greater than the number of individuals predicted by Gleadow and Woodrow (2000a) and Goodger et al. (2002) (n = 95–100) that would need to be sampled to capture an acyanogenic individual assuming a similar genetic system for cyanogenesis to Trifolium repens (Hughes et al., 1988) and an estimate of the rarity of a species (or polymorph) (McArdle, 1990). The floristic heterogeneity of the rainforest makes sampling large populations challenging; it is noteworthy, however, that others have detected polymorphism for cyanogenesis in tropical studies based on very small sample sizes (e.g. n = 2) (Kaplan et al., 1983; Thomsen and Brimer, 1997). These acyanogenic morphs, determined only by indicator paper tests in these studies, were not verified by quantitative assay as for B. platynema here.
Conclusions
In summary, the findings of this survey indicate that cyanogenesis is an important, yet little studied, chemical defence in tropical rainforests. The identification of specific cyanogens in but a few of the cyanogenic species first reported here has yielded novel findings. Given the large number of new reports for species belonging to plant families or orders in which cyanogenesis has been little reported, the ongoing characterization of cyanogenic constituents in these species will potentially be of both phytochemical and chemotaxonomic significance. In addition, preliminary data on intra-population variation in cyanogenesis here suggest that ontogenetic variation in cyanogenesis, and polymorphism for cyanogenesis merit further investigation in tropical rainforest species.
Acknowledgments
The authors thank Professor Eric Conn (University of California, Davis) for sharing unpublished data on the Proteaceae family, Nicole Middleton and volunteers at the University of Melbourne Herbarium for mounting and databasing specimens, Andrew Ford (CSIRO Tropical Forest Research Centre, Atherton, Queensland) for assistance with species name changes, and Wendy Cooper and Steve McKenna for additional taxonomic assistance during field work. We also thank Dr John Kanowski (Griffith University, Queensland) and Andrew Graham (TFRC, Atherton) for advice in the selection of field sites, and Marisa Collins, Bruce Webber, Ross Waller, Jennifer Fox, Gavin Smith and Joel Youl for assistance in the field. We thank the Australian Canopy Crane Research Facility for access to forest on the site. Field work was supported by an Individual Award from the Queen's Trust for Young Australians to R.E.M., and was conducted under the Queensland Department of Environment and Heritage Scientific Purposes Permit number F1/000270/99/SAA, and Department of Natural Resources Permits to Collect numbers 1542, 1716, 1409 and 1663.
APPENDIX
Summary of all species tested for cyanogenesis within 6 × 200 m2 plots at five sites in upland/highland (U) or lowland (L) tropical rainforest, on sites contrasting in soil type: basalt sites at Lamins Hill (B1) and Longlands Gap (B2), and sites on granite at Mt Nomico (G), on rhyolite at Longlands Gap (R) and on metamorphic substrate near Cape Tribulation (M). Species are listed in alphabetical order. Life forms: tree (T), shrub (SH), herb (H), vine (V), treefern (TF), palm (P) and hemi-epiphyte (HE). The results (+ or –) for tests using Feigl–Anger papers and approx. 1 g f. wt leaf tissue, with and without the addition of pectinase (+/– enz) for n individuals of each species are listed. All tests were carried out using most recently fully expanded leaves and in most instances also using young leaves. In some cases, leaf tips (tips), only a few fruit (ft) or flowers (flwr) were tested. Previous findings for species and in some cases genera are noted (e.g. Proteaceae species tested by E. E. Conn, University of California, Davis, CA, USA, pers. comm. based on herbarium specimens). Species included in phytochemical screenings of Queensland rainforest taxa (alkaloids, CN; Webb, 1948, Webb, 1949) are noted, most were not tested for CN (DNT). Canopy species or rare species for which no sample was obtained were included in floristic analysis, but were not tested for cyanogenesis (DNT). Lodgement numbers at Brisbane (BRI) and The University of Melbourne (MELU) herbaria are given.
Table 2.
Appendix
| Lifeform | Species | Family | HCN +/– enz (n tested) | Upland/lowland | Site (substrate) | Previous reports | Lodgement no. |
|---|---|---|---|---|---|---|---|
| T | Acacia celsa Tindale | Mimosaceae | −/− (3) | U | R | Webb (1949) DNT | |
| T | Acmena graveolens (F.M.Bailey) L.S.Sm. | Myrtaceae | −/− (3) | L | M | MELU102318 | |
| T | Acmena resa B.Hyland | Myrtaceae | −/− (2) | U | B2, R | ||
| T | Acronychia acidula F.Muell. | Rutaceae | −/− (2) | U | G | Webb (1949) DNT | |
| T | Acronychia acronychioides (F.Muell.) T.G.Hartley | Rutaceae | −/− (3) | U | G | ||
| T | Acronychia crassipetala T.G.Hartley | Rutaceae | −/− (1) | U | B2 | ||
| SH | Acronychia parviflora C.T.White | Rutaceae | −/− (5) | U | B1, R | ||
| SH | Actephila sp. (Wooroonooran NP P.I.Forster+ PIF17151) | Euphorbiaceae | −/− (5) | U | B1 | ||
| T | Adenanthera pavonina L. | Mimosaceae | −/− (1) | L | M | ||
| T | Agathis atropurpurea B.Hyland | Araucariaceae | −/− (3) | U | R | ||
| T | Agathis microstachya J.F.Bailey & C.T.White | Araucariaceae | −/− (1) | U | G | ||
| T | Agathis robusta (C.Moore ex F.Muell.) F.M.Bailey | Araucariaceae | −/− (1) | U | B1 | ||
| SH | Aglaia meridionalis C.M.Pannell | Meliaceae | −/− (13) | U, L | B1, M | MELU102332 | |
| T | Aglaia sapindina (F.Muell.) Harms | Meliaceae | −/− (2) | L | M | ||
| T | Aglaia tomentosa Teijsm. & Binn. | Meliaceae | −/− (5) | U, L | B1, B2, G, M | MELU102275 | |
| T | Alangium villosum subsp. polyosmoides (F.Muell.) Bloemb. | Alangiaceae | −/− (3) | U | B1 | Webb (1949) DNT | MELU102329 |
| T | Alloxylon flammeum P.H.Weston & Crisp | Proteaceae | −/− (2) | U | B2 | ||
| T | Alloxylon wickhamii (W.Hill ex F.Muell.) P.H.Weston & Crisp | Proteaceae | −/− (4) | U | G, B2 | ||
| H | Alocasia brisbanensis (F.M.Bailey) Domin‡ | Araceae | +/+ | U | B1 | Hurst (1942) | |
| T | Alphitonia whitei Braid | Rhamnaceae | −/− (3) | U | G | MELU102103 | |
| H | Alpinia arctiflora (F.Muell.) Benth. | Zingiberaceae | −/− (1) | U | B1 | ||
| H | Alpinia modesta F.Muell. ex K.Schum. | Zingiberaceae | −/− (1) | U | B2 | ||
| T | Alstonia scholaris (L.) R.Br. | Apocynaceae | −/− (10) | L | M | Gibbs (1974) reported +CN; Webb (1949) DNT | BRI 578809, MELU102127, 102257 |
| SH | Alyxia ilicifolia subsp. ilicifolia F.Muell. | Apocynaceae | −/− (3) | U | G | ||
| V | Alyxia spicata R.Br. | Apocynaceae | −/− (2) | U | B1, R | Webb (1949) DNT | |
| T | Antidesma erostre F.Muell. ex Benth. | Euphorbiaceae | −/− (6) | U, L | B1, G, R, M | MELU102113 | |
| SH | Antirhea sp. (Mt Missery L.W.Jessup+ GJD 3136) | Rubiaceae | −/− (3) | U | B2 | ||
| T | Antirhea tenuiflora F.Muell. ex Benth. | Rubiaceae | −/− (4) | U, L | B1, G, M | ||
| T | Apodytes brachystylis F.Muell. | Icacinaceae | −/− (6) | U | B1, G, R | MELU102188 | |
| T | Archidendron ramiflorum (F.Muell.) Kosterm. | Mimosaceae | −/− (4) | U, L | B1, M | ||
| T | Archidendron vaillantii (F.Muell.) F.Muell. | Mimosaceae | −/− (3) | U | B2, G | MELU102186 | |
| T | Archidendron whitei I.C.Nielsen | Mimosaceae | −/− (1) | U | B1 | ||
| SH | Ardisia bifaria C.T.White & W.D.Francis | Myrsinaceae | −/− (2) | U | B1 | ||
| SH | Ardisia brevipedata F.Muell. | Myrsinaceae | −/− (7); frt – | U, L | B1, B2, G, R, M | MELU102148 | |
| T | Argyrodendron sp. (Boonjee BH 2139RFK) | Sterculiaceae | −/− (9) | U | B1 | ||
| T | Arytera pauciflora S.T.Reynolds | Sapindaceae | −/− (4) | U, L | B1, M | ||
| SH | Atractocarpus hirtus (F.Muell.) Puttock | Rubiaceae | −/− (7) | U, L | B1, M | Webb (1949) DNT | |
| SH | Atractocarpus merikin (F.M.Bailey) Puttock | Rubiaceae | −/− (3) | U | B1, B2 | ||
| T | Auranticarpa papyracea L.Cayzer, Crisp & I.Telford | Pittosporaceae | −/− (2) | U | B2, G | ||
| V | Austrobaileya scandens C.T.White | Austrobaileyaceae | −/− (6); flwr – | U | B1 | ||
| SH | Austromatthaea elegans L.S.Sm. | Monimiaceae | −/− (3) | U | G, R | ||
| T | Austromuellera trinervia C.T.White | Proteaceae | −/− (4) | U, L | B1, M | ||
| V | Austrosteenisia stipularis (C.T.White) Jessup | Fabaceae | −/− (4) | U | B1, G | ||
| T | Balanops australiana F.Muell. | Balanopaceae | −/− (3) | U | R, G | Webb (1949) DNT | MELU102216, 102108 |
