Abstract
• Background and Aims Aerenchyma formation in maize adventitious roots is induced in nutrient solution by the deprivation of sulfate (S) under well-oxygenated conditions. The aim of this research was to examine the extent of aerenchyma formation in the cortex of sulfate-deprived adventitious roots along the root axis, in correlation with the presence of reactive oxygen species (ROS), calcium levels and pH of cortex cells and root lignification.
• Methods The morphometry of the second whorl of adventitious (W2) roots, subject to S-deprivation conditions throughout development, was recorded in terms of root length and lateral root length and distribution. W2 roots divided into sectors according to the mean length of lateral roots, and cross-sections of each were examined for aerenchyma. In-situ detection of alterations in ROS presence, calcium levels and pH were performed by means of fluorescence microscopy using H2DCF-DA, fluo-3AM and BCECF, respectively. Lignification was detected using the Wiesner test.
• Key Results S-deprivation reduced shoot growth and enhanced root proliferation. Aerenchyma was found in the cortex of 77 % of the root length, particularly in the region of emerging or developing lateral roots. The basal and apical sectors had no aerenchyma and no aerenchyma connection was found with the shoot. S-deprivation resulted in alterations of ROS, calcium levels and pH in aerenchymatous sectors compared with the basal non-aerenchymatous region. Lignified epidermal layers were located at the basal and the proximal sectors. S-deprivation resulted in shorter lateral roots in the upper sectors and in a limited extension of the lignified layers towards the next lateral root carrying sector.
• Conclusions Lateral root proliferation is accompanied by spatially localized induced cell death in the cortex of developing young maize adventitious roots during S-deprivation.
Keywords: Adventitious root, aerenchyma, calcium, lateral root, lignification, reactive oxygen species, sulfate deprivation, Zea mays
INTRODUCTION
Maize (Zea mays) forms a complex root system that is divided into an embryonic root system consisting of a single primary root and a variable number of seminal roots, and a post-embryonic root system, which is made up of shoot-borne roots. The latter, formed at consecutive underground nodes, are called crown roots or adventitious roots. The roots formed at consecutive above-ground nodes of the shoot are called brace roots. Lateral roots, which emerge from all major root-types, belong to the post-embryonic root system (Hochholdinger et al., 2004). Intrinsic and environmental response pathways that regulate root system architecture have been reviewed recently (Malamy, 2005).
Sulfur is an essential macronutrient (Hawkesford, 2005). S-deprivation limits growth and inhibits energy assimilation and, therefore, it is necessary for the plant to adapt to maintain viability. It is suggested that there is a systemic internal rebalancing of metabolism, where the system changes priorities, aiming at economizing resources for survival (Nikiforova et al., 2005). S-deficiency has become an increasing problem for agriculture resulting in decreasing crop quality parameters and yield, and various effects of S-deficiency in plants have been described (Hawkesford, 2000). In response, plants possess mechanisms to adapt to low sulfate availability (Hawkesford, 2005). In maize, adaptations to increase sulfate acquisition include modifications of root architecture to maximize sulfate-acquisition efficiency; removal of the S-source from the medium of young maize seedlings resulted in a 3·8-fold overall increased capacity for sulfate uptake, which coincided with an increased root length, increased root mass, increased root : shoot ratio and lateral root proliferation. In addition, plants respond to limited S-supply by increasing expression of genes encoding components of the sulfur-uptake and -assimilation pathway (Hopkins et al., 2004). Under sulfate deprivation, aerenchyma is formed in adventitious maize roots beginning with the death and lysis of cells of the mid-cortex and spreading radially to form spaces separated by radial bridges of living cells linking the stele and epidermis. In addition to the effect of sulfate deprivation on root anatomy, the presence and location of superoxide anions and hydrogen peroxide, and changes in calcium and pH have been examined (Bouranis et al., 2003). Aerenchyma is tissue containing intercellular spaces that aid in the transfer of gases from the stem to the root and vice versa. In many species, root aerenchyma is a component of the pathway of gas-conducting tissue extending to the shoot. Gas transport in the pathway may be by diffusion or due to pressure flow (Colmer, 2003; Evans, 2003). The importance of enhanced gas transport by aerenchymatous tissue under nutrient stress, as well as the adaptive significance of aerenchyma formation under S-deprivation is unknown. When roots are nutrient stressed but not hypoxic, oxygen movement may not be a crucial factor. A model of induction of programmed cell death in cortical cells of maize roots by environmental factors is provided by Drew et al. (2000). Lignification is a tightly regulated and dynamic process subject to modulation at different levels during normal development and in response to stress (Boudet, 2000), and the lignification process requires sulfur compounds, i.e. S-adenosyl-methionine and CoASH.
