Abstract
• Background and Aims This study investigates 47 taxonomically related species (Gentianales), all native to a tropical montane forest in southern Ecuador, in terms of nectar chemistry and nectar volumes in relation to pollination biology.
• Methods Nectar volumes of covered (24-h production) and uncovered (standing crop) flowers were measured in the natural habitat. Sucrose, fructose and glucose were quantified in the nectar using high performance liquid chromatography. Flower visitors were observed.
• Key Results Nectar sugar concentration did not differ significantly among the pollination syndromes. Regarding sugar composition, the only significant differences were found in chiropterophilous and myiophilous flowers, which had a significantly lower sugar ratio than sphingophilous flowers. A separation of chiropterophilous and myiophilous flowers from the other pollination syndromes is further substantiated by non-linear multidimensional scaling using the chord-normalized expected species shared index of dissimilarity based on nectar sugar compositions. The matrix test revealed no correlation of observed floral visitors to nectar concentrations; however, a weak significant correlation was found between floral visitors and nectar sugar compositions. The nectar volumes of covered and uncovered flowers are related to, and differ significantly among, pollination syndromes. Matrix tests revealed correlation between floral visitors and nectar volume of covered flowers and, to a lesser extent, of uncovered flowers.
• Conclusions Sucrose is the predominant floral nectar sugar in the order Gentianales, suggesting that nectar sugar composition is a conservative characteristic. However, some degree of an adaptive convergence of floral nectar compositions to principal pollinator type within the constraints set by phylogenetic history is likely. The driving force to visitation appears to be the volume of nectar the visitor can expect to consume.
Keywords: Nectar sugar composition, nectar volume, nectar standing crop, pollination syndrome, Rubiaceae, Gentianaceae, tropical montane forest, Ecuador
INTRODUCTION
Floral nectar is the most important reward offered to pollinators in angiosperms (Simpson and Neff, 1983). The major sugars in nectar are the disaccharide sucrose and the hexose monosaccharides glucose and fructose (Baker and Baker, 1983). Floral nectar characteristics such as sugar composition, sucrose–hexose proportions, concentration, volume, time of nectar secretion and nectar dynamics are often related to the interaction of flowers and pollinators (Baker and Baker, 1983; Freeman et al., 1984; Baker and Baker, 1990; Stiles and Freeman, 1993; Galetto et al., 1998; Perret et al., 2001; Pacini et al., 2003; Wolff et al., 2003, 2006). There are similarities in nectar features between taxonomically unrelated species in connection with the pollinator type. These convergences are often seen as a result of plant adaptation to preferences, digestive abilities, or sugar intake efficiencies of specific pollinators (Stiles, 1976; Haber and Frankie, 1989; Martínez del Rio et al., 1992; Baker et al., 1998). Other studies show homogeneity of nectar sugar composition among phylogenetically related taxa over various pollination syndromes (Galetto et al., 1998; Perret et al., 2001; Galetto and Bernardello, 2003). Whether nectar features are related to the type of pollinator, or whether nectar sugar composition is a conservative feature relatively constant within taxonomically related species, or both, still remains uncertain.
Many field studies of the nectar characteristics of flowering species sharing a single pollination syndrome, carried out in natural plant communities, reveal adaptation to this specific syndrome, such as hummingbird flowers (Stiles and Freeman, 1993; Sazima et al., 1996; Dziedzioch, 2001; McDade and Weeks, 2004a, b), moth flowers (Haber and Frankie, 1989), or bat flowers (Sazima et al., 1999). Previous studies focusing on nectar sugar composition in phylogenetically related taxa comprising a large variety of pollination syndromes in, for example, Asteraceae (Baker and Baker, 1982), Scrophulariaceae (Elisens and Freeman, 1988), Fabaceae (Van Wyk, 1993), Solanaceae (Galetto et al., 1998), Caryophyllaceae (Witt et al., 1999) and Gesneriaceae (Perret et al., 2001) were based primarily upon plant material from greenhouses or botanical gardens, such that the flower visitor impacts on nectar standing crop were unobserved. In an ecological context, however, decisions made by foragers are based upon rewards actually encountered (i.e. standing crop), and those are quite different from nectar volumes protected from flower visitors (McDade and Weeks, 2004a, b). Field observations are necessary to determine the role of nectar features in the interactions between plants and flower visitors. This study investigates taxonomically related species (Gentianales) all native to a tropical montane forest in southern Ecuador, under natural conditions. The monophyletic order Gentianales includes Apocynaceae, Gelsemiaceae, Gentianaceae, Loganiaceae and Rubiaceae (Backlund et al., 2000). Rubiaceae range among the most predominant Andean families in floristic studies (e.g. Gentry, 1988; Madsen and Øllgaard, 1994; Jørgensen and León-Yánez, 1999; Dorr et al., 2000; Webster and Rhode, 2001). According to Grant and Struwe (2003), the Podocarpus National Park presents one of the greatest species diversity in Macrocarpaea (Gentianaceae). Besides the large number of species existing at the study site, Gentianales exhibit flowers visited by bees, flies, butterflies, hummingbirds and bats, so this order is ideal for testing nectar features. Nectar composition, volume of covered and uncovered flowers, and flower visitors of 47 taxonomically related plant species from such a hitherto data-scarce region are presented here.
MATERIALS AND METHODS
Study site and plant material
The study site ‘Estación Científica San Fransisco’(03°58′S, 79°04′W; 1800–3150 m a.s.l.) is located within the Eastern Cordillera of the southern Ecuadorian Andes, bordering the Podocarpus National Park, which is known as an outstanding biodiversity hotspot (Barthlott et al., 1996). Most parts are covered with undisturbed or slightly disturbed montane rain forest. Detailed information on the floristic composition of the study site is provided in Bussmann (2001), Paulsch (2002) and Homeier (2004). Mean annual temperatures range from 15·5 °C in the lower areas to 9 °C at higher elevations. Annual rainfall increases from about 2000 mm in lower areas to >5000 mm in higher areas (P. Emck, University of Erlangen, Germany, unpubl. res.). Fieldwork was carried out from March to July 2000, September 2000 to February 2001 and from August to December 2001. All members of the order found at the study site were investigated except nine species of the subfamily Asclepiadoideae (Apocynaceae), which are treated in a separate paper, because their highly derived floral structure, their pollinia-forming habit demands and special pollination mechanisms. Gelsemiaceae and Loganiaceae did not occur at the study site. For Rubiaceae, the taxonomic classification of Andersson (1993) was followed and Gentianaceae were classified following Struwe et al. (2002). Voucher specimens are housed at MO and UBT.
Characterization of flower syndromes and observation of flower visitors
Considering the floral morphology of Gentianales, there is great variability in floral displays (corolla size, colour, scent) and nectar accessibility (corolla shape, corolla opening, tube length). The notion of pollination syndrome (Vogel, 1969; Faegri and van der Pijl, 1980; Proctor et al., 1996) was used to group the species. Classification was based on a set of morphological characteristics such as corolla shape, corolla colour, scent, pattern of floral anthesis and nectar secretion. Additionally, flower visitors were observed in the field. Each plant species considered to belong to the melittophilous, myiophilous or ornithophilous syndrome was observed for at least 12 h from 0600 h to 1800 h in blocks of 4 h. Night-flowering species were observed during the day and from 1800 h until midnight.
Species were classified as myiophilous when they were visited exclusively by diptera. The criteria for melittophily were: flowers open during the day, corolla white, cream, yellow or light blue, in some cases sweet diurnal scent emission (Faramea coerulescens, F. glandulosa, Arcytophyllum macbridei), small corolla tubes (<15 mm) and no visitation by hummingbirds. The criteria for ornithophily were: corolla or inflorescence branch red, yellow, blue or violet, no scent and frequent visitation by hummingbirds. ‘Sphingophily’ is used as a generic term for all species morphologically adapted to pollination by lepidopterans (including psychophily; Arachnothryx lojensis). The criteria for sphingophily in the narrow sense were: synchronized anthesis at night, corolla coloured white to cream, very narrow corolla tube, sweet fragrance and scent emission beginning or becoming more intense in the evening. Finally, chiropterophily was assigned by bell-shaped corolla, mushroom-like scent being more intensive during the night, and visitation by bats.
