Abstract
• Background and Aims Success during the early stages of the life-history of alien plants is essential for invasion to occur. The reproductive components of plant invaders have mostly been studied in species reproducing sexually but little is known about invaders that depend exclusively on vegetative reproduction. In this paper, the importance of the different recruitment stages on population growth is quantified and, thus, the invasion potential of the South African annual geophyte Oxalis pes-caprae invading Mediterranean ecosystems is assessed.
• Methods Tests and experiments were conducted across Menorca (Balearic Islands) to analyse the spatial variability of Oxalis pes-caprae reproductive components (i.e. bulb production, bulb bank, bulb predation, bulb mortality, bulb dormancy, bulb germination, plant establishment and survival).
• Key Results Oxalis pes-caprae has a transient bulb bank that remains dormant in the soil during summer. High levels of bulb predation after dispersal, followed by bulb mortality during summer or a failure to germinate in autumn were the most critical factors limiting plant establishment. Bulb germination was high. However, plant establishment and bulb production is constrained by intraspecific competition, but is not affected by soil disturbance. No symptoms of spatial discordance could be found between recruitment stages because the spatial variability of the life cycle was extremely low at all the scales examined (i.e. among populations, habitats and microsites). It was estimated that, on average, 4 % of bulbs can become plants the following year and the field rate of population increase (λ) to be 0·08.
• Conclusions The results suggest that invasion is constrained by post-dispersal bulb predation, loss of viability of the propagule bank due to summer drought and high intraspecific competition. However, a high spatial concordance between recruitment stages and probably a high propagule pressure due to human and livestock bulb dispersal determine the success of this invader across Menorca Island.
Keywords: Alien plant, asexual reproduction, Bermuda butter-cup (soursob), bulb production, disturbance, geophyte, Menorca Island, microsite, Oxalis pes-caprae, post-dispersal bulb predation, plant establishment and survival
INTRODUCTION
Plant fitness depends on a chain of events from propagule production to seedling survival. As expected from the customary independent factors that affect each stage of the life cycle, there is great spatio-temporal variability (i.e. discordance or uncoupling) between the different stages of recruitment, making predictions about population dynamics difficult (Houle, 1998). Therefore, to understand better the critical stages modulating plant fitness it is necessary to identify and quantify the ecological factors influencing each single stage and to test how consistent they are in space and time. For example, seed deposition patterns may be obscured by factors acting later in the recruitment process such as predation (García-Fayos and Verdú, 1998). Indeed, pre- and post-dispersal seed predation is different among vegetation types and does not necessarily match seedling abundance patterns (Vilà and Gimeno, 2003). This uncoupling of phases is known as seed–seedling conflict and is found in diverse taxa and environments (Jordano and Herrera, 1995; Schupp, 1995; Ibáñez and Schupp, 2002; Traveset et al., 2003).
High reproduction and seedling recruitment which ultimately determine a positive rate of population increase have been related to the success of alien species invading new biogeographical areas (Baker, 1974; Lodge, 1993). However, at the local scale, early stages of the life cycle of invasive plants face ecological constraints in the receptive community, limiting their colonization and spread. Therefore, the success of the invader, like that of most species, is directly coupled to the community context, and a number of studies have shown that invader fitness has a high spatial variability. The spatial variability of invader reproductive components is evident both among and within the communities (i.e. microhabitat) (Vilà and D'Antonio, 1998b; Grigulis et al., 2001; Lambrinos, 2002) suggesting that a realistic view of an invader's performance should encompass multiple sites within the area of introduction.
Detailed exploration of the recruitment dynamics of native plant species has mainly focused on long-lived species reproducing sexually, especially those with seeds dispersed by birds (Herrera et al., 1994; Schupp, 1995; Rey and Alcántara, 2000; Traveset et al., 2003) or by wind (Houle and Payette, 1990; Vilà and Lloret, 2000). Similarly, even though clonal plants are well represented in the alien constituent of worldwide floras, the fitness components of these plant invaders have mostly been studied in species reproducing sexually rather than in invaders depending exclusively on vegetative reproduction. In fact, vegetative reproduction is one of the traits related to invasion success in temperate semi-natural habitats (Pyšek et al., 1995), in subalpine communities (Godfrey et al., 2004) and in Mediterranean islands (Lloret et al., 2005). Therefore, a better knowledge of the propagule dynamics of invasive species is necessary to improve weed management models such as those existing for certain weeds interfering with crops (Swinton and King, 1994).
Oxalis pes-caprae (Bermuda butter-cup or soursob) is a South African annual geophyte. In invaded habitats of the Mediterranean Basin it reproduces only asexually by bulbs. It was introduced into Mediterranean islands at the beginning of the nineteenth century, and is now invading ruderal, agricultural, grasslands and old-field habitats (Gimeno et al., in press). It can cause oxalate poisoning to livestock if eaten in large quantities (Hulme, 2004).
Here regional-scale data on the bulb dynamics of O. pes-caprae invading Menorca Island (Balearic Islands, western Mediterranean Basin) is reported in order to quantify the importance of the different recruitment stages (i.e. bulb production, bulb bank, bulb predation, bulb mortality, bulb dormancy, bulb germination, plant establishment and survival) on population growth and, therefore, invasion potential. The following specific questions are addressed: (a) What are the critical recruitment stages likely to control invasion? (b) What is the overall probability of a bulb becoming an adult plant? (c) What is the rate of population increase? (d) Is there a spatial discordance for different stages of recruitment at either habitat or microhabitat scales? As far as is known, this is the first comprehensive study on the reproductive ecology of an alien geophyte relying completely on bulb production for population persistence.
