Abstract
• Background and Aims Ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) activase (RCA) is a nuclear-encoded chloroplast protein that modifies the conformation of Rubisco, releases inhibitors from active sites, and increases enzymatic activity. It appears to have other functions, e.g. in gibberellin signalling and as a molecular chaperone, which are related to its distribution within the chloroplast. The aim of this research was to resolve uncertainty about the localization of RCA, and to determine whether the distributions of Rubisco and RCA were altered when RCA content was reduced. The monocotyledon, Oryza sativa was used as a model species.
• Methods Gas exchange and Rubisco were measured, and the sub-cellular locations of Rubisco and RCA were determined using immunogold-labelling electron microscopy, in wild-type and antisense rca rice plants.
• Key Results In antisense rca plants, net photosynthetic rate and the initial Rubisco activity decreased much less than RCA content. Immunocytolocalization showed that Rubisco in wild-type and antisense plants was localized in the stroma of chloroplasts. However, the amount of Rubisco in the antisense rca plants was greater than in the wild-type plants. RCA was detected in both the chloroplast stroma and in the thylakoid membranes of wild-type plants. The percentage of RCA labelling in the thylakoid membrane was shown to be substantially decreased, while the fraction in the stroma was increased, by the antisense rca treatment.
• Conclusions From the changes in RCA distribution and alterations in Rubisco activity, RCA in the stroma of the chloroplast probably contributes to the activation of Rubisco, and RCA in thylakoids compensates for the reduction of RCA in the stroma, allowing steady-state photosynthesis to be maintained when RCA is depleted. RCA may also have a second role in protecting membranes against environmental stresses as a chaperone.
Keywords: Antisense rca rice plants, Rubisco, Rubisco activase (RCA), cellular localization
INTRODUCTION
In green plants, ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco, EC 4.1.1.39) catalyses the irreversible carboxylation of ribulose-1,5-bisphosphate and CO2 to form two 3-phosphoglyceric acid molecules. However, the rate of the reaction is extremely slow, and Rubisco must be activated and carbamylated to become catalytically competent. Activation is achieved by Rubisco activase (RCA), which can remove inhibitors from Rubisco's catalytic sites, alter the conformation, and activate Rubisco in vivo (Andrews et al., 1995). RCA is a nuclear-encoded chloroplast protein, and is essential for plants (Portis, 1995). Komatsu et al. (1996) reported that a gibberellin-binding protein in rice was homologous to RCA. Sharma and Komatsu (2002), using an in-gel protein kinase assay, suggested that RCA was associated with Ca2+-dependent protein kinases in gibberellin signalling. These studies suggest some additional role for RCA beyond Rubisco regulation (Portis, 2003). Possibly, the role of RCA depends on its location within the chloroplast, as functions of other proteins are known to be related to their cellular localization. Immunogold labelling for electron microscopy has been widely used to identify the position of macromolecules in plant tissues. Therefore, one aim of this study was to establish where RCA is located in higher plants.
A second aim of this study was to determine whether Rubisco and RCA contents were altered, or the proportions in different parts of the chloroplast changed, when RCA content was reduced by use of antisense rca. This was made possible by using genetically modified rice plants with antisense-RNA to RCA (Jin et al., 2004a, b). The photosynthetic rate of such plants was largely unaffected by RCA concentration until it was reduced below approx. 35 % of that of wild-type plants (Jin et al., 2004a). These results were similar to results obtained in transgenic tobacco (Mate et al., 1993, 1996; Hammond et al., 1998) and Arabidopsis thaliana (Eckardt et al., 1997; Zhang et al., 2002). In these plants, modest reduction (32–49 %) in Rubisco activation did not mirror the large decrease (from 0·02 to 0·0025) in the RCA : Rubisco ratio that occurred (He et al., 1997). Although the reduced amount of RCA in the anti-activase plants might be partially compensated by an increase in ATP or in the ATP : ADP ratio in vivo, in no case was compensation by ATP sufficient to explain the relative insensitivity of photosynthesis to loss of RCA. Other factors, such as increased amount of Rubisco protein, or re-location of sequestered RCA to maintain Rubisco activity might also explain the insensitivity of photosynthesis to loss of RCA. It is therefore hypothesized that changes in amount and distribution of RCA and Rubisco are responsible for the discrepancy between changes in RCA and gas exchange and Rubisco activity, and test this by examining the localization of Rubisco and RCA in the wild-type and antisense rca rice plants.