To examine the effect of S-deprivation on the anatomy of the shoot–root connection, the extent of aerenchyma formation along root axes in the second whorl of adventitious (W2) roots was investigated. In the experimental system, the first whorl of adventitious roots experienced a transition from complete to S-deprived nutrient solution, while the second whorl experienced S-deprivation throughout. Both control and –S nutrient solutions remained well aerated throughout the experiments. Surprisingly, no aerenchyma formed at the root base of the W2 roots. The dynamics of aerenchyma distribution along the W2 roots are presented in correlation with (a) the lateral root proliferation observed under the treatment; (b) the alterations in the presence of reactive oxygen species (ROS), calcium levels, and pH in situ by means of the changes in the fluorescence provided by indicators applied to transverse sections from aerenchymatous and non-aerenchymatous sectors, i.e. the basal (forming no aerenchyma) and the middle (carrying aerenchyma) part of W2 roots; and (c) the extent of lignification by means of the Wiesner test.
MATERIALS AND METHODS
Plant material and growth conditions
Maize (Zea mays L., hybrid LG-2447) seeds were kept on wet filter paper, in the dark and under constant conditions (24 °C, relative humidity 40 %) until their germination. Three days later, the most uniform plants were selected and maintained in a hydroponic batch culture system for 5 h in distilled H2O, 1 d in one-tenth-strength complete nutrient solution, 1 d in half-strength complete nutrient solution and 3 d in full-strength complete nutrient solution containing 7 mm KNO3, 1 mm KH2PO4, 2·15 mm Mg(NO3)2, 0·1 mm NaCl, 2·5 mm MgSO4, 0·074 mm EDTA FeNa, 5 mm Ca(NO3)2, 0·95 μm Zn-acetate, 25·1 μm H3BO3, 0·5 μm Cu(NO3)2, 0·081 μm (NH4)6Mo7O24. Growth conditions were 24/18 °C, relative humidity 40 %, 250 µmol photon m−2 s−1 and a 16-h photoperiod. With the third leaf fully appeared (day 0), one half of the plants were maintained in the complete nutrient solution (C) and the other half in nutrient solution lacking  (–S, omitting MgSO4) for the next 18 d. Nutrient solutions were changed every day, and were continuously aerated.
(–S, omitting MgSO4) for the next 18 d. Nutrient solutions were changed every day, and were continuously aerated.
Sampling and observations
Free-hand transverse sections were made of the W2 roots of C and –S plants at days 6, 12 and 18. Sections were viewed and photographed using a Zeiss Axiolab HBO 50 light microscope, equipped with a G-450-490 (blue) excitation filter (barrier filter at 520 nm) for use as a fluorescence microscope. Sections were observed for aerenchyma formation in distilled H2O under a light microscope. To depict and study blue-excited autofluorescence, sections were viewed in distilled H2O under a fluorescence microscope. The excitation wavelength was 450–490 nm.
Detection of ROS
Intracellular production of ROS was monitored using 2′,7′-dichlorodihydro fluorescein diacetate (H2DCF-DA; Molecular Probes). This non-polar compound is converted to the membrane-impermeable polar derivative H2DCF by esterases following entry into the cell. H2DCF in non-fluorescent, but is rapidly oxidized to the highly fluorescent DCF by intracellular H2O2 and other peroxides. Stocks of H2DCF-DA (5 mm) were made in absolute ethanol. Sections were incubated in H2DCF-DA at a final concentration of 5 μm (in distilled H2O) for 1 h at 25 °C, rinsed with distilled H2O and observed in distilled H2O by means of fluorescence microscopy. The excitation and the emission wavelengths were 450–490 nm and 520 nm (green), respectively (Bouranis et al., 2003). Alternatively, another set of sections was pre-incubated in the presence of an inhibitor of CuZn-SOD (1 mm N,N-diethyldithiocarbamate; DDC) for 10 min and then incubated in H2DCF-DA for 1 h. Finally, a third set of sections was pre-incubated for 10 min in 200 U mL−1 catalase solution (CAT) prior to the addition of H2DCF-DA (Ros Barceló, 1998). One unit was defined as the amount that decomposes 1·0 µmol of H2O2 min−1 at pH 7·0, according to the supplier (Serva).
pH estimation
The polar fluorescein derivative 2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxy fluorescein (dextran BCECF anionic; Molecular Probes) is a fluorescent indicator for estimating intracellular pH, whose fluorescence excitation profile is pH-dependent. Sections were incubated in BCECF at a final concentration of 0·5 μm (in distilled H2O) for 1 h at 25 °C, rinsed with distilled H2O and observed in distilled H2O by means of fluorescence microscopy. The excitation and the emission wavelengths were 450–490 nm and 532 nm (green), respectively. Stocks of BCECF (2 mm) were made in high-purity water (Bouranis et al., 2003).