| T | Beilschmiedia bancroftii (F.M. Bailey) C.T.White | Lauraceae | −/− (3) | U, L | B1, G, M | Webb (1949) DNT | MELU102219 |
| T | Beilschmiedia castrisinensis B.Hyland | Lauraceae | −/− (2) | L | M | ||
| T | Beilschmiedia collina B.Hyland | Lauraceae | +/+ | U | B1, B2, G, R | BRI 578804, MELU102299, 102300 | |
| T | Beilschmiedia oligandra L.S.Sm. | Lauraceae | −/− (3) | U | G | ||
| T | Beilschmiedia recurva B.Hyland | Lauraceae | −/− (4) | U | B1, G | ||
| T | Beilschmiedia tooram (F.M.Bailey ) B.Hyland | Lauraceae | −/− (10) | U | B1, B2, G | MELU102211 | |
| T | Bobea myrtoides (F.Muell.) Valeton | Rubiaceae | −/− (8) | U | G, R | MELU102105, 102110 | |
| SH | Bowenia spectabilis Hook. ex Hook.f. | Stangeriaceae | −/− (6) | U, L | B1, M | ||
| T | Brachychiton acerifolius (A.Cunn. ex G.Don.) Macarthur | Sterculiaceae | −/− (3) | U, L | B2, M | ||
| T | Brackenridgea australiana F.Muell. | Ochnaceae | −/− (4) | U, L | G, R, M | MELU102322 | |
| SH/T | Breynia cernua (Poir.) Muell.Arg | Euphorbiaceae | −/− (2) | U | B2, G | Webb (1949) 1948 B. oblongifolia Muell. Arg. +CN | |
| T | Brombya platynema F.Muell. | Rutaceae | +/+ | L | M | Webb (1949) DNT | BRI 578818. MELU102283, 102313 |
| T | Bubbia semecarpoides (F.Muell.) B.L.Burtt | Winteraceae | −/− (5) | U | B1, G | ||
| V (P) | Calamus australis Mart. | Arecaceae | −/− (1); | U | B1 | ||
| V | Calamus moti F.M.Bailey | Arecaceae | −/− (1) | U | B1 | ||
| T | Caldcluvia australiensis (Schltr.) Hoogland | Cunoniaceae | −/− (1) | U | B1 | ||
| T | Calophyllum costatum F.M.Bailey | Clusiaceae | −/− (3) | U | B2, R | ||
| T | Cananga odorata (Lam.) Hook.f. & Thomson | Annonaceae | −/− (2) | L | M | ||
| T | Canarium muelleri F.M.Bailey | Burseraceae | −/− (1) | U | G | MELU102250 | |
| T | Canthium sp. | Rubiaceae | −/− (1) | U | G | ||
| T | Carallia brachiata (Lour.) Merr. | Rhizophoraceae | −/− (1) | L | M | ||
| T | Cardwellia sublimis F.Muell. | Proteaceae | +/+ | U, L | B1, B2, G, R, M | E. E. Conn (pers. comm.) 1 of 7 +ve | BRI 578806, MELU102280, 102281 |
| T | Carnarvonia araliifolia var. montana B.Hyland | Proteaceae | −/− (3) | U | B2, G, R | E. E. Conn (pers. comm.) –ve | MELU102282 |
| V | Carronia protensa (F.Muell.) Diels | Menispermaceae | −/− (5) | U, L | B1, B2, M | MELU102213 | |
| T | Casearia costulata L.W.Jessup | Salicaceae† | −/− (4) | U | B1, B2, G, R | Webb (1949) DNT | |
| T | Casearia dallachii F.Muell. | Salicaceae† | −/− (1) | L | M | ||
| T | Casearia grayi L.W.Jessup | Salicaceae† | −/− (2) dry* | U | B2 | ||
| T | Castanospermum australe A.Cunn. & C.Fraser ex Hook. | Fabaceae | −/− (3) | L | M | Webb (1949) DNT | |
| T | Castanospora alphandii (F.Muell.) F.Muell. | Sapindaceae | −/− (3) | U | B1, B2 | MELU102130 | |
| V | Cayratia saponaria (Seem. & Benth.) Domin | Vitaceae | −/− (1) | L | M | C. acrisGibbs (1974) –CN | |
| T | Celtis paniculata (Endl.) Planch. | Ulmaceae | −/− (1) | L | M | ||
| V | Cephalaralia cephalobotrys (F.Muell.) Harms | Araliaceae | −/− (2) | U | B1, B2 | ||
| T | Ceratopetalum succirubrum C.T.White | Cunoniaceae | −/− (4) | U | B2, G | Webb (1949) DNT | |
| T | Cerbera floribunda K.Schum. | Apocynaceae | −/− (1) | U, L | G, M | Webb (1949) DNT | |
| T | Chionanthus axillaris R.Br. | Oleaceae | −/− (4) | U | B1, G | MELU102327 | |
| T | Cinnamomum laubatii F.Muell. | Lauraceae | −/− (3) | U | B1, G, R | Webb (1949) DNT | MELU102210 |
| V | Cissus hypoglauca A.Gray | Vitaceae | −/− (2) | U | G | ||
| V | Cissus penninervis (F.Muell.) Planch. | Vitaceae | −/− (1) | U, L | R, M | ||
| V | Cissus sterculiifolia (F.Muell. ex Benth.) Planch. | Vitaceae | −/− (4) | U | B1, B2, G | MELU102325 | |
| V | Cissus vinosa Jackes | Vitaceae | −/− (3) | U | B1, B2, G | MELU102195 | |
| T | Citronella smythii (F.Muell.) R.A.Howard | Icacinaceae | −/− (4) | U, L | B1, G, M | MELU102143 | |
| T | Claoxylon tenerifolium (Baill.) F.Muell. | Euphorbiaceae | −/− (1) | U | B1 | ||
| T | Cleistanthus myrianthus (Hassk.) Kurz | Euphorbiaceae | +/+ | L | M | BRI 578811, MELU102122, 102123, 102258 | |
| T | Clerodendrum grayi Munir | Verbenaceae | +/+ | U | B1, B2, G | Other Clerodendrum spp. +CN (Gibbs, 1974; Tjon Sie Fat, 1979a; Adsersen et al., 1988) | BRI 578815, MELU102115, 102116, 102117 |
| T | Cnesmocarpon dasyantha (Radlk.) Adema | Sapindaceae | −/− (2) | U | G | ||
| V | Connarus conchocarpus F.Muell. | Connaraceae | −/− (1) | L | M | ||
| SH | Cordyline petiolaris (Domin) Pedley | Laxmanniaceae | −/− (2) | U | B1 | ||
| SH | Cordyline sp. | Laxmanniaceae | −/− (1) | U | G | ||
| T | Corynocarpus cribbianus (F.M.Bailey) L.S.Sm. | Corynocarpaceae | −/− (1) | U | B1 | C. laevigata +CN (Webb, 1948) | |
| SH | Crispiloba disperma (S.Moore) Steenis | Alseuosmiaceae | −/− (4) | U | G | ||
| T | Cryptocarya angulata C.T.White | Lauraceae | −/− (4) | U | B1, G, R | MELU102102 | |
| T | Cryptocarya cocosoides B.Hyland | Lauraceae | −/− (3) | U | B2, G | MELU102157, 102268 | |
| T | Cryptocarya corrugata C.T.White & W.D.Francis | Lauraceae | −/− (3) | U | B1, B2, G, R | MELU102147 | |
| T | Cryptocarya densiflora Blume | Lauraceae | −/− (2) | U | G, R | MELU102104, 102269 | |
| T | Cryptocarya grandis B.Hyland | Lauraceae | −/− (3); | U, L | B1, B2, G, M | MELU102247 | |
| T | Cryptocarya hypospodia F.Muell. | Lauraceae | −/− (1) | L | M | ||
| T | Cryptocarya laevigata Blume | Lauraceae | −/− (2) | U, L | G, M | ||
| T | Cryptocarya leucophylla B.Hyland | Lauraceae | −/− (1) | U | B2, R | MELU102248 | |
| T | Cryptocarya lividula B.Hyland | Lauraceae | −/− (2) | U | G, R | MELU102095 | |
| T | Cryptocarya mackinnoniana F.Muell. | Lauraceae | −/− (5) | U, L | B1, G, M | MELU102212 | |
| T | Cryptocarya melanocarpa B.Hyland | Lauraceae | −/− (5) | U | B1, B2 | ||
| T | Cryptocarya murrayi F.Muell. | Lauraceae | −/− (2) | U, L | B1, M | ||
| T | Cryptocarya oblata F.M.Bailey | Lauraceae | −/− (2) | U, L | B1, M | Webb (1949) DNT | MELU102099 |
| T | Cryptocarya pleurosperma C.T.White & W.D.Francis | Lauraceae | −/− (4) | U | B1, G | Webb (1949) DNT | MELU102270 |
| T | Cryptocarya putida B.Hyland | Lauraceae | −/− (4) | U | G, R | MELU102144 | |
| T | Cryptocarya saccharata B.Hyland | Lauraceae | −/− (3) | U | B2, R | ||
| T | Cryptocarya smaragdina B.Hyland | Lauraceae | −/− (3) | U | B2, R | MELU102193 | |
| T | Cupaniopsis flagelliformis (F.M.Bailey) Radlk. var. flagelliformis | Sapindaceae | −/− (4) | U | B1 | MELU102129 | |
| T | Cupaniopsis sp. | Sapindaceae | −/− (1) | L | M | ||
| TF | Cyathea rebeccae (F.Muell.) Domin | Cyatheaceae | −/− (2) | U | G, R | ||
| T | Cyclophyllum multiflorum S.T.Reynolds & R.J.F.Hend. | Rubiaceae | −/− (1) | U | B1 | Canthium vaccinifolium F. Muell. +CN Webb (1949) 1948 | |
| T | Daphnandra repandula (F.Muell.) F.Muell. | Monimiaceae | −/− (2) | U | B1, B2, G | ||
| T | Darlingia darlingiana (F.Muell.) L.A.S.Johnson | Proteaceae | −/− (2) | U | G | E. E. Conn (pers. comm.) –ve | |
| T | Darlingia ferruginea J.F.Bailey | Proteaceae | −/− (3)– | U | B1, B2 | E. E. Conn (pers. comm.) –ve | |
| T | Davidsonia pruriens F.Muell. | Davidsoniaceae | −/− (2) | U | G | Webb (1949) DNT; +CN (Rosenthaler, 1929; Hurst, 1942); –CN Gibbs (1974) | MELU102151, 102152 |
| T | Decaspermum humile (G.Don.) A.J.Scott | Myrtaceae | −/− (1) dry* | L | M | ||
| T | Delarbrea michieana (F.Muell.) F.Muell. | Araliaceae | −/− (4) | U | B1, G | ||
| V | Dendrotrophe varians (Blume) Miq. | Santalaceae | −/− (1) | U | R | ||
| V | Derris sp. (Daintree D.E. Boyland+469) | Fabaceae | −/− (1) dry* | L | M | ||
| V | Desmos goezeanus (F.Muell.) Jessup | Annonaceae | −/− (2) | U | G | ||
| V | Dichapetalum papuanum (Becc.) Boerl. | Dichapetalaceae | −/− (4) | U | B1 | MELU102326 | |
| T | Dinosperma stipitatum (C.T.White & W.D.Francis) T.G. Hartley | Rutaceae | −/− (3) | U | B1 | ||
| T | Diospyros cupulosa F.Muell. | Ebenaceae | −/− (1) | L | M | ||
| T | Diospyros hebecarpa A.Cunn. ex Benth. | Ebenaceae | −/− (1) dry* | U | B1 | MELU102307 | |
| T | Diploglottis bernieana S.T.Reynolds | Sapindaceae | −/− (2) | L | M | ||
| T | Diploglottis bracteata Leenh. | Sapindaceae | −/− (1) | U | G | ||
| T | Doryphora aromatica (F.M.Bailey) L.S.Sm. | Monimiaceae | −/− (5) | U, L | B1, B2, M | Webb (1949) DNT | |
| T | Dysoxylum alliaceum Blume (Blume) | Meliaceae | −/− (1) | L | M | ||
| T | Dysoxylum arborescens (Blume) Miq. | Meliaceae | −/− (2) | U, L | B1, M | ||