MATERIALS AND METHODS
Study species and study sites
Oxalis pes-caprae L. (Oxalidaceae) is an annual bulbous herb from South Africa invading temperate and Mediterranean habitats of the world (Peirce, 1997). This geophyte, up to 30–40 cm in height reproduces and disperses vegetatively by bulbs formed from the underground stems, maturing in spring, remaining dormant in summer and sprouting in autumn (Galil, 1968). Therefore, plants are of vegetative origin. It is a persistent and pernicious weed in cultivated areas as well as in ruderal and disturbed habitats. In the Balearic Islands it also invades shrublands, grasslands and the coastal vegetation (Gimeno et al., in press).
The study was conducted at several sites in Menorca (Balearics Islands, Spain). The island's surface area is about 700 km2 and it has relatively low elevations, the highest being 357 m. The north of the island has soils from different geological origins, the greater part being silicic, whereas the south is totally calcareous. The climate is characterized by moderate temperatures throughout the year. The average annual temperature is 16–17° C. The annual mean precipitation is 642 mm, concentrated mainly in late summer and autumn, especially in October.
Potential plant bulb production
In summer 2001 healthy and mature bulbs were unearthed and collected from ten populations by digging holes 20 × 20 × 20 cm in the soil. Each population was separated by >5 km from each other. Bulbs from each population were stored at room temperature in paper envelopes until experimentation. In October 2001, the month when bulbs usually sprout in natural conditions, ten bulbs were randomly selected from each population. Each bulb was weighed and sown in a glasshouse in a 5-L plastic pot (11 cm diameter × 13 cm high) containing a 4 : 2 : 1 mixture of peat : vermiculite : sand with the slow release fertilizer Osmocote Plus (N : P : K in proportion 15 : 10 : 12 + 2 MgO). Soil acidity due to the presence of peat in the pot soil was neutralized with 0·7 g CaCO3 L–1. Pots were watered liberally and equally during the course of the experiment. In June 2002, when plants had senesced, the number of bulbs produced per plant was counted.
Differences in bulb production per plant among populations were analysed with an ANCOVA with population as a fixed effect and initial bulb weight as the covariate. Data were log-transformed to meet the assumptions of parametric analysis.
Effect of intraspecific competition on bulb production
In October 2004, a random sample of second generation bulbs that had been produced in homogeneous glasshouse conditions the previous spring, and had been stored in paper envelopes in laboratory conditions for 4 months, was planted in the glasshouse in the following intraspecific treatments: 1 (no competition), 2, 5, 10 and 15 (maximum competition) bulbs per pot. Five-litre pots were again used containing rinsed sand with a slow release fertilizer (N : P : K, in proportion 15 : 10 : 12 + 2 MgO). Soil acidity was neutralized with 0·7 g CaCO3 L–1. Pots were watered liberally and equally during the course of the experiment. Each treatment was replicated ten times.
Before sowing, each group of bulbs per pot was weighed. The number of plants that emerged per pot was counted during the next 3 months after planting. In February 2005, when plants were in full above-ground development the total number of vegetative and flowering stalks per pot were counted. At this stage, plants were very vigorous and it was not possible to distinguish individual plants within pots. In June 2005, when plants had senesced, the number of bulbs per pot was counted. Also the average number of bulbs per emerged plant from each pot was calculated. Differences in the percentage of plant establishment per pot and bulb production per plant among treatments were compared with an ANCOVA with initial total bulb weight as the covariate.
Analysis of the bulb bank
In January 2002, eight ruderal, 8-old-fields and eight shrublands invaded by O. pes-caprae across Menorca Island were selected. The sites were at least 5 km apart. In each site a 2-m plot which was highly invaded (>90 % cover) by O. pes-caprae was selected. In January 2002, May 2002, September 2002, January 2003 and May 2003 all bulbs in a different randomly placed 20 × 20 cm subplot were collected to a 20-cm depth within each plot. Sampled subplots were at least 20 cm apart from each other to avoid the effects of disturbance made when bulbs from an adjacent subplot were removed. Furthermore, trampling was avoided within the plot. In order to estimate bulb production per plant, in January 2002 the number of O. pes-caprae plants was counted in the same subplot where the number of bulbs was going to be counted in May 2002. To ensure similar soil volume sampling in each subplot, the soil was sieved to discard stones larger than 0·5 cm diameter and the total soil volume sampled was 1 L. Bulbs were classified as viable and non-viable. Non-viable bulbs were distinguished as being dry and empty.
A repeated-measures ANOVA was conducted to test seasonal differences in the bulb bank with number of bulbs per plot as the dependent variable. Since many sites were disturbed or lost during the course of the survey due to human trampling and vandalism, it was not possible to compare differences among habitat types.
Field bulb germination and predation
In early September 2004, 96 groups of ten bulbs were randomly selected from a stock of bulbs that had grown in glasshouse conditions for two generations and were placed in 7 × 7 cm white nylon bags made from a sufficiently fine gauge (0·5 mm) to retain bulbs. In each site where the bulb bank was studied, four areas were randomly selected, separarated by at least 4 m from each other, two in the open and two beneath a perennial shrub. In each microsite a bag was buried at a depth of about 5 cm, the depth at which most bulbs are found. A total of 960 bulbs i.e. 2 microsites × 2 bags × 10 bulbs per bag × 24 replicates, were placed. In December 2004, at the end of the plant establishment season, bulbs were retrieved from their bags and the percentage of germinating bulbs was quantified. All bulbs were alive at the end of the test. For analysis, the percentage germination was calculated as the average germination between the pair of bags within a microsite type.