MATERIALS AND METHODS
Plant materials and growth
Transgenic rice with reduced amounts of RCA was grown from seed collected from selfed R1 progeny of rice (Oryza sativa L. ‘ZhongHua 11’) transformed with an antisense gene directed against RCA by the CaMV35S promoter using the Agrobacterium tumefaciens system (Jin et al., 2004b). The R1 seeds of the transformant with 30 % of wild-type RCA were used for this study to test the effects of a large but not damaging change in RCA. Untransformed ‘ZhongHua11’ rice plants were used as controls. About 50 R1 seeds and 30 wild-type seeds were germinated, and the seedlings were grown in 15-L pots containing paddy soil, in a shaded greenhouse with natural sunlight during the day (maximum of 800 µmol photons m−2 s−1) at the Huajiachi Campus of the Zhejiang University. The greenhouse temperature was 28 ± 3 °C during the day and 25 ± 2 °C at night.
Gas exchange measurements
Gas exchange was measured with a portable photosynthesis system (LiCor-6400; LiCor Inc., Lincoln, NB, USA) and an LED light source, 6400-02. This experiment was conducted at a light intensity of 1500 µmol m−2 s−1, a leaf temperature of 28 °C and CO2 at 380 ± 5 μmolCO2 mol−1 in the sample chamber. Measurements were made on the uppermost youngest, fully expanded mature leaves of the main stem of 50-d-old antisense rca and wild-type rice plants, and were repeated at least six times on each. After measurements, the leaves were excised, frozen in liquid N2 and stored at −80 °C for Rubisco and RCA assays. There were at least six replicate samples for each plant type.
Measurements of Rubisco content and activity
About 6–7 cm2 (0·10 g) of frozen rice leaves were ground to a powder using a chilled mortar and pestle with liquid N2, a small amount of quartz sand and insoluble polyvinylpolypyrrolidone (PVP), then homogenized with 1·9 mL cooled extraction buffer containing 50 mm Tris–HCl (pH 7·5), 1 mm EDTA, 10 mm MgCl2, 12 % (v/v) glycerol, 0·1 % (v/v) β-mercaptoethanol and 1 % (w/v) PVP-40 (soluble PVP) at 0–4 °C. The homogenate was centrifuged at 15 000 g for 15 min at 4 °C. The supernatant was used to determine the concentration and activity of Rubisco. The Rubisco concentrations were measured with the single radial immunodiffusion method as described by Huang et al. (2004).
Rubisco activity in the supernatant was assayed according to Sawada et al. (2003) with minor modifications. The initial Rubisco activity was measured at 30 °C by adding 100 μL of supernatant into 900 μL of assay buffer containing 50 mm HEPES–KOH (pH 8·0), 1 mm EDTA–2Na, 20 mm MgCl2, 2·5 mm dithioerythritol (DTT), 10 mm NaHCO3, 5 mm ATP, 0·15 mm NADH, 5 mm creatine phosphate, 0·6 mm RuBP, 10 units of phosphocreatine kinase, 10 units of glyceraldehydes-3-phosphate dehydrogenase and 10 units of phosphoglycerate kinase. The total Rubisco activity was assayed by adding 100 μL of the Rubisco-containing supernatant into 200 μL of an activation medium containing 33 mm Tris–HCl (pH 7·5), 0·67 mm EDTA-2Na, 33 mm MgCl2 and 10 mm NaHCO3, and then incubating the sample at 30 °C for 10 min prior to measurements.
Quantification of RCA content
The amount of RCA was quantified by the immuno-diffusion method using rabbit serum antibody and the purified RCA from rice leaves as a standard (Jiang et al., 2001).