Detection of Ca
Intracellular Ca was detected by using the Ca2+ indicator, fluo-3 AM (Molecular Probes). The acetoxymethyl (AM) ester derivative of fluo-3 is non-fluorescent. Fluo-3 AM is converted to fluo-3 by esterases. Fluo-3 is non-fluorescent, unless bound to Ca2+, thus being a Ca2+ indicator. Stocks of fluo-3 AM (5 mm) were made in dimethylsulfoxide. Sections were incubated in fluo-3 AM at a final concentration of 5 μm (in distilled H2O) for 1 h at 25 °C, rinsed with distilled H2O and observed in distilled H2O by means of fluorescence microscopy. The excitation and the emission wavelengths were 450–490 nm and 526 nm (green), respectively (Bouranis et al., 2003).
Detection of lignification
Lignification was detected using the Wiesner test (which is specific for cinnamaldehyde groups). For this, root sections were soaked in 1 % (w/v) phloroglucinol in 1 : 3 (v/v) HCl : ethanol for 15–30 min at 25 °C (Ros Barceló, 1998). This dye gave an orange to deep purple colour in the lignified areas, depending on the degree of lignification. The original image of the stained section was visualized with Gimp software (v.2.2.9) and an area negative to the Wiesner test was selected and marked. A pseudocolour image was produced by adjusting the magenta/green balance to green and the yellow/blue balance to blue, without modifying the cyan/red balance. Thus, the deep purple field of the pseudocolour image corresponded to lignified sites of the sections examined.
Image analysis
To obtain the percentage of area occupied by aerenchyma in the cortex, images were analysed by means of ImageJ (v.1.34s) software package. Scilab software package (v.2.7) was used for the analysis of photographs taken using the fluorescent microscope equipped with blue and UV filters. In each photograph (RGB image) the three channels (red, green, blue) were split and the desired channel was used for further processing, and the differences in the intensity of the desired colour were expressed using Scilab's colour map. The colour map ranged from blue to red and passed through the colours cyan, yellow and orange (as shown in the colour bars of Figs 5 and 6).
Statistical analysis
Five repetitions of the experiment were conducted. Three plants per sampling day and treatment (control or S-deprived) were measured; thus in each repetition 60 plants were examined and growth analysis (Fig. 1 and Table 1) was based on 300 seedlings. Data represent the mean values±s.e. (number of measurements n = 15) (Fowler et al., 2000). The W2 roots of each plant possessed three roots; thus nine adventitious roots were examined per day and per treatment. At least four cross-sections were taken and processed per representative image depicted in Figs 4–7.
Fig. 1.
Dry mass accumulation in S-deprived plants (–S) against the control plants. Open and shaded columns represent shoot and root dry mass accumulation (± s.e., n = 5), respectively. Corresponding means were compared by t-test (P = 0.05, d.f. = 8). S-deprived plants reduced the invested biomass to the shoot in favour of the root and statistically significant differences were found in the shoot after day 6 and in root after day 8.
Table 1.
Plant growth under sulfate deprivation in terms of dry mass accumulation rate, the percentage change in the dry shoot and root mass allocation in –S plants relative to control, and root : shoot ratio
| Accumulation rate (g d−1) |
% change in dry mass of –S relative to C |
Root : shoot ratio |
|||||||||||
| Day |
ShootC |
Shoot-S |
RootC |
Root-S |
Shoot |
Root |
C |
–S |
|||||
| 0 | 0 | 0 | 0·45 ± 0·10 | 0·45 ± 0·10 | |||||||||
| 6 | 0·028 ± 0·010 | 0·017 ± 0·010 | 0·012 ± 0·005 | 0·012 ± 0·004 | –18·9 | 0 | 0·43 ± 0·11 | 0·53 ± 0·11 | |||||
| 12 | 0·080 ± 0·018 | 0·040 ± 0·017 | 0·025 ± 0·008 | 0·025 ± 0·007 | –36·5 | 25·8 | 0·36 ± 0·07 | 0·72 ± 0·14 | |||||
| 18 | 0·095 ± 0·033 | 0·043 ± 0·027 | 0·017 ± 0·012 | 0·060 ± 0·017 | –43·7 | 63·4 | 0·29 ± 0·06 | 0·84 ± 0·19 | |||||
C, Control plant; –S: sulfate-deprived plant.
Growth analysis was based on 300 seedlings.
Data represent the mean values ± standard error (number of measurements n = 15).
