Abstract
• Background and Aims The basic regulatory mechanisms that control lateral root (LR) initiation are still poorly understood. An attempt is made to characterize the pattern and timing of LR initiation, to define a developmental window in which LR initiation takes place and to address the question of whether LR initiation is predictable.
• Methods The spatial patterning of LRs and LR primordia (LRPs) on cleared root preparations were characterized. New measures of LR and LRP densities (number of LRs and/or LRPs divided by the length of the root portions where they are present) were introduced and illustrate the shortcomings of the more customarily used measure through a comparative analysis of the mutant aux1-7. The enhancer trap line J0121 was used to monitor LR initiation in time-lapse experiments and a plasmolysis-based method was developed to determine the number of pericycle cells between successive LRPs.
• Key Results LRP initiation occurred strictly acropetally and no de novo initiation events were found between already developed LRs or LRPs. However, LRPs did not become LRs in a similar pattern. The longitudinal spacing of lateral organs was variable and the distance between lateral organs was proportional to the number of cells and the time between initiations of successive LRPs. There was a strong tendency towards alternation in LR initiation between the two pericycle cell files adjacent to the protoxylem poles. LR density increased with time due to the emergence of slowly developing LRPs and appears to be unique for individual Arabidopsis accessions.
• Conclusions. In Arabidopsis there is a narrow developmental window for LR initiation, and no specific cell-count or distance-measuring mechanisms have been found that determine the site of successive initiation events. Nevertheless, the branching density and lateral organ density (density of LRs and LRPs) are accession-specific, and based on the latter density the average distance between successive LRs can be predicted.
Keywords: Root, lateral root, patterning, density, development, initiation
INTRODUCTION
The root system in a higher plant is an essential part of the plant body. It not only anchors the plant in its substrate, mines water and minerals, and transports them above ground, but also is a site of synthesis of many metabolites that are essential for the plant as a whole. Among these metabolites, hormones have a significant role in almost all physiological processes, and it has been shown that cytokinins are synthesized in the root tip and transported to shoot tissues (Werner et al., 2001, 2003; Kieber, 2002; Aloni et al., 2005). In addition, root tips synthesize auxins (Ljung et al., 2005). In order to fulfil its functions the root, as an organ, has evolved an extensively branched system of lateral and adventitious roots. New root production within the root system occurs continuously during the life of a plant. Multiple processes are involved in lateral root (LR) formation operating on multiple levels of organization. One of these processes is LR initiation.
LR initiation is a cardinal point in LR development and forms the basis of root system formation in plants. Within a framework proposed to describe the regulation of root system development, intrinsic pathways determine particular features of a species' root architecture whereas response pathways co-ordinate various environmental stimuli that modulate intrinsic pathways (Malamy, 2005). Although there have been many efforts to understand these pathways (McCully, 1975; Peterson and Peterson, 1986; Torrey, 1986; Charlton, 1996; Malamy and Benfey, 1997b; Lloret and Casero, 2002; Casimiro et al., 2003; Dubrovsky and Rost, 2003; Barlow et al., 2004; Malamy, 2005), the basic regulatory mechanisms that control LR initiation in plants are still poorly understood. The exact mechanisms controlling LR initiation may vary between each plant species studied; nevertheless, LR initiation in general should have fundamental features that are common in large taxa.
With the aid of new experimental approaches, the current study attempts further to develop concepts that may be useful in understanding the intrinsic pathways of LR initiation, and to investigate how these pathways provide for the developmental plasticity to adapt to a heterogeneous environment. To gain insight into how these pathways may operate to control LR initiation, a detailed analysis of the spatial patterning of LR initiation is presented. Arabidopsis thaliana was used as a model plant because with relatively simple methods it permits various types of experimentation. Two wild-type accessions (Col-0 and C24) and an enhancer trap line J0121 in C24 background were used for this analysis. James Haseloff and co-workers (Department of Plant Sciences, University of Cambridge, UK) produced a collection of 250 Arabidopsis enhancer trap lines with distinct and stable patterns of GAL4-VP16 and green fluorescent protein (GFP) expression in the root (http://www.plantsci.cam.ac.uk/Haseloff/geneControl/GAL4/screen.html). In one of these lines, J0121, GFP expression in the root is found only in the pericycle cells adjacent to protoxylem poles. This tissue-specific pattern has been used as marker of pericycle cell identity. For example, it was demonstrated that GFP expression in J0121 roots did not change in the background of an Arabidopsis mutant that is deficient in LR primordium (LRP) initiation, alf4-1 (altered lateral root formation) (DiDonato et al., 2003), or in wild-type plants treated with the auxin transport inhibitor naphtylphtalamic acid (NPA; Casimiro et al., 2001).
In addition, new measures of LR and LRP density are introduced that more accurately represent the differences in LR development observed in mutants and among various Arabidopsis accessions. The study investigates whether LR initiation takes place within a given developmental window: a period of time, or a physiological state, within which a certain developmental process must be initiated and in some cases completed. Finally, the question of whether LR initiation is, on some level, predictable is addressed.
MATERIALS AND METHODS
Plant material and growth conditions
Seed of Arabidopsis thaliana accession Columbia (Col-0) was kindly donated by Gladys Cassab (Instituto de Biotecnología, UNAM, Mexico). The accessions C24 (CS906) and J0121 (CS9090) were obtained from the Arabidopsis Biological Resource Center, Ohio State University. The aux1-7 mutant was kindly donated by Luis Herrera-Estrella (CINVESTAV, Irapuato, Mexico).
Seeds of the Arabidopsis accessions were surface sterilized by incubation in folded Whatman filter paper at room temperature in 20 % bleach, 0·3 % triton X-100 for 10 min followed by four 10-min washes with copious amounts of sterile nanopure water. Seeds were then incubated at 4 °C for 2 d and plated on medium containing 0·2 × MS salts (pH 5·8), 1 % sucrose and 0·8 % agar. Plants were grown for various times at 22 °C under a light intensity of 105 µmol m−2 s−1.
Clearing of tissues, mounting, and microscopic analysis
Material was cleared by using the method of Malamy and Benfey (1997a) with minor modifications. Briefly, seedlings were incubated at 62 °C for 40 min in a solution of 0·24 n HCl and 20 % methanol and then at room temperature for 20 min in a solution of 7 % NaOH and 60 % ethanol. Plants were sequentially rehydrated via incubations in 40, 20 and 10 % ethanol, and in 25 % glycerol diluted in 5 % ethanol (2 h per step). Individual plants were numbered and a root with known growth increments from each plant was cleared and mounted separately from others.
Plants were mounted on slides in the following manner. First, a border of parafilm was made by folding a rectangle that was slightly smaller than the cover-slip and cutting out another rectangle starting from the folded edge. This border was placed on the slide and rubbed into place. Material was mounted in 50 % glycerol within the parafilm border, covered by the cover-slip and then sealed with clear fingernail polish. Samples were then visualized under a Zeiss Axiovert 200 microscope employing Nomarski differential interference contrast (DIC). Only roots that were laying on a slide in a protoxylem plane (see Fig. 3A) were analysed. When roots were twisted, only those that had 85–90 % of their length in the protoxylem plane were analysed; other root preparations were discarded from the analysis.
All measurements were made with an ocular micrometer. The following parameters were measured: distance between successive lateral organs (LRPs or LRs) along each protoxylem-adjacent pericycle cell file (rank), distance from the root tip (excluding the root cap) to the most distal LRP, the length from the most distal LRP to the first emerged LR, and the distance from the first emerged LR to the root base. Number of LRs and LRPs (starting from stage I) was also recorded.
Roots of the enhancer trap J0121 line were divided into 5–10-mm portions, stained with 10 µg mL−1 propidium iodide for 5 min and were then observed under an inverted Zeiss LSM 510 Meta confocal laser scanning microscope. Zeiss ×40 (NA 0·75, Plan Neofluar) dry and ×63 (NA 1·2, C-Apochromat) water-immersion objectives were used. The 543-nm line of an He/Ne laser and the 488-nm line of an Ar laser were used for propidium iodide and GFP excitation, respectively. GFP imaging was performed with a BP 500–530 filter. For image analysis, the Zeiss Image Examiner software, version 3·2, was used.