| T | Dysoxylum klanderi F.Muell. | Meliaceae | −/− (3) | U | B1, B2, R | ||
| T | Dysoxylum oppositifolium F.Muell. | Meliaceae | −/− (3) | U | B2, G | MELU102273 | |
| T | Elaeocarpus bancroftii F.Muell. & F.M.Bailey | Elaeocarpaceae | −/− (3) | L | M | ||
| T | Elaeocarpus eumundi F.M.Bailey | Elaeocarpaceae | −/− (1) | U | G, R | MELU102125 | |
| T | Elaeocarpus foveolatus F.Muell. | Elaeocarpaceae | −/− (2) | U | B1, R | ||
| T | Elaeocarpus grahamii F.Muell. | Elaeocarpaceae | −/− (1) | L | M | ||
| T | Elaeocarpus largiflorens C.T.White subsp. largiflorens | Elaeocarpaceae | −/− (4) | U | B1, G | ||
| T | Elaeocarpus ruminatus F.Muell. | Elaeocarpaceae | −/− (3) | U | B2, G | ||
| T | Elaeocarpus sericopetalus F.Muell. | Elaeocarpaceae | +/+ | U | G, R | BRI 578817, MELU102135, 102304 | |
| T | Elaeocarpus sp. (Mt Bellenden Ker LJB 18336) | Elaeocarpaceae | −/− (3) | U | B2, G, R | MELU102304 | |
| V | Embelia caulialata S.T.Reynolds | Myrsinaceae | −/− (1) dry* | L | M | ||
| V | Embelia grayi S.T.Reynolds | Myrsinaceae | +/+ | U | B1, B2 | BRI 578803, MELU102267 | |
| T | Endiandra bessaphila B.Hyland | Lauraceae | −/− (2) | U | B1 | ||
| T | Endiandra dielsiana Teschn. | Lauraceae | −/− (2) | U | G | ||
| T | Endiandra hypotephra F.Muell. | Lauraceae | DNT | L | M | ||
| T | Endiandra leptodendron B.Hyland | Lauraceae | −/− (7) | U, L | B1, M | MELU102293 | |
| T | Endiandra microneura C.T.White | Lauraceae | −/− (3) | L | M | ||
| T | Endiandra monothyra B.Hyland subsp. monothyra | Lauraceae | −/− (3) | U | B1, B2 | ||
| T | Endiandra montana C.T.White | Lauraceae | DNT | U | B2 | ||
| T | Endiandra palmerstonii (F.M.Bailey) C.T.White & W.D.Francis | Lauraceae | −/− (2) | U | B2, G | Webb (1949) DNT | MELU102294 |
| T | Endiandra sankeyana F.M.Bailey | Lauraceae | −/− (3) | U | B1, B2 | MELU102155 | |
| T | Endiandra sideroxylon B.Hyland | Lauraceae | −/− (3) | U | B2 | MELU102109 | |
| T | Endiandra wolfei B.Hyland | Lauraceae | −/− (3); sht – | U | B1, G | MELU102295 | |
| T | Endospermum myrmecophilum L.S.Sm. | Euphorbiaceae | −/− (1) dry * | L | M | ||
| V | Entada phaseoloides (L.) Merr. | Mimosaceae | −/− (1) | L | M | ||
| HE | Epipremnum pinnatum (L.) Engl. | Araceae | −/− (2) | L | M | ||
| V | Erycibe coccinea (F.M.Bailey) Hoogl. | Convolvulaceae | −/− (3) | L | M | ||
| T | Erythroxylum ecarinatum Burck ex Hoch. | Erythroxylaceae | −/− (2) | U | B1 | Webb (1949) DNT | MELU102225 |
| SH | Eupomatia barbata Jessup | Eupomatiaceae | −/− (2) dry* | U, L | B1, M | ||
| SH/T | Eupomatia laurina R.Br. | Eupomatiaceae | −/− (4) | U | B2, G | Gibbs (1974) ‘doubtfully cyanogenic’; Webb (1949) DNT | MELU102331 |
| V | Eustrephus latifolius R.Br. ex Ker Gawl. | Philesiaceae | −/− (2) | U, L | B1, M | ||
| T | Fagraea cambagei Domin | Gentianaceae | −/− (4) | L | M | MELU102317 | |
| T | Fagraea fagraeacea (F.Muell.) Druce | Gentianaceae | −/− (3) | U | B1, B2, G, R | MELU102323 | |
| T | Ficus congesta Roxb. | Moraceae | −/− (1) | L | M | ||
| T | Ficus crassipes F.M.Bailey | Moraceae | −/− (1) | U | B1 | ||
| T | Ficus destruens F.Muell. ex C.T.White | Moraceae | −/− (1) | U | B2, G | ||
| T | Ficus leptoclada Benth. | Moraceae | DNT | U | B1, B2 | MELU102107 | |
| V | Ficus pantoniana King | Moraceae | −/− (1) | L | M | ||
| T | Ficus pleurocarpa F.Muell. | Moraceae | −/− (1) | U | B1 | ||
| T | Ficus triradiata Corner | Moraceae | −/− (1) | L | M | ||
| V | Flagellaria indica L. | Flagellariaceae | +/+ | U, L | B1, G, M | +CN (1912) | BRI 578816, MELU102259, 102296 |
| T | Flindersia bourjotiana F.Muell. | Rutaceae | −/− (4) | U, L | B2, G, R, M | Webb (1949) DNT | |
| T | Flindersia brayleyana F.Muell. | Rutaceae | −/− (1) | U | B2 | Webb (1949) DNT | |
| T | Flindersia laevicarpa C.T.White & W.D.Francis | Rutaceae | −/− (2) | U | G | MELU102222 | |
| T | Flindersia pimenteliana F.Muell. | Rutaceae | −/− (2) | U | G, R | Webb (1949) DNT | MELU102132, 102106 |
| T | Fontainea picrosperma C.T.White | Euphorbiaceae | −/− (2) | U | B2 | ||
| T | Franciscodendron laurifolium (F.Muell.) B.Hyland & Steenis | Sterculiaceae | −/− (10) | U | B1, G | ||
| V | Freycinetia excelsa F.Muell. | Pandanaceae | −/− (3) | U | B1, B2 | ||
| V | Freycinetia scandens Gaudich. | Pandanaceae | −/− (1) | U | B1 | ||
| T | Galbulimima baccata F.M.Bailey | Himantandraceae | −/− (3) | U | B1, B2, G, R | Webb (1949) DNT; fruits –CN (Bailey, 1909 in Webb, 1948) | |
| T | Garcinia gibbsiae S.Moore | Clusiaceae | −/− (7) | U | B1 | MELU102126 | |
| T | Garcinia warrenii F.Muell. | Clusiaceae | −/− (4) | L | M | ||
| T | Gardenia ovularis F.M.Bailey | Rubiaceae | −/− (5) | U, L | G, M | Webb (1949) DNT | MELU102319 |
| T | Gevuina bleasdalei (F.Muell.) Sleumer | Proteaceae | −/− (3) | U | B1, G | E. E. Conn (pers. comm.) –ve | MELU102292 |
| T | Gillbeea adenopetala F.Muell. | Cunoniaceae | −/− (3) | U | B1, G | ||
| T | Glochidion harveyanum Domin var. harveyanum | Euphorbiaceae | −/− (1) | U | G | Webb (1949) DNT | |
| T | Glochidion hylandii Airy Shaw | Euphorbiaceae | −/− (1) | U | B2, G | Webb (1949) DNT | |
| T | Gmelina fasciculiflora Benth. | Lamiaceae | −/− (2) | U, L | G, M | Webb (1949) DNT | MELU102261, 102262,102263 |
| T | Gomphandra australiana F.Muell. | Icacinaceae | −/− (2) | L | M | MELU102333 | |
| T | Goniothalamus australis Jessup | Annonaceae | −/− (6) | U | B1 | MELU102185, 102290 | |
| T | Gossia dallachiana (F.Muell.) N.Snow & Guymer | Myrtaceae | −/− (5) | U | B1, B2, G | MELU102220 | |
| T | Gossia myrsinocarpa (F.Muell.) N.Snow & Guymer | Myrtaceae | −/− (1) | U | B2 | ||
| T | Gossia grayii N.Snow & Guymer | Myrtaceae | −/− (2) | U | G | MELU102146 | |
| T | Grevillea baileyana McGill. | Proteaceae | −/− (1) | L | M | Webb (1952); Hurst (1942)Grevillea spp. +CN; E. E. Conn (pers. comm.) –ve | |
| T | Guioa acutifolia Radlk. | Sapindaceae | −/− (2) | U, L | B2, M | ||
| T | Guioa lasioneura Radlk. | Sapindaceae | −/− (2) | U | B1, G | ||
| T | Guioa montana C.T.White | Sapindaceae | −/− (2) | U | R | MELU102150 | |
| SH | Gymnostachys anceps R.Br. | Araceae | −/− (2) | U, L | B2, M | ||
| V | Gynochtodes sp. (Lamb Range J.W.398) | Rubiaceae | −/− (2) | U | G, R | ||
| T | Halfordia kendack (Montrouz.) Guillaumin | Rutaceae | −/− (3) | U | B1, B2, G, R | Webb (1949) DNT | MELU102112 |
| SH | Haplostichanthus sp. (Coopers Creek B. Gray 2433) | Annonaceae | −/− (7) | L | M | MELU102291 | |
| SH | Haplostichanthus sp. (Topaz L.W.Jessup 520) | Annonaceae | −/− (7) | U | B1 | MELU102328 | |
| SH | Harpullia frutescens F.M.Bailey | Sapindaceae | −/− (1) | U | B2 | ||
| SH/T | Harpullia rhyticarpa C.T.White & W.D.Francis | Sapindaceae | −/− (10) | U, L | B1, G, M | Webb (1949) DNT | |
| T | Hedycarya loxocarya (Benth.) W.D.Francis | Monimiaceae | −/− (4) | U | G | ||
| T | Helicia australasica F.Muell. | Proteaceae | +/+ | L | M | E. E. Conn (pers. comm.) flwr +ve; H. robusta +CN Pammel, 1911 in Webb (1949) 1948; Gibbs (1974) | BRI 578805, MELU102284 |
| T | Helicia blakei Foreman | Proteaceae | +/+ | U | B1 | E. E. Conn (pers. comm.) –ve | BRI 578807, MELU102285 |
| T | Helicia lamingtoniana (F.M.Bailey) C.T.White ex L.S.Sm. | Proteaceae | −/− (3) | U | B2 | E. E. Conn (pers. comm.) –ve | MELU102214 |
| T | Hernandia albiflora (C.T.White) Kubitzki | Hernandiaceae | −/− (4) | L | M | Webb (1949) DNT | MELU102314 |
| V | Hibbertia scandens (Willd.) Dryand. | Dilleniaceae | −/− (1) | U | R | ||
| V | Hippocratea barbata F.Muell. | Celastraceae | −/− (4) | U, L | B1, M | ||
| V | Hoya sp. | Asclepiadaceae | −/− (1) | L | M | ||
| T | Hylandia dockrillii Airy Shaw | Euphorbiaceae | −/− (2) | U | B1 | ||
| V | Hypserpa decumbens (Benth.) Diels | Menispermaceae | −/− (4) | U | B1, G | MELU102316 | |
| V | Hypserpa laurina (F.Muell.) Diels | Menispermaceae | −/− (2) | L | M | Webb (1949) DNT | MELU102276 |
| V | Hypserpa smilacifolia Diels | Menispermaceae | −/− (1) | U | B2 | ||
| T | Hypsophila dielsiana Loes. | Celastraceae | −/− (3) | U | B1 | MELU102311 | |
| T | Irvingbaileya australis (C.T.White) R.A.Howard | Icacinaceae | −/− (2) | U | B1, G | MELU102324 | |
| T | Ixora biflora Fosberg | Rubiaceae | −/− (2) | L | M | ||
| T | Ixora sp. (North Mary LA B.P.Hyland 8618) | Rubiaceae | −/− (3) | U | B1, B2 | ||
| V | Jasminum didymum G.Forst. | Oleaceae | −/− (2) | U | B1, B2, G | ||