Nectar sampling and analysis
In order to measure the nectar volumes that legitimate flower visitors may obtain, the nectar standing crop was sampled at 0600 h, 1000 h, 1400 h and 1800 h for diurnal and nocturnal uncovered flowers, and at 1800 h, 2200 h, 0200 h, and 0600 h for nocturnal uncovered flowers. To determine the daily nectar production and nectar sugar concentrations, flowers were covered at the bud stage. The nectar of bee, fly and hummingbird flowers was sampled in the evening, and that of moth and bat flowers was sampled in the early morning by inserting microcapillaries and then recording the nectar volume. An aliquot of 2 μL nectar (or less if flowers contained <2 μL nectar) was injected into Eppendorf® caps with 70 % ethanol for each flower. Nectar taken at the same time and from the same species was pooled. The samples were frozen until determination of nectar concentration and composition. For analysis, samples were dried in a vacuum centrifuge, diluted with 200 μL water, and filtered using a WATERS™ high performance carbohydrate column to avoid contamination. The injection volume was 10 μL, and elution took place with an acetonitrile–water mixture (71 : 29) at a flow rate of 1·4 mL min−1 and a temperature of 35 °C. Glucose, fructose, and sucrose were detected with a refraction index detector and quantified with the WATERS Millenium Software™. Concentrations were converted from µg μL−1 to sucrose-equivalent, percentage weight per total weight, using table 63 in the 50th edition of the Handbook of Chemistry and Physics (Weast, 1969).
Statistical analysis
Data were tested for normality and homogeneity of variance. In order to meet these criteria, nectar volume of covered flowers was log (x + 1) transformed, and the sugar ratio was square root transformed. When data met the assumption for parametric statistics, ANOVA followed by Tukey–Kramer HSD for unequal N were used to test for differences of species means among classes of pollination. Because data on nectar standing crop violated the normality assumption for parametric statistics, the Kruskal–Wallis rank sums test followed by the Tukey–Kramer multiple comparison for non-parametric data were used to ascertain differences of species means among classes of pollination (Siegel and Castellan, 1988). The chord-normalized expected species shared (CNESS) distance index (Trueblood et al., 1994), ranging between 0 and the square root of 2, was used to determine differences between the sampled species' nectar sugar composition. CNESS is a metric version of Grassle and Smith's NESS similarity index (Grassle and Smith, 1976), and both can be regarded as more generalized forms of the Morisita index (Morisita, 1959). These are the most appropriate indices for analysing quantitative data (Wolda, 1981, 1983; Trueblood et al., 1994). Calculation of the CNESS index was performed using the updated version of the combinatorial polythetic agglomerative hierarchical clustering (COMPAH 96) program (Boesch, 1977) provided by Gallagher at UMASS/Boston (http://www.es.umb.edu/edgwebp.htm). Non-linear multidimensional scaling (NMDS) was used to visualize similarities among the species. Stress is a measurement that reflects the degree of deviation of NMDS distances from true matrix distances. According to Clarke (1993), stress values below 0·05 give an excellent representation with no prospect of misinterpretation. The Sørensen index, based on presence–absence data, was calculated for the floral visitors of each plant species. Euclidean distances were calculated for the nectar volumes of covered and uncovered flowers, as well as for nectar sugar concentrations. Matrix correlation tests were used to associate distance matrices (Mantel, 1967). For example, (1-Sørensen) the matrix of floral visitors can be directly compared with the dissimilarity (CNESS) matrix of nectar sugar composition, or nectar concentration (Euclidean) distances, or any other derived matrices (e.g. from nectar volume data). For the performance of matrix correlation tests, distance matrices were calculated for 46 plant species; Palicourea sp. was excluded because no floral visit was observed. Matrix correlation tests were performed by the program Primer™ Version 5 (Clarke and Gorley, 2001). To test correlation, Pearson correlation was used for parametric data (sugar ratio versus dimension 1 of the NMDS), and Spearman rank order correlation R was used for non-parametric data (nectar volumes of uncovered versus covered flowers; mean versus standard deviation of nectar volumes in covered and uncovered flowers). The data analysis software, STATISTICA™, Version 7·0 from StatSoft, Inc. (2004) was used.
RESULTS
Nectar sugar composition and concentration
Floral nectars were sucrose-dominant in all flowers classified as ornithophilous, as well as in the majority of flowers classified as sphingophilous, with sugar ratios ranging from 1·3 to 15·5 (the only exception was Isertia laevis with 0·7; see Table 1). Sucrose : hexose ratios below 1 were found in bat flowers all belonging to the tribe Helieae. The nectar sugar ratio ranged from 0·1 to 13·6 within the melittophilous syndrome. Sugar composition varied markedly among myiophilous species (Table 1) from hexose-dominant (Gentianella sp.) to hexose-rich (Halenia sp.) to sucrose-rich (Arcytophyllum filiforme, Psychotria aubletiana, Dioicodendron dioicum) and sucrose-dominant (Psychotria sp.). It is worth noting that the hexose-dominant and hexose-rich species occur at elevations above 3000 m (the only exception is Macrocarpaea harlingii).
Table 1.
Tribe, flower visitors of each species investigated, sample size Nf flowers, Ni individuals, Ns pooled nectar samples analysed, nectar sugar concentration and composition, daily nectar production of covered flowers and nectar standing crop grouped according to their pollination syndrome
| Species | Tribe* | Flower visitors† | Nf (Ni) | Ns | Conc. [%w/w] 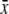 ± s.d. ± s.d. |
% Fructose 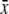 ± s.d. ± s.d. |
% Glucose 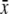 ± s.d. ± s.d. |