Fixation and immunolocalization
Sub-cellular protein distribution was analysed by electron microscopy, on sections from the middle portions (about 10 mm taken 100 mm away from the leaf tip) of fully expanded uppermost leaves of the main stem, similar to those used for gas exchange. Three leaves from different plants of each type were collected, and two pieces (1 × 3 mm) of each were taken and used for analysis. Small pieces of the leaves were fixed with 0·1 m phosphate buffer (pH 7·2) containing 3 % (v/v) paraformaldehyde and 1 % (v/v) glutaraldehyde for 2 h at 4 °C and washed in the buffer. The segments were dehydrated in an ethanol series and embedded in Lowicryl K4M according to the following protocol: 100 % ethanol/resin 1 : 1 (v/v) for1 h, 100 % ethanol/resin 1 : 2 (v/v) for 1 h, pure resin for 12 h at −20 °C. The embedded samples were transferred to 0·5-mL tubes filled with resin and polymerized completely under UV-radiation at −20 °C for 72 h, followed by 24 h at room temperature. Ultra-thin sections (70–90 nm) were cut with a diamond knife and placed on nickel grids. Two sections from each small piece were analysed. The sections were washed with distilled water for 15 min, and then incubated in blocking buffer [0·05 m phosphate-buffered saline (PBS) with 1 % (w/v) BSA, 0·02 % PEG20000, 0·1 m NaCl, 1 % (w/v) NaN3] for 1 h at room temperature. The sections were then incubated for 1 h at room temperature with anti-Rubisco, or anti-RCA serum applied at dilutions of 1 : 1000 and 1 : 200, respectively, in blocking buffer. For control sections, antiserum was replaced with non-immune serum. After washing with blocking buffer, the sections were incubated in blocking buffer containing protein A conjugated with 15-nm colloidal gold particles for 1 h, and were then washed in PBS and in deionized distilled water. Finally, the sections were stained with uranyl acetate and lead citrate, observed and photographed with an electron microscope (JEM-1200EX, JEOL, Japan) at 80 kV.
The labelling density was determined by counting the gold particles on electron micrographs at ×20 000 magnification and calculating the number per unit area (µm2). Between seven and ten individual cells from different immunolabelled sections for each cell type were examined. The areas occupied by starch grains were omitted from the calculation of chloroplast area. No significant labelling for Rubisco or RCA was present in the vacuole, cell wall, mitochondria or cytosol, and these labelling densities were taken as the background value. To obtain the proportions of Rubisco and RCA in the stroma and thylakoids, the average density of immunogold particles in the background was subtracted from the average density within the stroma and thylakoids.
Antibodies
Rabbit antibodies to the whole molecule of rice Rubisco and to rice RCA, which recognized both forms of RCA (Jiang et al., 2000), were used in this work.
Statistical methods
Data were analysed statistically by ANOVA, using Student's t-test for comparison of means. For these analyses SPSS 10·0 software (SPSS Inc., Chicago, IL, USA), with the statistical significance level set at P < 0·05, was used.
RESULTS
The RCA content of the antisense plants was about 30 % of that of the wild type. However, the antisense plants possessed much more (1·8-fold) Rubisco in their leaves (Table 1). The net photosynthetic rate (Pn) and the initial activity of Rubisco in the antisense plants were reduced by about 50 %, compared with the wild-type plants (Table 1). Thus, the magnitude of the decrease in the initial Rubisco activity and Pn was much less than that in RCA content. Nevertheless, the total activity of Rubisco in antisense plants exposed to light was significantly higher than that in the wild type (P < 0·05), correlating with the measured Rubisco concentrations (Table 1). The intercellular CO2 concentrations (Ci) of the antisense rca rice were higher than that in controls (P < 0·05), while there were no changes in stomatal conductance (gs) between the antisense and wild-type plants (Table 1), indicating that the reductions of photosynthetic rate of the selected antisense plants were not due to stomatal conductance.
Table 1.