Characterization of J0121
For Southern blot analysis, 2 µg of digested plant genomic DNA was electrophoresed and transferred to a Hybond-N+ membrane (Amersham Life Sciences, Little Chalfont, UK). To obtain the probe for hybridization, a 0·7-kb fragment of the GAL::VP16 sequence was amplified from DNA of J0121 using the 5′-AAGGAAGTTCATTTCATTTGG-3′ and 5′-CAAGGGCATCGGTAAACATC-3′ primer pair. The amplified fragment was cloned into pCR2·1 (Invitrogen, Carlsbad, CA, USA), thus generating pCR-GAL4 plasmid. Hybridization was performed at 65 °C using a 32P-labelled 0·5-kb BamHI–SstI fragment of the GAL::VP16 gene from the pCR-GAL4 plasmid [P in part A of the figure presented in Supplementary Information, available on the journal website (http://aob.oxfordjournals.org)]. For analysis of kanamycin resistance, plants were grown on solid MS medium supplemented with 25 mg L−1 kanamycin.
Plasmolysis and pericycle cell counts in J0121
Seeds of J0121 were surface sterilized and germinated as described above. In some cases, root length was recorded daily from 4 d after germination in order to access the growth rate at 1-d intervals. In these cases, at 8 d the whole root portion formed from day 4 to the root tip was taken for analysis. For non-measured roots, root sections 3–13 mm from the root tip were harvested. Root portions were placed in plasmolysing solution containing 1 m sorbitol in 100 mm sodium phosphate buffer, pH 7·4, for 1 h at room temperature and subsequently transferred to the same solution supplemented with 4 % paraformaldehyde for an additional 2–3 h at room temperature. Root portions were mounted on slides in plasmolysing solution with fixative and analysed immediately under an epifluorescence microscope.
Cells were counted within a single cell file from the cells directly adjacent to each primordium and only unambiguous measurements were taken. Interprimordial distances were measured with an ocular micrometer from the midline of each LRP, and the approximate stage of LRP development was recorded for both LRPs.
Root mapping experiment
Seeds of J0121 were surface sterilized as described above. Seeds were then incubated at 4 °C for 2 d and plated to special window plates containing the same growth medium as in other experiments. The window plates were prepared by gluing a 24 × 50-mm cover-slip over two windows cut in the bottom of a square 10 × 10-cm plate, constructed in house, and sterilized by X-ray irradiation. Seeds were sown directly above the cover-slip and two additional seeds were sown in between to provide conditioning. Plates were orientated at 45° to the horizon and plants were grown under the same conditions as in other experiments for 8 d. The position and distances between each LR and LRP were recorded for each individual root. These same root portions were mapped again after an additional 7 d of growth (see Fig. 2).
Time parameters
Time parameters were determined essentially by the method described in Dubrovsky et al. (2000). Time passing from one initiation event to the next (between two successive primordia) along one rank [time between lateral organ formation, Tlf (h)] was determined from the equation:
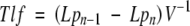 |
where Lpn is the distance from the root tip (excluding the root cap) to the youngest detected primordium in a root, Lpn–1 is the distance to the previously initiated primordium along each rank and V is the rate of root growth (µm h−1).
As no new initiation events took place between already initiated primordia (see Results below) the distance between each successive primordium and/or LR (referred to collectively hereafter as ‘lateral organs’) along each rank was recorded. Tlf was then estimated by dividing this distance by V. In all cases, V was estimated as the rate of root growth during the 24-h period corresponding to the day of growth of that individual root (growth increments were recorded in each individual root and time was calculated separately for each root after clearing) when the respective lateral organs in it were formed. Given the relatively short time interval, steady-state growth conditions in each 24-h interval were assumed.
RESULTS
The enhancer trap line, J0121, is a useful tool for studies of lateral root initiation
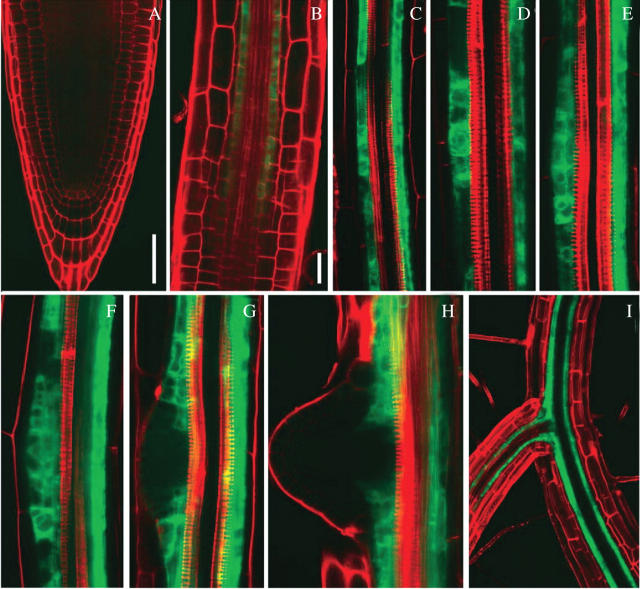
In J0121 plants, GFP expression was absent in the pericycle within the root apical meristem (Fig. 1A). It first appeared in the pericycle within the zone where rapid elongation starts (Fig. 1B) and was maintained in protoxylem-adjacent pericycle cells throughout the root (Fig. 1C). Interestingly, when LR initiation takes place, GFP expression was detected in the primordia up to stage III (classification of Malamy and Benfey, 1997a), as can bee seen in Fig. 1D–F,and at later developmental stages was absent in LRP meristem (Fig. 1G and H). After LR emergence, GFP expression was again detected in protoxylem-adjacent pericycle cells of a LR (Fig. 1I).
Fig. 1.
Expression pattern of green fluorescent protein (GFP) in the enhancer trap line J0121 observed on roots orientated in the protoxylem plane. (A) Root apical meristem. (B) Portion of the elongation zone; note, in the upper root portion expression is detected in the pericycle (4th cell layer). (C–I) Root portions in the differentiation zone. (D–F) Stages I, II and III of lateral root primordium development, respectively. (G) Stage IV of primordium development. (H) Recently emerged lateral root. (I) Junction between primary and lateral root. Before observations, roots of 8 d (A, B, D–H), 16 d (C) and 30 d (I) after germination were divided into 5–10-mm portions and then stained with propidium iodide. Green, GFP fluorescence; red, propidium iodide. Scale bars: A, I = 50 µm; B–H = 20 µm.
To assess the number of T-DNA insertions in J0121, total DNA was digested with either SstI or EcoRI and probed with a BamHI–SstI GAL4::VP16 gene fragment. The hybridization pattern indicated that this line could have two insertions (see Supplementary Information). However, when the F2 progeny of a J0121 × C24 backcross was scored for kanamycin resistance, it showed a 3 : 1 monogenic ratio of resistant versus susceptible plants (n = 200, χ2 = 0·24) rather than a 15 : 1 digenic ratio. F2 kanamycin-resistant plants showed either a high GFP expression similar to that of the parent J0121 line, or a lower level of GFP expression, in a 1 : 2 ratio (n = 58, χ2 = 0·14). Plants with high GFP expression were assumed to be homozygous for the insertion, and plants with lower GFP expression to be heterozygous. As expected, no kanamycin-sensitive F2 plant analysed (n = 21) showed GFP expression in roots. Although it is possible that inactivation of the kanamycin resistance gene may have resulted from recombination or partial insertion, together the data suggest that the J0121 line has either one or two tightly linked T-DNA insertions. In conclusion, the enhancer trap line J0121 has a reproducible and non-segregating GFP expression pattern and can be a useful tool for detection of LR initiation sites.
Developmental windows and growth-control points during LR development
It is generally accepted that LR initiation along the root obeys an acropetal pattern; the primordia are initiated in a more distal root portion relative to already initiated primordia. Although this is in general true, earlier stages of LR primordium development can be found basipetally to more developed LR primordia (Charlton, 1975; MacLeod, 1990; Dubrovsky et al., 2000). This, however, does not exclude the possibility of the simultaneous existence of two phenomena: an acropetal pattern of initiation and a non-acropetal sequence in the development of primordia. This question was addressed through analysing the pattern of LR initiation and development in J0121.