% Sucrose 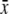 ±s.d. ±s.d. |
S/(F + G) 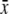 ± s.d. ± s.d. |
Nectar production 24 h covered 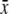 ± s.d. (μL) (n) ± s.d. (μL) (n) |
Standing crop 1800–0600 h 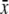 ± s.d. (μL) (n) ± s.d. (μL) (n) |
Standing crop 0600–1800 h 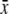 ± s.d. (μL) (n) ± s.d. (μL) (n) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Myiophily | ||||||||||||
| Arcytophyllum filiforme (Ruiz & Pav.) Standl. | R2 | Dip | 3 (1) | 1 | 32·0 | 30·8 | 36·3 | 33·0 | 0·5 | 0·3 ± 0·2 (3) | – | 0·1 ± 0·03 (7) |
| Dioicodendron dioicum (K. Schum. & K. Krause) Taylor | R8 | Dip | 13 (2) | 1 | 31·0 | 20·0 | 34·1 | 46·0 | 0·9 | No data | – | 0·3 ± 0·1 (13) |
| Gentianella sp. 1 | G1 | Dip | 15 (15) | 1 | 59·0 | 45·8 | 54·2 | 0·0 | 0·0 | 0·7 ± 0·5 (6) | – | 0·1 ± 0·1 (15) |
| Halenia sp. 1 | G1 | Dip | 12 (10) | 2 | 26·8 ± 14·5 | 44·1 ± 9·7 | 42·8 ± 8·9 | 13·1 ± 18·5 | 0·2 | 1·1 ± 0·4 (10) | – | 0·1 ± 0·1 (12) |
| Psychotria aubletiana Steyerm. | R1 | Dip | 3 (1) | 1 | 13·0 | 25·5 | 28·6 | 45·9 | 0·8 | No data | – | 0·5 ± 0·1 (3) |
| Psychotria sp. 1 | R1 | Dip | 16 (6) | 2 | 25·0 ± 8·5 | 16·2 ± 13·8 | 10·5 ± 8·1 | 73·3 ± 21·9 | 4·7 ± 4·6 | 0·7 ± 0·4 (9) | – | 0·2 ± 0·2 (14) |
| Melittophily | ||||||||||||
| Faramea uniflora Dwyer & M. V. Hayden | R3 | Hym, Dip | 9 (6) | 2 | 2·3 ± 2·5 | 0 | 0 | 100 | Not defined | 0·2 ± 0·2 (7) | – | 0·2 ± 0·2 (11) |
| Psychotria acuminata Benth. | R1 | Hym, Dip | 34 (6) | 1 | 9·0 | 16·2 | 17·3 | 66·5 | 2·0 | 1·9 ± 0·8 (18) | – | 0·8 ± 0·4 (19) |
| Psychotria tinctoria Ruiz & Pav. | R1 | Hym | 6 (4) | 2 | 49·5 ± 3·5 | 18·3 ± 2·5 | 21·3 ± 3·0 | 60·4 ± 5·4 | 1·5 ± 0·3 | 4·9 ± 3·4 (6) | – | 0·8 ± 0·7 (6) |
| Palicourea sp. nov. ined. C.M. Taylor | R1 | Hym | 78 (8) | 3 | 24 ± 5·6 | 16·6 ± 1·4 | 16·5 ± 1·3 | 66·9 ± 2·5 | 2·0 ± 0·2 | 5·8 ± 2·3 (78) | – | 0·1 ± 0·3 (25) |
| Coccocypselum condalia Pers. | R7 | Hym | 27 (8) | 2 | 13·7 ± 11·7 | 5·7 ± 5·2 | 6·8 ± 6·0 | 87·5 ± 11·2 | 4·5 ± 1·2 | 0·8 ± 0·4 (15) | – | 0·3 ± 0·2 (27) |
| Manettia sp. 2 | R2 | Hym | 23 (3) | 1 | 22·0 | 9·0 | 8·4 | 82·6 | 4·7 | 3·1 ± 1·4 (23) | – | 0·8 ± 1·2 (23) |
| Rudgea ciliata (Ruiz & Pav.) Spreng | R1 | Hym | 25 (4) | 2 | 40·8 ± 14·5 | 12·7 ± 4·8 | 13·3 ± 7·6 | 73·9 ± 12·4 | 3·3 ± 2·0 | 2·1 ± 0·9 (11) | – | 0·6 ± 0·5 (22) |
| Stilpnophyllum oellgaardii L. Andersson | R4 | Hym | 45 (7) | 6 | 27·4 ± 4·5 | 4·9 ± 1·4 | 15·8 ± 1·2 | 79·2 ± 2·5 | 3·9 ± 0·6 | 3·9 ± 2·2 (62) | – | 1·2 ± 0·9 (20) |
| Arcytophyllum macbridei Standl. | R2 | Hym | 27 (6) | 3 | 20·8 ± 11·6 | 30·9 ± 14·7 | 30·9 ± 19·7 | 38·1 ± 34·2 | 0·6 ± 1·0 | 0·5 ± 0·2 (12) | – | 0·3 ± 0·3 (35) |
| Arcytophyllum capitatum (Benth.) K. Schum. | R2 | Hym, Col | 40 (14) | 5 | 38·3 ± 12·9 | 38·8 ± 3·3 | 44·0 ± 7·0 | 17·2 ± 10·2 | 0·2 ± 0·1 | 1·0 ± 0·5 (24) | – | 0·2 ± 0·1 (40) |
| Arcytophyllum ciliolatum Standl. | R2 | Hym, Col | 45 (12) | 6 | 41·5 ± 20·8 | 31·8 ± 5·9 | 32·0 ± 5·7 | 36·1 ± 11·5 | 0·6 ± 0·3 | 1·4 ± 0·8 (13) | – | 0·6 ± 0·7 (31) |
| Arcytophyllum thymifolium (Ruiz & Pav.) Standl. | R2 | Hym, Col | 8 (4) | 2 | 22 ± 21·2 | 44·8 ± 0·4 | 42·8 ± 1·5 | 12·4 ± 1·9 | 0·1 ± 0·02 | 0·4 ± 0·2 (7) | – | 0·3 ± 0·2 (8) |
| Arcytophyllum vernicosum Standl. | R2 | Hym, Col, Dip | 12 (3) | 2 | 22 ± 2·8 | 22·5 ± 2·1 | 24·0 ± 0·2 | 53·5 ± 2·2 | 1·2 ± 0·1 | 0·4 ± 0·4 (13) | – | 0·4 ± 0·2 (12) |
| Notopleura vargasiana sp. nov. ined. C.M. Taylor | R1 | Hym, Lep, Dip | 21 (7) | 3 | 26·5 ± 25·3 | 6·4 ± 4·3 | 5·6 ± 3·8 | 88·0 ± 8·0 | 13·6 ± 14·5 | 0·8 ± 0·3(10) | – | 0·3 ± 0·3 (21) |
| Faramea cf. glandulosa Poepp. & Endl. | R3 | Hym, Lep | 8 (4) | 2 | 41·5 ± 9·2 | 14·3 ± 9·8 | 13·6 ± 9·7 | 72·1 ± 19·5 | 3·8 ± 3·3 | 1·6 ± 0·8 (12) | – | 0·4 ± 0·2 (12) |
| Psychotria reticulata Ruiz & Pav. | R1 | Hym, Lep | 19 (5) | 3 | 25·3 ± 14·0 | 12·7 ± 0·5 | 16·4 ± 2·6 | 70·9 ± 2·1 | 2·4 ± 0·3 | 1·0 ± 0·5 (27) | – | 0·3 ± 0·2 (12) |
| Faramea coerulescens K. Schum. & K. Krause | R3 | Hym, Lep, T3 | 8 (3) | 1 | 14·5 | 23·2 | 17·1 | 59·8 | 1·5 | 4·7 ± 2·6 (8) | – | 0·9 ± 0·7 (10) |
| Ornithophily | ||||||||||||
| Palicourea angustifolia Kunth | R1 | T, Hym, Lep | 24 (6) | 8 | 19·8 ± 5·9 | 14·4 ± 2·2 | 12·4 ± 2·7 | 73·2 ± 4·7 | 2·8 ± 0·7 | 7·7 ± 2·6 (24) | – | 2·2 ± 1·7 (53) |
| Palicourea calycina Benth. | R1 | T, Hym, Lep | 44 (12) | 9 | 24·9 ± 10·5 | 18·3 ± 5·9 | 16·0 ± 7 5 | 65·8 ± 13·2 | 2·2 ± 0·8 | 16·1 ± 5·1 (44) | – | 1·1 ± 1·0 (49) |
| Palicourea canarina C.M. Taylor | R1 | T, Hym, Lep | 106 (14) | 3 | 14·0 ± 2·0 | 29·3 ± 3·7 | 15·1 ± 3·8 | 55·6 ± 6·3 | 1·3 ± 0·3 | 14·4 ± 9·6 (106) | – | 5·6 ± 7·0 (62) |
| Palicourea heterochroma K. Schum. & K. Krause | R1 | T, Hym, Lep | 21 (6) | 13 | 15·3 ± 4·9 | 18·2 ± 5·4 | 14·4 ± 5·3 | 67·4 ± 10·6 | 2·3 ± 0·9 | 43·1 ± 16·3 (21) | – | 4·0 ± 4·8 (29) |
| Palicourea luteonivea C.M. Taylor | R1 | T, Hym, Lep | 26 (8) | 16 | 15·9 ± 4·0 | 14·8 ± 4·0 | 10·8 ± 8·6 | 74·4 ± 12·5 | 3·3 ± 0·8 | 15·9 ± 7·1 (26) | – | 2·8 ± 3·4 (92) |
| Palicourea subtomentosa (Ruiz & Pav.) DC | R1 | T2, Hym, Lep | 15 (4) | 2 | 17·0 ± 5·7 | 14·2 ± 3·8 | 12·2 ± 4·8 | 73·6 ± 8·6 | 3·0 ± 1·3 | 1·6 ± 0·6 (15) | – | 0·6 ± 0·5 (15) |
| Palicourea c.f. weberbaueri K. Krause | R1 | T, Hym, Lep | 33 (10) | 23 | 21·8 ± 8·4 | 20·4 ± 7·5 | 20·8 ± 11·2 | 58·9 ± 18·0 | 1·7 ± 0·8 | 7·5 ± 3·8 (45) | – | 1·6 ± 2·9 (92) |
| Palicourea lobbii Standl. | R1 | T, Hym | 13 (5) | 3 | 17·7 ± 2·1 | 11·8 ± 0·8 | 7·7 ± 0·7 | 80·5 ± 0·8 | 4·1 ± 0·2 | 10·6 ± 3·7 (15) | – | 3·6 ± 4·0 (15) |
| Palicourea lyristipula Wernham | R1 | T, Hym | 49 (10) | 14 | 26·1 ± 11·7 | 18·0 ± 6·6 | 17·6 ± 8·3 | 64·3 ± 14·7 | 2·1 ± 1·0 | 4·8 ± 2·0 (57) | – | 0·8 ± 1·3 (154) |
| Palicourea thyrsiflora SW | R1 | T, Hym | 35 (6) | 13 | 10·6 ± 2·6 | 22·8 ± 6·7 | 17·7 ± 5·8 | 59·5 ± 12·3 | 2·1 ± 2·6 | 30·6 ± 15·7 (35) | – | 2·8 ± 2·9 (51) |
| Manettia sp. 1 | R2 | T | 12 (4) | 4 | 8·3 ± 2·4 | 15·9 ± 6·5 | 7·3 ± 4·9 | 76·8 ± 11·3 | 4·1 ± 2·5 | 51·9 ± 7·1 (12) | – | 1·7 ± 2·4 (38) |
| Symbolanthus calygonus (Ruiz & Pav.) Griseb. ex Gilg | G2 | T | 10 (5) | 1 | 15·5 | 20·1 | 8 | 71·9 | 2·6 | 48·9 ± 16·5 (10) | – | 6·8 ± 5·7 (15) |