The net photosynthetic rate (Pn), intercellular CO2 concentration (Ci), stomatal conductance (gs), RCA contents, Rubisco contents, initial and total Rubisco activity of the leaves of the wild-type and antisense rca plants
| Parameter | Wild type | Anti-rca |
|---|---|---|
| Pn (µmol m−2 s−1) | 18·9 ± 0·7a | 9·68 ± 0·5b |
| Ci (µmol mol−1) | 311 ± 8b | 369 ± 5a |
| gs (mol m−2 s−1) | 0·46 ± 0·09a | 0·44 ± 0·07a |
| RCA (mg m−2) | 20·1 ± 0·6a | 6·11 ± 0·9b |
| Rubisco (g m−2) | 1·68 ± 0·20b | 2·97 ± 0·57a |
| Initial activity (µmol m−2 s−1) | 32·7 ± 3·3a | 17·2 ± 2·58b |
| Total activity (µmol m−2 s−1) | 42·4 ± 6·4b | 70·8 ± 13·84a |
The light-saturated net photosynthesis (A), intercellular CO2 concentrations (Ci) and stomatal conductance (gs) were determined at 30 °C, an ambient CO2 concentration (Ca) of 380 µmol mol−1, 21 % O2 and a PPFD of 1500 µmol m−2 s−1.
Values represent the mean ± s.e. of six replicates.
Values in the same line followed by different letters are significantly different at P < 0·05.
In the wild-type and antisense rca rice plants, when the thin sections were treated with antibody directed against Rubisco of rice, almost all of the immunogold was in the stroma of the chloroplasts (Fig. 1A, B). However, the labelling density in the stroma was related to the RCA content; in the antisense plants small RCA content resulted in larger densities of particles in the stroma (Table 2), consistent with the increased Rubisco content measured in vitro (Table 1). There were only a few particles over the thylakoid in both types of plant (Fig. 1A, B and Table 2).
Fig. 1.
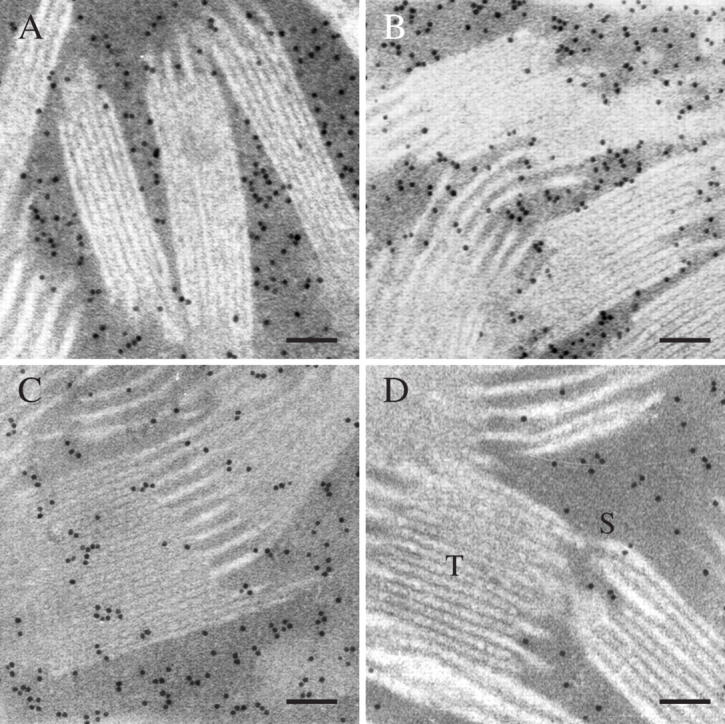
Immunogold labelling of Rubisco (A and B) and RCA (C and D) in mesophyll cell chloroplasts of leaves of the wild-type (A and C) and rca1 (B and D). T, Thylakoid; S, stroma. Scale bars = 0.1 μm.
Table 2.
Immunogold labelling of Rubisco and RCA in the chloroplast organelles of the mature leaves of the wild-type and antisense rca plants
| Label density of Rubisco (µm2) |
Label density of RCA (µm2) |
|||||
|---|---|---|---|---|---|---|
| Strain | Stroma | Thylakoid | Rubisco in stroma (%)* | Stroma | Thylakoid | RCA in stroma (%)† |
| Wild type | 736 ± 82b | 29 ± 7c | 96·2 | 389 ± 62a | 132 ± 36b | 74·7 |
| Anti-rca | 867 ± 97a | 33 ± 11c | 96·3 | 161 ± 47b | 12 ± 4c | 93·8 |
Between seven and ten individual cells were examined in several immunolabelled sections for each cell type.