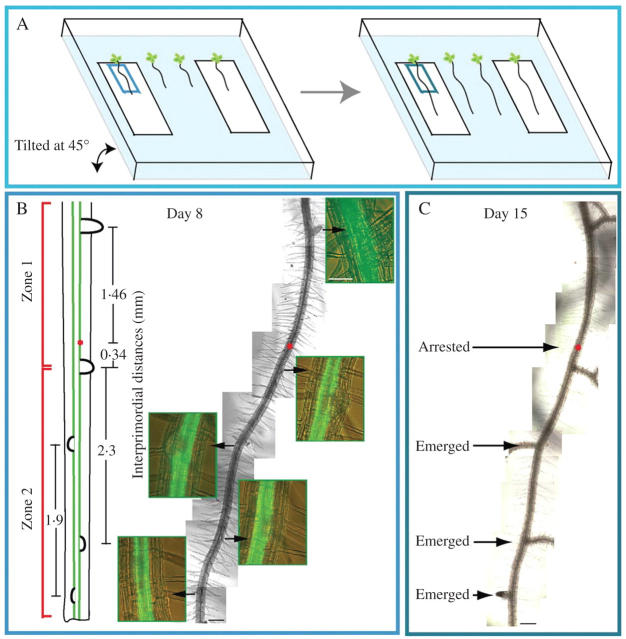
Direct observations were made on live J0121 roots that grew in sterile custom-made Petri dishes with cover-slips on the bottom. In 8-d plants observed under an inverted microscope, using a combination of Nomarski bright-field and epifluorescence, the position of each detectable primordium and LR within the region above the cover-slip was recorded. This method permitted detection of LRP from stage I. To make these observations more accurate and to follow the fate of each primordium under observation, the primary root was divided into two zones. Zone one, ontogenetically formed first, was defined as the root portion from the root base to the most distal emerged LR that can be detected by eye (usually about 0·5 mm). In the same root, zone two was defined as the zone of the root where LR initiation and development takes place. We defined this zone as the root portion that extended from the youngest LR primordium detected in the distal root portion (closest to the root tip) to the first emerged LR (Fig. 2B, left side). To localize each root or primordium later and to define whether new initiation events take place between already developed primordia, the distance between each successive lateral organ along each rank was recorded and a position map was constructed. One week later, the same root portion was analysed and developmental changes were recorded (Fig. 2).
Fig. 2.
Summary of the experimental method for the mapping of lateral root formation. (A) Diagram illustrating the experimental method allowing for analysis of the same root portion (blue rectangles) at day 8 and later at day 15. (B) At day 8, live roots (right) were examined and lateral roots and lateral root primordia were visualized using a combination of epifluorescence and Nomarski optics (green insets). Schematic representations (left) were rendered of each root including inter-lateral organ distance (mm) and zonation. Red dot indicates the presence of a stage I primordium. (C) At day 15, live roots were examined again and primordium initiation and lateral root formation were analysed. Scale bars = 200 µm (for root portion) and 50 µm (for all green insets).
This analysis demonstrated that in roots of 8-d plants, within zone one, 34 % of lateral organs were primordia. This indicated that at least in J0121 the developmental sequence of primordium formation was not strictly acropetal given that primordia were found between emerged roots. Seven days later, only 37·5 % of these primordia emerged as LRs and the remaining 62·5 % of primordia did not (Table 1). This observation confirmed the conclusion that the development of primordia was not strictly acropetal. Primordia in zone two followed a similar emergence pattern. About two-thirds of all primordia in zone two (defined at 8 d) subsequently formed mature LRs, whereas the others remained unemerged during the time interval studied (Table 1). Most importantly, we did not find any de novo LR initiation events taking place between already initiated LR primordia or LRs (Table 1). This strongly suggests that the pattern of LR initiation was acropetal.
Table 1.
Time-lapse analysis of lateral root development in line J0121
| Day 8 |
Day 15 |
||||
|---|---|---|---|---|---|
| LRs | LRPs | % LRPs emerged | % LRPs un-emerged | Incidence of de novo LR development | |
| Zone 1 | 62 | 32 | 37.5 | 62.5 | 0 |
| Zone 2 | – | 67 | 71.6 | 28.4 | 0 |
In total, 24 plants were analysed. Zones are indicated as explained in the text and shown in Fig. 2B. LRs, lateral roots; LRPs, lateral root primordia.
In Arabidopsis wild-type 8-d plants, the earliest LR primordia were found at a distance ranging from 2·05 to 5·42 mm (C24, average 3·54 mm, n = 14) and from 2·68 to 6·20 mm (Col-0, average 3·95 mm, n = 16) from the root tip (excluding the root cap). Based on these data and on the average rate of root growth between days 7 and 8, it was estimated that the developmental window was ‘opened’ for 15·7 and 10·4 h in C24 and Col-0, respectively. This period of time was calculated in the same way as that between two successive lateral organs along one rank (see Materials and methods), but only considering minimum and maximum distances from the tip to the youngest primordium found in a sample of roots and irrespective of rank. The most distal primordia were found at stage I in 82 and 56 % of cases in C24 and Col-0, respectively. Because the most distal primordia in both accessions were found also at stages II and III, this time period represents an overestimation and the developmental window should be operating over an even shorter time. These results demonstrate that in Arabidopsis LR initiation takes place in a relatively narrow developmental window.
Longitudinal spacing of lateral organs within the same rank of the root
The position of each primordium is an indication of where it has been initiated with respect to others. Thus, an analysis of the spatial patterning of root lateral organs can help to determine whether spatial cues dictate where or when a primordium has to be initiated.
Each rank represents a unit of spatial regulation of LR initiation along the root (Mallory et al., 1970; Charlton, 1983; Barlow and Adam, 1988). It has been proposed that primordium initiation sites are determined by a pattern resulting from the history of previous cell divisions (Charlton, 1983; Barlow and Adam, 1988). If this is the case for Arabidopsis, then the rank-specific distance between nearest lateral organs should follow a pattern. The distance between successive lateral organs within a rank in roots of 8-d plants of J0121, in the wild-type accession of this line, C24, and in the widely used wild-type accession Col-0 was analysed. Roots with known growth rates were treated individually, cleared and distances were measured.
A summary of the distributions of inter-lateral organ distance is given in Table 2. The average distance was 47, 23 and 25 % of the maximum distance for J0121, C24 and Col-0, respectively, indicating that overall distribution was skewed to the left. This distribution thus indicated that smaller distances were found more frequently than larger distances within a rank. No apparent modality was found in the lines studied (data not shown).
Table 2.
Summary of the distributions of distances between nearest lateral organs within one rank in the roots of Arabidopsis 8 d after germination within zones one and two
| Line or accession | Minimum distance (mm) | Maximum distance (mm) | Median distance (mm) | Average distance (mm) | No. of distances measured/no. of plants |
|---|---|---|---|---|---|
| J0121 | 0.20 | 4.4 | 2.00 | 2.07 | 105/75 |
| C24 | 0.22 | 6.34 | 1.27 | 1.46 | 109/14 |
| Col-0 | 0.30 | 7.54 | 1.44 | 1.87 | 112/19 |
Data for C24 and Col-0 were collected from cleared preparations of whole roots, whereas data for J0121 were collected on plasmolysed root portions 3–13 mm from the root tip or on the root portions formed between days 4 and 8 (see Materials and methods). As smaller root portions were analysed in J0121, existing maximum distances were underestimated. In addition, only those distances where pericycle cell number was possible to score between lateral organs were taken for analysis, so the number of J0121 plants analysed was much greater than for C24 or Col-0. This explains why the data for J0121 and C24 are slightly different.
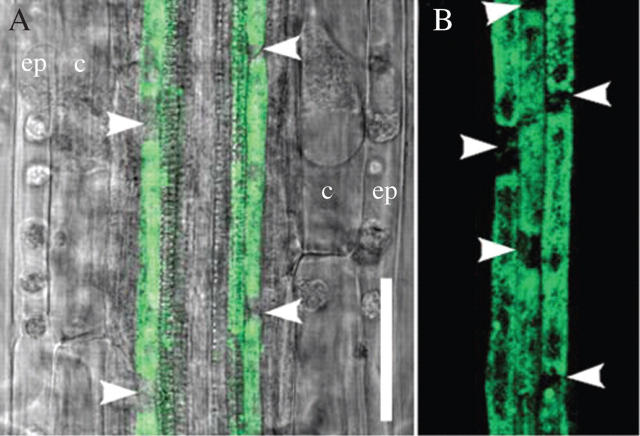
The number of pericycle cells between successive primordia is rarely determined (Hinchee and Rost, 1992) because of technical difficulties; however, we have developed a method to plasmolyse pericycle cells in J0121 and at the same time to maintain GFP fluorescence (see Materials and methods). After plasmolysis, individual pericycle cells were clearly visible regardless of root orientation (Fig. 3), allowing estimation of the distance in units of length, and also in the number of pericycle cells. This and other analyses provided correlations between inter-lateral organ distance and time required for initiation of successive primordia; between distance and the number of cells (for J0121) an increase in the time, or in cell number, between primordia was proportional to the distance between successive lateral organs (Fig. 4).