In early September 2004, bulb predation was also estimated by individually gluing average-weight bulbs to a nylon fishing line 25 cm long, which was tied to a wire stake. A total of 480 bulbs were glued, separated by 4 m from each other, and superficially buried in the soil at a depth of 4 cm. As for bulb germination, in each site two microsite types were selected. Ten open areas and ten areas beneath a perennial shrub, in which a randomly chosen bulb was buried, were randomly chosen. The number of predated bulbs was assessed in early December 2004.
Differences in percentage germination and percentage predation between habitats and microsites were tested with a two-way ANOVA. Data were normalized with the angular transformation to meet the assumptions of parametric analysis.
Effect of disturbance on plant establishment
In October 2001, 11 old-field sites that were not infested, that were at least 5 km apart, were selected. In each site, two adjacent 0·5 × 0·5 m plots that were 1 m apart were chosen. One plot in each site was randomly selected and the soil was slightly disturbed by manually excavating the top 3 cm. Tenty-five bulbs were superficially buried to <2 cm in depth in both plots. Bulbs were interspaced regularly within the plot. Plant establishment was counted in January 2002, 2003, 2004 and 2005. In January 2005 all emerged plants were pulled up and killed. It is planned to revisit all study sites during the next 2 years and remove all new plants to prevent an invasion of such sites by O. pes-caprae.
A paired t-test was conducted to compare differences in percentage plant establishment between control and disturbed plots for 2002 and plant density for each year. Data was log(x + 1) transformed to meet the assumptions of parametric analysis. Data were not analysed with repeated measures ANOVA because several plots were destroyed during the course of the experiment.
Viability of 1-year-old bulbs
In September 2004, bulbs produced under glasshouse conditions were tested for viability. These bulbs had been stored for 1 year in paper envelopes in laboratory conditions. For this purpose, 50 bulbs were planted in individual small plastic pots (5 cm diameter × 15 cm depth) in the same soil and glasshouse conditions as for the potential plant bulb production test. Fifty bulbs produced under glasshouse conditions in the current year were also planted as controls. Before planting, the largest diameter of each bulb was measured. Plant establishment was surveyed for 5 months. Differences in percentage germination between current and 1-year-old bulbs were compared with an ANCOVA using bulb diameter as the covariate.
Transition probabilities between reproduction stages and population growth
The following estimations of bulb production (BPs) were calculated:
BP1: potential bulb production. Average production of bulbs per plant in the glasshouse test.
BP2: field bulb production. Number of bulbs in the soil in May 2002/number of plants in January 2002 in the 20 × 20 × 20 cm field subplots.
BP3: density-dependent bulb production. Average production of bulbs per plant when grown in the high intraspecific competition treatment.
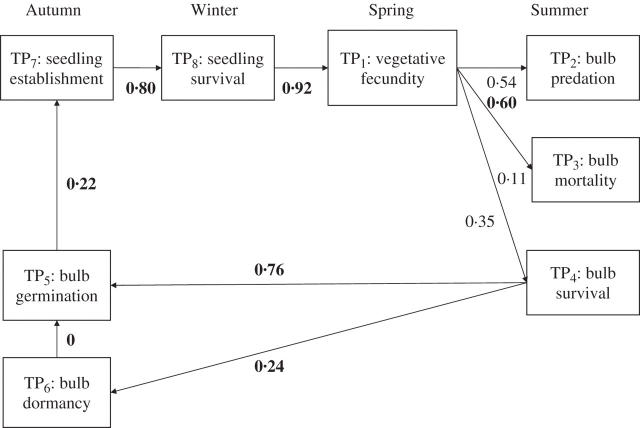
Also the following transition probabilities (TPs) between different reproduction stages from vegetative fecundity to plant survival were calculated. In general, each TP was calculated as the ratio between the number of individuals completing a stage and the number of individuals entering that stage (Fig. 1).
TP1: vegetative fecundity. Proportion of plants producing bulbs at the end of the life cycle in the glasshouse test (see Potential plant bulb production section).
TP2: bulb predation. Estimated in two different ways: (1) as the proportion of bulbs which disappeared in the field during summer [(bulbs in May 2002 – bulbs in September 2002) × 100/bulbs in May 2002] and (2) calculated directly as the average proportion of bulbs disappearing from the experimental test of predation.
TP3: bulb mortality during summer (non-viable bulbs in September 2002 × 100/total number of bulbs in September 2002).
TP4: bulb survival during summer [1 – (bulb predation TP2 + bulb mortality TP3)].
TP5: bulb germination. Proportion of germinated bulbs in the nylon bags placed in the field.
TP6: bulb dormancy. Proportion of non-germinated bulbs in the nylon bags placed in the field.
TP7: plant establishment. Proportion of bulbs emerging in the field (see Effect of disturbance on plant establishment section).
TP8: plant survival. Proportion of established plants which survived to the end of the life cycle in the glasshouse test (see Potential plant bulb production section).
Fig. 1.
Diagram of recruitment dynamics of Oxalis pes-caprae invading Menorca Island. Values correspond to the estimated process-specific transition probabilities (TPs) according to observation analysis (in normal type) or specific experimental tests conducted in the field or in the glasshouse (in bold type).
Assuming that the processes are independent, the product of the process-specific TPs occurring before each stage will determine the cumulative probability (CP) up until that particular stage (Rey and Alcántara, 2000).