Numbers of gold particles per unit area (µm2) on an individual basis are given as means ± standard error.
Values from the same protein followed by different letters are significantly different at P < 0·05.
s.e. > 0·05 for all values;
s.e. < 0·05 for all values.
When sections of rice leaves were treated with antibody directed against rice RCA, most of the immunogold particles in the wild type were heavily concentrated in the stroma (dark part), and some in the thylakoid membranes (white part) (Fig. 1C). Gold particle densities were 389 ± 62 µm−2 in the stroma, and 132 ± 36 µm−2 in the thylakoid membranes (Table 2). In contrast, less RCA labelling was observed in the chloroplasts of antisense rca plants than in the wild type, which correlates with the RCA concentrations previously observed in vivo (Jin et al., 2004a; Table 1). The densities of gold particles in the antisense plants were 161 ± 47 and 12 ± 4 µm−2 in the stroma and thylakoid membrane of chloroplasts (Table 2), respectively. Interestingly, the percentage of the immunogold RCA labelling in the chloroplast stroma depended on the RCA concentration. There was a significantly higher percentage of immunogold particles in the rca chloroplast stroma than in the wild type (93 compared with 74 %) (P < 0·05) (Fig. 1D and Table 2). It is clear that almost all of the RCA was in the chloroplast stroma of antisense plants. When sections were incubated with non-immune serum, they showed only non-specific and negligible labelling with gold particles (data not shown).
DISCUSSION
Although RCA is an important enzyme activating Rubisco in vivo (Spreitzer and Salvucci, 2002; Portis, 2003), the cellular localization of RCA is not well established. Anderson et al. (1996) found that RCA was in the stroma of chloroplasts in pea plants. Recently, Hong et al. (2005) demonstrated that RCA was in the chloroplast of the bundle sheath and mesophyll cells in the C4 plant, Amaranthus tricolor. It is shown here that RCA is mainly in the stroma, and to a smaller extent in the thylakoid membranes, of chloroplasts (Fig. 1C). Rokka et al. (2001) reported that most RCA in spinach was sequestered in the thylakoid membrane region, and that the amount of RCA associated with the thylakoid membrane increased with the temperature and duration of the heat treatment. Interestingly, a reduction of RCA in tobacco plants increased sensitivity of photosynthesis to heat (Sharkey et al., 2001). Therefore, RCA appears to be a multifunction enzyme, regulating Rubisco activity and photosynthetic rate, and also involved in protection against heat damage in chloroplasts. The RCA in stroma of chloroplasts contributes to the activation of Rubisco, a process dependent on ATP hydrolysis (Spreitzer and Salvucci, 2002; Portis, 2003) and it is possible that the RCA in thylakoid membranes has a second, protective role there. RCA has been likened to a molecular chaperone (Sánchez de Jiménez et al., 1995). Neuwald et al. (1999) reported that RCA is related to an AAA family of proteins, a class of chaperone-like ATPases associated with a variety of cellular activities. They are a novel type of molecular chaperone, typically acting as disruptors of molecular or macromolecular structures (Ogura and Wilkinson, 2001). This describes the role of RCA in disrupting Rubisco-inhibitor complexes. AAA+ modules are also often linked covalently to other protein domains that mediate transporting to, and positioning in, cellular membranes. It is also possible that RCA is sequestered to the thylakoid membrane due to its redox regulation, since the activity of the large RCA isoform is regulated by redox changes via the ferredoxin/thioredoxin system at physiological ATP/ADP ratios (Zhang and Portis, 1999; Zhang et al., 2002). Reduction of RCA by thioredoxin could lead to inactivation and sequestration in thylakoid membranes.