RESULTS
Root growth under S-deprivation conditions
S-deprivation significantly reduced shoot growth and enhanced root proliferation (Fig. 1). By day 6, the accumulation rate of dry mass in the shoot was reduced, resulting in a 44 % decrease of the S-deprived shoot at the end of the experiment. In contrast, dry mass accumulation in the root system was enhanced by day 18, resulting in a 63 % increase of the S-deprived root compared with the control (Table 1). As a consequence, the root : shoot ratio of S-deprived plants increased progressively from 0·53 at day 6 to 0·84 at day 18.
Morphometry of the W2 roots
Adventitious roots emerged in whorls containing three roots each (Fig. 2). There was a precise timing in the appearance of the whorls: each whorl emerged every 6 d. In this report, the morphometry of the W2 roots after 6, 12 and 18 d of sulfate deprivation are described.
Fig. 2.
The order of the emergence of the four whorls (W1–W4) of adventitious roots at day 12. W2 stands for the second whorl of adventitious roots, which was studied in detail, being the first one that faced sulfate starvation from the beginning. M stands for mesocotyl.
Growth of the W2 roots of the control plants remained stable at 2·2 cm d−1 for the 18 d of the experiment. By contrast, sulfate deprivation resulted in an initial decrease of the growth rate to 2 cm d−1 in the first 6 d followed by an increase to 3·5 cm d−1 up to day 12 and to 4·1 cm d−1 up to day 18. W2 roots possessed two end sectors without lateral roots (B, the basal sector proximal to the shoot; A, the apical sector that included tip and expanding zone) and intermediate sectors with lateral roots of various lengths. The intermediate sector was divided into subsectors according to the mean length of the lateral roots.
At day 6, no lateral roots and no differences between control and S-deprived W2 roots were observed. Lateral roots started to form after day 6, and by day 12 three groups of lateral roots (LR20, LR10 and ELR; Fig. 3) were distinguishable (W2 sector LR20 and LR10 represent groups of lateral roots on W2 root with a mean length of 20 or 10 mm, respectively, while ELR indicates the W2 sector carrying the emerging lateral roots). At day 12, S-deprived W2 root length was increased by 22 % compared with the control. Each of sectors B, LR20, LR10, ELR and A occupied 5·9 %, 13·6 %, 20·8 %, 26·7 %, 33 % in control and 3·3 %, 6·6 %, 24·7 %, 35·3 %, 30·1 % in S-deprived W2 roots, respectively. Corresponding sectors had similar mean lengths for the lateral roots. Thus, under S-deprivation the length of sectors LR10 and ELR increased, while B, LR20 and A decreased. From day 12 to day 18, W2 roots of the control and S-deprived W2 roots enlarged in length by 51·3 %, and 74·3 %, respectively. At day 18, S-deprived W2 root length was 40·2 % longer than the control. Sectors B, LR65, LR25, LR8, ELR and A on the control W2 root occupying 3·4 %, 31·5 %, 20·6 %, 28 %, 9 % and 7·5 % and sectors B, LR40, LR20, LR8, ELR and A on W2 S-deprived root occupying 1·2 %, 9·7 %, 27·6 %, 22·5 %, 24·7 % and 14·3 % were distinguished, respectively. S-deprived W2 roots had a reduced basal sector and mean length of the lateral roots of the proximal sector, but showed no difference in the next two sectors, whilst the sector with the emerging lateral roots and the apical part increased by factors of 3·9 and 2·7, respectively (Fig. 3).
Fig. 3.

Schematic of the development of adventitious root belonging to the second whorl at days 6, 12 and 18 in a complete (C) or lacking sulfate (–S) nutrient solution. In black, root length (± s.e.) is depicted. In green, the various root sectors possessing lateral roots. For each sector, the mean lateral root length (in mm) and the percentage of the total root length it occupies are given. In red, the beginning and the end of aerenchyma formation are given, and within this range, the percentage of aerenchyma measured in the cortex of each root section included is provided. Dashed lines indicate the middle (m) and the basal (b) part of the root, where cross sections were taken for Figs 5 and 6, respectively. The second whorl of adventitious roots of each plant possessed three roots; thus nine adventitious roots were examined per day and treatment, and therefore root growth analysis was based on 54 roots. Data represent the mean values ± s.e. (number of measurements n = 9).
Aerenchyma distribution
To reveal the distribution of aerenchyma along the root, successive cross-sections were performed in each root sector to locate precisely the beginning and the end of the aerenchyma. Axial aerenchyma distribution was expressed as a percentage of the total root length and radially as a percentage of the area of the cortex. At day 6 no aerenchyma was found in W2 roots of either control or S-deprived plants. Aerenchyma formation was triggered some days later and by the 12th day, 24 % of the length of control and 66 % of S-deprived W2 roots contained aerenchyma. In the control W2 roots, aerenchyma extended from the middle of the LR10 sector to the middle of the ELR sector, occupying 1 % of the cortex. In the S-deprived W2 roots, aerenchyma covered almost the entire LR10 and ELR sectors and occupied 14 % of the cortex in both.