The rate of population increase (λ) was estimated as λ = BPs × TP1 × TP4 × TP5 × TP7 × TP8 (Harper, 1977; Silvertown, 1987).
Throughout the paper means are accompanied by their standard errors.
RESULTS
Bulb production per plant
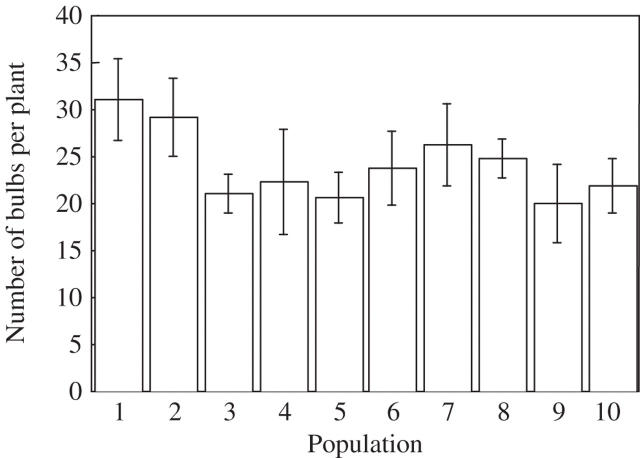
Plant establishment in the glasshouse was very high (81 %) and almost all plants (92 %) produced bulbs. Potential bulb production (BP1) was on average 24·00 ± 1·24 bulbs per plant and there were only marginally significant differences among populations (F9,52 = 1·86, P = 0·08) (Fig. 2). Differences in total bulb weight among populations were non-significant (F9,52 = 1·35, P = 0·23). Potential bulb production appeared to depend upon initial bulb weight (F1,52 = 4·3, P = 0·04). Large maternal bulbs produced fewer bulbs but of a similar weight as those produced by small maternal bulbs (F9,52 = 1·53, P = 0·23).
Fig. 2.
Average (± s.e.) bulb production per plant of Oxalis pes-caprae populations from Menorca Island growing in a glasshouse.
In contrast, field bulb production (BP2) was low. In January 2002, there were on average 15·46 ± 3·04 plants per subplot (20 × 20 × 20 cm) and it was estimated that field bulb production was only 2·46 ± 0·65 bulbs per plant.
Effect of intraspecific competition on bulb production
Eighty-six per cent of the planted bulbs emerged. Plant establishment decreased with intraspecific competition, being higher in pots with one or two bulbs planted than in the other treatments (F4,40 = 9·99, P < 0·001), although this was independent of initial bulb weight (F1,4 = 0·23, P = 0·64; Fig. 3). The average number of bulbs per plant also decreased with intraspecific competition (F4,40 = 5·02, P = 0·0022) regardless of initial bulb weight (F1,4 = 0·55, P = 0·46). Plants subjected to strong intraspecific competition produced four times fewer bulbs than plants grown singly. Density-dependent bulb production (BP3) was on average 12·24 ± 1·38 bulbs per plant compared with 50·6 ± 3·99 when grown singly (Fig. 3).
Fig. 3.
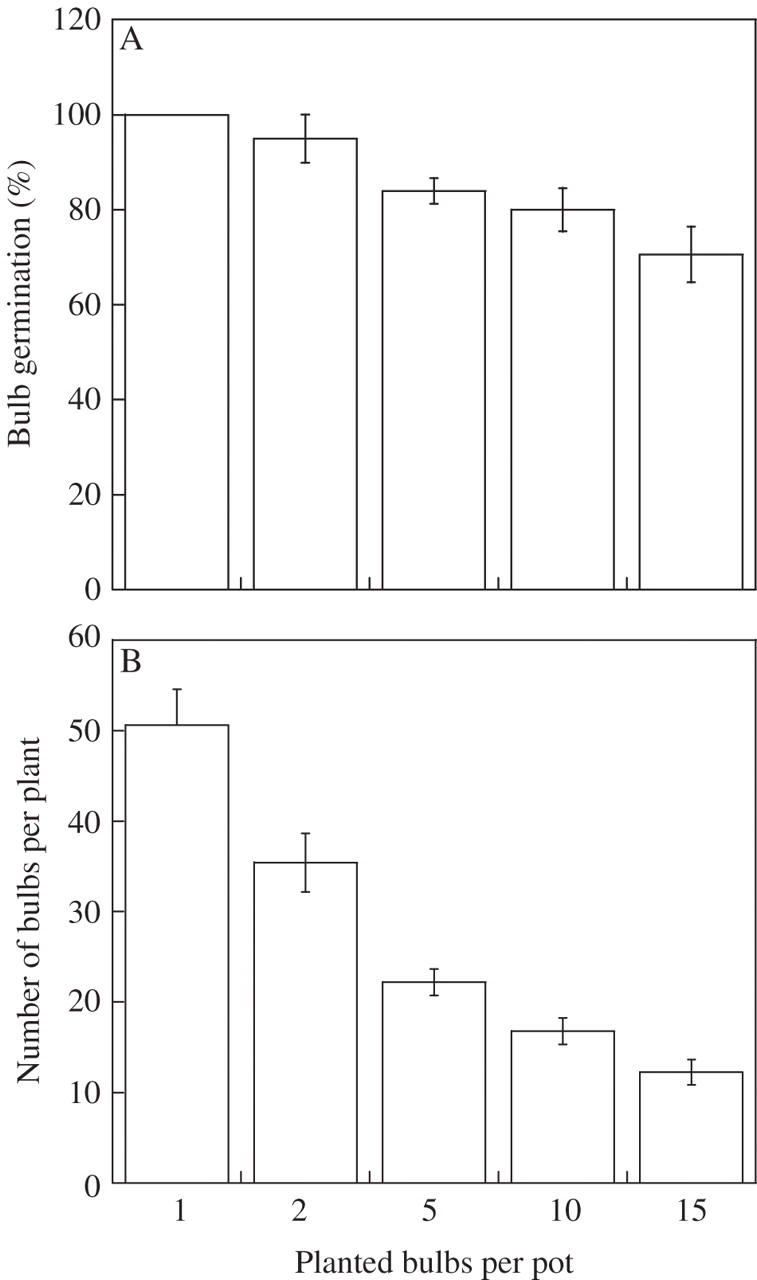
Average (± s.e.) bulb germination (A) and bulb production (B) in Oxalis pes-caprae plants subjected to intraspecific competition in glasshouse conditions.