| Palicourea sp. 1 | R1 | – | 11 (1) | 1 | 14 | 17·8 | 17·6 | 64·6 | 1·8 | 14·5 ± 4·5 (11) | – | 0·5 ± 0·7 (11) |
| Psycho-Sphingo-Phalaenophily | ||||||||||||
| Arachnothryx lojensis Steyerm. | R6 | Lep | 17 (5) | 11 | 15·5 ± 4·8 | 7·6 ± 3·6 | 3·0 ± 5·3 | 89·4 ± 8·3 | 10·3 ± 4·6 | 1·8 ± 0·9 (17) | – | 0·6 ± 1·1 (248) |
| Ladenbergia sp. 1 | R4 | Noc, Sph, T | 3 (2) | 1 | 22 | 12·2 | 6·4 | 81·4 | 4·4 | 45·2 ± 9·7 (3) | 41·3 ± 15·3 (3) | 1·1 ± 1·0 (5) |
| Palicourea andrei Standl. | R1 | Noc, Sph, T | 5 (2) | 1 | 17 | 16 | 20·2 | 63·7 | 1·8 | 14·7 ± 3·1 (5) | 13·3 ± 3·3 (11) | 1·5 ± 1·0 (4) |
| Isertia laevis (Triana) B. M. Boom | R9 | Noc, Sph, T, Hym | 28 (6) | 18 | 17·9 ± 6·8 | 33·6 ± 9·0 | 31·3 ± 7·1 | 35·1 ± 12·2 | 0·7 ± 0·9 | 39·3 ± 19·6 (28) | 18·5 ± 14·3 (163) | 4·7 ± 7·2 (119) |
| Ladenbergia sp. 2 | R4 | Noc, Sph, Hym | 10 (1) | 1 | 9·5 | 14·5 | 17·6 | 67·9 | 2·1 | 5·4 ± 1·7 (10) | 6·4 ± 1·9 (15) | 0·2 ± 0·3 (15) |
| Hillia parasitica Jacquin | R5 | Sph, Hym | 4 (2) | 1 | 16 | 2·5 | 3·6 | 93·9 | 15·5 | 41·0 ± 9·9 (3) | 32 ± 11·9 (6) | 24·3 (1) |
| Hillia wurdackii Steyerm. | R5 | Sph, | 3 (2) | 1 | 19·5 | 6·5 | 2·5 | 91 | 10·1 | 38·3 ± 15·2 (4) | 36·7 ± 13·1 (7) | 29·5 (1) |
| Chiropterophily | ||||||||||||
| Macrocarpaea arborescens Gilg | G2 | G1, T, Hym | 7 (4) | 1 | 23 | 43·5 | 29·6 | 26·9 | 0·4 | 67·9 ± 15·8 (7) | 51·7 ± 18·7 (15) | 1·6 ± 1·7 (14) |
| Macrocarpaea harlingii J. S. Pringle | G2 | G1, T, Hym, Noc | 13 (6) | 1 | 11 | 36·3 | 35·3 | 28·4 | 0·4 | 73·1 ± 27·8 (13) | 60·6 ± 27·7 (18) | 2·5 ± 2·5 (19) |
| Macrocarpaea noctiluca J. R. Grant & Struwe | G2 | G1, T, Hym, Spi, Noc, Lep, Dip | 12 (6) | 1 | 10·5 | 33.9 | 25·8 | 40·3 | 0·7 | 98·8 ± 28·3 (12) | 58·7 ± 33·8 (32) | 4·4 ± 12·6 (26) |
| Symbolanthus sp. nov. ined. | G2 | G1, T, Hym, Spi, Noc | 12 (5) | 1 | 12 | 34·9 | 19·2 | 45·9 | 0·8 | 102·9 ± 42·1 (15) | 83·9 ± 28·0 (12) | 9·5 ± 6·2 (15) |
Flower visitors were observed by the author with the exception of those observed by 1 Matt (2001), 2 Dziedzioch (2001) and 3 A. Paulsch (pers. comm.).
 = mean; s.d. = standard deviation; n = number of flowers sampled for nectar volumes.
= mean; s.d. = standard deviation; n = number of flowers sampled for nectar volumes.
G, Gentianaceae; R, Rubiaceae. Tribal affiation is designated by the following numerals: G1, Gentianeae; G2, Helieae; R1, Psychotrieae; R2, Hedyotideae; R3, Coussareae; R4, Cinchoneae; R5, Hillieae; R6, Rondeletieae; R7, Coccocypseleae; R8, Condamineeae; R9, Isertieae.
G, Glossophagidae; T, Trochilidae; Hym, Hymenoptera (mainly Apidae); Lep, Lepidoptera except Sphingidae, Noctuideae, Geometridae; Sph, Sphingidae; Noc, Noctuidae and Geometridae; Dip, Diptera; Col, Coleoptera.
There is a significant sugar ratio difference between sphingophilous and myiophilous species and between sphingophilous and chiropterophilous species (ANOVA with a following post-hoc test; see Table 2).
Table 2.
Means and standard deviation of nectar sugar concentration and sugar ratio, nectar volume covered and nectar standing crop of flowers in different pollination syndromes
| Syndrome | n | Conc. (%w /w) 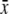 ± s.d. ± s.d. |
Sugar ratio S/(F + G) 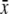 ± s.d. ± s.d. |
Nectar volume 24 h covered (μL) 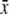 ± s.d. ± s.d. |
Standing crop 0600–1800 h (μL) 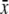 ± s.d. ± s.d. |
Mann–Whitney U Nectar volume covered vs. standing crop |
|---|---|---|---|---|---|---|
| Myiophilous | 61 | 31·1 ± 15·2 | 1·2 ± 1·8 a | 0·7 ± 0·3 a,b,c | 0·2 ± 0·2 a,b,c | z = 2·2, P = 0·025 |
| Melittophilous | 172 | 25·9 ± 12·8 | 2·9 ± 3·2 | 2·0 ± 1·8 e,g,h | 0·5 ± 0·3 d,e | z = 2·3, P = 0·021 |
| Ornithophilous | 13 | 17·0 ± 5·1 | 2·6 ± 0·9 | 20·6 ± 17·2 a,d,e | 2·6 ± 1·9 a,d | z = 3·4, P = 0·000 |
| Sphingophilous (including one psychophilous species) | 7 | 16·8 ± 3·9 | 6·4 ± 5·6 a,b | 26·5 ± 18·5 b,f,g | 8·8 ± 12·5 b | z = 3·8, P = 0·000 |
| Chiropterophilous | 4 | 14·1 ± 5·9 | 0·6 ± 0·2 b | 85·7 ± 17·7 c,d,f,h | 4·5 ± 3·5 c,e | z = –2·2, P = 0·025 |
| ANOVA/K–W ANOVA | F4,42 = 3·7 P = 0·011 | F4,41 = 4·3 P = 0·005 | F4,40 = 29·3 P = 0·000 | H(4, 7) = 27·4 P = 0·000 |
Mann–Whitney U-test of significant differences between covered nectar volumes and standing crop.
n = number of species, 1number of species nectar production: n = 4 myiophilous syndrome; 2number of species sugar ratio: n = 16 melittophilous syndrome.
A same letter following the values indicates significantly different pairs after ANOVA with following Tukey–Kramer HDS post-hoc test (α = 0.05) or Kruskall–Wallis ANOVA with Tukey–Kramer post-hoc test (α = 0.05).
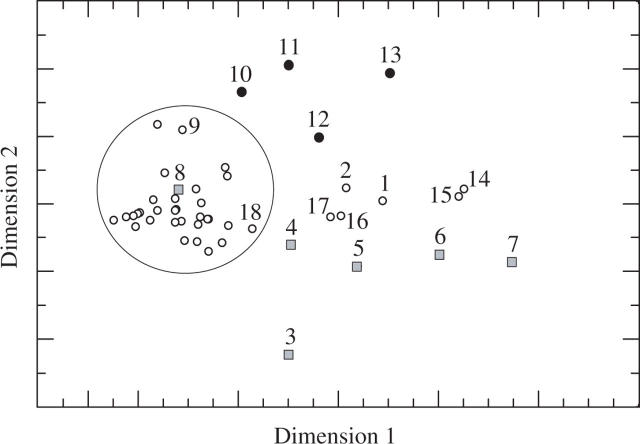
Differing nectar compositions among species, based on the CNESS index are visualized using non-linear multidimensional scaling (stress = 0·014; Fig. 1). The dominant cluster was characterized by species belonging to the melitto-, ornitho- and sphingophilous syndrome; only sphingophilous Isertia laevis, ornithophilous Palicourea canarina and melittophilous members of the genus Arcytophyllum are separated from this cluster. Species receiving visits exclusively from dipters are well separated from the main cluster (only myiophilous Psychotia sp. is located within the main cluster). Chiropterophilous species belonging to the tribe Helieae are further separated from the main cluster. There is a significant negative correlation (r = 0·395, t = −2·8, P = 0·007; Pearson) between dimension 1 and the sugar ratio.
Fig. 1.
Non-linear multidimensional scaling (NMDS) of the nectar sugar composition of 47 species based on the CNESS index. 1, Isertia laevis, 2; Palicourea canarina, 3; Dioicodendron dioicum, 4; Psychotria aubletiana, 5; Arcytophyllum filiforme, 6; Halenia; 7, Gentianella; 8, Psychotria sp.; 9, Symbolanthus calygonous; 10, Macrocarpaea harlingii; 11, Sym. sp.; 12, M. noctiluca; 13, M. arborescens; 14, A. thymifolium; 15, A. capitatum; 16, A. ciliolatum; 17, A. macbridei; 18, A. vernicosum. Squares, myiophilous species; filled circles, chiropterophilous species.