By day 18, aerenchyma occurrence along the length of the root was 79 % in control and 77 % in S-deprived W2 roots, and there were differences in its location between treatments. Aerenchyma was shifted towards the basal part of the control W2 root and towards the apical part in S-deprived W2 roots. Aerenchyma of control covered a part of sector LR65, and the entire LR25 and LR8 sectors occupying 5 %, 9 % and 12 % of their cortex, respectively. In contrast, aerenchyma of S-deprived W2 roots completely covered all three LR20, LR8 and ELR sectors, occupying 19 %, 26 % and 19 % of the cortex, respectively. Accordingly, coverage of the cortex of S-deprived W2 roots by aerenchyma was greater compared with control roots by a factor of 2·8, 1·9, 0·6 for each sector, respectively. The basal and apical sectors had no aerenchyma at all (Figs 3 and 4).
Fig. 4.

Cross-sections of the root sectors examined providing views of both the aerenchyma in the cortex cells and the extent of lignification according to the Wiesner test. Areas appearing as deep purple (pseudocolour) are indicative of lignification as indicated by the Wiesner test. LRx, Root sector carrying lateral roots with mean length of x mm; ELR, root sector with emerging lateral roots; B and A, the basal and apical root sectors, respectively. The percentage number in each photograph represents the percentage of aerenchyma measured in the cortex. Panels a and b at bottom right are magnifications of the boxed areas in sector B of control and –S roots, respectively, at day 18. Scale bar = 250 μm.
Extent of lignification in cortex cell walls
By means of the Wiesner test, the extent of lignification of cortex cell walls was examined in cross-sections of all sectors. Cortex cell walls of sector B (Fig. 4) were positive for the test, i.e. lignified at day 18 in both control and S-deprived plants compared with day 12. At day 18, the cortex cell walls of sector LR40 of S-deprived roots were more lignified compared with the corresponding LR65 sector of control roots.
In situ detection of alterations in ROS presence, pH and calcium levels
(i) In the aerenchymatous sectors of the root under sulfate-deprivation
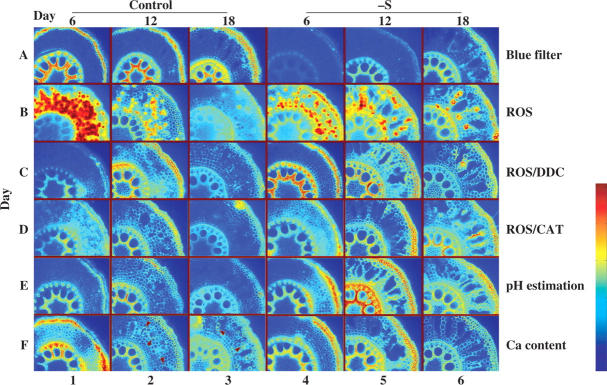
Xylem elements and the cells of hypodermis layers at days 6, 12 and 18 fluoresced intensely under the blue filter in the middle of the root of control plants (Fig. 5A1–3). Fluorescence was weaker in the S-deprived tissues and was only evident in xylem at day 12 and in the xylem and hypodermis at day 18. The ROS analysis (Fig. 5B) indicated that in the cortex of both control and S-deprived tissues, intact cells and plasma membranes of intact cells (not cell walls due to the mode of action of the dye) of the cortex fluoresced more at day 6 (Fig. 5B1 and 4) and less at day 12 (Fig. 5B2 and 5). S-deprived conditions resulted in a reduced number of fluorescing cells (compare B1 and B4 in Fig. 5).
Fig. 5.
Processed images for in situ detection of alterations in ROS, pH and calcium levels in the middle of the root. Corresponding levels increase from blue to red (see colour bar). Cross-sections 1–3, from roots of control plants at days 6, 12 and 18, respectively; 4–6, from the corresponding roots of –S plants at days 6, 12 and 18, respectively. A, Blue filter (control); B, ROS; C, ROS/DDC; D, ROS/CAT; E, pH estimation; F, Ca detection. Each individual image is 1125 μm in width.
With DDC (Fig. 5C1–6), plasma membranes of intact cortex cells fluoresced more intensely in S-deprived (Fig. 5C5–6) sections compared with control at days 12 and 18 (Fig. 5C2 and 3). The presence of catalase (CAT, Fig. 5D1–6) provided almost the same pattern at days 12 and 18.
Fig. 6.