Fig. 3.
Roots of J0121 subjected to plasmolysis with 1 m sorbitol. Root in protoxylem (A) and protophloem (B) plane. (A) Merged image of green fluorescent protein (GFP) fluorescence and Nomarski bright-field; note, plasmolysed cells in epidermis (ep) and cortex (c). Arrowheads indicate approximate position of pericycle end-walls. Scale bars = 50 µm.
Fig. 4.
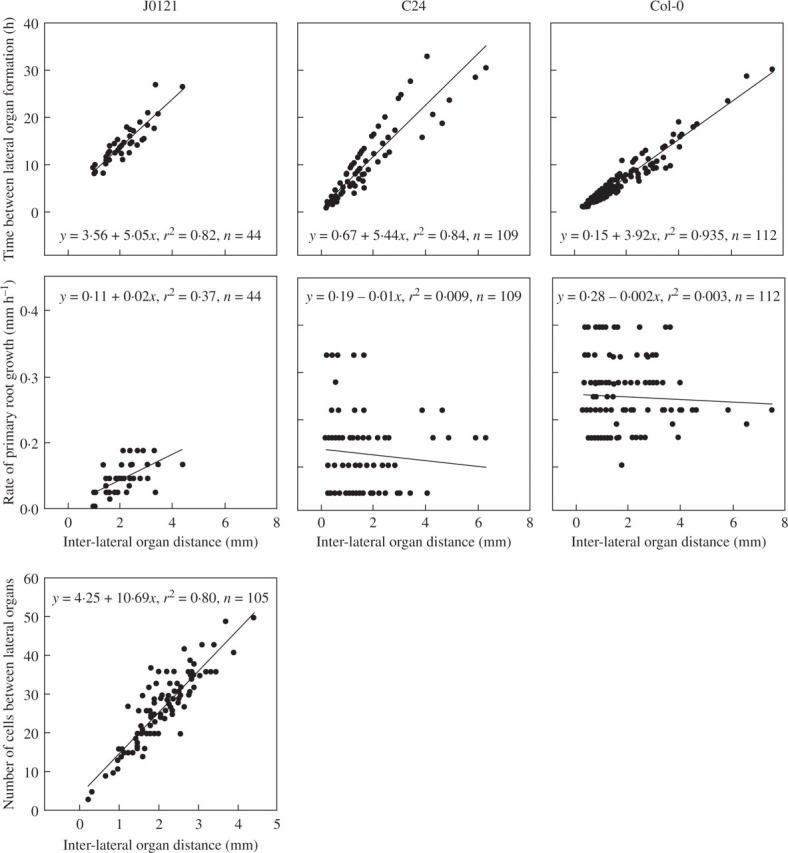
Correlation analysis of interactions between inter-lateral organ distance and time required for initiation of successive lateral organs, between inter-lateral organ distance and the rate of root growth at time of initiation (C24, Col-0 and J0121), and between inter-lateral organ distance and the number of pericycle cells (J0121). All data are shown and regression lines are drawn. Equations and correlation coefficients, and number of measurements (n) are given.
Co-ordination of initiation of LRPs between two ranks
In a diarch root of A. thaliana it is relatively simple to analyse whether there is a certain order in initiation of successive primordia in an acropetal pattern between the two ranks of the protoxylem-adjacent pericycle cell files. In the majority of cases, successive lateral organs were arranged in an alternating pattern. When lateral organs were formed in a non-alternating pattern the majority were grouped in twos, and the numbers of grouped non-alternating lateral organs significantly decreased (Table 3). These data clearly indicated that there was a strong tendency towards alternation when successive primordia are initiated. We hypothesized that when lateral organs were initiated in an alternating pattern, the distance between them should be shorter than the distance between nearest primordia initiated in a non-alternating pattern. For all three lines analysed this hypothesis was rejected (data not shown), indicating that the distance between successive primordia does not depend on the spatial pattern of initiation established between two ranks in the Arabidopsis root.
Table 3.
Two-rank spatial analysis of initiation of lateral organs formed over a period of 15 d (J0121) and 16 d (C24 and Col-0) after germination in the roots of Arabidopsis plants
| Percentage of cases of non-alternating lateral organs that are grouped in: |
||||||||
|---|---|---|---|---|---|---|---|---|
| Accession | Percentage of cases where lateral organs are in an alternating pattern | No. of cases/roots analysed | 2 | 3 | 4 | 5 | 6 | 7 |
| J0121 | 71.7 | 60/13 | 58.3 | 33.3 | 9.3 | 0.0 | 0.0 | 0.0 |
| C24 | 63.1 | 594/38 | 69.0 | 22.8 | 6.3 | 2.0 | 0.0 | 0.0 |
| Col-0 | 62.2 | 370/31 | 70.1 | 21.8 | 5.7 | 0.0 | 1.2 | 1.2 |
The experiments demonstrated that along the same rank in 80 % of cases (excluding the smallest distances) the minimal distance between primordia was equal to or greater than 1·45, 0·56 and 0·80 mm in J0121, C24 and Col-0, respectively, and on average 66 % (all lines analysed) of lateral organs in the root were found in an alternating pattern. It was estimated that in the majority of cases successive groups of founder cells were activated at distances that were greater than approximately 5–14 diameters of established primordia. This indicates that some mechanism exists that determines a minimum distance between successive primordia.
Density of LRs and LRPs
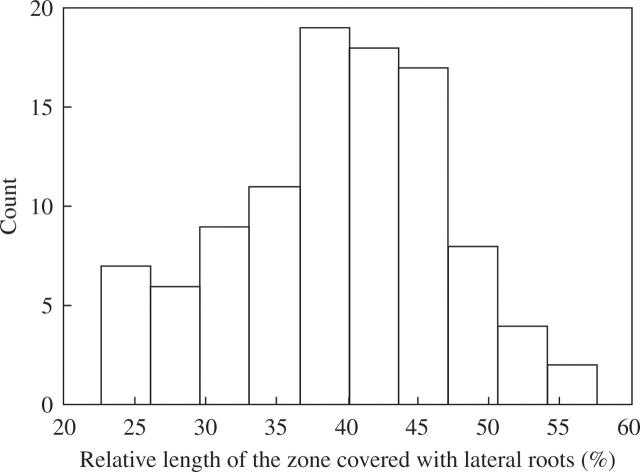
The lack of an acropetal pattern of primordia development described above prompted us to analyse this phenomenon quantitatively. LR density is customarily calculated as a ratio between the number of LRs and the total length of the primary root, and most studies do not include LRPs in their analyses. This approach, widely used in current literature, represents an estimation of overall compactness of the root system (Cernac et al., 1997; Marchant et al., 2002; Chevalier et al., 2003; Gray et al., 2003; Oono et al., 2003; Bao et al., 2004; Hawker and Bowman, 2004; Nodzon et al., 2004; López-Bucio et al., 2005; Loudet et al., 2005) and is hereafter termed the ‘customary density’. These overall estimations can be applied only when in all ecotypes, and at all ages, the ratio between the root portion covered by LRs and the total primary root length remains constant, a condition that is rarely fulfilled. Even in plants of the same age, this ratio is variable in a population of 101 wild-type plants of the Col-0 Arabidopsis (Fig. 5). In this example, in 8-d plants, relative length of the root covered by LRs varied from 24·3 to 55·8 % of total root length. Therefore, it is important to estimate LR density not per total root length but per portion of the primary (or parent) root where LRs are present (within zone one). The term ‘branching density’ is used frequently in the description of root system architecture of field-grown plants and in the development of root architecture models (Johnson and Aguirre, 1991; Pagès and Pellerin, 1994; Mollier and Pellerin, 1999; Freixes et al., 2002; Pagès et al., 2004). By definition, branching density is the density of lateral roots formed on the parent axis. This term was adopted here and branching density was calculated as the number of LRs divided by the length of only the portion of the parent root where LRs emerged. In addition, changes in LRP density were analysed in the zone where LRs emerged (zone one) as defined earlier (see exact definitions of the zones in the Developmental Window subsection). A new measure of density, lateral organ density, was introduced which reflects the density of all lateral organs (LRs and LRPs) per portion of the primary (or parent) root where these lateral organs are present. This analysis was undertaken in plants of 8 and 16 d after germination. To make this analysis more accurate, the data were obtained on cleared roots (see Materials and methods), and LRPs starting from stage I were scored.
Fig. 5.