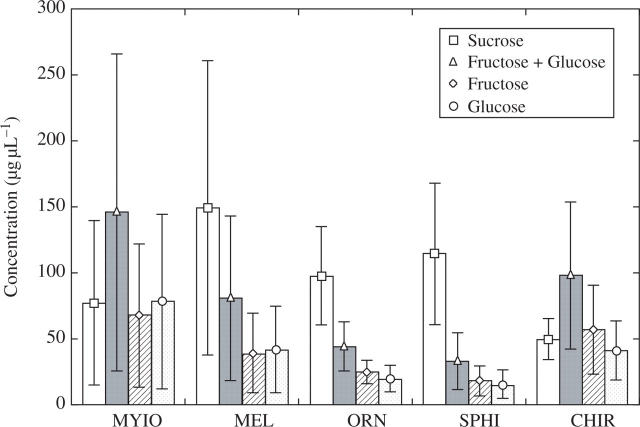
Sucrose concentration averaged 149 µg μL−1 in species with the melittophilous syndrome, compared with 50 µg μL−1 in those with the chiropterophilous syndrome, whereas hexose concentration was similar 81 µg μL−1 in bee flowers and 98 µg μL−1 in bat flowers (Fig. 2). Sugar proportions between ornithophilous and sphingophilous flowers are more or less equal, amounting to 98 µg μL−1 sucrose in the former and 114 µg μL−1 sucrose in the latter, while hexose concentration was 44 µg μL−1 in hummingbird flowers and 33 µg μL−1 in moth flowers (Fig. 2). Within these two types of flowers, the hexose proportion was clearly lower than in bee, bat and fly flowers. The highest hexose concentration of 146 µg μL−1 was found in flowers of the myiophilous syndrome, whose sugar proportions were the inverse to those of the melittophilous syndrome. Considering hexose only, the proportion of fructose to glucose was more or less balanced across all species. Total sugar concentrations were variable among species (Table 1), and no significant differences could be detected among syndromes (ANOVA with following Tukey–Kramer post-hoc test, Table 2).
Fig. 2.
Mean proportion of sucrose and hexose (fructose + glucose) in the nectar of 47 Gentianales species arranged according to their pollination syndromes: MYIO, myiophilous syndrome (n = 6); MEL, melittophilous syndrome (n = 17); ORN, ornithophilous snydrome (n = 13); SPHI, sphingophilous syndrome (n = 7); CHIR, chiropterophilous syndrome (n = 4). Vertical bars represent s.d.
Nectar sugar concentration was not significantly correlated with floral visitors (R = 0·097, P = 0·077, matrix correlation). There was, however, a slight significant correlation between nectar sugar composition and floral visitors (R = 0·197, P = 0·043, matrix correlation).
Nectar volume and standing crop
In covered flowers, nectar volumes varied markedly among the pollination syndromes and among species with the same syndrome, ranging from 0·3 to 1·1 μL in fly, 0·2 to 5·8 μL in bee, 1·6 to 51·9 μL in hummingbird, 67·9 to 102·9 μL in bat and 1·8 to 45·2 μL in moth flowers (Table 1). Daily nectar production differed significantly among the pollination syndromes, except for ornithophilous versus sphingophilous and myiophilous versus melittophilous (ANOVA, with following Tukey–Kramer post-hoc test; see Table 2). In uncovered flowers sampled during the day, nectar volumes among the pollination syndromes and among species with the same syndrome were less variable, and ranged from 0·1 to 0·5 μL in fly, 0·1 to 1·2 μL in bee, 0·5 to 6·8 μL in hummingbird, 1·6 to 9·5 μL in bat and 0·2 to 29·5 μL in moth flowers (Table 1). The nectar standing crop measured during the day differed significantly between myiophilous flowers versus ornithophilous, sphingophilous, and chiropterophilous flowers, and between melittophilous flowers versus ornithophilous and chiropterophilous flowers (Table 2).
A significant correlation was found between the nectar volumes of covered flowers and floral visitors (R = 0·228, P = 0·007, matrix correlation test). Standing crop and floral visitors were also significantly correlated (R = 0·157, P = 0·028, matrix correlation test).
The nectar standing crop sampled during the day was significantly correlated with nectar volumes of covered flowers (Spearman coefficient R = 0·83, P = 0·000). Conversely, diurnal standing crop values differed significantly from covered nectar volumes in all syndromes (Mann–Whitney U-test; Table 2). The nectar standing crop of bat and moth flowers sampled at night did not differ significantly (Mann–Whitney U-test: bat flowers z = −1·7, P = 0·08, moth flowers z = 0·8, P = 0·42) from those of covered flowers, which indicates a low nocturnal visitation rate.
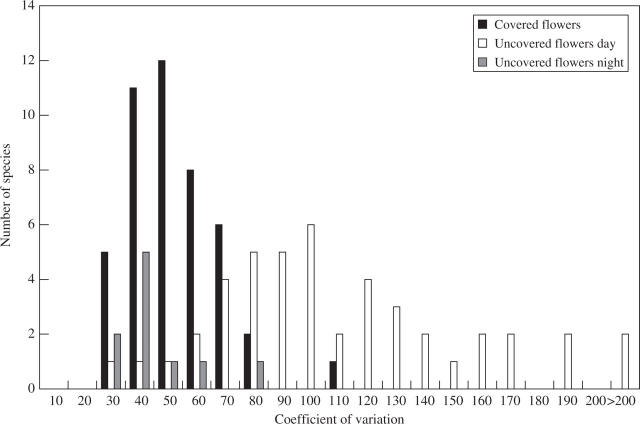
Among species, the distribution of variability of nectar volumes measured by the coefficient of variation (standard deviation/mean) is shown in Fig. 3. Nectar volumes of uncovered flowers sampled during the day were more variable than the nectar volumes sampled at night and the nectar volumes of covered flowers. Coefficients of variation among nectar volumes in uncovered nocturnal flowers ranged within those for covered flowers, further indicating a low nocturnal visitation rate. There was a significant linear correlation between the means and standard deviations of nectar volumes of covered and uncovered flowers sampled during the day (Spearman coefficient R = 0·98, P = 0·000; R = 0·94, P = 0·000, respectively).
Fig. 3.
Distribution of variability, measured by the coefficient of variation of nectar volume of covered flowers (45 plant species) and uncovered flowers (ten species probed during the night, and 45 species probed during the day).
DISCUSSION
Nectar sugar composition and concentration
The main goal of this study was to determine whether nectar features are related to the type of pollinator. Regarding the pollination syndromes, no nectar sugar concentration correlation was found. Regarding sugar composition, the only significant differences were found in chiropterophilous and myiophilous flowers, which had a significantly lower sugar ratio than sphingophilous flowers. This is further substantiated by the NMDS of the CNESS dissimilarity index based on nectar sugar compositions (Fig. 1). Nectars from flowers visited by hummingbirds, bees, butterflies and moths formed one homogenous cluster, and nectar from flowers visited exclusively by flies (except Psychotria sp.) formed a second group. A third group included nectar from flowers visited by bats. Although sucrose is the predominant floral nectar sugar in 41 out of 47 investigated species, nectars from species within each pollination syndrome tend to have characteristic sugar compositions (Fig. 2). In this study, sucrose was the dominant constituent in all flowers of the ornithophilous syndrome. Ornithophilous flowers of several taxonomic groups have sucrose-dominant nectar (Baker and Baker, 1983; Freeman et al., 1984; Gottsberger et al., 1984; Stiles and Freeman, 1993; Dziedzioch, 2001; Perret et al., 2001; Galetto and Bernardello, 2003). Nectar of the ornithophilous plants investigated contained, on average, 68·2 % sucrose, agreeing with the results of Nicolson and Fleming (2003), who showed that hummingbird nectars cluster around 64 % sucrose. Considering the sugar concentration in the nectar of Gentianales, values for hummingbird flowers (17·0 %) were slightly lower than those found in the literature, citing a range from 21 to 26 % (Baker, 1975; Waser and Pyke, 1981; Heyneman, 1983; Stiles and Freeman, 1993; Sazima et al., 1996; Kraemer, 1998; Perret et al., 2001; McDade and Weeks, 2004a). However, feeding experiments with hummingbirds show, that even more concentrated sugar solutions (31–45 %) are preferred (Pyke and Waser, 1981; Kingslover and Daniel, 1983; Tamm and Gass, 1986; Roberts, 1996; Nicolson and Fleming, 2003).
This study's data on the sugar composition of sphingophilous Rubiaceae agree with the studies of several other families (Baker and Baker, 1983; Haber and Frankie, 1989; Schwerdtfeger, 1996), showing sucrose-dominant nectar presence in the majority of sphingophilous flowers. Sphingophilous flowers produced less concentrated nectar (16·8 %) than the reported mean of 21 % (Haber and Frankie, 1989), and 19 % (hawkmoths) and 22 % (settling moths) reviewed by Heyneman (1983).