Processed images for in situ detection of alterations in ROS, pH and calcium levels in the root base. Corresponding levels increase from blue to red (see colour bar). Cross-sections 1–3, from roots of control plants at days 6, 12 and 18, respectively; 4–6, from the corresponding roots of –S plants at days 6, 12 and 18, respectively. A, Blue filter (control); B, ROS; C, ROS/DDC; D, ROS/CAT; E, pH estimation; F, Ca detection. Each individual image is 1125 μm in width.
When cross-sections were treated for pH estimation (Fig. 5E1–6), cell walls and/or plasma membranes of S-deprived tissues at days 12 and 18 (Fig. 5E5 and 6) fluoresced intensely, indicating higher pH. In the assay for calcium (Fig. 5F1–6), intense fluorescence indicating high calcium levels was observed in control roots, cell walls and/or plasma membranes of intact cells near aerenchyma at days 12 and 18 (Fig. 5F2 and 3, arrows). In S-deprived tissues at both days 12 and 18, fluorescence was observed in cell walls and/or plasma membranes of all intact cortex cells (Fig. 5F5 and 6).
(ii) In the non-aerenchymatous basal sector under sulfate-deprivation
With the blue filter (Fig. 6A1–6), the base of the root of control and S-deprived plants, xylem elements, endodermis and lignified hypodermis cells showed fluorescence apart from –S plants at day 6 (Fig. 6A6). In addition, fluorescence also occurred in the cell walls of cortex cells at days 12 and 18 for control plants and at day 18 for S-deprived plants. These cell walls were positive with the Wiesner test for lignification. In control plants, the cortex fluorescence intensity increased from day 12 to 18 (Fig. 6A2 and 3), while the highest fluorescence was observed in S-deprived plants at day 18. When stained for ROS (Fig. 6B1–6), plasma membranes of intact cortex cells fluoresced with increased intensity from days 6 to 18 for S-deprived plants and from days 12 to 18 for control plants. Using DDC (Fig. 6C1–6) the pattern was the same, with a remarkably intense fluorescence at the cortex of control plants at day 18. The treatment with CAT showed no specific pattern (Fig. 6D1–6). After treatment of root sections for pH estimation (Fig. 6E1–6) or calcium detection (Fig. 6F1–6), sections from the base fluoresced intensively in the cell walls and/or plasma membranes of intact cells in both cortex (lignified) cells and xylem elements of both control and S-deprived plants at all time points.
Lateral root formation in relation to aerenchyma
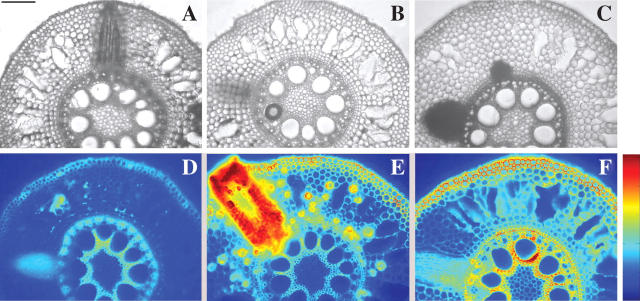
Up to four or five layers of cortex cells around the developing lateral root contained intact cells (Fig. 7A and B), as was also the case for the cells of the cortex between the apex of the emerging lateral root and the hypodermis of the W2 root (Fig. 7C). Comparing these layers with the cell layers between aerenchyma spaces, the latter were occupied mostly by one or two intact cortex cells. ROS were detected in lateral root tissues (Fig. 7E).
Fig. 7.
Lateral root formation in relation to aerenchyma under –S conditions. Cross-sections were taken from the middle of the root. (A–C) Observation in water; (D) blue filter; (E) ROS; (F) ROS/DDC. Corresponding levels increase from blue to red (see colour bar). Cross-sections at day 12. Scale bar = 250 μm.
DISCUSSION
Combining the data on aerenchyma distribution with those on lateral root proliferation suggests that the presence of aerenchyma coincides with the presence of developing lateral roots. At day 6 no aerenchyma and no lateral roots were found. By day 12, S-deprived roots have more biomass and have increased elongation and lateral root production, with aerenchyma being remarkably developed in the W2 roots. Notably, aerenchyma was located in the root sectors that possessed developing lateral roots, covering 66 % of the root length compared with 24 % of control roots, at the equivalent day. Within the next 6 d, an enhancement in aerenchyma formation was observed in S-deprived roots to the extent that at day 18, aerenchyma occupied 77 % of their length, comprising 19–26 % of the cortex. Surprisingly, aerenchyma in the W2 roots did not extend to the shoot. Aerenchyma distribution was characterized by start and end points, with basal and apical root sectors lacking aerenchyma.