| V | Jasminum kajewskii C.T.White | Oleaceae | −/− (2) | U | B2, G | ||
| SH | Lasianthus strigosus Wight | Rubiaceae | −/− (2) | L | M | ||
| SH/T | Leea indica (Burm.f.) Merr. | Leeaceae | −/− (1) | L | M | ||
| SH/T | Lepidozamia hopei (W.Hill) Regel | Zamiaceae | −/− (3) | L | M | ||
| T | Lethedon setosa (C.T.White) Kosterm. | Thymelaeaceae | −/− (2) | U | G | ||
| P(T) | Licuala ramsayi (F.Muell.) Domin | Arecaceae | −/− (3) | L | M | ||
| P(SH) | Linospadix minor (W.Hill) F.Muell. | Arecaceae | −/− (2) | L | M | ||
| T | Litsea bindoniana F.Muell. (F.Muell.) | Lauraceae | −/− (2) | U | G | MELU102097 | |
| T | Litsea connorsii B.Hyland | Lauraceae | −/− (2) | U | G, R | ||
| T | Litsea leefeana (F.Muell.) Merr. | Lauraceae | −/− (7) | U,L | B1, B2, M | MELU102145 | |
| T | Loganiaceae sp. | Loganiaceae | −/− (1) | U | G | ||
| T | Lomatia fraxinifolia F.Muell. ex Benth. | Proteaceae | −/− (2) | U | B2, G | E. E. (Conn (pers. comm.) –ve; L. silaifolia R.Br. ft, flwr +CN Webb (1949) 1948 | MELU102091 |
| T | Macaranga inamoena F.Muell. ex Benth. | Euphorbiaceae | −/− (7) | U | B1 | Webb (1949) DNT | MELU102156 |
| T | Macaranga subdentata Benth | Euphorbiaceae | −/− (1) | L | M | ||
| SH | Mackinlaya confusa Hemsl. | Araliaceae | −/− (1) | U | R | ||
| SH | Mackinlaya macrosciadea (F.Muell.) F.Muell. | Araliaceae | −/− (4) | U | B1, G | Webb (1949) DNT | |
| V | Maesa dependens F.Muell. | Myrsinaceae | −/− (1) | U | R | ||
| SH/T | Melicope broadbentiana F.M.Bailey | Rutaceae | −/− (3) | U | B1, G | MELU102131 | |
| T | Melicope jonesii T.G.Hartley | Rutaceae | −/− (1) | U | B2 | ||
| T | Melicope vitiflora (F.Muell.) T.G.Hartley | Rubiaceae | −/− (1) | U, L | B1, G, M | ||
| V | Melodinus acutiflorus F.Muell. | Apocynaceae | −/− (1) | L | M | Webb (1949) DNT | |
| V | Melodinus australis (F.Muell.) Pierre | Apocynaceae | −/− (4) | U | B1, G, R | ||
| V | Melodinus baccellianus (F.Muell.) S.T.Blake | Apocynaceae | −/− (3) | U | B1, B2 | MELU102230 | |
| V | Melodorum uhrii F.Muell. | Annonaceae | −/− (1) dry* | L | M | ||
| T | Mischarytera lautereriana (F.M.Bailey) H.Turner | Sapindaceae | −/− (2) | U | B2, G, R | MELU102286 | |
| T | Mischocarpus exangulatus (F.Muell.) Radlk. | Sapindaceae | +/+ | U | B1 | BRI 578801, MELU102190, 102288 | |
| T | Mischocarpus grandissimus (F.Muell.) Radlk. | Sapindaceae | +/+ | U, L | G, M | BRI 578802, MELU102287 | |
| T | Mischocarpus lachnocarpus (F.Muell.) Radlk. | Sapindaceae | −/− (3) | U | B2, G | ||
| T | Mischocarpus macrocarpus S.T.Reynolds | Sapindaceae | −/− (3) | U | B1, B2 | ||
| T | Mischocarpus pyriformis (F.Muell.) Radlk. subsp. pyriformis | Sapindaceae | −/− (1) | U | B2 | ||
| T | Monimiaceae Gen.(AQ63687) sp. (Davies Creek L.J.Webb+ 6430) | Monimiaceae | −/− (3) | U | B2, R | ||
| V | Morinda Gen.(AQ124851) sp. (Boonjie L.J.Webb+ 6837A) | Rubiaceae | −/− (3) | U | B1 | ||
| V | Morinda jasminoides A.Cunn. ex Hook. | Rubiaceae | −/− (1) | U | B2, G | Webb (1949)–CN | |
| V | Morinda sp. | Rubiaceae | −/− (1) | U | G | ||
| V | Morinda umbellata L. | Rubiaceae | −/− (1) | U | G | ||
| T | Musgravea heterophylla L.S.Sm. | Proteaceae | −/− (2) | L | M | E. E. Conn (pers. comm.) 1 of 8 +ve | |
| T | Musgravea stenostachya F.Muell. | Proteaceae | −/− (4) | U | G, R | E. E. Conn (pers. comm.) –ve | |
| T | Myristica globosa subsp. muelleri (Warb.) W.J.de Wilde | Myristicaceae | −/− (3) | U | B1 | ||
| T | Myristica insipida R.Br. | Myristicaceae | −/− (3) | L | M | MELU102312 | |
| T | Neisosperma poweri (F.M.Bailey) Fosberg & Sachet | Apocynaceae | −/− (2) | U | B2 | ||
| T | Neolitsea dealbata (R.Br.) Merr. | Lauraceae | −/− (4) | U | B1, B2 | Gibbs (1974) –CN | |
| V | Neosepicaea jucunda (F.Muell.) Steenis | Bignoniaceae | −/− (2) | L | M | ||
| T | Niemeyera prunifera (F.Muell.) F.Muell. | Sapotaceae | −/− (4) | U, L | B1, G, M | MELU102149 | |
| P(T) | Normanbya normanbyi (W.Hill) L.H.Bailey | Arecaceae | −/−(4) | L | M | ||
| T | Opisthiolepis heterophylla L.S.Sm. | Proteaceae | +/+ | U | B1, B2 | E.E. Conn (pers. comm.) flwr and lf +ve | BRI 578808, MELU102298 |
| P(T) | Oraniopsis appendiculata (F.M.Bailey) J.Dransf., A.K.Irvine & N.W.Uhl | Arecaceae | −/− (1) | U | B1 | ||
| V | Pachygone longifolia F.M.Bailey | Menispermaceae | −/− (2) | L | M | ||
| T | Palaquium galactoxylon (F.Muell.) H.J.Lam | Sapotaceae | −/− (1) dry* | L | M | ||
| V | Palmeria scandens F.Muell. | Monimiaceae | −/− (4) | U | G | MELU102194 | |
| SH/T | Pandanus monticola F.Muell. | Pandanaceae | −/− (1) | U | G | ||
| V | Pandorea pandorana (Andrews) Steenis | Bignoniaceae | −/− (1) | U | B2 | Webb (1949) DNT | |
| V | Pararistolochia australopithecurus (F.Muell.) Michael J.Parsons | Aristolochiaceae | −/− (3) | U | B1, B2, G | ||
| V | Parsonsia latifolia (Benth.) S.T.Blake | Apocynaceae | +/+ | U | B1, G, R | Webb (1949) DNT; other Parsonsia spp. –CN | BRI 578800, MELU102128 |
| V | Parsonsia sp. 1 | Apocynaceae | −/− (1) | U | G | ||
| V | Parsonsia langiana F.Muell | Apocynaceae | −/− (1) | U | R | ||
| V | Passiflora sp. (Kuranda BH12896) | Passifloraceae | +/+ | L | M | BRI 578813, MELU102297 | |
| T | Perrottetia arborescens (F.Muell.) Loes. | Celastraceae | −/− (1) | U | G | ||
| SH/T | Pilidiostigma papuanum (Lauterb.) A.J.Scott | Myrtaceae | −/− (3) | L | M | ||
| SH | Pilidiostigma tetramerum L.S.Sm. | Myrtaceae | −/− (4) | U | B1 | MELU102274 | |
| T | Pilidiostigma tropicum L.S.Sm. | Myrtaceae | −/− (2) | U | B1 | ||
| V | Piper caninum Blume | Piperaceae | −/− (3) | L | M | MELU102265 | |
| V | Piper novae-hollandiae Miq. | Piperaceae | −/− (3) | U | B1, G | Webb (1949) DNT | |
| T | Pitaviaster haplophyllus (F.Muell.) T.G.Hartley | Rutaceae | −/− (12) | U, L | B1, G, M | MELU102100, 102187 | |
| SH/T | Pittosporum rubiginosum A.Cunn. | Pittosporaceae | −/− (3) | U, L | B1, B2, G, R, M | P. undulatum (Bailey, 1909 in Webb, 1949) 1948 | MELU102192 |
| T | Pittosporum wingii F.Muell. | Pittosporaceae | −/− (2) | U | G | ||
| T | Placospermum coriaceum C.T.White & W.D.Francis | Proteaceae | −/− (2) | U | G | ||
| T | Podocarpus dispermus C.T.White | Podocarpaceae | −/− (2) | U | B1 | ||
| T | Polyalthia michaelii C.T.White | Annonaceae | DNT | U | B1 | ||
| T | Polyosma alangiacea F.Muell. | Grossulariaceae | −/− (3) | U | G, R | ||
| T | Polyosma hirsuta C.T.White | Grossulariaceae | −/− (1) | U | B1, B2 | ||
| T | Polyosma rhytophloia C.T.White & W.D.Francis | Grossulariaceae | −/− (1) | U | B2, R | Webb (1949) DNT | |
| T | Polyosma rigidiuscula F.Muell. & F.M.Bailey ex F.M. Bailey | Grossulariaceae | −/− (3) | U | B1 | ||
| T | Polyscias australiana (F.Muell.) Philipson | Araliaceae | +/+ | U, L | B1, B2, G, R, M | BRI 578812, MELU102301 | |
| T | Polyscias mollis (Benth.) Harms & C.T.White | Araliaceae | −/− (1) | U | B1 | ||
| T | Polyscias murrayi (F.Muell.) Harms | Araliaceae | −/− (1) | U | B1 | Webb (1949) DNT | |
| SH | Polyscias purpurea C.T.White | Araliaceae | −/− (4) | U | G | MELU102154 | |
| HE | Pothos longipes Schott | Araceae | −/− (4) | U, L | B1, M | ||
| T | Pouteria asterocarpon (P.Royen) Jessup | Sapotaceae | −/− (1) | U | B2 | ||
| T | Pouteria brownlessiana (F.Muell.) Baehni | Sapotaceae | −/− (3) | U, L | B1, B2, G, M | ||
| T | Pouteria castanosperma (C.T.White) Baehni | Sapotaceae | −/− (4) | U | B1, G | ||
| T | Pouteria chartacea (F.Muell. ex Benth.) Baehni | Sapotaceae | −/− (3) 2 dry* | L | M | ||
| T | Pouteria myrsinodendron (F.Muell.) Jessup | Sapotaceae | −/− (7) | U, L | G, M | MELU102221 | |
| T | Pouteria papyracea (P.Royen) Baehni | Sapotaceae | −/− (2) | U | B2 | MELU102308, 102309 | |
| T | Pouteria pearsoniorum Jessup | Sapotaceae | −/− (1) | U | R | ||
| T | Prunus turneriana (F.M.Bailey) Kalkman | Rosaceae | +/+ | U, L | B1, M | BRI 578814, MELU102133, 102134 | |
| H | Pseuderanthemum variabile (R.Br.) Radlk. | Acanthaceae | −/− (1) | L | M | Webb (1949) DNT | |
| T | Pseuduvaria froggattii (F.Muell.) Jessup | Annonaceae | −/− (3) | L | M | MELU102310 | |
| T | Pseuduvaria mulgraveana Jessup var. glabrescens | Annonaceae | −/− (1) | U | G | ||
| SH | Psychotria dallachiana Benth. | Sapindaceae | −/− (1) | U | B2 | ||
| SH | Psychotria loniceroides Sieber ex DC. | Rubiaceae | −/− (2) | U | G | Webb (1949) DNT | |
| SH | Psychotria nematopoda F.Muell. | Rubiaceae | −/− (2) | L | M | ||
| SH | Psychotria sp. | Rubiaceae | −/− (1) | L | M | ||