The nectar of the majority (13 of 17 species) of melittophilous flowers is sucrose-dominant. This agrees with previous observations for melittophilous flowers of the Antirrhineae (Scrophulariaceae; Elisens and Freeman, 1988), Iridaceae (Goldblatt et al., 1998) and Sinningieae (Gesneriaceae; Perret et al., 2001). Hexose-dominant to sucrose-rich nectar is found in four species of Arcytophyllum (Rubiaceae). According to the phylogeny of the genus Arcytophyllum provided by Andersson et al. (2002) and Wolff and Liede-Schumann (2006), the most derived species of A. macbridei and A. vernicosum have higher sucrose proportions, whereas the basal A. thymifolium has a very low sucrose/hexose ratio. This suggests a tendency towards a higher percentage of sucrose in the genus. Nectar concentration of the flowers of the melittophilous syndrome studied here are lower (25·9 %) than the corresponding values in the temperate and tropical regions reported by Pyke and Waser (1981) (36 %) and Galetto et al. (1998) (48 %), but are close to the value (29 %) for melittophilous Gesneriaceae (Perret et al., 2001). Bees prefer very concentrated nectar to guarantee energetically profitable foraging (Bolten and Feinsinger, 1978).
Distinctive nectar composition is associated with the chiropterophilous syndrome, in which particularly low sucrose production is responsible for hexose dominance (Fig. 2). The high hexose proportion found in flowers of Macrocarpaea corresponds well with other bat flowers (Baker and Baker, 1983; Baker et al., 1998; Perret et al., 2001). Nectar concentration (14 %) of this study's bat pollinated Gentianaceae corresponds with the chiropterophilous Gentianaceae (10–15 %) reported by Machado et al. (1998), as well as with the results from Sazima et al. (1999) who reported an average sugar concentration of bat-pollinated flower assemblages of 15 % (lowland) and 18 % (highland). These values are close to the median range of the frequency distribution reviewed by Helversen (1993) for 33 species of neotropical bat-pollinated flowers. Roces et al. (1993), however, showed in a series of dual choice tests that glossophagine bats preferred higher nectar concentrations up to 50 %.
Flies prefer hexose-dominant and hexose-rich nectars (Baker and Baker, 1983). High hexose proportions are found in flowers exclusively visited by flies. Sugar concentration (31 %) varied markedly within the myiophilous syndrome (range 13–59 %). Pombal and Morellato (1995) found very low sugar concentrations (2 %) in fly-pollinated Araliaceae. Machado and Loiola (2000) report 16 % in Cordia (Boraginaceae) and 30 % in Borreria (Rubiaceae).
Except for the nectar of Psychotria aubletiana, nectars analysed for 21 species of tribe Psychotrieae are quite homogenous and sucrose-dominant, even though species are morphologically classified as ornithophilous, melittophilous, sphingophilous and myiophilous. In contrast, working with two other ornithophilous Ecuadorian species of the tribe, Bernardello et al. (1994) found hexose-rich and hexose-dominant nectar. The sugar compositions among Gentianales reported here indicate sucrose-dominant (53·5–100 % sucrose) or sucrose-rich (33–46 % sucrose) nectars predominate, even though flies, bees, beetles, diurnal and nocturnal butterflies, hummingbirds and bats were the principal floral visitors. Only Arcytophyllum capitatum, A. thymifolium, Macrocarpaea harlingii, M. arborescens, Halenia sp. and Gentianella sp. had hexose-rich to hexose-dominant nectar (0–28·4 % sucrose). The homogeneity of nectar sugar composition in the majority of species indicates that this is a conservative characteristic in the Gentianales investigated. The data support hypotheses of phylogenetic constraint on nectar sugar composition. Interestingly, similar results are found in different families if nectar sugar composition is compared with flower morphology and studied within small monophyletic groups (Elisens and Freeman, 1988; Galetto et al., 1998; Perret et al., 2001; Torres and Galetto, 2002).
In general, no correlation of floral visitors to nectar concentration was found (matrix correlation). A weak significant correlation was found, however, between floral visitors and nectar sugar composition (matrix correlation). It is likely that there has been some degree of an adaptive convergence of floral nectar compositions to principal pollinator type within the constraints set by phylogenetic history.
Nectar volume and standing crop
The nectar volumes of covered flowers are related to, and differ significantly among, pollination syndromes, with the exception of ornithophilous versus sphingophilous and myiophilous versus melittophilous flowers.
In this study, bat flowers contained about half of the average nectar volume found by Sazima et al. (1999) (151 μL lowland, 167 μL highland). The nectar volume of seven bat-visited flowers studied by Tschapka (2004) varied from 100 to 21 260 μL. However, Perret et al. (2001) reported an average amount of 89 μL for two chiropterophilous Sinningieae (Gesneriaceae), and Machado et al. (1998) reported for the Gentianaceae Irlbachia an average nectar amount of 43 μL. Nevertheless, the bat flowers investigated here contained four times as much nectar as the hummingbird flowers studied. Mean nectar amounts from the ornithophilous flowers fell within the range of other neotropical bird-visited flowers, with 16·9 μL (Sazima et al., 1996), 28·9 μL (Kraemer, 1998), 16·3 μL (Schmitt, 2000), 18·4 μL (Perret et al., 2001), 38·5 μL (Dziedzioch, 2001) and 8·8 to 72·7 μL (McDade and Weeks, 2004a) being reported. Haber and Frankie (1989) observed highly variable nectar volumes among sphingophilous species with a mean of approx. 60 μL, which is twice the mean nectar volume found in this study. Low nectar volumes have generally been found in melittophilous flowers; however, Perret et al. (2001) found more than ten times more nectar (15·4 μL) in flowers of bee-pollinated Gesneriaceae than in the bee flowers studied here. Mean nectar volumes below 1 μL were found in myiophilous species. In addition, there is a significant correlation between floral visitors and covered and uncovered nectar volumes (matrix correlation).
The nectar volumes of covered flowers have little relation to the standing crop quantities actually offered to potential flower visitors (as this study shows by the significantly lower values in standing crop compared with the cumulative nectar of covered flowers, and by the results of McDade and Weeks, 2004b), but even the standing crop nectar volumes differed among the syndromes. On the other hand, there is a positive correlation between nectar sampled during the day in covered and uncovered flowers. According to Zimmermann (1988), there must be a significant relationship between nectar production and standing crop if pollinators are to exert any selective pressure on the rate of nectar production. The amount of nectar obtained by the pollinators from the standing crop is determined by nectar production, as well as by depletion and by the morphological match between the pollinator and the flower (Rathcke, 1992). Environmental factors such as temperature, relative humidity and soil moisture also affect standing crop nectar. The data reveal great variability in the coefficients of variation (CV) for nectar volume among plant species, and even greater variability in the CV for the diurnal nectar standing crop. Variability in nectar amount is quite common (Rathcke, 1992; Petanidou and Smets, 1995; Cresswell, 1998; McDade and Weeks, 2004a, b). Foragers are sensitive to the CV of the reward (review in Real and Caraco, 1986; Kacelnik and Bateson, 1996; Bateson, 2002; Shafir et al., 2003). Among other things, a pollinator's behaviour is influenced by the CV of the nectar standing crop, i.e. the higher the CV, the stronger the risk-aversion (Bateson, 2002; Shafir et al., 2003). The linear correlation between the mean and standard deviation of nectar volume and standing crop between plant species found in this study is in accordance with the findings of Petanidou and Smets (1995) and McDade and Weeks (2004a, b).
Nectar volume influence pollinators' behaviour, which governs pollen receipt and donation (for example, see Ladio and Aizen, 1999; Manetas and Petropoulou, 2000; Lasso and Naranjo, 2003; Wolff et al., 2006). Effective pollination is guaranteed when nectar reward is abundant enough to attract the pollinator but small enough to force the pollinator to visit various individuals. Nectar volume production is therefore important in floral evolution and probably influenced by the most effective pollinator.
In summary, sucrose is the predominant floral nectar sugar in the order Gentianales. The homogeneity of nectar sugar composition in the majority of species indicates that this is a conservative characteristic in the Gentianales investigated. There is no correlation between sugar concentration and pollination syndromes. Nectar sugar composition does not differ significantly among the pollination syndromes (two exceptions being sphingophilous versus chiropterophilous and myiophilous nectars); only nectar volumes are related to pollination syndromes. Although certain nectar compositions and concentrations may be preferred by a given visitor, the results of the study show that various compositions and concentrations are accepted and tolerated by the visitor, not unlike the feeding behaviour of other species, including our own. However, some degree of an adaptive convergence of floral nectar compositions to principal pollinator type within the constraints set by phylogenetic history is likely. The driving force to visitation appears to be the volume of nectar the visitor can expect to consume. As the data on nectar volumes disclose, nectar production is important in floral evolution and influenced by the predominant pollinator.