Histogram of the distribution of the relative length of the branching root zone (%) in 8-d Col-0 plants (n = 101). The relative length of this zone was calculated for each plant as a percentage of the total root length.
As discussed above, in 8-d plants, zone one contained both LRPs and emerged LRs, and a given percentage of arrested or slowly developing LRPs later emerged in 15-d plants. In an independent analysis, it was found that the density of LRPs in zone one in 8-d plants was relatively high and comparable with the branching density (Fig. 6). The branching density increased significantly between 8 and 16 d in both J0121 (Student's t-test, P < 0·05) and C24 (Student's t-test, P < 0·001), although no significant increase was found in Col-0. The density of LRPs in zone one decreased 4·2-, 3·9- and 2·7-fold during the same period for J0121, C24, and Col-0, respectively (Student's t-test, P < 0·001; Fig. 6). These changes in densities most likely resulted from the emergence of those LRPs that were found in zone one at day 8.
Fig. 6.
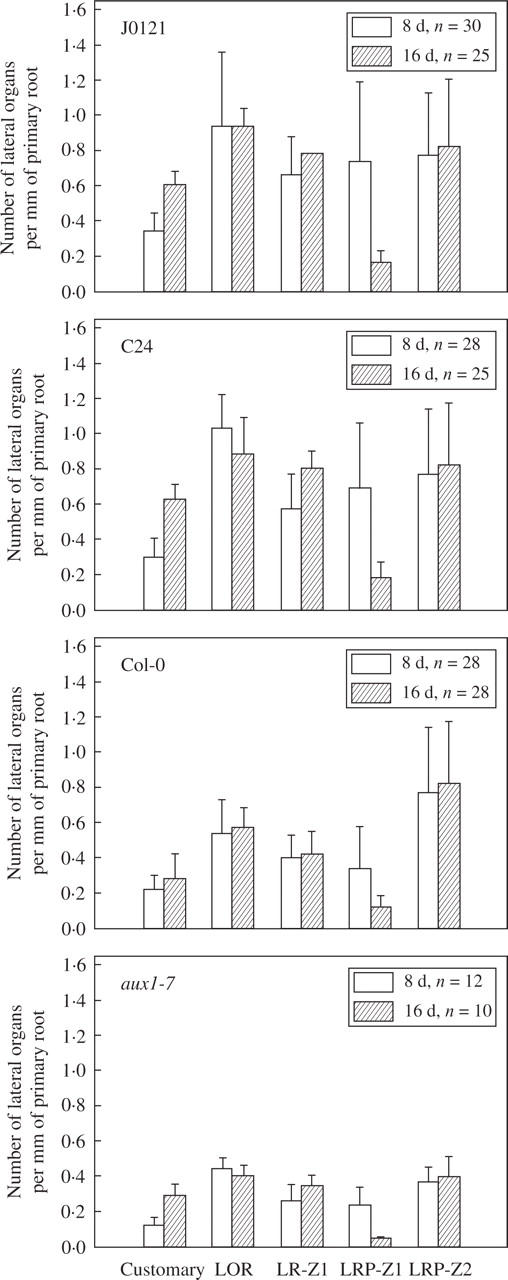
Changes in the density of lateral organs in J0121, C24, Col-0 and aux1-7 plants 8 and 16 d after germination. Customary density was calculated as the number of emerged lateral roots divided by the total root length. The lateral organ density (LOR) was calculated as the number of all lateral organs divided by the root portion where they are present. The branching density (LR-Z1) was calculated as the number of emerged lateral roots divided by the length of the root portion where LRs are emerged (zone one). The density of primordia within this zone (LRP-Z1) was calculated as the number of primordia within the zone divided by the length of the zone. The density of primordia within zone two (LRP-Z2) was calculated as the number of primordia within the zone (see Fig. 2B) divided by the length of the zone. Combined data of at least two independent experiments; n is indicated in each case.
LRP density in zone two did not change between days 8 and 16 in J0121 and C24 (Student's t-test, P > 0·05). In Col-0 it increased 49 % (Student's t-test, P < 0·001) during this period, indicating that there was an increase in the rate of LRP initiation. Such an increase in LRP initiation can be assumed under the condition that the average length of fully elongated cells did not change during this period. Indeed, in this case average cortex cell length was not changed (data not shown). Under conditions of severe water stress in desert Cactaceae, lateral organ density increases proportionally to a decrease in the epidermis cell length, indicating there is no actual acceleration in LRP initiation even when density increased and demonstrating that LRP initiation in the studied species is not affected by water stress (Dubrovsky and Gómez-Lomelí, 2003). This example illustrates that when estimating the rate of LR initiation it is important to consider possible changes in average cell length.
Lateral organ densities determined by the novel method were compared with those as customarily determined. In plants of 8 and 16 d, the lateral organ density remained relatively constant in J0121 and Col-0 but significantly decreased in C24 (Student's t-test, P < 0·05; Fig. 6). Densities measured customarily were always less that the branching densities (at day 8, they were 2·0, 1·9 and 1·8 times less than the branching density in J0121, C24 and Col-0, respectively; Student's t-test, P < 0·001). Furthermore, customary density always increased significantly between 8 and 16 d (Student's t-test, P < 0·05 for all lines).
To provide a comparison of the densities proposed here with the customary method lateral organ density was measured in the mutant aux1-7 deficient in lateral root formation (Marchant et al., 2002). These authors showed that customary density gradually increased between 5 and 13 d after germination and that it was significantly lower in the mutant. Similar results showing a gradual increase in customary density were obatined here (Fig. 6). In experiments described here, branching density in 8-d aux1-7 plants was 1·5 times lower than in Col-0 (Student's t-test, P < 0·01), but in 16-d aux1-7 plants it was similar to that in Col-0 (Student's t-test, P > 0·05). As the density of LRPs in zone one significantly decreased between 8 and 16 d (Fig. 6), this indicated that primordia arrested (or slowly developing) in 8-d plants emerged later. Thus, AUX1 is seemingly not involved in the emergence of slowly developing primordia. The lateral organ density in this mutant was similar to that of Col-0 at 8 d but at 16 d became significantly lower (Student's t-test, P < 0·001), indicating a decrease in overall LRP initiation. To verify this, the density of LRPs in zone two was analysed. Although there were no differences in 8-d plants, at 16 d the LRP density in aux1-7 zone two decreased almost two-fold compared with Col-0 (Student's t-test, P < 0·002; Fig. 6). The length of fully elongated cortical cells in 16-d aux1-7 plants was similar to that in Col-0 (data not shown). Analysis of LRP density in zone two demonstrated that AUX1 is required for LRP initiation. This same conclusion was reached previously based on analysis of LRP developmental stages on root sections (Marchant et al., 2002). This example illustrates that important aspects of LR development are revealed with the method proposed here and that it permits quantitative and adequate estimation of LR initiation.
DISCUSSION
Developmental window and acropetal pattern of root initiation
Various growth control points operate in root development (Laskowski et al., 1995; Malamy and Benfey, 1997a, b; Dubrovsky et al., 2000; De Smet et al., 2003), but whether or not specific developmental windows exist for processes such as LR initiation and/or emergence remains unknown. In most plants LR initiation begins with the activation of pericycle founder cells that give rise to a population of actively dividing cells of the LRP. A developmental window defining cell competence to produce LRs has been established for pea roots (Gladish and Rost, 1993), although LRP initiation was not studied in that work.
The fact that LR initiation in Arabidopsis is restricted to a developmental window implies that there is a regulatory mechanism to control such a pattern. Most probably, nutritional and hormonal factors play a significant role in this control. Many studies have demonstrated that this pattern can be broken with external hormonal treatment. Exogenous application of the auxins induces initiation of LR primordia between already formed LRs (Blakely et al., 1982; Lloret and Pulgarín, 1992; Laskowski et al., 1995; Kerk et al., 2000). Thus, the concept of a developmental window can be applied only to intact untreated roots. Our research suggests that these regulatory mechanisms determine the competence of pericycle cells to produce (or activate already determined) founder cells, maintain an acropetal pattern of LR initiation, and operate during a relatively short time within the zone distal to the youngest LRP.
The acropetal pattern of LR initiation found in this study of Arabidopsis roots is not necessarily present in other plants. For example, MacLeod (1990) reported that in maize roots, there is a gradual increase in the number of lateral organs in each of 5-mm portions up to 8 cm from the tip (from on average 0·4 to 4·6 lateral organs per portion). This clearly indicates that LR initiation does not proceed acropetally in maize roots and that new initiation takes place between already initiated primordia during normal development. In this case, the developmental window for LR initiation appears to be relatively wide.