| SH | Psychotria sp. (Utchee Creek H. Flecker NQNC 5313) | Rubiaceae | −/− (3) | U | G | ||
| SH | Psychotria submontana Domin | Rubiaceae | −/− (2) | U | B1, G | ||
| T | Psydrax laxiflorens S.T.Reynolds & R.J.F.Hend. | Rubiaceae | −/− (2) | U | G | ||
| T | Pullea stutzeri (F.Muell.) Gibbs | Cunoniaceae | −/− (3) | U | G | ||
| SH | Randia sp. (Boonjie L.W.Jessup+ GJM264) | Rubiaceae | −/− (1) | U | B1 | ||
| SH | Randia tuberculosa F.M.Bailey | Rubiaceae | −/− (7) | U | B1, G | MELU102158 | |
| T | Rapanea achradifolia (F.Muell.) Mez | Myrsinaceae | −/− (3) | U | B2, G, R | MELU102217 | |
| SH/T | Rapanea porosa (F.Muell.) Mez | Myrsinaceae | −/− (3) | U, L | R, M | ||
| SH | Rapanea sp. (Atherton K.J.White AQ91778) | Myrsinaceae | −/− (2) | U | R | MELU102260 | |
| HE | Rhaphidophora petrieana A.Hay | Araceae | −/− (3) | L | M | Webb (1949) DNT | |
| T | Rhodamnia blairiana F.Muell. | Myrtaceae | −/− (2) | U | G | ||
| T | Rhodamnia spongiosa (F.M.Bailey) Domin | Myrtaceae | −/− (2) | U | R | ||
| T | Rhodomyrtus pervagata Guymer | Myrtaceae | −/− (3) | U | B2, G, R | ||
| T | Rhysotoechia mortoniana (F.Muell.) Radlk. | Sapindaceae | −/− (1) | U | B2 | ||
| T | Rhysotoechia robertsonii (F.Muell.) Radlk. | Sapindaceae | −/− (1) | L | M | ||
| V | Ripogonum album R.Br. | Smilacaceae | −/− (3) | U | B1, B2, G | ||
| V | Ripogonum elseyanum F.Muell. | Smilacaceae | −/− (3) | U | B1 | ||
| V | Rourea brachyandra F.Muell. | Connaraceae | −/− (1) | L | M | ||
| SH | Rubus queenslandicus A.R.Bean | Rosaceae | −/− (1) | U | B2 | ||
| T | Ryparosa javanica (Blume) Kurz ex Koord. & ValetonΔ | Achariaceae† | +/+ | L | M | Pammel (1911)R. caesia +CN; Rosenthaler (1919)Ryparosa spp. +CN | MELU102277 |
| V | Sarcopetalum harveyanum F.Muell. | Menispermaceae | −/− (1) | B | B1 | Webb (1949) DNT; Hurst (1942) –CN | |
| T | Sarcopteryx martyana (F.Muell.) Radlk. | Sapindaceae | DNT | U | G | ||
| T | Sarcopteryx reticulata S.T.Reynolds | Sapindaceae | −/− (2) | L | M | ||
| T | Sarcotoechia lanceolata (C.T.White) S.T.Reynolds | Sapindaceae | −/− (2) | U | G | ||
| T | Sarcotoechia protracta Radlk. | Sapindaceae | −/− (3) | U | B1, G | ||
| T | Sarcotoechia sp. (Mt Carbine L.W.Jessup+ GJM995) | Sapindaceae | −/− (2) | U | R | MELU102303 | |
| V | Scaevola enantophylla F.Muell. | Goodeniaceae | −/− (4) | U | B1, G | Webb (1949) DNT | |
| T | Schistocarpaea johnsonii F.Muell. | Rhamnaceae | −/− (5) | U | B1 | ||
| T | Schizomeria whitei Mattf. | Cunoniaceae | −/− (1) | U | G | ||
| T | Scolopia braunii (Klotzsch) Sleumer | Salicaceae† | −/− (1) | U | B2 | ||
| T | Siphonodon membranaceus F.M.Bailey | Celastraceae | −/− (3) | U, L | B1, M | ||
| T | Sloanea australis subsp. parviflora Coode | Elaeocarpaceae | −/− (4) | U | B1 | ||
| T | Sloanea langii F.Muell. | Elaeocarpaceae | −/− (5) | U, L | B2, G, M | ||
| T | Sloanea macbrydei F.Muell. | Elaeocarpaceae | −/− (3) | U | B1, G | ||
| V | Smilax aculeatissima Conran | Smilacaceae | −/− (3) | U | B1 | ||
| V | Smilax calophylla Wall. ex A.DC. | Smilacaceae | −/− (1) | L | M | ||
| V | Smilax glauca Walter | Smilacaceae | −/− (2) | U | B1 | ||
| V | Smilax glyciphylla Sm. | Smilacaceae | −/− (2) | U | B2, R | ||
| SH | Solanum macoorai F.M.Bailey | Solanaceae | −/− (1) | U | B2 | ||
| SH | Solanum sp. | Solanaceae | −/− (2) | U | B1 | ||
| SH | Solanum viridifolium Dunal | Solanaceae | −/− (1) | U | G | ||
| T | Sphenostemon lobosporus (F.Muell.) L.S.Sm. | Sphenostemonaceae | −/− (3) | U | G, R | MELU102111 | |
| SH/T | Steganthera australiana C.T.White | Monimiaceae | −/− (2) | U | B2 | ||
| T | Steganthera macooraia (F.M.Bailey) P.K.Endress | Monimiaceae | −/− (4) | U | R | MELU102231 | |
| T | Stenocarpus reticulatus C.T.White | Proteaceae | −/− (1) | U | R | E. E. Conn (pers. comm.) –ve | MELU102196 |
| T | Stenocarpus sinuatus (Loudon) Endl. | Proteaceae | −/− (1); flwr – | U | B2 | E. E. Conn (pers. comm.) –ve | |
| V | Stephania japonica (Thunb.) Miers | Menispermaceae | DNT | L | M | ||
| T | Storckiella australiensis J.H.Ross & B.Hyland | Caesalpiniaceae | −/− (3) | L | M | MELU102279 | |
| T | Streblus glaber var. australianus (C.T.White) Corner | Moraceae | −/− (3) | U | B2, R | ||
| V | Strychnos minor Dennst. | Strychnaceae | −/− (1) | L | M | ||
| T | Symplocos cochinchinensis subsp. 1 | Symplocaceae | −/− (2) | U | G | ||
| T | Symplocos cochinchinensis var. gittonsii Noot. | Symplocaceae | −/− (2) | U | B2, R | ||
| T | Symplocos cochinchinensis var. pilosiuscula Noot. | Symplocaceae | −/− (3) | U | B2, G, R | MELU102218 | |
| SH | Symplocos hayesii C.T.White & W.D.Francis | Symplocaceae | −/− (3) | U | B1, B2, R | MELU102320 | |
| SH/T | Symplocos paucistaminea F.Muell. & F.M.Bailey | Symplocaceae | −/− (3) | U, L | B1, G, M | ||
| T | Synima cordierorum (F.Muell.) Radlk. | Sapindaceae | −/− (1) | U, L | B2, G, M | ||
| T | Synima macrophylla S.T.Reynolds | Sapindaceae | −/− (4) | U | B1, B2, G | MELU102289 | |
| T | Synoum muelleri C.DC. | Meliaceae | −/− (2) | U | B2 | MELU102306 | |
| T | Syzygium canicortex B.Hyland | Myrtaceae | −/− (2) | U | B2, G | MELU102272 | |
| T | Syzygium cormiflorum (F.Muell.) B.Hyland | Myrtaceae | −/− (3) | U, L | B1, B2, G, M | MELU102224 | |
| T | Syzygium endophloium B.Hyland | Myrtaceae | −/− (4) | U | B1, B2, G, R | MELU102153 | |
| T | Syzygium gustavioides (F.M.Bailey) B.Hyland | Myrtaceae | −/− (1) | U | B1 | ||
| T | Syzygium johnsonii (F.Muell.) B.Hyland | Myrtaceae | −/− (3) | U | B2, G | ||
| T | Syzygium kuranda (F.M.Bailey) B.Hyland | Myrtaceae | −/− (4) | U, L | B1, G, M | ||
| T | Syzygium luehmannii (F.Muell.) L.A.S.Johnson | Myrtaceae | −/− (1) | U | R | ||
| T | Syzygium monospermum Craven | Myrtaceae | −/− (1) | L | M | ||
| T | Syzygium papyraceum B.Hyland | Myrtaceae | −/− (3) | U | B1, G | MELU102159 | |
| T | Syzygium trachyphloium (C.T.White) B.Hyland | Myrtaceae | −/− (1) | U | B1 | ||
| T | Syzygium wesa B.Hyland | Myrtaceae | −/− (3) | U | B2, G | MELU102096, 102249 | |
| T | Syzygium wilsonii subsp. cryptophlebium (F.Muell.) B.Hyland | Myrtaceae | −/− (3) | U | B1, G | MELU102223 | |
| SH | Tabernaemontana pandacaqui Lam. | Apocynaceae | −/− (1) | L | M | ||
| SH | Tapeinosperma sp. (Cedar Bay J.G.Tracey 14780) | Myrsinaceae | −/− (4) | U | G | ||
| SH | Tasmannia insipida R.Br. ex DC. | Winteraceae | −/− (3) dry* | U | R | ||
| T | Ternstroemia cherryi (F.M.Bailey) Merr. ex J.F.Bailey & C.T.White | Theaceae | −/− (2) | L | M | ||
| V | Tetracera nordtiana var. nordtiana F.Muell. | Dilleniaceae | −/− (2) | U, L | G, M | ||
| T | Tetrasynandra laxiflora (Benth.) J.R.Perkins | Monimiaceae | −/− (6) | U, L | B1, G, M | MELU102229 | |
| T | Timonius singularis (F.Muell.) L.S.Sm. | Rubiaceae | −/− (1) | U | B1 | ||
| T | Toechima erythrocarpum (F.Muell.) Radlk. | Sapindaceae | −/− (3) | U, L | B1, G, M | MELU102321 | |
| T | Toechima monticola S.T.Reynolds | Sapindaceae | −/− (1) | U | G, R | MELU102098 | |
| T | Triunia erythrocarpa Foreman | Proteaceae | −/− (3) | U | B1 | E. E. Conn (pers. comm.) –ve | |
| V | Trophis scandens (Lour.) Hook. & Arn. subsp. scandens | Moraceae | −/− (1) | L | M | MELU102271 | |
| V | Uncaria lanosa var. appendiculata (Benth.) Ridsdale | Rubiaceae | −/− (1) | L | M | ||
| T | Uromyrtus metrosideros (F.M.Bailey) A.J.Scott | Myrtaceae | −/− (1) | U | R | MELU102315 | |
| V | Ventilago ecorollata F.Muell. | Rhamnaceae | DNT | L | M | ||
| T | Wilkiea angustifolia (F.M.Bailey) J.R.Perkins | Monimiaceae | −/− (1) | U | B1 | MELU102330 | |
| SH | Wilkiea sp. | Monimiaceae | −/− (2) | U | B1 | ||
| SH | Wilkiea sp. (Barong L.W.Jessup 719) | Monimiaceae | −/− (14) | U | B1, B2, G | ||
| SH | Wilkiea sp. Gen. (AQ63687) sp. (Davies Creek L.J.Webb+ 6430) | Monimiaceae | −/− (3) | U | B1 | MELU102302 | |
| SH | Wilkiea wardellii (F.Muell.) J.R.Perkins | Monimiaceae | −/− (3) | U | R | ||
| T | Xanthophyllum octandrum (F.Muell.) Domin | Xanthophyllaceae | −/− (3) | U, L | B1, B2, G, M | MELU102101 | |
| T | Xylopia maccreae (F.Muell.) L.S.Sm. | Annonaceae | −/− (1) | L | M | ||
| T | Zanthoxylum veneficum F.M.Bailey | Rutaceae | −/− (2) | U | B2, G | Webb (1949) DNT | MELU102215 |
Species in the families Salicaceae and Achariaceae which, until recent revisions, were in the Flacourtiaceae (Chase et al., 2002)
Non-woody Alocasia brisbanensis was not included in the analysis of the frequency of cyanogenic species.