Acknowledgments
I thank S. Liede-Schumann and H. Döring for valuable comments on early drafts, G. Gottsberger for the use of HPLC equipment, C. M. Taylor for identifying the plant species, A. Täuber for technical assistance, S. Dötterl for performing matrix correlation tests, and the Ministerio del Ambiente for permission to work at Parque Nacional Podocarpus (0010-PNPZA). Comments of two anonymous referees were especially helpful in order to improve the manuscript. This research was supported by DFG grant Li 496/11-1, FOR 402.
LITERATURE CITED
- Andersson L. 1993. Rubiaceae – introduction. In: Harling G, Andersson L, eds. Flora of Ecuador No 47. Nordic Journal of Botany, 3–11.
- Andersson L, Rova JHE, Guarin FA. 2002. Relationships, circumscription, and biogeography of Arcytophyllum (Rubiaceae) based on evidence from cpDNA. Brittonia 54: 40–49. [Google Scholar]
- Backlund M, Oxelman B, Bremer B. 2000. Phylogenetic relationships within the Gentianales based on ndhF and rbcL sequences, with particular reference to the Loganiaceae. American Journal of Botany 87: 1029–1043. [PubMed] [Google Scholar]
- Baker HG. 1975. Sugar concentration in nectar from hummingbird flowers. Biotopica 7: 37–41. [Google Scholar]
- Baker HG, Baker I. 1982. Chemical constituents in nectar in relation to pollination mechanisms and phylogeny. In: Nitecki MH, ed. Biochemical aspects of evolutionary biology. Chicago, IL: University of Chicago Press, 131–171.
- Baker HG, Baker I. 1983. Floral nectar constituents in relation to pollinator type. In: Jones CE, Little RJ, eds. Handbook of experimental pollination biology. New York, NY: Van Nostrand Reinhold, 117−141.
- Baker HG, Baker I. 1990. The predictive value of nectar chemistry to the recognition of pollinator types. Israel Journal of Botany 39: 157–166. [Google Scholar]
- Baker HG, Baker I, Hodges SA. 1998. Sugar composition of nectar and fruits consumed by birds and bats in the tropics and subtropics. Biotropica 30: 559–586. [Google Scholar]
- Barthlott W, Lauer W, Placke A. 1996. Global distribution of species diversity in vascular plants: towards a world map of phytodiversity. Erdkunde 50: 317–327. [Google Scholar]
- Bateson M. 2002. Recent advances in our understanding of risk-sensitive foraging preferences. Proceedings of the Nutrition Society 61: 509–516. [DOI] [PubMed] [Google Scholar]
- Bernardello G, Galetto G, Jaramillo L, Grijalba E. 1994. Floral nectar chemical composition of some species from Reserva Río Guajalito, Ecuador. Biotropica 26: 113–116. [Google Scholar]
- Boesch DF. 1977. Application of numerical classification in ecological investigations of water pollution. Special Scientific Report, Institute of Marine Science, Virginia.
- Bolten AB, Feinsinger P. 1978. Why do hummingbird flowers secrete dilute nectar? Biotropica 10: 307–308. [Google Scholar]
- Bussmann RW. 2001. The montane forests of Reserva Biológica San Francisco (Zamora-Chinchipe, Ecuador). Die Erde 132: 9–25. [Google Scholar]
- Clarke KR. 1993. Non-parametric multivariate analyses of changes in community structure. Australian Journal of Ecology 18: 117–143. [Google Scholar]
- Clarke KR, Gorley RN. 2001. PRIMER v5: user manual/tutorial. Plymouth: PRIMER-E, 1–91.
- Cresswell JE. 1998. Stabilizing selection and the structural variability of flowers within species. Annals of Botany 81: 463–473. [Google Scholar]
- Dorr LJ, Stergios B, Smith AR, Cuello ANL. 2000. Catalogue of the vascular plants of Guaramacal National Park, Portuguesa and Trujillo states, Venezuela. Contributions from the United States National Herbarium, Smithsonian Institution, Washington 40.
- Dziedzioch C. 2001. Artenzusammensetzung und Ressourcenangebot kolibribesuchter Pflanzen im Bergwald Südecuadors. Doctoral Thesis, University of Ulm, Germany.
- Elisens WJ, Freeman CE. 1988. Floral nectar sugar composition and pollinator type among New World genera in tribe Antirrhineae (Scrophulariaceae). American Journal of Botany 75: 971–978. [Google Scholar]
- Faegri K, van der Pijl L. 1980. The principles of pollination ecology. Oxford: Pergamon Press.
- Freeman CE, Reid WH, Becvar JE, Scogin R. 1984. Similarity and apparent convergence in the nectar-sugar composition of some hummingbird-pollinated flowers. Botanical Gazette 145: 132–135. [Google Scholar]
- Galetto L, Bernardello G. 2003. Nectar sugar composition in angiosperms from Chaco and Patagonia (Argentina): do animal visitors matter? Plant Systematics and Evolution 238: 69–86. [Google Scholar]
- Galetto L, Bernadello G, Sosa CA. 1998. The relationship between floral nectar composition and visitors in Lycium (Solanaceae) from Argentina and Chile: what does it reflect? Flora 193: 303–314. [Google Scholar]
- Gentry AH. 1988. Tree species richness of upper Amazonian forests. Proceedings of the National Academy of Sciences of the USA 85: 156–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldblatt P, Manning JC, Bernhardt P. 1998. Adaptive radiation of bee-pollinated Gladiolus species (Iridaceae) in Southern Africa. Annals of the Missouri Botanical Garden 85: 492–517. [Google Scholar]
- Gottsberger G, Schrauwen J, Linskens HF. 1984. Amino acids and sugars in nectar, and their putative evolutionary significance. Plant Systematics and Evolution 145: 55–77. [Google Scholar]
- Grant JR, Struwe L. 2003. De Macrocarpaeae Grisebach (ex Gentianaceis) speciebus novis III: six new species of moon-gentians from Parque Nacional Podokarpus, Ecuador. Havard Papers in Botany 8: 61–81. [Google Scholar]
- Grassle JF, Smith W. 1976. A similarity measure sensitive to the contribution of rare species and its use in investigation of variation in marine benthic communities. Oecologia 25: 13–22. [DOI] [PubMed] [Google Scholar]
- Haber WA, Frankie GW. 1989. A tropical hawkmoth community: Costa Rican dry forest Sphingidae. Biotropica 21: 155–172. [Google Scholar]
- Helversen Ov. 1993. Adaptation of flowers to pollination by glossophagine bats. In: Barthlott W, Naumann CM, Schmidt-Loske K, Schumann KL, eds. Animal–plant interaction in tropical environments. Bonn: Zoologisches Forschungsinstitut und Museum Alexander König, 41–59.
- Heyneman AJ. 1983. Optimal sugar concentration of floral costs. Oecologia 60: 198–213. [DOI] [PubMed] [Google Scholar]
- Homeier J. 2004. Baumdiversität, Waldstrucktur und Wachstumsdynamik zweier tropischer Bergregenwälder in Ecuador und Costa Rica. Doctoral Thesis, University of Bielefeld, Germany.
- Jørgensen PM, León-Yánez S. 1999. Catalogue of the Vascular Plants of Ecuador. St Louis, MO: Missouri Botanical Garden Press.
- Kacelnik A, Bateson M. 1996. Risky theories – the effects of variance on foraging decisions. American Zoologist 36: 402–434. [Google Scholar]
- Kingslover JG, Daniel TL. 1983. Mechanical determination of nectar feeding strategy in hummingbirds: energetics, tongue morphology, and licking behavior. Oecologia 60: 214–226. [DOI] [PubMed] [Google Scholar]
- Kraemer M. 1998. Struktur und Dynamik der Pflanzen-Kolibri-Gemeinschaft von Bajo Calama, Kolumbien. Doctoral Thesis, University of Bonn, Germany.
- Ladio AH, Aizen MA. 1999. Early reproductive failure increases nectar production and pollination success of late flowers in Alstroemeria aurea (Alstromeriaceae). Oecologia 120: 235–241. [DOI] [PubMed] [Google Scholar]
- Lasso E, Naranjo ME 2003. Effect of pollinators and nectar robbers on nectar production and pollen deposition in Hamelia patens (Rubiaceae). Biotropica 35: 57–66. [Google Scholar]
- McDade LA, Weeks J. 2004a Nectar in hummingbird-pollinated Neotropical plants. I. Patterns of production and variability in 12 species. Biotropica 36: 196–215.
- McDade LA, Weeks J. 2004b Nectar in hummingbird-pollinated Neotropical plants. II. Interactions with flower visitors. Biotropica 36: 216–230.