Cell death occurs as an integral and necessary component in the development of a variety of tissues and organs of maize. Various cells, tissues or organs undergo cell death at predictable times and each of these cell-death events occurs at a specific developmental stage or under specific environmental conditions (Buckner et al., 1998, 2000). In response to oxygen, N, P and S deficiencies, the cortical cells of the root can undergo cell death to produce lysigenous aerenchyma. The role of ethylene as the signal for the spatially selective programmed cell death (PCD) in the cortex of roots of Zea mays during formation of lysigenous aerenchyma has been studied extensively, and aerenchyma formation as an adaptive, localized response to low nutrient availability seems to be mediated by ethylene (Drew et al., 1989; He et al., 1992, 1994; Lynch and Brown, 1997; Morgan and Drew, 1997). Plants use ROS as second messengers in signal transduction cascades in diverse processes including cell death (Jabs, 1999; Foyer and Noctor, 2005). Thus, ROS accumulation is crucial to tissue development. Plants possess an NADP oxidase which stimulates the production of superoxide dismutase, a multiprotein complex consisting of two intrinsic plasma membrane proteins, and three cytosolic proteins including Rac, a G protein. One of the two plasma membrane proteins contains calcium-binding motifs, which suggests that the NADP oxidase complex may be regulated by calcium, a common feature of PCD (Keller et al., 1998). Rac may play a role in regulating ROS formation and cell death. In addition to NADPH-oxidase, pH-dependent cell wall peroxidases, germin-like oxidases and amine oxidases have been proposed as sources of hydrogen peroxide in the apoplast. Cell wall pH-dependent peroxidases are activated by alkaline pH and, in the presence of a reductant, hydrogen peroxide is formed. Hydrogen peroxide formed by the oxidation of amines may be directly utilized by wall-bound peroxidases in lignification and cell wall strengthening, both during normal growth and in response to external stimuli (Neill et al., 2002; Vranová et al., 2002).
At days 6, 12 and 18, fluorescence was apparent in the inside and in the plasma membranes of whole cells of the cortex in the middle of W2 roots. The intensity of fluorescence diminished at days 12 and 18 in both control and S-deprived W2 roots, but in a treatment-specific manner. H2DCFDA (2,7-dichlorodihydrofluorescein diacetate) enters the cell as a non-polar compound and esterases produce the polar product H2DCF, which does not either fluoresce or pass through the plasma membrane. It is oxidized in the cortex cell by peroxides and it is the oxidized DCF form that fluoresces. Thus, it must be the intact cortex cells and the internal sides of plasma membranes that produce the fluorescence. After treatment with H2DCFDA plus DDC (a CuZn-SOD inhibitor), no fluorescence was observed inside cortex cells. In contrast, the inside of plasma membranes fluoresced in both control and S-deprived cortex cells. In the treatment with H2DCFDA plus catalase, almost the same picture as in the treatment with DDC was observed. The present data provide evidence that ROS-monitored inside cortex cells may be due to hydrogen peroxide produced by superoxide anions, while ROS that fluoresce at the plasma membranes are other peroxides or hydrogen peroxide produced by other sources. With the anionic BCECF, S-deprived roots fluoresced more intensively at days 12 and 18 at the plasma membranes or in cell walls, indicating a shift to higher pH. Fluo-3 AM fluoresced more intensively in intact cortex cells, plasma membranes and cell walls located near to aerenchyma in control roots, compared with only plasma membranes and cell walls of all intact cortex cells in the middle of S-deprived W2 roots.
ROS were involved in all three processes that were examined, i.e. aerenchyma formation, lateral root formation and lignification, indicating multiple roles which were spatially and developmentally separated. All these processes were affected by S-deprivation. The present data provide evidence that the presence of ROS alters in the aerenchymatous sectors of the root, and the same holds true for pH and calcium levels, compared with the basal non-aerenchymatous sector. Furthermore, the basal and its proximal non-aerenchymatous sector carried more lignified cortex cell walls below the hypodermis. In the appearance of aerenchyma, no lignified cortex cell walls were detected. Lytic processes did not affect the inner- and outermost parenchyma interconnected with unaffected radial files of cells. The presence of a developing lateral root inhibited cell breakdown. Activity of ROS was detected in lateral root tissues and it was observed that, as a rule, cortex cells surrounding the developing lateral root contained intact cells in four or five layers.
It is concluded that lateral root proliferation is accompanied by spatially localized induced cell death in the cortex of developing young maize adventitious roots during S-deprivation, mediated by alterations in the activity of ROS, pH and calcium levels.
Acknowledgments
Rothamsted Research receives grant-aided support from the Biotechnology and Biological Sciences Research Council of the UK.