Analysis of the bulb bank
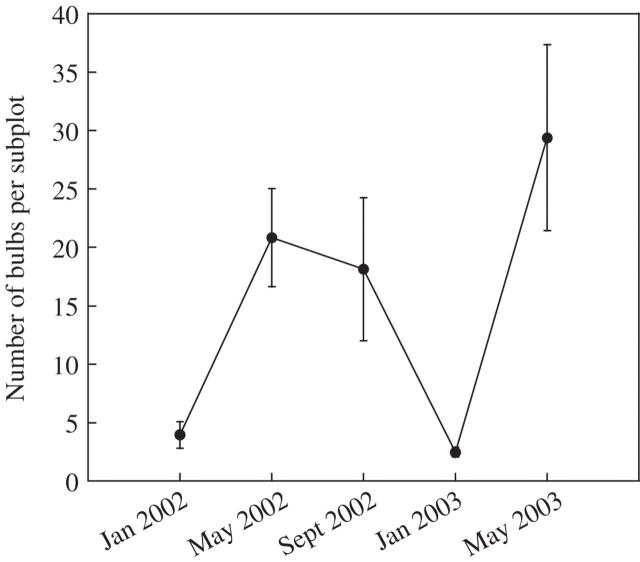
There was a significant seasonal variation in bulb bank density (F4,28 = 3·85, P = 0·01). Bulb production per subplot was 20·82 ± 4·20 in May 2002 and 29·40 ± 7·96 in May 2003 (Fig. 4). After summer, the number of bulbs per subplot was lower than before summer, indicating that some bulbs had disappeared (53·89 ± 11·46 % for 2002) and some lost viability (10·79 ± 3·78 % for 2002). Not all of the remaining viable bulbs emerged in autumn despite being viable in early winter: an average of 3·95 ± 1·13 and 2·47 ± 0·41 bulbs were found dormant per subplot in January 2002 and January 2003, respectively.
Fig. 4.
Seasonal bulb bank dynamics of Oxalis pes-caprae populations invading Menorca Island. Number of bulbs per subplot corresponds to the average (± s.e.) number of bulbs in 20 × 20 × 20 cm of soil.
Field bulb germination and predation
On average, 75·56 ± 4·19 % of bulbs placed in nylon bags germinated (Fig. 5) and there were no significant differences among habitats (F2,33 = 0·47, P = 0·63) or microhabitats (F1,33 = 0·08, P = 0·78).
Fig. 5.
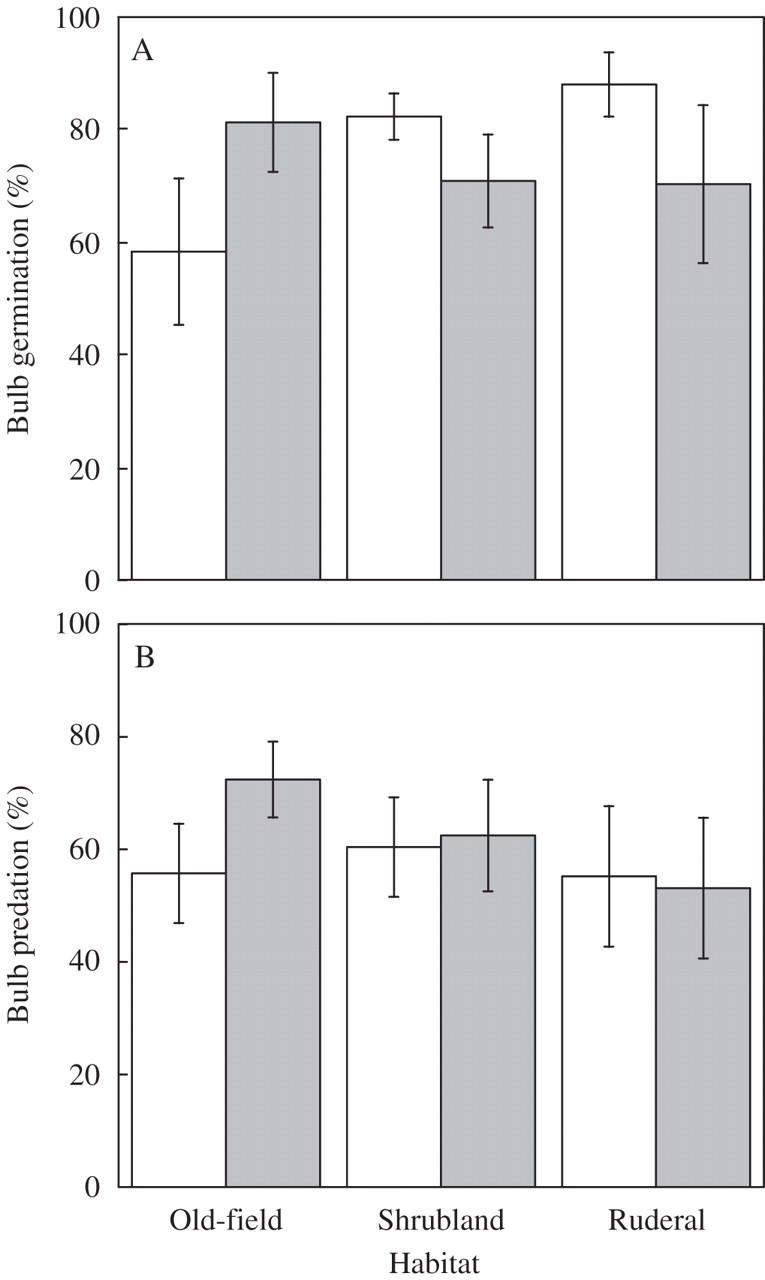
Percentage (mean ± s.e.) bulb germination (A) and predation (B) of Oxalis pes-caprae in open areas (white) and beneath a shrub (grey) in different habitats across Menorca Island.