RCA facilitates the dissociation of inhibitors from Rubisco, so it must bind to Rubisco and induce a conformational change at the active site. RCA was chemically cross-linked to the Rubisco (Yokota and Tsujimoto, 1992) and co-immunoprecipitation of the two proteins was reported (Sánchez de Jiménez et al., 1995; Zhang and Komatsu, 2000). Portis (2003) suggested that RCA encircles the Rubisco molecule. However, no RCA–Rubisco complex has been isolated. A double immunogold labelling study suggested that most of the RCA is associated with Rubisco, forming complexes in the chloroplast, while a large part of the Rubisco is not associated (Anderson and Carol, 2004). In the present study, approx. 75 % of RCA was in the stroma, and 25 % in the thylakoids (Table 2). By contrast, 96 % or more of the Rubisco was in the stroma (Table 2). These results imply that 75 % or less of the RCA interacts with Rubisco.
Rubisco occurs in the chloroplasts of higher plants (Catherine et al., 1984; Nishioka et al., 1996; Miyake et al., 2001) with most immunogold particles from anti-Rubisco antibodies in the stroma. In the antisense rca rice, it was found that the Rubisco content was substantially increased (Table 1), although its location was unaltered (Fig. 1 and Table 2). This effect of reduced RCA content upon Rubisco concentration is consistent with work using tobacco plants (Mate et al., 1993, 1996). The observed increase in Rubisco concentration tempts speculation that Rubisco accumulation counteracts the RCA deficit. Therefore, an increased requirement for RCA in the stroma was required to activate the increased Rubisco. It was demonstrated that labelling for RCA decreased, as did the percentage of RCA labelling in thylakoid membranes (Fig. 1D and Table 2). Mate et al. (1993), Eckardt et al. (1997) and Hammond et al. (1998) showed that RCA was largely saturating for the steady-state concentration of active Rubisco. This suggests that the changes in subcellular RCA distribution in the antisense plants might be related to compensation for the loss of RCA in the stroma and to the increase in Rubisco. This process may be linked to the redox regulation of large RCA isoform. This may explain why steady-state photosynthesis is largely unaffected until RCA concentration is reduced substantially. RCA in the antisense plants may then be transferred from the thylakoid membranes to the stroma to compensate for the reduced RCA, which activates Rubisco. However, transgenic plants with reduced RCA (Mott et al., 1997; Hammond et al., 1998) have a slower rate of photosynthetic induction following a rapid increase in light intensity, suggesting that RCA was not present in excess under those conditions; whether RCA in thylakoid membrane is transferred to the stroma is unclear. Changes in protein distribution following a stress have already been reported. For instance, mechanical stimulation induces a change in the location of GPXle-1 protein from the wall to the cytosol in collenchyma (Herbette et al., 2004). As far as is known, a change of protein location induced by reduction of protein content has not been reported previously. However, a decrease in the binding affinity of the protein for the thylakoid membrane in the antisense rca plants is not ruled out; more detailed biochemical characterization of these changes will be required to establish if this occurs.
Acknowledgments
This work was partly supported by the National Natural Science Foundation (30471051) and the Doctoral Foundation of Education Department (20020335043) of P. R. China.