Ryparosa javanica is currently the subject of taxonomic review; this Queensland Ryparosa sp. is likely to be renamed (Webber, 2005).
Due to limited access to foliage, tests for cyanogenesis using fresh tissue were not conducted, instead, freeze-dried samples were assayed quantitatively for the presence of CN indicated as ‘dry’.
LITERATURE CITED
- Adsersen A, Adsersen H. 1993. Cyanogenic plants in the Galapagos Islands: ecological and evolutionary aspects. OIKOS 66: 511–520. [Google Scholar]
- Adsersen A, Adsersen H, Brimer L. 1988. Cyanogenic constituents in plants from the Galapagos Islands. Biochemical Systematics and Ecology 16: 65–77. [Google Scholar]
- Aikman K, Bergman D, Ebinger J, Seigler D. 1996. Variation of cyanogenesis in some plant species of the Midwestern United States. Biochemical Systematics and Ecology 24: 637–645. [Google Scholar]
- Brimer L, Cicalini AR, Federici F, Petruccioli M. 1995. Beta-glycosidase as a side activity in commerical pectinase preparations of fungal origin. The hydrolysis of cyanogenic glycosides. Italian Journal of Food Sciences 4: 387–394. [Google Scholar]
- Brinker AM, Seigler DS. 1989. Methods for the detection and quantitative determination of cyanide in plant materials. Phytochemical Bulletin 21: 24–31. [Google Scholar]
- Buhrmester RA, Ebinger JE, Seigler DS. 2000. Sambunigrin and cyanogenic variability in populations of Sambucus canadensis L. Caprifoliaceae. Biochemical Systematics and Ecology 28: 689–695. [DOI] [PubMed] [Google Scholar]
- Cantino PD. 1992. Evidence for a polyphyletic origin of the Labiatae. Annals of the Missouri Botanical Garden 79: 361–379. [Google Scholar]
- CFSAN Centre for Food Safety and Applied Nutrition. 2003. US Department of Health and Human Services Poisonous Plant Database. http://www.cfsan.fda.gov/~djw/plantox.html. Accessed: 16 October 2003.
- Chase MW, Zmarzty S, Lledo MD, Wurdack KJ, Swensen SM, Fay MF. 2002. When in doubt, put it in Flacourtiaceae: a molecular phylogenetic analysis based on plastid rbcL DNA sequences. Kew Bulletin 57: 141–181. [Google Scholar]
- Chassagne D, Crouzet J, Bayonove CL, Baumes RL. 1996. Identification and quantification of passion fruit cyanogenic glycosides. Journal of Agricultural and Food Chemistry 44: 3817–3820. [Google Scholar]
- Coley PD. 1983. Herbivory and defensive characteristics of tree species in a lowland tropical rainforest. Ecological Monographs 53: 209–233. [Google Scholar]
- Coley PD. 1988. Effects of plant growth rate and leaf lifetime on the amount and type of anti-herbivore defense. Oecologia 74: 531–536. [DOI] [PubMed] [Google Scholar]
- Coley PD, Barone JA. 1996. Herbivory and plant defenses in tropical forests. Annual Review of Ecology and Systematics 27: 305–335. [Google Scholar]
- Coley PD, Kursar TA. 1996. Anti-herbivore defenses of young tropical leaves: physiological constraints and ecological trade-offs. In: Mulkey SS, Chazdon RL, Smith AP, eds. Tropical forest plant ecophysiology. New York: Chapman and Hall, 305–336.
- Coley PD, Bryant JP, Chappin FS, III. 1985. Resource availability and plant antiherbivore defense. Science 230: 895–899. [DOI] [PubMed] [Google Scholar]
- Collins DJ, Culvenor CCJ, Lamberton JA, Loder JW, Price JR. 1990. Plants for medicines: a chemical and pharmacological survey of plants in the Australian region. Melbourne: CSIRO Publications.
- Conn EE. 1981. Cyanogenic glycosides. In: Conn EE, ed. The biochemistry of plants. New York: Academic Press Inc., 479–499.
- Conn EE. 1991. The metabolism of a natural product: lessons learned from cyanogenic glycosides. Planta Medica 57: S1–S9. [DOI] [PubMed] [Google Scholar]
- Conn EE, Maslin BR, Curry S, Conn ME. 1985. Cyanogenesis in Australian species of Acacia. Survey of herbarium specimens and living plants. Western Australian Herbarium Research Notes 10: 1–13. [Google Scholar]
- Cooper W, Cooper WT. 1994. Fruits of the rain forest. A guide to fruits in Australian tropical rain forests. Surry Hills, NSW, Australia: RD Press.
- Cooper-Driver GA, Finch S, Swain T. 1977. Seasonal variation in secondary plant compounds in relation to the palatability of Pteridium aquilinum. Biochemical Systematics and Ecology 5: 177–183. [Google Scholar]
- Cronquist A. 1981. An integrated system of classification of flowering plants. New York: Columbia University Press.
- Dahler JM, McConchie CA, Turnbull CGN. 1995. Quantification of cyanogenic glycosides in seedlings of three Macadamia Proteaceae. species. Australian Journal of Botany 43: 619–628. [Google Scholar]
- Davies AG, Bennett EL, Waterman PG. 1988. Food selection by two South-east Asian colobine monkeys Preshytis rubicunda and Presbytis melalophos in relation to plant chemistry. Biological Journal of the Linnean Society 34: 33–56. [Google Scholar]
- Dement WA, Mooney HA. 1974. Seasonal variation in the production of tannins and cyanogenic glycosides in the Chaparral shrub, Heteromeles arbutifolia. Oecologia 15: 65–76. [DOI] [PubMed] [Google Scholar]
- Dickenmann R. 1982. Cyanogenesis in Ranunculus montanus s.l. from the Swiss Alps. Bericht des Geobotanischen Institutes ETH 49: 56–75. [Google Scholar]
- Everist SL. 1981. Poisonous plants of Australia. Sydney, Australia: Angus and Robertson.
- Eyjólfsson R. 1971. Constitution and stereochemistry of lucumin, a cyanogenic glycoside from Lucuma mammosa Gaertn. Acta Chemica Scandanavia 25: 1898–1900. [DOI] [PubMed] [Google Scholar]
- Feeny P. 1976. Plant apparency and chemical defense. In: Wallace JW, Mansell RL, eds. Biochemical interaction between plants and insects. New York: Plenum Press, 1–40.
- Feigl F, Anger V. 1966. Replacement of benzidine by copper ethylacetoacetate and tetra base as spot-test reagent for hydrogen cyanide and cyanogen. Analyst 91: 282–284. [DOI] [PubMed] [Google Scholar]
- Fikenscher LH, Hegnauer R. 1977. Cyanogenese bei den Cormophyten. 12. Mitteilung. Chaptalia nutans, eine stark cyanogene Planze Brasiliens. Planta Medica 31: 266–269. [DOI] [PubMed] [Google Scholar]
- Finnemore H, Cooper JM. 1936. Cyanogenetic glucosides in Australian plants. Part 4—Zieria laevigata. Journal of the Royal Society of New South Wales 70: 175–182. [Google Scholar]
- Finnemore H, Cooper JM. 1938. The cyanogenic constituents of Australian and other plants. Journal of the Society of Chemistry and Industry 57: 167–169. [Google Scholar]
- Finnemore H, Cox CB. 1928. Cyanogenetic glucosides in Australian plants. Journal of the Royal Society of New South Wales 62: 369–378. [Google Scholar]
- Finnemore H, Cox CB. 1929. Cyanogenic glucosides in Australian plants Part II. A. Eremophila maculata, the native fuchsia. Journal of the Royal Society of New South Wales 63: 172–178. [Google Scholar]
- Foreman DB. 1995. Helicia. Flora of Australia 16: 393–399. [Google Scholar]
- Forster PI, Williams JB. 1996. Apocynaceae. Flora of Australia 28: 104–196. [Google Scholar]
- Francisco IA, Pinotti MHP. 2000. Cyanogenic glycosides in plants. Brazilian Archives of Biology and Technology 43: 487–492. [Google Scholar]
- Gardner CA, Bennetts HW. 1956. The toxic plants of Western Australia. Perth, Australia: West Australian Newpapers Ltd.
- Gartlan JS, McKey DB, Waterman PG, Mbi CN, Struhsaker TT. 1980. A comparative study of the phytochemistry of two African rain forests. Biochemical Systematics and Ecology 8: 401–422. [Google Scholar]
- Gibbs RD. 1974. Chemotaxonomy of flowering plants. Vol. 1–3. Montreal and London: Queen's University Press.
- Gleadow RM, Woodrow IE 2000a. Polymorphism in cyanogenic glycoside content and cyanogenic β-glucosidase activity in natural populations of Eucalyptus cladocalyx. Australian Journal of Plant Physiology 27: 693–699. [Google Scholar]
- Gleadow RM, Woodrow IE. 2000b. Temporal and spatial variation in cyanogenic glycosides in Eucalyptus cladocalyx. Tree Physiology 20: 591–598. [DOI] [PubMed] [Google Scholar]
- Gleadow RM, Woodrow IE. 2002. Constraints on effectiveness of cyanogenic glycosides in herbivore defence. Journal of Chemical Ecology 28: 1297–1309. [DOI] [PubMed] [Google Scholar]
- Gleadow RM, Foley WJ, Woodrow IE. 1998. Enhanced CO2 alters the relationship between photosynthesis and defence in cyanogenic Eucalyptus cladocalyx F. Muell. Plant Cell and Environment 21: 12–22. [Google Scholar]
- Goodger JQD, Woodrow IE. 2002. Cyanogenic polymorphism as an indicator of genetic diversity in the rare species Eucalyptus yarraensis Myrtaceae. Functional Plant Biology 29: 1445–1452. [DOI] [PubMed] [Google Scholar]
- Goodger JQD, Capon RJ, Woodrow IE. 2002. Cyanogenic polymorphism in Eucalyptus polyanthemos Schauer subsp. vestita L. Johnson and K. Hill Myrtaceae. Biochemical Systematics and Ecology 30: 617–630. [Google Scholar]
- Gowrikumar G, Mani VVS, Lakshminarayana G 1976. Cyanolipids in Sapinus emarginatus seed oil. Phytochemistry 15: 1566–1567. [Google Scholar]
- Graham AW, Hopkins MS, Maggs J. 1995. Succession and disturbance in the remnant rainforest type complex notophyll vine forest on basalt type 5b. I. Vegetation map and explanatory notes. Atherton, Australia: CSIRO Division of Wildlife and Ecology, Tropical Research Centre, VM/1/1295-6.
- Hadi S, Bremmer JB. 2001. Initial studies on alkaloids from Lombok medicinal plants. Molecules 6: 117–129. [Google Scholar]
- Hall N, Wainwright RW, Wolf LJ. 1981. Summary of meteorological data in Australia no. 6. CSIRO Australian Division of Forest Research.