In Arabidopsis once LR primordia are initiated, their pace of development can be variable even in two successively initiated primordia. In the current study, the common occurrence of arrested or slowly developing primordia at various stages during normal development indicates that LRP initiation, LR meristem establishment and root emergence can be interrupted. Primordia present between already emerged LRs were also observed in other studies (Dubrovsky et al., 1998; Zhang et al., 1999; Deak and Malamy, 2005). The fact that in Arabidopsis some of these primordia later emerge and grow indicates that after initiation takes place no other strict developmental window exists that prohibits primordium meristem establishment or LR emergence under the conditions studied. These results support that LR initiation, meristem establishment and LR emergence represent growth-control points that operate during normal root development. These appear to be controlled independently, providing a level of plasticity during root system formation.
Spatial patterning
It is unknown whether in Arabidopsis the number of pericycle cells between successive primordia in one rank is a factor important for the determination of the site of LRP initiation. Answering this question in wild-type plants is difficult because pericycle cells are long vacuolated cells that can be up to 300 µm in length (Dubrovsky et al., 2000). The dye propidium iodide, commonly used for root studies, does not penetrate differentiated tissues of living roots (J. G. Dubrovsky, personal observation), and even when excised root portions are stained as in this study, pericycle end-walls cannot be detected easily (Fig. 1). Because only protoxylem-adjacent pericycle cells can be counted, extensive histological work is required. The plasmolysis-based method here permitted identification of end cell walls in the pericycle, but even with this method vacuolization made some intervals between lateral organs impossible to score. This analysis demonstrated high variability in the number of cells between successive lateral organs.
In J0121 plants the number of cells between successive lateral organs increased in proportion to the distance, an this can be extrapolated to C24 and Col-0 plants. The data presented in Fig. 4 indicate that neither time nor cell number was deterministic for defining the site of new primordium initiation. Interestingly, in all three lines analysed there was no correlation found between inter-lateral organ distance and the rate of root growth. This may indicate that plant nutritional status affecting the rate of root growth does not have a role in the determination of the site of a new initiation event. Both slowly and rapidly growing roots may have shorter and longer distances between successive primordia (Fig. 4). Similar findings that inter-lateral distances are independent of root growth rate were reported for banana roots (Draye, 2002).
A certain level of regularity in the spacing of LRs has been demonstrated in some species (Mallory et al., 1970; Charlton, 1983; Barlow and Adam, 1988). Analysis of the spatial distribution of primordium initiation within a rank in Arabidopsis demonstrated no regular patterning. The ratio between maximum and minimum distances within one rank was equal to 29 and 25 in C24 and Col-0, respectively. The absence of any modality in inter-lateral organ distances indicates the complex regulation of determination of LR initiation site. The analysis presented here is based on the supposition that one rank represents a unit of spatial regulation of LR initiation (see Results). However, we cannot exclude the possibility that in diarch roots such as those of Arabidopsis spatial patterning of inter-lateral organ distance may be established irrespective of rank. Further work is required to solve this problem. One indication that this is the case is a strong tendency to alternation in initiation sites of successive lateral organs between two ranks found in the current study. This tendency demonstrates that some level of regular patterning exists, and that some as yet unknown mechanisms operate to determine founder cell activation for LRP formation.
Independent of the high variability in inter-lateral organ distance along one rank, the average distance between nearest lateral organs appears to be a general characteristic of each accession. To evaluate the general pattern of primordium initiation in a species or an accession, LR and LRP densities may serve as important quantitative parameters describing the general ability of a parent root to form lateral organs.
Density of LRs and LRPs
There are two fundamental reasons why the customary means of measuring LR densities does not reflect the actual LR density or changes in this value during development. First, by including the variable non-LR-bearing root portion, noise is inadvertently introduced and actual density values that reflect the true distances between successive LRs will always be underestimated. Second, the customary density will always tend to increase artificially with developmental time because existing LRPs not included in the measure at an earlier developmental stage later emerge, thus resulting in an increase in density. Compounding the problem is that as the root grows longer the non-LR-bearing root portion always makes up a smaller percentage of the overall root length. The result is that with developmental time the customary density increasingly underestimates actual LR density. This is reflected in the data presented here; in all lines examined, customary density increases between 8 and 16 d of growth whereas lateral organ density stays the same for J0121 and Col-0 or decreases for C24 (Fig. 6). Similar increases in customary density over time have also been reported for both wild-type and aux1-7 plants (Marchant et al., 2002).
Branching density and lateral organ density are apparently accession-specific. Lateral organ density was the same in J0121 (which is in a C24 background) and C24 both in 8-d and 16-d plants (P > 0·05, Student's t-test), but was about two-fold lower in Col-0. Comparison of both spatial patterning and the densities of primordia in J0121 and C24 demonstrated no differences for most of the parameters studied. This indicates that in J0121, T-DNA insertion apparently did not affect root phenotype and thus the observations for J0121 could be valid for plants with C24 genetic background. F2 progeny segregation after backcrosses indicated that kanamycin resistance and GFP expression co-segregate in a monogenic mode. Together with results of Southern hybridization, this suggests that J0121 has only one functional insertion and could easily be used for pericycle and LRP developmental analyses in the genetic background of various mutants.
It was of interest to evaluate density as a measure of LR initiation, and in this regard, as noted in the Results, the length of fully elongated cells should be considered. The pericycle cells in the young differentiation zone maintain proliferation and become shorter, whereas in the more differentiated zone their proliferation activity is decreased (Dubrovsky et al., 2000). As there is no sliding growth in plants (Sinnot and Bloch, 1939; Brumfield, 1942), it can be assumed that in an accession, or under certain growth conditions, the ratio between average cell length on non-pericycle cells and that of the protoxylem-adjacent pericycle cells is constant. Interestingly, whereas in 16-d plants there was a difference in lateral organ density in C24 and Col-0, there were no differences in the average length of elongated cortical cells in these accessions. Therefore, these density differences are real and indicate that C24 initiated more LRPs per unit length than Col-0, and that in C24 more LRPs were produced per average number of pericycle cells in a rank. To make this analysis more conclusive, it is necessary to evaluate the length of pericycle cells in the differentiation zone. These measurements would need to be made on histological sections and are extremely time consuming. Nevertheless, we consider that for evaluation of the rate of LRP initiation, density data lacking an evaluation of cell length would be insufficient.
With the approach here, the branching density in roots was calculated based on data on the length of the branching root zone and the number of LRs reported in Chevalier et al. (2003). Branching density in the Arabidopsis accessions Cvi-0, Col-4, Ler-1 and Ws-1 were 2·85, 3·70, 4·14 and 3·29 LRs per cm, respectively, and branching density was 1·4- to 2-fold greater that the customary density reported here for these accessions. Thus, these estimations and our data demonstrate that there are, apparently, intrinsic differences in branching density between different accessions. This example also shows that branching density can be a sufficient parameter to discern differences between different accessions, as with C24 and Col-0 here, and that it is an important descriptive element of root architecture in general. In other species, branching density (although not always termed as such) was also shown to be a relatively stable parameter in isolated and intact roots (Barlow and Adam, 1988; Mollier and Pellerin, 1999; Freixes et al., 2002). Customary density when used in complex analyses such as of quantitative trait loci (Loudet et al., 2005) or large-scale analysis of the effect of phosphate availability on root system architecture (Chevalier et al., 2003) may introduce undesirable errors.
Predictability of primordium initiation
In Pteridophytes, a repetitive and relatively regular pattern of LR spacing has been reported that can be attributed to a merophytic organization of the root apical meristem (reviewed in Charlton 1996). A regular pattern was also found to exist in some Angiosperms (Mallory et al., 1970; Charlton, 1983) and can be determined within the root apical meristem post-germination (e.g. in banana, Musa acuminata, Charlton, 1982) or even during embryogenesis (e.g. in cucumber, Cucumis sativus, Dubrovsky, 1987). In most Angiosperms, however, initiation takes place in the differentiation zone of the growing root. It is generally considered that there is no pattern of LR spacing and thus that LR initiation is an unpredictable, stochastic process. In most studies, usually only developed lateral organs (lateral roots) have been analysed. To determine if there is a pattern of LR spacing, and whether LR initiation is predictable, it is crucial to consider both primordia and developed roots. It is known that in stressful environmental conditions, such as drought, primordia can be arrested and subsequently emerge very rapidly when water becomes available (Dubrovsky et al., 1998). In Arabidopsis, there is a fraction of LRPs that are arrested (or slowly developing) and subsequently emerge as the plants age (Table 1). LR initiation occurs during a relatively narrow developmental window (this study), so in assessing the predictability of LR initiation it is not critical to have information on the subsequent fate of initiated primordia (whether they developed into LRs or not).