The predation field test showed that, on average, more than half (59·64 ± 4·13 %) of the bulbs were predated (Fig. 5), probably by rodents as indicated by the presence of bulb peels next to some predated bulbs and the fact that some of the nylon bags were torn. As for bulb germination, differences among habitats (F2,42 = 0·096, P = 0·909) and microhabitats were non-significant (F1,42 = 0·603, P = 0·44). Such predation values were similar to the proportion of bulbs disappearing during summer (53·89 ± 11·46 %) according to the bulb bank survey (see above).
Effect of disturbance on plant establishment
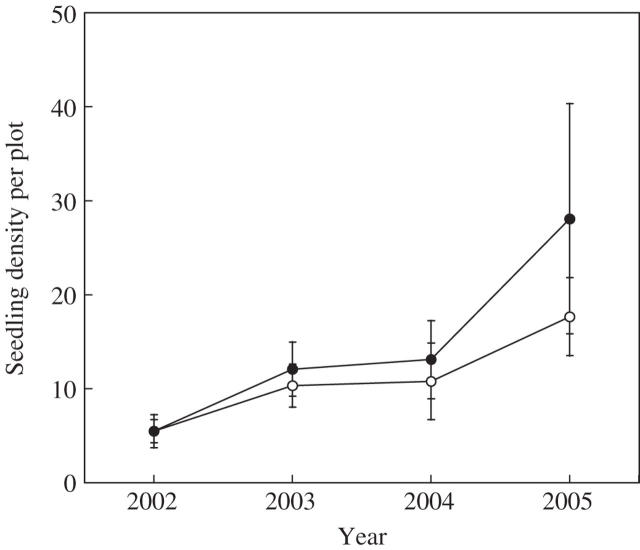
Plant establishment in the field experiment showed no differences (paired t-test = 0·09, P = 0·93) between control (22 ± 4·93 %) and disturbed plots (22 ± 7·04). Therefore, only an average of 5·5 ± 1·05, out of the 25 bulbs planted per site and treatment, became established plants. There was an annual increase in plant density, doubling the following 2 years and tripling in the fourth year (Fig. 6). However, the effect of disturbance appeared to be non-significant (paired t-test = 0·00, P = 0·99 in 2002; 0·93, P = 0·38 in 2003; 0·62, P = 0·55 in 2004; 1·36, P = 0·21 in 2005).
Fig. 6.
Oxalis pes-caprae plant recruitment in disturbed (closed circles) and non-disturbed soil (open circles) across Menorca Island. Plant density per plot corresponds to the average (± s.e.) number of plants present in a 0·5 × 0·5 m area where 25 bulbs had been sown.
Viability of 1-year-old bulbs
Not a single bulb produced the previous year germinated in the glasshouse, indicating that bulbs that do not germinate in the first year die.
Transition probabilities between reproduction stages and population growth
Transition probabilities (TP) of O. pes-caprae recruitment dynamics are shown in Fig. 1. The TP from bulb survival after summer to bulb germination in autumn was very high (0·76). However, plant establishment was low (0·22) but counterbalanced by a high plant survival (0·80) and vegetative fecundity (0·92). Because all bulbs remaining dormant in winter died, the TP from bulb dormancy to bulb germination was zero. Overall, the cumulative probability (CP) for bulb loss (i.e. bulb predation plus bulb mortality during summer or winter) was high: 0·99 according to the bulb bank survey and 0·95 according to the predation field test. The CP from plant fecundity to plant survival was 0·92 (vegetative fecundity) × 0·35 (bulb survival) × 0·76 (bulb germination) × 0·22 (plant establishment) × 0·8 (plant survival) = 0·04.
The potential rate of population increase (λ) was 0·96. However, λ declined to 0·48 when density-dependent effects were considered, and even more when bulb production was estimated in the field across the island (λ = 0·08).