- Hartley TG, Dunstone EA, Fitzgerald JS, Johns SR, Lamberton JA. 1973. A survey of New Guinea plants for alkaloids. Lloydia 36: 217–319. [PubMed] [Google Scholar]
- Hartmann T, Witte I, Ehmke A, Theuring C, Rowell-Rahier M, Pasteels JM. 1997. Selective sequestration and metabolism of plant derived pyrrolizidine alkaloids by chrysomelid leaf beetles. Phytochemistry 45: 489–497. [Google Scholar]
- Haskins FA, Gorz HJ, Johnson BE. 1987. Seasonal variation in leaf hydrocyanic acid potential of low- and high-dhurrin Sorghums. Crop Science 27: 903–906. [Google Scholar]
- Hegnauer R. 1966. Chemotaxonomie der pflanzen. Eine Ubersicht uber die Verbreitung und die systematische Bedeutung der Pflanzenstoffe. Vol. 4. Basel: Birkhauser Verlag.
- Hegnauer R. 1973. Chemotaxonomie der pflanzen. Eine Ubersicht uber die Verbreitung und die systematische Bedeutung der Pflanzenstoffe. Vol. 6. Basel: Birkhauser Verlag.
- Hegnauer R. 1986. Chemotaxonomie der pflanzen. Eine Ubersicht uber die Verbreitung und die systematische Bedeutung der Pflanzenstoffe. Vol. 7. Basel: Birkhauser Verlag.
- Hegnauer R. 1989. Chemotaxonomie der pflanzen. Eine Ubersicht uber die Verbreitung und die systematische Bedeutung der Pflanzenstoffe. Vol. 8. Basel: Birkhauser Verlag.
- Hegnauer R. 1990. Chemotaxonomie der pflanzen. Eine Ubersicht uber die Verbreitung und die systematische Bedeutung der Pflanzenstoffe. Vol. 9. Basel: Birkhauser Verlag.
- Hickel A, Hasslacher M, Griengl H. 1996. Hydroxynitrile lyases: functions and properties. Physiologia Plantarum 98: 891–898. [Google Scholar]
- Hoot SB, Douglas AW. 1998. Phylogeny of the Proteaceae based on atpB and atpB–rbcL intergenic spacer region sequences. Australian Sytematic Botany 11: 301–320. [Google Scholar]
- Hübel W, Nahrstedt A. 1975. Ein neues cyanglykosid aus Heterodendron oleaefolium. Phytochemistry 14: 2723–2725. [Google Scholar]
- Hübel W, Nahrstedt A. 1979. Cardiosperminsulfate—a sulfur containing cyanogenic glucoside from Cardiospermum grandiflorum. Tetrahedron Letters 45: 4395–4396. [Google Scholar]
- Hughes MA. 1991. The cyanogenic polymorphism in Trifolium repens L. white clover. Heredity 66: 105–115. [Google Scholar]
- Hughes MA, Sharif AL, Alison-Dunn M, Oxtoby E. 1988. The molecular biology of cyanogenesis. In: Evered D, Harnett S, eds. Cyanide compounds in biology. Chichester: John Wiley & Sons, 111–130.
- Hurst E. 1942. The poison plants of New South Wales. Sydney, Australia: Poison Plants Committee NSW.
- Huxley M. 2003. Ngadjon names and uses of some rainforest plants. http://www.koori.usyd.edu.au/ngadjonji/Food/table1.html. Accessed: 16 February 2004.
- Hyland BPM, Whiffin T, Christophel DC, Gray B, Elick RW. 2003. Australian rain forest plants: trees, shrubs, vines. Collingwood: CSIRO Publishing Australia.
- Janzen DH, Waterman PG. 1984. A seasonal census of phenolics, fibre, and alkaloids in foliage of forest trees in Costa Rica: some factors influencing their distribution and relation to host selection by Sphingidae and Saturniidae. Biological Journal of the Linnean Society 21: 439–454. [Google Scholar]
- Janzen DH, Doerner ST, Conn EE. 1980. Seasonal constancy of intra-population variation of HCN content of Costa Rican Acacia farnesiana foliage. Phytochemistry 19: 2022–2023. [Google Scholar]
- Jaroszewski JW, Olafsdottir ES. 1987. Monohydroxylated cyclopentenone cyanohydrin glucosides of Flacourtiaceae. Phytochemistry 26: 3348–3349. [Google Scholar]
- Jensen SR, Nielsen BJ. 1973. Cyanogenic glucosides in Sambucus nigra L. Acta Chemica Scandanavia 27: 2661–2662. [DOI] [PubMed] [Google Scholar]
- Jensen SR, Nielsen BJ. 1986. Gynocardin in Ceratiosicyos laevis Achariaceae. Phytochemistry 25: 2349–2350. [Google Scholar]
- Jones DA. 1972. Cyanogenic glycosides and their function. In: Harborne JB, ed. Phytochemical ecology. London: Academic Press, 103–124.
- Jones DA. 1998. Why are so many food plants cyanogenic? Phytochemistry 47: 155–162. [DOI] [PubMed] [Google Scholar]
- Kaplan MAC, Figueiredo MR, Gottlieb OR. 1983. Variation in cyanogenesis in plants with season and insect pressure. Biochemical Systematics and Ecology 11: 367–370. [Google Scholar]
- Kojima M, Poulton JE, Thayer SS, Conn EE. 1979. Tissue distributions of dhurrin and of enzymes involved in its metabolism in leaves of Sorghum bicolor. Plant Physiology 63: 1022–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kursar TA, Coley PD. 2003. Convergence in defense syndromes of young leaves in tropical rainforests. Biochemical Systematics and Ecology 31: 929–949. [Google Scholar]
- Lamont BB. 1993. Injury-induced cyanogenesis in vegetative and reproductive parts of two Grevillea species and their F1 hybrid. Annals of Botany 71: 537–542. [Google Scholar]
- Lechtenberg M, Nahrstedt A. 1999. Cyanogenic glycosides. In: Ikan R, ed. Naturally occurring glycosides. Chichester, UK: John Wiley & Sons, 147–191.
- Levin DA. 1976. Alkaloid-bearing plants: an ecogeographic perspective. American Naturalist 110: 261–284. [Google Scholar]
- Levin DA, York BM, Jr. 1978. The toxicity of plant alkaloids: an ecogeographic perspective. Biochemical Systematics and Ecology 6: 61–76. [Google Scholar]
- Lewis CE, Zona S. 2000. A survey of cyanogenesis in palms Arecaceae. Biochemical Systematics and Ecology 28: 219–228. [Google Scholar]
- Mabberley DJ. 1990. The plant book. Cambridge, UK: Cambridge University Press.
- Mali S, Borges RM. 2003. Phenolics, fibre, alkaloids, saponins, and cyanogenic glycosides in a seasonal cloud forest in India. Biochemical Systematics and Ecology 31: 1221–1246. [Google Scholar]
- Martin JH, Couch JF, Briese RR. 1938. Hydrocyanic acid content of different parts of the sorghum plant. Journal of the American Society of Agronomy 30: 725–734. [Google Scholar]
- Maslin BR, Dunn JE, Conn EE. 1988. Cyanogenesis in Australian species of Acacia. Phytochemistry 27: 421–428. [Google Scholar]
- McArdle BH. 1990. When are rare species not there? OIKOS 57: 276–277. [Google Scholar]
- McKey D. 1974. Adaptive patterns in alkaloid physiology. American Naturalist 108: 305–320. [Google Scholar]
- McKey D, Waterman PG, Gartlan JS, Struhsaker TT. 1978. Phenolic content of vegetation in two African rain forests: ecological implications. Science 202: 61–64. [Google Scholar]
- Mikolajczak KL, Smith CR, Jr, Tjarks LW. 1970. Cyanolipids of Cardiospermum halicacabum L. and other Sapindaceous seed oils. Lipids 5: 812–817. [Google Scholar]
- Miller RE. 2004. Cyanogenesis in Australian tropical rainforests: resource allocation to a nitrogen-based defence. PhD Thesis, The University of Melbourne, Australia.
- Miller RE, Gleadow RM, Woodrow IE. 2004. Cyanogenesis in tropical Prunus turneriana: characterisation, variation and response to low light. Functional Plant Biology 31: 491–503. [DOI] [PubMed] [Google Scholar]
- Miller RE, McConville MJ, Woodrow IE. 2006a. Cyanogenic glycosides from the rare Australian endemic rainforest tree Clerodendrum grayi (Lamiaceae). Phytochemistry 67: 43–51. [DOI] [PubMed] [Google Scholar]
- Miller RE, Simon J, Woodrow IE. 2006b. Cyanogenesis in the Australian tropical rainforest endemic Brombya platynema (Rutaceae): chemical characterisation and polymorphism. Functional Plant Biology (in press). [DOI] [PubMed]
- Møller BL, Seigler DS. 1999. Biosynthesis of cyanogenic glycosides, cyanolipids, and related compounds. In: Singh KK, ed. Plant amino acids: biochemistry and biotechnology. New York: Marcel Dekker, 563–609.
- Morley BD, Toelken HRE. 1983. Flowering plants in Australia. Adelaide, Australia: Rigby Publishers.
- Munir AA. 1989. A taxonomic revision of the genus Clerodendrum L. Verbenaceae. in Australia. Journal of the Adelaide Botanic Gardens 11: 101–173. [Google Scholar]
- Nahrstedt A. 1975. Verteilung von prunasin in fruchtstielen von Prunus avium L. und der einfluß von IES auf den gesamtgehalt an prunasin. Biochemie und Physiologie der Pflanzen 167: 597–600. [Google Scholar]
- Nahrstedt A. 1980. Absence of cyanogenesis from Droseraceae. Phytochemistry 19: 2757–2758. [Google Scholar]
- Nahrstedt A. 1987. Recent developments in the chemistry, distribution and biology of the cyanogenic glycosides. In: Hostettmann K, Lea PJ, eds. Biologically active natural products. Oxford: Clarendon Press, 213–234.
- Nahrstedt A, Hübel W. 1978. Die cyanogenen verbindungen von Heterodendron oleaefolium. Phytochemistry 17: 314–315. [Google Scholar]
- Nix HA. 1991. Biogeography: pattern and process. In: Nix HA, Switzer MA, Kowari I, eds. Rainforest animals. Atlas of vertebrates endemic to Australia's wet tropics. Canberra: Australian National Parks and Wildlife Service, 11–40.
- Olafsdottir ES, Andersen JV, Jaroszewski JW. 1989. Cyanohydrin glycosides of Passifloraceae. Phytochemistry 28: 127–132. [Google Scholar]
- Osunkoya OO, Ash JE, Graham AW, Hopkins MS. 1993. Growth of tree seedlings in tropical rain forests of North Queensland, Australia. Journal of Tropical Ecology 9: 1–18. [Google Scholar]
- Pammel LH. 1911. A manual of poisonous plants. Chiefly of eastern North America, with brief notes on economic and medicinal plants, and numerous illustrations. Cedar Rapids, Iowa, USA: The Torch Press.
- Patton CA, Ranney TG, Burton JD, Walgenbach JF. 1997. Natural pest resistance of Prunus taxa to feeding by adult Japanese beetles: role of endogenous allelochemicals in host plant resistance. Journal of the American Society for Horticultural Science 122: 668–672. [Google Scholar]
- Petrie JM. 1912. Hydrocyanic acid in plants. Part I. Its distribution in the Australian flora. Proceedings of the Linnean Society of New South Wales 37: 220–234. [Google Scholar]
- Petrie JM. 1913. Hydrocyanic acid in plants I. Proceedings of the Linnean Society of New South Wales 37: 220–234. [Google Scholar]
- Petrie JM. 1914. Hydrocyanic acid in plants II. Proceedings of the Linnean Society of New South Wales 38: 624–638. [Google Scholar]
- Petrie JM. 1920. Cyanogenesis in plants. IV. The hydrocyanic acid of Heterodendron—a fodder plant of New South Wales. Proceedings of the Linnean Society of New South Wales 45: 447–459. [Google Scholar]
- Plouvier V. 1964. Investigation of polyalcohols and cyanogenic heterosides in certain Proteaceae. Compte Rendu Hebdomadaire des Séances de L'Académie des Sciences 259: 665–666. [PubMed] [Google Scholar]
- Poulton JE. 1983. Cyanogenic compounds in plants and their toxic effects. In: Keeler RF, Tu WT, eds. Plant and fungal toxins. New York: Dekker, 117–157.