LITERATURE CITED
- Anderson LE, Carol AA. 2004. Enzyme co-localization with Rubisco in pea leaf chloroplasts. Photosynthesis Research 82: 49–58. [DOI] [PubMed] [Google Scholar]
- Anderson LE, Gibbons JT, Wu WX. 1996. Distribution of ten enzymes of carbon metabolism in pea (Pisum sativum) chloroplasts. International Journal of Plant Sciences 157: 525–538. [Google Scholar]
- Andrews TJ, Hudson GS, Mate CJ, von Caemmerer S, Evans JR, Arvidsson YBC. 1995. Rubisco: the consequences of altering its expression and activation in transgenic plants. Journal of Experimental Botany 46: 1293–1300. [Google Scholar]
- Catherine PR, Raymond C, Pierre G. 1984. In situ immunofluorescent localization of phosphoenolpyruvate and ribulose- 1,5-bisphosphate carboxylase in leaves of C3, C4, and C3-C4 intermediate Panicum species. Planta 161: 266–271. [DOI] [PubMed] [Google Scholar]
- Eckardt AN, Snyder GW, Portis AR, Ogren WL. 1997. Growth and photosynthesis under high and low irradiance of Arabidopsis thaliana antisense mutants with reduced ribulose-1,5-bisphosphate carboxylase/oxygenase activase content. Plant Physiology 113: 575–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond ET, Andrews TJ, Mott KA. 1998. Regulation of Rubisco activation in antisense plants of tobacco containing reduced levels of Rubisco activase. The Plant Journal 4: 101–110. [DOI] [PubMed] [Google Scholar]
- He Z, von Caemmerer S, Hudson GS, Price GD, Badger MR, Andrews TJ. 1997. Ribulose-1,5-bisphosphate carboxylase/oxygenase activase deficiency delays senescence of ribulose-1,5-bisphosphate carboxylase/oxygenase but progressively impairs its catalysis during tobacco leaf development. Plant Physiology 115: 1569–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbette S, Brunel N, Prensier C, Julien JL, Drevet JR, Roeckel-Drevet P. 2004. Immunolocalization of a plant glutathione peroxidase-like protein. Planta 219: 784–789. [DOI] [PubMed] [Google Scholar]
- Hong J, Jiang DA, Weng XY, Wang WB, Hu DW. 2005. Leaf anatomy, chloroplast ultrastructure and Rubisco and Rubisco activase cellular localization in Amaranthus tricolor L. Photosynthetica 43: 519–528. [Google Scholar]
- Huang ZA, Jiang DA, Yang Y, Sun JW, Jin SH. 2004. Effects of nitrogen deficiency on gas exchange, chlorophyll fluorescence and antioxidant enzymes in leaves of rice plants. Photosynthetica 42: 357–364. [Google Scholar]
- Jiang DA, Lu Q, Weng XY, Zheng BS, Xi HF. 2000. The regulation of Rubisco carboxylation activity and photosynthetic rate by Rubisco activase during leaf senescence in rice. Journal of Zhejiang University (Agriculture and Life Sciences) 26: 119–124. [Google Scholar]
- Jiang DA, Weng XY, Lu Q. 2001. Quantitation of Rubisco activase by single radial immunodiffusion. Journal of Zhejiang University (Agriculture and Life Sciences) 27: 255–258. [Google Scholar]
- Jin S, Jiang D, Li X, Sun J. 2004a Characteristics of photosynthesis in rice plants transformed with an antisense Rubisco activase gene. Journal of Zhejiang University Science 5: 897–899. [DOI] [PubMed]
- Jin SH, Weng XY, Wang NY, Li XQ, Mao WH, Jiang DA 2004b Construction of expression vector with antisense Rubisco activase gene and its genetic transformation in rice. Hereditas (Beijing) 26: 881–886. [PubMed]
- Komatsu S, Masuda T, Hirano H. 1996. Rice gibberellin-binding phosphoprotein structurally related to ribulose-1,5-bisphosphate carboxylase-oxygenase activase. FEBS Letters 384: 167–171. [DOI] [PubMed] [Google Scholar]
- Mate CJ, Hudson GS, von Caemmerer S, Evans JR, Andrews TJ. 1993. Reduction of ribulose bisphosphate carboxylase activase levels in tobacco (Nicotiana tabacum) by antisense RNA reduces ribulose bisphosphate carboxylase carbamylation and impairs photosynthesis. Plant Physiology 102: 1119–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mate CJ, Hudson GS, von Caemmerer S, Evans JR, Andrews TJ. 1996. The relationship between CO2-assimilation rate, rubisco carbamylation and rubisco activase content in activase-deficient transgenic tobacco suggests a sample model of activase action. Planta 198: 604–613. [DOI] [PubMed] [Google Scholar]