Spatial and temporal information regarding primordia initiation for two accessions of Arabidopsis permitted us to address the problem of predictability. We have shown that determination of the site of primordium initiation is highly variable. Inter-lateral organ distance was not dependent on the rate of root growth or on the number of pericycle cells between successive primordia within the same rank. As a result, the time that passed between the initiation of one primordium and a new initiation event along one rank ranged from 1·8 to 17·2 h (C24) or from 2·3 to 13·7 h (Col-0) in 80 % of cases (excluding the 10 % lowest and 10 % highest values). The experiments described here show that hypothetical internal mechanisms controlling LR patterning, such as the strict counting of pericycle cells or the perception of a particular distance or time interval, do not function in this system.
In spite of the highly variable behaviour of LR initiation, several independent lines of evidence provide a foundation from which a level of predictability can still be established. Each accession of Arabidopsis analysed here has its own average lateral organ density (considering the density of both LRPs and LRs per parent root where these lateral organs are present). From the density data a theoretical (predicted) average distance can be deduced. For example, for 8-d C24 plants the average lateral organ density was 1·03 mm−1. Considering that the Arabidopsis root is a diarch, the lateral organ density along one rank should be 0·515 mm−1 (two-fold lower than the lateral organ density). From this the theoretical (predictable) distance between successive lateral organs can be estimated. A lateral organ density of 0·515 mm−1 is the same as one lateral organ per 1·94 mm. In reality, the average inter-lateral organ distance was equal to 1·46 mm (Table 2), which is similar. Similar estimations for predictable distance in J0121 and Col-0 would be 2·13 and 3·70 mm, again relatively close to real average distance of 2·07 and 1·87 mm, respectively (Table 2). Interestingly, in J0121 the data on root density (n = 30) and on inter-lateral organ distances (n = 75) were collected from different samples, but the predicted distance was very close to the estimated distance. For Col-0, the predicted distance was almost twice that of the estimated distance. However, considering that the maximum measured distance in Col-0 was 25-fold greater than the minimum, this prediction is still relatively accurate. As the density represents an average for the root, this can produce mathematical noise, which may explain why in some cases the predicted distance is different from the estimated average distance. Using this approach, only average distances can be estimated.
In addition, it was demonstrated here that in the majority of cases there is an alternation in the position of initiation between ranks. The findings that (a) there is a strong tendency towards an alternation of LR position between ranks and (b) no new LR initiation events took place between already initiated primordia allowed us to make general predictions. The fact that there is to some degree predictability makes it possible to visualize and model the general pattern of LR initiation. In polyarch roots, this concept may have certain limitations. However, there are cases where even in polyarch roots a pattern of initiation can be followed in time (Charlton, 1983) and thus can be predicted. The analysis here demonstrates that a level of predictability is operative and can be applied within the context of developmental plasticity.
In conclusion, through applying the concepts of developmental window, spatial patterning, density and predictability LR initiation in Arabidopsis plants was analysed. The relatively narrow developmental window for LRP initiation found in this study permitted a quantitative analysis of LRP initiation to be made. Simple evaluation of the number of roots does not permit evaluation of the rate of initiation. However, comparing the density of all lateral organs (LRs and LRPs), lateral organ density and the density of LRPs in zone two during plant ontogenesis provides an adequate means of analysing how LRP initiation may change in the same plant species over time, or under different environmental conditions. High plasticity in lateral root development certainly should be functional for ecological fitness. In Arabidopsis, no specific cell-count or distance-measuring mechanisms have been found that function for determination of the site of successive initiation events. Nevertheless, branching density and lateral organ density are apparently accession-specific in Arabidopsis, and perhaps species-specific in different taxa, indicating the existence of certain intrinsic control mechanisms and parameters operating during LR initiation. The finding that there was no de novo initiation events and the introduction of a measure of lateral organ density allowed us to predict an average distance at which new initiation events should occur in a growing root. Further studies are required to answer many questions about how developmental plasticity in LR initiation is maintained within certain developmental restrictions, and how these parameters are established in different plant species.
SUPPLEMENTARY INFORMATION
Information on Southern blot hybridization is available online at http://aob.oxfordjournals.org, consisting of a schematic representation of T-DNA integrated into plant DNA of the J0121 line and a visualization of restriction fragments.
Acknowledgments
We thank Drs G. Cassab, J. Haseloff, L. Herrera-Estrella and M. Estelle, and the Arabidopsis Biological Resource Center, Ohio State University, for donation of seeds of Col-0, J0121 (CS9090), C24 (CS906) and aux7-1 (CS3074). We also thank S. Napsucialy-Mendivil, A. Saralegui and A. Ocádiz-Ramírez for their excellent technical help, and N. Doktor for her help with some figures. J.G.D. was partially supported by Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica (PAPIIT), Universidad Nacional Autónoma de México, UNAM (Projects IN 210202 and IX225304), and by Fondo Sectorial CONACYT-SEMARNAT (Project SEMARNAT-2004-C01-80). G.A.G. was supported by a US Department of State Fulbright-García Robles Scholarship.
LITERATURE CITED
- Aloni R, Langhans M, Aloni E, Dreieicher E, Ullrich CI. 2005. Root-synthesized cytokinin in Arabidopsis is distributed in the shoot by the transpiration stream. Journal of Experimental Botany 56: 1535–1544. [DOI] [PubMed] [Google Scholar]
- Bao F, Shen J, Brady SR, Muday GK, Asami T, Yang Z. 2004. Brassinosteroids interact with auxin to promote lateral root development in Arabidopsis. Plant Physiology 134: 1624–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow PW, Adam JS. 1988. The position and growth of lateral roots on cultured root axes of tomato, Lycopersicon esculentum (Solanaceae). Plant Systematics and Evolution 158: 141–154. [Google Scholar]
- Barlow PW, Volkmann D, Baluška F. 2004. Polarity in roots. In: Lindsey K, ed. Polarity in plants. Oxford: Blackwell Publishing, 192–241.
- Blakely LM, Durham M, Evans TA, Blakely RM. 1982. Experimental studies on lateral root formation in radish seedling roots. I. General methods, developmental stages, and spontaneous formation of laterals. Botanical Gazette 143: 341–352. [Google Scholar]
- Brumfield RT. 1942. Cell growth and division in living root meristems. American Journal of Botany 29: 533–543. [Google Scholar]
- Casimiro I, Marchant A, Bhalerao RP, Beeckman T, Dhooge S, Swarup R, et al. 2001. Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell 13: 843–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casimiro I, Beeckman T, Graham N, Bhalerao R, Zhang H, Casero P, et al. 2003. Dissecting Arabidopsis lateral root development. Trends in Plant Science 8: 165–171. [DOI] [PubMed] [Google Scholar]
- Cernac A, Lincoln C, Lammer D, Estelle M. 1997. The SAR1 gene of Arabidopsis acts downstream of the AXR1 gene in auxin response. Development 124: 1583–1591. [DOI] [PubMed] [Google Scholar]
- Charlton WA. 1975. Distribution of lateral roots and pattern of lateral initiation in Pontederia cordata L. Botanical Gazette 36: 225–235. [Google Scholar]
- Charlton WA. 1982. Distribution of lateral root primordia in root tips of Musa acuminata Colla. Annals of Botany 49: 509–520. [Google Scholar]
- Charlton WA. 1983. Patterns of distribution of lateral root primordia. Annals of Botany 51: 417–427. [Google Scholar]
- Charlton WA. 1996. Lateral root initiation. In: Waisel Y, Eshel A, Kafkaf U, eds. Plant roots: hidden half. New York: Marcel Dekker, 149–173.