- Poulton JE. 1990. Cyanogenesis in plants. Plant Physiology 94: 401–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulton JE, Li CP. 1994. Tissue level compartmentation of R.-amygdalin and amygdalin hydrolase prevents large-scale cyanogenesis in undamaged Prunus seeds. Plant Physiology 104: 29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JR. 1963. The distribution of alkaloids in the Rutaceae. In: Swain T, ed. Chemical plant taxonomy. London: Academic Press, 429–452.
- Rhoades DF. 1979. Evolution of plant chemical defense against herbivores. In: Rosenthal GA, Janzen DH, eds. Herbivores: their interaction with secondary plant metabolites. New York: Academic Press, 3–54.
- Rosenthaler L. 1919. Die Verbreitung der Blausäure im Pflanzenreich. Journal Suisse de Pharmacie 57: 279–283, 307–313, 324–329, 341–346. [Google Scholar]
- Rosenthaler L. 1929. Beiträge zur Blausäure-Frage. Neue Blausäure-Pflanzen. Pharmaceutica Acta Helvetiae 4: 196–199. [Google Scholar]
- Santamour Jr FS. 1998. Amygdalin in Prunus leaves. Phytochemistry 47: 1537–1538. [Google Scholar]
- Saupe SG, Seigler DS, Escalante-Semerena J. 1982. Bacterial contamination as a cause of spurious cyanide tests. Phytochemistry 21: 2111–2112. [Google Scholar]
- Schappert PJ, Shore JS. 1999. Effects of cyanogenesis polymorphism in Turnera ulmifolia on Euptoieta hegesia and potential Anolis predators. Journal of Chemical Ecology 25.
- Schuster JL, James LF. 1988. Some other major poisonous plants of the Western United States. In: James LF, Ralphs MH, Darwin DB, eds. The ecology and economic impact of poisonous plants on livestock production. London: Westview Press, 295–307.
- Seigler DS. 1976a. Plants of Oklahoma and Texas capable of producing cyanogenic compounds. Proceedings of the Oklahoma Academy of Science 56: 95–100. [Google Scholar]
- Seigler DS. 1976b. Plants of the Northeastern United States that produce cyanogenic compounds. Economic Botany 30: 395–407. [Google Scholar]
- Seigler DS. 1991. Cyanide and cyanogenic glycosides. In: Rosenthal GA, Berenbaum MR, eds. Herbivores: their interactions with secondary plant metabolites. Academic Press: San Diego, 35–77.
- Seigler DS. 1994. Phytochemistry and systematics of the Euphorbiaceae. Annals of the Missouri Botanical Garden 81: 380–401. [Google Scholar]
- Seigler DS, Kawahara W. 1976. New reports of cyanolipids from Sapindaceous plants. Biochemical Systematics and Ecology 4: 263–265. [Google Scholar]
- Seigler DS, Seaman F, Mabry TJ. 1971. New cyanogenetic lipids from Ungnadia speciosa. Phytochemistry 10: 485–487. [Google Scholar]
- Seigler DS, Eggerding C, Butterfield C. 1974. A new cyanogenic glycoside from Cardiospemum hirsutum. Phytochemistry 13: 2330–2332. [Google Scholar]
- Seigler DS, Coussio JD, Rondina RVD. 1979. Cyanogenic plants from Argentina. Journal of Natural Products 42: 179–182. [DOI] [PubMed] [Google Scholar]
- Seigler DS, Spencer KC, Statler WS, Conn EE, Dunn JE. 1982. Tetraphyllin B and epitetraphyllin B sulphates: novel cyanogenic glucosides from Passiflora caerulea and P. alato-caerulea. Phytochemistry 21: 2277–2285. [Google Scholar]
- Seigler DS, Pauli GF, Nahrstedt A, Leen R. 2002. Cyanogenic allosides and glucosides from Passiflora edulis and Carica papaya. Phytochemistry 60: 873–882. [DOI] [PubMed] [Google Scholar]
- Selmar D. 1993a. Apoplastic occurrence of cyanogenic β-glucosidases and consequences for the metabolism of cyanogenic glucosides. In: Esen A, ed. The biochemistry and molecular biology of β-glucosidases. American Chemical Society: Washington, DC, 191–204.
- Selmar D. 1993b. Transport of cyanogenic glucosides: linustatin uptake by Hevea cotyledons. Planta 191: 191–199. [Google Scholar]
- Selmar D, Lieberei R, Junqueira N, Biehl B. 1991. Changes in cyanogenic glucoside content in seeds and seedlings of Hevea species. Phytochemistry 30: 2135–3140. [Google Scholar]
- Shore JS, Obrist CM. 1992. Variation in cyanogenesis within and among populations and species of Turnera series Canaligerae Turneraceae. Biochemical Systematics and Ecology 20: 9–16. [Google Scholar]
- Smith F, White CT. 1918. An interim census of cyanophoric plants in the Queensland flora. Proceedings of the Royal Society of Queensland 30: 84–90. [Google Scholar]
- Smolenski SJ, Silinis H, Farnsworth NR. 1974. Akaloid screening III. Lloydia 36: 359. [PubMed] [Google Scholar]
- Spencer KC, Seigler DS 1983. Cyanogenesis of Passiflora edulis. Journal of Agricultural and Food Chemistry 31: 794–796. [DOI] [PubMed] [Google Scholar]
- Spencer KC, Seigler DS. 1985. Cyanogenic glycosides and the systematics of the Flacourtiaceae. Biochemical Systematics and Ecology 13: 421–431. [Google Scholar]
- Spencer KC, Seigler DS, Nahrstedt A. 1986. Linamarin, lotaustralin, linustatin and neolinustatin from Passiflora species. Phytochemistry 25: 645–647. [Google Scholar]
- Swanholm CE, St John H, Scheuer PJ. 1960. A survey for alkaloids in Hawaiian plants II. Pacific Science 14: 68–74. [Google Scholar]
- Swenson WK, Dunn JE, Conn EE. 1989. Cyanogenesis in the Proteaceae. Phytochemistry 28: 821–823. [Google Scholar]
- Taskova R, Mitova M, Evstatieva L, Ancev M, Peev D, Handjieva N, Bankova V, Popov S. 1997. Iridoids, flavonoids and terpenoids as taxonomic markers in Lamiaceae, Scrophulariaceae and Rubiaceae. Bocconea 5: 631–636. [Google Scholar]
- Thomsen K, Brimer L. 1997. Cyanogenic constituents in woody plants in natural lowland rain forest in Costa Rica. Biological Journal of the Linnean Society 121: 273–291. [Google Scholar]
- Tjon Sie Fat L. 1978. Contribution to the knowledge of cyanogenesis in Angiosperms. 2. Communicatioin. Cyanogenesis in Campanulaceae. Proceedings of the Koninklijke Nederlandse Akadamie Van Wetenschappen Series C 81: 126–131. [Google Scholar]
- Tjon Sie Fat L. 1979a. Contribution to the knowledge of cyanogenesis in angiosperms Cyanogene Verbindingen bij Poaceae, Commelinaceae, Ranunculaceae en Campanulaceae. PhD. Thesis, Leiden University, The Netherlands.
- Tjon Sie Fat L. 1979b. Contribution to the knowledge of cyanogenesis in Angiosperms. 14. Communication. Cyanogenesis in Ranunculaceae. Proceedings of the Koninklijke Nederlandse Akadamie Van Wetenschappen Series C 82: 197–209. [Google Scholar]
- Tracey JC. 1982. The vegetation of the humid tropical region of North Queensland. Melbourne, Australia: CSIRO.
- Tracey JG, Webb LJ. 1975. Vegetation of the humid tropical region of North Queensland. Indooroopilly, Queensland: Rainforest Ecology Unit, CSIRO Australia, Division of Plant Industry.
- Urbanska K. 1982. Polymorphism of cyanogenesis in Lotus alpinus in Switzerland. I. Small-scale variability in phenotypic frequencies upon acidic silicate and carbonate. Bericht des Geobotanischen Institutes ETH 49: 35–55. [Google Scholar]
- van Valen F. 1978. Contribution to the knowledge of cyanogenesis in angiosperms. Planta Medica 34: 408–413. [DOI] [PubMed] [Google Scholar]
- van Wyk B-E, Whitehead CS. 1990. The chemotaxonomic significance of prunasin in Buchenroedera Fabaceae-Crotalarieae. South African Journal of Botany 56: 68–70. [Google Scholar]
- Wagstaff SJ, Olmstead RG. 1997. Phylogeny of Labiatae and Verbenaceae inferred from rbcL sequences. Systematic Botany 22: 165–179. [Google Scholar]
- Wagstaff SJ, Hickerson L, Spangler R, Reeves PA, Olmstead RG. 1998. Phylogeny in Labiatae s. l., inferred from cpDNA sequences. Plant Systematics and Evolution 209: 265–274. [Google Scholar]
- Wajant H, Riedel D, Benz S, Mundry K-W. 1994. Immunocytological localization of hydroxynitrile lyases from Sorghum bicolor L. and Linum usitatissimum L. Plant Science 103: 145–154. [Google Scholar]
- Waterman PG, Ross AM, Jr, Bennett EL, Davies AG. 1988. A comparison of the floristics and leaf chemistry of the tree flora in two Malaysian rain forests and the influence of leaf chemistry on populations of colobine monkeys in the Old World. Biological Journal of the Linnean Society 34: 1–32. [Google Scholar]
- Webb LJ. 1948. Guide to the medicinal and poisonous plants of Queensland. CSIRO Bulletin 232: 1–202. [Google Scholar]
- Webb LJ. 1949. An Australian phytochemical survey I. Alkaloids and cyanogenetic compounds in Queensland plants. CSIRO Bulletin 241: 1–56. [Google Scholar]
- Webb LJ. 1952. An Australian phytochemical survey II. Alkaloids in Queensland flowering plants. CSIRO Bulletin 268: 1–99. [Google Scholar]
- Webb LJ, Tracey JG. 1981. The rainforests of northern Australia. In: Groves RH, ed. Australian vegetation. Cambridge: Cambridge University Press, 67–101.
- Webb LJ, Tracey JG, Williams WT. 1972. Regeneration and pattern in the sub-tropical rainforest. Journal of Ecology 60: 675–95. [Google Scholar]
- Webber BL. 1999. Cyanogenesis in the tropical rainforest tree, Ryparosa javanica. Honours Thesis, The University of Melbourne.
- Webber BL. 2005. Plant–animal interactions and plant defence in the rainforest tree, Ryparosa. PhD Thesis, The University of Melbourne, Australia.
- Webber BL, Woodrow IE. 2004. Cassowary frugivory, seed defleshing and fruit fly infestation influence the transition from seed to seedling in the rare Australian rainforest tree, Ryparosa sp. nov. 1 Achariaceae. Functional Plant Biology 31: 505–516. [DOI] [PubMed] [Google Scholar]
- Young RL, Hamilton RA. 1966. A bitter principle in Macadamia nuts. In: Proceedings of the 6th annual meeting. Hawaii Macadamia Producers Association, 27–30.
- Zheng L, Poulton JE. 1995. Temporal and spatial expression of amygdalin hydrolase and R.-+.-mandelonitrile lygase in black cherry seeds. Plant Physiology 109: 31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]