DISCUSSION
The critical stages of recruitment appear to be bulb predation and summer mortality, since a large fraction of the bulbs produced are predated by vertebrates during summer, over 10 % of bulbs die during summer, and one-third which germinate in autumn do not persist to the next year. It is believed that rodents were involved in bulb predation because all bulbs were removed from the tied lines. Such a high probability of bulb predation calculated by observational analysis is probably quite realistic as it coincided with that estimated by field experiments. On the other hand, mortality of bulbs during summer might be related to environmental stress because dead bulbs were empty and dry. Bulbs were never seen to have been attacked by pathogens. However, as found in other studies (Lonsdale et al., 1988; Vilà and Gimeno, 2003), even if such propagule loss could reduce plant establishment in alien species it would be unlikely to represent a bottleneck for invasion to occur.
Bulbs of O. pes-caprae remain dormant in summer and sprout in autumn. For bulbous plants, a regular hot dry summer period such as in the Mediterranean climate is of fundamental importance for bulb dormancy. Dormancy in O. pes-caprae can be overcome by chilling (Chawdhry and Sagar, 1974). In regions where autumns are very mild, the chilling requirements of the bulbs may not be fully met, resulting in delayed, irregular and incomplete bulb sprouting (Lane, 1984). Early studies on the bulb dynamics of Oxalis spp. mentioned that bulbs remaining in the soil replenished the bulb-bank (Hunter and Over de Linden, 1958). However, the present study suggests that bulbs produced in summer do not persist in a viable form for >1 year. Therefore, according to Thompson and Grime's scheme (Thompson and Grime, 1979), O. pes-caprae has a Type I transient bulb bank characterized by a short-lived bulb bank present during summer.
It was found that an average of 4 % of the bulbs produced can become an established plant in the following cohort. This CP estimation is several orders of magnitude higher than the values found for other Mediterranean species (Rey and Alcántara, 2000; Traveset et al., 2003; Gulías et al., 2004) including invaders (Vilà and D'Antonio, 1998a; Vilà and Lloret, 2000).
The CP from plant fecundity to plant survival is likely to be overestimated and the interpretation of the estimated TP in the present study has some limitations. First, the same study sites for all the reproduction processes studied were not used, therefore TPs were not calculated for the same cohorts. For example, due to the impossibility of removing all bulbs from the soil, the plant establishment test was conducted in non-invaded areas. Secondly, some processes were studied in controlled glasshouse conditions (i.e. vegetative fecundity, plant survival) and, therefore, their magnitude was overestimated. For instance, bulb production in the glasshouse (24 bulbs per plant) was eight times greater than in the field (three bulbs per plant). Finally, some stages and processes in the field were estimated by different observational and experimental methodologies.
Still another caveat of the present study is that the same type of spatial variability was not analysed in all reproduction stages, and thus the spatial dynamics of TPs for O. pes-caprae could not be calculated as has been conducted for other Mediterranean species (Rey and Alcántara, 2000; Vilà and Lloret, 2000; Traveset et al., 2003). Instead, the average TPs and CPs were calculated without weighting for differences in the relative abundance of spatial units.
Furthermore, the selected areas where the bulb dynamics were estudied were located at the centre of highly invaded stands. This implies that the estimation of field bulb production did not consider differences between high-density and low-density stands (the latter usually located at the edge of the invasion front). The present estimation did not account for the spatial spread of bulbs because bulb production was estimated for the same area as plant density. For these reasons, the rate of population increase was very low in the field (λ = 0·08) compared with the potential rate of population increase (λ = 0·96). This difference can also be related to environmental and biotic constraints such as those posed by density-dependent effects (Harper, 1977; Silvertown, 1987). However, these constraits could be counterbalanced by dispersal of the numerous bulbs via biological vectors such as on livestock hoofs or due to human activities related to the transfer of contaminated soil (Hulme, 2004). Despite all these study limitations, however, our approach to analyse the recruitment dynamics of an invader is to date the first one conducted on a broad regional scale.
Large spatial variability is of paramount importance in the recruitment dynamics of plant species. In the Mediterranean, the cumulative probability from seed production to plant establishment is usually greater beneath adult plants than in open microhabitats (Rey and Alcántara, 2000; Vilà and Lloret, 2000; Traveset et al., 2003; Gulías et al., 2004) because the vegetation offers protection from browsing and ameliorates water stress. However, no differences in bulb germination or predation were found between either microhabitats or habitats. Nor were differences found in plant establishment between disturbed and non-disturbed microsites, which suggests that for a geophyte relying completely on vegetative reproduction such as O. pes-caprae, the early stages of the life cycle are spatially more concordant than on seeder species.
Acknowledgments
We thank J. Rita and M. Mus for assistance on field site selection, and S. Johnson and E. Winkler for comments on an earlier version of the manuscript. This study is part of EPIDEMIE (Exotic Plant Invasions: Deleterious Effects on Mediterranean Island Ecosystems), a research project supported by the European Commission under the 5th Framework, contributing to the implementation of Key Action 2.2.1 (Ecosystem Vulnerability) within the Energy, Environment and Sustainable Development thematic programme (contract no. EVK2-CT-2000-00074). Further details of the project can be found at www.ceh.ac.uk/epidemie.
LITERATURE CITED
- Baker HG. 1974. The evolution of weeds. Annual Review of Ecology and Systematics 5 1–24. [Google Scholar]
- Chawdhry MA, Sagar GR. 1974. Dormancy and sprouting of bulbs in Oxalis latifolia H.B.K. and O. pes-caprae L. Weed Research 14: 349–354.