- Chevalier F, Pata M, Nacry P, Doumas P, Rossignol M. 2003. Effects of phosphate availability on the root system architecture: large-scale analysis of the natural variation between Arabidopsis accessions. Plant, Cell and Environment 26: 1839–1850. [Google Scholar]
- Deak KI, Malamy J. 2005. Osmotic regulation of root system architecture. Plant Journal 43: 17–28. [DOI] [PubMed] [Google Scholar]
- De Smet I, Signora L, Beeckman T, Inze D, Foyer CH, Zhang H. 2003. An abscisic acid-sensitive checkpoint in lateral root development of Arabidopsis. Plant Journal 33: 543–555. [DOI] [PubMed] [Google Scholar]
- DiDonato RJ, Arbuckle E, Buker S, Sheets J, Tobar J, Totong R, et al. 2003. Arabidopsis ALF4 encodes a nuclear-localized protein required for lateral root formation. Plant Journal 327: 340–353. [DOI] [PubMed] [Google Scholar]
- Draye X. 2002. Consequences of root growth kinetics and vascular structure on the distribution of lateral roots. Plant, Cell and Environment 25: 1463–1474. [Google Scholar]
- Dubrovsky JG. 1987. Latent embryonic root system of the cucumber. Botanicheskii Zhurnal (Botanical Journal) Leningrad 72: 171–176.
- Dubrovsky JG, Gómez-Lomelí LF. 2003. Water deficit accelerates determinate developmental program of the primary root and does not affect lateral root initiation in a Sonoran desert Cactaceae, Pachycereus pringlei. American Journal of Botany 90 823–831. [DOI] [PubMed] [Google Scholar]
- Dubrovsky JG, Rost TL. 2003. Lateral root initiation. In: Thomas B, Murphy DJ, Murray BG, eds. Encyclopedia of applied plant sciences. New York: Academic Press, 1101–1107.
- Dubrovsky JG, North GB, Nobel PS. 1998. Root growth, developmental changes in the apex, and hydraulic conductivity for Opuntia ficus-indica during drought. New Phytologist 138: 75–82. [Google Scholar]
- Dubrovsky JG, Doerner PW, Colón-Carmona A, Rost TL. 2000. Pericycle cell proliferation and lateral root initiation in Arabidopsis. Plant Physiology 124: 1648–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freixes S, Thibaud M-C, Tardieu F, Muller B. 2002. Root elongation and branching is related to local hexose concentration in Arabidopsis thaliana seedlings. Plant, Cell and Environment 25: 1357–1366. [Google Scholar]
- Gladish DK, Rost TL. 1993. The effects of temperature on primary root growth dynamics and lateral root distribution in garden pea (Pisum sativum L, cv. Alaska). Environmental and Experimental Botany 33: 243–258. [Google Scholar]
- Gray WM, Muskett PR, Chuang H-w, Parker JE. 2003. Arabidopsis SGT1b is required for SCFTIR1-mediated auxin response. Plant Cell 15: 1310–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawker NP, Bowman JL. 2004. Roles for class III H-Zip and KANADI genes in Arabidopsis root development. Plant Physiology 135: 2261–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinchee MAW, Rost TL. 1992. The control of lateral root development in cultured pea seedlings. III. Spacing intervals. Botanica Acta 105: 127–131. [Google Scholar]
- Johnson DA, Aguirre L. 1991. Effect of water on morphological development in seedlings of three range grasses: root branching patterns. Journal of Range Management 44: 355–360. [Google Scholar]
- Kerk NM, Jiang K, Feldman LJ. 2000. Auxin metabolism in the root apical meristem. Plant Physiology 122: 925–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieber JJ. 2002. Cytokinins. In: Somerville CR, Meyerowitz EM, eds. The Arabidopsis book. Rockville, MD: American Society of Plant Biologists. http://www.aspb.org/publications/arabidopsis/toc.cfm.
- Laskowski MJ, Williams ME, Nusbaum HC, Sussex IM. 1995. Formation of lateral root meristems is a two-stage process. Development 121: 3303–3310. [DOI] [PubMed] [Google Scholar]
- Ljung K, Hull AK, Celenza J, Yamada M, Estelle M, Normanly J, et al. 2005. Sites and regulation of auxin biosynthesis in Arabidopsis roots. The Plant Cell 17: 1090–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloret PG, Casero PJ. 2002. Lateral root initiation. In: Waisel Y, Eshel A, Kafkafi U, eds. Plant roots: hidden half, 3rd edn. New York: Marcel Dekker, Inc., 127–155.
- Lloret PG, Pulgarín A. 1992. Effect of naphthaleneacetic acid on the formation of lateral roots in the adventitious root of Allium cepa: number and arrangement of laterals along the parent root. Canadian Journal of Botany 70: 1891–1896. [Google Scholar]
- López-Bucio J, Hernández-Abreu E, Sánchez-Calderón L, Pérez-Torres A, Rampey RA, Bartel B, et al. 2005. An auxin transport independent pathway is involved in phosphate stress-induced root architectural alterations in Arabidopsis. Identification of BIG as a mediator of auxin in pericycle cell activation. Plant Physiology 137: 681–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loudet O, Gaudon V, Trubuil A, Daniel-Vedele F. 2005. Quantitative trait loci controlling root growth and architecture in Arabidopsis thaliana confirmed by heterogeneous inbred family. Theoretical and Applied Genetics 110: 742–753. [DOI] [PubMed] [Google Scholar]
- MacLeod RD. 1990. Lateral root primordium inception in Zea mays L. Environmental and Experimental Botany 30: 225–234. [Google Scholar]
- Malamy JE. 2005. Intrinsic and environmental response pathways that regulate root system architecture. Plant, Cell and Environment 28: 67–77. [DOI] [PubMed] [Google Scholar]
- Malamy JE, Benfey PN. 1997a Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development 124: 33–44. [DOI] [PubMed] [Google Scholar]
- Malamy JE, Benfey PN. 1997b Down and out in Arabidopsis: the formation of lateral roots. Trends in Plant Science 2: 390–396. [Google Scholar]
- Mallory TE, Chiang S, Cutter EG, Jr, Gifford EM. 1970. Sequence and pattern of lateral root formation in five selected species. American Journal of Botany 57: 800–809. [Google Scholar]
- Marchant A, Bhalerao R, Casimiro I, Eklof J, Casero PJ, Bennett M, et al. 2002. AUX1 promotes lateral root formation by facilitating indole-3-acetic acid distribution between sink and source tissues in the Arabidopsis seedling. Plant Cell 14: 589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCully ME. 1975. The development of lateral roots. In: Torrey JG, Clarkson DT, eds. The development and functions of roots. London: Academic Press, 105–124.
- Mollier A, Pellerin S. 1999. Maize root system growth and development as influenced by phosphorus deficiency. Journal of Experimental Botany 50: 487–497. [Google Scholar]
- Nodzon LA, Xu WH, Wang Y, Pi LY, Chakrabarty PK, Song WY. 2004. The ubiquitin ligase XBAT32 regulates lateral root development in Arabidopsis. Plant Journal 40: 996–1006. [DOI] [PubMed] [Google Scholar]
- Oono Y, Ooura C, Rahman A, Aspuria ET, Hayashi KI, Tanaka A, et al. 2003. p-Chlorophenoxyisobutyric acid impairs auxin response in Arabidopsis root. Plant Physiology 133: 1135–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagès L, Pellerin S. 1994. Evaluation of parameters describing the root system architecture of field grown maize plants (Zea mays L.) II. Density, length, and branching of first-order lateral roots. Plant and Soil 164: 169–176. [Google Scholar]
- Pagès L, Vercambre G, Drouet J-L, Lecompte F, Collet C, Le Bot J. 2004. Root Typ: a generic model to depict and analyse the root system architecture. Plant and Soil 258: 103–119. [Google Scholar]
- Peterson RL, Peterson CA. 1986. Ontogeny and anatomy of lateral roots. In: Jackson MB, ed. New root formation in plants and cuttings. Dordrecht: Martinus Nijhoff, 1–30.
- Sinnot EW, Bloch R. 1939. Changes in intercellular relationships during the growth and differentiation in living plant tissues. American Journal of Botany 26: 625–634. [Google Scholar]
- Torrey JG. 1986. Endogenous and exogenous influences on the regulation of lateral root formation. In: Jackson MB, ed. New root formation in plants and cuttings. Dordrecht: Martinus Nijhoff, 31–66.
- Werner T, Motyka V, Strnad M, Schmulling T. 2001. Regulation of plant growth by cytokinin. Proceedings of the National Academy of Sciences of the USA 98: 10487–10492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner T, Motyka V, Laucou V, Smets R, Van Onckelen H, Schmülling T. 2003. Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell 15: 2532–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Jennings A, Barlow PW, Forde BG. 1999. Dual pathways for regulation of root branching by nitrate. Proceedings of the National Academy of Sciences of the USA 96: 6529–6534. [DOI] [PMC free article] [PubMed] [Google Scholar]