LITERATURE CITED
- Boudet A. 2000. Lignins and lignification: selected issues. Plant Physiology and Biochemistry 38: 81–96. [Google Scholar]
- Bouranis DL, Chorianopoulou SN, Siyiannis VF, Protonotarios VE, Hawkesford MJ. 2003. Aerenchyma formation in roots of maize during sulphate starvation. Planta 217: 382–391. [DOI] [PubMed] [Google Scholar]
- Buckner B, Janick-Buckner D, Gray J, Johal GS. 1998. Cell-death mechanisms in maize. Trends in Plant Science 3: 218–223. [Google Scholar]
- Buckner B, Johal GS, Janick-Buckner D. 2000. Cell death in maize. Physiologia Plantarum 108: 231–239. [Google Scholar]
- Colmer TD. 2003. Long-distance transport of gases in plants: a perspective on internal aeration and radial oxygen loss from roots. Plant, Cell and Environment 26: 17–36. [Google Scholar]
- Drew MC, He CJ, Morgan PW. 1989. Decreased ethylene biosynthesis, and induction of aerenchyma, by nitrogen- or phosphate-starvation in adventitious roots of Zea mays L. Plant Physiology 91: 266–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew MC, He C, Morgan P. 2000. Programmed cell death and aerenchyma formation in roots. Trends in Plant Science 5: 123–127. [DOI] [PubMed] [Google Scholar]
- Evans DE. 2003. Aerenchyma formation. New Phytologist 161: 35–49. [Google Scholar]
- Fowler J, Cohen L, Jarvis P. 2000. Practical statistics for field biology, 2nd edn. New York: Wiley.
- Foyer CH, Noctor G. 2005. Oxidant and antioxidant signalling in plants: a re-evaluation of the concept of oxidative stress in a physiological context. Plant, Cell and Environment 28: 1056–1071. [Google Scholar]
- Hawkesford MJ. 2000. Plant responses to sulphur deficiency and the genetic manipulation of sulphate transporters to improve S-utilization efficiency. Journal of Experimental Botany 51: 131–138. [PubMed] [Google Scholar]
- Hawkesford MJ. 2005. Sulphur. In: Broadley MR, White P, eds. Nutritional genomics. Blackwell Publishers, Oxford, 87–111.
- He CJ, Morgan PW, Drew MC. 1992. Enhanced sensitivity to ethylene in nitrogen-starved or phosphate-starved roots of Zea mays L during aerenchyma formation. Plant Physiology 98: 137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He CJ, Drew MC, Morgan PW. 1994. Induction of enzymes associated with lysigenous aerenchyma formation in roots of Zea mays during hypoxia or nitrogen starvation. Plant Physiology 105:861–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochholdinger F, Woll K, Sauer M, Dembinsky D. 2004. Genetic dissection of root formation in maize (Zea mays) reveals root-type specific developmental programmes. Annals of Botany 93: 359–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins L, Parmar S, Bouranis DL, Howarth JR, Hawkesford MJ. 2004. Coordinated expression of sulfate uptake and components of the sulfate assimilatory pathway in maize. Plant Biology 6: 408–414. [DOI] [PubMed] [Google Scholar]
- Jabs T. 1999. Reactive oxygen intermediates as mediators of programmed cell death in plants and animals. Biochemical Pharmacology 57: 231–245. [DOI] [PubMed] [Google Scholar]
- Keller T, Damude HG, Werner D, Doerner P, Dixon RA, Lamb C. 1998. A plant homolog of the neutrophil NADPH oxidase gp91ph°x subunit gene encodes a plasma membrane protein with Ca2+ binding motifs. The Plant Cell 10: 255–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch J, Brown KM. 1997. Ethylene and plant responses to nutritional stress. Physiologia Plantarum 100: 613–619. [Google Scholar]
- Malamy JE. 2005. Intrinsic and environmental response pathways that regulate root system architecture. Plant, Cell and Environment 28: 67–77. [DOI] [PubMed] [Google Scholar]
- Morgan PW, Drew MC. 1997. Ethylene and plant responses to stress. Physiologia Plantarum 100: 620–630. [Google Scholar]
- Neill S, Desikan R, Hancock J. 2002. Hydrogen peroxide signalling. Current Opinion in Plant Biology 5: 388–395. [DOI] [PubMed] [Google Scholar]
- Nikiforova VJ, Kopka J, Tolstikov V, Fiehn O, Hopkins L, Hawkesford MJ, et al. 2005. Systems rebalancing of metabolism in response to sulfur deprivation, as revealed by metabolome analysis of Arabidopsis plants. Plant Physiology 138: 304–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ros Barceló A. 1998. The generation of H2O2 in the xylem of Zinnia elegans is mediated by an NADPH-oxidase-like enzyme. Planta 207: 207–216. [Google Scholar]
- Vranová E, Inzé D, Van Breusegem F. 2002. Signal transduction during oxidative stress. Journal of Experimental Botany 53: 1227–1236. [PubMed] [Google Scholar]