- Galil J. 1968. Vegetative dispersal in Oxalis cernua. American Journal of Botany 55 68–73. [Google Scholar]
- García-Fayos P, Verdú M. 1998. Soil seed bank, factors controlling germination and establishment of a Mediterranean shrub: Pistacia lentiscus L. Acta Oecologica 19 357–366. [Google Scholar]
- Gimeno I, Vilà M, Hulme PH. Are islands more susceptible to plant invasion than continents? A test using Oxalis pes-caprae in the western Mediterranean. Journal of Biogeography (in press)
- Godfrey R, Lepschi B, Mallinson D 2004. Ecological filtering of exotic plants in an Australian sub-alpine environment. Journal of Vegetation Science 15 227–236. [Google Scholar]
- Grigulis K, Sheppard AW, Ash JE, Groves RH. 2001. The comparative demography of the pasture weed Echium plantagineum between its native and invaded ranges. Journal of Applied Ecology 38 281–290. [Google Scholar]
- Gulias J, Traveset A, Riera N, Mus M. 2004. Critical stages in the recruitment process of Rhamnus alaternus L. Annals of Botany 93 723–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper JL. 1977. Population biology of plants. London: Academic Press.
- Herrera CM, Jordano P, Lopez-Soria LL, Amat JA. 1994. Recruitment of a mast-fruiting, bird-dispersed tree: bridging frugivore activity and seedling establishment. Ecological Monographs 64 315–344. [Google Scholar]
- Houle G. 1998. Seed dispersal and seedling recruitment of Betula alleghaniensis: spatial inconsistency in time. Ecology 79 807–818. [Google Scholar]
- Houle G, Payette S. 1990. Seed dynamics of Betula alleghaniensis in a deciduous forest of north-eastern North America. Journal of Ecology 78 677–690. [Google Scholar]
- Hulme PE. 2004. Invasions, islands and impacts: a Mediterranean perspective. In: Fernandez-Palacios JM, Morici C, eds. Island ecology. Madrid: Asociación Española de Ecología Terrestre, 359–383.
- Hunter JA, Over de Linden AJ. 1958. The Oxalis problem, growth characteristics and control of the two species. New Zealand Gardening 15: 23–31.
- Ibáñez I, Schupp EW. 2002. Effect of litter, soil surface conditions, and microhabitat on Cercocarpus ledifolius Nutt. seedling emergence and establishment. Journal of Arid Environments 52 209–221. [Google Scholar]
- Jordano P, Herrera CM. 1995. Shuffling the offspring: uncoupling and spatial discordance of multiple stages in vertebrate seed dispersal. Ecoscience 2 230–237. [Google Scholar]
- Lambrinos JG. 2002. The variable invasive success of Cortaderia species in a complex landscape. Ecology 83 518–529. [Google Scholar]
- Lane D. 1984. Factors affecting the development of populations of Oxalis pes-caprae L. Weed Research 24: 219–225.
- Lloret F, Médail F, Brundu G, Camarda I, Moragues E, Rita J, et al. 2005. Species attributes and invasion success by alien plants on Mediterranean islands. Journal of Ecology 93 512–520. [Google Scholar]
- Lodge D. 1993. Biological invasions: lessons for ecology. Trends in Ecology and Evolution 8 133–137. [DOI] [PubMed] [Google Scholar]
- Lonsdale WM, Harley KLS, Gillett JD. 1988. Seed bank dynamcis in Mimosa pigra, an invasive tropical shrub. Journal of Ecology 25 963–976. [Google Scholar]
- Peirce JR. 1997. The biology of Australian weeds. 31. Oxalis pes-caprae L. Plant Protection Quarterly 12 110–119. [Google Scholar]
- Pyšek P, Prach K, Šmilauer P. 1995. Relating invasion success to plant traits: an analysis of the Czech alien flora. In: Pyšek P, Prach K, Rejmánek M, Wade M, eds. Plant invasions: general aspects and special problems. Amsterdam: SPB Academic Publishing, 39–60.
- Rey PJ, Alcántara JM. 2000. Recruitment dynamics of a fleshy-fruited plant (Olea europaea): connecting patterns of seed dispersal to seedling establishment. Journal of Ecology 88 622–633. [Google Scholar]
- Schupp E. 1995. Seed–seedling conflicts, habitat choice, and patterns of plant recruitment. American Journal of Botany 82 399–409. [Google Scholar]
- Silvertown JW. 1987. Introduction to plant population biology. New York, NY: Longman.
- Swinton SM, King RP. 1994. A bioeconomic model for weed management in corn and soybean. Agricultural Systematics 44 313–335. [Google Scholar]
- Thompson K, Grime JP. 1979. Seasonal variation in the seed banks of herbaceous species in ten contrasting habitats. Journal of Ecology 67 893–921. [Google Scholar]
- Traveset A, Gulías J, Riera N, Mus M. 2003. Transition probabilities from pollination to establishment in a rare dioecious shrub species (Rhamnus ludovici-salvatoris) in two habitats. Journal of Ecology 91 427–437. [Google Scholar]
- Vilà M, D'Antonio CM. 1998a. Fitness of invasive Carpobrotus (Aizoaceae) hybrids in coastal California. Ecoscience 5 191–199. [Google Scholar]
- Vilà M, D'Antonio CM. 1998b. Hybrid vigor in Carpobrotus in coastal California: effects of herbivory and water availability on clonal growth. Ecological Aplications 8 1196–1205. [Google Scholar]
- Vilà M, Gimeno I. 2003. Seed predation of two alien Opuntia species in Mediterranean communities. Plant Ecology 167 1–8. [Google Scholar]
- Vilà M, Lloret F. 2000. Seed dynamics of the mast seedling tussock grass Ampelodesmos mauritanica in Mediterranean shrublands. Journal of Ecology 88 1–15. [Google Scholar]