- Miyake H, Nishimura M, Takeoka Y. 2001. Immunogold labeling of Rubisco in C4 plant for scanning electron microscopy. Plant Production Science 4: 41–49. [Google Scholar]
- Mott KA, Snyder GW, Woodrow IE. 1997. Kinetics of Rubisco activation as determined from gas exchange measurements in antisense plants of Arabidopsis thaliana containing reduced levels of Rubisco activase. Australian Journal of Plant Physiology 24: 811–818. [Google Scholar]
- Neuwald AF, Aravind L, Spouge JL, Koonin EV. 1999. AAA +: a class of chaperone-like ATPases associated with the assembly, operation, and disassembly of protein complexes. Genome Research 9: 27–43. [PubMed] [Google Scholar]
- Nishioka D, Miyake H, Taniguchi T. 1996. Suppression of granal development and accumulation of Rubisco in different bundle sheath chloroplasts of C4 succulent plant Portulaca grandiflora. Annals of Botany 77: 629–637. [Google Scholar]
- Ogura T, Wilkinson AJ. 2001. AAA+ superfamily ATPases: common structure—diverse function. Genes to Cells 6: 575–597. [DOI] [PubMed] [Google Scholar]
- Portis Jr AR. 1995. The regulation of Rubisco by Rubisco activase, Journal of Experimental Botany 46: 1285–1291. [DOI] [PubMed] [Google Scholar]
- Portis Jr AR. 2003. Rubisco activase—Rubisco's catalytic chaperone. Photosynthesis Research 75: 11–27. [DOI] [PubMed] [Google Scholar]
- Rokka A, Zhang L, Aro EN. 2001. Rubisco activase: an enzyme with a temperature-dependent dual function. The Plant Journal 25: 463–471. [DOI] [PubMed] [Google Scholar]
- Sánchez de Jiménez E, Medrano L, Martínez-Barajas E. 1995. Rubisco activase, a possible new member of the molecular chaperone family. Biochemistry 34: 2826–2831. [DOI] [PubMed] [Google Scholar]
- Sawada SS, Sato M, Kasai A, Yaochi D, Kameya Y, Matsumoto L, et al. 2003. Analysis of the feed-forward effects of sink activity on the photosynthetic source-sink balance in sigle-rooted sweet potato leaves. I. Activation of RuBPacse through the development of sinks. Plant and Cell Physiology 44: 190–197. [DOI] [PubMed] [Google Scholar]
- Sharma A, Komatsu S. 2002. Involvement of a Ca2+-dependent protein kinase component downstream to the gibberellin-binding phosphoprotein, RuBisCO activase, in rice. Biochemical and Biophysical Research Communications 290: 690–695. [DOI] [PubMed] [Google Scholar]
- Sharkey TD, Badger MR, von Caemmerer S, Andrews TJ. 2001. Increased heat sensitivity of photosynthesis in tobacco plant with reduced Rubisco activase. Photosynthesis Research 67: 147–156. [DOI] [PubMed] [Google Scholar]
- Spreitzer RJ, Salvucci ME. 2002. Rubisco: structure, regulatory interactions, and possibilities for a better enzyme. Annual Review of Plant Biology 53: 449–485. [DOI] [PubMed] [Google Scholar]
- Yokota A, Tsujimoto N. 1992. Characterization of ribulose-1,5-bisphosphate carboxylase/oxygenase carrying ribulose-1,5-bisphosphate at its regulatory sites and the mechanism of interaction of this form the enzyme with ribulose-1,5-bisphosphate carboxylase/oxygenase activase. European Journal of Biochemistry 204: 901–909. [DOI] [PubMed] [Google Scholar]
- Zhang N, Portis Jr AR. 1999. Mechanism of light regulation of Rubisco activase isoform involving reductive activation by thioredoxin-f. Proceedings of the National Academy of Sciences of the USA 96: 9438–9443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Kallis RP, Ewy RG, Portis Jr AR. 2002. Light modulation of Rubisco in Arabidopsis requires a capacity for redox regulation of the larger Rubisco activase isoform. Proceedings of the National Academy of Sciences of the USA 99: 3330–3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZL, Komatsu S. 2000. Molecular cloning and characterization of cDNAs encoding two isoforms of ribulose-1,5-bisphosphate carboxylase/oxygenase activase in rice (Oryza sativa L.). Journal of Biochemistry 128: 383–389. [DOI] [PubMed] [Google Scholar]