- Machado IC, Loiola MI. 2000. Fly pollination and pollinator sharing in two synchronopatric species: Cordia multispicata (Boraginaceae) and Borreria alata (Rubiaceae). Revista Brasileira de Botânica 23: 305–311. [Google Scholar]
- Machado IC, Sazima I, Sazima M. 1998. Bat pollination of the terrestrial herb Irlbachia alata (Gentianaceae) in northeastern Brazil. Plant Systematics and Evolution 209: 231–237. [Google Scholar]
- Madsen JE, Øllgaard B. 1994. Floristic composition, structure, and dynamics of an upper montane forest in southern Ecuador. Nordic Journal of Botany 14: 403–423. [Google Scholar]
- Manetas Y, Petropoulou Y. 2000. Nectar amount, pollinator visit duration and pollination success in the Mediterranean shrub Cistus creticus. Annals of Botany 86: 815–820. [Google Scholar]
- Mantel N. 1967. The detection of disease clustering and generalized regression approach. Cancer Research 27: 209–220. [PubMed] [Google Scholar]
- Martínez del Rio C, Baker HG, Baker I. 1992. Ecological and evolutionary implications of digestive processes: bird preferences and sugar constituents of floral nectar and fruit pulp. Experimentia 48: 544–551. [Google Scholar]
- Matt F. 2001. Pflanzenbesuchende Fledermäuse im tropischen Bergregenwald: Diversität, Einnischung und Gildenstruktur. Doctoral Thesis, University of Erlangen-Nürnberg, Germany.
- Morisita M. 1959. Measuring interspecific association and similarity between communities. Memoirs of the Faculty of Science, Kyushu University, Series E (Biology) 3: 65–80. [Google Scholar]
- Nicolson SW, Fleming PA. 2003. Nectar as food for birds: the physiological consequences of drinking dilute sugar solutions. Plant Systematics and Evolution 238: 139–153. [Google Scholar]
- Pacini E, Nepi M, Vesprini JL. 2003. Nectary biodiversity: a short review. Plant Systematics and Evolution 238: 7–21. [Google Scholar]
- Paulsch A. 2002. Development and application of a classification system for undisturbed and disturbed tropical montane forests based on vegetation structure. Doctoral Thesis, University of Bayreuth, Germany.
- Perret M, Chautems A, Spichiger R, Peixoto M, Savolainen V. 2001. Nectar sugar composition in relation to pollination syndromes in Sinningieae (Gesneriaceae). Annals of Botany 87: 267–273. [DOI] [PubMed] [Google Scholar]
- Petanidou T, Smets E. 1995. The potential of marginal lands for bees and apiculture – nectar secretion in Mediterranean shrublands. Apidologie 26: 39–52. [Google Scholar]
- Pombal ECP, Morellato LPC. 1995. Polinização por moscas em Dendropanax cuneatum Decne. & Planch. (Araliaceae) em floresta semidecídua no sudeste do Brasil. Revista Brasileira de Botânica 18: 157–162. [Google Scholar]
- Proctor M, Yeo P, Lack A. 1996. The natural history of pollination. Portland, OH: Timber Press.
- Pyke GH, Waser NM. 1981. The production of dilute nectars by hummingbird and honeyeater flowers. Biotropica 13: 260–270. [Google Scholar]
- Rathcke BJ. 1992. Nectar distributions, pollinator behavior, and plant reproductive success. In: Hunter MD, Ohgushi T, Price PW, eds. Effects of resource distribution on animal–plant interactions. San Diego, CA: Academic Press, 113–138.
- Real LA, Caraco T. 1986. Risk and foraging in stochastic environments: theory and evidence. Annual Review of Ecology and Systematics 17: 371–390. [Google Scholar]
- Roberts WM. 1996. Hummingbirds' nectar concentration preferences at low volume: the importance of time scale. Animal Behaviour 52: 361–370. [Google Scholar]
- Roces F, Winter Y, Helversen O v. 1993. Nectar concentration preference and water balance in Glossophaga soricina antillarum. In: Barthlott W, Naumann CM, Schmidt-Loske K, Schumann KL, eds. Animal–plant interaction in tropical environments. Bonn: Zoologisches Forschungsinstitut und Museum Alexander König, 159–165.
- Sazima I, Buzato S, Sazima M. 1996. An assemblage of hummingbird-pollinated flowers in a montane forest in southeastern Brazil. Botanica Acta 109: 149–160. [Google Scholar]
- Sazima M, Buzato S, Sazima I. 1999. Bat-pollinated flower assemblages and bat visitors at two Atlantic forest sites in Brazil. Annals of Botany 83: 705–712. [Google Scholar]
- Schmitt U. 2000. Die Pflanzen-Kolibri-Gemeinschaft im Bergregenwald der Farallones de Cali, Reserva Natural Hato viejo, Kolumbien. Doctoral Thesis, University of Bonn, Germany.
- Schwerdtfeger M. 1996. Die Nektarzusammensetzung der Asteridae und ihre Beziehung zu Blütenökologie und Systematik. Doctoral Thesis, University of Göttingen, Germany.
- Shafir S, Bechar A, Weber EU. 2003. Cognition-mediated coevolution—context-dependent evaluations and sensitivity of pollinators to variability in nectar rewards. Plant Systematics and Evolution 238: 195–209. [Google Scholar]
- Siegel S, Castellan NJ. 1988. Nonparametric statistics for the behavioural sciences. New York, NY: McGraw-Hill.
- Simpson BB, Neff JL. 1983. Evolution and diversity of floral rewards. In: Jones CE, Little RJ, eds. Handbook of experimental pollination biology. New York, NY: Van Nostrand Reinhold, 142–159.
- Stiles FG. 1976. Taste preferences, color preferences, and flower choice in hummingbirds. Condor 78: 10–26. [Google Scholar]
- Stiles FG, Freeman CE. 1993. Patterns in floral nectar characteristics of some bird-visited plant species from Costa Rica. Biotropica 25: 191–205. [Google Scholar]
- Struwe L, Kadereit J, Klackenberg J, Nilsson S, Thiv M, von Hagen KB. 2002. Systematics, character evolution, and biogeography of Gentianaceae, including a new tribal and subtribal classification. In: Struwe L, Albert VA, eds. Gentianaceae—systematics and natural history. Cambridge: Cambridge University Press, 21–309.
- Tamm S, Gass CL. 1986. Energy intake rates and nectar concentration preferences by hummingbirds. Oecologia 70:20–23. [DOI] [PubMed] [Google Scholar]
- Torres C, Galetto L. 2002. Are nectar sugar composition and corolla tube length related to the diversity of insects that visit Asteraceae flowers? Plant Biology 4: 360–366. [Google Scholar]
- Trueblood DD, Gallagher ED, Gould SM. 1994. Three stages of seasonal succession on the Savin Hill Cove mudflat, Boston Harbor. Limnology and Oceanography 39: 1440–1454. [Google Scholar]
- Tschapka M. 2004. Energy density patterns of nectar resources permit coexistence within a guild of Neotropical flower-visiting bats. Journal of Zoology 263: 7–21. [Google Scholar]
- Van Wyk BE. 1993. Nectar sugar composition in Southern African Papilionoideae (Fabaceae). Biochemical Systematics and Ecology 21: 271–277. [Google Scholar]
- Vogel S. 1969. Chiropterophilie in der neotropischen Flora (Neue Mitteilungen II) Flora 158: 185–222. [Google Scholar]
- Waser NM, Pyke, GH. 1981. The production of dilute nectars by hummingbird and honeyeater flowers. Biotropica 13: 260–270. [Google Scholar]
- Weast RC. 1969. Handbook of chemistry and physics, 50th (1969–1970) edition. Cleveland, OH: The Chemical Rubber Co., D-207–208.
- Webster GL, Rhode RM. 2001. Plant diversity of an Andean cloud forest. Checklist of vascular flora of Maquipucuna, Ecuador. University of California publications in Botany. Berkeley and Los Angeles, CA: University of California Press.
- Witt T, Jürgens A, Geyer R, Gottsberger G. 1999. Nectar dynamics and sugar composition in flowers of Silene and Saponaria species (Caryophyllaceae). Plant Biology 1: 334–345. [Google Scholar]
- Wolda H. 1981. Similarity indices, sampling size and diversity. Oecologia 50: 296–302. [DOI] [PubMed] [Google Scholar]
- Wolda H. 1983. Diversity, diversity indices and tropical cockroaches. Oecologia 58: 290–298. [DOI] [PubMed] [Google Scholar]
- Wolff D, Braun M, Liede S. 2003. Nocturnal versus diurnal pollination success in Isertia laevis (Rubiaceae): a sphingophilous plant visited by hummingbirds. Plant Biology 5: 71–78. [Google Scholar]
- Wolff D, Witt T, Jürgens A, Gottsberger G. 2006. Nectar dynamics and reproductive success in Saponaria officinalis (Caryophyllaceae) in southern Germany. Flora (in press).
- Wolff D, Liede-Schumann S. 2006. Evolution of flower morphology, pollen dimorphism, and nectar composition in Arcytophyllum, a distylous genus of Rubiaceae. Organisms, Diversity and Evolution (in press).
- Zimmermann M. 1988. Nectar production, flowering phenology and strategies for pollination. In: Lovett Doust J, Lovett Doust L, eds. Plant reproductive ecology. New York: Oxford University Press, 157–178.